Effect of Commonly Used Cosmetic Preservatives on Healthy Human Skin Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. In Vitro Antimicrobial Assay
2.3. Cell Viability Assay
2.4. Sircol Collagen Assay
2.5. Western Blot Analysis
2.6. Zymographic Determination of MMP-2 Activity
2.7. Statistics
3. Results
3.1. Antibacterial Activity
3.2. Antifungal Activity
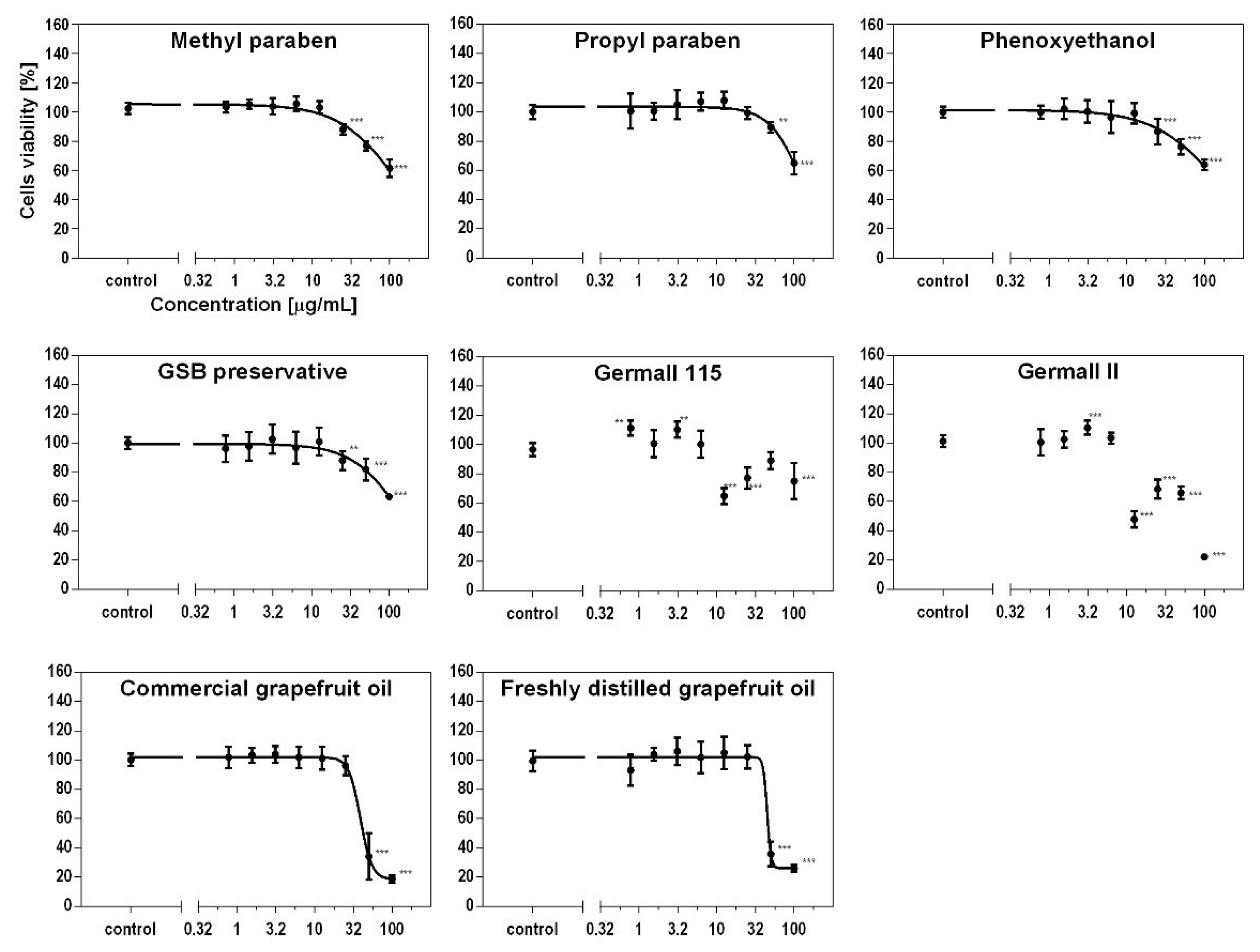
3.3. Effect of Preservatives on Cell Viability
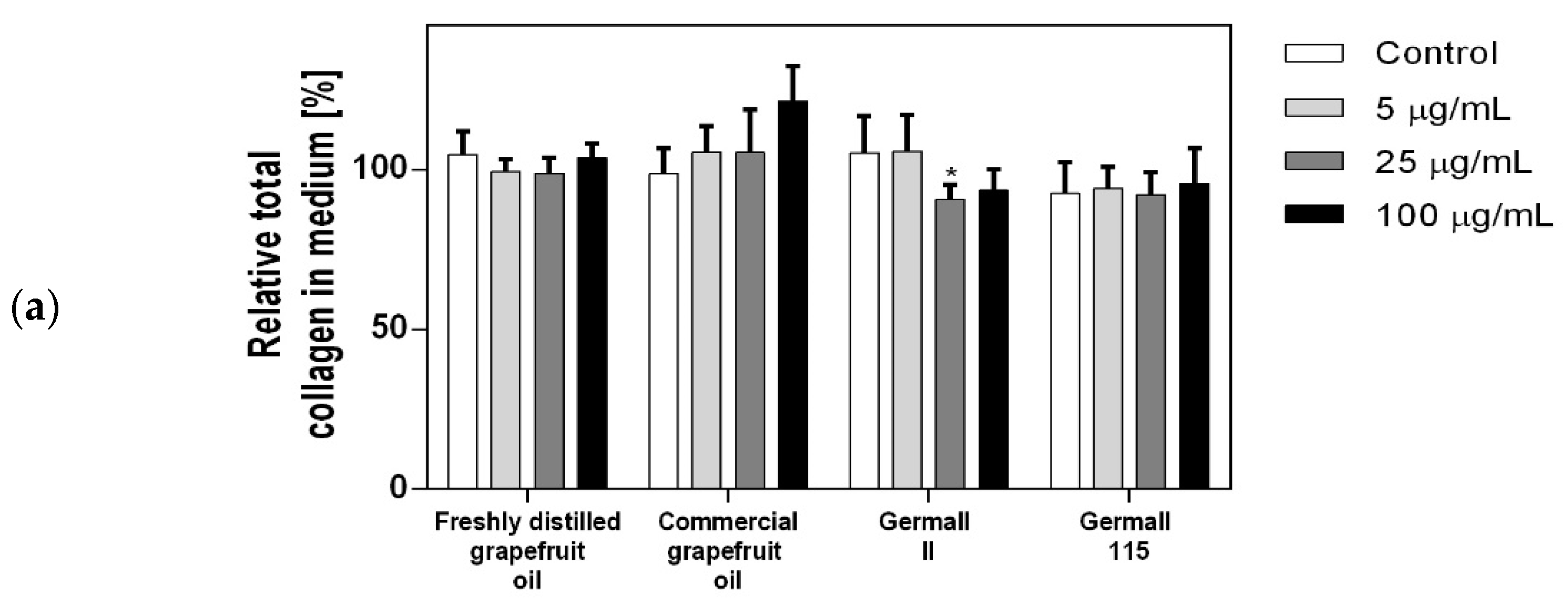
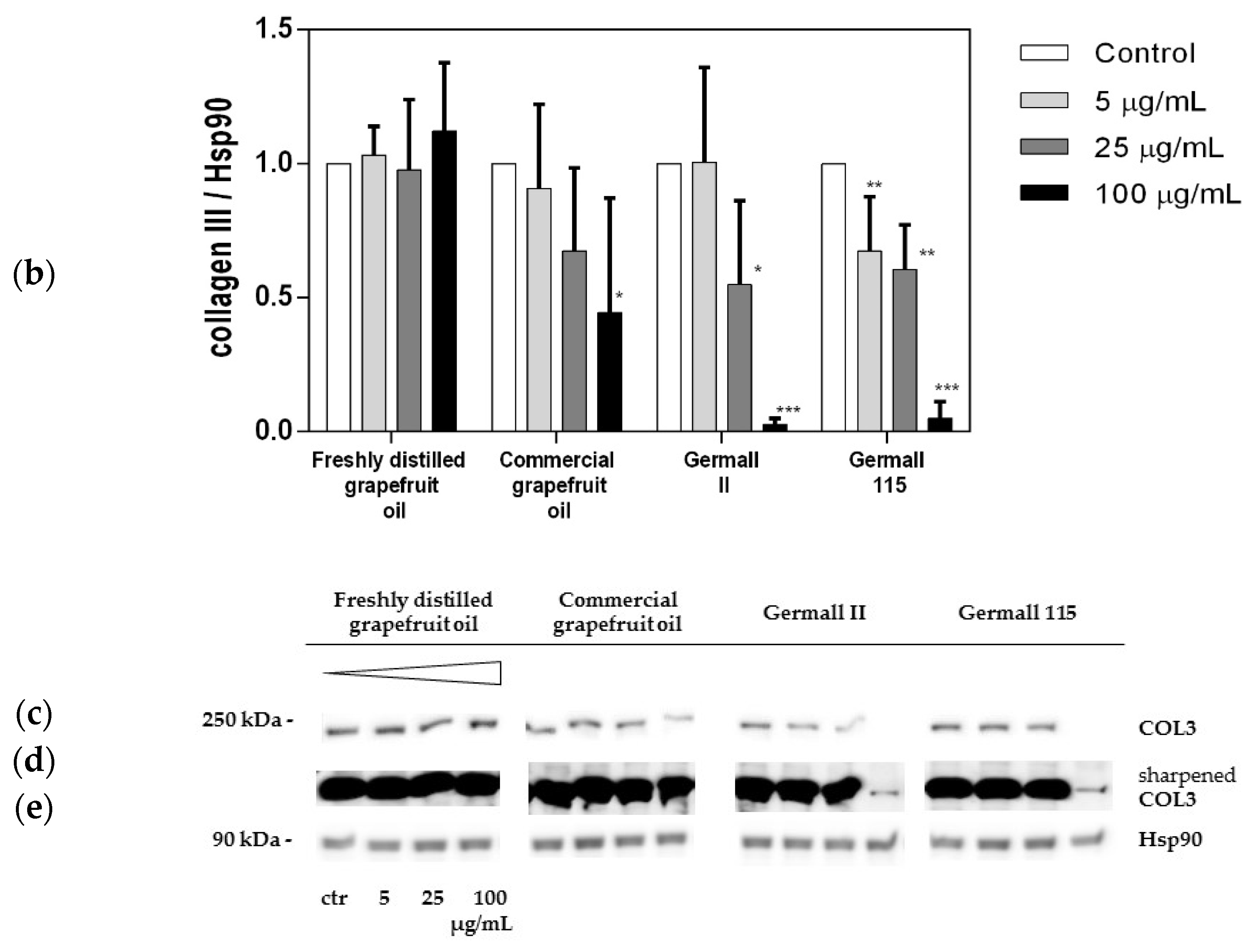
3.4. Influence of Preservatives on Collagen Synthesis
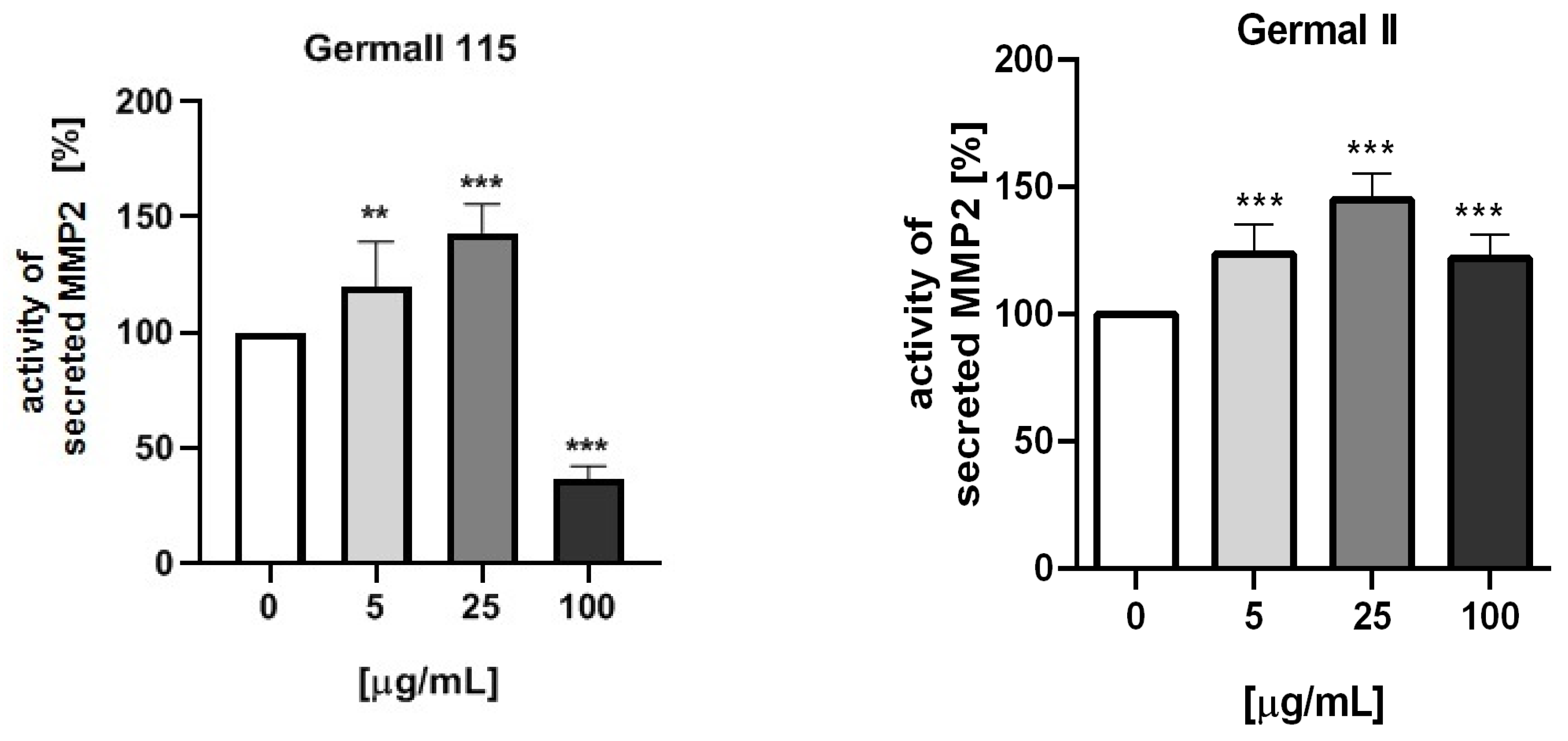
3.5. MMP-2 Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lane, M.E. Skin Penetration Enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- García-Gavín, J.; González-Vilas, D.; Fernández-Redondo, V.; Toribo, J. Allergic Contact Dermatitis in a Girl Due to Several Cosmetics Containing Diazolidinyl-Urea or Imidazolidinyl-Urea. Contact Dermatitis 2010, 63, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Ponec, M. In Vitro Cultured Human Skin Cells as Alternatives to Animals for Skin Irritancy Screening. Int. J. Cosmet. Sci. 1992, 14, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Miyazawa, M.; Yoshida, Y.; Ito, Y.; Suzuki, H. Prediction of Preservative Sensitization Potential Using Surface Marker CD86 and/or CD54 Expression on Human Cell Line, THP-1. Arch. Dermatol. Res. 2007, 298, 427–437. [Google Scholar] [CrossRef]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety Assessment of Esters of P-Hydroxybenzoic Acid (Parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy around Parabens: Alternative Strategies for Preservative Use in Cosmetics and Personal Care Products. Environ. Res. 2021, 198, 1–46. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, H.Y. Escherichia Coli β-Galactosidase-Catalyzed Synthesis of 2-Phenoxyethanol Galactoside and Its Characterization. Bioprocess Biosyst. Eng. 2015, 38, 365–372. [Google Scholar] [CrossRef]
- Uysal, B.; Sozmen, F.; Aktas, O.; Oksal, B.S.; Kose, E.O. Essential Oil Composition and Antibacterial Activity of the Grapefruit (Citrus Paradisi. L) Peel Essential Oils Obtained by Solvent-Free Microwave Extraction: Comparison with Hydrodistillation. Int. J. Food Sci. Technol. 2011, 46, 1455–1461. [Google Scholar] [CrossRef]
