Venom Peptides, Polyphenols and Alkaloids: Are They the Next Antidiabetics That Will Preserve β-Cell Mass and Function in Type 2 Diabetes?
Abstract
1. Introduction
2. Peptides and Substances Stimulating Insulin Secretion
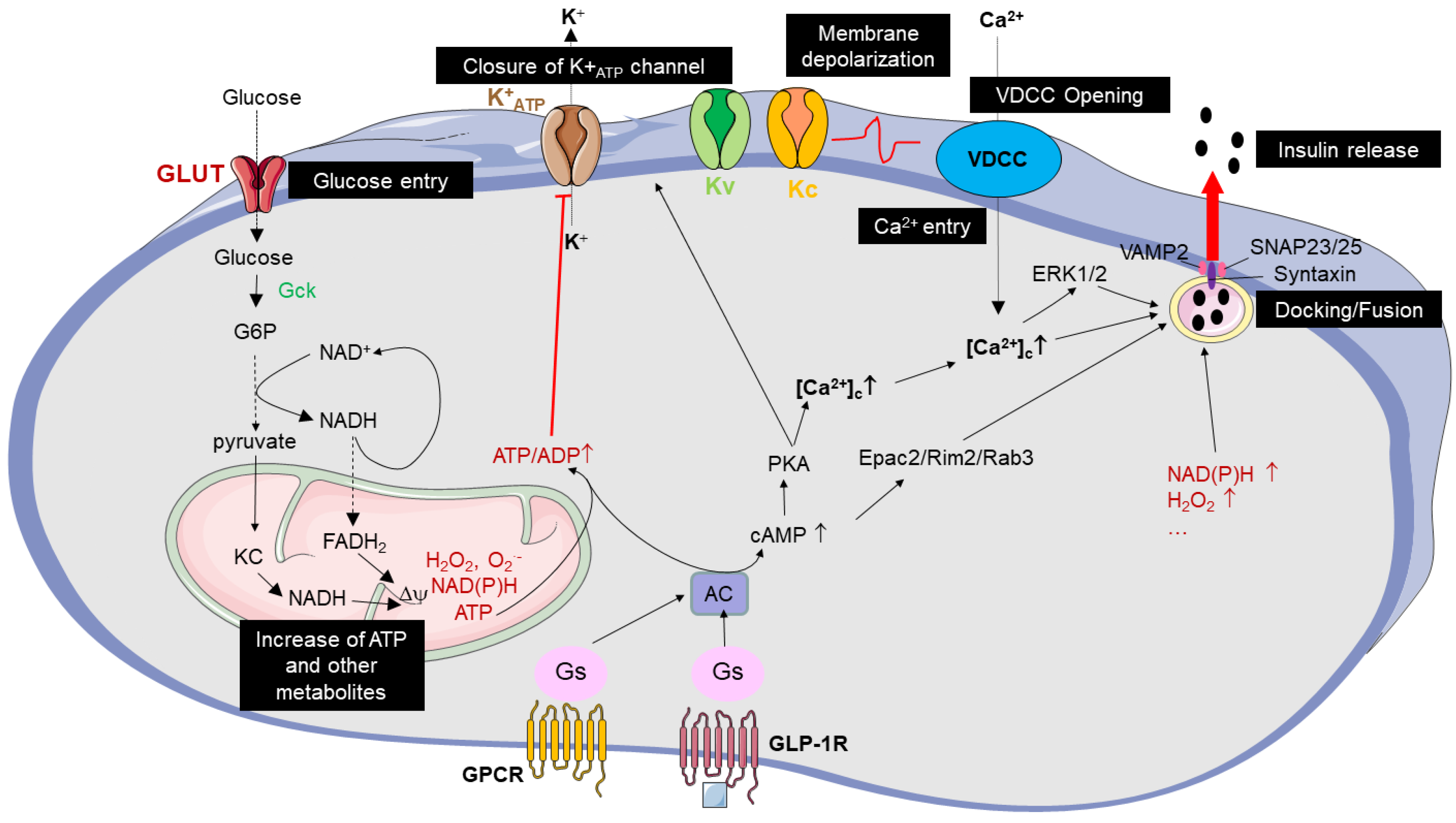
2.1. Key Pathways Regulating Glucose-Induced Insulin Secretion
2.2. Peptides from Animal Venoms That Act as Insulin Secretagogues
2.2.1. Venom Peptides as New GLP-1RAs
2.2.2. K+ATP Channel Inhibitor Peptides from Venom
2.2.3. Venom Peptides Inhibiting Voltage-Dependent (Kv) and Calcium-Activated (Kc) Potassium Channels
2.2.4. Peptides That Stimulate Insulin Secretion in a Not Yet Identified Mechanisms
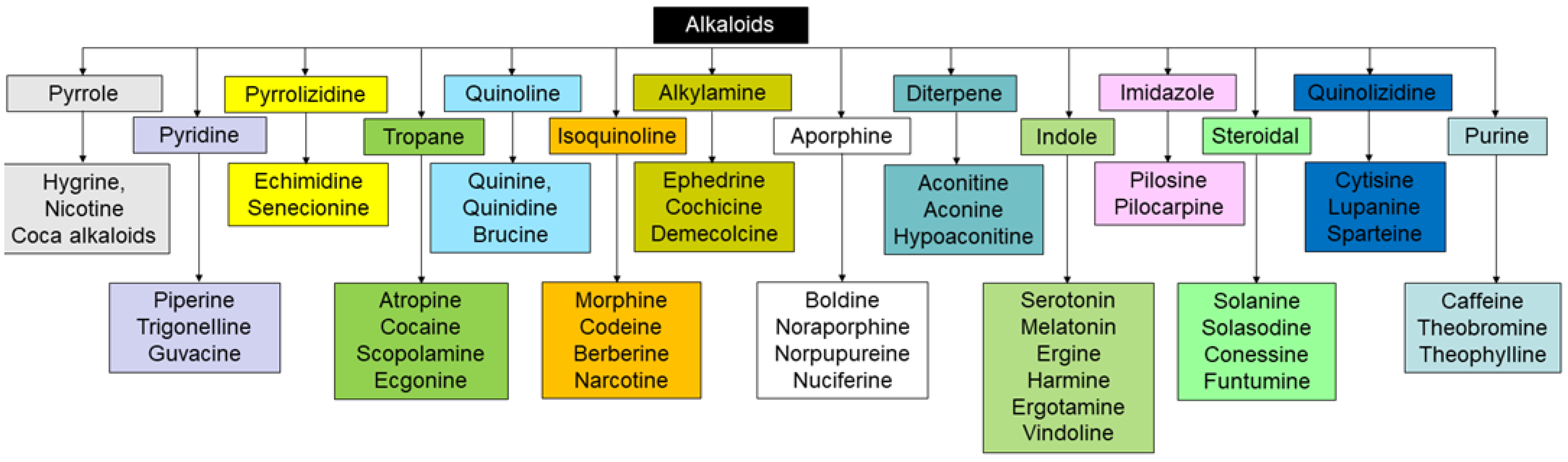
2.3. Polyphenols and Alkaloids from Plants Stimulating Insulin Secretion
3. Venom Peptides, Polyphenols and Alkaloids Protecting β-Cells against Death Induced by Diabetogenic Environments
3.1. Preserving β-Cell Mass in T2D by Antagonizing ER Stress, Oxidative Stress and Autophagy as the Paradigm for Achieving Long-Term Glycemic Control in T2D
3.2. Survival Proteins of β-Cells Revealed by GLP-1RAs
3.3. Peptides from Venoms That Protect β-Cells against Death by Targeting β-Cell Survival Proteins
3.4. Polyphenols and Alkaloids That Protect β-Cells against Death by Targeting the β-Cell Survival Proteins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bungau, S.; Popa, V.-C. Between Religion and Science: Some Aspects: Concerning Illness and Healing in Antiquity. Transylv. Rev. 2015, XXIV, 3–19. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Merchant, J. Frederick Banting (1891–1941): Discoverer of Insulin. Singap. Med. J. 2017, 58, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Historical Overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Drzewoski, J.; Hanefeld, M. The Current and Potential Therapeutic Use of Metformin—The Good Old Drug. Pharmaceuticals 2021, 14, 122. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Z.; Zhang, C.; Cai, Z.; Zhang, J. Metformin, beyond an Insulin Sensitizer, Targeting Heart and Pancreatic β Cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1984–1990. [Google Scholar] [CrossRef]
- Bays, H. Sodium Glucose Co-Transporter Type 2 (SGLT2) Inhibitors: Targeting the Kidney to Improve Glycemic Control in Diabetes Mellitus. Diabetes Ther. 2013, 4, 195–220. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 Inhibitors in Patients with Heart Failure with Reduced Ejection Fraction: A Meta-Analysis of the EMPEROR-Reduced and DAPA-HF Trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT-2 Inhibitors in Patients with Heart Failure: A Comprehensive Meta-Analysis of Five Randomised Controlled Trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef]
- White, J.R., Jr. Apple Trees to Sodium Glucose Co-Transporter Inhibitors: A Review of SGLT2 Inhibition. Clin. Diabetes 2010, 28, 5–10. [Google Scholar] [CrossRef]
- Stiles, P.G.; Lusk, G. On the Action of Phlorhizin. Am. J. Physiol.-Leg. Content 1903, 10, 67–79. [Google Scholar] [CrossRef]
- Abderrahmani, A.; Jacovetti, C.; Regazzi, R. Lessons from Neonatal β-Cell Epigenomic for Diabetes Prevention and Treatment. Trends Endocrinol. Metab. 2022, S1043-2760(22)00054-6. [Google Scholar] [CrossRef]
- Iorga, R.A.; Bacalbasa, N.; Carsote, M.; Bratu, O.G.; Stanescu, A.M.A.; Bungau, S.; Pantis, C.; Diaconu, C.C. Metabolic and Cardiovascular Benefits of GLP-1 Agonists, besides the Hypoglycemic Effect (Review). Exp. Ther. Med. 2020, 20, 2396–2400. [Google Scholar] [CrossRef]
- Yusta, B.; Baggio, L.L.; Estall, J.L.; Koehler, J.A.; Holland, D.P.; Li, H.; Pipeleers, D.; Ling, Z.; Drucker, D.J. GLP-1 Receptor Activation Improves Beta Cell Function and Survival Following Induction of Endoplasmic Reticulum Stress. Cell Metab. 2006, 4, 391–406. [Google Scholar] [CrossRef]
- Rahier, J.; Guiot, Y.; Goebbels, R.M.; Sempoux, C.; Henquin, J.C. Pancreatic Beta-Cell Mass in European Subjects with Type 2 Diabetes. Diabetes Obes. Metab. 2008, 10 (Suppl. S4), 32–42. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-Cell Deficit and Increased Beta-Cell Apoptosis in Humans with Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Garvey, W.T.; Olefsky, J.M.; Griffin, J.; Hamman, R.F.; Kolterman, O.G. The Effect of Insulin Treatment on Insulin Secretion and Insulin Action in Type II Diabetes Mellitus. Diabetes 1985, 34, 222–234. [Google Scholar] [CrossRef]
- Raufman, J.P.; Jensen, R.T.; Sutliff, V.E.; Pisano, J.J.; Gardner, J.D. Actions of Gila Monster Venom on Dispersed Acini from Guinea Pig Pancreas. Am. J. Physiol. 1982, 242, G470–G474. [Google Scholar] [CrossRef]
- Göke, R.; Fehmann, H.C.; Linn, T.; Schmidt, H.; Krause, M.; Eng, J.; Göke, B. Exendin-4 Is a High Potency Agonist and Truncated Exendin-(9-39)-Amide an Antagonist at the Glucagon-like Peptide 1-(7-36)-Amide Receptor of Insulin-Secreting Beta-Cells. J. Biol. Chem. 1993, 268, 19650–19655. [Google Scholar] [CrossRef]
- Abderrahmani, A.; Szunerits, S.; Dalle, S.; Boukherroub, R. Chapter 3: Optimizing the Current Type 2 Diabetes Antidiabetics with Nanotechnologies: Where Do We Stand? In Nanotechnology for Diabetes Management; Abderrahmani, A., Dalle, S., Szunerits, S., Boukherroub, B., El Ouaamari, A., Eds.; Royal Society of Chemistry: London, UK, 2022; pp. 92–112. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes–State-of-the-Art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Grant, R.W.; Pirraglia, P.A.; Meigs, J.B.; Singer, D.E. Trends in Complexity of Diabetes Care in the United States from 1991 to 2000. Arch. Intern. Med. 2004, 164, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- MacLean, C.D.; Littenberg, B.; Kennedy, A.G. Limitations of Diabetes Pharmacotherapy: Results from the Vermont Diabetes Information System Study. BMC Fam. Prac. 2006, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Tsujimoto, T.; Goto, A.; Goto, M.; Kishimoto, M.; Yamamoto-Honda, R.; Noto, H.; Kajio, H.; Noda, M. Prediction of Response to GLP-1 Receptor Agonist Therapy in Japanese Patients with Type 2 Diabetes. Diabetol. Metab. Syndr. 2014, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, L.; Rosenfeld, N.K.; Fuller, C.S.; Gallop, R.; Schutta, M.H.; Rickels, M.R. Effect of Exenatide, Sitagliptin, or Glimepiride on β-Cell Secretory Capacity in Early Type 2 Diabetes. Diabetes Care 2014, 37, 2451–2458. [Google Scholar] [CrossRef]
- Kahn, S.E.; Carr, D.B.; Faulenbach, M.V.; Utzschneider, K.M. An Examination of Beta-Cell Function Measures and Their Potential Use for Estimating Beta-Cell Mass. Diabetes Obes. Metab. 2008, 10 (Suppl. S4), 63–76. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Calderara, A.; Pozza, G. Secondary Failure of Oral Hypoglycaemic Agents: Frequency, Possible Causes, and Management. Diabetes Metab. Rev. 1994, 10, 31–43. [Google Scholar] [CrossRef]
- U.K. Prospective Diabetes Study Group. U.K. Prospective Diabetes Study 16. Overview of 6 Years’ Therapy of Type II Diabetes: A Progressive Disease. Diabetes 1995, 44, 1249–1258. [Google Scholar] [CrossRef]
- Hambrock, A.; de Oliveira Franz, C.B.; Hiller, S.; Osswald, H. Glibenclamide-Induced Apoptosis Is Specifically Enhanced by Expression of the Sulfonylurea Receptor Isoform SUR1 but Not by Expression of SUR2B or the Mutant SUR1(M1289T). J. Pharmacol. Exp. Ther. 2006, 316, 1031–1037. [Google Scholar] [CrossRef]
- Iwakura, T.; Fujimoto, S.; Kagimoto, S.; Inada, A.; Kubota, A.; Someya, Y.; Ihara, Y.; Yamada, Y.; Seino, Y. Sustained Enhancement of Ca(2+) Influx by Glibenclamide Induces Apoptosis in RINm5F Cells. Biochem. Biophys. Res. Commun. 2000, 271, 422–428. [Google Scholar] [CrossRef]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. Sulfonylureas and Their Use in Clinical Practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef]
- Khunti, K.; Chatterjee, S.; Gerstein, H.C.; Zoungas, S.; Davies, M.J. Do Sulphonylureas Still Have a Place in Clinical Practice? Lancet Diabetes Endocrinol. 2018, 6, 821–832. [Google Scholar] [CrossRef]
- Del Prato, S.; Pulizzi, N. The Place of Sulfonylureas in the Therapy for Type 2 Diabetes Mellitus. Metabolism 2006, 55, S20–S27. [Google Scholar] [CrossRef]
- Corrêa, A.S.; Vinson, C.C.; Braga, L.S.; Guedes, R.N.C.; Oliveira, L.O. de Ancient Origin and Recent Range Expansion of the Maize Weevil Sitophilus Zeamais, and Its Genealogical Relationship to the Rice Weevil S. Oryzae. Bull. Entomol. Res. 2017, 107, 9–20. [Google Scholar] [CrossRef]
- Henquin, J.-C. Glucose-Induced Insulin Secretion in Isolated Human Islets: Does It Truly Reflect β-Cell Function in Vivo? Mol. Metab. 2021, 48, 101212. [Google Scholar] [CrossRef]
- Williams, D.; Vicôgne, J.; Zaitseva, I.; McLaughlin, S.; Pessin, J.E. Evidence That Electrostatic Interactions between Vesicle-Associated Membrane Protein 2 and Acidic Phospholipids May Modulate the Fusion of Transport Vesicles with the Plasma Membrane. Mol. Biol. Cell. 2009, 20, 4910–4919. [Google Scholar] [CrossRef]
- Gaisano, H.Y. Recent New Insights into the Role of SNARE and Associated Proteins in Insulin Granule Exocytosis. Diabetes Obes. Metab. 2017, 19 (Suppl. S1), 115–123. [Google Scholar] [CrossRef]
- Abderrahmani, A.; Plaisance, V.; Lovis, P.; Regazzi, R. Mechanisms Controlling the Expression of the Components of the Exocytotic Apparatus under Physiological and Pathological Conditions. Biochem. Soc. Trans. 2006, 34, 696–700. [Google Scholar] [CrossRef]
