Tsc1 Loss in VIP-Lineage Cortical Interneurons Results in More VIP+ Interneurons and Enhanced Excitability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antibodies
2.3. Dual RNAscope (Fluorescent In Situ Hybridization) and IHC Labeling
2.4. Cell and Synapse Counting
2.5. Electrophysiology
2.6. Immunohistochemistry
2.7. In Situ Hybridization
2.8. Image Acquisition and Analysis
2.9. Statistics
3. Results
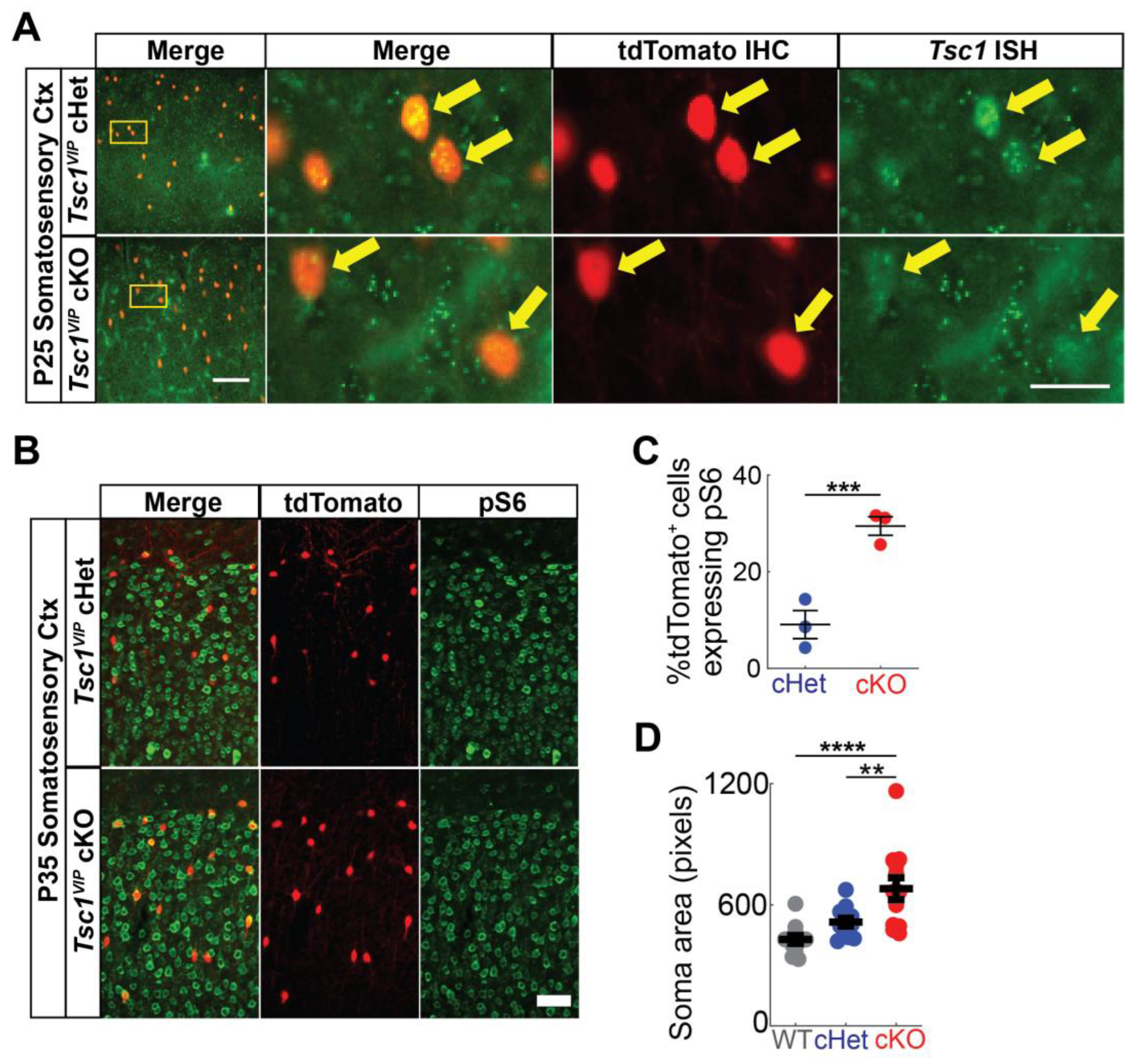
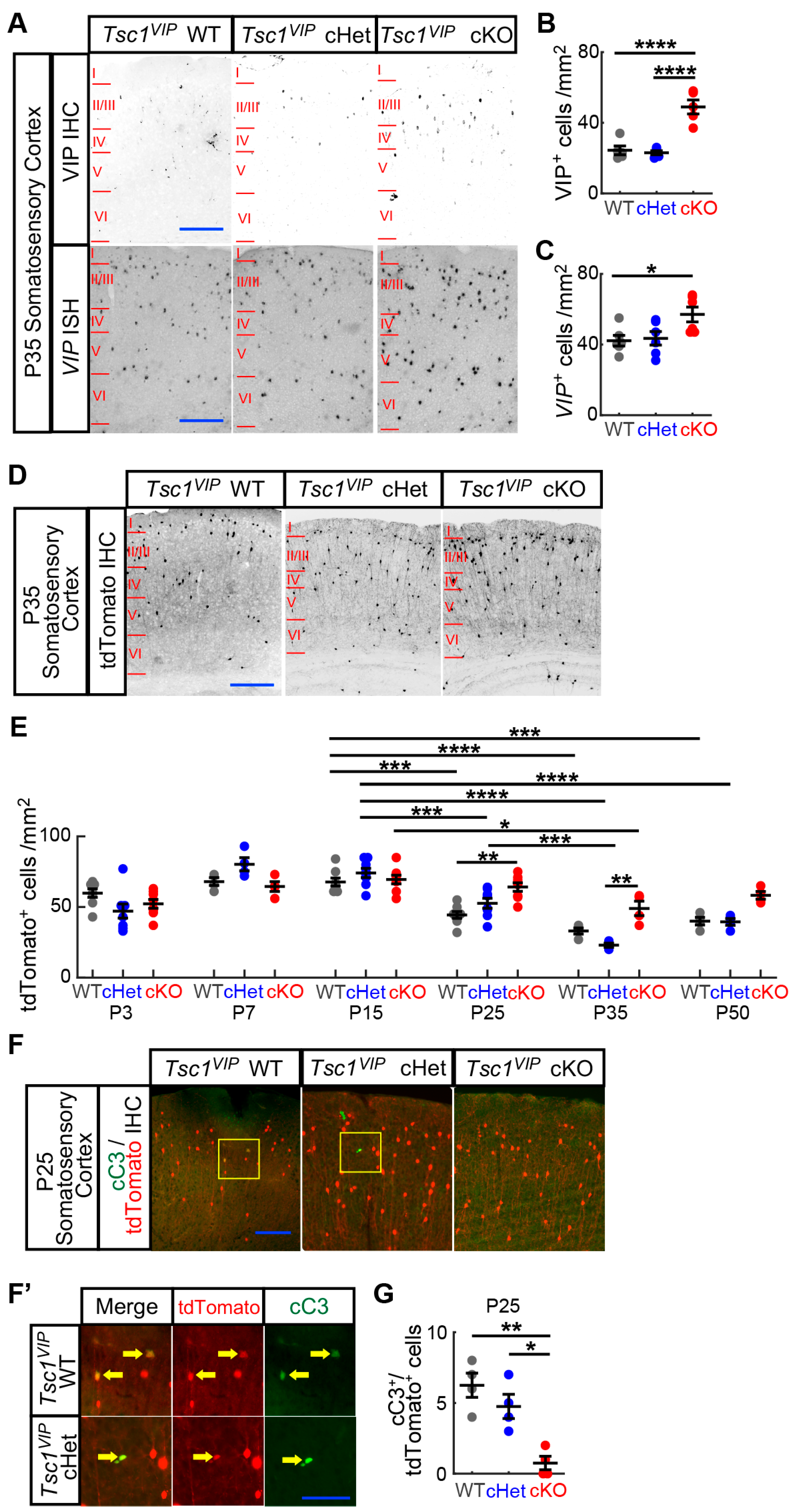
3.1. Conditional Deletion of Tsc1 Increases VIP+ CIN Density in Postnatal Cortex
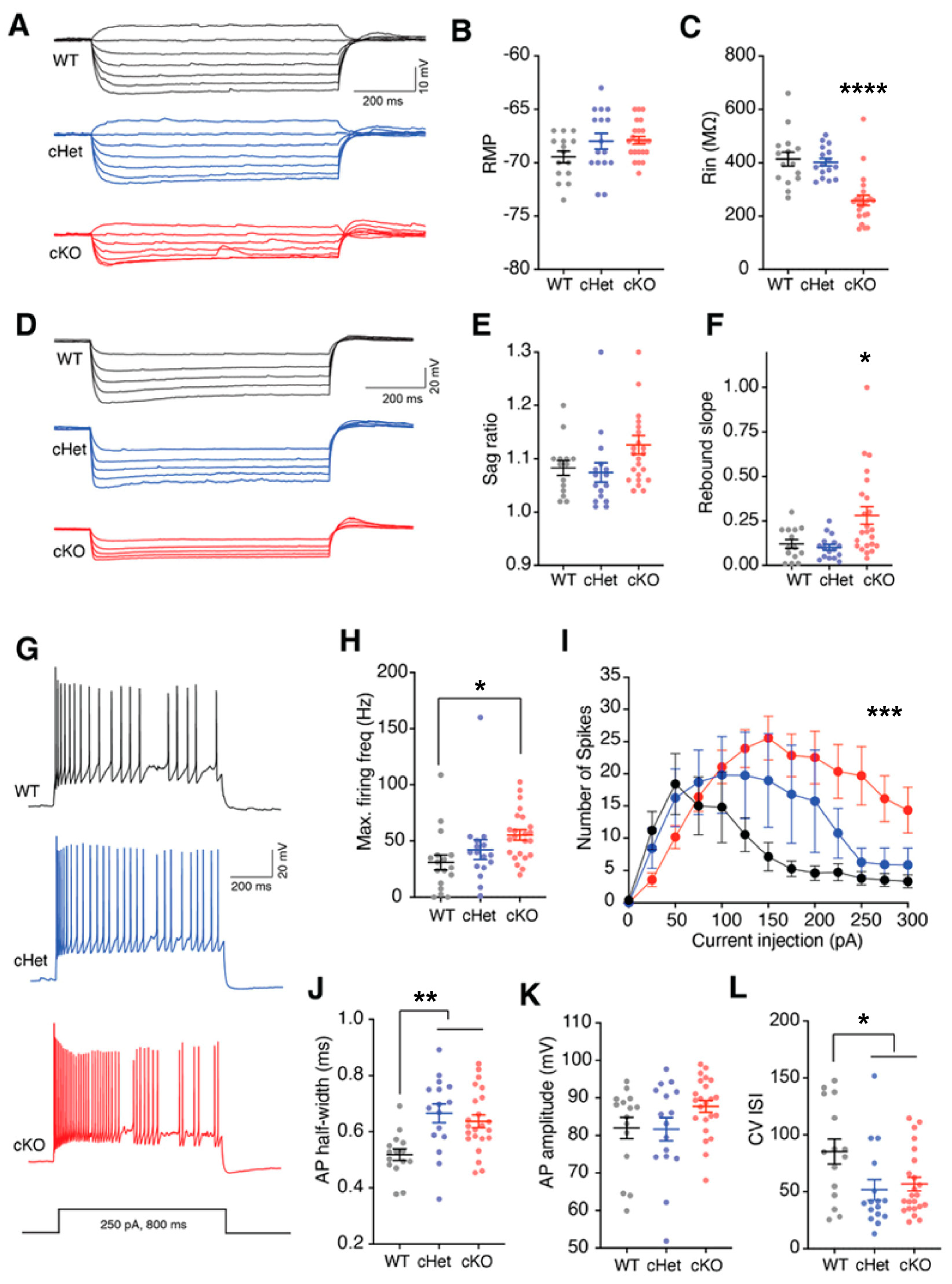
3.2. Conditional Deletion of Tsc1 Affects the Intrinsic and Synaptic Properties of VIP+ CINs
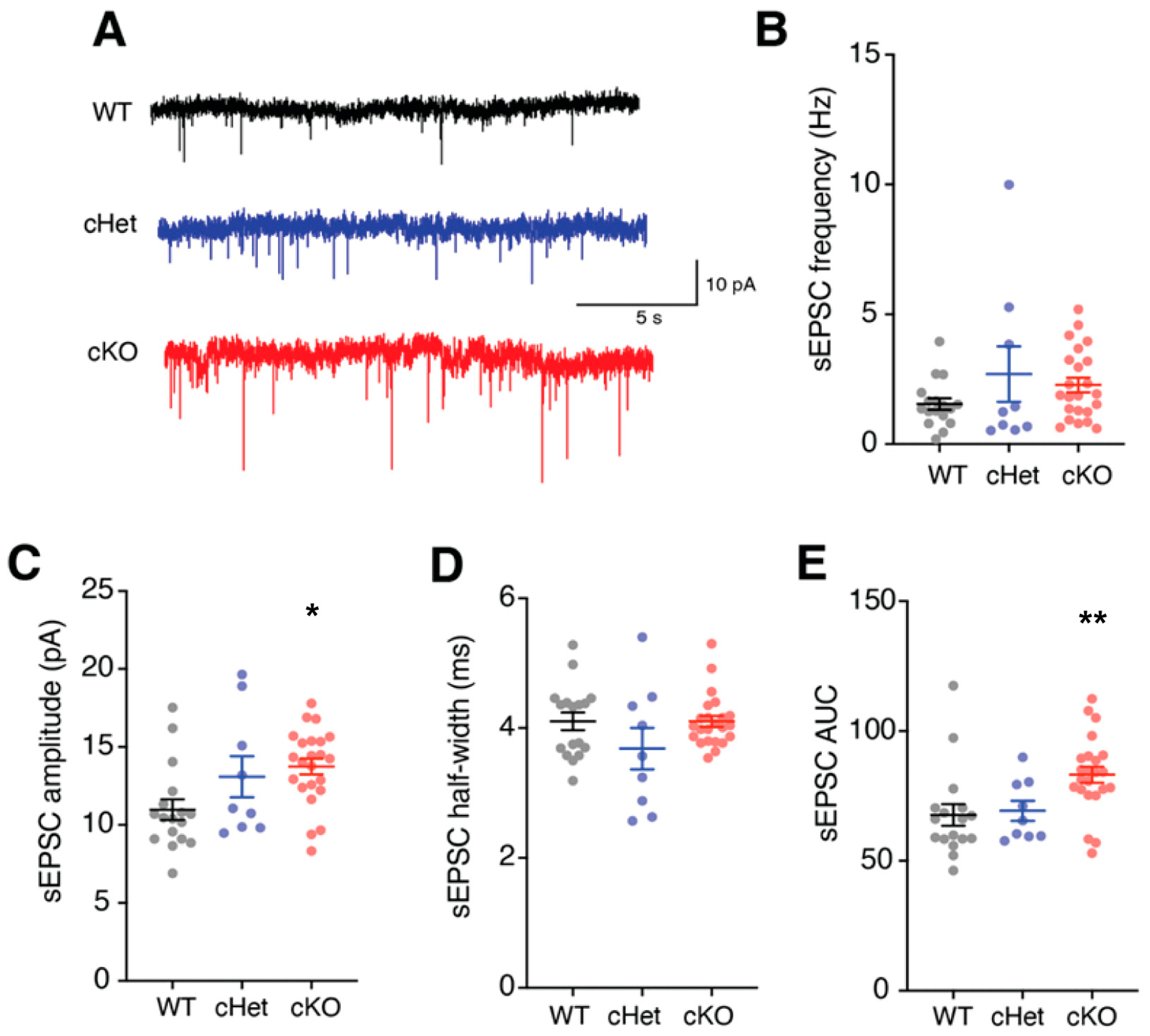
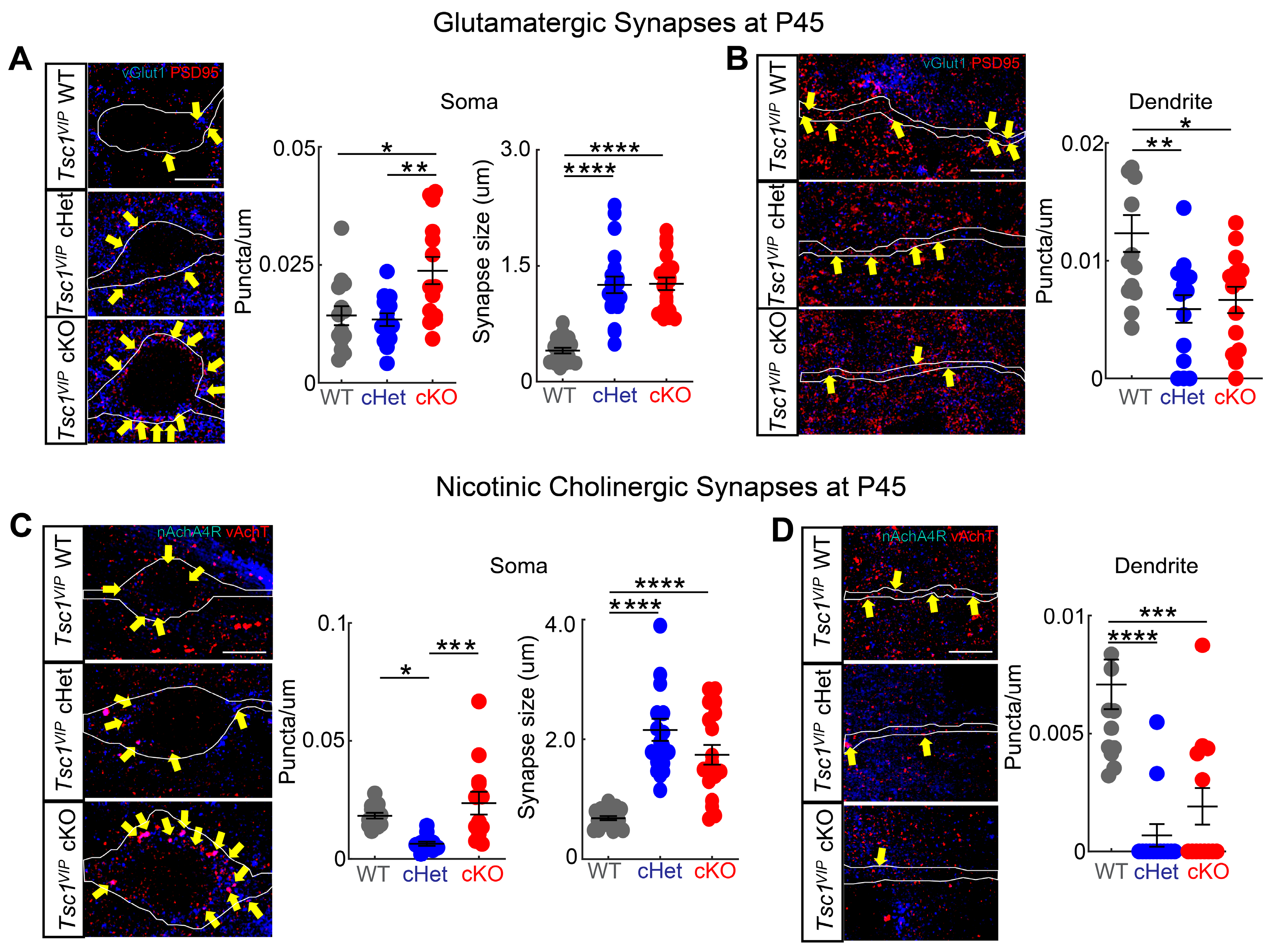
3.3. Conditional Deletion of Tsc1 Affects Synaptic Inputs onto VIP+ CINs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Chromosome 16 Tuberous Sclerosis Consortium Identification and Characterization of the Tuberous Sclerosis Gene on Chromosome 16. Cell 1993, 75, 1305–1315. [CrossRef]
- van Slegtenhorst, M.; de Hoogt, R.; Hermans, C.; Nellist, M.; Janssen, B.; Verhoef, S.; Lindhout, D.; van den Ouweland, A.; Halley, D.; Young, J.; et al. Identification of the Tuberous Sclerosis Gene TSC1 on Chromosome 9q34. Science 1997, 277, 805–808. [Google Scholar] [CrossRef]
- Curatolo, P.; Moavero, R.; de Vries, P.J. Neurological and Neuropsychiatric Aspects of Tuberous Sclerosis Complex. Lancet Neurol. 2015, 14, 733–745. [Google Scholar] [CrossRef]
- Winterkorn, E.B.; Pulsifer, M.B.; Thiele, E.A. Cognitive Prognosis of Patients with Tuberous Sclerosis Complex. Neurology 2007, 68, 62–64. [Google Scholar] [CrossRef]
