Imaging of Light-Enhanced Extracellular Vesicle-Mediated Delivery of Oxaliplatin to Colorectal Cancer Cells via Laser Ablation, Inductively Coupled Plasma Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. CRC Cell Culture
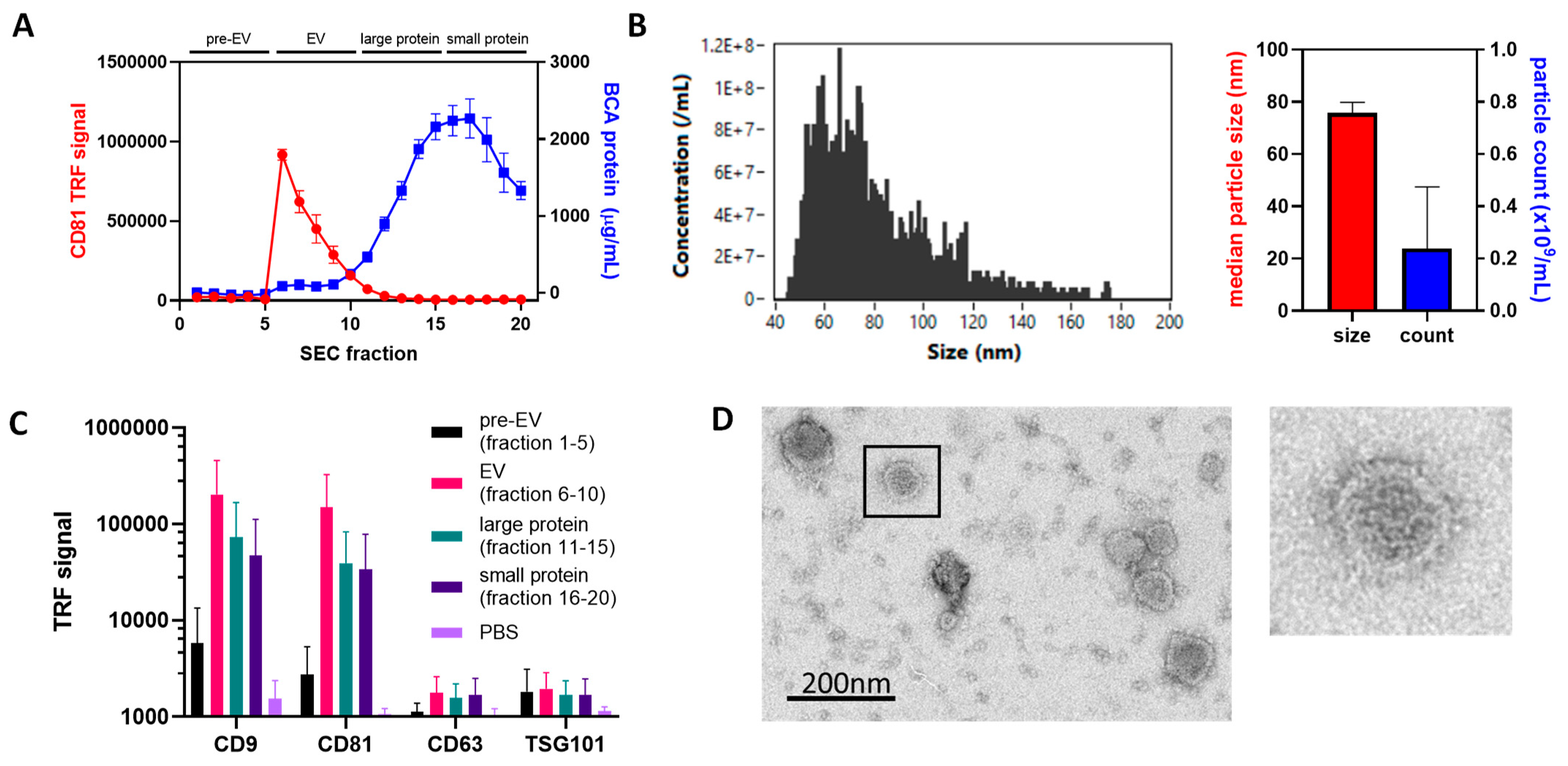
2.2. EV Isolation and Characterization
2.3. Chemotherapeutic Loading of EVs
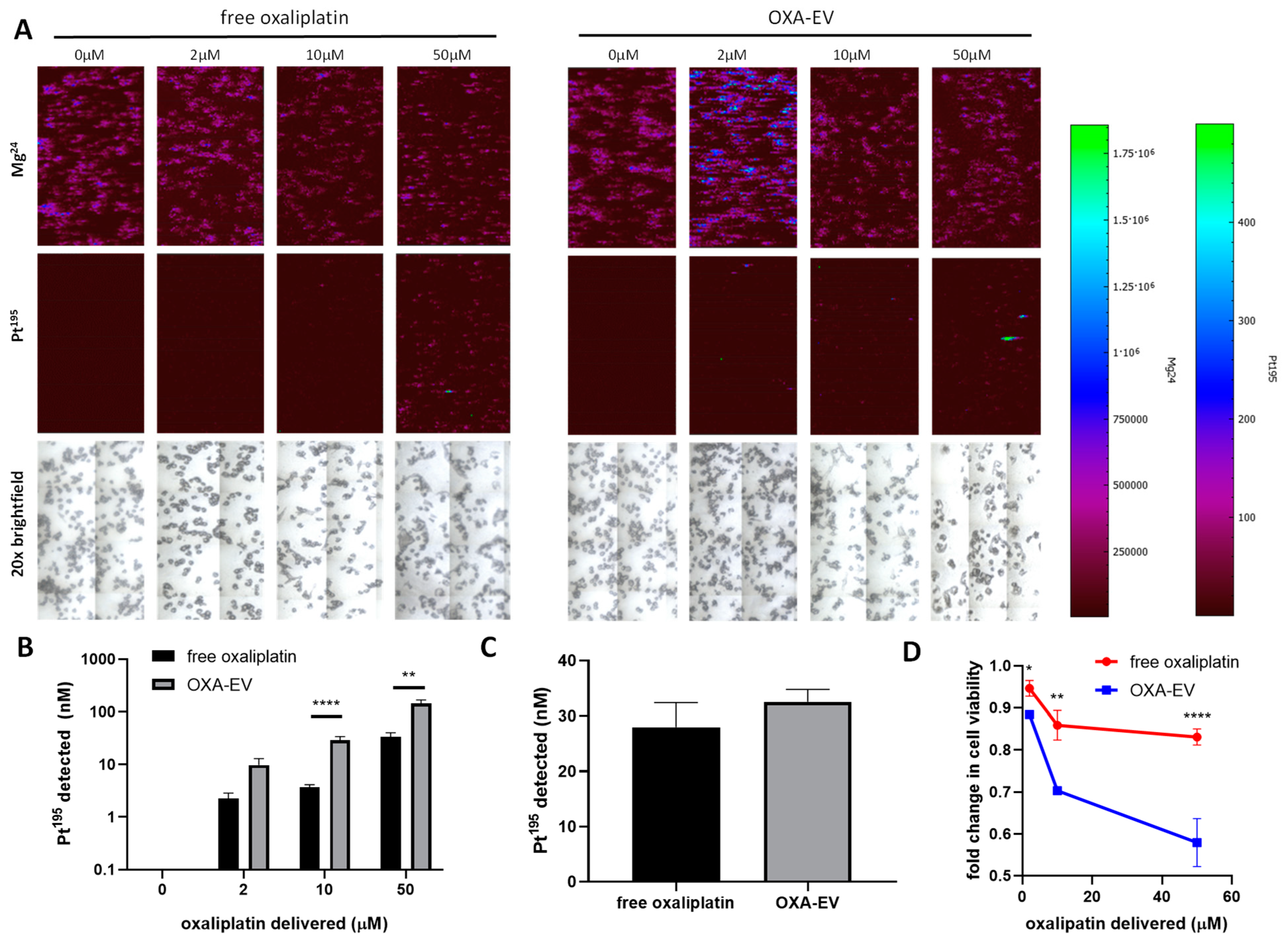
2.4. Cell Treatment and Drug Delivery Analysis
2.5. Cytotoxicity Assays
2.6. Statistical Analysis
3. Results
3.1. Isolation of EVs via SEC from SW480 Bioreactor Culture Produces EVs with Expected Size, Structure and Marker Profile
3.2. ICP-MS Shows Oxaliplatin Loading into EVs at Biologically Relevant Concentrations
3.3. LA-ICP-MS Imaging Indicates Enhanced Delivery of Oxaliplatin into CRC Cells
3.4. Loading of Porphyrins into EVs Is Concentration-Dependent and Biases towards Larger EVs
3.5. Light Exposure and C5 Both Alter Oxa-EV Delivery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Samuel, M.; Kumar, S.; Mathivanan, S. Ticket to a bubble ride: Cargo sorting into exosomes and extracellular vesicles. Biochem. Biophys. Acta—Proteins Proteom. 2019, 1867, 140203. [Google Scholar] [CrossRef]
- Yates, A.G.; Pink, R.C.; Erdbrugger, U.; Siljander, P.R.M.; Dellar, E.R.; Pantazi, P.; Akbar, N.; Cooke, W.R.; Vatish, M.; Dias-Neto, E.; et al. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo. J. Extracell. Vesicles 2022, 11, e12151. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Kotelevets, L.; Chastre, E. Extracellular Vesicles in Colorectal Cancer: From Tumor Growth and Metastasis to Biomarkers and Nanomedications. Cancers 2023, 15, 1107. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, L.; Perazzoli, G.; Pena, M.; Cepero, A.; Luque, C.; Melguizo, C.; Prados, J. Cancer therapy based on extracellular vesicles as drug delivery vehicles. J. Control. Release 2020, 327, 296–315. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przegląd Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Ko, C.Y. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J. Natl. Cancer Inst. 2004, 96, 1420–1425. [Google Scholar] [CrossRef]