- Kalwat, M.A.; Cobb, M.H. Mechanisms of the Amplifying Pathway of Insulin Secretion in the β Cell. Pharmacol. Ther. 2017, 179, 17–30. [Google Scholar] [CrossRef]
- Mobli, M.; Undheim, E.A.B.; Rash, L.D. Modulation of Ion Channels by Cysteine-Rich Peptides: From Sequence to Structure. Adv. Pharmacol. 2017, 79, 199–223. [Google Scholar] [CrossRef]
- Bordon, K.d.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Coulter-Parkhill, A.; McClean, S.; Gault, V.A.; Irwin, N. Therapeutic Potential of Peptides Derived from Animal Venoms: Current Views and Emerging Drugs for Diabetes. Clin. Med. Insights Endocrinol. Diabetes 2021, 14, 11795514211006072. [Google Scholar] [CrossRef] [PubMed]
- Prezoto, B.C.; Kato, E.E.; Gonçalves, L.R.C.; Sampaio, S.C.; Sano-Martins, I.S. Elevated Plasma Levels of Hepatocyte Growth Factor in Rats Experimentally Envenomated with Bothrops Jararaca Venom: Role of Snake Venom Metalloproteases. Toxicon 2019, 162, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.G.; Araújo, T.G.; Carvalho, B.D.M.; Rocha, G.Z.; Santos, A.; Saad, M.J.A. The Role of Hepatocyte Growth Factor (HGF) in Insulin Resistance and Diabetes. Front. Endocrinol. (Lausanne) 2018, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. The Development of Byetta (Exenatide) from the Venom of the Gila Monster as an Anti-Diabetic Agent. Toxicon 2012, 59, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Tomas, A.; Jones, B.; Leech, C. New Insights into Beta-Cell GLP-1 Receptor and CAMP Signaling. J. Mol. Biol. 2020, 432, 1347–1366. [Google Scholar] [CrossRef]
- Dalle, S.; Burcelin, R.; Gourdy, P. Specific Actions of GLP-1 Receptor Agonists and DPP4 Inhibitors for the Treatment of Pancreatic β-Cell Impairments in Type 2 Diabetes. Cell. Signal. 2013, 25, 570–579. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.A.; Marenah, L.; Orr, D.F.; Shaw, C.; Flatt, P.R. Isolation and Structural Characterisation of a Novel 13-Amino Acid Insulin-Releasing Peptide from the Skin Secretion of Agalychnis Calcarifer. Diabetologia 2005, 386, 581–587. [Google Scholar] [CrossRef]
- Jones, B.; McGlone, E.R.; Fang, Z.; Pickford, P.; Corrêa, I.R.; Oishi, A.; Jockers, R.; Inoue, A.; Kumar, S.; Görlitz, F.; et al. Genetic and Biased Agonist-Mediated Reductions in β-Arrestin Recruitment Prolong CAMP Signalling at Glucagon Family Receptors. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Tsend-Ayush, E.; He, C.; Myers, M.A.; Andrikopoulos, S.; Wong, N.; Sexton, P.M.; Wootten, D.; Forbes, B.E.; Grutzner, F. Monotreme Glucagon-like Peptide-1 in Venom and Gut: One Gene-Two Very Different Functions. Sci. Rep. 2016, 6, 37744. [Google Scholar] [CrossRef]
- Tucker, S.J.; Gribble, F.M.; Zhao, C.; Trapp, S.; Ashcroft, F.M. Truncation of Kir6.2 Produces ATP-Sensitive K+ Channels in the Absence of the Sulphonylurea Receptor. Nature 1997, 387, 179–183. [Google Scholar] [CrossRef]
- Kamp, F.; Kizilbash, N.; Corkey, B.E.; Berggren, P.-O.; Hamilton, J.A. Sulfonylureas Rapidly Cross Phospholipid Bilayer Membranes by a Free-Diffusion Mechanism. Diabetes 2003, 52, 2526–2531. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Sulphonylurea Action Revisited: The Post-Cloning Era. Diabetologia 2003, 46, 875–891. [Google Scholar] [CrossRef]
- Seltzer, H.S. Efficacy and Safety of Oral Hypoglycemic Agents. Annu. Rev. Med. 1980, 31, 261–272. [Google Scholar] [CrossRef]
- Kreisberg, R.A. The Second-Generation Sulfonylureas: Change or Progress? Ann. Intern. Med. 1985, 102, 125–126. [Google Scholar] [CrossRef]
- Campbell, R.K. Glimepiride: Role of a New Sulfonylurea in the Treatment of Type 2 Diabetes Mellitus. Ann. Pharmacother. 1998, 32, 1044–1052. [Google Scholar] [CrossRef]
- Dornhorst, A. Insulinotropic Meglitinide Analogues. Lancet 2001, 358, 1709–1716. [Google Scholar] [CrossRef]
- Eddlestone, G.T.; Komatsu, M.; Shen, L.; Sharp, G.W. Mastoparan Increases the Intracellular Free Calcium Concentration in Two Insulin-Secreting Cell Lines by Inhibition of ATP-Sensitive Potassium Channels. Mol. Pharmacol. 1995, 47, 787–797. [Google Scholar]
- Ojo, O.O.; Srinivasan, D.K.; Owolabi, B.O.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Beneficial Effects of Tigerinin-1R on Glucose Homeostasis and Beta Cell Function in Mice with Diet-Induced Obesity-Diabetes. Biochimie 2015, 109, 18–26. [Google Scholar] [CrossRef]
- Juhl, K.; Efanov, A.M.; Olsen, H.L.; Gromada, J. Secretory Phospholipase A2 Is Released from Pancreatic β-Cells and Stimulates Insulin Secretion via Inhibition of ATP-Dependent K+ Channels. Biochem. Biophys. Res. Commun. 2003, 310, 274–279. [Google Scholar] [CrossRef]
- Ramu, Y.; Xu, Y.; Lu, Z. A Novel High-Affinity Inhibitor against the Human ATP-Sensitive Kir6.2 Channel. J. Gen. Physiol. 2018, 150, 969–976. [Google Scholar] [CrossRef]
- Ramu, Y.; Yamakaze, J.; Zhou, Y.; Hoshi, T.; Lu, Z. Blocking Kir6.2 Channels with SpTx1 Potentiates Glucose-Stimulated Insulin Secretion from Murine Pancreatic β Cells and Lowers Blood Glucose in Diabetic Mice. Elife 2022, 11, e77026. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.H.; Lee, J.; Choi, S.; Vandermeer, B.; Abdelmoneim, A.S.; Featherstone, T.R. Mortality Risk among Sulfonylureas: A Systematic Review and Network Meta-Analysis. Lancet Diabetes Endocrinol. 2015, 3, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Straub, S.G.; James, R.F.; Dunne, M.J.; Sharp, G.W. Glucose Augmentation of Mastoparan-Stimulated Insulin Secretion in Rat and Human Pancreatic Islets. Diabetes 1998, 47, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; McDermott, A.M.; Gillison, S.L.; Sharp, G.W. Mastoparan Stimulates Exocytosis at a Ca(2+)-Independent Late Site in Stimulus-Secretion Coupling. Studies with the RINm5F Beta-Cell Line. J. Biol. Chem. 1993, 268, 23297–23306. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.H.; Chen, H.-Q.; Veluthakal, R.; Silver, R.B.; Li, J.; Li, G.; Kowluru, A. Mastoparan-Induced Insulin Secretion from Insulin-Secreting BetaTC3 and INS-1 Cells: Evidence for Its Regulation by Rho Subfamily of G Proteins. Endocrinology 2003, 144, 4508–4518. [Google Scholar] [CrossRef]
- Herrington, J.; Zhou, Y.-P.; Bugianesi, R.M.; Dulski, P.M.; Feng, Y.; Warren, V.A.; Smith, M.M.; Kohler, M.G.; Garsky, V.M.; Sanchez, M.; et al. Blockers of the Delayed-Rectifier Potassium Current in Pancreatic Beta-Cells Enhance Glucose-Dependent Insulin Secretion. Diabetes 2006, 55, 1034–1042. [Google Scholar] [CrossRef]
- Ojo, O.O.; Srinivasan, D.K.; Owolabi, B.O.; McGahon, M.K.; Moffett, R.C.; Curtis, T.M.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Molecular Mechanisms Mediating the Beneficial Metabolic Effects of [Arg4]Tigerinin-1R in Mice with Diet-Induced Obesity and Insulin Resistance. Biol. Chem. 2016, 397, 753–764. [Google Scholar] [CrossRef]
- MacDonald, P.E.; Wheeler, M.B. Voltage-Dependent K(+) Channels in Pancreatic Beta Cells: Role, Regulation and Potential as Therapeutic Targets. Diabetologia 2003, 46, 1046–1062. [Google Scholar] [CrossRef]
- Dwenger, M.M.; Raph, S.M.; Baba, S.P.; Moore, J.B.; Nystoriak, M.A. Diversification of Potassium Currents in Excitable Cells via Kvβ Proteins. Cells 2022, 11, 2230. [Google Scholar] [CrossRef]
- Patel, N.H.; Johannesen, J.; Shah, K.; Goswami, S.K.; Patel, N.J.; Ponnalagu, D.; Kohut, A.R.; Singh, H. Inhibition of BKCa Negatively Alters Cardiovascular Function. Physiol. Rep. 2018, 6, e13748. [Google Scholar] [CrossRef]
- Finol-Urdaneta, R.K.; Remedi, M.S.; Raasch, W.; Becker, S.; Clark, R.B.; Strüver, N.; Pavlov, E.; Nichols, C.G.; French, R.J.; Terlau, H. Block of Kv1.7 Potassium Currents Increases Glucose-Stimulated Insulin Secretion. EMBO Mol. Med. 2012, 4, 424–434. [Google Scholar] [CrossRef]
- Herrington, J.; Sanchez, M.; Wunderler, D.; Yan, L.; Bugianesi, R.M.; Dick, I.E.; Clark, S.A.; Brochu, R.M.; Priest, B.T.; Kohler, M.G.; et al. Biophysical and Pharmacological Properties of the Voltage-Gated Potassium Current of Human Pancreatic Beta-Cells. J. Physiol. 2005, 567, 159–175. [Google Scholar] [CrossRef]
- Braun, M.; Ramracheya, R.; Bengtsson, M.; Zhang, Q.; Karanauskaite, J.; Partridge, C.; Johnson, P.R.; Rorsman, P. Voltage-Gated Ion Channels in Human Pancreatic Beta-Cells: Electrophysiological Characterization and Role in Insulin Secretion. Diabetes 2008, 57, 1618–1628. [Google Scholar] [CrossRef]
- MacDonald, P.E.; Sewing, S.; Wang, J.; Joseph, J.W.; Smukler, S.R.; Sakellaropoulos, G.; Wang, J.; Saleh, M.C.; Chan, C.B.; Tsushima, R.G.; et al. Inhibition of Kv2.1 Voltage-Dependent K+Channels in Pancreatic β-Cells Enhances Glucose-Dependent Insulin Secretion*. J. Biol. Chem. 2002, 277, 44938–44945. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.-M.; Fajloun, Z. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of Action of the Antimicrobial Peptide Buforin II: Buforin II Kills Microorganisms by Penetrating the Cell Membrane and Inhibiting Cellular Functions. Biochem. Biophys. Res. Commun. 1998, 244, 253–257. [Google Scholar] [CrossRef]
- Lewis, R.J.; Garcia, M.L. Therapeutic Potential of Venom Peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef]
- Damasceno, A.; Ferreira, B.; Patel, S.; Sevene, E.; Polónia, J. Efficacy of Captopril and Nifedipine in Black and White Patients with Hypertensive Crisis. J. Hum. Hypertens. 1997, 11, 471–476. [Google Scholar] [CrossRef]
