Resistin Contribution to Cardiovascular Risk in Chronic Kidney Disease Male Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Patients
2.3. Defining the Cardiovascular Risk
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holcomb, I.N.; Kabakoff, R.C.; Chan, B.; Baker, T.W.; Gurney, A.; Henzel, W.; Nelson, C.; Lowman, H.B.; Wright, B.D.; Skelton, N.J.; et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000, 19, 4046–4055. [Google Scholar] [CrossRef] [PubMed]
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A reappraisal. Mech. Ageing Dev. 2019, 178, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Bergsten, A.; Qureshi, A.R.; Heimbürger, O.; Bárány, P.; Lönnqvist, F.; Lindholm, B.; Nordfors, L.; Alvestrand, A.; Stenvinkel, P. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int. 2006, 69, 596–604. [Google Scholar] [CrossRef]
- Tarkowski, A.; Bjersing, J.; Shestakov, A.; Bokarewa, M.I. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J. Cell. Mol. Med. 2010, 14, 1419–1431. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.-C.; Kwon, Y.-W.; Lee, S.E.; Cho, Y.; Kim, J.; Lee, S.; Kim, J.-Y.; Lee, J.; Yang, H.-M.; et al. Adenylyl Cyclase-Associated Protein 1 Is a Receptor for Human Resistin and Mediates Inflammatory Actions of Human Monocytes. Cell Metab. 2014, 19, 484–497. [Google Scholar] [CrossRef]
- Wolf, G. Insulin Resistance and Obesity: Resistin, A Hormone Secreted by Adipose Tissue. Nutr. Rev. 2004, 62, 389–394. [Google Scholar] [CrossRef]
- Filková, M.; Haluzík, M.; Gay, S.; Šenolt, L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin. Immunol. 2009, 133, 157–170. [Google Scholar] [CrossRef]
- Koch, A.; Gressner, O.A.; Sanson, E.; Tacke, F.; Trautwein, C. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Crit. Care 2009, 13, R95. [Google Scholar] [CrossRef]
- Schäffler, A.; Hamer, O.; Dickopf, J.; Goetz, A.; Landfried, K.; Voelk, M.; Herfarth, H.; Kopp, A.; Büchler, C.; Schölmerich, J.; et al. Admission Resistin Levels Predict Peripancreatic Necrosis and Clinical Severity in Acute Pancreatitis. Am. J. Gastroenterol. 2010, 105, 2474–2484. [Google Scholar] [CrossRef]
- Hutcheson, J.; Ye, Y.; Han, J.; Arriens, C.; Saxena, R.; Li, Q.-Z.; Mohan, C.; Wu, T. Resistin as a potential marker of renal disease in lupus nephritis. Clin. Exp. Immunol. 2015, 179, 435–443. [Google Scholar] [CrossRef]
- Degawa-Yamauchi, M.; Bovenkerk, J.E.; Juliar, B.E.; Watson, W.; Kerr, K.; Jones, R.; Zhu, Q.; Considine, R.V. Serum Resistin (FIZZ3) Protein Is Increased in Obese Humans. J. Clin. Endocrinol. Metab. 2003, 88, 5452–5455. [Google Scholar] [CrossRef]
- Fang, W.Q.; Zhang, Q.; Peng, Y.B.; Chen, M.; Lin, X.P.; Wu, J.H.; Cai, C.H.; Mei, Y.F.; Jin, H. Resistin levelispositively correlated with thrombotic complications in Southern Chinese metabolic syndrome patients. J. Endocrinol. Investig. 2011, 34, e36–e42. [Google Scholar] [CrossRef]
