Up-Regulation of Astrocytic Fgfr4 Expression in Adult Mice after Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
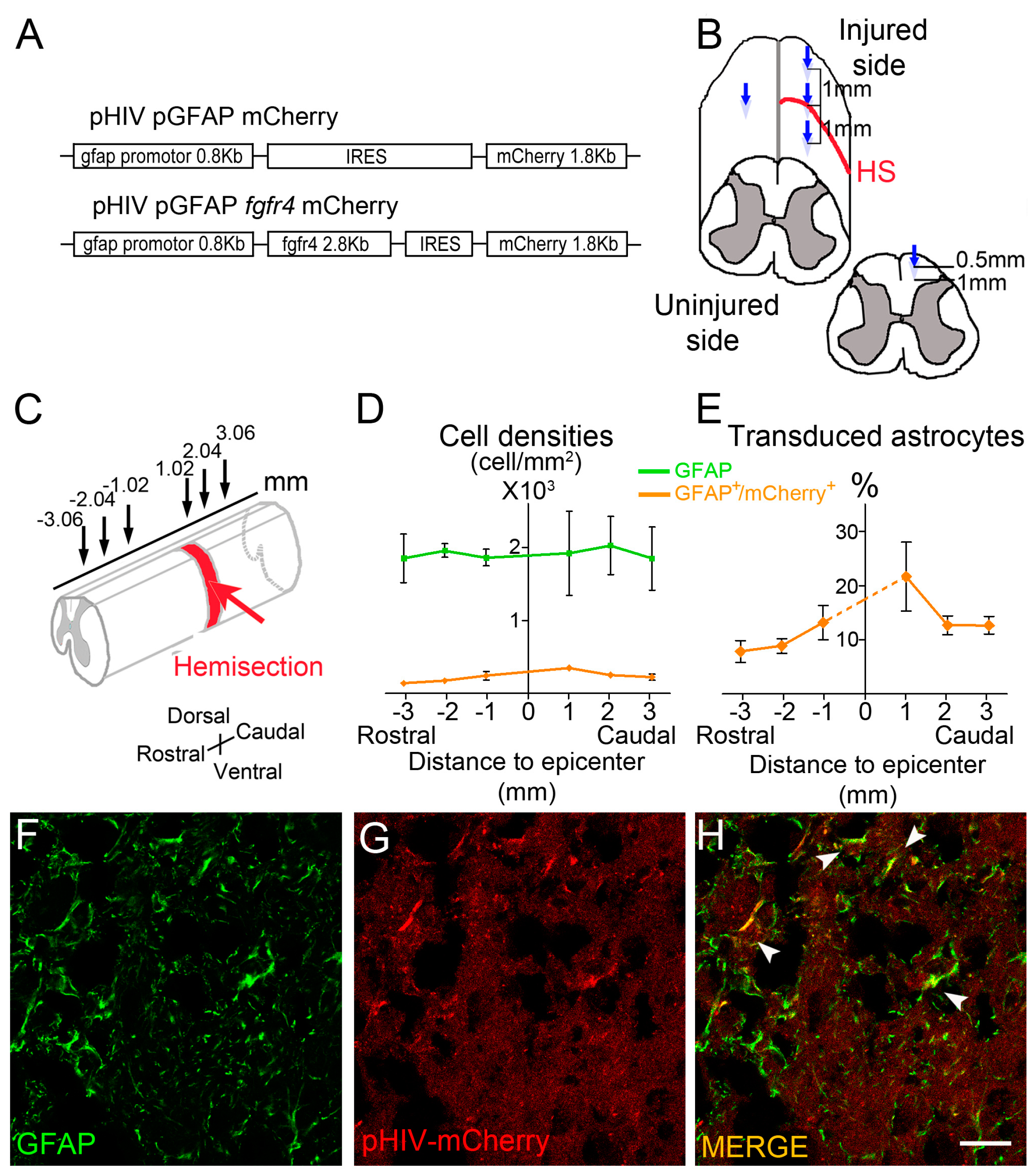
2.2. Viral Constructs
2.3. Spinal Cord Injury and Viral Vector Injections
2.4. Behavioral Analysis
- Open field: Animals were placed in a 50 × 50 cm square arena. Spontaneous motor activity was video-recorded over 10 min. The first two minutes were considered habituation time and were systematically excluded from analysis. Recordings were first performed on a smooth ground and then repeated on sandpaper surfaces with two types of granularities, i.e., 50 and 240 mean grain size/µm (adapted from [34,35]). The following parameters were analyzed: time spent by zone (s), speed by zone (cm/s), and time of immobility (s) (defined as no movement for more than 2 s). Zones were set as follow: total arena, arena center, arena periphery for smooth surface and zone of granularity 50, zone of granularity 240 for sandpaper surface. Center region size was set as 20 × 20 cm, and the periphery corresponds to the remaining area. Zones of granularities 50 and 240 were designed as 25 × 25 cm square and placed in alternance. Ethotrack software (Innovation Net, Tiranges, France) was used for the automatized video tracking and analysis.
- CatWalkTM: A dynamic walking pattern was analyzed using the CatWalk™ test (CatWalk XT™, Noldus, Wageningen, The Netherlands). Animals walked through a corridor on a backlighted glass plate. Paw placements were recorded by a camera placed under the glass plate. Six runs per sessions were recorded. Runs were analyzed only if the following criteria were met: average speed comprised between 5 and 30 cm/s, and combined to a maximum speed variation of 70% (adapted from [36]). Several parameters were analyzed, including base of support, print position, and max contact. Animals included in the experimental and control groups had similar weights and average motion speed, allowing for equivalent detection and comparison between groups, as previously described [36]. Additionally, we have quantified the percentage of detected ipsilateral hind paws prior traumatism and over the first week after SCI.
