Protective Effect of NO2-OA on Oxidative Stress, Gliosis, and Pro-Angiogenic Response in Müller Glial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Line and Culture Reagents
2.3. Cell Viability Assay
2.4. Western Blot Assay
2.5. Dichlorofluorescein Assay
2.6. Quantitative Real-Time Reverse-Transcription PCR (qRT-PCR)
2.7. Hypoxic Assays
2.8. Tube Formation Assay
2.9. Statistical Analysis
3. Results
3.1. NO2-OA Induces HO-1 Expression in MGCs
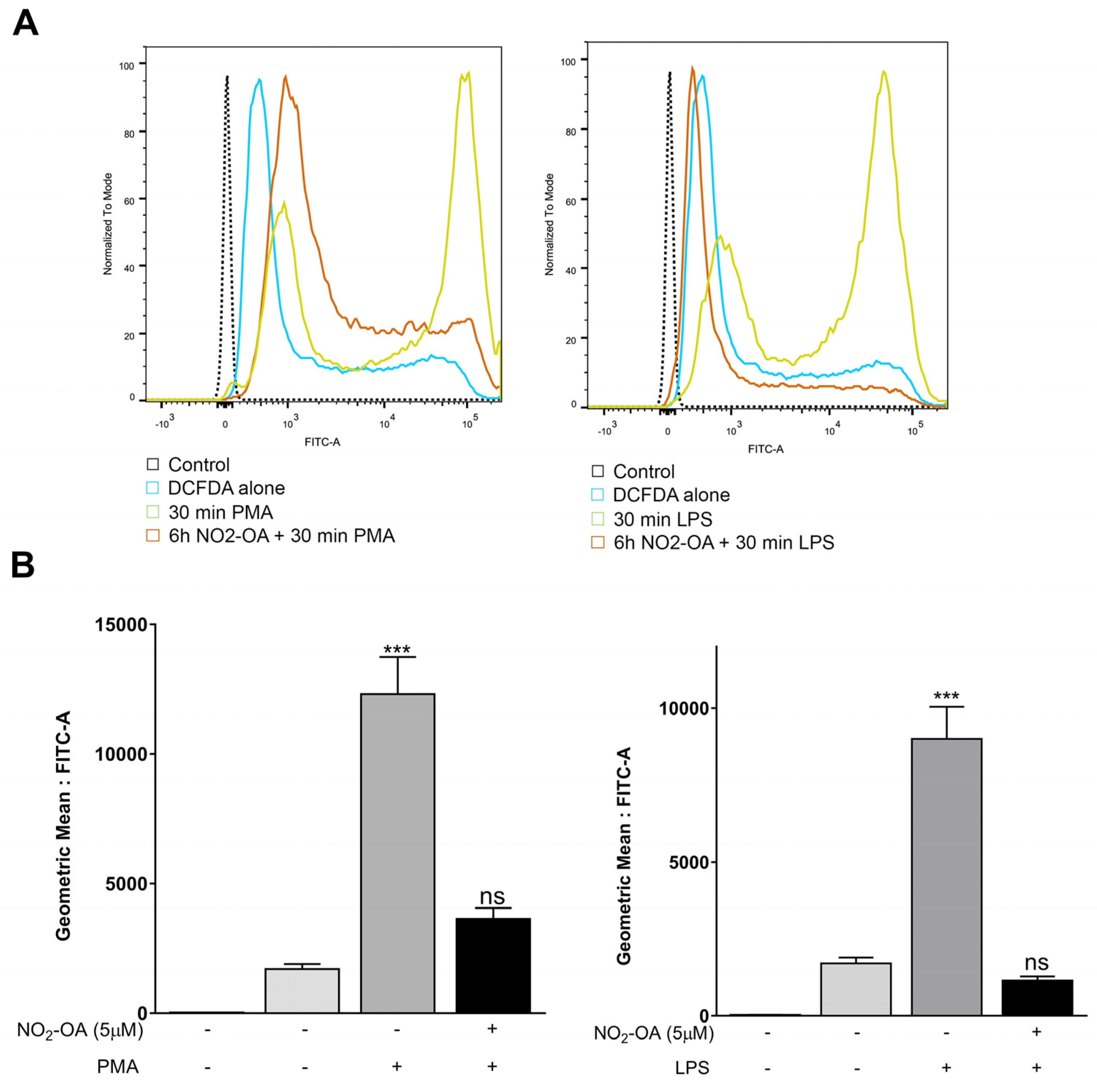
3.2. NO2-OA Prevents the Increase in ROS Levels Induced by LPS or PMA in MGCs
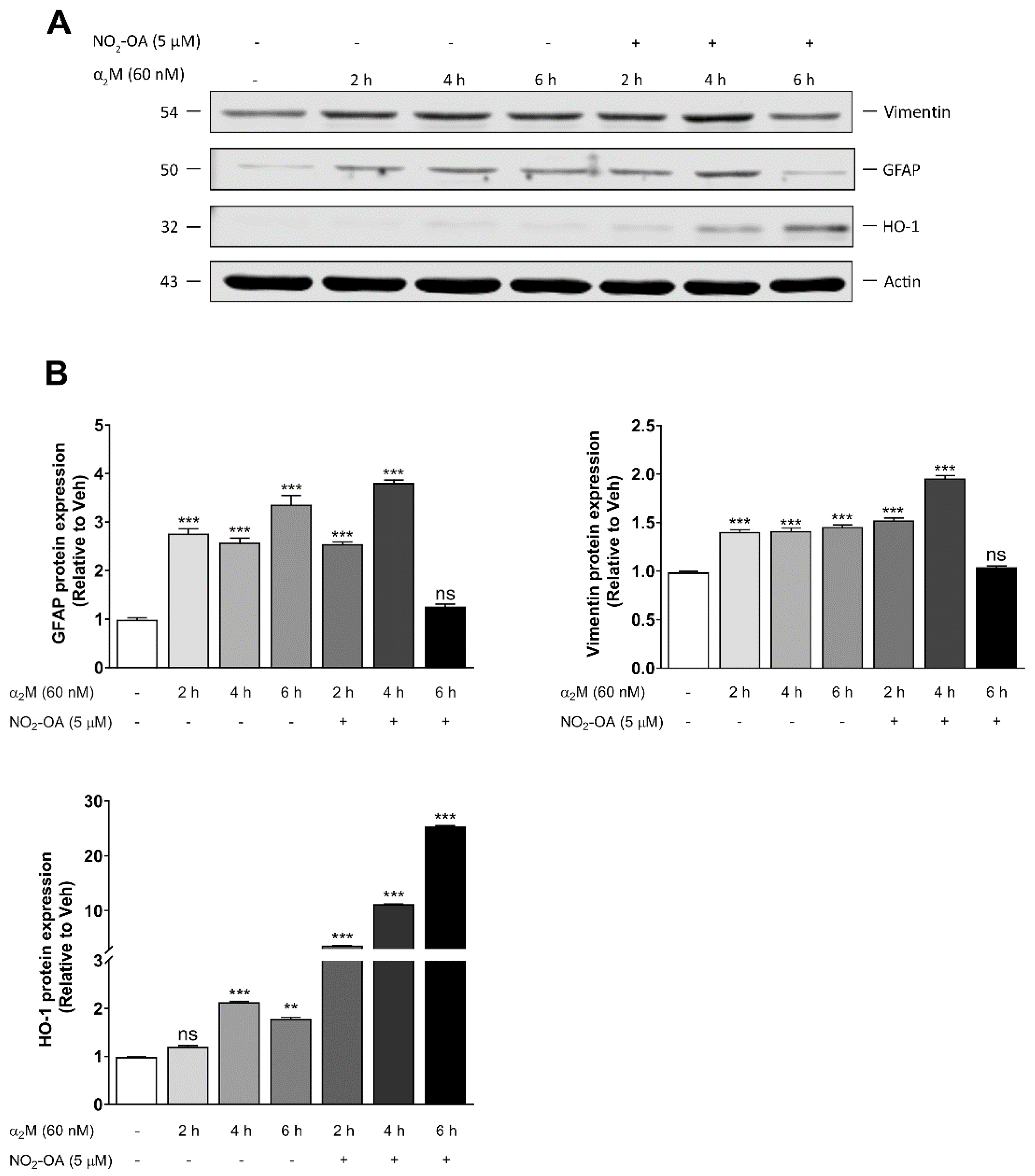
3.3. NO2-OA Reverts Glial Stress Induced by α2M* in MGCs
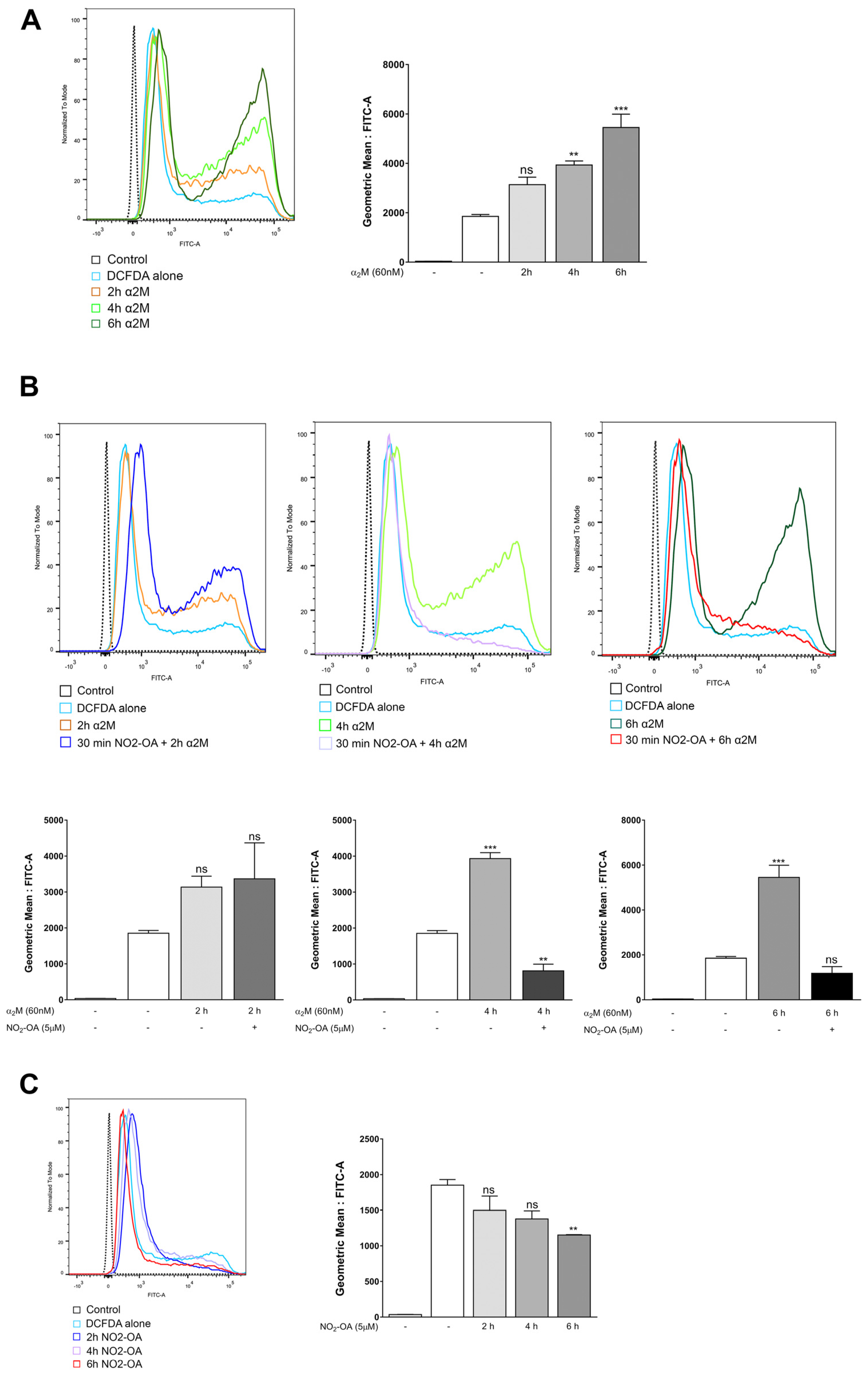
3.4. NO2-OA Inhibits ROS Induced by α2M* in MGCs