- Van den Berg, I.; van den Braak, R.J.C.; van Vugt, J.L.A.; Ijzermans, J.N.M.; Buettner, S. Actual survival after resection of primary colorectal cancer: Results from a prospective multicenter study. World J. Surg. Oncol. 2021, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Sawayama, H.; Miyamoto, Y.; Hiyoshi, Y.; Ogawa, K.; Kato, R.; Akiyama, T.; Kiyozumi, Y.; Yoshida, N.; Baba, H. Overall survival after recurrence in stage I–III colorectal cancer patients in accordance with the recurrence organ site and pattern. Ann. Gastroenterol. Surg. 2021, 5, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, S.M.; Galjart, B.; Verhoef, C.; Slooter, G.D.; Koopman, M.; Verhoeven, R.H.A.; de Wilt, J.H.W.; van Erning, F.N. Disease recurrence after colorectal cancer surgery in the modern era: A population-based study. Int. J. Color. Dis. 2021, 36, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Cunningham, D. Adjuvant therapy in colon cancer—What, when and how? Ann. Oncol. 2006, 17, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.T.; Eng, C. Neoadjuvant Chemotherapy for Colon Cancer. Cancers 2020, 12, 2368. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.M.; Kuryba, A.; Cowling, T.E.; van der Meulen Fearnhead, N.S.; Walker, K.; Braun, M.S.; Aggarwal, A. Survival outcomes associated with completion of adjuvant oxaliplatin-based chemotherapy for stage III colon cancer: A national population-based study. Int. J. Cancer 2022, 150, 335–346. [Google Scholar] [CrossRef]
- Xu, M.; Chen, Q.; Peng, L.; Ding, L. Dendritic cell-derived exosome-entrapped fluorouracil can enhance its anti-colon cancer effect. J. BUON 2020, 25, 1413–1422. [Google Scholar]
- Go, G.; Park, H.J.; Lee, J.H.; Yun, C.W.; Lee, S.H. Inhibitory Effect of Oxaliplatin-loaded Engineered Milk Extracellular Vesicles on Tumor Progression. Anticancer Res. 2022, 42, 857–866. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhury, A.; Dehari, D.; Shekher, A.; Gupta, S.C.; Majumdar, S.; Krishnamurthy, S.; Singh, S.; Kumar, D.; Agrawal, A.K. Combination Therapy Comprising Paclitaxel and 5-Fluorouracil by Using Folic Acid Functionalized Bovine Milk Exosomes Improves the Therapeutic Efficacy against Breast Cancer. Life 2022, 12, 1143. [Google Scholar] [CrossRef]
