Impact of Synthesized Indoloquinoline Analog to Isolates from Cryptolepis sanguinolenta on Tumor Growth Inhibition and Hepatotoxicity in Ehrlich Solid Tumor-Bearing Female Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Modeling Study
2.3. In Vivo Biological Assays
2.3.1. Animals
2.3.2. Ehrlich Solid Tumor (EST) Induction
2.3.3. Experimental Design
2.3.4. Tumor Weight and Volume
2.3.5. Samples
2.3.6. Histopathological Examination
2.3.7. Immunohistochemical Determination of Tumor Cell Proliferation and Apoptosis
2.3.8. Biochemical Assays
2.4. Statistical Analysis
3. Results
3.1. Chemistry
3.2. Modeling Studies
3.3. Tumor Weight and Volume
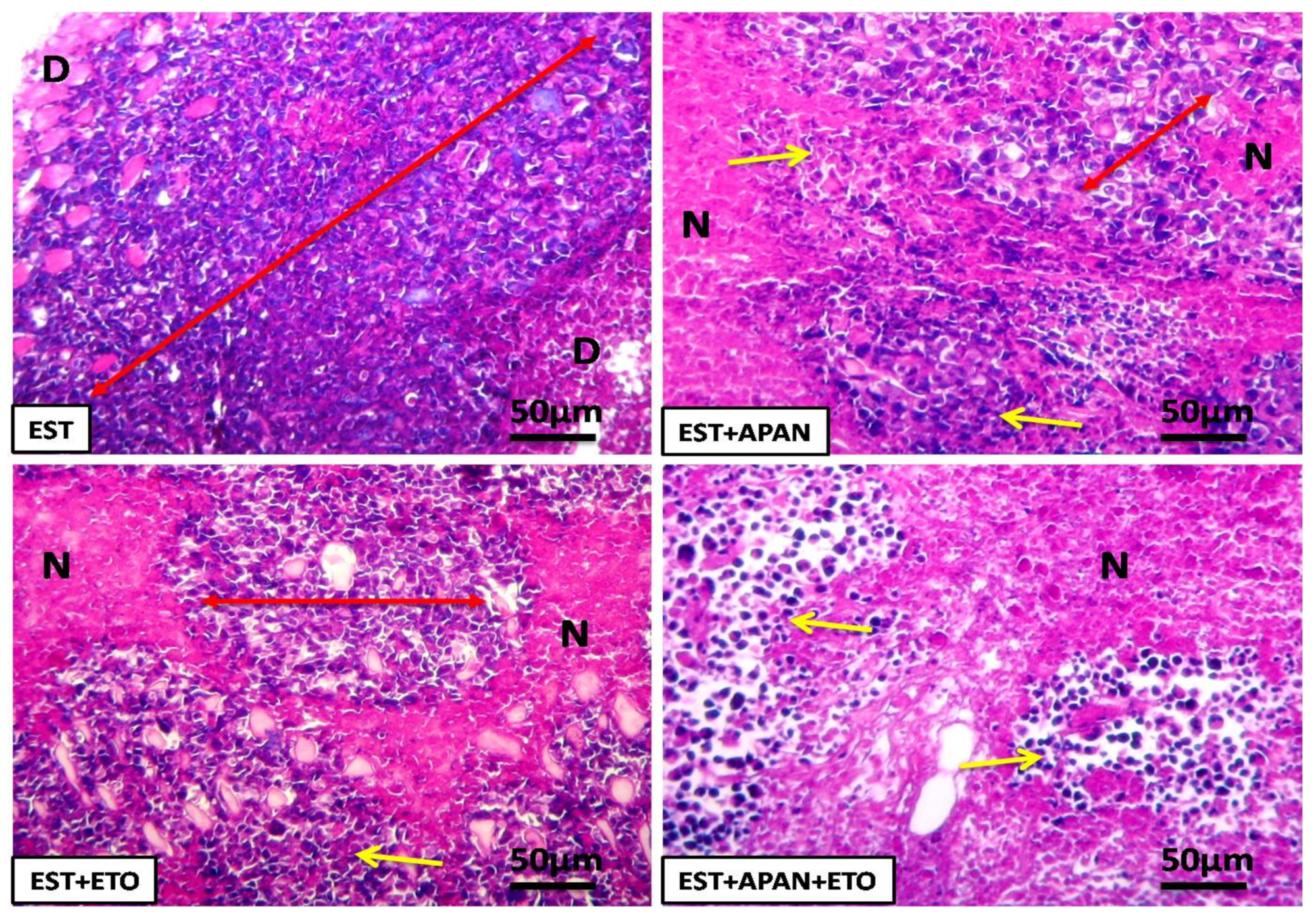
3.4. Histopathological Observations
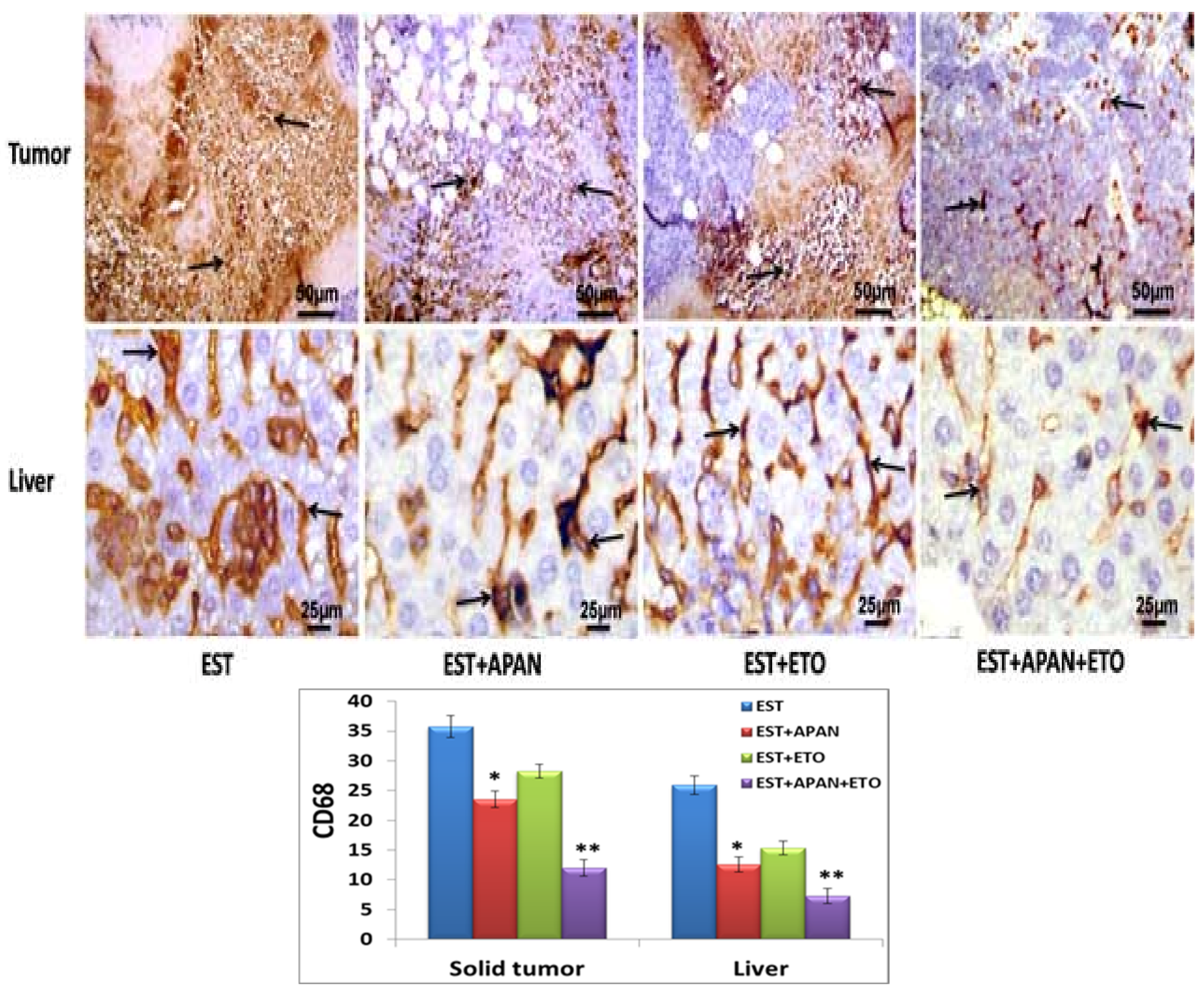
3.5. Immunoreactivity of CD68, TNF-α, and Survivin
3.6. Serum Tumor Marker
3.7. Liver Function Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soto, A.; Sonnenschein, C. Environmental causes of cancer: Endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 2010, 6, 363. [Google Scholar] [CrossRef] [PubMed]
- Tousson, E.; Hafez, E.; Zaki, S.; Gad, A. The cardioprotective effects of L-carnitine on rat cardiac injury, apoptosis, and oxidative stress caused by amethopterin. Environ. Sci. Pollut. Res. 2016, 23, 20600–20608. [Google Scholar] [CrossRef] [PubMed]
- Nofal, A.E.; Shatla, I.M.; Abdelhafeez, D.; Mustafa, M.; Aly, O.M. OMA1520 and OMA1774, novel 1,2,4-triazole bearing analogs of combretastatin A-4,inhibit hepatocellular carcinoma: Histological and immunohistochemical studies. Biomed. Pharmacother. 2021, 138, 111417. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, T.A.E.; Shaalan, N.H.A.; Tousson, E.; Buabeid, M.; Alyousef, A.M. The therapeutic and antineoplastic effects of vitamin B17 against the growth of solid-form Ehrlich tumors and the associated changes in oxidative stress, DNA damage, apoptosis, and proliferation in mice. Pak. J. Pharm. Sci. 2019, 32, 2801. [Google Scholar]
- Mutar, T.F.; Tousson, E.; Hafez, E.; Gazia, M.A.; Salem, S.B. Ameliorative effects of vitamin B17 on the kidney against Ehrlich ascites carcinoma induced renal toxicity in mice. Environ. Toxicol. 2020, 35, 528–537. [Google Scholar] [CrossRef]
- Tousson, E.; Hafez, E.; Gazia, M.M.; Salem, S.B.; Mutar, T.F. Hepatic ameliorative role of vitamin B17 against Ehrlich ascites carcinoma–induced liver toxicity. Environ. Sci. Pollut. Res. 2020, 27, 9236–9246. [Google Scholar] [CrossRef]
- Papież, M.A.; Krzyściak, W.; Szade, K.; Bukowska-Straková, K.; Kozakowska, M.; Hajduk, K.; Bystrowska, B.; Dulak, J.; Jozkowicz, A. Curcumin enhances the cytogenotoxic effect of Etoposide in leukemia cells through induction of reactive oxygen species. Drug Des. Dev. Ther. 2016, 10, 557. [Google Scholar] [CrossRef][Green Version]