- Chu-Shore, C.J.; Major, P.; Camposano, S.; Muzykewicz, D.; Thiele, E.A. The Natural History of Epilepsy in Tuberous Sclerosis Complex. Epilepsia 2010, 51, 1236–1241. [Google Scholar] [CrossRef]
- Numis, A.L.; Major, P.; Montenegro, M.A.; Muzykewicz, D.A.; Pulsifer, M.B.; Thiele, E.A. Identification of Risk Factors for Autism Spectrum Disorders in Tuberous Sclerosis Complex. Neurology 2011, 76, 981–987. [Google Scholar] [CrossRef]
- Spurling Jeste, S.; Wu, J.Y.; Senturk, D.; Varcin, K.; Ko, J.; McCarthy, B.; Shimizu, C.; Dies, K.; Vogel-Farley, V.; Sahin, M.; et al. Early Developmental Trajectories Associated with ASD in Infants with Tuberous Sclerosis Complex. Neurology 2014, 83, 160–168. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Xu, T.; Guan, K.-L. Rheb GTPase Is a Direct Target of TSC2 GAP Activity and Regulates mTOR Signaling. Genes Dev. 2003, 17, 1829–1834. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- O’Roak, B.J.; Vives, L.; Fu, W.; Egertson, J.D.; Stanaway, I.B.; Phelps, I.G.; Carvill, G.; Kumar, A.; Lee, C.; Ankenman, K.; et al. Multiplex Targeted Sequencing Identifies Recurrently Mutated Genes in Autism Spectrum Disorders. Science 2012, 338, 1619–1622. [Google Scholar] [CrossRef]
- Richards, C.; Jones, C.; Groves, L.; Moss, J.; Oliver, C. Prevalence of Autism Spectrum Disorder Phenomenology in Genetic Disorders: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2015, 2, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Kepecs, A.; Fishell, G. Interneuron Cell Types Are Fit to Function. Nature 2014, 505, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kessaris, N.; Magno, L.; Rubin, A.N.; Oliveira, M.G. Genetic Programs Controlling Cortical Interneuron Fate. Curr. Opin. Neurobiol. 2014, 26, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.S.; Vogt, D.; Sandberg, M.; Rubenstein, J.L. Cortical Interneuron Development: A Tale of Time and Space. Development 2017, 144, 3867–3878. [Google Scholar] [CrossRef] [PubMed]