2.5. Tissue Processing
2.6. Ex Vivo Diffusion Weighted Magnetic Resonance Imaging (DW-MRI)
2.7. Immunohistochemistry
- Immunoperoxidase: Sections were washed in 0.1 M PBS and incubated for 15 min in hydrogen peroxide solution (H2O2, 1% in 0.1 M PBS, Sigma Aldrich, Gilligham, UK), washed in 0.1 M PBS for 10 min and incubated in lysine 20 mM, pH 7.4 for 20 min. After two washes in 0.1 M PBS, sections were incubated in blocking buffer (BSA 1%, triton ×100 0.1%, PBS 0.1 M) for 2 h, at room temperature (RT). Sections were then incubated for 48 h at 4 °C with the primary antibody, washed three times in 0.1 M PBS and incubated in the corresponding secondary antibody for 2 h at RT. Then, sections were washed with 0.1 M Trizma Base Saline (TRIS) for 3 × 10 min and the peroxidase reaction product was revealed using DAB (3,3′-Diaminobenzidine) substrate kit (Vector Labs, Burlingame, CA, USA). The reaction was stopped by washing sections in 0.1 M TRIS for 3 × 10 min. Sections were then dehydrated with increasing concentrations of ethanol (70, 80, 90, and 100%), cleared using xylene and coversliped (Eukitt® Mounting Medium, Sigma Aldrich, Gilligham, UK). To limit bias due to experimentation, staining of all sections was conducted simultaneously for a given protein and time point.
- Immunofluorescence: Sections were washed in 0.1 M PBS, incubated in lysine 20 mM, pH 7.4 for 20 min. After two washes in 0.1 M PBS, sections were incubated in blocking buffer (BSA 1%, triton ×100 0.1%, PBS 0.1 M) for 2 h at RT. Sections were then incubated for 24 or 48 h at 4 °C with the primary antibody, washed three times in 0.1 M PBS and incubated in the corresponding secondary antibody (2 h, RT). Sections were washed 3 × 10 mn in 0.1 M PBS and coversliped using fluorescent mounting medium (Dako, Glostrup, Denmark). Fluorescent sections were kept away from light and stored at 4 °C.
- Myelin staining: we used fluoromyelin as previously described in [38,39]; in short, sections were rinsed in 0.1 M PBS for 1 min and then incubated in FluoroMyelin™ (1:200, Thermofisher Scientific, Waltham, MA, USA) for 20 min at RT. Sections were washed 3 × 10 mn in 0.1 M PBS, coversliped using fluorescent mounting medium (Dako, Glostrup, Denmark), were kept away from light, and stored at 4 °C.
- Neuromuscular junctions: staining on transverse cryosection (16 µm) of the gastrocnemius–soleus–plantaris muscular complex was carried out (Microm HM550, Thermofisher Scientific, Waltham, MA, USA) using enzymatic method [40]. Sections were washed in 0.1 M PBS and incubated for 30 min in a solution containing: 0.5% acetylthiocholine (Sigma Aldrich, Saint Louis, MO, USA), 0.1 M sodium acetate pH 6, 0.1 M sodium citrate pH 6, 30 mM CuSO4, and 5 mM potassium ferricyanide. Sections were then washed in 0.1 M PBS and dehydrated with increasing concentrations of ethanol (70, 80, 90, and 100%) followed by xylene before applying coverslips (Eukitt® Mounting Medium, Sigma Aldrich, Gilligham, UK).
- Antibodies: Primary antibodies: rat anti mCherry monoclonal antibody 16D7 (1:500, Invitrogen, Carlsbad, CA, USA), rabbit anti GFAP (1:1000, Dako, Glostrup, Denmark), rabbit anti Iba1 (1:1000, Wako Pure Chemical Industries, Osaka, Japan), rabbit anti GAD65/67 (1:500, Abcam, Cambridge, UK), rabbit anti GAP 43 (1:1000, Milliport, Dramstadt, Germany), and mouse anti βIII-tubulin (1:100; MAB1195; R&D Systems, Minneapolis, MS, USA).
- Secondary antibodies: donkey anti rat alexa 594 (1:1000, Invitrogen, Carlsbad, CA, USA), APC-conjugated donkey anti mouse (1:100; Invitrogen, Carlsbad, CA, USA), donkey anti rabbit peroxidase (1:500, Jackson Immunoresearch, Stratech Scientific Ltd., Soham, UK), and goat anti-mouse alexa 350 (1:1000, Invitrogen, Carlsbad, CA, USA).
2.8. Microscopy and Quantifications
- Immunohistochemistry: Transverse sections were scanned using NanoZoomer RS slide scanner (NanoZoomer Digital Pathology System and NDP view software, Hamamatsu City, Japan). All images were acquired at the same light exposure and exported with identical parameters. Optical density (OD) was measured using Image J software (National Institutes of Health, Bethesda, MD, USA). Background (OD without tissue) was subtracted for each section. Number of mice included in the study: 5 C57BL6/6J female experimental mice; 6 C57BL6/6J female control mice. Animals were sacrificed at 6 weeks after SCI.
- Quantifications of vector transduction in GFAP positive astrocytes: longitudinal sections of spinal cords were imaged with upright fluorescence microscope Axio Imager M1 (Zeiss, Oberkochen, Germany) at 1020 µm, 2040 µm, and 3060 µm, rostral and caudal to the lesion site. Settings were kept constant for all acquisitions. GFAP+ cells, mCherry+ cells, and GFAP+/mCherry+ cells were manually quantified using Multi-Point tool in Image J software (). Number of mice included in the quantifications: three injured male mice injected with pHIV-mCherry. Animals were sacrificed at 2 weeks after SCI.