- Wang, L.-K.; Wang, H.; Wu, X.-L.; Shi, L.; Yang, R.-M.; Wang, Y.-C. Relationships among resistin, adiponectin, and leptin and microvascular complications in patients with type 2 diabetes mellitus. J. Int. Med. Res. 2020, 48, 0300060519870407. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Yu, L.; Zhou, L. Association between serum resistin concentration and hypertension: A systematic review and meta-analysis. Oncotarget 2017, 8, 41529–41537. [Google Scholar] [CrossRef]
- Xu, W.; Yu, L.; Zhou, W.; Luo, M. Resistin increases lipid accumulation and CD36 expression in human macrophages. Biochem. Biophys. Res. Commun. 2006, 351, 376–382. [Google Scholar] [CrossRef]
- Calabrò, P.; Cirillo, P.; Limongelli, G.; Maddaloni, V.; Riegler, L.; Palmieri, R.; Pacileo, G.; De Rosa, S.; Pacileo, M.; De Palma, R.; et al. Tissue Factor Is Induced by Resistin in Human Coronary Artery Endothelial Cells by the NF-ĸB-Dependent Pathway. J. Vasc. Res. 2011, 48, 59–66. [Google Scholar] [CrossRef]
- Mu, H.; Ohashi, R.; Yan, S.; Chai, H.; Yang, H.; Lin, P.; Yao, Q.; Chen, C. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc. Res. 2006, 70, 146–157. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, J.; Lu, J.-M.; Chai, H.; Wang, X.; Lin, P.H.; Yao, Q. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H193–H201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, H.; Zhang, W.; Kong, W.; Zhu, Y.; Zhang, H.; Xu, Q.; Li, Y.; Wang, X. Homocysteine promotes vascular smooth muscle cell migration by induction of the adipokine resistin. Am. J. Physiol.-Cell Physiol. 2009, 297, C1466–C1476. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.-Y.; He, P.-P.; Yu, X.-H.; Tang, C.-K. Resistin: Potential biomarker and therapeutic target in atherosclerosis. Clin. Chim. Acta 2021, 512, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Weikert, C.; Westphal, S.; Berger, K.; Dierkes, J.; Möhlig, M.; Spranger, J.; Rimm, E.B.; Willich, S.N.; Boeing, H.; Pischon, T. Plasma Resistin Levels and Risk of Myocardial Infarction and Ischemic Stroke. J. Clin. Endocrinol. Metab. 2008, 93, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, E.R.; Hadri, L.; Zhang, S.; Kim, M.; Kohlbrenner, E.; Sheng, J.; Liang, L.; Chen, J.; K-Raman, P.; Hajjar, R.J.; et al. Long-term in vivo resistin overexpression induces myocardial dysfunction and remodeling in rats. J. Mol. Cell. Cardiol. 2011, 51, 144–155. [Google Scholar] [CrossRef]
- Frankel, D.S.; Vasan, R.S.; D’Agostino, R.B.; Benjamin, E.J.; Levy, D.; Wang, T.J.; Meigs, J.B. Resistin, Adiponectin, and Risk of Heart Failure: The Framingham Offspring Study. J. Am. Coll. Cardiol. 2009, 53, 754–762. [Google Scholar] [CrossRef]