- Jaramillo-Lambert, A.; Fabritius, A.S.; Hansen, T.J.; Smith, H.E.; Golden, A. The identification of a novel mutant allele of topoisomerase II in Caenorhabditis elegans reveals a unique role in chromosome segregation during spermatogenesis. Genetics 2016, 204, 1407–1422. [Google Scholar] [CrossRef]
- Tousson, E.; Bayom, M.F.; Ahmed, A.A. Rosemary extract modulates fertility potential, DNA fragmentation, injury, KI67 and P53 alterations induced by etoposide in rat testes. Biomed. Pharmacother. 2018, 98, 769–774. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.; International Natural Product Sciences Taskforc. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Wang, N.; Świtalska, M.; Wang, L.; Shaban, E.; Hossain, M.I.; El Sayed, I.E.T.; Wietrzyk, J.; Inokuchi, T. Structural Modifications of Nature-Inspired Indoloquinolines: A Mini Review of Their Potential Antiproliferative Activity. Molecules 2019, 24, 2121. [Google Scholar] [CrossRef]
- Eldaim, M.A.A.; Tousson, E.; Sayed, I.E.T.E.; Elmaksoud, A.Z.A.; Ahmed, A.A.S. Ameliorative effects of 9-diaminoacridine derivative against Ehrlich ascites carcinoma–induced hepatorenal injury in mice. Environ. Sci. Pollut. Res. 2021, 28, 21835. [Google Scholar] [CrossRef]
- Altwaijry, N.; El-Ghlban, S.; El Sayed, I.E.-T.; El-Bahnsawye, M.; Bayomi, A.I.; Samaka, R.M.; Shaban, E.; Elmongy, E.I.; El-Masry, T.A.; Ahmed, H.; et al. In Vitro and In Vivo antitumor activity of indolo [2,3-b] quinolines, natural product analogs from neocryptolepine alkaloid. Molecules 2021, 26, 754. [Google Scholar] [CrossRef]
- El-Boraey, H.A.L.; El-Gokha, A.A.A.; El-Sayed, I.E.T.; Azzam, M.A. Synthesis, characterization and anticancer activity of new Schiff bases bearing neocryptolepine. Med. Chem. Res. 2015, 24, 2142. [Google Scholar] [CrossRef]
- Abd Eldaim, M.A.; Tousson, E.; Soliman, M.M.; El Sayed, I.E.; Aleem, A.A.; Elsharkawy, H.N. Grape seed extract ameliorated Ehrlich solid tumor-induced hepatic tissue and DNA damage with reduction of PCNA and P53 protein expression in mice. Environ. Sci. Pollut. Res. 2021, 28, 44226–44238. [Google Scholar] [CrossRef]
- Sebeka, A.A.H.; Osman, A.M.A.; El Sayed, I.E.T.; El Bahanasawy, M.; Tantawy, M.A. Synthesis and antiproliferative activity of novel neocryptolepine-hydrazides hybrids. J. Appl. Pharm. Sci. 2017, 7, 9. [Google Scholar]
- Ahmed, A.A.S.; Awad, H.M.; El-Sayed, I.E.T.; El Gokha, A.A. Synthesis and antiproliferative activity of new hybrids bearing neocryptolepine, acridine and α-aminophosphonate scaffolds. J. Iran. Chem. Soc. 2020, 17, 1211. [Google Scholar] [CrossRef]
- El-Sayed, I.E.T.; Ullah, S.; Al-Hartomy, O.A.; Hasanein, A.M.; Ahmed, A.A.S.; Kahilo, K.A.; El-Naggar, M.E. Synthesis, Nanoformulations, and In Vitro Anticancer Activity of N-Substituted Side Chain Neocryptolepine Scaffolds. Molecules 2022, 27, 1024. [Google Scholar] [CrossRef]
