Abstract
Epigenetic deregulation is a critical theme which needs further investigation in bladder cancer research. One of the most highly mutated genes in bladder cancer is KDM6A, which functions as an H3K27 demethylase and is one of the MLL3/4 complexes. To decipher the role of KDM6A in normal versus tumor settings, we identified the genomic landscape of KDM6A in normal, immortalized, and cancerous bladder cells. Our results showed differential KDM6A occupancy in the genes involved in cell differentiation, chromatin organization, and Notch signaling depending on the cell type and the mutation status of KDM6A. Transcription factor motif analysis revealed HES1 to be enriched at KDM6A peaks identified in the T24 bladder cancer cell line; moreover, it has a truncating mutation in KDM6A and lacks a demethylase domain. Our co-immunoprecipitation experiments revealed TLE co-repressors and HES1 as potential truncated and wild-type KDM6A interactors. With the aid of structural modeling, we explored how truncated KDM6A could interact with TLE and HES1, as well as RUNX and HHEX transcription factors. These structures provide a solid means of studying the functions of KDM6A independently of its demethylase activity. Collectively, our work provides important contributions to the understanding of KDM6A malfunction in bladder cancer.
1. Introduction
Chromatin deregulation is regarded as one of the hallmarks of cancer; it is reflected at multiple different levels and affects many processes that drive tumorigenesis [1]. Malfunction in epigenetic regulatory mechanisms is especially noteworthy in bladder cancer considering the prominently high rate of mutations in chromatin-modifier genes [2], which underscores the importance of studying epigenetic regulation in this cancer type.
Bladder cancer is one of the most common and lethal diseases worldwide. Each year, there are 430,000 cases of patients diagnosed with bladder cancer, 170,000 of which result in death [3]. In total, 75–80% of bladder cancers show non-muscle-invasive characteristics, while the remaining are muscle-invasive [2]. Despite recent genomic and molecular characterizations, cisplatin-based chemotherapy and radiotherapy-based approaches still remain the main treatment options for this cancer type [4]. Thus, within the context of high-chromatin-modifier mutations in this cancer [2], the identification of epigenetic signatures implicated in pathogenesis of this disease is crucial.
Among the chromatin-modifier mutations observed in bladder cancer, a few of them are especially high, including KMT2D (28%), KDM6A (26%), and ARID1A (25%), which are observed in muscle-invasive bladder cancer [2]. Mutations identified in KDM6A in bladder cancer have loss-of-function characteristic [5], and there are many mutations identified within the intrinsically disordered region (IDR) of KDM6A, with one major mutation hotspot at position Q555 [5]. In this study, our focus is on identifying the differential function of KDM6A in normal, immortalized, and cancerous bladder cell lines. KDM6A is an H3K27 (lysine27 on histone H3) demethylase [6,7] that functions as a crucial chromatin modifier required for various developmental processes and for cellular differentiation [8]. Importantly, KDM6A is mutated in Kabuki syndrome, leading to facial abnormalities and cognitive dysfunction [8]. KDM6A is also required for embryonic development [9] and is involved in the resolution of bivalent chromatin domains [10]. KDM6A’s role in the regulation of Hox genes is well characterized [6]. In addition, KDM6A function has been identified to be important for heart development [11] and hematopoiesis [12]. On a biochemical level, KDM6A has been shown to be associated with the evolutionarily conserved MLL3/MLL4 COMPASS complex, which is involved in the methylation of H3K4 and active chromatin organization in several cell lines [7,8]. KDM6A is directly bound to the C-terminal of MLL3/MLL4 [13]. This indicates that KDM6A may play a role in regulating the catalytic activity of MLL3/4.
KDM6A is mutated in 26% of muscle-invasive bladder cancer cases, whereas its mutation frequency is higher in non-muscle-invasive bladder cancer (47.6%) (cBioPortal TCGA2017, MSK2017). However, the exact molecular mechanisms underlying the functions of KDM6A in a normal cellular setting and its promotion of tumorigenesis are currently open questions that need to be addressed in detail. Expanding on this, in this study, we generated KDM6A ChIP-seq data for one normal urothelial cell line (BdEC), one immortalized non-tumorigenic urothelial cell line (SV-HUC-1), and one urothelial cancer cell line (T24). The hypotheses we developed from the ChIP-seq data were further explored using biochemical assays and structural modeling. As a result, we show that KDM6A has a differential occupancy profile in the analyzed cell lines, and it shows distinct behavior when it is mutated. We also identify the involvement of KDM6A in the wide-spread regulation of Notch signaling in bladder cell lines. Furthermore, we structurally model the interaction of truncated KDM6A with repressors and co-repressors. Collectively, these results provide clear insights into previously uncovered roles of KDM6A in gene regulation.
2. Materials and Methods
2.1. Experimental Procedures
2.1.1. Cell Culture
The BdEC (primary bladder epithelial cells) (ATCC® PCS-420-010™), SV-HUC-1 (immortalized uroepithelial cells) (ATCC® CRL-9520™), T24 (urinary bladder transitional cell carcinoma) (ATCC® HTB-4™) and HEK293T cell lines were used for the experiments. The BdEC cell line was grown in the appropriate medium (ATCC® PCS-440-030™) and supplemented with the Prostate Epithelial Cell Growth Kit (ATCC PCS-440-040), as recommended by the ATCC, and Primocin (Invivogen). SV-HUC-1 was grown with RPMI-1640, and T24 and HEK293T were grown with DMEM, supplemented with 10% FBS and 1% penicillin. The cells were maintained in a humidified incubator at 37 °C and 5% CO2.
2.1.2. Chromatin Immunoprecipitation
The chromatin immunoprecipitation assays were performed by following the protocol of Weber et al., 2007 [14]. Briefly, the cells were fixed with 1% formaldehyde for 15 min when they reached 70–80% confluence. A final concentration of 0.125 M glycine was used to prevent crosslinking. After the pellet was lysed with lysis buffer, the chromatin was fragmented using a S220 Covaris Ultrasound Sonicator. With the intent of determining the optimum sonication for 200–500 bp fragmented DNA, a 50 µL input sample was taken from the lysate. The rest of the chromatin was used for the immunoprecipitation (IP). For IP, the chromatin was first pre-cleared using Dynabeads (Invitrogen, Waltham, MA, USA, 11203D) blocked with BSA and tRNA, and then, bound with 5 µL KDM6A antibody (Cell Signaling Technology, Danvers, MA, USA, 33510) overnight at 4 °C. The next day, the chromatin–antibody complex was bound with pre-blocked Dynabeads for 3 h at 4 °C, and after the washing steps, the chromatin was eluted. For both the input and IP DNA, following the RNase and proteinase K treatment, the samples were incubated overnight at 65 °C for reversal of the crosslink. The DNA was eluted using a DNA Clean & Concentrator kit (Zymo Research, Orange, CA, USA, D4034).
2.1.3. ChIP-Seq
For the T24, BdEC, and SV-HUC-1 cell lines, ChIP and input DNA were sent to the EMBL GeneCore facility for library preparation and sequencing. A NEBNext DNA Ultra II kit was used for library preparation. The libraries were sequenced on a Nextseq 500 platform using 75bp SE high-output mode.
2.1.4. RT-qPCR
Total RNAs were isolated using an MN Nucleospin RNA kit from each cell line. A total of 0.25 µg RNA was used for cDNA conversion using a Maxima First Strand cDNA Synthesis Kit. PCR was performed using the Applied Biosystems 7500 Fast Real-Time machine with a Roche FastStart Essential DNA Green Master (SYBR, Roche, Penzberg, Germany) kit for the selected genes. The experiments were performed as three technical replicates.
2.1.5. ChIP-qPCR
After performing the KDM6A ChIP experiments for the T24, SV-HUC-1, and BdEC cell lines, qPCR was performed using the Roche FastStart Essential DNA Green Master (SYBR) kit on an Applied Biosystems 7500 Fast Real-Time machine, and the results are presented as % input IP. The experiments were performed as three technical replicates. The ChIP-qPCR results were calculated using the ΔΔct method. ChIP Ct values were normalized to the input.
2.1.6. Primer Design
The primers for RT-qPCR and ChIP-qPCR were designed using the primer designing tool Primer-Blast (NCBI). A list of all the primers can be found in Table S3.
2.1.7. Western Blotting
The cells were washed with ice-cold PBS and scraped with 1× RIPA buffer supplemented with a protease inhibitor cocktail. After vortexing and incubating for 10 min on ice three times, the lysates were centrifuged at max speed at 4 °C for 20 min and the supernatants were collected. A Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA, 23225) was used to determine the protein concentrations. A total of 30 µg proteins were loaded onto 8% acrylamide:bis-acrylamide gel. The gel was run at 90 V for 30 min and at 120 V for one and a half hours. Gel transfer was performed at 350 mA for one and a half hours in a box full of ice. Non-fat dried milk powder was used for blocking the membrane for one hour at room temperature. KDM6A (CST 33510), HES1 (CST 11988), TLE1/2/3/4 (CST 4681S), and b-actin (CST 3700) primary antibodies were used with a 1/1000 dilution, and CST 5151S and LICOR 926-68072 secondary antibodies were used with a 1/30,000 dilution. Nitrocellulose membrane was incubated overnight at 4 °C with the KDM6A primary antibody, and for one and a half hours at room temperature with the b-actin antibody. Secondary antibody incubations were performed for one hour at room temperature. The membrane was imaged using the Li-COR ODYSSEY Clx machine (LiCOR, Lincoln, NE, USA) in auto mode.