- Bourgou, S.; Rahali, F.Z.; Ourghemmi, I.; Tounsi, M.S. Changes of Peel Essential Oil Composition of Four Tunisian Citrus during Fruit Maturation. Sci. World, J. 2012, 2012. [Google Scholar] [CrossRef]
- Droby, S.; Eick, A.; Macarisin, D.; Cohen, L.; Rafael, G.; Stange, R.; McColum, G.; Dudai, N.; Nasser, A.; Wisniewski, M.; et al. Role of Citrus Volatiles in Host Recognition, Germination and Growth of Penicillium Digitatum and Penicillium Italicum. Postharvest Biol. Technol. 2008, 49, 386–396. [Google Scholar] [CrossRef]
- Fuselli, S.R.; García De La Rosa, S.B.; Eguaras, M.J.; Fritz, R. Chemical Composition and Antimicrobial Activity of Citrus Essences on Honeybee Bacterial Pathogen Paenibacillus Larvae, the Causal Agent of American Foulbrood. World J. Microbiol. Biotechnol. 2008, 24, 2067–2072. [Google Scholar] [CrossRef]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial Activity of Flavonoids Extracted from Bergamot (Citrus Bergamia Risso) Peel, a Byproduct of the Essential Oil Industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A.; Bourgou, S.; Rahali, F.Z.; et al. Distinct Fibroblast Lineages Determine Dermal Architecture in Skin Development and Repair. Curr. Opin. Cell Biol. 2020, 9, 277–281. 1198–1205. [Google Scholar] [CrossRef]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular Matrix Regulation of Fibroblast Function: Redefining Our Perspective on Skin Aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef]
- Haydont, V.; Neiveyans, V.; Perez, P.; Busson, É.; Lataillade, J.J.; Asselineau, D.; Fortunel, N.O. Fibroblasts from the Human Skin Dermo-Hypodermal Junction Are Distinct from Dermal Papillary and Reticular Fibroblasts and from Mesenchymal Stem Cells and Exhibit a Specific Molecular Profile Related to Extracellular Matrix Organization and Modeling. Cells 2020, 9, 368. [Google Scholar] [CrossRef]
- Bella, J.; Eaton, M.; Brodsky, B.; Berman, H.M. Crystal and Molecular Structure of a Collagen-like Peptide at 1.9 A Resolution. Science 1994, 266, 75–81. [Google Scholar] [CrossRef]