- Dhar, S.; Datta, A.; Brosh, R.M., Jr. DNA helicases and their roles in cancer. DNA Repair 2020, 96, 102994. [Google Scholar] [CrossRef]
- Elmongy, E.I.; Altwaijry, N.; Attallah, N.G.M.; AlKahtani, M.M.; Henidi, H.A. In-Silico Screening of Novel Synthesized Thienopyrimidines Targeting Fms Related Receptor Tyrosine Kinase-3 and Their In-Vitro Biological Evaluation. Pharmaceuticals 2022, 15, 170. [Google Scholar] [CrossRef]
- Elmongy, E.I.; Henidi, H.A. In Silico Evaluation of a Promising Key Intermediate Thieno [2,3-d] Pyrimidine Derivative with Expected JAK2 Kinase Inhibitory Activity. Molbank 2022, 2022, M1352. [Google Scholar] [CrossRef]
- Kamble, P.; Kulkarni, S.; Bhiwgade, D.A. Ultrastructural and antioxidant studies of Etoposide treated kidney of rat. J Cancer Sci Ther. 2013, 5, 137. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971; p. 1432. [Google Scholar]
- Bahr, H.I.; Toraih, E.A.; Mohammed, E.A.; Mohammad, H.M.; Ali, E.A.; Zaitone, S.A. Chemopreventive effect of leflunomide against Ehrlich’s solid tumor grown in mice: Effect on EGF and EGFR expression and tumor proliferation. Life Sci. 2015, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Nishi, T.; Tamura, T.; Dev, S.; Takeshima, H.; Kochi, M.; Yoshizato, K.; Kuratsu, J.; Sakata, T.; Hofmann, G.; et al. Highly efficient electro-gene therapy of solid tumor by using an expression plasmid for the herpes simplex virus thymidine kinase gene. Proc. Natl. Acad. Sci. USA 2000, 97, 354. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, L.; Mironova, N.; Brenner, E.; Kurilshikov, A.; Patutina, O.; Zenkova, M. Alteration of the exDNA profile in blood seum of LLC-bearing mice under the decrease of tumour invasion potential by bovine pancreatic DNase I treatment. PLoS ONE 2017, 12, e01711988. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.; Layton, C.; Bancroft, J. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: London, UK, 2018; pp. 386–431. [Google Scholar]
- Tousson, E.; Hafez, E.; Zaki, S.; Gad, A. P53, Bcl-2 and CD68 expression in response to amethopterin-induced lung injury and ameliorating role of l-carnitine. Biomed. Pharmacother. 2014, 68, 631–639. [Google Scholar] [CrossRef]
- Calabrese, F.; Carturan, E.; Chimenti, C. Overexpression of tumor necrosis factor (TNF)α and TNFα receptor I in human viral myocarditis: Clinicopathologic correlations. Mod. Pathol. 2004, 17, 1108. [Google Scholar] [CrossRef]
- Das, A.; Tan, W.L.; Teo, J. Expression of Survivin in primary glioblastomas. J. Cancer Res. Clin. Oncol. 2002, 128, 302. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ, National Institutes of Health, Bethesda, MD, USA, 1997–2012. Available online: http://imagej.nih.gov/ij (accessed on 20 March 2023).