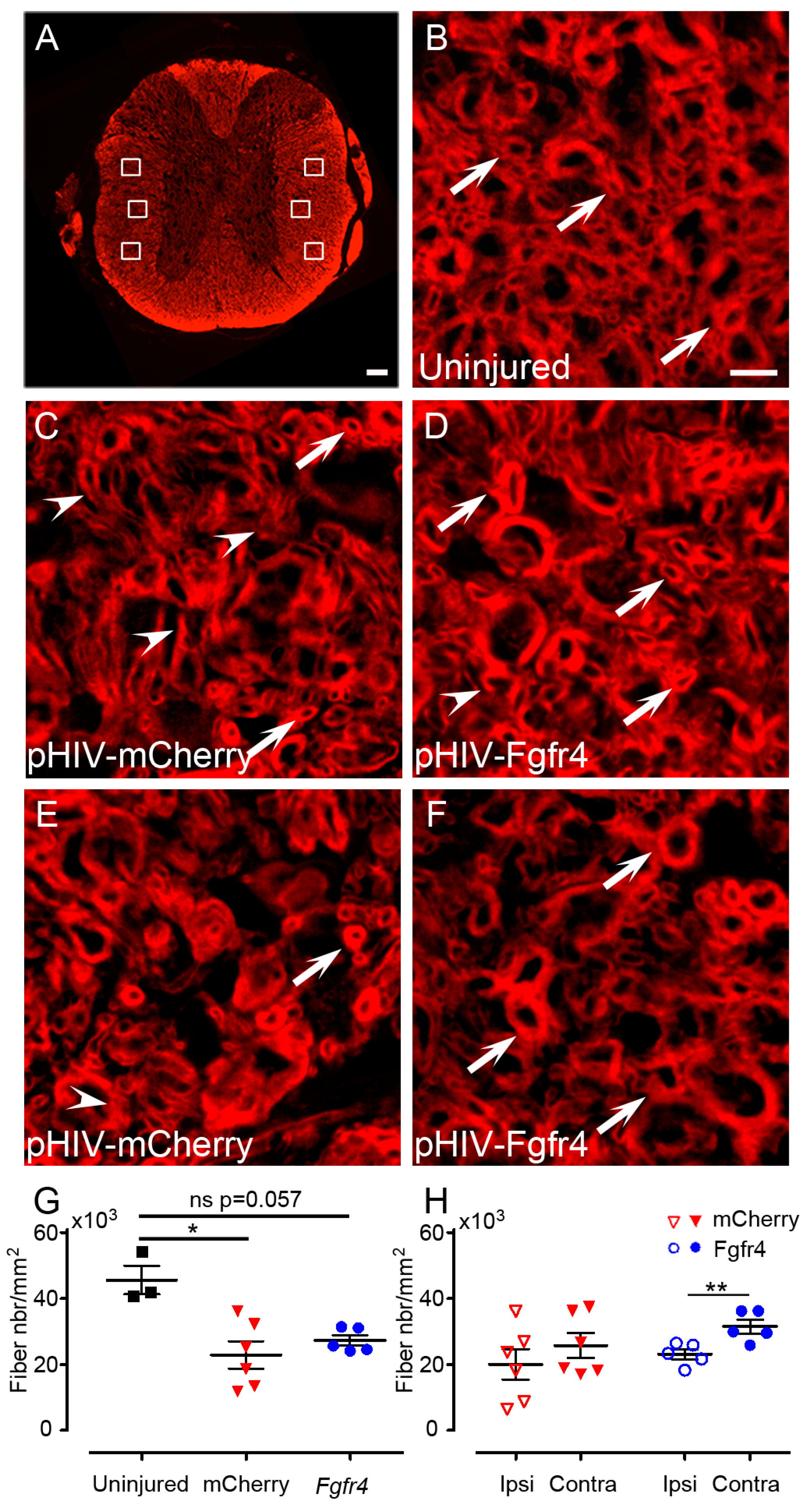
- Quantifications of spared myelin: images of 14 µm axial spinal cords cryosections were acquired with THUNDER Imager 3D (Leica, Wetzlar, Germany; lens × 63) 3150 µm, rostral and caudal to the lesion site. One field of 600 µm × 400 µm was acquired in both lateral funiculi and 3 images of 40 µm × 40 µm located dorsal, central, and ventral to the spinal cord were taken for quantification. Spared myelinated fibers were quantified using multi-point tool of ImageJ software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, MD, USA, https://imagej.nih.gov/ij/, 1997–2018) with numeric zoom to reach 200% of the original image. Number of mice included in the study: 6 C57BL6/6J female experimental mice; 6 C57BL6/6J female control mice. Animals were sacrificed at 6 weeks after SCI.
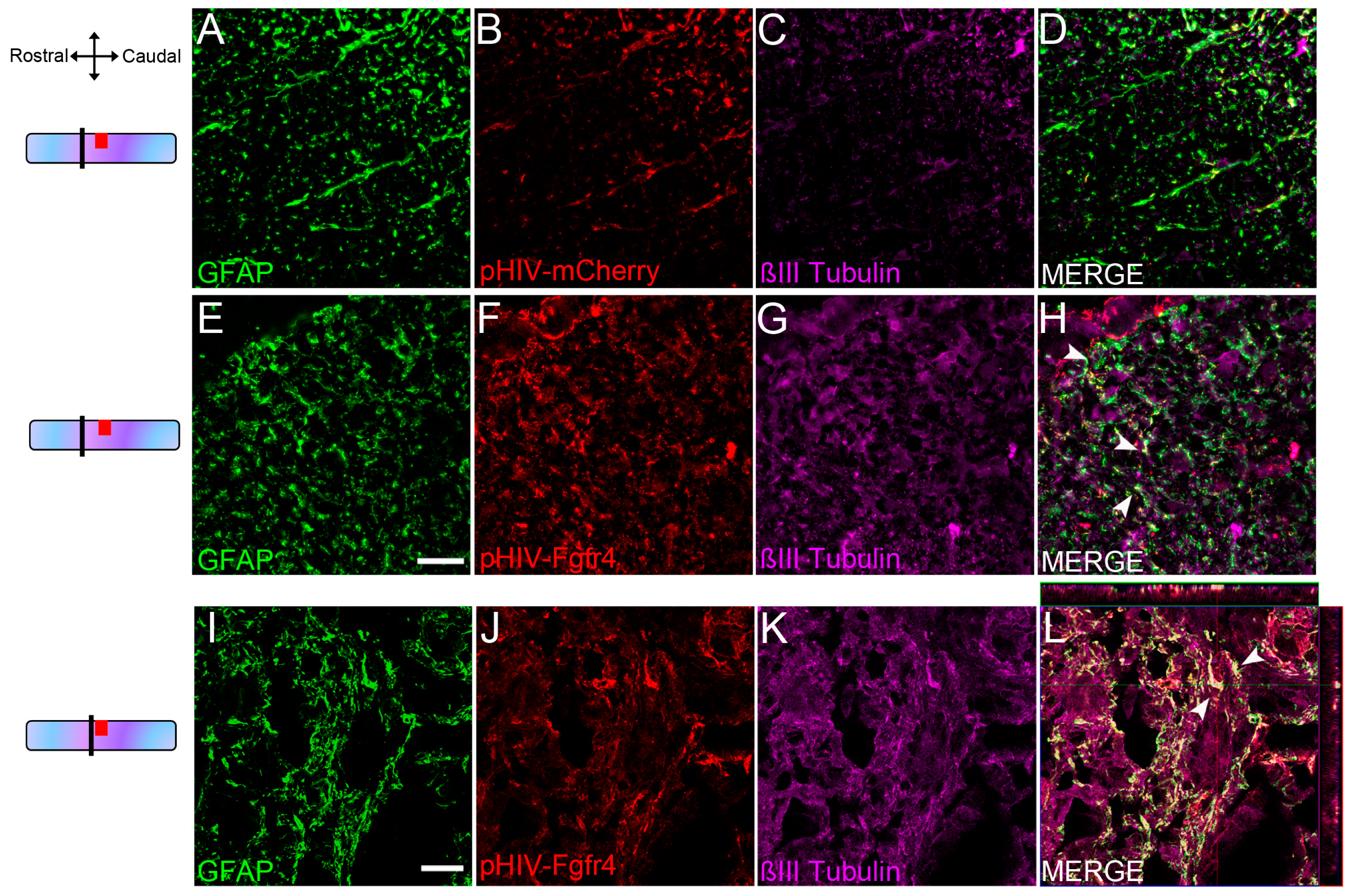
- In Figure 2, fluorescent THUNDER Imager 3D (Leica, Wetzlar, Germany; lens × 40) images were taken rostral and caudal to the lesion. Confocal images: sections were imaged with a laser scanning confocal microscope (Leica SPE, Mannheim, Germany) associated with a Leica LAS AF interface. Settings were kept constant for all acquisitions.