- Wonders, C.P.; Anderson, S.A. The Origin and Specification of Cortical Interneurons. Nat. Rev. Neurosci. 2006, 7, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, G.; Hjerling-Leffler, J.; Karayannis, T.; Sousa, V.H.; Butt, S.J.B.; Battiste, J.; Johnson, J.E.; Machold, R.P.; Fishell, G. Genetic Fate Mapping Reveals That the Caudal Ganglionic Eminence Produces a Large and Diverse Population of Superficial Cortical Interneurons. J. Neurosci. 2010, 30, 1582–1594. [Google Scholar] [CrossRef]
- Abs, E.; Poorthuis, R.B.; Apelblat, D.; Muhammad, K.; Pardi, M.B.; Enke, L.; Kushinsky, D.; Pu, D.-L.; Eizinger, M.F.; Conzelmann, K.-K.; et al. Learning-Related Plasticity in Dendrite-Targeting Layer 1 Interneurons. Neuron 2018, 100, 684–699.e6. [Google Scholar] [CrossRef]
- Huang, Z.J.; Di Cristo, G.; Ango, F. Development of GABA Innervation in the Cerebral and Cerebellar Cortices. Nat. Rev. Neurosci. 2007, 8, 673–686. [Google Scholar] [CrossRef]
- Pfeffer, C.K.; Xue, M.; He, M.; Huang, Z.J.; Scanziani, M. Inhibition of Inhibition in Visual Cortex: The Logic of Connections between Molecularly Distinct Interneurons. Nat. Neurosci. 2013, 16, 1068–1076. [Google Scholar] [CrossRef]
- Pi, H.-J.; Hangya, B.; Kvitsiani, D.; Sanders, J.I.; Huang, Z.J.; Kepecs, A. Cortical Interneurons That Specialize in Disinhibitory Control. Nature 2013, 503, 521–524. [Google Scholar] [CrossRef]
- Malik, R.; Pai, E.L.-L.; Rubin, A.N.; Stafford, A.M.; Angara, K.; Minasi, P.; Rubenstein, J.L.; Sohal, V.S.; Vogt, D. Tsc1 Represses Parvalbumin Expression and Fast-Spiking Properties in Somatostatin Lineage Cortical Interneurons. Nat. Commun. 2019, 10, 4994. [Google Scholar] [CrossRef] [PubMed]