- Jiménez-Mancilla, N.; Ferro-Flores, G.; Santos-Cuevas, C.; Ocampo-García, B.; Luna-Gutiérrez, M.; Azorín-Vega, E.; Isaac-Olivé, K.; Camacho-López, M.; Torres-García, E. Multifunctional Targeted Therapy System Based on (99m) Tc/(177) Lu-Labeled Gold Nanoparticles-Tat(49-57)-Lys(3) -Bombesin Internalized in Nuclei of Prostate Cancer Cells. J. Labelled Comp. Radiopharm. 2013, 56, 663–671. [Google Scholar] [CrossRef]
- Kerkis, A.; Kerkis, I.; Rádis-Baptista, G.; Oliveira, E.B.; Vianna-Morgante, A.M.; Pereira, L.V.; Yamane, T. Crotamine Is a Novel Cell-Penetrating Protein from the Venom of Rattlesnake Crotalus Durissus Terrificus. FASEB J. 2004, 18, 1407–1409. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M.; Abdel-Wahab, Y.H.; Flatt, P.R. Peptides from Frog Skin with Potential for Development into Agents for Type 2 Diabetes Therapy. Peptides 2018, 100, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Amatya, R.; Park, T.; Hwang, S.; Yang, J.; Lee, Y.; Cheong, H.; Moon, C.; Kwak, H.D.; Min, K.A.; Shin, M.C. Drug Delivery Strategies for Enhancing the Therapeutic Efficacy of Toxin-Derived Anti-Diabetic Peptides. Toxins 2020, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Saidemberg, N.B.; Saidemberg, D.M.; Ribeiro, R.A.; Arcuri, H.A.; Palma, M.S.; Carneiro, E.M. Agelaia MP-I: A Peptide Isolated from the Venom of the Social Wasp, Agelaia Pallipes Pallipes, Enhances Insulin Secretion in Mice Pancreatic Islets. Toxicon 2012, 60, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.O.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Conlon, J.M. Insulinotropic Actions of the Frog Skin Host-Defense Peptide Alyteserin-2a: A Structure-Activity Study. Chem. Biol. Drug. Des. 2013, 82, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Mo, G.-X.; Bai, X.-W.; Li, Z.-J.; Yan, X.-W.; He, X.-Q.; Rong, M.-Q. A Novel Insulinotropic Peptide from the Skin Secretions of Amolops Loloensis Frog. Nat. Prod. Bioprospect. 2014, 4, 309–313. [Google Scholar] [CrossRef]
- Swope, S.L.; Schonbrunn, A. Bombesin Stimulates Insulin Secretion by a Pancreatic Islet Cell Line. Proc. Natl. Acad. Sci. USA 1984, 81, 1822–1826. [Google Scholar] [CrossRef]
- Woods, S.C.; Stein, L.J.; Figlewicz, D.P.; Porte, D. Bombesin Stimulates Insulin Secretion and Reduces Food Intake in the Baboon. Peptides 1983, 4, 687–691. [Google Scholar] [CrossRef]
- Martindale, R.; Levin, S.; Alfin-Slater, R. Effects of Caerulein and Bombesin on Insulin and Glucagon Secretion from the Isolated, Perfused Rat Pancreas. Regul. Pept. 1982, 3, 313–324. [Google Scholar] [CrossRef]
- Swope, S.L.; Schonbrunn, A. The Biphasic Stimulation of Insulin Secretion by Bombesin Involves Both Cytosolic Free Calcium and Protein Kinase C. Biochem. J. 1988, 253, 193–202. [Google Scholar] [CrossRef]
- Schöfl, C.; Rössig, L.; Leitolf, H.; Mader, T.; von zur Mühlen, A.; Brabant, G. Generation of Repetitive Ca2+ Transients by Bombesin Requires Intracellular Release and Influx of Ca2+ through Voltage-Dependent and Voltage Independent Channels in Single HIT Cells. Cell. Calcium 1996, 19, 485–493. [Google Scholar] [CrossRef]
- Greeley, G.H.; Spannagel, A.; Trowbridge, J.; Thompson, J.C. Effect of Bombesin and Gastrin-Releasing Peptide on the Release of Gastric Inhibitory Polypeptide and Insulin in Rats. Proc. Soc. Exp. Biol. Med. 1986, 182, 540–542. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.A.; Patterson, S.; Flatt, P.R.; Conlon, J.M. Brevinin-2-Related Peptide and Its [D4K] Analogue Stimulate Insulin Release In Vitro and Improve Glucose Tolerance in Mice Fed a High Fat Diet. Horm. Metab. Res. 2010, 42, 652–656. [Google Scholar] [CrossRef]
- Zahid, O.K.; Mechkarska, M.; Ojo, O.O.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Meetani, M.A.; Conlon, J.M. Caerulein-and Xenopsin-Related Peptides with Insulin-Releasing Activities from Skin Secretions of the Clawed Frogs, Xenopus Borealis and Xenopus Amieti (Pipidae). Gen. Comp. Endocrinol. 2011, 172, 314–320. [Google Scholar] [CrossRef]
- Toyama, M.H.; Carneiro, E.M.; Marangoni, S.; Barbosa, R.L.; Corso, G.; Boschero, A.C. Biochemical Characterization of Two Crotamine Isoforms Isolated by a Single Step RP-HPLC from Crotalus Durissus Terrificus (South American Rattlesnake) Venom and Their Action on Insulin Secretion by Pancreatic Islets. Biochim. Biophys. Acta 2000, 1474, 56–60. [Google Scholar] [CrossRef]
- Marenah, L.; Mcclean, S.; Flatt, P.R.; Orr, D.F.; Shaw, C.; Abdel-wahab, Y.H.A. Novel Insulin-Releasing Peptides in the Skin of Phyllomedusa trinitatis Frog Include 28 Amino Acid Peptide From Dermaseptin Biv Precursor. Pancreas 2004, 29, 110–115. [Google Scholar] [CrossRef]
- Vasu, S.; McGahon, M.K.; Moffett, R.C.; Curtis, T.M.; Conlon, J.M.; Abdel-Wahab, Y.H.A.; Flatt, P.R. Esculentin-2CHa(1-30) and Its Analogues: Stability and Mechanisms of Insulinotropic Action. J. Endocrinol. 2017, 232, 423–435. [Google Scholar] [CrossRef]
- Ojo, O.O.; Srinivasan, D.K.; Owolabi, B.O.; Vasu, S.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Esculentin-2CHa-Related Peptides Modulate Islet Cell Function and Improve Glucose Tolerance in Mice with Diet-Induced Obesity and Insulin Resistance. PLoS ONE 2015, 10, e0141549. [Google Scholar] [CrossRef]
- Owolabi, B.O.; Ojo, O.O.; Srinivasan, D.K.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. In Vitro and in Vivo Insulinotropic Properties of the Multifunctional Frog Skin Peptide Hymenochirin-1B: A Structure-Activity Study. Amino Acids 2016, 48, 535–547. [Google Scholar] [CrossRef]
- Owolabi, B.O.; Ojo, O.O.; Srinivasan, D.K.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Glucoregulatory, Endocrine and Morphological Effects of [P5K]Hymenochirin-1B in Mice with Diet-Induced Glucose Intolerance and Insulin Resistance. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 769–781. [Google Scholar] [CrossRef]
- Ojo, O.O.; Srinivasan, D.K.; Owolabi, B.O.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Magainin-Related Peptides Stimulate Insulin-Release and Improve Glucose Tolerance in High Fat Fed Mice. Protein Pept. Lett. 2014, 22, 256–263. [Google Scholar] [CrossRef]
- Morgan, N.G.; Rumford, G.M.; Montague, W. Studies on the Mechanism by Which Melittin Stimulates Insulin Secretion from Isolated Rat Islets of Langerhans. Biochim. Biophys. Acta 1985, 845, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.S. Glucose Dose-Related Influence of Phospholipase C and Melittin on Insulin Release from Perifused Rat Islets. Res. Commun. Chem. Pathol. Pharmacol. 1988, 62, 295–308. [Google Scholar] [PubMed]
- Conlon, J.M.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Leprince, J.; Vaudry, H.; Jouenne, T.; Condamine, E. A Glycine-Leucine-Rich Peptide Structurally Related to the Plasticins from Skin Secretions of the Frog Leptodactylus Laticeps (Leptodactylidae). Peptides 2009, 30, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Mechkarska, M.; Ojo, O.O.; Meetani, M.A.; Coquet, L.; Jouenne, T.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; King, J.D.; Conlon, J.M. Peptidomic Analysis of Skin Secretions from the Bullfrog Lithobates Catesbeianus (Ranidae) Identifies Multiple Peptides with Potent Insulin-Releasing Activity. Peptides 2011, 32, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Musale, V.; Moffett, R.C.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H. Beneficial Actions of the [A14K] Analog of the Frog Skin Peptide PGLa-AM1 in Mice with Obesity and Degenerative Diabetes: A Mechanistic Study. Peptides 2021, 136, 170472. [Google Scholar] [CrossRef]
- Owolabi, B.O.; Musale, V.; Ojo, O.O.; Moffett, R.C.; McGahon, M.K.; Curtis, T.M.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Actions of PGLa-AM1 and Its [A14K] and [A20K] Analogues and Their Therapeutic Potential as Anti-Diabetic Agents. Biochimie 2017, 138, 1–12. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.A.; Power, G.J.; Ng, M.T.; Flatt, P.R.; Conlon, J.M. Insulin-Releasing Properties of the Frog Skin Peptide Pseudin-2 and Its [Lys18]-Substituted Analogue. Biol. Chem. 2008, 389, 143–148. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.A.; Marenah, L.; Flatt, P.R.; Conlon, J.M. Insulin Releasing Properties of the Temporin Family of Antimicrobial Peptides. Protein Pept. Lett. 2007, 14, 702–707. [Google Scholar] [CrossRef]
- Kumar, S.; Mittal, A.; Babu, D.; Mittal, A. Herbal Medicines for Diabetes Management and Its Secondary Complications. Curr. Diabetes Rev. 2020, 17, 437–456. [Google Scholar] [CrossRef]
- Beckman, C.H. Phenolic-Storing Cells: Keys to Programmed Cell Death and Periderm Formation in Wilt Disease Resistance and in General Defence Responses in Plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Sureda, A.; Tejada, S.; Bibiloni, d.M.; Tur, J.A.; Pons, A. Polyphenols: Well beyond the Antioxidant Capacity: Polyphenol Supplementation and Exercise-Induced Oxidative Stress and Inflammation. Curr. Pharm. Biotechnol. 2014, 15, 373–379. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Souza, C.R.M.; Bezerra, W.P.; Souto, J.T. Marine Alkaloids with Anti-Inflammatory Activity: Current Knowledge and Future Perspectives. Mar. Drugs 2020, 18, 147. [Google Scholar] [CrossRef]