- Sugita, S.; Suzumura, T.; Nakamura, A.; Tsukiji, S.; Ujihara, Y.; Nakamura, M. Second Harmonic Generation Light Quantifies the Ratio of Type III to Total (I + III) Collagen in a Bundle of Collagen Fiber. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Tromp, G. Type III Collagen (COL3A1): Gene and Protein Structure, Tissue Distribution, and Associated Diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef]
- McCawley, L.J.; Matrisian, L.M. Matrix Metalloproteinases: They’re Not Just for Matrix Anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Kurzepa, J.; Biała, G.; Kaszubska, K.; Grot, K.; Tarkowski, P.; Kowalczyk, J.; Silvestro, S.; Faggio, C.; Budzyńska, B. Mephedrone Impact on Matrix Metalloproteinases Activity-Do They Influence the Memory Processes? Curr. Mol. Pharmacol. 2019, 12, 115–121. [Google Scholar] [CrossRef]
- Drankowska, J.; Kos, M.; Kościuk, A.; Marzęda, P.; Boguszewska-Czubara, A.; Tylus, M.; Święch-Zubilewicz, A. MMP Targeting in the Battle for Vision: Recent Developments and Future Prospects in the Treatment of Diabetic Retinopathy. Life Sci. 2019, 229, 149–156. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clin. Microbiol. Infect. 2003, 9, 1–7. [CrossRef]
- Pfaller, M.A.; Haturvedi, V.; Espinel-Ingroff, A.; Ghannoum, M.A.; Gosey, L.L.; Odds, F.C.; Rex, J.H.; Rinaldi, M.G.; Sheehan, D.J.; Walsh, T.J.; et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition Serving the World’s Medical Science Community Through Voluntary Consensus; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2002. [Google Scholar]
- Popiołek, Ł.; Biernasiuk, A.; Malm, A. Synthesis and Antimicrobial Activity of New 1,3-Thiazolidin-4-One Derivatives Obtained from Carboxylic Acid Hydrazides. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 251–260. [Google Scholar] [CrossRef]
- O’Donnell, F.; Smyth, T.J.P.; Ramachandran, V.N.; Smyth, W.F. A Study of the Antimicrobial Activity of Selected Synthetic and Naturally Occurring Quinolines. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Wnorowski, A.; Sadowska, M.; Paul, R.K.; Singh, N.S.; Boguszewska-Czubara, A.; Jimenez, L.; Abdelmohsen, K.; Toll, L.; Jozwiak, K.; Bernier, M.; et al. Activation of Β2-Adrenergic Receptor by (R,R’)-4’-Methoxy-1-Naphthylfenoterol Inhibits Proliferation and Motility of Melanoma Cells. Cell. Signal. 2015, 27, 997–1007. [Google Scholar] [CrossRef]