- Wundrach, D.; Martinetti, L.E.; Stafford, A.M.; Bilinovich, S.M.; Angara, K.; Prokop, J.W.; Crandall, S.R.; Vogt, D. A Human TSC1 Variant Screening Platform in Gabaergic Cortical Interneurons for Genotype to Phenotype Assessments. Front. Mol. Neurosci. 2020, 13, 573409. [Google Scholar] [CrossRef] [PubMed]
- Southwell, D.G.; Paredes, M.F.; Galvao, R.P.; Jones, D.L.; Froemke, R.C.; Sebe, J.Y.; Alfaro-Cervello, C.; Tang, Y.; Garcia-Verdugo, J.M.; Rubenstein, J.L.; et al. Intrinsically Determined Cell Death of Developing Cortical Interneurons. Nature 2012, 491, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A Robust and High-Throughput Cre Reporting and Characterization System for the Whole Mouse Brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, D.J.; Zhang, H.; Bandura, J.L.; Heiberger, K.M.; Glogauer, M.; el-Hashemite, N.; Onda, H. A Mouse Model of TSC1 Reveals Sex-Dependent Lethality from Liver Hemangiomas, and up-Regulation of p70S6 Kinase Activity in Tsc1 Null Cells. Hum. Mol. Genet. 2002, 11, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; He, M.; Wu, P.; Kim, S.; Paik, R.; Sugino, K.; Kvitsiani, D.; Kvitsani, D.; Fu, Y.; Lu, J.; et al. A Resource of Cre Driver Lines for Genetic Targeting of GABAergic Neurons in Cerebral Cortex. Neuron 2011, 71, 995–1013. [Google Scholar] [CrossRef] [PubMed]
- Stanco, A.; Pla, R.; Vogt, D.; Chen, Y.; Mandal, S.; Walker, J.; Hunt, R.F.; Lindtner, S.; Erdman, C.A.; Pieper, A.A.; et al. NPAS1 Represses the Generation of Specific Subtypes of Cortical Interneurons. Neuron 2014, 84, 940–953. [Google Scholar] [CrossRef]