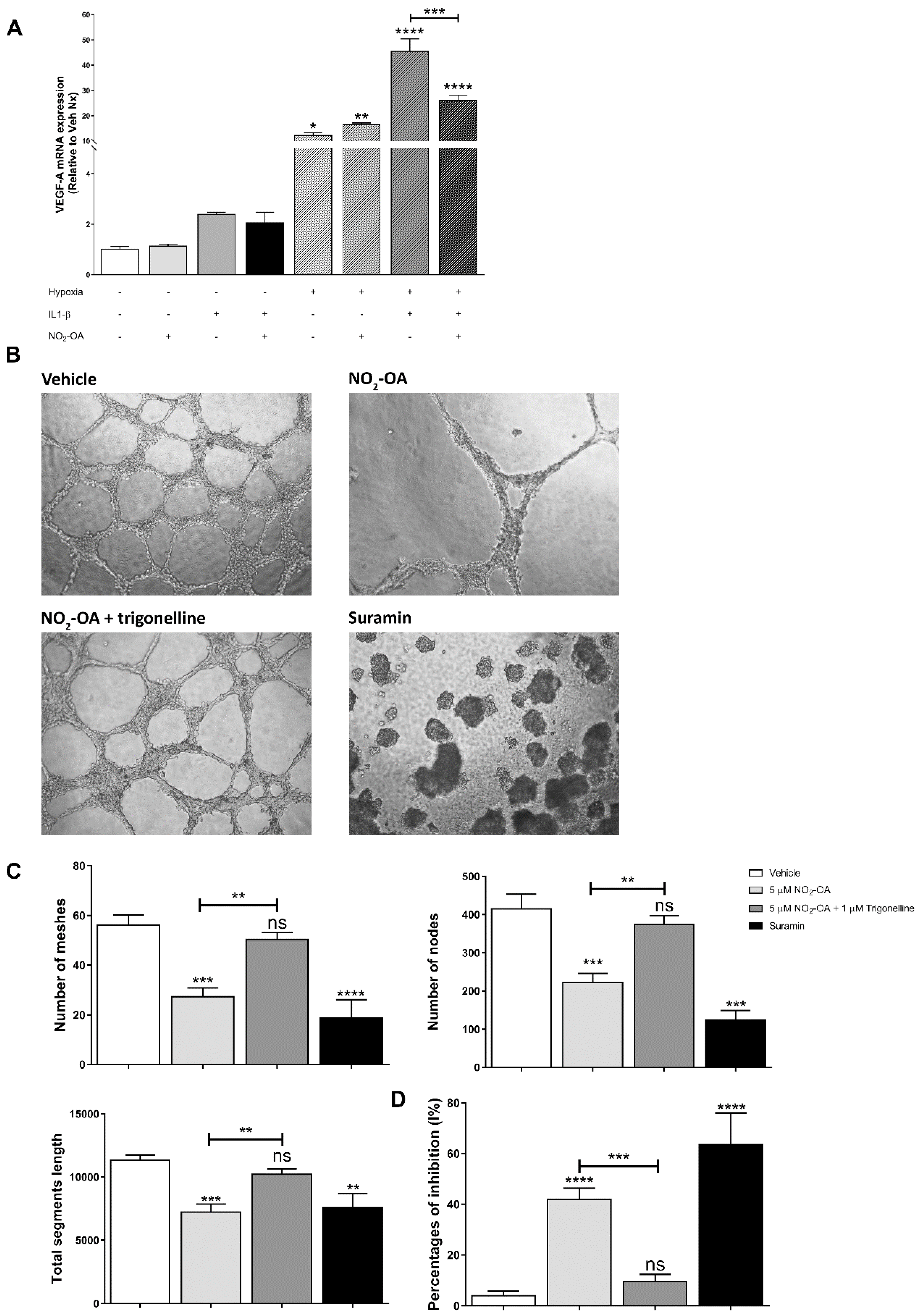
3.5. Effect of NO2-OA on Angiogenesis in MGCs and ECs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedlander, M.; Dorrell, M.I.; Ritter, M.R.; Marchetti, V.; Moreno, S.K.; El-Kalay, M.; Bird, A.C.; Banin, E.; Aguilar, E. Progenitor cells and retinal angiogenesis. Angiogenesis 2007, 10, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.T.; Herman, I.M. Microvascular modifications in diabetic retinopathy. Curr. Diab. Rep. 2011, 11, 253–264. [Google Scholar] [CrossRef]
- Chung, A.S.; Ferrara, N. Developmental and pathological angiogenesis. Annu. Rev. Cell. Dev. Biol. 2011, 27, 563–584. [Google Scholar] [CrossRef]