2.9. Fluorescence-Activated Cell Sorting (FACS)
2.10. RNA-Seq ANALYSIS
2.11. Statistics
3. Results
3.1. Lentiviral Mediated Over-Expression of Fgfr4 in Astrocytes Persists after SCI and Is Associated with βIII-Tubulin Expression
3.2. Lentiviral-Mediated Over-Expression of Fgfr4 in Astrocytes Improves Functional Recovery after SCI in a Gender-Dependent Manner
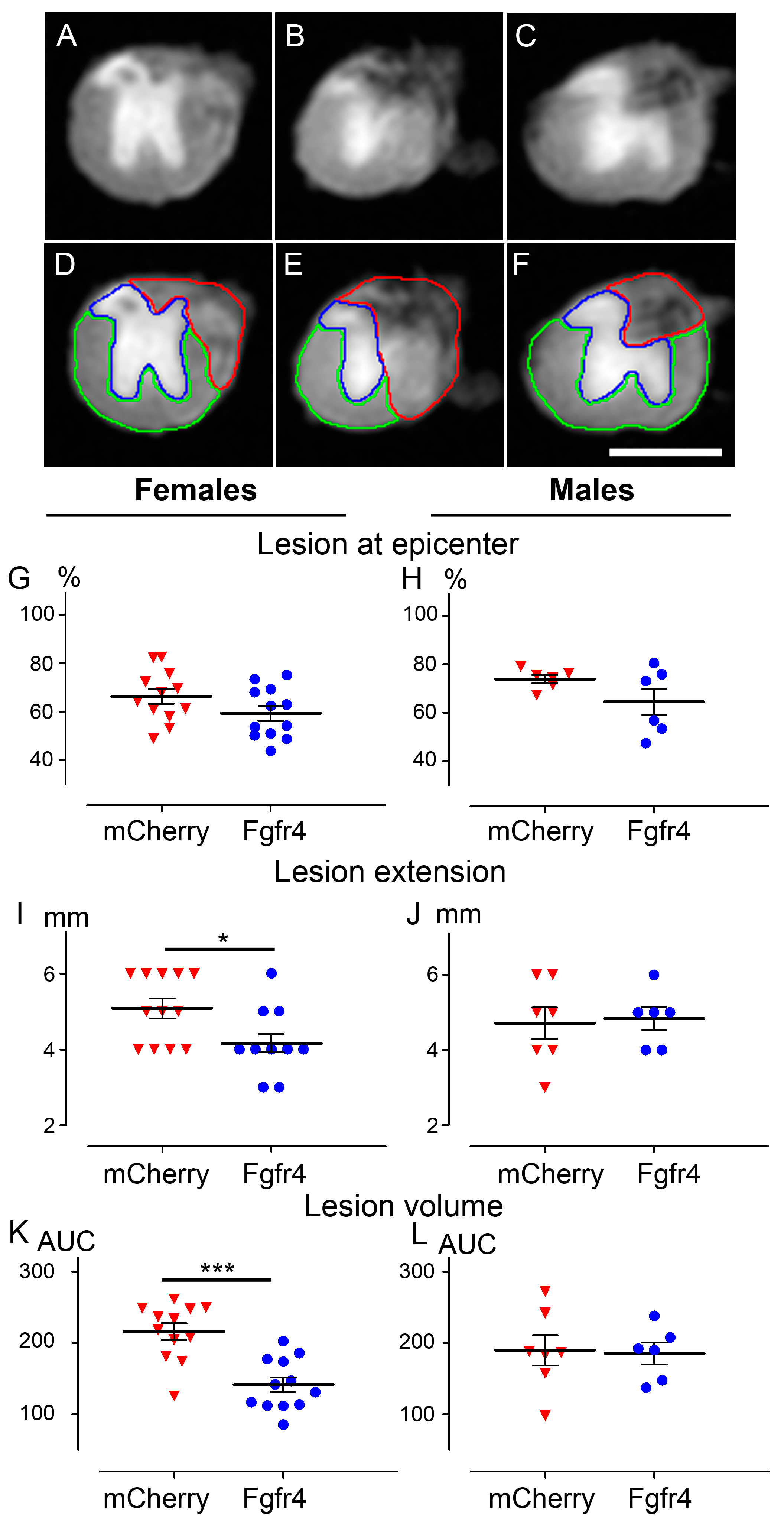
3.3. pHIV-Fgfr4 Condenses Lesion Volume Specifically in Female Mice after SCI
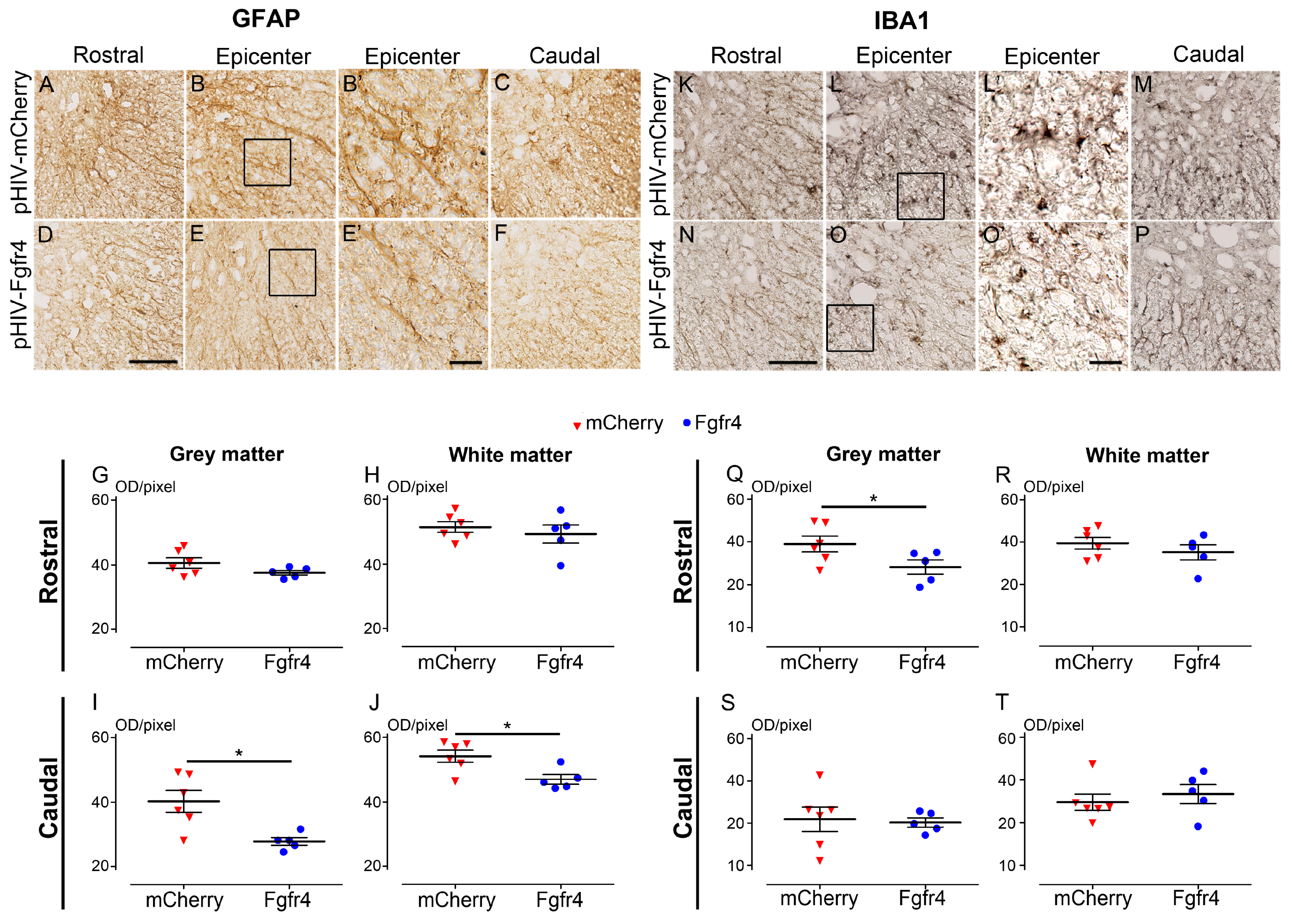
3.4. pHIV-Fgfr4 Reduces Glial Reactivity in Female Mice after SCI
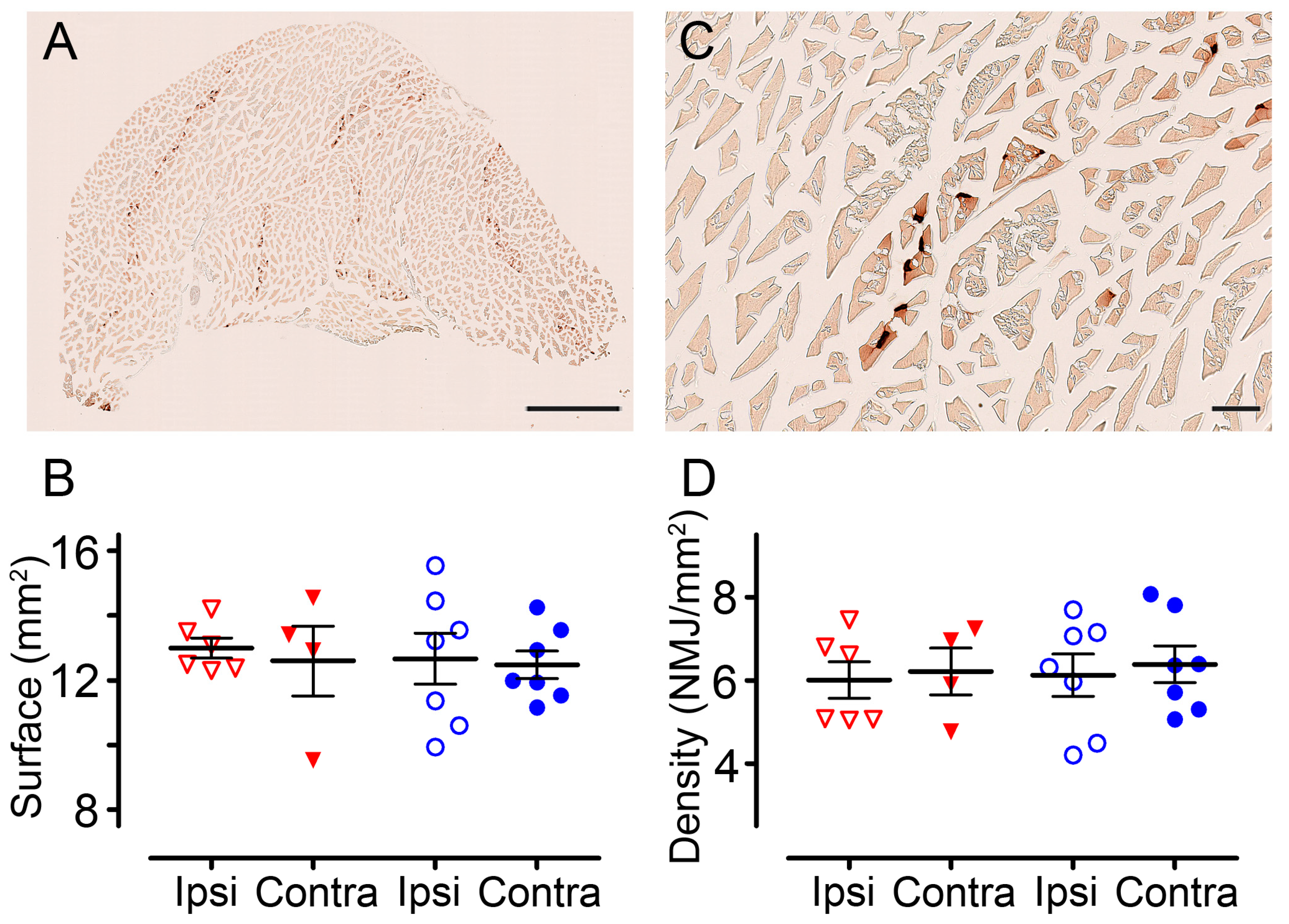
3.5. Positive Effect of pHIV-Fgfr4 on Motor Recovery after SCI Is Not Reflected by Muscles’ Surface and Neuromuscular Junction Density
3.6. Molecular Signature of SCI-Induced βIII-Tubulin-Expressing Astrocytes Is Consistent with Their Transdifferentiation State into Neuron-like Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef] [PubMed]
- Kroner, A.; Almanza, J.R. Role of microglia in spinal cord injury. Neurosci. Lett. 2019, 709, 134370. [Google Scholar] [CrossRef] [PubMed]
- Cregg, J.M.; DePaul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar] [CrossRef]
- Karimi-Abdolrezaee, S.; Billakanti, R. Reactive Astrogliosis after Spinal Cord Injury—Beneficial and Detrimental Effects. Mol. Neurobiol. 2012, 46, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A Transcriptome Database for Astrocytes, Neurons, and Oligodendrocytes: A New Resource for Understanding Brain Development and Function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef]
- Lin, C.-C.J.; Yu, K.; Hatcher, A.; Huang, T.-W.; Lee, H.K.; Carlson, J.; Weston, M.C.; Chen, F.; Zhang, Y.; Zhu, W.; et al. Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci. 2017, 20, 396–405. [Google Scholar] [CrossRef]
- Boisvert, M.M.; Erikson, G.A.; Shokhirev, M.N.; Allen, N.J. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep. 2018, 22, 269–285. [Google Scholar] [CrossRef]
- Simpson, J.E.; Ince, P.G.; Shaw, P.J.; Heath, P.R.; Raman, R.; Garwood, C.J.; Gelsthorpe, C.; Baxter, L.; Forster, G.; Matthews, F.E.; et al. Microarray analysis of the astrocyte transcriptome in the aging brain: Relationship to Alzheimer’s pathology and APOE genotype. Neurobiol. Aging 2011, 32, 1795–1807. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Noristani, H.N.; Perrin, F.E. Astrocyte-to-neuron conversion induced by spinal cord injury. Oncotarget 2016, 7, 83831–83832. [Google Scholar] [CrossRef]
- Noristani, H.N.; Sabourin, J.C.; Boukhaddaoui, H.; Chan-Seng, E.; Gerber, Y.N.; Perrin, F.E. Spinal cord injury induces astroglial conversion towards neuronal lineage. Mol. Neurodegener. 2016, 11, 68. [Google Scholar] [CrossRef]
- Guillemot, F.; Zimmer, C. From Cradle to Grave: The Multiple Roles of Fibroblast Growth Factors in Neural Development. Neuron 2011, 71, 574–588. [Google Scholar] [CrossRef]
- Kunath, T.; Saba-El-Leil, M.K.; Almousailleakh, M.; Wray, J.; Meloche, S.; Smith, A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 2007, 134, 2895–2902. [Google Scholar] [CrossRef]