- Corteen, N.L.; Cole, T.M.; Sarna, A.; Sieghart, W.; Swinny, J.D. Localization of GABA-A Receptor Alpha Subunits on Neurochemically Distinct Cell Types in the Rat Locus Coeruleus. Eur. J. Neurosci. 2011, 34, 250–262. [Google Scholar] [CrossRef]
- Hoch, R.V.; Clarke, J.A.; Rubenstein, J.L.R. Fgf Signaling Controls the Telencephalic Distribution of Fgf-Expressing Progenitors Generated in the Rostral Patterning Center. Neural Dev. 2015, 10, 8. [Google Scholar] [CrossRef]
- Hoch, R.V.; Lindtner, S.; Price, J.D.; Rubenstein, J.L.R. OTX2 Transcription Factor Controls Regional Patterning within the Medial Ganglionic Eminence and Regional Identity of the Septum. Cell Rep. 2015, 12, 482–494. [Google Scholar] [CrossRef]
- Gingras, A.C.; Raught, B.; Gygi, S.P.; Niedzwiecka, A.; Miron, M.; Burley, S.K.; Polakiewicz, R.D.; Wyslouch-Cieszynska, A.; Aebersold, R.; Sonenberg, N. Hierarchical Phosphorylation of the Translation Inhibitor 4E-BP1. Genes Dev. 2001, 15, 2852–2864. [Google Scholar] [CrossRef] [PubMed]
- Guet-McCreight, A.; Skinner, F.K.; Topolnik, L. Common Principles in Functional Organization of VIP/Calretinin Cell-Driven Disinhibitory Circuits Across Cortical Areas. Front. Neural Circuits 2020, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.L.; Gallopin, T.; Férézou, I.; Cauli, B.; Rossier, J.; Schweitzer, P.; Lambolez, B. Functional CB1 Receptors Are Broadly Expressed in Neocortical GABAergic and Glutamatergic Neurons. J. Neurophysiol. 2007, 97, 2580–2589. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.T.; Cauli, B.; Staiger, J.F.; Lambolez, B.; Rossier, J.; Audinat, E. Properties of Bipolar VIPergic Interneurons and Their Excitation by Pyramidal Neurons in the Rat Neocortex. Eur. J. Neurosci. 1998, 10, 3617–3628. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, R.; Guet-McCreight, A.; Amalyan, S.; Hui, C.W.; Topolnik, D.; Michaud, F.; Marino, B.; Tremblay, M.-È.; Skinner, F.K.; Topolnik, L. Alterations in Intrinsic and Synaptic Properties of Hippocampal CA1 VIP Interneurons During Aging. Front. Cell Neurosci. 2020, 14, 554405. [Google Scholar] [CrossRef] [PubMed]