- Majewska, N.; Zaręba, I.; Surażyński, A.; Galicka, A. Methylparaben-Induced Decrease in Collagen Production and Viability of Cultured Human Dermal Fibroblasts. J. Appl. Toxicol. 2017, 37, 1117–1124. [Google Scholar] [CrossRef]
- Strzemski, M.; Wojnicki, K.; Sowa, I.; Wojas-Krawczyk, K.; Krawczyk, P.; Kocjan, R.; Such, J.; Latalski, M.; Wnorowski, A.; Wójciak-Kosior, M. In Vitro Antiproliferative Activity of Extracts of Carlina Acaulis Subsp. Caulescens and Carlina Acanthifolia Subsp. Utzka. Front. Pharmacol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Wnorowski, A.; Wnorowska, S.; Wojas-Krawczyk, K.; Grenda, A.; Staniak, M.; Michalak, A.; Woźniak, S.; Matosiuk, D.; Biała, G.; Wójciak, M.; et al. Toxicity of Carlina Oxide—A Natural Polyacetylene from the Carlina Acaulis Roots—In Vitro and in Vivo Study. Toxins (Basel) 2020, 12, 239. [Google Scholar] [CrossRef]
- Gołąb, P.; Boguszewska-Czubara, A.; Kiełbus, M.; Kurzepa, J. The RtPA Increases MMP-9 Activity in Serum during Ischaemic Stroke. Neurol. Neurochir. Pol. 2014, 48, 309–314. [Google Scholar] [CrossRef]
- Veeramani kandan, P.; Balupillai, A.; Kanimozhi, G.; Khan, H.A.; Alhomida, A.S.; Prasad, N.R. Opuntiol Prevents Photoaging of Mouse Skin via Blocking Inflammatory Responses and Collagen Degradation. Oxid. Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef]
- Jacintho, A.P.P.; Melo, G.D.; Machado, G.F.; Bertolo, P.H.L.; Moreira, P.R.R.; Momo, C.; Souza, T.A.; Vasconcelos, R.D.O. Expression of Matrix Metalloproteinase-2 and Metalloproteinase-9 in the Skin of Dogs with Visceral Leishmaniasis. Parasitol. Res. 2018, 117, 1819–1827. [Google Scholar] [CrossRef]
- Ishiwatari, S.; Suzuki, T.; Hitomi, T.; Yoshino, T.; Matsukuma, S.; Tsuji, T. Effect of Methyl Paraben on Skin Keratinocytes. J. Appl. Toxicol. 2007, 1–9. [Google Scholar] [CrossRef]
- Dubey, D.; Chopra, D.; Singh, J.; Srivastav, A.K.; Kumari, S.; Verma, A.; Ray, R.S. Photosensitized Methyl Paraben Induces Apoptosis via Caspace Dependet Pathway under Ambient UVB Exposurce in Human Skin Cells. Food Chem. Toxicol. 2017, 108, 171–185. [Google Scholar] [CrossRef]
- Weinkauf, R.; Santhanam, U.; Palanker, L.; Donald, R.; Bartolone, J. Gluconolactone or Glucarolactone as an Anti-irritant in Cosmetic Skin Care Methods and Compositions. U.S. Patent Number: 6036963, 14 March 2000. [Google Scholar]