- Hay, S.I.; Jayaraman, S.P.; Truelsen, T.; Sorensen, R.J.D.; Millear, A.; Giussani, G.; Beghi, E. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Mafham, M.; Emberson, J.; Landray, M.J.; Wen, C.-P.; Baigent, C. Estimated Glomerular Filtration Rate and the Risk of Major Vascular Events and All-Cause Mortality: A Meta-Analysis. PLoS ONE 2011, 6, e25920. [Google Scholar] [CrossRef]
- Ortiz, P.A.; Covic, A.; Fliser, D.; Fouque, D.; Goldsmith, D.; Kanbay, M.; Mallamaci, F.; Massy, Z.A.; Rossignol, P.; Vanholder, R.; et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014, 383, 1831–1843. [Google Scholar] [CrossRef]
- Cebeci, E.; Cakan, C.; Gursu, M.; Uzun, S.; Karadag, S.; Koldas, M.; Calhan, T.; Helvaci, S.A.; Ozturk, S. The Main Determinants of Serum Resistin Level in Type 2 Diabetic Patients are Renal Function and Inflammation not Presence of Microvascular Complication, Obesity and Insulin Resistance. Exp. Clin. Endocrinol. Diabetes 2019, 127, 189–194. [Google Scholar] [CrossRef]
- Dan, S.; Aditya, P.; Banerjee, P.; Bal, C.; Roy, H.; Banerjee, I. Effect of chronic kidney disease on serum resistin level. Niger. J. Clin. Pract. 2014, 17, 735–738. [Google Scholar] [CrossRef]
- Liu, G.; Deng, Y.; Sun, L.; Ye, X.; Yao, P.; Hu, Y.; Wang, F.; Ma, Y.; Li, H.; Liu, Y.; et al. Elevated plasma tumor necrosis factor-α receptor 2 and resistin are associated with increased incidence of kidney function decline in Chinese adults. Endocrine 2016, 52, 541–549. [Google Scholar] [CrossRef]
- Munjas, J.; Sopić, M.; Bogavac-Stanojević, N.; Kravljača, M.; Miljković, M.; Simić-Ogrizović, S.; Spasojević-Kalimanovska, V.; Jelić-Ivanović, Z. Serum Resistin, Adenylate Cyclase-Associated Protein 1 Gene Expression, and Carotid Intima-Media Thickness in Patients with End-Stage Renal Disease and Healthy Controls. Cardiorenal. Med. 2020, 10, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Marouga, A.; Dalamaga, M.; Kastania, A.N.; Kroupis, C.; Lagiou, M.; Saounatsou, K.; Dimas, K.; Vlahakos, D.V. Circulating resistin is a significant predictor of mortality independently from cardiovascular comorbidities in elderly, non-diabetic subjects with chronic kidney disease. Biomarkers 2016, 21, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Fouque, D.; Kalantar-Zadeh, K. Kidney cachexia or protein-energy wasting in chronic kidney disease: Facts and numbers. J. Cachexia Sarcopenia Muscle 2019, 10, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Marouga, A.; Dalamaga, M.; Kastania, A.N.; Antonakos, G.; Thrasyvoulides, A.; Kontelia, G.; Dimas, C.; Vlahakos, D.V. Correlates of serum resistin in elderly, non-diabetic patients with chronic kidney disease. Clin. Lab. 2013, 59, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Rae, C.; Robertson, S.A.; Taylor, J.M.W.; Graham, A. Resistin induces lipolysis and re-esterification of triacylglycerol stores, and increases cholesteryl ester deposition, in human macrophages. FEBS Lett. 2007, 581, 4877–4883. [Google Scholar] [CrossRef]