2.1.8. Identification of KDM6A Mutation
DNA was extracted from the cell lines BdEC, T24, and SV-HUC-1 using a ZymoResearch (Irvine, CA, USA) Quick DNA Miniprep Plus kit (D4068). Mutation analysis was performed using the Archer VariantPlex Myeloid Panel (Diagnostica Longwood, Zaragoza, Spain), an NGS panel containing the KDM6A gene. Mutation calling was performed using ArcherDx Analysis (Version 6.2.7) with default settings using the Gen-ERA NGS service.
2.1.9. Plasmid Constructs and Transfection
To generate the FLAG-KDM6A construct, a pCMV_HA_UTX (Addgene, Plasmid #24168, Watertown, MA, USA) vector was digested for 2 h at 37 °C using Kpn1 and Not1 restriction enzymes, and the KDM6A gene was removed from this vector. In order to add a FLAG-tag to KDM6A, 2-step PCR was set up using the Q5 polymerase (M0494S, Neb, Ipswich, MA, USA) with designed primers (see primer list, Table S3). The PCR product was cut from the agarose gel and isolated using NucleoSpin® Gel and a PCR Clean-up kit (MN, 740609, Düren, Germany). The digested FLAG-tag KDM6A gene and the digested pcDNA3.1(+)/myc-HisA (Invitrogen) vector were ligated for 2 h at room temperature using T4 Ligase (Neb, M0202S) in accordance with the Neb Ligation Calculator (https://nebiocalculator.neb.com/#!/ligation, accessed on 1 April 2022). The ligation product (pcDNA3.1_FLAG_KDM6A) was transformed into an E. coli 10beta bacterial strain. Growing colonies were isolated using the plasmid isolation kit (MN, 740588) and validation was performed via Sanger sequencing. A total of 80% confluent 10cm plate HEK293T cells were transfected with 7.5 µg plasmid DNA using Lipofectamine 3000 (Invitrogen, L3000015) reagent. The medium was changed 24 h after transfection. To generate the KDM6A truncated mutation like in the T24 cell line, a pcdna3.1_FLAG-KDM6A plasmid was digested for 2 h at 37 °C using Kpn1 and Not1 restriction enzymes. After being run on 1% agarose gel at 100 V for 45 min, the FLAG-KDM6A band was isolated using NucleoSpin Gel and the PCR Clean-up kit (MN, Düren, Germany) from the gel. two-step PCR was set up using the Q5 polymerase with designed primers for E895* mutation (see primer list, Table S3). After this, the same steps as for the generation of the pcDNA3.1_FLAG_KDM6A plasmid were followed.
2.1.10. Co-IP
Proteins from untransfected and pcDNA3.1_FLAG_KDM6A or pcDNA3.1_FLAG_E895*mutant_KDM6A transfected HEK293T cells (48 h post transfection) were isolated using 300 µL lysis buffer (50 mM Tris-HCl pH:7.4, 150 mM NaCl, 1 mM EDTA, 1% TritonX-100, Protease Inhibitor Cocktail (Roche, Indianapolis, IN, USA)). For input controls 20 µL untransfected and transfected HEK293T cell lysates were used. Remaining proteins were bound overnight at 4 °C on the rotator with Flag affinity gel (Sigma, A2220, St. Louis, MO, USA) in accordance with the manufacturer’s instructions. After the incubation, IP proteins were centrifuged, and pellets were washed with TBS three times. Proteins for both input and IP fractions were boiled with SDS Sample Buffer (62.5 nM Tris-HCl pH:6.8, 2%SDS, 10% (v/v) Glycerol and 0.002% Bromophenol Blue) at 95 °C for 3 min and analyzed by Western blotting. KDM6A (CST 33510), HES1 (CST 11988) and TLE1/2/3/4 (CST 4681S) antibodies were used for Western blotting.
2.2. Data Analysis and Structural Modeling Procedures
All the bioinformatics analyses were performed at IBG Bioinformatics Platform.
2.2.1. Alignment and Processing of Next-Generation Sequencing Data
The ChIP-seq data were aligned to the human genome (hg38) using the nf-core/chip-seq pipeline (https://nf-co.re/chipseq/1.2.1, accessed on 5 February 2021). Using this pipeline, KDM6A ChIP peaks were called, peak annotation was performed, and KDM6A signals on peaks were quantified in CPM.
2.2.2. Peak Calling and Peak Overlap
KDM6A peak calling was performed using MACS2 (embedded in the nf-core chip-seq pipeline) using the parameters: p < 10−5 and FDR < 0.1 and a filter with the narrow option. For the set of peaks called for the T24, SV-HUC-1, and BdEC cell lines, the overlap among the peaks was checked using the “VennDiagram package” in R.
2.2.3. Clustering of KDM6A Peaks
Only the peaks which were located within a ±2 kb distance of the transcriptional start site (TSS) of genes and that had an fc value > 3 (value coming from MACS) were used for the clustering analysis. Before clustering, CPM counts were normalized for the library sizes, and the log2 of the normalized values were used for plotting. Hierarchical clustering was performed using the “pheatmap package” (https://cran.r-project.org/web/packages/pheatmap/index.html, accessed on 15 March 2021) in R. Clusters were determined and visualized using the cutree = 4 option in the package.
2.2.4. Analysis of T24 KDM6A Peaks
The TSS ±2 kb filtered peaks in the Venn Diagram were used for the analysis. Peaks were ranked according to their CPM normalized log2 values for each cluster. The pheatmap R package was used for visualization.
2.2.5. Transcription Factor Motif Finding
Transcription factor motif finding for different KDM6A clusters was performed using the findMotifsGenome.pl command of HOMER (http://homer.ucsd.edu/homer/motif/, accessed on 17 March 2021), with a size parameter of 300, using genomic positions.
2.2.6. Gene Ontology Analysis
Gene Ontology (GO) term analysis was performed using ConsensusPathDB (http://cpdb.molgen.mpg.de/, accessed on 22 March 2021) with a GO Level of 3 and biological process (BP) options. The top 10 terms for ‘IH-MULTI’, ‘normal’, and ‘normal-immortal’ clusters were identified, and for those terms, GO Level 3 was visualized using the dotplot function of the ggplot2 package (https://cran.r-project.org/web/packages/ggplot2/index.html, accessed on 5 August 2021). Only the terms with a qval < 0.01 filter were visualized.
2.2.7. Data Visualization
ChIP-seq signal data visualization was performed using the Bioconductor Gviz package (https://bioconductor.org/packages/release/bioc/html/Gviz.html, accessed on 1 June 2021). “BW” files were obtained from CPM-normalized BAM and BAI files using deeptools “BamCoverage” functions’ default parameters. Visualization was performed with “BW” files using the ‘horizon’ type of GViz package. GENCODE hg38 Comprehensive Gene Annotation Version 30 data were used as the GeneRegionTrack for the annotation.
2.2.8. String Protein Interaction Data Visualization
For protein interaction data visualization, Cytoscape (version 3.8.2)’s default style was used with String data.
2.2.9. HHEX and HES1 Gene Expression-Related Associations
To obtain correlation heatmaps between the expressions of HHEX and HES1, primary bladder cancer Hiseq data from TCGA BLCA 2017 (n = 426) and bladder cancer cell line RNAseq data (n = 25) from CCLE (https://depmap.org/portal/download/all/(CCLE_RNAseq_genes_rpkm_20180929.gct, accessed on 17 December 2021) were used. Pearson correlation coefficients for the correlation of the expression of HHEX and HES1 and the genes associated with the term ‘regulation of the developmental process’ within the normal-immortal cluster (n = 34) were calculated using RPKM-normalized log2 expression values. The ggplot2 (version 3.3.5) and pheatmap (version 1.10.12) packages in R were used for visualization.
2.2.10. Survival Analysis
To generate Kaplan–Meier graphs showing patients survival times according to different expression levels of HHEX and HES1, gene expression RNAseq IlluminaHiSeq* (n = 426) data and phenotype-curated survival data (n = 436) belonging to a TCGA Bladder Cancer (BLCA) cohort from Xena Browser Datasets [15] was used. Patients with survival data of OS.time > 120 (n = 382) were included in the Kaplan–Meier graphs. Low/high expression groups were formed by calculating the median values for HES1 and HHEX genes separately. Kaplan–Meier graphs were created using a log-rank test according to OS.time values. The coin (https://cran.r-project.org/web/packages/coin/index.html, accessed on 4 January 2022) (version 1.4-2) and survival (https://cran.r-project.org/web/packages/survival/index.html, accessed on 4 January 2022) (version 3.2-7) R packages were used for the analysis.
2.2.11. Comparison of T24 KDM6A Peaks with Published Data
To compare the KDM6A peak profile in the T24 cell line to a wild-type KDM6A peak profile called in a bladder cancer cell line, published WT KDM6A-expressing UMUC-1 bladder cancer cell line’s narrowpeak data (Barrows et al., GSE157091) were used. Peak overlaps were performed using the ‘findOverlaps’ function and the rtracklayer (https://bioconductor.org/packages/release/bioc/html/rtracklayer.html, accessed on 15 January 2023) and Genomic Ranges R packages (https://bioconductor.org/packages/release/bioc/html/GenomicRanges.html, accessed on 15 January 2023), and subsequently visualized using a Venn diagram. A snapshot showing KDM6A occupancy at HES1 and TLE3 loci in wild-type KDM6A-expressing UMUC-1 cells was created using the IGV genome browser (https://software.broadinstitute.org/software/igv/, accessed on 15 January 2023).
2.2.12. Structural Modeling
To support our in vitro findings, we modeled potentially interacting protein pairs linked to the truncated KDM6A interaction network, i.e., TLE1, HES1, RUNX1, and RUNX2. For this, first, we retrieved the available relevant PDB structures from the Protein Data Bank [16]. We also gathered the structures of homologous protein complex systems that would help us model the unknown protein complexes. For the proteins with unknown structures, we used the deep learning-based protein modeling tool AlphaFold2 (AF2) [17].