- Daly, J.W.; Garraffo, H.M.; Spande, T.F.; Decker, M.W.; Sullivan, J.P.; Williams, M. Alkaloids from Frog Skin: The Discovery of Epibatidine and the Potential for Developing Novel Non-Opioid Analgesics. Nat. Prod. Rep. 2000, 17, 131–135. [Google Scholar] [CrossRef]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Boyd, A.E.; Bolton, W.E.; Brinkley, B.R. Microtubules and Beta Cell Function: Effect of Colchicine on Microtubules and Insulin Secretion in Vitro by Mouse Beta Cells. J. Cell. Biol. 1982, 92, 425–434. [Google Scholar] [CrossRef]
- Malta, A.; de Souza, A.A.; Ribeiro, T.A.; Francisco, F.A.; Pavanello, A.; Prates, K.V.; Tófolo, L.P.; Miranda, R.A.; de Oliveira, J.C.; Martins, I.P.; et al. Neonatal Treatment with Scopolamine Butylbromide Prevents Metabolic Dysfunction in Male Rats. Sci. Rep. 2016, 6, 30745. [Google Scholar] [CrossRef]
- Grill, V.; Ostenson, C.G. Muscarinic Receptors in Pancreatic Islets of the Rat. Demonstration and Dependence on Long-Term Glucose Environment. Biochim. Biophys. Acta 1983, 756, 159–162. [Google Scholar] [CrossRef]
- Tuomi, T.; Nagorny, C.L.F.; Singh, P.; Bennet, H.; Yu, Q.; Alenkvist, I.; Isomaa, B.; Östman, B.; Söderström, J.; Pesonen, A.-K.; et al. Increased Melatonin Signaling Is a Risk Factor for Type 2 Diabetes. Cell. Metab. 2016, 23, 1067–1077. [Google Scholar] [CrossRef]
- Picinato, M.C.; Haber, E.P.; Cipolla-Neto, J.; Curi, R.; de Oliveira Carvalho, C.R.; Carpinelli, A.R. Melatonin Inhibits Insulin Secretion and Decreases PKA Levels without Interfering with Glucose Metabolism in Rat Pancreatic Islets. J. Pineal Res. 2002, 33, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.R.; Jefferys, D.B.; Jones, R.H.; Stanley, D. The Effect of atropine on the insulin release caused by oral and intravenous glucose in human subjects. Eur. J. Endocrinol. 1976, 83, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Hellström-Lindahl, E.; Grill, V. Evidence for Functional Nicotinic Receptors on Pancreatic Beta Cells. Metabolism 2005, 54, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zern, R.T.; Bird, J.L.; Feldman, J.M. Effect of Increased Pancreatic Islet Norepinephrine, Dopamine and Serotonin Concentration on Insulin Secretion in the Golden Hamster. Diabetologia 1980, 18, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Tjälve, H.; Popov, D. Effect of Nicotine and Nicotine Metabolites on Insulin Secretion from Rabbit Pancreas Pieces. Endocrinology 1973, 92, 1343–1348. [Google Scholar] [CrossRef]
- Chapal, J.; Loubatières-Mariani, M.M. [Effect of nicotine on insulin secretion in the isolated perfused rat pancreas]. C. R. Seances Soc. Biol. Fil. 1978, 172, 156–160. [Google Scholar]
- Henquin, J.C.; Horemans, B.; Nenquin, M.; Verniers, J.; Lambert, A.E. Quinine-Induced Modifications of Insulin Release and Glucose Metabolism by Isolated Pancreatic Islets. FEBS Lett. 1975, 57, 280–284. [Google Scholar] [CrossRef]
- Herchuelz, A.; Lebrun, P.; Carpinelli, A.; Thonnart, N.; Sener, A.; Malaisse, W.J. Regulation of Calcium Fluxes in Rat Pancreatic Islets. Quinine Mimics the Dual Effect of Glucose on Calcium Movements. Biochim. Biophys. Acta 1981, 640, 16–30. [Google Scholar] [CrossRef]
- Bai, M.; Liu, Y.; Zhou, F.; Zhang, Y.; Zhu, Q.; Zhang, L.; Zhang, Q.; Wang, S.; Zhu, K.; Wang, X.; et al. Berberine Inhibits Glucose Oxidation and Insulin Secretion in Rat Islets. Endocr. J. 2018, 65, 469–477. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, Y.; Yang, X.; Han, H.; Ge, Y.; Zhang, M.; Zhang, H.; Zhang, M.; Chen, L. Berberine Potentiates Insulin Secretion and Prevents β-Cell Dysfunction Through the MiR-204/SIRT1 Signaling Pathway. Front. Pharmacol. 2021, 12, 720866. [Google Scholar] [CrossRef]
- Zhao, M.-M.; Lu, J.; Li, S.; Wang, H.; Cao, X.; Li, Q.; Shi, T.-T.; Matsunaga, K.; Chen, C.; Huang, H.; et al. Berberine Is an Insulin Secretagogue Targeting the KCNH6 Potassium Channel. Nat. Commun. 2021, 12, 5616. [Google Scholar] [CrossRef]
- Suantawee, T.; Elazab, S.T.; Hsu, W.H.; Yao, S.; Cheng, H.; Adisakwattana, S. Cyanidin Stimulates Insulin Secretion and Pancreatic β-Cell Gene Expression through Activation of l-Type Voltage-Dependent Ca2+ Channels. Nutrients 2017, 9, 814. [Google Scholar] [CrossRef]
- Kappel, V.D.; Frederico, M.J.S.; Postal, B.G.; Mendes, C.P.; Cazarolli, L.H.; Silva, F.R.M.B. The Role of Calcium in Intracellular Pathways of Rutin in Rat Pancreatic Islets: Potential Insulin Secretagogue Effect. Eur. J. Pharmacol. 2013, 702, 264–268. [Google Scholar] [CrossRef]
- Vetterli, L.; Brun, T.; Giovannoni, L.; Bosco, D.; Maechler, P. Resveratrol Potentiates Glucose-Stimulated Insulin Secretion in INS-1E Beta-Cells and Human Islets through a SIRT1-Dependent Mechanism. J. Biol. Chem. 2011, 286, 6049–6060. [Google Scholar] [CrossRef]
- Rouse, M.; Younès, A.; Egan, J.M. Resveratrol and Curcumin Enhance Pancreatic β-Cell Function by Inhibiting Phosphodiesterase Activity. J. Endocrinol. 2014, 223, 107–117. [Google Scholar] [CrossRef]
- Liu, D.; Zhen, W.; Yang, Z.; Carter, J.D.; Si, H.; Reynolds, K.A. Genistein Acutely Stimulates Insulin Secretion in Pancreatic Beta-Cells through a CAMP-Dependent Protein Kinase Pathway. Diabetes 2006, 55, 1043–1050. [Google Scholar] [CrossRef]
- Pavan, B.; Capuzzo, A.; Forlani, G. Quercetin and Quercetin-3-O-Glucoside Interact with Different Components of the CAMP Signaling Cascade in Human Retinal Pigment Epithelial Cells. Life Sci. 2015, 121, 166–173. [Google Scholar] [CrossRef]
- Green, I.C.; Ray, K.; Perrin, D. Opioid Peptide Effects on Insulin Release and C-AMP in Islets of Langerhans. Horm. Metab. Res. 1983, 15, 124–128. [Google Scholar] [CrossRef]
- Rey, D.; Miranda Sulis, P.; Alves Fernandes, T.; Gonçalves, R.; Silva Frederico, M.J.; Costa, G.M.; Aragon, M.; Ospina, L.F.; Mena Barreto Silva, F.R. Astragalin Augments Basal Calcium Influx and Insulin Secretion in Rat Pancreatic Islets. Cell. Calcium 2019, 80, 56–62. [Google Scholar] [CrossRef]
- Mao, X.; Chai, Y.; Lin, Y.-F. Dual Regulation of the ATP-Sensitive Potassium Channel by Caffeine. Am. J. Physiol. Cell. Physiol. 2007, 292, C2239–C2258. [Google Scholar] [CrossRef]
- Shi, C.L. Effects of Caffeine and Acetylcholine on Glucose-Stimulated Insulin Release from Islet Transplants in Mice. Cell. Transplant. 1997, 6, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee Acutely Modifies Gastrointestinal Hormone Secretion and Glucose Tolerance in Humans: Glycemic Effects of Chlorogenic Acid and Caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Hafizur, R.M.; Hameed, A.; Ahmed, S.; Nisar, M.; Kabir, N. Ellagic Acid in Emblica Officinalis Exerts Anti-Diabetic Activity through the Action on β-Cells of Pancreas. Eur. J. Nutr. 2017, 56, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.J.; Raj, N.N.; Sugappriya, M.; Priyadarshini, S.M. Modeling of ATP-Sensitive Inward Rectifier Potassium Channel 11 and Inhibition Mechanism of the Natural Ligand, Ellagic Acid, Using Molecular Docking. Adv. Exp. Med. Biol. 2010, 680, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D. Flavonol Kaempferol Improves Chronic Hyperglycemia-Impaired Pancreatic Beta-Cell Viability and Insulin Secretory Function. Eur. J. Pharmacol. 2011, 670, 325–332. [Google Scholar] [CrossRef]
- García López, P.M.; de la Mora, P.G.; Wysocka, W.; Maiztegui, B.; Alzugaray, M.E.; Del Zotto, H.; Borelli, M.I. Quinolizidine Alkaloids Isolated from Lupinus Species Enhance Insulin Secretion. Eur. J. Pharmacol. 2004, 504, 139–142. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Ta, T.N.; Pham, T.H.M.; Nguyen, Q.T.; Pham, H.D.; Mishra, S.; Nyomba, B.L.G. Nuciferine Stimulates Insulin Secretion from Beta Cells—An in Vitro Comparison with Glibenclamide. J. Ethnopharmacol. 2012, 142, 488–495. [Google Scholar] [CrossRef]
- Kittl, M.; Beyreis, M.; Tumurkhuu, M.; Fürst, J.; Helm, K.; Pitschmann, A.; Gaisberger, M.; Glasl, S.; Ritter, M.; Jakab, M. Quercetin Stimulates Insulin Secretion and Reduces the Viability of Rat INS-1 Beta-Cells. CPB 2016, 39, 278–293. [Google Scholar] [CrossRef]
- Chen, W.-P.; Chi, T.-C.; Chuang, L.-M.; Su, M.-J. Resveratrol Enhances Insulin Secretion by Blocking K(ATP) and K(V) Channels of Beta Cells. Eur. J. Pharmacol. 2007, 568, 269–277. [Google Scholar] [CrossRef]
- Lee, D.; Kim, Y.-M.; Kim, H.W.; Choi, Y.-K.; Park, B.J.; Joo, S.H.; Kang, K.S. Schisandrin C Affects Glucose-Stimulated Insulin Secretion in Pancreatic β-Cells and Glucose Uptake in Skeletal Muscle Cells. Molecules 2021, 26, 6509. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Kerr, A.J.; Gibson, J.S.; Williams, B.A. Amantadine and Sparteine Inhibit ATP-Regulated K-Currents in the Insulin-Secreting Beta-Cell Line, HIT-T15. Br. J. Pharmacol. 1991, 104, 579–584. [Google Scholar] [CrossRef]
- Balamurugan, R.; Vendan, S.E.; Aravinthan, A.; Kim, J.-H. Isolation and Structural Characterization of 2R, 3R Taxifolin 3-O-Rhamnoside from Ethyl Acetate Extract of Hydnocarpus Alpina and Its Hypoglycemic Effect by Attenuating Hepatic Key Enzymes of Glucose Metabolism in Streptozotocin-Induced Diabetic Rats. Biochimie 2015, 111, 70–81. [Google Scholar] [CrossRef]
- Yao, X.; Chen, F.; Li, P.; Quan, L.; Chen, J.; Yu, L.; Ding, H.; Li, C.; Chen, L.; Gao, Z.; et al. Natural Product Vindoline Stimulates Insulin Secretion and Efficiently Ameliorates Glucose Homeostasis in Diabetic Murine Models. J. Ethnopharmacol. 2013, 150, 285–297. [Google Scholar] [CrossRef]