- Jang, E.S.; Jeong, S.H.; Kim, J.W.; Choi, Y.S.; Leissner, P.; Brechot, C. Diagnostic performance of alpha-fetoprotein, protein induced by vitamin K absence, osteopontin, Dickkopf-1 and its combinations for hepatocellular carcinoma. PLoS ONE 2016, 11, e0151069. [Google Scholar] [CrossRef]
- Kuusela, P.; Haglund, C.; Roberts, P.J. Comparison of a new tumour marker CA242 with CA19-9, CA50 and carcinoembryonic antigen (CEA) in digestive tract diseases. Br. J. Cancer 1991, 63, 636. [Google Scholar] [CrossRef]
- Eriksson, C.; Kokkonen, H.; Johansson, M.; Hallmans, G.; Wadell, G.; Rantapää-Dahlqvist, S. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res. Ther. 2011, 13, 1. [Google Scholar] [CrossRef]
- Saggu, S.; Sakeran, M.I.; Zidan, N.; Tousson, E.; Mohan, A.; Rehman, H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem. Toxicol. 2014, 72, 138–146. [Google Scholar] [CrossRef]
- Moss, D.; Henderson, A. Clinical enzymology. In Tietz Textbook of Clinical Chemistry; WB Saunders Company: Philadelphia, PA, USA, 1999; p. 37. [Google Scholar]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1997, 258, 21. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Mishra, S.; Tamta, A.K.; Sarikhani, M. Subcutaneous Ehrlich Ascites Carcinoma mice model for studying cancer-induced cardiomyopathy. Sci. Rep. 2018, 8, 5599. [Google Scholar] [CrossRef]
- Akkachairin, B.; Rodphon, R.W.O.; Mungthin, M.; Tummatorn, J.; Thongsornkleeb, C.; Ruchirawat, S. Synthesis of neocryptolepines and carbocycle-fused quinolines and evaluation of their anticancer and antiplasmodial activities. Bioorg. Chem. 2020, 1, 103732. [Google Scholar] [CrossRef]
- Riechert-Krause, F.; Weisz, K. Indoloquinolines as DNA binding ligands. Heterocycl. Commun. 2013, 19, 145. [Google Scholar] [CrossRef]
- Zhang, W.; Gou, P.; Dupret, J.M.; Chomienne, C.; Rodrigues-Lima, F. Etoposide, an anticancer drug involved in therapy-related secondary leukemia: Enzymes at play. Etoposide, an anticancer drug involved in therapy-related secondary leukemia: Enzymes at play. Transl. Oncol. 2021, 14, 101169. [Google Scholar] [CrossRef]
- Gupta, S.; Mathur, R.; Dwarakanath, B. The glycolytic inhibitor 2-deoxy-D-glucose enhances the efficacy of etoposide in ehrlich ascites tumor bearing mice. Cancer Biol. Ther. 2005, 4, 94. [Google Scholar] [CrossRef]
- El-Naa, M.M.; Othman, M.; Younes, S. Sildenafil potentiates the antitumor activity of cisplatin by induction of apoptosis and inhibition of proliferation and angiogenesis. Drug Des. Dev. Ther. 2016, 10, 3661. [Google Scholar] [CrossRef]