- Greaves, M.; Camp, R. Prostaglandins, Leukotrienes, Phospholipase, Platelet Activating Factor, and Cytokines: An Integrated Approach to Inflammation of Human Skin. Arch. Dermatol Res. 1988, 280, 33–41. [Google Scholar]
- Arabsolghar, R.; Saberzadeh, J.; Khodaei, F.; Borojeni, R.; Khorsand, M.; Rashedinia, M. The Protective Effect of Sodium Benzoate on Aluminum Toxicity in PC12 Cell Line. Res. Pharm. Sci. 2017, 12, 391–400. [Google Scholar] [CrossRef]
- Park, H.W.; Park, E.H.; Yun, H.M.; Rhim, H. Sodium Benzoate-Mediated Cytotoxicity in Mammalian Cells. J. Food Biochem. 2011, 35, 1034–1046. [Google Scholar] [CrossRef]
- Heggers, J.P.; Cottingham, J.; Gusman, J.; Reagor, L.; McCoy, L.; Carino, E.; Cox, R.; Zhao, J.G. The Effectiveness of Processed Grapefruit-Seed Extract as an Antibacterial Agent: II. Mechanism of Action and in Vitro Toxicity. J. Altern. Complement. Med. 2002, 8, 333–340. [Google Scholar] [CrossRef]
- Harvey, P.W.; Everett, D.J. Significance of the Detection of Esters of P-Hydroxybenzoic Acid (Parabens) in Human Breast Tumours. J. Appl. Toxicol. 2004, 24, 1–4. [Google Scholar] [CrossRef]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 1. [Google Scholar] [CrossRef]


| Compound Name | Chemical Structure |
|---|---|
| Phenoxyethanol | 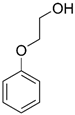 |
| Germall II Diazolidinyl urea 1-(1,3-Bis(hydroxymethyl)-2,5-dioxoimidazolidin-4-yl)-1,3-bis(hydroxymethyl)urea | 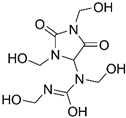 |
| Germall 115 Imidazolidinyl urea 1,1′-Methylenebis(3-(3-(hydroxymethyl)-2,5-dioxoimidazolidin-4-yl)urea) | 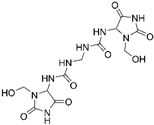 |
| GSB Gluconolactone (70–80%) and sodium benzoate (22–28%) |  |
| Methyl paraben | 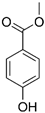 |
| Propyl paraben | 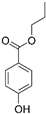 |
| Grapefruit oil Citrus grandis oil | Undetermined composition. The main substances are flavonoids and ascorbic acid. |
| Species | MIC (MBC) and {MBC/MIC Ratio} of Compounds against Gram-Positive Bacteria | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Commercial Grapefruit Oil | Freshly Made Grapefruit Oil | Germall II | Germall 115 | Phenoxy-Ethanol | GSB Preservative | Propyl Paraben | Methyl Paraben | CIP/ VA | |