- Horiuchi, H.; Usami, A.; Shirai, R.; Harada, N.; Ikushiro, S.; Sakaki, T.; Nakano, Y.; Inui, H.; Yamaji, R. S-Equol Activates CAMP Signaling at the Plasma Membrane of INS-1 Pancreatic β-Cells and Protects against Streptozotocin-Induced Hyperglycemia by Increasing β-Cell Function in Male Mice. J. Nutr. 2017, 147, 1631–1639. [Google Scholar] [CrossRef]
- Jonas, J.C.; Plant, T.D.; Gilon, P.; Detimary, P.; Nenquin, M.; Henquin, J.C. Multiple Effects and Stimulation of Insulin Secretion by the Tyrosine Kinase Inhibitor Genistein in Normal Mouse Islets. Br. J. Pharmacol. 1995, 114, 872–880. [Google Scholar] [CrossRef]
- Ohno, T.; Kato, N.; Ishii, C.; Shimizu, M.; Ito, Y.; Tomono, S.; Kawazu, S. Genistein Augments Cyclic Adenosine 3′5′-Monophosphate(CAMP) Accumulation and Insulin Release in MIN6 Cells. Endocr. Res. 1993, 19, 273–285. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, D. Long-Term Exposure to Genistein Improves Insulin Secretory Function of Pancreatic Beta-Cells. Eur. J. Pharmacol. 2009, 616, 321–327. [Google Scholar] [CrossRef]
- Green, I.C.; Perrin, D.; Pedley, K.C.; Leslie, R.D.; Pyke, D.A. Effect of Enkephalins and Morphine on Insulin Secretion from Isolated Rat Islets. Diabetologia 1980, 19, 158–161. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Yi, X.; Liu, C.; Kong, D.; Zhang, J.; Gong, M. Myricetin: A Potent Approach for the Treatment of Type 2 Diabetes as a Natural Class B GPCR Agonist. FASEB J. 2017, 31, 2603–2611. [Google Scholar] [CrossRef]
- Mahendra, V.P.; Haware, D.J.; Kumar, R. CAMP-PKA Dependent ERK1/2 Activation Is Necessary for Vanillic Acid Potentiated Glucose-Stimulated Insulin Secretion in Pancreatic β-Cells. J. Funct. Foods 2019, 56, 110–118. [Google Scholar] [CrossRef]
- Rhodes, C.J. Type 2 Diabetes-a Matter of Beta-Cell Life and Death? Science 2005, 307, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Mazzucato, C.; Andle, J.; Lee, T.B.; Midha, A.; Talemal, L.; Chipashvili, V.; Hollister-Lock, J.; van Deursen, J.; Weir, G.; Bonner-Weir, S. Acceleration of β Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes. Cell. Metab. 2019, 30, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic β-Cell Dedifferentiation As Mechanism Of Diabetic β-Cell Failure. Cell. 2012, 150, 1223–1234. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Endoplasmic Reticulum (ER) Stress and Its Role in Pancreatic β-Cell Dysfunction and Senescence in Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 4843. [Google Scholar] [CrossRef] [PubMed]
- Ježek, P.; Jabůrek, M.; Plecitá-Hlavatá, L. Contribution of Oxidative Stress and Impaired Biogenesis of Pancreatic β-Cells to Type 2 Diabetes. Antioxid. Redox Signal. 2019, 31, 722–751. [Google Scholar] [CrossRef]
- Masini, M.; Bugliani, M.; Lupi, R.; del Guerra, S.; Boggi, U.; Filipponi, F.; Marselli, L.; Masiello, P.; Marchetti, P. Autophagy in Human Type 2 Diabetes Pancreatic Beta Cells. Diabetologia 2009, 52, 1083–1086. [Google Scholar] [CrossRef]
- Bugliani, M.; Mossuto, S.; Grano, F.; Suleiman, M.; Marselli, L.; Boggi, U.; De Simone, P.; Eizirik, D.L.; Cnop, M.; Marchetti, P.; et al. Modulation of Autophagy Influences the Function and Survival of Human Pancreatic Beta Cells Under Endoplasmic Reticulum Stress Conditions and in Type 2 Diabetes. Front. Endocrinol. (Lausanne) 2019, 10, 52. [Google Scholar] [CrossRef]
- Cerf, M.E. Developmental Programming and Glucolipotoxicity: Insights on Beta Cell Inflammation and Diabetes. Metabolites 2020, 10, 444. [Google Scholar] [CrossRef]
- Lytrivi, M.; Castell, A.-L.; Poitout, V.; Cnop, M. Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes. J. Mol. Biol. 2020, 432, 1514–1534. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.-S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- Zummo, F.P.; Cullen, K.S.; Honkanen-Scott, M.; Shaw, J.A.M.; Lovat, P.E.; Arden, C. Glucagon-Like Peptide 1 Protects Pancreatic β-Cells From Death by Increasing Autophagic Flux and Restoring Lysosomal Function. Diabetes 2017, 66, 1272–1285. [Google Scholar] [CrossRef]
- Montvida, O.; Klein, K.; Kumar, S.; Khunti, K.; Paul, S.K. Addition of or Switch to Insulin Therapy in People Treated with Glucagon-like Peptide-1 Receptor Agonists: A Real-World Study in 66 583 Patients. Diabetes Obes. Metab. 2017, 19, 108–117. [Google Scholar] [CrossRef]
- Xu, G.; Kaneto, H.; Laybutt, D.R.; Duvivier-Kali, V.F.; Trivedi, N.; Suzuma, K.; King, G.L.; Weir, G.C.; Bonner-Weir, S. Downregulation of GLP-1 and GIP Receptor Expression by Hyperglycemia: Possible Contribution to Impaired Incretin Effects in Diabetes. Diabetes 2007, 56, 1551–1558. [Google Scholar] [CrossRef]
- Mitchell, R.K.; Mondragon, A.; Chen, L.; Mcginty, J.A.; French, P.M.; Ferrer, J.; Thorens, B.; Hodson, D.J.; Rutter, G.A.; Da Silva Xavier, G. Selective Disruption of Tcf7l2 in the Pancreatic β Cell Impairs Secretory Function and Lowers β Cell Mass. Hum. Mol. Genet. 2015, 24, 1390–1399. [Google Scholar] [CrossRef]
- Ezanno, H.; Pawlowski, V.; Abdelli, S.; Boutry, R.; Gmyr, V.; Kerr-Conte, J.; Bonny, C.; Pattou, F.; Abderrahmani, A. JNK3 Is Required for the Cytoprotective Effect of Exendin 4. J. Diabetes Res. 2014, 2014, 814854. [Google Scholar] [CrossRef]
- Kapodistria, K.; Tsilibary, E.-P.; Kotsopoulou, E.; Moustardas, P.; Kitsiou, P. Liraglutide, a Human Glucagon-like Peptide-1 Analogue, Stimulates AKT-Dependent Survival Signalling and Inhibits Pancreatic β-Cell Apoptosis. J. Cell. Mol. Med. 2018, 22, 2970–2980. [Google Scholar] [CrossRef]
- Camaya, I.; Donnelly, S.; O’Brien, B. Targeting the PI3K/Akt Signaling Pathway in Pancreatic Β-cells to Enhance Their Survival and Function: An Emerging Therapeutic Strategy for Type 1 Diabetes. J. Diabetes 2022, 14, 247–260. [Google Scholar] [CrossRef]
- Weston, C.R.; Davis, R.J. The JNK Signal Transduction Pathway. Curr. Opin. Cell. Biol. 2007, 19, 142–149. [Google Scholar] [CrossRef]
- Tenenbaum, M.; Plaisance, V.; Boutry, R.; Pawlowski, V.; Jacovetti, C.; Sanchez-Parra, C.; Ezanno, H.; Bourry, J.; Beeler, N.; Pasquetti, G.; et al. The Map3k12 (Dlk)/JNK3 Signaling Pathway Is Required for Pancreatic Beta-Cell Proliferation during Postnatal Development. Cell. Mol. Life Sci. 2021, 78, 287–298. [Google Scholar] [CrossRef]
- Ferdaoussi, M.; Abdelli, S.; Yang, J.-Y.; Cornu, M.; Niederhauser, G.; Favre, D.; Widmann, C.; Regazzi, R.; Thorens, B.; Waeber, G.; et al. Exendin-4 Protects Beta-Cells from Interleukin-1 Beta-Induced Apoptosis by Interfering with the c-Jun NH2-Terminal Kinase Pathway. Diabetes 2008, 57, 1205–1215. [Google Scholar] [CrossRef]
- Jhala, U.S.; Canettieri, G.; Screaton, R.A.; Kulkarni, R.N.; Krajewski, S.; Reed, J.; Walker, J.; Lin, X.; White, M.; Montminy, M. CAMP Promotes Pancreatic Beta-Cell Survival via CREB-Mediated Induction of IRS2. Genes. Dev. 2003, 17, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Blandino-Rosano, M.; Perez-Arana, G.; Mellado-Gil, J.M.; Segundo, C.; Aguilar-Diosdado, M. Anti-Proliferative Effect of pro-Inflammatory Cytokines in Cultured Beta Cells Is Associated with Extracellular Signal-Regulated Kinase 1/2 Pathway Inhibition: Protective Role of Glucagon-like Peptide -1. J. Mol. Endocrinol. 2008, 41, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Costes, S.; Broca, C.; Bertrand, G.; Lajoix, A.-D.; Bataille, D.; Bockaert, J.; Dalle, S. ERK1/2 Control Phosphorylation and Protein Level of CAMP-Responsive Element-Binding Protein: A Key Role in Glucose-Mediated Pancreatic Beta-Cell Survival. Diabetes 2006, 55, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Quoyer, J.; Longuet, C.; Broca, C.; Linck, N.; Costes, S.; Varin, E.; Bockaert, J.; Bertrand, G.; Dalle, S. GLP-1 Mediates Antiapoptotic Effect by Phosphorylating Bad through a Beta-Arrestin 1-Mediated ERK1/2 Activation in Pancreatic Beta-Cells. J. Biol. Chem. 2010, 285, 1989–2002. [Google Scholar] [CrossRef]
- Tong, X.; Kono, T.; Anderson-Baucum, E.K.; Yamamoto, W.; Gilon, P.; Lebeche, D.; Day, R.N.; Shull, G.E.; Evans-Molina, C. SERCA2 Deficiency Impairs Pancreatic β-Cell Function in Response to Diet-Induced Obesity. Diabetes 2016, 65, 3039–3052. [Google Scholar] [CrossRef]
- Lee, W.-Y. New Potential Targets of Glucagon-Like Peptide 1 Receptor Agonists in Pancreatic β-Cells and Hepatocytes. Endocrinol. Metab. 2017, 32, 1–5. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.; Li, L.-X.; Brubaker, P.L.; Edlund, H.; Drucker, D.J. Beta-Cell Pdx1 Expression Is Essential for the Glucoregulatory, Proliferative, and Cytoprotective Actions of Glucagon-like Peptide-1. Diabetes 2005, 54, 482–491. [Google Scholar] [CrossRef]
- Aigha, I.I.; Abdelalim, E.M. NKX6.1 Transcription Factor: A Crucial Regulator of Pancreatic β Cell Development, Identity, and Proliferation. Stem Cell. Res. Ther. 2020, 11, 459. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Y.; Shilun, L.; Xue, P. Liraglutide Inhibits Endoplasmic Reticulum Stress in Pancreatic Beta Cells via Regulation of the Homeodomain Transcription Factor Nkx6.1. Diabetes 2018, 67, 2146-P. [Google Scholar] [CrossRef]
- Buteau, J.; Spatz, M.L.; Accili, D. Transcription Factor FoxO1 Mediates Glucagon-like Peptide-1 Effects on Pancreatic Beta-Cell Mass. Diabetes 2006, 55, 1190–1196. [Google Scholar] [CrossRef]