- Ray, T.; Chakrabarti, M.K.; Pal, A. Hemagglutinin protease secreted by V. cholerae induced apoptosis in breast cancer cells by ROS mediated intrinsic pathway and regresses tumor growth in mice model. Apoptosis 2016, 21, 143. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; El-Masry, T.A.; Tousson, E.; Hassan, H.M.; Al-Ghadeer, A. Hepatic protective effect of grape seed proanthocyanidin extract against Gleevec-induced apoptosis, liver Injury and Ki67 alterations in rats. Braz. J. Pharm. Sci. 2018, 54, e17391. [Google Scholar] [CrossRef]
- Amin, D.M.; Abaza, M.T.; Sarhaan, W.M.; Ahmed, A.I.; Moustafa, A.A. The ameliorative effect of L-arginin and omega-3 fatty acid against sodium valproate induced hepatotoxicity. J. Toxicol. Environ. Health 2018, 10, 20. [Google Scholar] [CrossRef]
- Singh, L.; Shrivastav, A.; Verma, N. Effect of L-arginine amino acid on liver regeneration after hepatocyte damage in rats: An experimental study. Drug Deliv. Ther. 2019, 9, 470–476. [Google Scholar] [CrossRef]
- Abd Eldaim, M.A.; Tousson, E.; El Sayed, I.E.T.; Abd, E.A.E.; Elsharkawy, H.N. Grape seeds proanthocyanidin extract ameliorates Ehrlich solid tumor induced renal tissue and DNA damage in mice. Biomed. Pharmacother. 2019, 115, 108908. [Google Scholar] [CrossRef]
- Neamatallah, T.; El-Shitany, N.; Abbas, A.; Eid, B.; Harakeh, S.; Ali, S.; Mousa, S. Nano ellagic acid counteracts cisplatin-induced upregulation in OAT1 and OAT3: A possible nephroprotection mechanism. Molecules 2020, 25, 3031. [Google Scholar] [CrossRef]
- Meshcheryakova, A.; Svoboda, M.; Jaritz, M.; Mungenast, F.; Salzmann, M.; Pils, D.; Mechtcheriakova, D. Interrelations of sphingolipid and lysophosphatidate signaling with immune system in ovarian cancer. Comput. Struct. Biotechnol. J. 2019, 17, 537. [Google Scholar] [CrossRef]
- Lanaya, H.; Natarajan, A.; Komposch, K.; Li, L.; Amberg, N.; Chen, L.; Sibilia, M. EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nat. Cell Biol. 2014, 16, 972–981. [Google Scholar] [CrossRef]
- Chun, S.H.; Lee, H.A.; Lee, K.B.; Kim, S.H.; Park, K.Y.; Lee, K.W. Effects of glycated whey protein concentrate on pro-inflammatory cytokine expression and phagocytic activity in RAW264. 7 macrophages. Biol. Pharm. Bull. 2016, 39, 199. [Google Scholar] [CrossRef]
- Ruder, B.; Atreya, R.; Becker, C. Tumour Necrosis Factor Alpha in Intestinal Homeostasis and Gut Related Diseases. Int. J. Mol. Sci. 2019, 16, 1887. [Google Scholar] [CrossRef]
- Turculeanu, A.; Mogoanta, C.A.; IoniTa, E.; Avrămescu, C.S.; Afrem, M.C.; Costache, A. TNF-alpha evaluation in tonsil cancer. Rom. J. Morphol. Embryol. 2015, 56, 101. [Google Scholar]