KDM6A:TLE1 Interaction Modeling
KDM6A folds into the tetratricopeptide repeat (TPR) and jumanji family (JmjC) domains. These two domains are connected by long-disordered regions between the 437th and 1079th residues. Resolved KDM6A structures start from the 877th residue; therefore, they lack the N-terminal TPR domain (PDB IDs: 3AVR, 3AVS, 6FUK, and 6FUL) [18,19] The full-length TLE1 is a 770-amino acid-long protein containing a glutamine-rich (Q), a glycine–proline-rich (GP), a nuclear localization signal-containing-central (CcN), a serine–proline-rich (SP), and a C-ter WD40 repeat domain (WDR). Among these domains, only the Q and WDR domains were experimentally determined for the TLE1 protein (PDB IDs: 1GXR, 2CE8, 2CE9, 4OM2, 4OM3, and 5MWJ) [20,21,22,23]. To model the KDM6A:TLE1 complex, we focused on the TPR and Q domains. The TPR motif is formed of 34-amino acid-long alpha-helix pair repeats and possesses a helical curvature. The structure of the Q domain is represented in the 4OM2 and 4OM3 PDB entries [22]. In these structures, TLE1-Q forms a coiled-coil dimer through the interaction of 70-amino acid-long alpha helices belonging to different TLE1s.
For the interaction modeling, initially, we modeled the TPR domain with AF2 using default parameters [17]. Then, we modeled KDM6A-TPR:TLE1-Q with an AlphaFold-multimer (data not shown here). Assessment of the AlphaFold models was carried out by analyzing: (i) pLDDT scores showing the predicted confidence level of each residue, (ii) predicted aligned error (PAE) graphs indicating the predicted position errors of each residue, and (iii) predicted TM (PTM) and interface predicted TM (iPTM) scores representing the overall accuracy of the complex and interface, respectively.
To generate good-quality models, we further searched the PDB Data Bank to find complexes containing TPR and coiled-coil interactions. The 6EJN PDB structure was chosen as a reference here [24]. In this structure, the leucine zipper-like domain of JNK-interacting protein 3 (JIP3, a cargo protein) is found in a coiled-coil dimer form. The cargo interacts with two different mouse kinesin light chain 2 proteins (KLC2, a transport protein) through their first TPR motifs. The overall KLC2:JIP3 structure has a stoichiometry of 2:2. As this structure provides the binding pose for TPR and the coiled-coil interaction, we generated our KDM6A-TPR:TLE1-Q model based on the 6EJN complex. To build the targeted complex, we used the best-ranked KDM6A-TPR AF2 model. The coiled-coil TLE1 dimer was retrieved from the 4OM3 PDB entry [22]. We used the flexible structural alignment server FATCAT to superimpose the first three TPRs of KLC2 and KDM6A [25]. The obtained superimposed models were further used during structural fitting via PyMOL along with coiled-coil alignments, leading to our final model (The PyMOL Molecular Graphics System, Version 2.0, Schrödinger, LLC, 1 December 2022).
TLE1:HES1 Interaction Modeling
HES1 contains basic helix–loop–helix (bHLH) and orange domains and a Trp-Arg-Pro-Trp (WRPW) motif located at its C-terminal end. The WRPW motif is required for binding to Groucho/TLE family members. The PDB entry 2CE9 contains an interaction between the WRPW peptide and the Human Groucho/TLE WDR domain. In this structure, the WDR domain interacts with the WRPW peptide in a 2:1 fashion. This TLE1:HES1 complex has been further refined using the HADDOCK 2.4 webserver [26,27]. HADDOCK is a user-friendly tool for performing biomolecular docking. After carrying out docking protocols, it also performs short molecular dynamics simulations to refine the interface by enabling sidechain movements. When turning off the docking procedures, HADDOCK completes the missing atoms in the input structures and improves the interface. To preserve the binding mode, we only applied the refinement step with unambiguous distance restraints to keep the peptide at its binding position (see advanced refinement protocol of HADDOCK 2.4 for details) [28].
TLE1:RUNX1/2 Interaction Modeling
RUNX1 and RUNX2, hereinafter referred to as RUNX in the text, are characterized by an approx. 120-amino acid-long RUNT domain, which is required for DNA recognition. The C-ter Trp-Arg-Pro-Tyr (WRPY) motif is known to interact with TLE1-WDR. We modeled this complex by taking the 2CE9 PDB as a template. During modeling, we mutated the last amino acid in the WRPW peptide to a tyrosine to match the WRPY motif of RUNX, and refined it using HADDOCK2.4, as described above.
TLE1:HHEX Modeling
The primary structure of HHEX, also known as the proline-rich homeodomain protein (PRH), consists of three domains: an N-terminal proline-rich (P-rich) domain, a central homeodomain that interacts with DNA, and a C-terminal acidic domain that regulates transcription activity. The 137-amino acid-long P-rich domain includes an EH1 motif (FxIxxIL) that provides an interaction surface for TLE1. From the Protein Data Bank, we retrieved the 2CE8 structure, showing the interaction of TLE1-WDR with the EH1 peptide (FSIDNIL) from human Goosecoid (GSC), another homeobox protein. To mimic the sequence of the EH1 motif in HHEX and FYIEDIL, we mutated the mismatched amino acids. Mutation and refinement procedures were performed as outlined above.
Further, we used AlphaFold-multimer to model the interaction between TLE1-Q and HHEX [29]. AlphaFold-multimer allows for the prediction of multimeric protein complexes with a known stoichiometry. Here, our input sequences contained the N-terminal 131 and 98 amino acids of TLE1 and HHEX, respectively. We modeled the TLE1:HHEX complex with a stoichiometry of 2:1. The default parameters were used for multimer modeling in our local machine. Assessment of the models was performed as described above.
2.2.13. Statistical Analysis
The R environment was used for all data analysis and plots. Kaplan–Meier graphs were created using a log-rank test according to OS.time values. ChIP-qPCR and gene expression data were visualized using GraphPad Prism 9. Pearson correlation coefficients were calculated to determine the correlation between gene expression values. For RT-qPCR’s significance scores, one-way ANOVA and Tukey’s multiple comparison test were used.
3. Results
3.1. KDM6A Mutation Status, Identification, and Classification of KDM6A Peaks in Bladder Cell Lines
We performed KDM6A ChIP-seq for three different cell lines: BdEC (normal primary bladder epithelial cells), SV-HUC-1 (immortalized bladder epithelial cells), and T24 (the muscle-invasive bladder cancer cell line). Among the cell lines analyzed, T24 had a known stop-gain mutation (position: E895). Prior studies showed that this mutation was homozygous, and Western blot analysis revealed a protein product about 110 kDa in size [30]. Our own Western blot analysis also confirmed the presence of KDM6A in all three cell lines, and a truncated mutated product for the T24 cell line (Figure 1A). The mutant KDM6A observed for the T24 cell line lacked the histone demethylase domain. To be thorough about identifying the mutation status of the cell lines used in the study, we also determined the mutation status of KDM6A in all three cell lines ourselves. As expected, the T24 cell line had a homozygous truncating mutation at position 895 for KDM6A. The SV-HUC-1 cell line had missense mutations at positions 1238 and 1239 with allele frequencies of 0.029 and 0.0282, while BdEC was a wild type for KDM6A (Figure 1B). We considered the missense mutation observed for SV-HUC-1 to be a reflection of a clonal mosaic pattern and to have a negligible effect on chromatin binding.
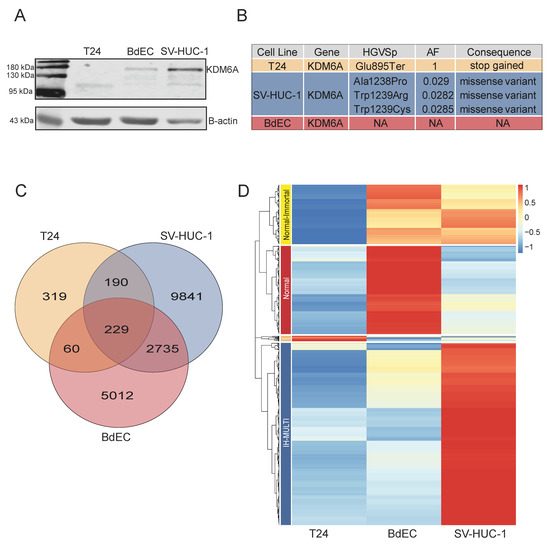
Figure 1.
Occupancy and mutation profile of KDM6A in different bladder cell lines. (A) Western blot image displaying expression of KDM6A at protein level. (B) Image represents the details concerning depth, allele frequency, and alternative alleles related to mutation status of KDM6A. (C) Venn diagram showing the overlap among KDM6A peaks called in T24, SV-HUC-1, and BdEC cell lines. (D) Heatmap shows the hierarchical clustering of KDM6A peaks, resulting in 4 clusters: normal-immortal (n = 235), normal (n = 349), cancer (n = 21), and IH-MULTI (n = 721).
We called KDM6A peaks in the three cell lines from our ChIP-seq data, resulting in 8050, 13,039, and 819 f peaks in the BdEC, SV-HUC-1, and T24 cell lines, respectively. A total of 16% of peaks were shared between the BdEC and SV-HUC-1 cell lines, whereas the total number of peaks called for the T24 cell lines was rather limited. Here, only 229 peaks were common among the all three cell lines used (Figure 1C). We then proceeded with the identification of KDM6A peaks with differential enrichment of the KDM6A signal. This analysis resulted in four distinct clusters, which we named ‘normal-immortalized shared’ (normal-immortal) (n = 235), ‘normal’ (n = 349), ‘cancer’ (n = 21), and ‘immortalized high-multiform in cancer and normal’ (IH-MULTI) (n = 721) (Figure 1D).