- Feng, G.-D.; He, B.-R.; Lu, F.; Liu, L.-H.; Zhang, L.; Chen, B.; He, Z.-P.; Hao, D.-J.; Yang, H. Fibroblast Growth Factor 4 Is Required but not Sufficient for the Astrocyte Dedifferentiation. Mol. Neurobiol. 2014, 50, 997–1012. [Google Scholar] [CrossRef]
- Chen, J.; Poskanzer, K.E.; Freeman, M.R.; Monk, K.R. Live-imaging of astrocyte morphogenesis and function in zebrafish neural circuits. Nat. Neurosci. 2020, 23, 1297–1306. [Google Scholar] [CrossRef]
- Laywell, E.D.; Rakic, P.; Kukekov, V.G.; Holland, E.C.; Steindler, D.A. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl. Acad. Sci. USA 2000, 97, 13883–13888. [Google Scholar] [CrossRef] [PubMed]
- Torper, O.; Pfisterer, U.; Wolf, D.A.; Pereira, M.; Lau, S.; Jakobsson, J.; Björklund, A.; Grealish, S.; Parmar, M. Generation of induced neurons via direct conversion in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 7038–7043. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Birtele, M.; Shrigley, S.; Benitez, J.A.; Hedlund, E.; Parmar, M.; Ottosson, D.R. Direct Reprogramming of Resident NG2 Glia into Neurons with Properties of Fast-Spiking Parvalbumin-Containing Interneurons. Stem Cell Rep. 2017, 9, 742–751. [Google Scholar] [CrossRef]
- Torper, O.; Ottosson, D.R.; Pereira, M.; Lau, S.; Cardoso, T.; Grealish, S.; Parmar, M. In Vivo Reprogramming of Striatal NG2 Glia into Functional Neurons that Integrate into Local Host Circuitry. Cell Rep. 2015, 12, 474–481. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, Q.; Yuan, J.; Han, S.; Zhang, P.; Li, S.; Rao, Z.; Zhao, W.; Ye, Q.; Geng, J.; et al. Ascl1 Converts Dorsal Midbrain Astrocytes into Functional Neurons In Vivo. J. Neurosci. 2015, 35, 9336–9355. [Google Scholar] [CrossRef]
- Niu, W.; Zang, T.; Smith, D.K.; Vue, T.Y.; Zou, Y.; Bachoo, R.; Johnson, J.E.; Zhang, C.-L. SOX2 Reprograms Resident Astrocytes into Neural Progenitors in the Adult Brain. Stem Cell Rep. 2015, 4, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Zang, T.; Zou, Y.; Fang, S.; Smith, D.K.; Bachoo, R.; Zhang, C.-L. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nature 2013, 15, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Brulet, R.; Matsuda, T.; Zhang, L.; Miranda, C.; Giacca, M.; Kaspar, B.K.; Nakashima, K.; Hsieh, J. NEUROD1 Instructs Neuronal Conversion in Non-Reactive Astrocytes. Stem Cell Rep. 2017, 8, 1506–1515. [Google Scholar] [CrossRef]
- Zheng, J.; Li, T.; Qi, S.; Qin, B.; Yu, J.; Chen, G. Neuroregenerative gene therapy to treat temporal lobe epilepsy in a rat model. Prog. Neurobiol. 2021, 208, 102198. [Google Scholar] [CrossRef]
- Rivetti Di Val Cervo, P.; Romanov, R.A.; Spigolon, G.; Masini, D.; Martín-Montañez, E.; Toledo, E.M.; Manno, G.L.; Feyder, M.; Pifl, C.; Ng, Y.-H.; et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat. Biotechnol. 2017, 35, 444–452. [Google Scholar] [CrossRef]