- Yu, Y.; Ke, L.; Xia, W.X.; Lv, Y.; Xiang, X. Elevated Levels of TNF-α and decreased levels of CD68-positive macrophages in primary tumor tissues are unfavorable for the survival of patients with nasopharyngeal carcinoma. J. Bu Technol. Cancer Res. Treat. 2019, 18, 1533033819874807. [Google Scholar] [CrossRef] [PubMed]
- Aldubayan, M.A.; Elgharabawy, R.M.; Ahmed, A.S.; Tousson, E. Antineoplastic activity and curative role of avenanthramides against the growth of ehrlich solid tumors in mice. Oxid. Med. Cell. Longev. 2019, 2019, 5162687. [Google Scholar] [CrossRef]
- Lechler, P.; Wu, X.; Bernhardt, W.; Campean, V.; Gastiger, S.; Hackenbeck, S.; Klanke, B.; Weidemann, A.; Warnecke, C.; Amann, K.; et al. The Tumor Gene Survivin Is Highly Expressed in Adult Renal Tubular Cells. Am. J. Pathol. 2007, 171, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, H.; Zhou, Z.; Tian, Y.; Du, K.; Zhang, H.; Jiang, X.; Lu, J.; Niu, Y.; Tu, L.; et al. CFNC, a neocryptolepine de-rivative, inhibited the growth of gastric cancer AGS cells by inhibiting PI3K/AKT signaling pathway. Eur. J. Pharmacol. 2023, 938, 175408. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.D.; Tang, A.; Li, K.F.; Li, P.; Wang, H.L.; Yi, J.P.; Yan, J.B. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang province, China, 2020. Emerg. Infect. Dis. 2020, 26, 1052. [Google Scholar] [CrossRef] [PubMed]
- Oshiba, E.; Touson, E.; Elsherbini, Y.; Abdraboh, M.E. Melatonin: A regulator of the interplay between FoxO1, miR96, and miR215 signaling to diminish the growth, survival, and metastasis of murine adenocarcinoma. BioFactors 2021, 47, 740–753. [Google Scholar] [CrossRef]
- Dolai, N.; Karmakar, I.; Kumar, R.S.; Kar, B.; Bala, A.; Haldar, P.K. Evaluation of antitumor activity and in vivo antioxidant status of Anthocephaluscadamba on Ehrlich ascites carcinoma treated mice. J. Ethnopharmacol. 2012, 142, 865. [Google Scholar] [CrossRef]
- Abou Zaid, O.A.; Hassanein, M.R.; EL-Senosi, Y.A.; EL-Shiekha, M.F. Ameliorative effect of curcumin and tannic acid on tumor-induced in female mice. World Journal of Pharmaceutical Sciences. World J. Pharm. Res. 2014, 1, 259. [Google Scholar]
- Badr, O.M.; Abd-Eltawab, H.M.; Sakr, S.A. Ameliorative effect of ginger extract against pathological alterations induced in mice bearing solid tumors. J. Biosci. Appl. Res. 2016, 1, 185. [Google Scholar] [CrossRef]
- Morsi, D.S.; Salem, M.L.; Ibrahim, H.M.; Osman, G.Y.; Mohamed, A.H.; Nofal, A.E. Synergistic and chemosensitizing effects of bovine lactoferrin or muramyl dipeptide in Ehrlich solid tumor-bearing mice treated with cisplatin. Int. J. Cancer Biomed. Res. (IJCBR) 2020, 5, 75–94. [Google Scholar]