3.2. Characterization of Genomic Loci with Differential KDM6A Signals
After determining the genomic regions with differential KDM6A signals in the cell lines analyzed, we annotated them with functional features. First, we identified the genes associated with the peaks located in the clusters we defined (Figure 1D) (peaks located within a ±2kb distance of the transcriptional start sites (TSS); see Methods), and performed GO term enrichment analysis for the genes identified in different clusters. Genes associated with the ‘cancer’ cluster were not significantly enriched in the GO analysis. For the other three clusters, terms related to general developmental functions showed similar enrichment patterns for all three clusters. The ‘Normal’ and ‘normal-immortal’ clusters were similarly enriched for ‘cell junction organization’ (Figure 2A, Table S1). On the other hand, we identified ‘chromatin organization’-related terms to be significantly enriched only in the ‘normal’ cluster (Figure 2A). These genes, exceptionally, mainly consisted of replication-dependent histone genes (Figure S1A,B). In contrast, non-canonical histones did not have a KDM6A signal in any of the three cell lines (Figure S1C,D).
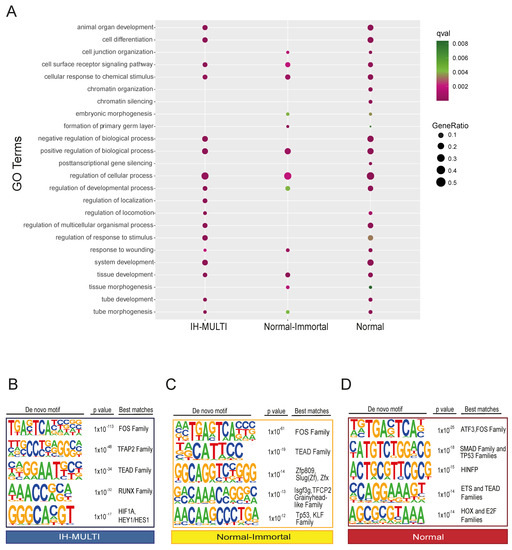
Figure 2.
Functional analysis of differential KDM6A peak sets identified across the cell lines. (A) Dot plot image displaying the GO term analysis performed for the genes associated with different clusters. (B–D) Transcription factor motif analysis performed for IH-MULTI (B), normal-immortal (C), and normal (D) clusters. Up to 3 different transcription factors/transcription factor families are shown with the best motif matching scores.
Next, we performed transcription factor motif analysis for the peaks belonging to different clusters (Figure 1C). All clusters were similarly enriched for FOS and TEAD family transcription factor motifs (Figure 2B–D). We identified enrichment in several members of the zinc finger family, grainyhead-like family, and p53 transcription factor motifs at peaks located within the ‘normal-immortal’ cluster, while the RUNX and bHLH families of transcription factors (HIF1A/HEY1) were enriched at peaks within the ‘IH-MULTI’ cluster (Figure 2B,C). The ‘Normal’ cluster showed enrichment in the Smad family, p53, and HINFP, a transcription factor involved in transcriptional regulation of histone H4 [31] (Figure 2D). As the identification of transcription motifs only does not directly provide information about the functional involvement of these factors, we checked the expression of several transcription factors with motifs enriched in these clusters, which might be relevant for bladder cancer biology. Combined with the motif finding results, these data (Figure S2) suggest that KDM6A might potentially cooperate with the TP53 family and the GRHL2 and FOSL2 transcription factors to drive gene expression associated with epithelial characteristics; meanwhile, coordination with transcription factors such as FOSL1, previously described to be involved in the invasive characteristic of bladder cancer [32,33], might contribute to the gene expression programs that drive tumorigenesis.
3.3. KDM6A Occupies the Genes Involved in Notch Signaling
To decipher the signaling pathways associated with KDM6A function, we analyzed the term ‘cell surface receptor signaling pathways’, which are similarly enriched for different clusters. The ‘IH-MULTI’ class showed enrichment in the Raf/MAP kinase cascade and VEGF signaling (Figure 3A). Surprisingly, genes involved in cell surface receptor signaling showed clustering of the genes associated with Notch signaling for both the ‘IH-MULTI’ and ‘normal-immortal’ clusters (Figure 3A,B). The ‘Normal’ class also had enrichment in Notch signaling. However, we discovered that these mostly consisted of histone genes, reflecting enrichment of the genes involved in chromatin organization in the ‘normal’ cluster (Figure S3).
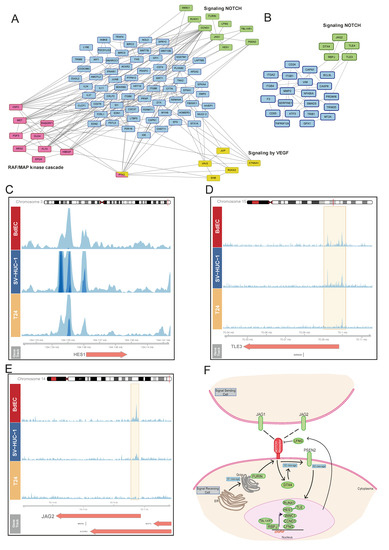
Figure 3.
Genes regulated by KDM6A are implicated in notch signaling. (A,B) Analysis of the genes involved in cell surface receptor signaling for IH-MULTI (A) and normal-immortal clusters (B). Interaction data obtained from String was visualized using Cytoscape. (C–E) Snapshot images showing KDM6A signal in BdEC, SV-HUC-1, and T24 cell lines at HES1 (C), TLE3 (D), and JAG2 (E) loci. (F) Manually created cartoon showing the genes involved in Notch signaling and associated with KDM6A peaks (A,B). Genes marked with KDM6A signal are highlighted in green.
Notch signaling has a key role in normal urothelium development, and its deregulation is implicated in bladder tumorigenesis [34,35]. We identified a KDM6A signal at the promoter regions of several prominent Notch signaling-related genes, such as HES1, a transcriptional repressor, and TLE3/4, a transcriptional co-repressor, both critical for cell fate decisions [36]; JAG1 and JAG2, ligands of the NOTCH receptor genes [37]; and RBPJ, a DNA binding protein which can complex with cleaved Notch to activate transcription [38] in all three cell lines with different signal intensities (Figure 3C–E and Figure S4A,B), covering all prominent components of Notch signaling (Figure 3F). Upon checking the expression of KDM6A-occupied Notch signaling genes in the analyzed cell lines, we identified that the occupancy level of KDM6A correlates with the expression level of the genes overall (Figure S4C), supporting the idea of a relationship between active gene expression and chromatin organization by KDM6A. However, it should be noted that relative levels of transcription factors and repressive chromatin remodeler complexes might be critical in the regulation of gene expression by KDM6A, as has been shown in the literature before [10,39]. Collectively, our results underscore the role of KDM6A in the regulation of Notch signaling and its potential deregulation in bladder cancer.
3.4. Truncated KDM6A Associates with Genes Deregulated in Cancer
We were able to find only a limited number of peaks annotated with a gene for the ‘cancer’ cluster (n = 20) (Figure 1D). Performing a GO enrichment analysis for this set of genes did not result in any significant GO term. However, as we have shown, the truncating mutation at position 895 resulted in a protein product, and this product still showed some chromatin binding profiles. Thus, we re-inspected all T24 KDM6A peaks within a ±2 kb distance from TSS (Figure 1C), independently of their cell line specificity (n = 50), (Table S2) to decipher more mechanistic insights which might be associated with truncated KDM6A. In relation to our initial clustering analysis (Figure 1D), most T24 KDM6A peaks were either located in the ‘IH-MULTI’ (42%) or ‘cancer’ (40%) clusters. The peaks with the highest signal intensity were also found in these respective clusters (Figure S5A). Among the genes occupied by KDM6A in the T24 cell line were Notch signaling family members, such as HES1 and TLE3 (as we have already shown in Figure 3), and genes that are deregulated in cancer, such as RUNX1, HHEX, and MET [40,41,42] (Figure 4A,B, Table S2). Additionally, ChIP-qPCR validation showed the occupancy of mutant KDM6A at these loci, confirming the presence of truncated KDM6A at these genomic regions, rather than being an artifact (Figure 4C).
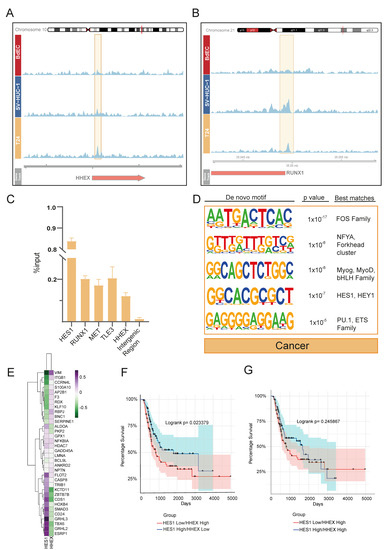
Figure 4.
Genomic occupancy profile of truncated KDM6A in T24 bladder cancer cell line. (A,B) Snapshot images showing KDM6A signal in BdEC, SV-HUC-1, and T24 cell lines at HHEX (A) and RUNX1 (B) loci. (C) ChIP-qPCR analysis of KDM6A enrichment in T24 cell line at selected loci. (D) Transcription factor motif enrichment analysis of T24 KDM6A peaks linked with genes. (E) Heatmap demonstrates the correlation between the expression of HES1, HHEX, and the genes involved in the regulation of developmental processes in primary bladder cancer. (F) Kaplan–Meier graph shows the overall survival of primary bladder cancer patients, grouped according to the expression status of HES1 and HHEX. (G) Kaplan–Meier graph shows the overall survival of primary bladder cancer patients, grouped according to the expression status of HES1 with high ‘HHEX’ criteria.