| Staphylococcus aureus ATCC 25923 | 16,000 (>16,000) {>1} | 8000 (>16,000) {>2} | 250 (500) {2} | 250 (1000) {4} | >4000 (>4000) {>1} | 4000 (>4000) {>1} | 500 (4000) {8} | 2000 (>4000) {>2} | 0.48 (0.48) {1} |
| Staphylococcus aureus ATCC 6538 | 16,000 (>16,000) {>1} | 8000 (>16,000) {>2} | 250 (500) {2} | 250 (1000) {4} | 4000 (>4000) {>1} | 4000 (>4000) {>1} | 2000 (2000) {1} | 2000 (>4000) {>2} | 0.24 (0.24) {1} |
| Staphylococcus aureus ATCC 43300 | 16,000 (>16,000) {>1} | 16,000 (>16,000) {>1} | 250 (500) {2} | 250 (500) {2} | >4000 (>4000) {>1} | 4000 (>4000) {>1} | 2000 (4000) {2} | 2000 (>4000) {>2} | 0.24 (0.24) {1} |
| Staphylococcus aureus ATCC 29213 | 16,000 (>16,000) {>1} | 16,000 (>16,000) {>1} | 250 (1000) {4} | 500 (1000) {2} | >4000 (>4000) {>1} | 4000 (>4000) {>1} | 1000 (4000) {4} | 2000 (>4000) {>2} | 0.48 (0.48) {1} |
| Staphylococcus epidermidis ATCC 12228 | 16,000 (>16,000) {>1} | 16,000 (>16,000) {>1} | 250 (500) {2} | 500 (1000) {2} | >4000 (>4000) {>1} | 4000 (>4000) {>1} | 500 (2000) {4} | 2000 (2000) {1} | 0.12 (0.12) {1} |
| Enterococcus faecalis ATCC 29212 | 16,000 (>16,000) {>1} | 16,000 (>16,000) {>1} | 250 (1000) {4} | 500 (1000) {2} | >4000 (>4000) {>1} | 4000 (>4000) {>1} | 1000 (2000) {2} | 1000 (>4000) {>4} | 0.98 (1.95) {2} |
| Streptococcus pyogenes ATCC 19615 | 8000 (8000) {1} | 4000 (8000) {2} | 125 (125) {1} | 250 (250) {1} | 4000 (4000) {1} | 4000 (>4000) {>1} | 250 (250) {1} | 2000 (4000) {2} | 0.24 (0.24) {1} |
| Micrococcus luteus ATCC 10240 | 8000 (16,000) {2} | 8000 (>16,000) {>2} | 125 (500) {2} | 250 (500) {2} | 2000 (4000) {2} | 2000 (>4000) {>2} | 500 (2000) {4} | 1000 (>4000) {>4} | 0.98 (1.95) {2} |
| Bacillus subtilis ATCC 6633 | 8000 (>16,000) {>2} | 8000 (>16,000) {>2} | 250 (500) {2} | 500 (500) {1} | 4000 (>4000) {>1} | 4000 (4000) {1} | 500 (2000) {4} | 2000 (4000) {2} | 0.03 (0.03) {1} |
| Bacillus cereus ATCC 10876 | 16,000, (>16,000) {>1} | 8000 (>16,000) {>2} | 250 (1000) {4} | 500 (2000) {4} | >4000 (>4000) {>1} | 4000 (>4000) {>1} | 500 (4000) {8} | 2000 (>4000) {>2} | 0.06 (0.12) {2} |
| Propionibacterium acnes ATCC 11827 | 8000 (8000) {1} | 4000 (4000) {1} | 125 (125) {1} | 250 (250) {1} | >4000 (>4000) {>1} | 4000 (>4000) {>1} | 500 (1000) {1} | 2000 (4000) {2} | – |