- Tavazoie, S.F.; Alvarez, V.A.; Ridenour, D.A.; Kwiatkowski, D.J.; Sabatini, B.L. Regulation of Neuronal Morphology and Function by the Tumor Suppressors Tsc1 and Tsc2. Nat. Neurosci. 2005, 8, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.; Ge, H.; Dimitroff, B.D.; Ren, Y.; Howe, K.A.; Arsham, A.M.; Easterday, M.C.; Neufeld, T.P.; O’Connor, M.B.; Selleck, S.B. Mechanisms of TSC-Mediated Control of Synapse Assembly and Axon Guidance. PLoS ONE 2007, 2, e375. [Google Scholar] [CrossRef]
- Bateup, H.S.; Takasaki, K.T.; Saulnier, J.L.; Denefrio, C.L.; Sabatini, B.L. Loss of Tsc1 in Vivo Impairs Hippocampal mGluR-LTD and Increases Excitatory Synaptic Function. J. Neurosci. 2011, 31, 8862–8869. [Google Scholar] [CrossRef]
- Karnani, M.M.; Jackson, J.; Ayzenshtat, I.; Hamzehei Sichani, A.; Manoocheri, K.; Kim, S.; Yuste, R. Opening Holes in the Blanket of Inhibition: Localized Lateral Disinhibition by VIP Interneurons. J. Neurosci. 2016, 36, 3471–3480. [Google Scholar] [CrossRef]
- Mossner, J.M.; Batista-Brito, R.; Pant, R.; Cardin, J.A. Developmental Loss of MeCP2 from VIP Interneurons Impairs Cortical Function and Behavior. Elife 2020, 9, e55639. [Google Scholar] [CrossRef] [PubMed]
- Batista-Brito, R.; Vinck, M.; Ferguson, K.A.; Chang, J.T.; Laubender, D.; Lur, G.; Mossner, J.M.; Hernandez, V.G.; Ramakrishnan, C.; Deisseroth, K.; et al. Developmental Dysfunction of VIP Interneurons Impairs Cortical Circuits. Neuron 2017, 95, 884–895.e9. [Google Scholar] [CrossRef] [PubMed]