As the bladder cancer cell line included in our study, T24 is not a wild type for KDM6A; thus, we additionally compared our data to published ChIP-seq data for the bladder cancer cell line KDM6A. The used data were for the wild-type KDM6A-expressing UMUC-1 luminal bladder cancer cell line, on which we performed ectopic expression of wild-type KDM6A on UMUC-1 cells, which is normally a KDM6A mutant and lacks KDM6A expression [43]. This comparison revealed that overall, 114 out of 798 KDM6A peaks identified for the T24 cell line overlapped the KDM6A peaks called for wild-type KDM6A-expressing UMUC-1 cells (Figure S6A). The low rate of intersection might be attributed to either the wild-type vs. truncated KDM6A function or the different characteristics of these two cancer cell lines. However, the analysis of genes associated with overlapping peaks also pointed to the occupancy of KDM6A in wild-type KDM6A-expressing UMUC-1 cells at two key Notch signaling genes: HES1, and TLE3 (Figure S6B).
After performing a transcription factor motif analysis of T24 KDM6A peaks linked with genes, similar to other clusters, we identified FOS family members at the top. We also found HES1 and other bHLH family motifs to be enriched (Figure 4D). This result suggests that KDM6A is involved in the transcriptional regulation of HES1, and in turn, the interaction between KDM6A and HES1 might be implicated in the regulation of genes in bladder cancer. Although we determined the HES1 motif to be enriched primarily in KDM6A peak regions identified in the T24 cell line, our transcription finding motif analysis of the IH-MULTI cluster also showed the potential enrichment of the HEY/HES1 family for this cluster. Additionally, although it ranked low, we identified the HES1 motif to be enriched in the normal-immortal cluster (data not shown). Together, these data suggest that HES/HEY transcription factors are critical players in KDM6A-dependent gene regulation with varying degrees.
Among the genes occupied by truncated KDM6A in the T24 cell line, HHEX especially gained our attention. Like HES1, HHEX is also a transcriptional repressor involved in many developmental processes [44]. HHEX has the highest expression pattern in the T24 cell line among the cell lines analyzed, in contrast to the lowest expression of HES1 in the same cell line (Figure S5B). We wondered whether these two KDM6A-occupied repressors, HHEX and HES1, act differentially in the regulation of genes that are critical to urothelium development. Therefore, we plotted the correlation between HHEX, HES1 expression, and the expression of genes associated with the term ‘regulation of developmental process’ in the normal-immortal cluster (Figure 1D) for primary bladder cancer [45,46,47,48]. This analysis revealed a striking positive and corresponding negative correlation for the expression of developmental transcription factors such as GRHL2, GRHL3, ZBTB7B, and TBX6 [49,50,51], and the expression of HES1 and HHEX, respectively (Figure 4E). Conducting a similar analysis using CCLE bladder cancer cell line data showed comparable patterns for these factors, albeit with slightly more scattered clustering (Figure S5C). Based on this contrasting association observed for HES1 and HHEX, we next individually classified the MIBC patients (TCGA, 2017) according to the expression status of HES1 and HHEX and checked their survival. This analysis revealed statistically significant better overall survival in the patients expressing high levels of HES1 (Figure S5D), while the patients expressing high levels of HHEX showed worse prognoses, without statistical significance (Figure S5E). We further classified the patients according to the co-expression status of HHEX and HES1 as ‘HES1-high/HHEX-low’ and ‘HES1-low/HHEX-high’. Our analysis showed that patients who were in the ‘HES1-high/HHEX-low’ group showed significantly better prognoses compared to the patients belonging to the ‘HES1-low/HHEX-high’ group, especially regarding improved midterm survival (1000–3000 days) (Figure 4F). On the other hand, with a high HHEX expression level, high HES1 expression does not bring improved survival compared to low HES1 expression (Figure 4G). These results suggest that although HES1 expression level seems to be the main player in HES1-HHEX interplay with regard to patient survival, a higher expression level of HHEX disrupts the beneficial effects of high HES1 expression.
3.5. Structural Modeling and Co-IP Illustrate Previously Uncovered KDM6A-TLE-HES1 Interactions
We identified that the truncating mutation at position 895 of KDM6A still results in a protein product and is associated with chromatin. We also saw that the truncated KDM6A might still interact with the transcription factors, with HES1 determined as one prominent candidate. Therefore, we concentrated on understanding the effect of KDM6A truncation on its chromatin and transcription factor binding landscape.
The full-length KDM6A is 1401 amino acids long, containing eight TPR motifs at its N-terminal and a JmjC domain at its C-terminal region. The function of KDM6A-TPR is yet to be determined. The JmjC domain, on the other hand, was demonstrated to regulate KDM6A’s enzymatic activity in demethylating H3K27 [52]. TPR and JmjC are connected through an approx. 600-amino acid-long intrinsically disordered region (IDR) (Figure 5A). There is no structural information available on the full-length KDM6A, except its AlphaFold 2 (AF2) model deposited at the EBI’s database (Figure S7A) [53]. The prediction quality of this model shows that AF2 could predict the fold of TPR and JmjC domains accurately, whereas it could not predict the organization of the IDR or the orientation of the N- and C-terminal domains with respect to each other (Figure S7B).
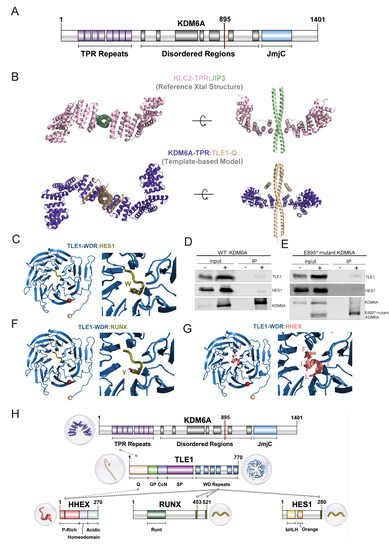
Figure 5.
KDM6A TPR motif related interactions. (A) Domain illustration of KDM6A. (B) Modeling of KDM6A-TPR:TLE1-Q. The reference structure, containing KLC2-TPR (pink) and JIP3 (light green), is given in the first row (PDB ID: 6EJN). Our KDM6A-TPR (purple) and TLE1-Q (wheat) interaction model is shown in the second row. (C,F,G) Refined structures for TLE1-WDR containing interactions, together with the interacting motif closeups. N-ter (wheat) and C-ter (red) are represented by spheres. (D,E) Immunoprecipitation using anti-FLAG affinity gel in HEK293T cells expressing wild-type FLAG-tagged KDM6A (D) and E895* mutant FLAG-tagged KDM6A (E). Western blot images show the bands detected for WT-KDM6A, truncated KDM6A, TLE1, and HES1. ‘−’ and ‘+’ denote the untransfected and transfected HEK293T cells, respectively, for the input and IP samples. (H) Overall representation of interactions of co-repressors with KDM6A and TLE1 at domain level. Gray lines represent interacting regions between proteins, the source of the interaction is grouped as literature information and structural evidence, shown with dotted and solid lines, respectively. Three-dimensional structures are also provided for each interacting region.
Upon investigating the interacting partners of KDM6A in the BioGRID database (https://thebiogrid.org/, accessed on 7 October 2022.), we realized that one interacting partner is TLE1. TLE1, belonging to the Groucho/transducin-like enhancer of split (TLE) family, is a co-repressor involved in diverse developmental functions [54]. Further, it is also known that TLEs can interact with HES1 [54]. Previously, it was shown that the first three TPRs of KDM6A interact with the N-terminal Q domain of TLE1 [55]. Expanding on this information from the literature, we explored the means of KDM6A:TLE1 interaction.
TLE family co-repressors (TLE1-4) all have a conserved N-terminal Q domain and C-terminal WDR domain, which are structurally resolved as isolated domains (Figure S8) [54]. From these structures, we know that the Q domain is responsible for the tetramerization of TLEs by forming parallel coiled-coil dimers (Figure S8, [22,56]). To model the KDM6A-TPR:TLE1-Q interaction, we looked for homologous systems, though we could not obtain any structure with meaningful sequence homology. Therefore, we checked all the available TPR structures that bind to a coiled-coil. As a result, we found the PDB entry 6EJN, a mouse KLC2 TPR domain that binds to the JIP3 leucine zipper domain [24]. In this structure, KLC2-TPR carries its cargo, JIP3, in a 2:2 fashion, where two independent TPRs bind to the coiled-coil dimer of JIP3 through their first TPR (Figure 5B). As this TPR interaction information overlaps with that of KDM6A:TLE1, we decided to use 6EJN as a template for our KDM6A-TPR:TLE1-Q complex modeling. Although there are no significant sequence similarities between the KDM6A-TPR:TLE1-Q interaction system and KLC2-TPR:JIP3, upon one-to-one structural alignment (using FATCAT [25]), we were able to build a working structural model for the KDM6A-TPR:TLE1-Q complex (Figure 5B). This model portrays the first structural hypothesis on how KDM6A can carry out its non-enzymatic tasks in the absence of its JmjC domain. It also suggests that the KDM6A-TPR:TLE1-Q complex is formed in a 2:2 fashion, which warrants further experimental validation.