- Kawanami, D.; Maemura, K.; Takeda, N.; Harada, T.; Nojiri, T.; Imai, Y.; Manabe, I.; Utsunomiya, K.; Nagai, R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: A new insight into adipocytokine–endothelial cell interactions. Biochem. Biophys. Res. Commun. 2004, 314, 415–419. [Google Scholar] [CrossRef]
- Asgary, S.; Samsamshariat, S.Z.A.; Sakhaei, F.; Salehizadeh, L.; Keshvari, M. Relationship between Resistin, Endothelin-1, and Flow-Mediated Dilation in Patient with and without Metabolic Syndrome. Adv. Biomed. Res. 2019, 8, 16. [Google Scholar] [CrossRef]
- Hsu, W.-Y.; Chao, Y.-W.; Tsai, Y.-L.; Lien, C.-C.; Chang, C.-F.; Deng, M.-C.; Ho, L.-T.; Kwok, C.F.; Juan, C.-C. Resistin induces monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathway. J. Cell. Physiol. 2011, 226, 2181–2188. [Google Scholar] [CrossRef]
- Subodh, V.; Shu-Hong, L.; Chao-Hung, W.; Paul, W.M.; Ren-Ke, L.; Richard, D.W.; Donald, A.G. Resistin Promotes Endothelial Cell Activation. Circulation 2003, 108, 736–740. [Google Scholar] [CrossRef]
- Tisato, V.; Romani, A.; Tavanti, E.; Melloni, E.; Milani, D.; Bonaccorsi, G.; Sanz, J.M.; Gemmati, D.; Passaro, A.; Cervellati, C. Crosstalk Between Adipokines and Paraoxonase 1: A New Potential Axis Linking Oxidative Stress and Inflammation. Antioxidants 2019, 8, 287. [Google Scholar] [CrossRef]
- Melone, M.; Wilsie, L.; Palyha, O.; Strack, A.; Rashid, S. Discovery of a New Role of Human Resistin in Hepatocyte Low-Density Lipoprotein Receptor Suppression Mediated in Part by Proprotein Convertase Subtilisin/Kexin Type 9. J. Am. Coll. Cardiol. 2012, 59, 1697–1705. [Google Scholar] [CrossRef]
- Jurin, I.; Paić, F.; Bulimbašić, S.; Rudež, I.; Đerek, L.; Jurin, H.; Knežević, A.; Starcevic, B.; Ajduk, M. Association between Circulatory and Plaque Resistin Levels with Carotid Plaque Instability and Ischemic Stroke Events. Heart Surg. Forum 2018, 21, E448–E463. [Google Scholar] [CrossRef]
- Costandi, J.; Melone, M.; Zhao, A.; Rashid, S. Human Resistin Stimulates Hepatic Overproduction of Atherogenic ApoB-Containing Lipoprotein Particles by Enhancing ApoB Stability and Impairing Intracellular Insulin Signaling. Circ. Res. 2011, 108, 727–742. [Google Scholar] [CrossRef]
- Norman, G.; Woodiwiss, A.J.; Peterson, V.; Gomes, M.; Sareli, P.; Norton, G.R. Impact of metabolic and inflammatory changes on glomerular function beyond conventional risk factors in an urban South Africa community with prevalent obesity. Cardiovasc. J. Afr. 2020, 31, 91–102. [Google Scholar] [CrossRef]
- Karbach, S.; Wenzel, P.; Waisman, A.; Munzel, T.; Daiber, A. eNOS Uncoupling in Cardiovascular Diseases—The Role of Oxidative Stress and Inflammation. Curr. Pharm. Des. 2014, 20, 3579–3594. [Google Scholar] [CrossRef]