- Shek, R.; Ahmad, A.; Tiwari, R.K.; Saeed, M.; Shukla, R.; Al-Thubiani, W.S.; Ansari, I.A.; Ashfaque, M.; Bajpai, P. High therapeutic efficacy of 5-Fluorouracil-loaded exosomes against colon cancer cells. Chem. Biol. Drug Des. 2023, 101, 962–976. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Salarpour, S.; Forootanfar, H.; Pournamdari, M.; Ahmadi-Zeidabadi, M.; Esmaeeli, M.; Pardakhty, A. Paclitaxel incorporated exosomes derived from glioblastoma cells: Comparative study of two loading techniques. Daru 2019, 27, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Y.; Wang, Q.; Cai, N.; Wu, L.; Yan, X. Single-particle assessment of six different drug-loading strategies for incorporating doxorubicin into small extracellular vesicles. Anal. Bioanal. Chem. 2023, 415, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Gomari, H.; Moghadam, M.F.; Soleimani, M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. OncoTargets Ther. 2018, 11, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.; Collinson, A.; Matthews, C.; Pointon, A.; Jenkinson, L.; Minter, R.R.; Vaughan, T.J.; Tigue, N.J. Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency. PLoS ONE 2019, 14, e0214545. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Tian, J.; Wang, Z.; Gao, Y.; Wu, X.; Ding, X.; Qiang, L.; Li, G.; Han, Z.; Yuan, Y.; et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Pullan, J.; Dailey, K.; Bhallamudi, S.; Feng, L.; Alhalhooly, L.; Froberg, J.; Osborn, J.; Sarkar, K.; Molden, T.; Sathish, V.; et al. Modified Bovine Milk Exosomes for Doxorubicin Delivery to Triple-Negative Breast Cancer Cells. ACS Appl. Bio Mater. 2022, 5, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Farhat, W.; Yeung, V.; Kahale, F.; Parekh, M.; Cortinas, J.; Chen, L.; Ross, A.E.; Ciolino, J.B. Doxorubicin-Loaded Extracellular Vesicles Enhance Tumor Cell Death in Retinoblastoma. Bioengineering 2022, 9, 671. [Google Scholar] [CrossRef]