We also know that TLE1 interacts through its WDR domain with HES1’s C-terminal WRPW motif (2CE9) [21] (Figure 5C). This tells us that TLE1 interacts with KDM6A and HES1 via its different domains. Based on this, we can suggest that KDM6A, TLE1, and HES1 form a complex in which they function together. To probe such an interaction, we overexpressed FLAG-tagged full-length KDM6A and performed a co-immunoprecipitation experiment. The antibody used for the detection of TLE1 also recognizes the other TLE proteins (TLE2, TLE3, and TLE4), based on the conservation of all functional domains in these proteins [57]. However, for simplicity, we refer to the detected protein as ‘TLE1’. Our co-immunoprecipitation results demonstrate the potential presence of both TLE1 and HES1 in the same complex as KDM6A (Figure 5D). As this experiment was performed with wild-type KDM6A that possesses all the functional domains, to further illustrate that TLE1:KDM6A interaction occurs via the TPR domain of KDM6A, we carried out another co-immunoprecipitation experiment using FLAG-tagged truncated KDM6A, mimicking the E895* mutation observed in the T24 cell line. These results also revealed the association of truncated KDM6A with TLE1, as well as HES1, although the latter was slightly weaker compared to full-length KDM6A (Figure 5E). Collectively, these data emphasize the role of the TPR domain in these protein interactions.
Overall, the interaction between KDM6A, TLE1, and HES1 might be puzzling, as TLEs and HES1 are involved in transcriptional repression, and KDM6A is responsible for active chromatin organization. Several studies have shown that TLE/HES1-mediated repression can be resolved through the action of transcription factors, which can turn the repressive complex into an activator. For instance, RUNX2 has been determined to interact with the TLE-WDR domain, and thus, can interrupt the interaction of the TLE-WDR domain with HES1, resulting in transcriptional derepression [58]. RUNX proteins are 453 and 521 amino acid long, containing WRPY (WRPW-like) motifs in their C-termini. So, RUNX’s WRPY might compete with the WRPW of HES1 for the same binding site on TLE1 (Figure 5F). Another study also showed that HIPK2 can interact with the TLE1/HES1 complex and mediate transcriptional activation that is critical for cortical neurogenesis [59]. Indeed, our model of the interaction of RUNX family transcription factors, enriched at KDM6A peaks belonging to the IH-MULTI cluster (Figure 2B), with TLE1 illustrated this association (Figure 5F). Furthermore, additional repressors can interact with the TLE:HHEX transcriptional repressor, another KDM6A-regulated gene we identified (Figure 4A). HHEX consists of P-rich, homeo, and acidic domains. It has an FxIxxIL motif residing between 32 and 38 residues in the P-rich domain. HHEX has been suggested to interact with both TLE1-WDR and TLE1-Q via its 98 N-terminal residues [60]. The studies narrowed down the interface of HHEX with TLE1-WDR to the FxIxxIL motif in the N-terminal HHEX, as shown in our structural model [61] (Figure 5G). Though the N-terminal HHEX has been suggested to interact with TLE1-Q, we could not obtain a confident model showing this interaction. Therefore, our AF2 model is presented in the SI (Figure S9).
Collectively, all these results and this knowledge extend the interaction network of TLE1, suggesting a dynamic interaction network that depends on the domain characteristics of the interactors (Figure 5H). Based on this, we propose a regulatory mechanism where KDM6A associates with TLE co-repressor complexes, and upon the action of a transcriptional activator, KDM6A exerts its role in active chromatin organization and transcriptional activation.
4. Discussion
KDM6A is one of the most frequently mutated genes in bladder cancer [2]. Therefore, understanding the function of KDM6A in normal and tumorigenic bladders is essential. In this study, we performed KDM6A ChIP-seq in normal, immortalized, and tumorigenic bladder cell lines. Our results show differential KDM6A occupancy across the cell lines analyzed.
Among the KDM6A groups we defined, the ‘normal’ cluster was especially associated with the genes involved in chromatin organization, mainly consisting of replication-dependent histone genes. We identified the ‘normal-immortal’ and ‘IH-MULTI’ cluster to be enriched in the genes involved in Notch signaling, including the ligands and the repressor and co-repressor proteins critical for this pathway. Notch signaling is implicated in normal urothelium development [35] and is known to be deregulated in bladder cancer [62]. Our findings here, which show the relationship of KDM6A with Notch signaling, provide additional insights into what goes wrong in cancer samples when KDM6A is mutated. Previously, the association of KDM6A with Notch signaling has been shown for neural crest cells in connection to Kabuki syndrome [63], as well as the reprogramming of germ line cells to neurons [64].
The T24 cancer cell line we used in this study has a homozygous truncating mutation at position 895 for KDM6A. Overall, we identified a much lower number of KDM6A peaks for this cell line (Figure 1C); still, we realized that some loci showed strong KDM6A occupancy rather than being artifacts. This finding highly suggests that truncated KDM6A still preserves some of its chromatin binding abilities. Thus, we inspected this mutant product in more detail in terms of cooperation with transcription factors and structural modeling. Our transcription factor motif analysis implicated HES/HEY family as being associated with truncated KDM6A in the T24 cell line. KDM6A has TPR motifs located at the N-terminus and a demethylase domain at the C-terminus. Our modeling showed that truncated KDM6A in T24 has TPR motifs, which can fulfill the protein–protein interactions. In fact, using co-immunoprecipitation experiments, we show that KDM6A, TLE1, and HES1 are in the same complex. However, it should be noted that immunoprecipitation does not provide complete proof of the direct interaction of these proteins.
We propose that the TPR domain of KDM6A interacts with repressor regulatory factors, which also interacts with transcriptional activators. Upon receiving the correct signal, KDM6A loses its interaction with repressor regulatory proteins and activates transcription, potentially via its JmjC demethylase domain. In the case of truncated KDM6A, the TPR domain might interact with repressors and co-repressors such as HES1/TLE1. Additionally, the lack of a JmjC domain may result in repression of the key differentiation genes, while some oncogenic ones might be activated with the cooperation with transcription factors implicated in tumorigenesis.
HES1 is normally required for the timely differentiation of many cell types. It is known that function of HES1 in cancer might be abrogated, and high HES1 expression might be associated with less differentiated tumors [36]. Recently, a study investigating the expression of the genes involved in Notch signaling in normal bladder, papillary, and non-papillary bladder tumors showed that overall, the expression of HES1 is higher in both papillary and non-papillary tumors compared to normal, while its expression is higher in papillary tumors in comparison to non-papillary tumors [65]. Thus, we suggest that depending on the different mutations observed for KDM6A, and the presence of different activator proteins, the KDM6A-TLE1-HES1 regulatory axis can be manipulated in diverse ways, boosting tumorigenesis.
Our results further show that the interactions of TLE1 and, subsequently, its interaction with KDM6A might be quite dynamic, whereby associations and dissociations can be established depending on the domain organization and the relative number of interacting proteins. In the KDM6A-TLE1-HES1 regulatory axis, we suggest that another repressor, HHEX, which is highly expressed in the T24 cell line, comes into this regulatory scheme via its interaction with TLE1. The literature about the role of HHEX in bladder cancer is very limited. One study suggested that the expression level of HHEX in superficial bladder cancer is lower compared to that in the normal bladder [66]. Here, our results based on MIBC data (TCGA, 2017) suggest that high expression of HHEX shows more oncogenic behavior and is associated with worse survival. Checking the results of the published study actually shows that compared to superficial bladder cancer, invasive cancer shows a slightly higher level of HHEX expression [66]. Together, the existing literature and our data led us to propose a model in which the expression level of HHEX might show differential effects depending on the interplay with other repressors, such as HES1, and the status and the grade of bladder tumors.
KDM6A has a JmjC demethylase domain functioning in the demethylation of H3K27 [18,52]. However, so far, multiple studies have shown that the function of KDM6A in differentiation and development is largely independent of its demethylase activity [52,63]. A recent study also demonstrated that the expression of wild-type KDM6A or a KDM6A-demethylase-mutant in a KDM6A-null bladder cancer cell line resulted in similar KDM6A genomic localizations [43]. It was additionally shown that the IDR region of KDM6A is critical to the creation of KDM6A liquid condensates and the functioning of MLL4 [67]. The existing literature and our findings highly suggest that TPR motifs present at the N-terminus of the KDM6A are very critical in terms of the specification of the interaction partners of KDM6A.
Collectively, our results provide much critical information about the distinct KDM6A occupancy in bladder cell lines with different characteristics, and the possibility of interacting transcription factors. Importantly, we believe that the KDM6A-TLE1-HES1 regulatory axis we defined makes a valuable contribution to the understanding of epigenetic deregulation in bladder cancer with regard to the mutations observed in KDM6A.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells12060836/s1, Figure S1: KDM6A localizes to the replication-dependent histone genes in normal bladder cell line. Figure S2: Expression of transcription factors in BdEC, SV-HUC-1, and T24 cell lines. Figure S3: Analysis of the genes involved in cell surface receptor signaling for ‘normal’ cluster. Figure S4: Localization of KDM6A in genes involved in Notch signaling. Figure S5: HES1 and HHEX interplay in bladder cancer cell lines and their association with patient survival. Figure S6: Comparison of KDM6A peaks identified for T24 cell line with the peaks called in WT KDM6A-expressing UMUC-1 cells. Figure S7: AlphaFold models of KDM6A protein. Figure S8: Conserved domain structures in TLE1. Figure S9: AlphaFold model of TLE1-Q:HHEX complex. Figure S10: Original Western Blot images. Table S1: Results of gene ontology analysis performed for the clusters in Figure 1D. Table S2: Genes associated with all T24 KDM6A peaks within ±2kb distance from TSS. Table S3: List of primers used in the study.