- Priya, R.; Paredes, M.F.; Karayannis, T.; Yusuf, N.; Liu, X.; Jaglin, X.; Graef, I.; Alvarez-Buylla, A.; Fishell, G. Activity Regulates Cell Death within Cortical Interneurons through a Calcineurin-Dependent Mechanism. Cell Rep. 2018, 22, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.K.; Selten, M.; Rosés-Novella, C.; Sreenivasan, V.; Pallas-Bazarra, N.; Serafeimidou-Pouliou, E.; Hanusz-Godoy, A.; Oozeer, F.; Edwards, R.; Marín, O. Serotonergic Regulation of Bipolar Cell Survival in the Developing Cerebral Cortex. Cell Rep. 2022, 40, 111037. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Liao, J.; Li, Q.; Shi, J.; Zhang, H.; Guo, J.; Han, Q.; Hu, L.; Li, Y.; Pan, J.; et al. Copper Induces Mitochondria-Mediated Apoptosis via AMPK-mTOR Pathway in Hypothalamus of Pigs. Ecotoxicol. Environ. Saf. 2021, 220, 112395. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Yue, P.; Deng, X.; Khuri, F.R.; Sun, S.-Y. mTOR Complex 2 Stabilizes Mcl-1 Protein by Suppressing Its Glycogen Synthase Kinase 3-Dependent and SCF-FBXW7-Mediated Degradation. Mol. Cell. Biol. 2015, 35, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.C.; Holt, C.E. Local Translation and Directional Steering in Axons. EMBO J. 2007, 26, 3729–3736. [Google Scholar] [CrossRef] [PubMed]
- Raab-Graham, K.F.; Haddick, P.C.G.; Jan, Y.N.; Jan, L.Y. Activity- and mTOR-Dependent Suppression of Kv1.1 Channel mRNA Translation in Dendrites. Science 2006, 314, 144–148. [Google Scholar] [CrossRef]
- Meikle, L.; Talos, D.M.; Onda, H.; Pollizzi, K.; Rotenberg, A.; Sahin, M.; Jensen, F.E.; Kwiatkowski, D.J. A Mouse Model of Tuberous Sclerosis: Neuronal Loss of Tsc1 Causes Dysplastic and Ectopic Neurons, Reduced Myelination, Seizure Activity, and Limited Survival. J. Neurosci. 2007, 27, 5546–5558. [Google Scholar] [CrossRef]