- Kitamura, T.; Nakae, J.; Kitamura, Y.; Kido, Y.; Biggs, W.H.; Wright, C.V.E.; White, M.F.; Arden, K.C.; Accili, D. The Forkhead Transcription Factor Foxo1 Links Insulin Signaling to Pdx1 Regulation of Pancreatic Beta Cell Growth. J. Clin. Investig. 2002, 110, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Millán, E.; Martín, M.A.; Goya, L.; Lizárraga-Mollinedo, E.; Escrivá, F.; Ramos, S.; Álvarez, C. Glucagon-like Peptide-1 Improves Beta-Cell Antioxidant Capacity via Extracellular Regulated Kinases Pathway and Nrf2 Translocation. Free Radic. Biol. Med. 2016, 95, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, W.; Iwasa, H.; Tumurkhuu, M. Role of the Transcription Factor MAFA in the Maintenance of Pancreatic β-Cells. Int. J. Mol. Sci. 2022, 23, 4478. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.A.; Ladrière, L.; Ortis, F.; Igoillo-Esteve, M.; Gurzov, E.N.; Lupi, R.; Marchetti, P.; Eizirik, D.L.; Cnop, M. Glucagon-Like Peptide-1 Agonists Protect Pancreatic β-Cells From Lipotoxic Endoplasmic Reticulum Stress Through Upregulation of BiP and JunB. Diabetes 2009, 58, 2851–2862. [Google Scholar] [CrossRef]
- Lee, A.-H.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 Regulates a Subset of Endoplasmic Reticulum Resident Chaperone Genes in the Unfolded Protein Response. Mol. Cell. Biol. 2003, 23, 7448–7459. [Google Scholar] [CrossRef]
- Zhou, Z.; Ribas, V.; Rajbhandari, P.; Drew, B.G.; Moore, T.M.; Fluitt, A.H.; Reddish, B.R.; Whitney, K.A.; Georgia, S.; Vergnes, L.; et al. Estrogen Receptor α Protects Pancreatic β-Cells from Apoptosis by Preserving Mitochondrial Function and Suppressing Endoplasmic Reticulum Stress. J. Biol. Chem. 2018, 293, 4735–4751. [Google Scholar] [CrossRef]
- Fuselier, T.; Mota de Sa, P.; Qadir, M.M.F.; Xu, B.; Allard, C.; Meyers, M.M.; Tiano, J.P.; Yang, B.S.; Gelfanov, V.; Lindsey, S.H.; et al. Efficacy of Glucagon-like Peptide-1 and Estrogen Dual Agonist in Pancreatic Islets Protection and Pre-Clinical Models of Insulin-Deficient Diabetes. Cell. Rep. Med. 2022, 3, 100598. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, S.-J.; Park, S.-H.; Son, D.-G.; Bae, J.-H.; Kim, H.K.; Han, J.; Song, D.-K. Glucagon-Like Peptide-1 Enhances Glucokinase Activity in Pancreatic β-Cells through the Association of Epac2 with Rim2 and Rab3A. Endocrinology 2012, 153, 574–582. [Google Scholar] [CrossRef]
- Marinho, T.d.S.; Martins, F.F.; Cardoso, L.E.d.M.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Pancreatic Islet Cells Disarray, Apoptosis, and Proliferation in Obese Mice. The Role of Semaglutide Treatment. Biochimie 2022, 193, 126–136. [Google Scholar] [CrossRef]
- Gupta, D.; Kono, T.; Evans-Molina, C. The Role of Peroxisome Proliferator-Activated Receptor Gamma in Pancreatic Beta Cell Function and Survival: Therapeutic Implications for the Treatment of Type 2 Diabetes Mellitus. Diabetes Obes. Metab. 2010, 12, 1036–1047. [Google Scholar] [CrossRef]
- Rourke, J.L.; Hu, Q.; Screaton, R.A. AMPK and Friends: Central Regulators of β Cell Biology. Trends Endocrinol. Metab. 2018, 29, 111–122. [Google Scholar] [CrossRef]
- Li, R.; Sun, X.; Li, P.; Li, W.; Zhao, L.; Zhu, L.; Zhu, S. GLP-1-Induced AMPK Activation Inhibits PARP-1 and Promotes LXR-Mediated ABCA1 Expression to Protect Pancreatic β-Cells Against Cholesterol-Induced Toxicity Through Cholesterol Efflux. Front. Cell. Dev. Biol. 2021, 9, 646113. [Google Scholar] [CrossRef]
- Buteau, J.; El-Assaad, W.; Rhodes, C.J.; Rosenberg, L.; Joly, E.; Prentki, M. Glucagon-like Peptide-1 Prevents Beta Cell Glucolipotoxicity. Diabetologia 2004, 47, 806–815. [Google Scholar] [CrossRef]
- Musale, V.; Casciaro, B.; Mangoni, M.L.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Conlon, J.M. Assessment of the Potential of Temporin Peptides from the Frog Rana Temporaria (Ranidae) as Anti-Diabetic Agents. J. Pept. Sci. 2018, 24, e3065. [Google Scholar] [CrossRef]
- Vasu, S.; Ojo, O.O.; Moffett, R.C.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Anti-Diabetic Actions of Esculentin-2CHa(1-30) and Its Stable Analogues in a Diet-Induced Model of Obesity-Diabetes. Amino Acids 2017, 49, 1705–1717. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and Their Effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Mirisola, V.; Romeo, F.; Generoso, L.; Esposito, A.; Dell’eva, R.; Blengio, F.; Killian, P.H.; Albini, A.; Pfeffer, U. Reference Profile Correlation Reveals Estrogen-like Trancriptional Activity of Curcumin. Cell. Physiol. Biochem. 2010, 26, 471–482. [Google Scholar] [CrossRef]
- Desmawati, D.; Sulastri, D. Phytoestrogens and Their Health Effect. Open Access Maced. J. Med. Sci. 2019, 7, 495. [Google Scholar] [CrossRef]
- Le May, C.; Chu, K.; Hu, M.; Ortega, C.S.; Simpson, E.R.; Korach, K.S.; Tsai, M.-J.; Mauvais-Jarvis, F. Estrogens Protect Pancreatic β-Cells from Apoptosis and Prevent Insulin-Deficient Diabetes Mellitus in Mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9232–9237. [Google Scholar] [CrossRef]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of Resveratrol for 5 Wk Has No Effect on Glucagon-like Peptide 1 Secretion, Gastric Emptying, or Glycemic Control in Type 2 Diabetes: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Stone, S.G.; Mok, J.E.; Mhajan, R.K.; Fu, C.-I.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Apple and Blackcurrant Polyphenol-Rich Drinks Decrease Postprandial Glucose, Insulin and Incretin Response to a High-Carbohydrate Meal in Healthy Men and Women. J. Nutr. Biochem. 2017, 49, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Tasic, N.; Jakovljevic, V.L.J.; Mitrovic, M.; Djindjic, B.; Tasic, D.; Dragisic, D.; Citakovic, Z.; Kovacevic, Z.; Radoman, K.; Zivkovic, V.; et al. Black Chokeberry Aronia Melanocarpa Extract Reduces Blood Pressure, Glycemia and Lipid Profile in Patients with Metabolic Syndrome: A Prospective Controlled Trial. Mol. Cell. Biochem. 2021, 476, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Chavanelle, V.; Otero, Y.F.; Le Joubioux, F.; Ripoche, D.; Bargetto, M.; Vluggens, A.; Montaurier, C.; Pickering, G.; Ducheix, G.; Dubray, C.; et al. Effects of Totum-63 on Glucose Homeostasis and Postprandial Glycemia: A Translational Study. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E1119–E1137. [Google Scholar] [CrossRef]
- Derosa, G.; Cicero, A.F.G.; D’Angelo, A.; Maffioli, P. Ascophyllum Nodosum and Fucus Vesiculosus on Glycemic Status and on Endothelial Damage Markers in Dysglicemic Patients. Phytother. Res. 2019, 33, 791–797. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug. Res. (Stuttg) 2018, 68, 403–409. [Google Scholar] [CrossRef]
- Xu, X.; Yi, H.; Wu, J.; Kuang, T.; Zhang, J.; Li, Q.; Du, H.; Xu, T.; Jiang, G.; Fan, G. Therapeutic Effect of Berberine on Metabolic Diseases: Both Pharmacological Data and Clinical Evidence. Biomed. Pharmacother. 2021, 133, 110984. [Google Scholar] [CrossRef]
- Li, M.; She, J.; Ma, L.; Ma, L.; Ma, X.; Zhai, J. Berberine Protects against Palmitate Induced Beta Cell Injury via Promoting Mitophagy. Genes Genomics 2022, 44, 867–878. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of Berberine in Patients with Type 2 Diabetes Mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Moon, J.M.; Ratliff, K.M.; Hagele, A.M.; Stecker, R.A.; Mumford, P.W.; Kerksick, C.M. Absorption Kinetics of Berberine and Dihydroberberine and Their Impact on Glycemia: A Randomized, Controlled, Crossover Pilot Trial. Nutrients 2021, 14, 124. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, Z.; Duan, T.; Tang, C.; Huang, D.; Hu, X. Curcumin Ameliorates HO-Induced Injury through SIRT1-PERK-CHOP Pathway in Pancreatic Beta Cells. Acta Biochim. Biophys. Sin. (Shanghai) 2022, 54, 370–377. [Google Scholar] [CrossRef]
- Hao, F.; Kang, J.; Cao, Y.; Fan, S.; Yang, H.; An, Y.; Pan, Y.; Tie, L.; Li, X. Curcumin Attenuates Palmitate-Induced Apoptosis in MIN6 Pancreatic β-Cells through PI3K/Akt/FoxO1 and Mitochondrial Survival Pathways. Apoptosis 2015, 20, 1420–1432. [Google Scholar] [CrossRef]
- Cai, E.P.; Lin, J.-K. Epigallocatechin Gallate (EGCG) and Rutin Suppress the Glucotoxicity through Activating IRS2 and AMPK Signaling in Rat Pancreatic Beta Cells. J. Agric. Food Chem. 2009, 57, 9817–9827. [Google Scholar] [CrossRef]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary Anthocyanin-Rich Bilberry Extract Ameliorates Hyperglycemia and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic Mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef]
- Nizamutdinova, I.T.; Jin, Y.C.; Chung, J.I.; Shin, S.C.; Lee, S.J.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. The Anti-Diabetic Effect of Anthocyanins in Streptozotocin-Induced Diabetic Rats through Glucose Transporter 4 Regulation and Prevention of Insulin Resistance and Pancreatic Apoptosis. Mol. Nutr. Food Res. 2009, 53, 1419–1429. [Google Scholar] [CrossRef]
- Delphinidin-Induced Autophagy Protects Pancreatic <bold>β</Bold> Cells against Apoptosis Resulting from High-Glucose Stress via AMPK Signaling Pathway. ABBS 2019, 1242–1249. [CrossRef]
- Lee, J.S.; Kim, Y.R.; Park, J.M.; Kim, Y.E.; Baek, N.I.; Hong, E.K. Cyanidin-3-Glucoside Isolated from Mulberry Fruits Protects Pancreatic β-Cells against Glucotoxicity-Induced Apoptosis. Mol. Med. Rep. 2015, 11, 2723–2728. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.R.; Song, I.G.; Ha, S.-J.; Kim, Y.E.; Baek, N.-I.; Hong, E.K. Cyanidin-3-Glucoside Isolated from Mulberry Fruit Protects Pancreatic β-Cells against Oxidative Stress-Induced Apoptosis. Int. J. Mol. Med. 2015, 35, 405–412. [Google Scholar] [CrossRef]