- Harrison, D.G.; Guzik, T.J.; Lob, H.E.; Madhur, M.S.; Marvar, P.J.; Thabet, S.R.; Vinh, A.; Weyand, C.M. Inflammation, Immunity, and Hypertension. Hypertension 2011, 57, 132–140. [Google Scholar] [CrossRef]
- Ohara, Y.; Peterson, T.E.; Harrison, D.G. Hypercholesterolemia increases endothelial superoxide anion production. J. Clin. Investig. 1993, 91, 2546–2551. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2020, 49, 202–211. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Koenig, W.; Libby, P.; Everett, B.M.; Lefkowitz, M.; Thuren, T.; Cornel, J.H. Inhibition of Interleukin-1β by Canakinumab and Cardiovascular Outcomes in Patients with Chronic Kidney Disease. J. Am. Coll. Cardiol. 2018, 71, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 3), iii35–iii40. [Google Scholar] [CrossRef] [PubMed]
- Yaturu, S.; Reddy, R.D.; Rains, J.; Jain, S.K. Plasma and urine levels of resistin and adiponectin in chronic kidney disease. Cytokine 2007, 37, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, X. Resistin Promotes Thrombosis in Rats with Deep Vein Thrombosis via Up-Regulating MMP-2, MMP-9, and PAI-1. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef]
- Ikeda, Y.; Tsuchiya, H.; Hama, S.; Kajimoto, K.; Kogure, K. Resistin regulates the expression of plasminogen activator inhibitor-1 in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2014, 448, 129–133. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, J.; Li, H.; Yu, Z.; Ye, X.; Hu, F.B.; Franco, O.H.; Pan, A.; Liu, Y.; Lin, X. Associations of resistin with inflammatory and fibrinolytic markers, insulin resistance, and metabolic syndrome in middle-aged and older Chinese. Eur. J. Endocrinol. 2008, 159, 585–593. [Google Scholar] [CrossRef]
- Beier, J.I.; Guo, L.; von Montfort, C.; Kaiser, J.P.; Joshi-Barve, S.; Arteel, G.E. New Role of Resistin in Lipopolysaccharide-Induced Liver Damage in Mice. Experiment 2008, 325, 801–808. [Google Scholar] [CrossRef]
- Lijnen, H.R.; Bachmann, F.; Collen, D.; Ellis, V.; Pannekoek, H.; Rijken, D.C.; Thorsen, S. Mechanisms of plasminogen activation. J. Intern. Med. 1994, 236, 415–424. [Google Scholar] [CrossRef]
- Collen, D.; Lijnen, H.; Plow, E.F. The fibrinolytic system in man. Crit. Rev. Oncol. 1986, 4, 249–301. [Google Scholar] [CrossRef]
- Sprengers, E.D.; Kluft, C. Plasminogen activator inhibitors. Blood 1987, 69, 381–387. [Google Scholar] [CrossRef]
- Kohler, H.P.; Grant, P.J. Plasminogen-Activator Inhibitor Type 1 and Coronary Artery Disease. N. Engl. J. Med. 2000, 342, 1792–1801. [Google Scholar] [CrossRef]
- Thögersen, A.M.; Jansson, J.-H.; Boman, K.; Nilsson, T.K.; Weinehall, L.; Huhtasaari, F.; Hallmans, G. High Plasminogen Activator Inhibitor and Tissue Plasminogen Activator Levels in Plasma Precede a First Acute Myocardial Infarction in Both Men and Women: Evidence for the fibrinolytic system as an independent primary risk factor. Circulation 1998, 98, 2241–2247. [Google Scholar] [CrossRef]