- Su, Z.; Niu, W.; Liu, M.-L.; Zou, Y.; Zhang, C.-L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014, 5, 3338. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Su, Z.; Tai, W.; Zou, Y.; Xu, X.-M.; Zhang, C.-L. The p53 Pathway Controls SOX2-Mediated Reprogramming in the Adult Mouse Spinal Cord. Cell Rep. 2016, 17, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Puls, B.; Ding, Y.; Zhang, F.; Pan, M.; Lei, Z.; Pei, Z.; Jiang, M.; Bai, Y.; Forsyth, C.; Metzger, M.; et al. Regeneration of Functional Neurons After Spinal Cord Injury via in situ NeuroD1-Mediated Astrocyte-to-Neuron Conversion. Front. Cell Dev. Biol. 2020, 8, 591883. [Google Scholar] [CrossRef]
- Zarei-Kheirabadi, M.; Hesaraki, M.; Kiani, S.; Baharvand, H. In vivo conversion of rat astrocytes into neuronal cells through neural stem cells in injured spinal cord with a single zinc-finger transcription factor. Stem Cell Res. Ther. 2019, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Noristani, H.N.; Gerber, Y.N.; Sabourin, J.-C.; Le Corre, M.; Lonjon, N.; Mestre-Frances, N.; Hirbec, H.E.; Perrin, F.E. RNA-Seq Analysis of Microglia Reveals Time-Dependent Activation of Specific Genetic Programs following Spinal Cord Injury. Front. Mol. Neurosci. 2017, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Desclaux, M.; Perrin, F.E.; Do-Thi, A.; Prieto-Cappellini, M.; Ribotta, M.G.Y.; Mallet, J.; Privat, A. Lentiviral-mediated silencing of glial fibrillary acidic protein and vimentin promotes anatomical plasticity and functional recovery after spinal cord injury. J. Neurosci. Res. 2014, 93, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Noristani, H.N.; Saint-Martin, G.P.; Cardoso, M.M.; Sidiboulenouar, M.R.; Catteau, M.; Coillot, C.; Goze-Bac, C.; Perrin, F.E. Longitudinal Magnetic Resonance Imaging Analysis and Histological Characterization after Spinal Cord Injury in Two Mouse Strains with Different Functional Recovery: Gliosis as a Key Factor. J. Neurotrauma 2018, 35, 2924–2940. [Google Scholar] [CrossRef]
- Noristani, H.N.; They, L.; Perrin, F.E. C57BL/6 and Swiss Webster Mice Display Differences in Mobility, Gliosis, Microcavity Formation and Lesion Volume After Severe Spinal Cord Injury. Front. Cell. Neurosci. 2018, 12, 173. [Google Scholar] [CrossRef]
- Gerber, Y.N.; Saint-Martin, G.P.; Bringuier, C.M.; Bartolami, S.; Goze-Bac, C.; Noristani, H.N.; Perrin, F.E. CSF1R Inhibition Reduces Microglia Proliferation, Promotes Tissue Preservation and Improves Motor Recovery After Spinal Cord Injury. Front. Cell. Neurosci. 2018, 12, 368. [Google Scholar] [CrossRef]
- Coillot, C.; Sidiboulenouar, R.; Nativel, E.; Zanca, M.; Alibert, E.; Cardoso, M.; Saintmartin, G.; Noristani, H.; Lonjon, N.; Lecorre, M.; et al. Signal modeling of an MRI ribbon solenoid coil dedicated to spinal cord injury investigations. J. Sens. Sens. Syst. 2016, 5, 137–145. [Google Scholar] [CrossRef]
- Poulen, G.; Aloy, E.; Bringuier, C.M.; Mestre-Francés, N.; Artus, E.V.; Cardoso, M.; Perez, J.-C.; Goze-Bac, C.; Boukhaddaoui, H.; Lonjon, N.; et al. Inhibiting microglia proliferation after spinal cord injury improves recovery in mice and nonhuman primates. Theranostics 2021, 11, 8640–8659. [Google Scholar] [CrossRef]