- Choi, K.-H.; Park, M.H.; Lee, H.A.; Han, J.-S. Cyanidin-3-Rutinoside Protects INS-1 Pancreatic β Cells against High Glucose-Induced Glucotoxicity by Apoptosis. Z. Naturforsch. C 2018, 73, 281–289. [Google Scholar] [CrossRef]
- Suh, K.S.; Oh, S.; Woo, J.-T.; Kim, S.-W.; Kim, J.-W.; Kim, Y.S.; Chon, S. Apigenin Attenuates 2-Deoxy-D-Ribose-Induced Oxidative Cell Damage in HIT-T15 Pancreatic β-Cells. Biol. Pharm. Bull. 2012, 35, 121–126. [Google Scholar] [CrossRef]
- Kim, E.-K.; Kwon, K.-B.; Song, M.-Y.; Han, M.-J.; Lee, J.-H.; Lee, Y.-R.; Lee, J.-H.; Ryu, D.-G.; Park, B.-H.; Park, J.-W. Flavonoids Protect against Cytokine-Induced Pancreatic Beta-Cell Damage through Suppression of Nuclear Factor KappaB Activation. Pancreas 2007, 35, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yi, W.J.; Tan, L.; Zhang, J.H.; Xu, J.; Chen, Y.; Qin, M.; Yu, S.; Guan, J.; Zhang, R. Apigenin Attenuates Streptozotocin-Induced Pancreatic β Cell Damage by Its Protective Effects on Cellular Antioxidant Defense. In Vitro Cell. Dev.Biol.-Anim. 2017, 53, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, J.F.; Gross, R.; Petit, P.; et al. Quercetin Potentiates Insulin Secretion and Protects INS-1 Pancreatic β-Cells against Oxidative Damage via the ERK1/2 Pathway. Br. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, X.; Shuai, X.; Xu, Y.; Liu, Y.; Liang, X.; Wei, D.; Su, D. Luteolin Prevents Uric Acid-Induced Pancreatic β-Cell Dysfunction. J. Biomed. Res. 2014, 28, 292–298. [Google Scholar] [CrossRef]
- Ojo, O.A.; Grant, S.; Amanze, J.C.; Oni, A.I.; Ojo, A.B.; Elebiyo, T.C.; Obafemi, T.O.; Ayokunle, D.I.; Ogunlakin, A.D. Annona Muricata L. Peel Extract Inhibits Carbohydrate Metabolizing Enzymes and Reduces Pancreatic β-Cells, Inflammation, and Apoptosis via Upregulation of PI3K/AKT Genes. PLoS ONE 2022, 17, e0276984. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Ni, C.-C.; Yin, M.-C.; Lii, C.-K. Flavonoids Protect Pancreatic Beta-Cells from Cytokines Mediated Apoptosis through the Activation of PI3-Kinase Pathway. Cytokine 2012, 59, 65–71. [Google Scholar] [CrossRef]
- Karunakaran, U.; Elumalai, S.; Moon, J.S.; Jeon, J.H.; Kim, N.D.; Park, K.G.; Won, K.C.; Leem, J.; Lee, I.K. Myricetin Protects Against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5. Diabetes Metab. J. 2019, 43, 192–205. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Z.-F.; Dai, X.-Q.; Li, Y. Myricetin Protects against Cytokine-Induced Cell Death in RIN-M5f β Cells. J. Med. Food 2012, 15, 733–740. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, W.; Zhen, W.; Lum, H.; Nadler, J.; Bassaganya-Riera, J.; Jia, Z.; Wang, Y.; Misra, H.; Liu, D. Genistein Induces Pancreatic Beta-Cell Proliferation through Activation of Multiple Signaling Pathways and Prevents Insulin-Deficient Diabetes in Mice. Endocrinology 2010, 151, 3026–3037. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, M.; Liu, W.-W.; Hao, W.-B.; Tashiro, S.; Onodera, S.; Ikejima, T. In Vivo Recovery Effect of Silibinin Treatment on Streptozotocin-Induced Diabetic Mice Is Associated with the Modulations of Sirt-1 Expression and Autophagy in Pancreatic β-Cell. J. Asian Nat. Prod. Res. 2012, 14, 413–423. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Xu, F.; Liu, W.; Mai, Y.; Hayashi, T.; Hattori, S.; Ushiki-Kaku, Y.; Onodera, S.; Tashiro, S.-I.; et al. Silibinin Ameliorates Amylin-Induced Pancreatic β-Cell Apoptosis Partly via Upregulation of GLP-1R/PKA Pathway. Mol. Cell. Biochem. 2019, 452, 83–94. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, L.; He, H.; Wan, X.; Wang, F.; Mo, Z. Silibinin Protects β Cells from Glucotoxicity through Regulation of the Insig-1/SREBP-1c Pathway. Int. J. Mol. Med. 2014, 34, 1073–1080. [Google Scholar] [CrossRef]
- Keshtkar, S.; Kaviani, M.; Jabbarpour, Z.; Al-Abdullah, I.H.; Aghdaei, M.H.; Nikeghbalian, S.; Shamsaeefar, A.; Geramizadeh, B.; Azarpira, N.; Ghahremani, M.H. Significant Reduction of Apoptosis Induced via Hypoxia and Oxidative Stress in Isolated Human Islet by Resveratrol. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1216–1226. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Zheng, J.; Zeng, T.; Li, H.; Xiao, H.; Deng, X.; Hu, X. The Protective Effect of Resveratrol on Islet Insulin Secretion and Morphology in Mice on a High-Fat Diet. Diabetes Res. Clin. Pract. 2012, 97, 474–482. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, L.; Lu, Y.; Zhang, J.; Jian, F.; Liu, Y.; Li, F.; Li, W.; Wang, X.; Li, G. Activation of SIRT1 Protects Pancreatic β-Cells against Palmitate-Induced Dysfunction. Biochim. Biophys. Acta 2012, 1822, 1815–1825. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas 10th Edition Scientific Committee IDF Diabetes Atlas; IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 978-2-930229-98-0. [Google Scholar]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The Potential Health Effects of Dietary Phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Kang, N.J.; Shin, S.H.; Lee, H.J.; Lee, K.W. Polyphenols as Small Molecular Inhibitors of Signaling Cascades in Carcinogenesis. Pharmacol. Ther. 2011, 130, 310–324. [Google Scholar] [CrossRef]
- Sakle, N.S.; More, S.A.; Dhawale, S.A.; Mokale, S.N. Targeting Small Molecule Tyrosine Kinases by Polyphenols: New Move Towards Anti-Tumor Drug Discovery. Curr. Drug. Discov. Technol. 2020, 17, 585–615. [Google Scholar] [CrossRef]
- Wang, Y.; Alkhalidy, H.; Liu, D. The Emerging Role of Polyphenols in the Management of Type 2 Diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M.; et al. Recognizing the Benefits of Pre-/Probiotics in Metabolic Syndrome and Type 2 Diabetes Mellitus Considering the Influence of Akkermansia Muciniphila as a Key Gut Bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef]
- Anhê, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with Dietary Polyphenols: A New Weapon to Combat the Metabolic Syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid Metabolism: The Interaction of Metabolites and Gut Microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Martinoli, M.-G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Szunerits, S.; Melinte, S.; Barras, A.; Pagneux, Q.; Voronova, A.; Abderrahmani, A.; Boukherroub, R. The Impact of Chemical Engineering and Technological Advances on Managing Diabetes: Present and Future Concepts. Chem. Soc. Rev. 2021, 50, 2102–2146. [Google Scholar] [CrossRef]
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and Characterization of Polyphenol-Loaded Lipid Nanocapsules. Int. J. Pharm. 2009, 379, 270–277. [Google Scholar] [CrossRef]
- Hannon, T.; Kahn, S.; Utzschneider, K.; Buchanan, T.; Nadeau, K.; Zeitler, P.; Ehrmann, D.; Arslanian, S.; Caprio, S.; Edelstein, S.; et al. A Review of Methods for Measuring β-Cell Function: Design Considerations from the Restoring Insulin Secretion (RISE) Consortium. Diabetes Obes. Metab. 2018, 20, 14–24. [Google Scholar] [CrossRef]

| Compounds | Specie | In Vitro Models | In Vivo Models | Reference(s) |
|---|---|---|---|---|
| SpTx1 | Scolopendra polymorpha | Isolated mouse islets | Wild type mice | [62] |
| Mastoparan | Vespula lewisii | Rat RINm5F, hamster HIT-T15, mouse αTC3 cells, rat INS-1 cells isolated rat and human islets | ND | [58,64,65,66] |
| Secretory phospholipase 2 | Naja mossambica | Isolated mouse islets and single β-cells | ND | [60] |
| Tigerinin-1R and analogs | Indian frog Hoplobatrachus tigerinus | BRIN-BD11 cells | HFD-induced Swiss obese mice | [59,67,68] |
| Compounds | Specie | Class of the Active Substance | In Vitro Models | In Vivo Models | Reference(s) |
|---|---|---|---|---|---|
| Conkunitzin-S1 | Striated Cone (Conus striatus) | Inhibitor of KV1.7 | Isolated rat islets | ND | [72] |
| Guangxitoxin-1 (GxTX-1) | Chinese Fawn Tarentula (Chilobrachys Guangxiensis) | Inhibitor of KV2.1 and KV2.2 | Isolated mouse islets | ND | [67] |
| Hanatoxin (HaTX) | Chilean Rose Tarentula (Grammostola rosea) | Inhibitor of KV2.1 | Isolated human islets | ND | [73] |
| Iberiotoxin | Eastern Indian Scorpion (Hottentotta tamulus) | Inhibitor of KC | Isolated human islets, mouse MIN6 cells | ND | [74,75] |
| Compounds | Specie | In Vitro Models | In Vivo Models | Reference(s) |
|---|---|---|---|---|
| Agelaia MP-I (AMP-I) | Vespid wasp (Agelaia pallipes pallipes) | Isolated mouse islets | ND | [84] |
| Alyteserin-2a | Midwife toad (Alytes obstetricans) | BRIN-BD11 cells | High-fat-diet-induced obese Swiss mice | [85] |
| Amolopin | Frog (Amolops loloensis) | Rat INS-1 cells | ND | [86] |
| Bombesin | Frog (Bombina bombina) | HIT-T15 cells, isolated rat islets | Wild type baboon, wild type rats | [87,88,89,90,91,92] |
| Brevinin-2-related peptide (B2RP) | Mink frog (Lithobates septentrionalis) | BRIN-BD11 cells | HFD-induced obese Swiss mice | [93] |
| Caerulein-related peptides | Frog (Xenopus borealis and Xenopus amieti) | BRIN-BD11 cells | ND | [94] |
| Crotamine | Rattlesnake (Crotalus durrisus terrificus) | Isolated rat islets | ND | [95] |
| Dermaseptin B-IV | Frog (Phyllomedusa trinitatis) | BRIN-BD11 cells | ND | [96] |