Author Contributions
Conceptualization, G.Ö.-Y., S.S., E.K., G.K. and S.E.-O.; methodology, G.Ö.-Y., B.S. and A.B.; software, validation, formal analysis, investigation, data curation, and visualization, G.Ö.-Y., B.S., A.B., A.E. and A.A.; writing—review and editing, G.Ö.-Y., B.S., S.S., E.K., G.K. and S.E.-O.; supervision, project administration, and funding acquisition, S.E.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the L’Oreal-UNESCO National for Women in Science Program, the L’Oreal-UNESCO International Rising Talent Program, and the Scientific and Technological Research Council of Turkey (TÜBİTAK). S.E.-O. and E.K. were supported by the EMBO Installation Grant (Nos. 4148 and 4421, respectively).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
ChIP-seq data have been deposited in the NCBI Gene Expression Omnibus (GEO) database with accession number GSE216625. Structural modeling efforts have been deposited on GitHub (https://github.com/CSB-KaracaLab/KDM6A-interacting-models, accessed on 16 December 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bennett, R.L.; Licht, J.D. Targeting Epigenetics in Cancer. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Sinha, R.J.; Bhaskar, V.; Aeron, R.; Sharma, A.; Singh, V. Role of gemcitabine and cisplatin as neoadjuvant chemotherapy in muscle invasive bladder cancer: Experience over the last decade. Asian J. Urol. 2019, 6, 222–229. [Google Scholar] [CrossRef]
- Koch, J.; Lang, A.; Whongsiri, P.; Schulz, W.A.; Hoffmann, M.J.; Greife, A. KDM6A mutations promote acute cytoplasmic DNA release, DNA damage response and mitosis defects. BMC Mol. Cell Biol. 2021, 22, 54. [Google Scholar] [CrossRef]
- Agger, K.; Cloos, P.A.C.; Christensen, J.; Pasini, D.; Rose, S.; Rappsilber, J.; Issaeva, I.; Canaani, E.; Salcini, A.E.; Helin, K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 2007, 449, 731–734. [Google Scholar] [CrossRef]
- Hong, S.; Cho, Y.-W.; Yu, L.-R.; Yu, H.; Veenstra, T.D.; Ge, K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc. Natl. Acad. Sci. USA 2007, 104, 18439–18444. [Google Scholar] [CrossRef]
- Der Meulen, V.; Speleman, F.; Van Vlierberghe, P. The H3K27me3 demethylase UTX in normal development and disease. Epigenetics 2014, 9, 658–668. [Google Scholar] [CrossRef]
- Welstead, G.G.; Creyghton, M.P.; Bilodeau, S.; Cheng, A.W.; Markoulaki, S.; Young, R.A.; Jaenisch, R. X-linked H3K27me3 demethylase Utx is required for embryonic development in a sex-specific manner. Proc. Natl. Acad. Sci. USA 2012, 109, 13004–13009. [Google Scholar] [CrossRef]
- Dhar, S.S.; Lee, S.-H.; Chen, K.; Zhu, G.; Oh, W.; Allton, K.; Gafni, O.; Kim, Y.Z.; Tomoiga, A.S.; Barton, M.; et al. An essential role for UTX in resolution and activation of bivalent promoters. Nucleic Acids Res. 2016, 44, 3659–3674. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.W.; Lee, S.K. UTX, a histone H3-lysine 27 demethylase, acts as a critical switch to activate the cardiac developmental program. Dev. Cell 2012, 22, 25–37. [Google Scholar] [CrossRef]
- Thieme, S.; Gyárfás, T.; Richter, C.; Özhan, G.; Fu, J.; Alexopoulou, D.; Muders, M.H.; Michalk, I.; Jakob, C.; Dahl, A.; et al. The histone demethylase UTX regulates stem cell migration and hematopoiesis. Blood 2013, 121, 2462–2473. [Google Scholar] [CrossRef]
- Kim, J.-H.; Sharma, A.; Dhar, S.S.; Lee, S.-H.; Gu, B.; Chan, C.-H.; Lin, H.-K.; Lee, M.G. UTX and MLL4 coordinately regulate transcriptional programs for cell proliferation and invasiveness in breast cancer cells. Cancer Res. 2014, 74, 1705–1717. [Google Scholar] [CrossRef]
- Weber, M.; Hellmann, I.; Stadler, M.B.; Ramos, L.; Pääbo, S.; Rebhan, M.; Schübeler, D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat. Genet. 2007, 39, 457–466. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Sengoku, T.; Yokoyama, S. Structural basis for histone H3 Lys 27 demethylation by UTX/KDM6A. Genes Dev. 2011, 25, 2266–2277. [Google Scholar] [CrossRef]
- Esposito, C.; Wiedmer, L.; Caflisch, A. In Silico Identification of JMJD3 Demethylase Inhibitors. J. Chem. Inf. Model 2018, 58, 2151–2163. [Google Scholar] [CrossRef]
- Pickles, L.M.; Roe, M.; Hemingway, E.J.; Stifani, S.; Pearl, L.H. Crystal structure of the C-terminal WD40 repeat domain of the human Groucho/TLE1 transcriptional corepressor. Structure 2002, 10, 751–761. [Google Scholar] [CrossRef]
- Jennings, B.; Pickles, L.M.; Wainwright, S.M.; Roe, M.; Pearl, L.H.; Ish-Horowicz, D. Molecular recognition of transcriptional repressor motifs by the WD domain of the Groucho/TLE corepressor. Mol. Cell. 2006, 22, 645–655. [Google Scholar] [CrossRef]
- Chodaparambil, J.; Pate, K.T.; Hepler, M.R.D.; Tsai, B.P.; Muthurajan, U.M.; Luger, K.; Waterman, M.L.; Weis, W. Molecular functions of the TLE tetramerization domain in Wnt target gene repression. Embo J. 2014, 33, 719–731. [Google Scholar] [CrossRef]
- McGrath, S.; Tortorici, M.; Drouin, L.; Solanki, S.; Vidler, L.; Westwood, I.; Gimeson, P.; Van Montfort, R.; Hoelder, S. Structure-Enabled Discovery of a Stapled Peptide Inhibitor to Target the Oncogenic Transcriptional Repressor TLE1. Chemistry 2017, 23, 9577–9584. [Google Scholar] [CrossRef]
- Cockburn, J.J.; Hesketh, S.J.; Mulhair, P.; Thomsen, M.; O’Connell, M.J.; Way, M. Insights into Kinesin-1 Activation from the Crystal Structure of KLC2 Bound to JIP3. Structure 2018, 26, 1486–1498.e6. [Google Scholar] [CrossRef]
- Ye, Y.; Godzik, A. FATCAT: A web server for flexible structure comparison and structure similarity searching. Nucleic Acids Res. 2004, 32, W582–W585. [Google Scholar] [CrossRef]
- Honorato, R.V.; Koukos, P.I.; Jimenez-Garcia, B.; Tsaregorodtsev, A.; Verlato, M.; Giachetti, A.; Rosato, A.; Bonvin, A.M.J.J. Structural Biology in the Clouds: The WeNMR-EOSC Ecosystem. Front. Mol. Biosci. 2021, 8, 729513. [Google Scholar] [CrossRef]
- Van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; De Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
- Bonvin, A.M.J.J.; Karaca, E.; Kastritis, P.L.; Rodrigues, J.P.G.L.M. Defining distance restraints in HADDOCK. Nat. Protoc. 2018, 13, 1503. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T. Protein complex prediction with AlphaFold-Multimer. bioRxiv 2022. [Google Scholar] [CrossRef]
- Lang, A.; Yilmaz, M.; Hader, C.; Murday, S.; Kunz, X.; Wagner, N.; Wiek, C.; Petzsch, P.; Köhrer, K.; Koch, J.; et al. Contingencies of UTX/KDM6A Action in Urothelial Carcinoma. Cancers 2019, 11, 481. [Google Scholar] [CrossRef]
- Ghule, P.N.; Xie, R.L.; Colby, J.L.; Jones, S.N.; Lian, J.B.; Van Wijnen, A.J.; Stein, J.L.; Stein, G.S. p53 checkpoint ablation exacerbates the phenotype of Hinfp dependent histone H4 deficiency. Cell Cycle 2015, 14, 2501–2508. [Google Scholar] [CrossRef]
- Sayan, A.E.; Stanford, R.; Vickery, R.; Grigorenko, E.; Diesch, J.; Kulbicki, K.; Edwards, R.; Pal, R.; Greaves, P.; Jariel-Encontre, I.; et al. Fra-1 controls motility of bladder cancer cells via transcriptional upregulation of the receptor tyrosine kinase AXL. Oncogene 2012, 31, 1493–1503. [Google Scholar] [CrossRef]
- Guneri-Sozeri, P.Y.; Özden-Yılmaz, G.; Kisim, A.; Cakiroglu, E.; Eray, A.; Uzuner, H.; Karakülah, G.; Pesen-Okvur, D.; Senturk, S.; Erkek-Ozhan, S. FLI1 and FRA1 transcription factors drive the transcriptional regulatory networks characterizing muscle invasive bladder cancer. Commun. Biol. 2023, 6, 199. [Google Scholar] [CrossRef]
- Maraver, A.; Fernandez-Marcos, P.J.; Cash, T.P.; Mendez-Pertuz, M.; Dueñas, M.; Maietta, P.; Martinelli, P.; Muñoz-Martin, M.; Martínez-Fernández, M.; Cañamero, M.; et al. NOTCH pathway inactivation promotes bladder cancer progression. J. Clin. Investig. 2015, 125, 824–830. [Google Scholar] [CrossRef]