- El Nabi, S.E.; El-Garawani, I.M.; Salman, A.M.; Ouda, R.I. The Possible Antigenotoxic Potential of Ginger Oil on Etoposide–Treated Albino Rats. Saudi J. Med. Pharm. Sci. 2017, 3, 693. [Google Scholar] [CrossRef]
- Al-Ameri, A.S. Prevention of Etoposide induced kidney toxicity, electrolytes, injury and KI67 alternations in male rats treated with star anise. Biosci. Appl. Res. 2017, 1, 36. [Google Scholar] [CrossRef]
- Nofal, A.; Fayad, R. Enhanced Effect of Resveratrol on Hepatocellular Carcinoma of Rats Treated with 5-Fluorouracil. Adv. Anim. Vet. 2021, 9, 1978–1988. [Google Scholar] [CrossRef]

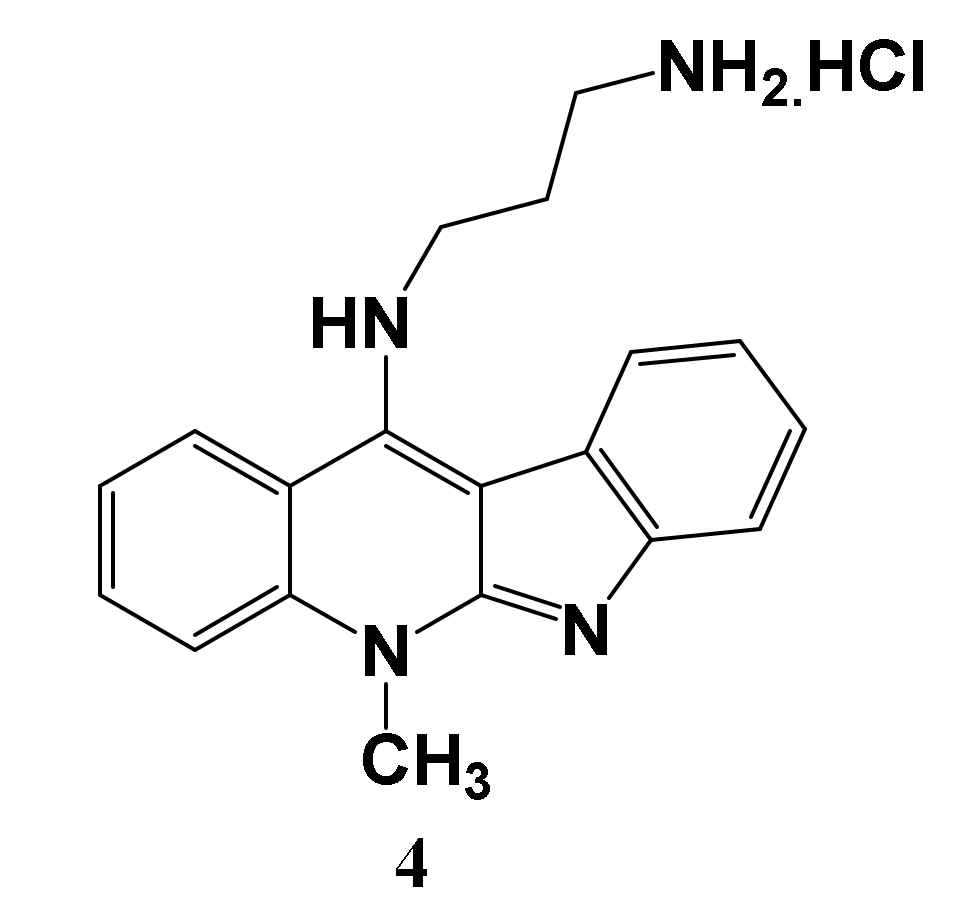
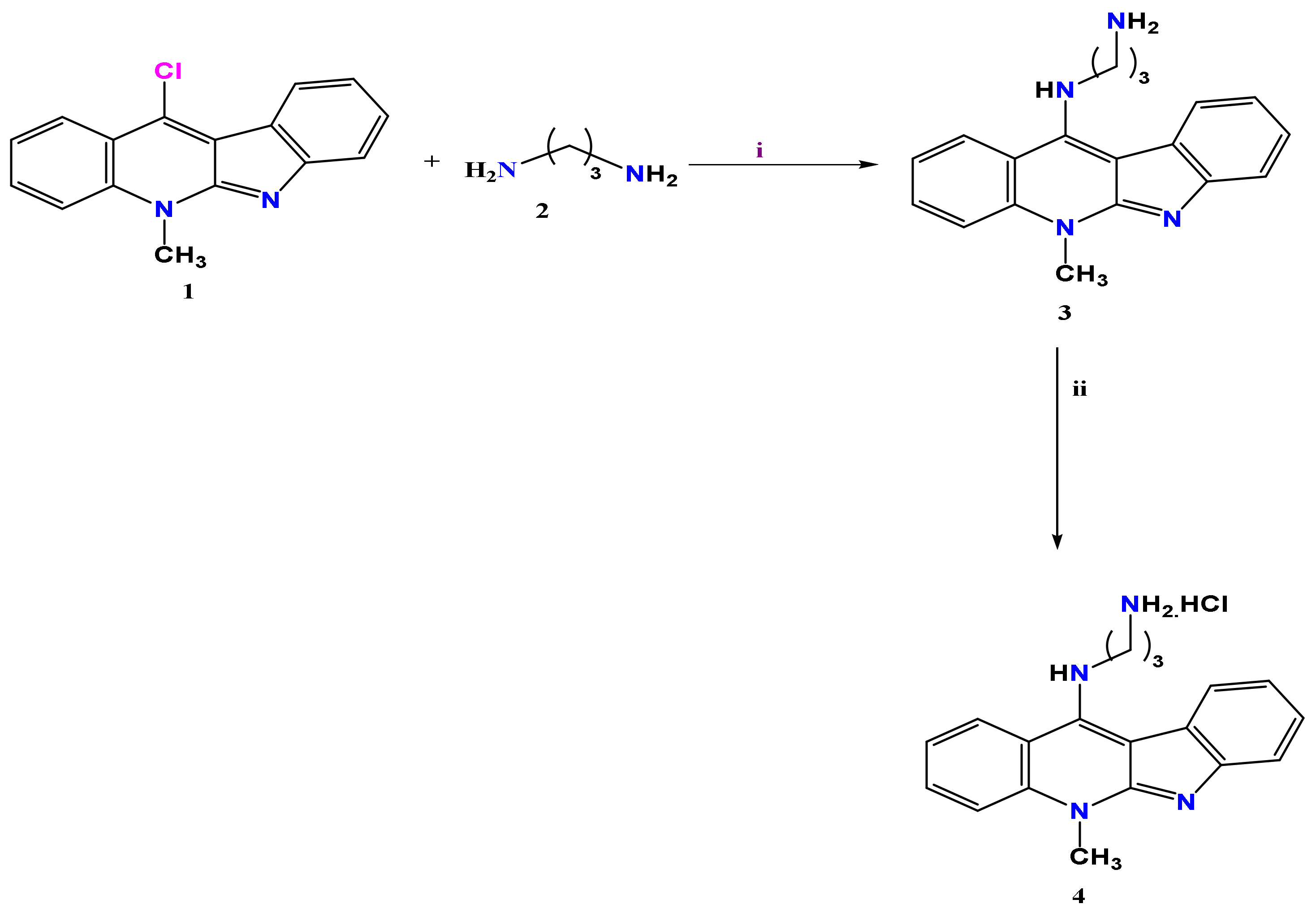
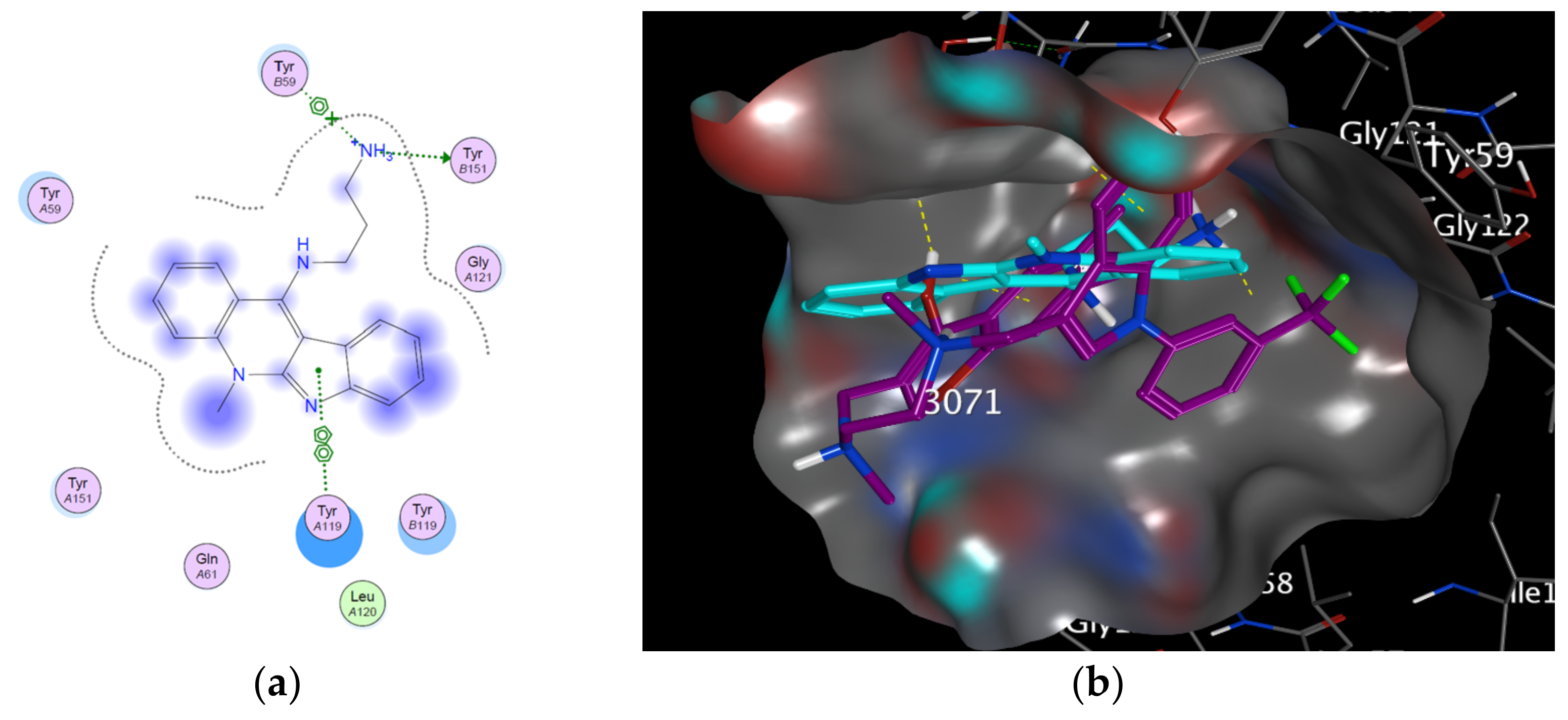
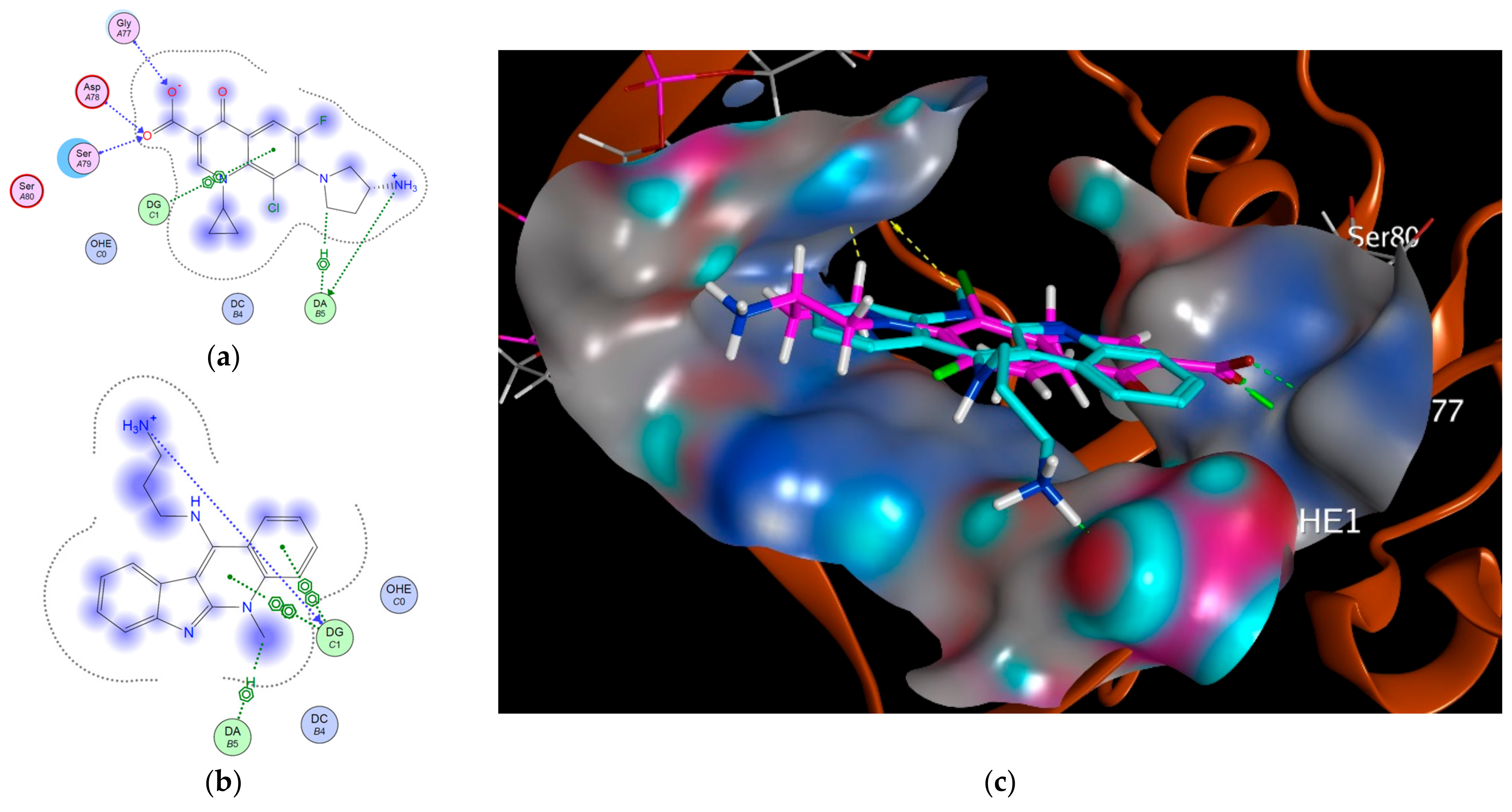




| Target | Protein Code | Binding Affinity | RMSD | Residues Involved in Interaction |
|---|---|---|---|---|
| TNF-α | 2AZ5 | −5.0704 | 2.08865 | TYR 151: H-donor TYR 59: π stacking (pi-cation). TYR 119: π–π |
| Topoisomerase II | 3FOE | −5.5996 | 1.1698 | DG: H-donor DG: Ionic DA: H–pi DG: π–π DG: π–π DG: π–π |
| Experimental Groups | Tumor Weight (g) | Tumor Volume (mm3) | Reduction Ratio (%) |
|---|---|---|---|
| EST | 1.2475 ± 0.0894 | 1.49 ± 0.23 | — |
| EST+APAN | 0.6367 ± 0.0410 * | 1.21 ± 0.36 | 19.8 * |
| EST+ETO | 0.5775 ± 0.0406 * | 1.06 ± 0.41 | 29.9 * |
| EST+APAN+ETO | 0.2522 ± 0.0086 ** | 0.82 ± 0.40 | 45 ** |
| Alterations | EST | EST+APAN | EST+ETO | EST+APAN+ETO |
|---|---|---|---|---|
| Cell degeneration | ++++ | ++ | +++ | + |
| Necrosis | ++++ | ++ | +++ | + |
| Cellular inflammatory infiltration | ++ | ++ | +++ | + |
| Tumor cell | ++++ | +++ | ++ | + |
| Intracytoplasmic vacuolation | ++ | + | +++ | + |
| Giant cell | +++ | ++ | + | + |
| Immuno-expression of CD68 | ++++ | ++ | +++ | + |
| Immuno-expression of TNF-α | ++++ | +++ | ++ | + |
| Immuno-expression of survivin | ++++ | +++ | ++ | + |
| Parameters | AFP (ng/mL) | CEA (ng/mL) | Anti-dsDNA (U/mL) | |
|---|---|---|---|---|
| Groups | ||||
| Control | 0.1043 ± 0.0156 | 0.3046 ± 0.0398 | 9.7937 ± 0.2028 | |
| APAN | 0.2030 ± 0.0065 | 0.3454 ± 0.0171 | 16.9925 ± 1.5185 | |
| ETO | 0.1341 ± 0.0113 | 0.1829 ± 0.0120 | 12.0663 ± 1.9776 | |
| APAN+ETO | 0.2203 ± 0.0053 | 0.2526 ± 0.0159 | 16.7925 ± 1.3601 | |
| EST | 0.9876 ± 0.0430 * | 1.2844 ± 0.1128 * | 36.2700 ± 1.7377 * | |
| EST+APAN | 0.3946 ± 0.0349 ** | 0.5754 ± 0.0319 ** | 17.1512 ± 1.8841 ** | |
| EST+ETO | 0.3628 ± 0.0400 ** | 0.4975 ± 0.0195 ** | 16.1287 ± 1.1369 ** | |
| EST+APAN+ETO | 0.1559 ± 0.0184 ** | 0.3011 ± 0.0429 ** | 13.7000 ± 0.5397 ** | |