- Mukhopadhya, A.; Tsiapalis, D.; McNamee, N.; Talbot, B.; O’Driscoll, L. Doxorubicin Loading into Milk and Mesenchymal Stem Cells’ Extracellular Vesicles as Drug Delivery Vehicles. Pharmaceutics 2023, 15, 718. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-encapsulated Paclitaxel to Overcome MDR in Cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Huang, Q.; Peng, C.; Yao, L.; Chen, H.; Qiu, Z.; Wu, Y.; Wang, L.; Chen, W. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics 2019, 9, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, K.; Zhang, T.; Gao, G.C.; Xu, H. Natural killer cell-derived exosome-entrapped paclitaxel can enhance its anti-tumor effect. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5703–5713. [Google Scholar]
- Kandimalla, R.; Aqil, F.; Alhakeem, S.S.; Jeyabalan, J.; Tyagi, N.; Agrawal, A.; Yan, J.; Spencer, W.; Bondada, S.; Gupta, R.C. Targeted Oral Delivery of Paclitaxel Using Colostrum-Derived Exosomes. Cancers 2021, 13, 3700. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, Y.; Hu, X.; Huang, S.; Xu, W.; Hao, X.; Zhou, M.; Wu, J.; Xiang, D. Paclitaxel-loaded hybrid exosome for targeted chemotherapy of triple-negative breast cancer. Res. Sq. 2022; preprint. [Google Scholar] [CrossRef]
- Sun, Z.; Zang, L.; Cheng, Y.; Qin, L. Cancer Exosome Loaded with Paclitaxel for Targeted Lung Cancer Therapy. J. Biomater. Tissue Eng. 2023, 13, 118–122. [Google Scholar] [CrossRef]
- Taffoli, G.; Hadla, M.; Corona, G.; Caligiuri, I.; Palazzolo, S.; Semeraro, S.; Gamini, A.; Canzonieri, V.; Rizzolio, F. Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine 2015, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Ip, V.; McKeage, M.J.; Thompson, P.; Damianovich, D.; Findlay, M.; Liu, J.J. Platinum-specific detection and quantification of oxaliplatin and Pt(R,R-diaminocyclohexane)Cl2 in the blood plasma of colorectal cancer patients. J. Anal. At. Spectrom. 2008, 23, 881–884. [Google Scholar] [CrossRef]
- Lavilla, I.; Costas, M.; Miguel, P.S.; Millos, J.; Bendicho, C. Elemental fingerprinting of tumorous and adjacent non-tumorous tissues from patients with colorectal cancer using ICP-MS, ICP-OES and chemometric analysis. Biometals 2009, 22, 863–875. [Google Scholar] [CrossRef]
- Theiner, S.; Kornauth, C.; Varbanov, H.P.; Galanski, M.; Van Schoonhoven, S.; Heffeter, P.; Berger, W.; Egger, A.E.; Keppler, B.K. Tumor microenvironment in focus: LA-ICP-MS bioimaging of a preclinical tumor model upon treatment with platinum(IV)-based anticancer agents. Metallomics 2015, 7, 1256–1264. [Google Scholar] [CrossRef]