- Poulen, G.; Gerber, Y.N.; Perez, J.-C.; Oubarrahou, K.; Lonjon, N.; Vachiery-Lahaye, F.; Boukhaddaoui, H.; Perrin, F.E. Coherent Anti-Stokes Raman Scattering Microscopy: A Label-Free Method to Compare Spinal Cord Myelin in Different Species. Front. Phys. 2021, 9, 665650. [Google Scholar] [CrossRef]
- Karnovsky, M.J.; Roots, L. A “Direct-colorING” thiocholine method for cholinesterases. J. Histochem. Cytochem. 1964, 12, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Noristani, H.N.; Sabourin, J.C.; Gerber, Y.N.; Teigell, M.; Sommacal, A.; Vivanco, M.D.; Weber, M.; Perrin, F.E. Brca1 is expressed in human microglia and is dysregulated in human and animal model of ALS. Mol. Neurodegener. 2015, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- See, P.; Lum, J.; Chen, J.; Ginhoux, F. A Single-Cell Sequencing Guide for Immunologists. Front. Immunol. 2018, 9, 2425. [Google Scholar] [CrossRef]
- Janowska, J.; Gargas, J.; Ziemka-Nalecz, M.; Zalewska, T.; Buzanska, L.; Sypecka, J. Directed glial differentiation and transdifferentiation for neural tissue regeneration. Exp. Neurol. 2018, 319, 112813. [Google Scholar] [CrossRef]
- Gascón, S.; Masserdotti, G.; Russo, G.L.; Götz, M. Direct Neuronal Reprogramming: Achievements, Hurdles, and New Roads to Success. Cell Stem Cell 2017, 21, 18–34. [Google Scholar] [CrossRef]
- Buffo, A.; Vosko, M.R.; Ertürk, D.; Hamann, G.F.; Jucker, M.; Rowitch, D.; Götz, M. Expression pattern of the transcription factor Olig2 in response to brain injuries: Implications for neuronal repair. Proc. Natl. Acad. Sci. USA 2005, 102, 18183–18188. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In Vivo Direct Reprogramming of Reactive Glial Cells into Functional Neurons after Brain Injury and in an Alzheimer’s Disease Model. Cell Stem Cell 2013, 14, 188–202. [Google Scholar] [CrossRef]
- Heinrich, C.; Bergami, M.; Gascón, S.; Lepier, A.; Viganò, F.; Dimou, L.; Sutor, B.; Berninger, B.; Götz, M. Sox2-Mediated Conversion of NG2 Glia into Induced Neurons in the Injured Adult Cerebral Cortex. Stem Cell Rep. 2014, 3, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Ma, N.-X.; Pei, Z.-F.; Wu, Z.; Do-Monte, F.H.; Keefe, S.; Yellin, E.; Chen, M.S.; Yin, J.-C.; Lee, G.; et al. A NeuroD1 AAV-Based Gene Therapy for Functional Brain Repair after Ischemic Injury through In Vivo Astrocyte-to-Neuron Conversion. Mol. Ther. 2019, 28, 217–234. [Google Scholar] [CrossRef]
- Wang, L.-L.; Serrano, C.; Zhong, X.; Ma, S.; Zou, Y.; Zhang, C.-L. Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell 2021, 184, 5465–5481.e16. [Google Scholar] [CrossRef] [PubMed]
- Farmer, W.T.; Murai, K. Resolving Astrocyte Heterogeneity in the CNS. Front. Cell. Neurosci. 2017, 11, 300. [Google Scholar] [CrossRef]
- Clarke, L.E.; Barres, B.A. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 2013, 14, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, N.C.; Sohrabji, F. Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol. Dis. 2015, 85, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Loram, L.C.; Sholar, P.W.; Taylor, F.R.; Wiesler, J.L.; Babb, J.A.; Strand, K.A.; Berkelhammer, D.; Day, H.E.; Maier, S.F.; Watkins, L.R. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 2012, 37, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Santos-Galindo, M.; Acaz-Fonseca, E.; Bellini, M.J.; Garcia-Segura, L.M. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol. Sex Differ. 2011, 2, 7. [Google Scholar] [CrossRef]