| Parameters | ALT (U/I) | AST (U/I) | ALB (gm/dl) | ALP (U/I) | Total Protein (gm/dL) | |
|---|---|---|---|---|---|---|
| Groups | ||||||
| Control | 46.3750 ± 2.6693 | 142.7500 ± 3.2404 | 4.4175 ± 0.0604 | 128.5000 ± 4.6904 | 5.9962 ± 0.0427 | |
| APAN | 66.3750 ± 2.6693 | 219.7500 ± 3.4949 | 3.2550 ± 0.0288 | 150.5000 ± 3.1623 | 6.3275 ± 0.0396 | |
| ETO | 73.1000 ± 2.9799 | 240.3750 ± 3.3780 | 3.8125 ± 0.0396 | 161.8750 ± 3.3139 | 5.6188 ± 0.0494 | |
| APAN+ETO | 75.2500 ± 3.1623 | 229.6250 ± 3.2486 | 3.3975 ± 0.0324 | 215.2500 ± 2.8158 | 6.0000 ± 0.0346 | |
| EST | 94.2500 ± 4.4641 * | 304.8750 ± 3.7961 * | 3.4750 ± 0.0374 * | 193.8750 ± 3.1820 * | 5.0075 ± 0.0427 * | |
| EST+APAN | 90.8750 ± 4.0156 ** | 160.3750 ± 3.5026 ** | 3.6850 ± 0.0245 ** | 142.8750 ± 2.9001 ** | 5.5625 ± 0.0377 ** | |
| EST+ETO | 78.3125 ± 3.1046 ** | 144.0000 ± 3.4641 ** | 3.5312 ± 0.0327 ** | 137.3750 ± 3.7009 ** | 5.3112 ± 0.03488 ** | |
| EST+APAN+ETO | 53.8750 ± 4.4861 ** | 104.1250 ± 2.9001 ** | 3.7538 ± 0.0463 ** | 101.2500 ± 4.0620 ** | 5.5375 ± 0.0420 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nofal, A.E.; Elmongy, E.I.; Hassan, E.A.; Tousson, E.; Ahmed, A.A.S.; El Sayed, I.E.T.; Binsuwaidan, R.; Sakr, M. Impact of Synthesized Indoloquinoline Analog to Isolates from Cryptolepis sanguinolenta on Tumor Growth Inhibition and Hepatotoxicity in Ehrlich Solid Tumor-Bearing Female Mice. Cells 2023, 12, 1024. https://doi.org/10.3390/cells12071024
Nofal AE, Elmongy EI, Hassan EA, Tousson E, Ahmed AAS, El Sayed IET, Binsuwaidan R, Sakr M. Impact of Synthesized Indoloquinoline Analog to Isolates from Cryptolepis sanguinolenta on Tumor Growth Inhibition and Hepatotoxicity in Ehrlich Solid Tumor-Bearing Female Mice. Cells. 2023; 12(7):1024. https://doi.org/10.3390/cells12071024
Chicago/Turabian StyleNofal, Amany E., Elshaymaa I. Elmongy, Engy Abo Hassan, Ehab Tousson, Abdullah A. S. Ahmed, Ibrahim El Tantawy El Sayed, Reem Binsuwaidan, and Manar Sakr. 2023. "Impact of Synthesized Indoloquinoline Analog to Isolates from Cryptolepis sanguinolenta on Tumor Growth Inhibition and Hepatotoxicity in Ehrlich Solid Tumor-Bearing Female Mice" Cells 12, no. 7: 1024. https://doi.org/10.3390/cells12071024
APA StyleNofal, A. E., Elmongy, E. I., Hassan, E. A., Tousson, E., Ahmed, A. A. S., El Sayed, I. E. T., Binsuwaidan, R., & Sakr, M. (2023). Impact of Synthesized Indoloquinoline Analog to Isolates from Cryptolepis sanguinolenta on Tumor Growth Inhibition and Hepatotoxicity in Ehrlich Solid Tumor-Bearing Female Mice. Cells, 12(7), 1024. https://doi.org/10.3390/cells12071024