- Karalis, V.; Caval-Holme, F.; Bateup, H.S. Raptor Downregulation Rescues Neuronal Phenotypes in Mouse Models of Tuberous Sclerosis Complex. Nat. Commun. 2022, 13, 4665. [Google Scholar] [CrossRef]
- Zhao, J.-P.; Yoshii, A. Hyperexcitability of the Local Cortical Circuit in Mouse Models of Tuberous Sclerosis Complex. Mol. Brain 2019, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Haji, N.; Riebe, I.; Aguilar-Valles, A.; Artinian, J.; Laplante, I.; Lacaille, J.-C. Tsc1 Haploinsufficiency in Nkx2.1 Cells Upregulates Hippocampal Interneuron mTORC1 Activity, Impairs Pyramidal Cell Synaptic Inhibition, and Alters Contextual Fear Discrimination and Spatial Working Memory in Mice. Mol. Autism 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.S.; Malik, R.; Sohal, V.S.; Rubenstein, J.L.; Vogt, D. Tsc1 Loss in VIP-Lineage Cortical Interneurons Results in More VIP+ Interneurons and Enhanced Excitability. Cells 2024, 13, 52. https://doi.org/10.3390/cells13010052
Hu JS, Malik R, Sohal VS, Rubenstein JL, Vogt D. Tsc1 Loss in VIP-Lineage Cortical Interneurons Results in More VIP+ Interneurons and Enhanced Excitability. Cells. 2024; 13(1):52. https://doi.org/10.3390/cells13010052
Chicago/Turabian StyleHu, Jia Sheng, Ruchi Malik, Vikaas S. Sohal, John L. Rubenstein, and Daniel Vogt. 2024. "Tsc1 Loss in VIP-Lineage Cortical Interneurons Results in More VIP+ Interneurons and Enhanced Excitability" Cells 13, no. 1: 52. https://doi.org/10.3390/cells13010052
APA StyleHu, J. S., Malik, R., Sohal, V. S., Rubenstein, J. L., & Vogt, D. (2024). Tsc1 Loss in VIP-Lineage Cortical Interneurons Results in More VIP+ Interneurons and Enhanced Excitability. Cells, 13(1), 52. https://doi.org/10.3390/cells13010052