| Esculentin-2CHa | Chiricahua leopard frog (Lithobates chiricahuensis) | BRIN-BD11 cells | Wild type and HFD-induced obese Swiss mice | [97,98] |
| Hymenochirin-1b | Frog Hymennochirus boettgeri | BRIN-BD11 cells | Wild type Swiss mice | [99,100] |
| Magainin–AM1 | Volcano clawed frog (Xenopus amieti) | BRIN-BD11 cells | Wild type and HFD-induced obese Swiss mice | [101] |
| Magainin–AM2 | Volcano clawed frog (Xenopus amieti) | BRIN-BD11 cells | Wild type and HFD-induced obese Swiss mice | [101] |
| Melittin | Honeybee (Apis mellifera) | Isolated mouse and rat islets | ND | [102,103] |
| Ocellatin-L2 | Bullfrog (Lithobates catesbeianus) | BRIN-BD11 cells | ND | [104] |
| Palustrin-2CBa | Bullfrog (Lithobates catesbeianus) | BRIN-BD11 cells | ND | [105] |
| Peptide Glycine-Leucine-Amide (PGLa)-AM1 | Frog (Xenopus amieti) | BRIN-BD11 cells and isolated mouse islets | ND | [106,107] |
| Plasticin-L1 | Frog (Leptodactylus laticeps) | BRIN-BD11 cells | ND | [82] |
| Pseudin-2 | frog (Pseudis paradoxa) | BRIN-BD11 cells | ND | [108] |
| Ranatuerin-2CBd | Bullfrog (Lithobates catesbeianus) | BRIN-BD11 cells | ND | [105] |
| Temporin-1OE, -1Va, -1Vb 1-Vc; -1DRb, -1TGb | Frog (Rana Ornativentris, Rana virgatipes, Rana draytonii, Rana Tagoi) | BRIN-BD11 cells | ND | [109] |
| Xenopsin and Xenopsin-AM2 | Frog (Xenopus borealis and Xenopus amieti) | BRIN-BD11 cells | ND | [94] |
| Compounds | Class | Group of the Active Substance | In Vitro Models | In Vivo Models | Reference(s) |
|---|---|---|---|---|---|
| Astragalin | Flavonol | Polyphenol | Isolated rat islets | ND | [140] |
| Caffeine | Purine | Alkaloid | ND | NMRI and BALB/c mice transplanted with mouse islets | [141,142,143] |
| Ellagic acid | Tannin | Polyphenol | Isolated mouse islets | ND | [144,145] |
| Kaempferol | Flavonol | Polyphenol | ND | ND | [146] |
| Lupanine | Quinolizidine | Alkaloid | Isolated rat islets | ND | [147] |
| Nuciferin | Aporphine | Alkaloid | Isolated mouse CD1 islets and INS-1 cells | ND | [148] |
| Quercetin | Flavonol | Polyphenol | Rat INS-1 cells | ND | [149] |
| Resveratrol | Stilbene | Polyphenol | MIN6 cells, HIT-T15, and RIN-m5F cells | Wistar Rats | [150] |
| Schisandrol A, schisandrol B and schisandrin C | Lignan | Polyphenol | Rat INS-1 cells | ND | [151] |
| Sparteine | Quinolizidine | Alkaloid | HIT-T15 cells | ND | [152] |
| 2R, 3R taxifolin 3-O-rhamnoside | Flavanonol | Polyphenol | ND | Wild type mice | [153] |
| Vindoline | Indole | Alkaloid | Mouse MIN6 cells and isolated mouse islets | db/db mice and STZ/HFD-induced type 2 diabetic rats | [154] |
| Compounds | Subclass | Class of the Active Substance | In Vitro Models | In Vivo Models | Reference(s) |
|---|---|---|---|---|---|
| Curcumin | Curcuminoid | Polyphenol | Mouse MIN6 cells and isolated human islets | ND | [136] |
| Daidzein | Isoflavone | Polyphenol | Rat INS-1 cells and isolated mouse islets | ND | [155,156] |
| Genistein | Isoflavone | Polyphenol | Rat INS-1 cells, mouse MIN6 cells and isolated mouse islets, isolated human islets | Streptozotocin-induced diabetic mice | [137,157,158] |
| Morphine | Isoquinoline | Alkaloid | Isolated rat islets | ND | [139,159] |
| Myricetin | Flavonol | Polyphenol | Isolated rat islets | Wistar Rats | [160] |
| Vanillic acid | Benzoic acid | Polyphenol | Rat INS-1 cells and isolated rat islets | ND | [161] |
| Protein Name | Protein Role | Reference(s) |
|---|---|---|
| AKT, also called PKB | AKT is a serine/threonine kinase which activates CREB, PDX1 and mammalian target of rapamycin (mTOR) complex 1. It inhibits glycogen synthase kinase 3 (GSK3β), caspase-9, FoxO1 and Bcl-2-associated death promoter (Bad) | [177,178] |
| MAK8IP1 also called Islet Brain 1/JIP1 | Scaffold protein that tethers MAP3K/MAP2K/JNK.MAPK8IP1 is involved in the anti-apoptotic JNK signaling pathway | [179,180,181] |
| MAPK10/JNK3 | Anti-apoptotic with unidentified targets. JNK3 is regulated by MAP8IP1/JIP-1/IB1 | [176] |
| CREB | Transcription factor that positively regulates the expression of insulin receptor substrate 2, a key component of IGF-1 and insulin receptor signaling leading to AKT activation | [182] |
| ERK1/2 | Ras-dependent extracellular signal-regulated kinase 1 (ERK1)/2 mitogen-activated protein (MAP) kinase pathway regulates cell survival | [183,184,185] |
| SERCA2b | P-type ATPase that regulates endoplasmic reticulum (ER) Ca2+ stores. | [186,187] |
| PDX-1 | Transcription factor that determines endocrine cell fate and controls β-cell differentiation | [188] |
| PKA | Protein kinase A that phosphorylates transcription factor CREB | [14,185] |
| NKX6.1 | Transcription factor that determines the specification of progenitor cells into mature functional β-cells. It maintains the function of adult pancreatic β-cells. | [189,190] |
| FoxO1 | Forkhead transcription factor (Fox) of the O subclass. FoxO1 is a transcriptional effector of IGF signaling that controls β-cell mass through Pdx1 | [191,192] |
| NRF2 | The nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) is a leucine zipper (bZip) transcription factor that regulates oxidant levels | [193,194] |
| MAFA | While v-Maf musculoaponeurotic fibrosarcoma transcription factor A (MAFA) controls β-cell differentiation, it maintains the mature phenotype and viability of β-cells | [195] |
| XBP-1 | X-box binding protein 1 (XBP1) is leucine zipper (bZIP) transcription factor that promotes ER biogenesis and activates the expression of ER chaperone genes | [196,197] |
| ERα | Estrogen receptor α (ERα) is a nuclear receptor that maintains the mitochondrial fission/fusion–mitophagy dynamics | [198,199] |
| Glucokinase | Transferase that phosphorylates glucose | [200] |
| PPARγ | Nuclear factor that regulates components of β-cell function and survival | [201,202] |
| AMPK | AMP-activated protein kinase that regulates β-cell survival via the mTOR pathway | [203,204] |
| Bcl2 | Mitochondrial membrane protein that inhibits apoptosis | [205] |
| Compounds | Class | Group of the Active Substance | Target Protein | In Vitro Models | In Vivo Models | Reference(s) |
|---|---|---|---|---|---|---|
| Curcumin | Curcuminoid | Polyphenol | AKT, FoxO1, SIRT1 | Mouse MIN6 cells | ND | [222,223] |
| Epigallocatchin (EGCG) | Flavonol | Polyphenol | AKT, PDX1 FoxO1, Bcl2, AMPK | Rat RINm5F | ND | [224] |
| Anthocyanins | Anthocyanidins | Polyphenol | AMPK, Bcl2, PDX1 | ND | KKAy diabetic mice STZ-induced diabetic rats | [225,226] |
| Dephinidin | Anthocyanidins | Polyphenol | AMPK | Rat RINm5F | ND | [227] |
| Cyanidin (Cyanidin-3-glucoside) | Anthocyanidins | Polyphenol | PPARγ, AKT, Bcl2 | Mouse MIN6 and rat INS-1 cells | ND | [228,229,230] |
| Apigenin | Flavone | Polyphenol | AKT | Hamster HIT-T15 and rat RINm5F cells | ND | [231,232,233] |
| Luteolin | Flavone | Polyphenol | AKT, MAFA | Rat INS-1 cells, MIN6 cells and isolated mouse islets | Alloxan-induced diabetic rats | [234,235,236] |
| Kaempferol | Flavonol | Polyphenol | AKT, Bcl2, PKA, PDX1 | Isolated human islets and INS-1 cells | ND | [146] |
| Quercetin | Flavonol | Polyphenol | ERK1/2, AKT | Rat RINm5F and INS-1 cells | ND | [232,237] |
| Myricetin | Flavonol | Polyphenol | PDX1, AKT | Rat RINm5F and INS-1 cells | ND | [238,239] |
| Naringenin | Flavonol | Polyphenol | AKT | INS-1 cells | ND | [237] |
| Genistein | Isoflavone | Polyphenol | ERK1/2, cAMP | Isolated human islets and INS-1 cells | STZ-induced diabetic rats | [240] |
| Silibinin | Flavinolignan | Polyphenol | PKA, PDX1 | ND | STZ-induced diabetic rats | [241,242,243] |
| Resveratrol | Stilbene | Polyphenol | PDX1, FoxO1 | Isolated human islets, isolated rat islets and INS-1 cells | HFD-induced diabetic mice | [244,245,246] |
| Berberine | Isoquinoline | Alkaloid | Nrf2 | Rat INS-1 cells, MIN6 cells | ND | [131,219] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lodato, M.; Plaisance, V.; Pawlowski, V.; Kwapich, M.; Barras, A.; Buissart, E.; Dalle, S.; Szunerits, S.; Vicogne, J.; Boukherroub, R.; et al. Venom Peptides, Polyphenols and Alkaloids: Are They the Next Antidiabetics That Will Preserve β-Cell Mass and Function in Type 2 Diabetes? Cells 2023, 12, 940. https://doi.org/10.3390/cells12060940
Lodato M, Plaisance V, Pawlowski V, Kwapich M, Barras A, Buissart E, Dalle S, Szunerits S, Vicogne J, Boukherroub R, et al. Venom Peptides, Polyphenols and Alkaloids: Are They the Next Antidiabetics That Will Preserve β-Cell Mass and Function in Type 2 Diabetes? Cells. 2023; 12(6):940. https://doi.org/10.3390/cells12060940
Chicago/Turabian StyleLodato, Michele, Valérie Plaisance, Valérie Pawlowski, Maxime Kwapich, Alexandre Barras, Emeline Buissart, Stéphane Dalle, Sabine Szunerits, Jérôme Vicogne, Rabah Boukherroub, and et al. 2023. "Venom Peptides, Polyphenols and Alkaloids: Are They the Next Antidiabetics That Will Preserve β-Cell Mass and Function in Type 2 Diabetes?" Cells 12, no. 6: 940. https://doi.org/10.3390/cells12060940
APA StyleLodato, M., Plaisance, V., Pawlowski, V., Kwapich, M., Barras, A., Buissart, E., Dalle, S., Szunerits, S., Vicogne, J., Boukherroub, R., & Abderrahmani, A. (2023). Venom Peptides, Polyphenols and Alkaloids: Are They the Next Antidiabetics That Will Preserve β-Cell Mass and Function in Type 2 Diabetes? Cells, 12(6), 940. https://doi.org/10.3390/cells12060940