- Christ, G.; Nikfardjam, M.; Huber-Beckmann, R.; Gottsauner-Wolf, M.; Glogar, D.; Binder, B.R.; Wojta, J.; Huber, K. Predictive value of plasma plasminogen activator inhibitor-1 for coronary restenosis: Dependence on stent implantation and antithrombotic medication. J. Thromb. Haemost. 2005, 3, 233–239. [Google Scholar] [CrossRef]
- Alessi, M.-C.; Nicaud, V.; Scroyen, I.; Lange, C.; Saut, N.; Fumeron, F.; Marre, M.; Lantieri, O.; Fontaine-Bisson, B.; Juhan-Vague, I.; et al. Association of vitronectin and plasminogen activator inhibitor-1 levels with the risk of metabolic syndrome and type 2 diabetes mellitus. Thromb. Haemost. 2011, 106, 416–422. [Google Scholar] [CrossRef]
- Van De Craen, B.; Declerck, P.J.; Gils, A. The Biochemistry, Physiology and Pathological roles of PAI-1 and the requirements for PAI-1 inhibition in vivo. Thromb. Res. 2012, 130, 576–585. [Google Scholar] [CrossRef]
- Shirakawa, J.; Togashi, Y.; Tajima, K.; Orime, K.; Kikuchi, K.; Miyazaki, T.; Sato, K.; Kimura, M.; Goshima, Y.; Terauchi, Y. Plasminogen activator inhibitor-1 is associated with renal dysfunction independent of BMI and serum lipid levels in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2012, 97, e9–e12. [Google Scholar] [CrossRef]
- Owecki, M.; Miczke, A.; Nikisch, E.; Pupek-Musialik, D.; Sowiński, J. Serum Resistin Concentrations are Higher in Human Obesity but Independent from Insulin Resistance. Exp. Clin. Endocrinol. Diabetes 2011, 119, 117–121. [Google Scholar] [CrossRef]
- Hasegawa, G.; Ohta, M.; Ichida, Y.; Obayashi, H.; Shigeta, M.; Yamasaki, M.; Fukui, M.; Yoshikawa, T.; Nakamura, N. Increased serum resistin levels in patients with type 2 diabetes are not linked with markers of insulin resistance and adiposity. Acta Diabetol. 2005, 42, 104–109. [Google Scholar] [CrossRef]
- Lee, J.H.; Chan, J.L.; Yiannakouris, N.; Kontogianni, M.; Estrada, E.; Seip, R.; Orlova, C.; Mantzoros, C.S. Circulating Resistin Levels Are Not Associated with Obesity or Insulin Resistance in Humans and Are Not Regulated by Fasting or Leptin Administration: Cross-Sectional and Interventional Studies in Normal, Insulin-Resistant, and Diabetic Subjects. J. Clin. Endocrinol. Metab. 2003, 88, 4848–4856. [Google Scholar] [CrossRef]
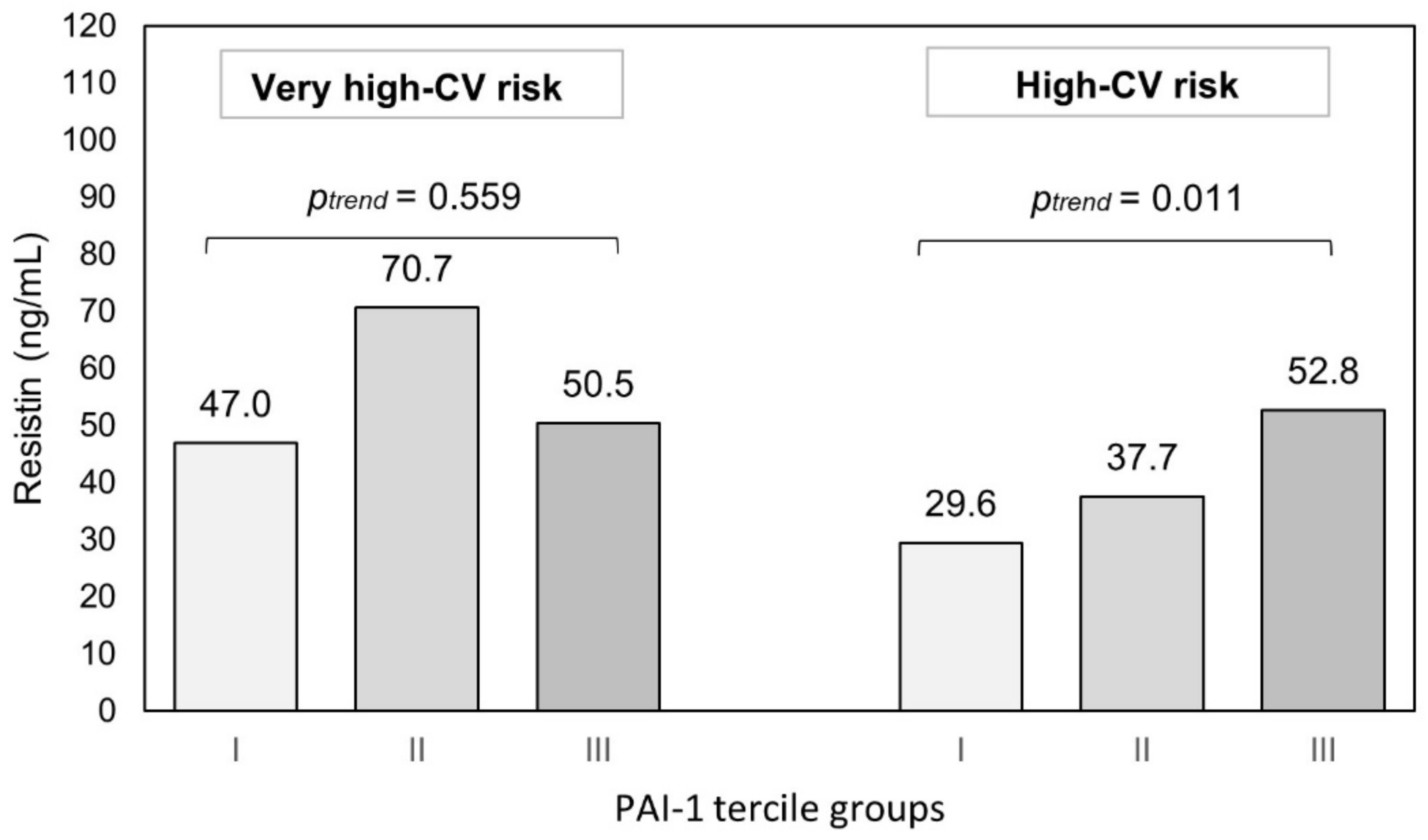
| eGFR < 60 mL/min/1.73 m2 n = 99 | eGFR ≥ 60 mL/min/1.73 m2 n = 43 | p-Value | |
|---|---|---|---|
| Age [years] | 66.0 (59.0–71.0) | 57.0 (41.0–70.0) | 0.005 |
| eGFR [mL/min/1.73 m2] | 36.0 (23.0–46.0) | 95.0 (76.0–110.0) | <0.001 |
| Total cholesterol [mg/dL] | 165.0 (143.0–207.0) | 196.0 (163.0–221.0) | 0.014 |
| Low-density lipoprotein cholesterol [mg/dL] | 103.0 (80.0–139.0) | 119.5 (93.7–159.1) | 0.084 |