- Gremonprez, F.; Descamps, B.; Izmer, A.; Vanhove, C.; Vanhaecke, F.; De Wever, O.; Ceelen, W. Pretreatment with VEGF(R)-inhibitors reduces interstitial fluid pressure, increases intraperitoneal chemotherapy drug penetration, and impedes tumor growth in a mouse colorectal carcinomatosis model. Oncotarget 2015, 6, 29889–29900. [Google Scholar] [CrossRef][Green Version]
- Bonsall, S.; Hubbard, S.; Jithin, U.; Anslow, A.; Todd, D.; Rowding, C.; Filarowski, T.; Duly, G.; Wilson, R.; Porter, J.; et al. Water-Soluble Truncated Fatty Acid-Porphyrin Conjugates Provide Photo-Sensitizer Activity for Photodynamic Therapy in Malignant Mesothelioma. Cancers 2022, 14, 5446. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.; Goberdhan, D.C.I.; O’Driscoll, L.; Thery, C.; Welsh, J.A.; Blenkiron, C.; Buzas, E.I.; Di Vizio, D.; Erdbrugger, U.; Falcon-Perez, J.M.; et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J. Extracell. Ves. 2021, 10, e12182. [Google Scholar] [CrossRef] [PubMed]
- Lees, R.; Tempest, R.; Law, A.; Aubert, D.; Davies, O.G.; Williams, S.; Peake, N.; Peacock, B. Single Extracellular Vesicle Transmembrane Protein Characterization by Nano-Flow Cytometry. J. Vis. Exp. 2022, 185, e64020. [Google Scholar] [CrossRef]
- Driver, M.D.; Williamson, M.J.; De Mitri, N.; Nikolov, T.; Hunter, C.A. SSIPTools: Software and Methodology for Surface Site Interaction Point (SSIP) Approach and Applications. J. Chem. Inf. Model. 2021, 61, 5331–5335. [Google Scholar] [CrossRef] [PubMed]
- Tomicic, M.T.; Kramer, F.; Nguyen, A.; Schwarzenbach, C.; Christmann, M. Oxaliplatin-Induced Senescence in Colorectal Cancer Cells Depends on p14ARF-Mediated Sustained p53 Activation. Cancers 2021, 13, 2019. [Google Scholar] [CrossRef] [PubMed]
- Slater, C.; de La Mare, J.; Edkins, A.L. In vitro analysis of putative cancer stem cell populations and chemosensitivity in the SW480 and SW620 colon cancer metastasis model. Oncol. Lett. 2018, 15, 8516–8526. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, M.; Wang, J.; Su, L.; Lin, J.; Yan, X. Active cargo loading into extracellular vesicles: Highlights the heterogeneous encapsulation behaviour. J. Extracell. Vesicles 2021, 10, e12163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, J.; Zhang, Z.; Duan, M.; Wang, G.; Qian, Y.; Zhao, H.; Yang, Z.; Jiang, X. Application of engineered extracellular vesicles for targeted tumor therapy. J. Biomed. Sci. 2022, 29, 14. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef]
- Theiner, S.; Schoeberl, A.; Schweikert, A.; Keppler, B.K.; Koellensperger, G. Mass spectrometry techniques for imaging and detection of metallodrugs. Curr. Opin. Chem. Biol. 2012, 61, 123–134. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandler, K.; Millar, J.; Ward, G.; Boyall, C.; White, T.; Ready, J.D.; Maani, R.; Chapple, K.; Tempest, R.; Brealey, J.; et al. Imaging of Light-Enhanced Extracellular Vesicle-Mediated Delivery of Oxaliplatin to Colorectal Cancer Cells via Laser Ablation, Inductively Coupled Plasma Mass Spectrometry. Cells 2024, 13, 24. https://doi.org/10.3390/cells13010024
Chandler K, Millar J, Ward G, Boyall C, White T, Ready JD, Maani R, Chapple K, Tempest R, Brealey J, et al. Imaging of Light-Enhanced Extracellular Vesicle-Mediated Delivery of Oxaliplatin to Colorectal Cancer Cells via Laser Ablation, Inductively Coupled Plasma Mass Spectrometry. Cells. 2024; 13(1):24. https://doi.org/10.3390/cells13010024
Chicago/Turabian StyleChandler, Kara, Josh Millar, George Ward, Christopher Boyall, Tom White, Joseph David Ready, Rawan Maani, Keith Chapple, Robert Tempest, Joseph Brealey, and et al. 2024. "Imaging of Light-Enhanced Extracellular Vesicle-Mediated Delivery of Oxaliplatin to Colorectal Cancer Cells via Laser Ablation, Inductively Coupled Plasma Mass Spectrometry" Cells 13, no. 1: 24. https://doi.org/10.3390/cells13010024
APA StyleChandler, K., Millar, J., Ward, G., Boyall, C., White, T., Ready, J. D., Maani, R., Chapple, K., Tempest, R., Brealey, J., Duckett, C., Haywood-Small, S., Turega, S., & Peake, N. (2024). Imaging of Light-Enhanced Extracellular Vesicle-Mediated Delivery of Oxaliplatin to Colorectal Cancer Cells via Laser Ablation, Inductively Coupled Plasma Mass Spectrometry. Cells, 13(1), 24. https://doi.org/10.3390/cells13010024