- Paraskevopoulou, V.; Bonis, V.; Dionellis, V.S.; Paschalidis, N.; Melissa, P.; Chavdoula, E.; Vasilaki, E.; Pateras, I.S.; Klinakis, A. Notch controls urothelial integrity in the mouse bladder. JCI Insight 2020, 5, e133232. [Google Scholar] [CrossRef]
- Sang, L.; Roberts, J.; Coller, H. Hijacking HES1: How tumors co-opt the anti-differentiation strategies of quiescent cells. Trends Mol. Med. 2010, 16, 17–26. [Google Scholar] [CrossRef]
- Choi, K.; Ahn, Y.-H.; Gibbons, D.L.; Tran, H.T.; Creighton, C.J.; Girard, L.; Minna, J.D.; Qin, F.X.-F.; Kurie, J.M. Distinct biological roles for the notch ligands Jagged-1 and Jagged-2. J. Biol. Chem. 2009, 284, 17766–17774. [Google Scholar] [CrossRef]
- Borggrefe, T.; Oswald, F. The Notch signaling pathway: Transcriptional regulation at Notch target genes. Cell Mol. Life Sci. 2009, 66, 1631–1646. [Google Scholar] [CrossRef]
- Seenundun, S.; Rampalli, S.; Liu, Q.-C.; Aziz, A.; Palii, C.; Hong, S.; Blais, A.; Brand, M.; Ge, K.; Dilworth, F.J. UTX mediates demethylation of H3K27me3 at muscle-specific genes during myogenesis. EMBO J. 2010, 29, 1401–1411. [Google Scholar] [CrossRef]
- Supernat, A.; Łapińska-Szumczyk, S.; Sawicki, S.; Wydra, D.; Biernat, W.; Żaczek, A.J. Deregulation of RAD21 and RUNX1 expression in endometrial cancer. Oncol. Lett. 2012, 4, 727–732. [Google Scholar] [CrossRef]
- Jankovic, D.; Gorello, P.; Liu, T.; Ehret, S.; La Starza, R.; Desjobert, C.; Baty, F.; Brutsche, M.; Jayaraman, P.S.; Santoro, A.; et al. Leukemogenic mechanisms and targets of a NUP98/HHEX fusion in acute myeloid leukemia. Blood 2008, 111, 5672–5682. [Google Scholar] [CrossRef]
- Minuti, G.; Landi, L. MET deregulation in breast cancer. Ann. Transl. Med. 2015, 3, 181. [Google Scholar]
- Barrows, D.; Feng, L.; Carroll, T.S.; Allis, C.D. Loss of UTX/KDM6A and the activation of FGFR3 converge to regulate differentiation gene-expression programs in bladder cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 25732–25741. [Google Scholar] [CrossRef]
- Gaston, K.; Tsitsilianos, M.-A.; Wadey, K.; Jayaraman, P.-S. Misregulation of the proline rich homeodomain (PRH/HHEX) protein in cancer cells and its consequences for tumour growth and invasion. Cell Biosci. 2016, 6, 12. [Google Scholar] [CrossRef]
- Matsumori, T.; Kodama, Y.; Takai, A.; Shiokawa, M.; Nishikawa, Y.; Matsumoto, T.; Takeda, H.; Marui, S.; Okada, H.; Hirano, T.; et al. Hes1 Is Essential in Proliferating Ductal Cell-Mediated Development of Intrahepatic Cholangiocarcinoma. Cancer Res. 2020, 80, 5305–5316. [Google Scholar] [CrossRef]
- Kuriyama, K.; Kodama, Y.; Shiokawa, M.; Nishikawa, Y.; Marui, S.; Kuwada, T.; Sogabe, Y.; Kakiuchi, N.; Tomono, T.; Matsumori, T.; et al. Essential role of Notch/Hes1 signaling in postnatal pancreatic exocrine development. J. Gastroenterol. 2021, 56, 673–687. [Google Scholar] [CrossRef]
- Villasenor, A.; Gauvrit, S.; Collins, M.M.; Maischein, H.-M.; Stainier, D.Y. Hhex regulates the specification and growth of the hepatopancreatic ductal system. Dev. Biol. 2020, 458, 228–236. [Google Scholar] [CrossRef]
- Evseeva, M.N.; Dyikanov, D.T.; Karagyaur, M.N.; Prikazchikova, T.A.; Sheptulina, A.F.; Balashova, M.S.; Zatsepin, T.S.; Rubtsov, Y.P.; Kulebyakin, K.Y. Hematopoietically-expressed homeobox protein HHEX regulates adipogenesis in preadipocytes. Biochimie 2021, 185, 68–77. [Google Scholar] [CrossRef]
- Boglev, Y.; Wilanowski, T.; Caddy, J.; Parekh, V.; Auden, A.; Darido, C.; Hislop, N.R.; Cangkrama, M.; Ting, S.B.; Jane, S.M. The unique and cooperative roles of the Grainy head-like transcription factors in epidermal development reflect unexpected target gene specificity. Dev. Biol. 2011, 349, 512–522. [Google Scholar] [CrossRef]
- Cheng, Z.-Y.; He, T.-T.; Gao, X.-M.; Zhao, Y.; Wang, J. ZBTB Transcription Factors: Key Regulators of the Development, Differentiation and Effector Function of T Cells. Front. Immunol. 2021, 12, 713294. [Google Scholar] [CrossRef]
- Wehn, A.K.; Farkas, D.R.; Sedlock, C.E.; Subedi, D.; Chapman, D.L. Functionally distinct roles for T and Tbx6 during mouse development. Biol. Open 2020, 9, bio054692. [Google Scholar] [CrossRef]
- Tran, N.; Broun, A.; Ge, K. Lysine Demethylase KDM6A in Differentiation, Development, and Cancer. Mol. Cell Biol. 2020, 40, e00341-20. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Agarwal, M.; Kumar, P.; Mathew, S. The Groucho/Transducin-like enhancer of split protein family in animal development. IUBMB Life 2015, 67, 472–481. [Google Scholar] [CrossRef]
- Grbavec, D.; Lo, R.; Liu, Y.; Greenfield, A.; Stifani, S. Groucho/transducin-like enhancer of split (TLE) family members interact with the yeast transcriptional co-repressor SSN6 and mammalian SSN6-related proteins: Implications for evolutionary conservation of transcription repression mechanisms. Biochem. J. 1999, 337 Pt 1, 13–17. [Google Scholar] [CrossRef]
- Chen, G.; Nguyen, P.; Courey, A. A role for Groucho tetramerization in transcriptional repression. Mol. Cell Biol. 1998, 18, 7259–7268. [Google Scholar] [CrossRef]
- Gasperowicz, M.; Otto, F. Mammalian Groucho homologs: Redundancy or specificity? J. Cell Biochem. 2005, 95, 670–687. [Google Scholar] [CrossRef]
- McLarren, K.W.; Theriault, F.M.; Stifani, S. Association with the nuclear matrix and interaction with Groucho and RUNX proteins regulate the transcription repression activity of the basic helix loop helix factor Hes1. J. Biol. Chem. 2001, 276, 1578–1584. [Google Scholar] [CrossRef]
- Ciarapica, R.; Methot, L.; Tang, Y.; Lo, R.; Dali, R.; Buscarlet, M.; Locatelli, F.; Del Sal, G.; Rota, R.; Stifani, S. Prolyl isomerase Pin1 and protein kinase HIPK2 cooperate to promote cortical neurogenesis by suppressing Groucho/TLE:Hes1-mediated inhibition of neuronal differentiation. Cell Death Differ. 2014, 21, 321–332. [Google Scholar] [CrossRef]
- Swingler, T.E.; Bess, K.L.; Yao, J.; Stifani, S.; Jayaraman, P.-S. The proline-rich homeodomain protein recruits members of the Groucho/Transducin-like enhancer of split protein family to co-repress transcription in hematopoietic cells. J. Biol. Chem. 2004, 279, 34938–34947. [Google Scholar] [CrossRef]
- Soufi, A.; Jayaraman, P. PRH/Hex: An oligomeric transcription factor and multifunctional regulator of cell fate. Biochem. J. 2008, 412, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Greife, A.; Jankowiak, S.; Steinbring, J.; Nikpour, P.; Niegisch, G.; Hoffmann, M.J.; A Schulz, W. Canonical Notch signalling is inactive in urothelial carcinoma. BMC Cancer 2014, 14, 628. [Google Scholar] [CrossRef] [PubMed]
- Shpargel, K.B.; Sengoku, T.; Yokoyama, S.; Magnuson, T. UTX and UTY demonstrate histone demethylase-independent function in mouse embryonic development. PLoS Genet. 2012, 8, e1002964. [Google Scholar] [CrossRef] [PubMed]
- Seelk, S.; Adrian-Kalchhauser, I.; Hargitai, B.; Hajduskova, M.; Gutnik, S.; Tursun, B.; Ciosk, R. Increasing Notch signaling antagonizes PRC2-mediated silencing to promote reprograming of germ cells into neurons. eLife 2016, 5, e15477. [Google Scholar] [CrossRef]
- Zhang, C.; Berndt-Paetz, M.; Neuhaus, J. A Comprehensive Bioinformatics Analysis of Notch Pathways in Bladder Cancer. Cancers 2021, 13, 3089. [Google Scholar] [CrossRef]
- Marfil, V.; Blazquez, M.; Serrano, F.; Castell, J.V.; Bort, R. Growth-promoting and tumourigenic activity of c-Myc is suppressed by Hhex. Oncogene 2015, 34, 3011–3022. [Google Scholar] [CrossRef]
- Shi, B.; Li, W.; Song, Y.; Wang, Z.; Ju, R.; Ulman, A.; Hu, J.; Palomba, F.; Zhao, Y.; Le, J.P.; et al. UTX condensation underlies its tumour-suppressive activity. Nature 2021, 597, 726–731. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).