| Species | MIC (MBC) and {MBC/MIC Ratio} of Compounds against Gram-Negative Bacteria | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Commercial Grapefruit Oil | Freshly Made Grapefruit Oil | Germall II | Germall 115 | Phenoxy-Ethanol | GSB Preservative | Propyl Paraben | Methyl Paraben | CIP | |
| Bordetella bronchiseptica ATCC 4617 | 16,000, (16,000) {1} | 16,000 (16,000) {1} | 125 (250) {2} | 125 (500) {4} | 2000 (4000) {2} | 2000 (4000) {2} | 500 (1000) {2} | 250 (2000) {8} | 0.98 (0.98) {1} |
| Klebsiella pneumoniae ATCC 13883 | 16,000, (>16,000) {>1} | 16,000 (>16,000) {>1} | 250 (500) {2} | 500 (500) {1} | 4000 (>4000) {>1} | 4000 (>4000) {>1} | 1000 (2000) {2} | 1000 (4000) {4} | 0.12 (0.24) {2} |
| Proteus mirabilis ATCC 12453 | 16,000, (>16,000) {>1} | 16,000 (>16,000) {>1} | 500 (500) {1} | 250 (500) {2} | 4000 (>4000) {>1} | 4000 (>4000) {>1} | 2000 (2000) {1} | 1000 (4000) {4} | 0.03 (0.03) {1) |
| Salmonella typhimurium ATCC 14028 | 16,000 (>16,000) {>1} | 16,000, (>16,000) {>1} | 250 (500) {2} | 500 (1000) {2} | 4000 (>4000) {>1} | 4000 (>4000) {>1} | 2000 (4000) {2} | 1000 (4000) {4} | 0.06 (0.06) {1} |
| Escherichia coli ATCC 25922 | 16,000 (>16,000) {>1} | 16,000 (>16,000) {>1} | 250 (500) {2} | 500 (500) {1} | 4000 (>4000) {>1} | 4000 (>4000) {>1} | 2000 (2000) {1} | 1000 (4000) {4} | 0.004 (0.008) {2} |
| Pseudomonas aeruginosa ATCC 9027 | 16,000 (>16,000) {>1} | 16,000 (>16,000) {>1} | 500 (500) {1} | 1000 (1000) {1} | 4000 (>4000) {>1} | 4000 (>4000) {>1} | 2000 (>4000) {>1} | 2000 (2000) {1} | 0.48 (0.98) {2} |
| Species | MIC (MFC) and {MFC/MIC Ratio} of Compounds against Fungi | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Commercial Grapefruit Oil | Freshly Made Grapefruit Oil | Germall II | Germall 115 | Phenoxy-Ethanol | GSB Preservative | Propyl Paraben | Methyl Paraben | NY | |
| Candida albicans ATCC 2091 | 4000 (8000) {2} | 2000 (16,000) {8} | 1000 (1000) {1} | 1000 (2000) {2} | 2000 (4000) {2} | 4000 (4000) {1} | 250 (500) {2} | 500 (1000) {2} | 0.24 (0.24) {1} |
| Candida albicans ATCC 10231 | 2000 (8000) {4} | 2000 (16,000) {8} | 1000 (2000) {2} | 2000 (4000) {2} | 2000 (4000) {2} | 4000 (4000) {1} | 250 (500) {2} | 500 (2000) {4} | 0.48 (0.48) {1} |
| Candida parapsilosis ATCC 22019 | 2000 (16,000) {8} | 2000 (16,000) {8} | 500 (500) {1} | 500 (500) {1} | 1000 (4000) {4} | 4000 (4000) {1} | 125 (250) {2} | 250 (500) {2} | 0.24 (0.48) {2} |