| High-density lipoprotein cholesterol [mg/dL] | 42.0 (35.0–54.0) | 46.1 (40.0–54.4) | 0.170 |
| Non-high-density lipoprotein cholesterol [mg/dL] | 123.0 (93.0–166.0) | 145.6 (116.5–173.9) | 0.011 |
| Triglycerides [mg/dL] | 144.0 (108.0–224.0) | 127.0 (93.0–161.0) | 0.024 |
| BMI [kg/m2] | 28.6 (25.4–33.4) | 28.4 (24.4–32.3) | 0.374 |
| HgbA1c ≥ 6.5 [%] | 24.2% | 11.9% | 0.097 |
| Serum glucose [mg/dL] | 97.5 (86.5–132.5) | 97.0 (89.0–103.0) | 0.833 |
| HOMA-IR | 3.8 (1.9–7.9) | 2.4 (1.5–6.8) | 0.189 |
| SBP [mmHg] | 130.0 (125.0–140.0) | 130.0 (113.8–135.0) | 0.006 |
| DBP [mmHg] | 80.0 (70.0–85.0) | 71.0 (69.5–80.0) | 0.001 |
| Smoking [%] | NA | 23.1% | - |
| Resistin [ng/mL] | 48.3 (35.2–66.4) | 35.2(26.1–54.4) | 0.003 |
| TNF-alpha [pg/mL] | 4.4 (3.5–5.6) | 3.0 (2.5–4.0) | <0.001 |
| CRP [mg/dL] | 0.2 (0.1–0.4) | 0.1 (0.1–0.4) | 0.020 |
| PAI-1 [ng/mL] | 92.4 (71.6–119.4) | 113.1 (85.8–149.8) | 0.008 |
| Cardiovascular Risk | eGFR < 60 mL/min/1.73 m2 n = 99 | eGFR ≥ 60 mL/min/1.73 m2 n = 43 | ||
|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | |
| Very high-risk | 70 | 52.1 (36.9–74.2) | 7 | 45.8 (30.8–73.4) |
| High-risk | 29 | 43.6 (29.1–54.4) | 19 | 37.1 (25.2–60.6) |
| Moderate-risk | - | - | 14 | 36.4 (29.9–52.6) |
| Low-risk | - | - | 3 | 22.4 (16.7–26.1) |
| p = 0.014 | ptrend = 0.087 | |||
| Variable | β Coefficient | 95% CI for β | p-Value |
|---|---|---|---|
| CV risk | |||
| very high | 36.38 | 4.53;68.24 | 0.026 |
| high | Ref. | - | - |
| TNF-alpha | −0.02 | −2.67;2.63 | 0.989 |
| TNF-alpha*very high CV risk | 1.02 | −1.83;3.87 | 0.478 |
| TNF-alpha*high CV risk | Ref. | - | - |
| PAI-1 | 0.30 | 0.07;0.53 | 0.012 |
| PAI-1*very high CV risk | −0.27 | −0.54;−0.003 | 0.048 |
| PAI-1*high CV risk | Ref. | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romejko, K.; Rymarz, A.; Szamotulska, K.; Bartoszewicz, Z.; Rozmyslowicz, T.; Niemczyk, S. Resistin Contribution to Cardiovascular Risk in Chronic Kidney Disease Male Patients. Cells 2023, 12, 999. https://doi.org/10.3390/cells12070999
Romejko K, Rymarz A, Szamotulska K, Bartoszewicz Z, Rozmyslowicz T, Niemczyk S. Resistin Contribution to Cardiovascular Risk in Chronic Kidney Disease Male Patients. Cells. 2023; 12(7):999. https://doi.org/10.3390/cells12070999
Chicago/Turabian StyleRomejko, Katarzyna, Aleksandra Rymarz, Katarzyna Szamotulska, Zbigniew Bartoszewicz, Tomasz Rozmyslowicz, and Stanisław Niemczyk. 2023. "Resistin Contribution to Cardiovascular Risk in Chronic Kidney Disease Male Patients" Cells 12, no. 7: 999. https://doi.org/10.3390/cells12070999
APA StyleRomejko, K., Rymarz, A., Szamotulska, K., Bartoszewicz, Z., Rozmyslowicz, T., & Niemczyk, S. (2023). Resistin Contribution to Cardiovascular Risk in Chronic Kidney Disease Male Patients. Cells, 12(7), 999. https://doi.org/10.3390/cells12070999