- Pinto-Benito, D.; Paradela-Leal, C.; Ganchala, D.; Castro-Molina, P.; Arevalo, M. IGF -1 regulates astrocytic phagocytosis and inflammation through the p110α isoform of PI3K in a sex-specific manner. Glia 2022, 70, 1153–1169. [Google Scholar] [CrossRef]
- Jullienne, A.; Salehi, A.; Affeldt, B.; Baghchechi, M.; Haddad, E.; Avitua, A.; Walsworth, M.; Enjalric, I.; Hamer, M.; Bhakta, S.; et al. Male and Female Mice Exhibit Divergent Responses of the Cortical Vasculature to Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S.; Loane, D.J.; Burns, M.P. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 2017, 65, 1423–1438. [Google Scholar] [CrossRef]
- Magnusson, J.P.; Göritz, C.; Tatarishvili, J.; Dias, D.O.; Smith, E.M.K.; Lindvall, O.; Kokaia, Z.; Frisén, J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science 2014, 346, 237–241. [Google Scholar] [CrossRef]
- Magnusson, J.P.; Zamboni, M.; Santopolo, G.; Mold, J.E.; Barrientos-Somarribas, M.; Talavera-Lopez, C.; Andersson, B.; Frisén, J. Activation of a neural stem cell transcriptional program in parenchymal astrocytes. Elife 2020, 9, e59733. [Google Scholar] [CrossRef]
- Voelkel, J.E.; Harvey, J.A.; Adams, J.S.; Lassiter, R.N.; Stark, M.R. FGF and Notch signaling in sensory neuron formation: A multifactorial approach to understanding signaling pathway hierarchy. Mech. Dev. 2014, 134, 55–66. [Google Scholar] [CrossRef]
- Haenzi, B.; Moon, L.D.F. The Function of FGFR1 Signalling in the Spinal Cord: Therapeutic Approaches Using FGFR1 Ligands after Spinal Cord Injury. Neural Plast. 2017, 2017, 2740768. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bringuier, C.M.; Noristani, H.N.; Perez, J.-C.; Cardoso, M.; Goze-Bac, C.; Gerber, Y.N.; Perrin, F.E. Up-Regulation of Astrocytic Fgfr4 Expression in Adult Mice after Spinal Cord Injury. Cells 2023, 12, 528. https://doi.org/10.3390/cells12040528
Bringuier CM, Noristani HN, Perez J-C, Cardoso M, Goze-Bac C, Gerber YN, Perrin FE. Up-Regulation of Astrocytic Fgfr4 Expression in Adult Mice after Spinal Cord Injury. Cells. 2023; 12(4):528. https://doi.org/10.3390/cells12040528
Chicago/Turabian StyleBringuier, Claire Mathilde, Harun Najib Noristani, Jean-Christophe Perez, Maida Cardoso, Christophe Goze-Bac, Yannick Nicolas Gerber, and Florence Evelyne Perrin. 2023. "Up-Regulation of Astrocytic Fgfr4 Expression in Adult Mice after Spinal Cord Injury" Cells 12, no. 4: 528. https://doi.org/10.3390/cells12040528
APA StyleBringuier, C. M., Noristani, H. N., Perez, J.-C., Cardoso, M., Goze-Bac, C., Gerber, Y. N., & Perrin, F. E. (2023). Up-Regulation of Astrocytic Fgfr4 Expression in Adult Mice after Spinal Cord Injury. Cells, 12(4), 528. https://doi.org/10.3390/cells12040528