| Candida glabrata ATCC 90030 | 4000 (16,000) {4} | 4000 (16,000) {4} | 1000 (1000) {1} | 1000 (1000) {1} | 4000 (4000) {1} | 4000 (4000) {1} | 250 (500) {2} | 500 (2000) {4} | 0.24 (0.48) {2} |
| Candida krusei ATCC 14243 | 2000 (2000) {1} | 2000 (2000) {1} | 500 (1000) {2} | 1000 (1000) {1} | 4000 (4000) {1} | 4000 (4000) {1} | 125 (250) {2} | 500 (2000) {4} | 0.24 (0.24) {1} |
| Aspergillus niger ATCC 16404 | 8000 (8000) {1} | 8000 (16,000) {2} | 500 (500) {1} | 2000 (4000) {2} | 2000 (4000) {2} | 4000 (4000) {1} | 250 (500) {2} | 500 (1000) {2} | – |
| Control | 5 µg/mL | 25 µg/mL | 100 µg/mL | |
|---|---|---|---|---|
| Germall 115 | 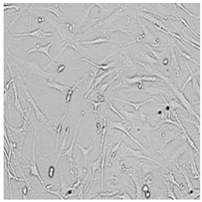 | 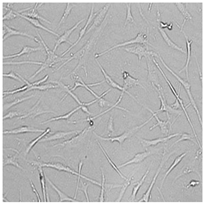 |  | 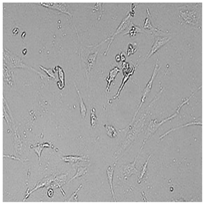 |
| Germall II | 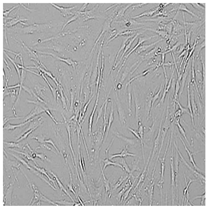 | 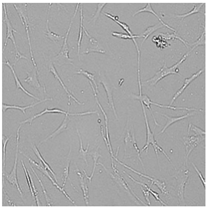 | 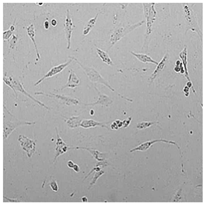 |  |
| Commercial grapefruit oil |  | 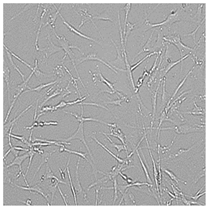 | 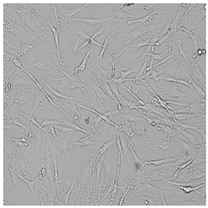 | 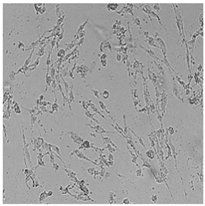 |
| Distilled grapefruit oil | 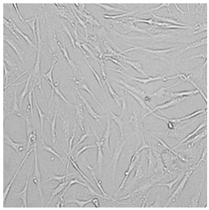 |  | 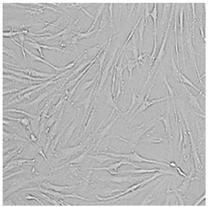 | 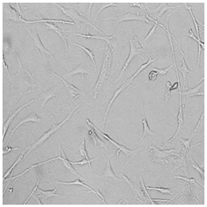 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głaz, P.; Rosińska, A.; Woźniak, S.; Boguszewska-Czubara, A.; Biernasiuk, A.; Matosiuk, D. Effect of Commonly Used Cosmetic Preservatives on Healthy Human Skin Cells. Cells 2023, 12, 1076. https://doi.org/10.3390/cells12071076
Głaz P, Rosińska A, Woźniak S, Boguszewska-Czubara A, Biernasiuk A, Matosiuk D. Effect of Commonly Used Cosmetic Preservatives on Healthy Human Skin Cells. Cells. 2023; 12(7):1076. https://doi.org/10.3390/cells12071076
Chicago/Turabian StyleGłaz, Patrycja, Agata Rosińska, Sylwia Woźniak, Anna Boguszewska-Czubara, Anna Biernasiuk, and Dariusz Matosiuk. 2023. "Effect of Commonly Used Cosmetic Preservatives on Healthy Human Skin Cells" Cells 12, no. 7: 1076. https://doi.org/10.3390/cells12071076
APA StyleGłaz, P., Rosińska, A., Woźniak, S., Boguszewska-Czubara, A., Biernasiuk, A., & Matosiuk, D. (2023). Effect of Commonly Used Cosmetic Preservatives on Healthy Human Skin Cells. Cells, 12(7), 1076. https://doi.org/10.3390/cells12071076