- Rodrigues, M.; Xin, X.; Jee, K.; Babapoor-Farrokhran, S.; Kashiwabuchi, F.; Ma, T.; Bhutto, I.; Hassan, S.J.; Daoud, Y.; Baranano, D.; et al. VEGF secreted by hypoxic Müller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes 2013, 62, 3863–3873. [Google Scholar] [CrossRef]
- Subirada, P.V.; Paz, M.C.; Ridano, M.E.; Lorenc, V.E.; Vaglienti, M.V.; Barcelona, P.F.; Luna, J.D.; Sánchez, M.C. A journey into the retina: Müller glia commanding survival and death. Eur. J. Neurosci. 2018, 47, 1429–1443. [Google Scholar] [CrossRef]
- Graca, A.B.; Hippert, C.; Pearson, R.A. Müller Glia Reactivity and Development of Gliosis in Response to Pathological Conditions. Adv. Exp. Med. Biol. 2018, 1074, 303–308. [Google Scholar]
- Liu, Q.; Ling, T.Y.; Shieh, H.S.; Johnson, F.E.; Huang, J.S.; Huang, S.S. Identification of the high affinity binding site in transforming growth factor-beta involved in complex formation with alpha 2-macroglobulin. Implications regarding the molecular mechanisms of complex formation between alpha 2-macroglobulin and growth factors, cytokines, and hormones. J. Biol. Chem. 2001, 276, 46212–46218. [Google Scholar] [PubMed]
- Garcia-Ferrer, I.; Marrero, A.; Gomis-Rüth, F.X.; Goulas, T. α 2-Macroglobulins: Structure and Function. Subcell. Biochem. 2017, 83, 149–183. [Google Scholar] [PubMed]
- Barcelona, P.F.; Ortiz, S.G.; Chiabrando, G.A.; Sánchez, M.C. alpha2-Macroglobulin induces glial fibrillary acidic protein expression mediated by low-density lipoprotein receptor-related protein 1 in Müller cells. Invest. Ophthalmol. Vis. Sci. 2011, 52, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Eastlake, K.; Banerjee, P.J.; Angbohang, A.; Charteris, D.G.; Khaw, P.T.; Limb, G.A. Müller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia 2016, 64, 495–506. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Hoh, J.; Liu, J. Müller cells in pathological retinal angiogenesis. Transl. Res. 2019, 207, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P.; Joyal, J.S.; Rivera, J.C.; Kermorvant-Duchemin, E.; Sennlaub, F.; Hardy, P.; Lachapelle, P.; Chemtob, S. Retinopathy of prematurity: Understanding ischemic retinal vasculopathies at an extreme of life. J. Clin. Investig. 2010, 120, 3022–3032. [Google Scholar] [CrossRef]
- Bonacci, G.; Baker, P.R.S.; Salvatore, S.R.; Shores, D.; Khoo, N.K.H.; Koenitzer, J.R.; Vitturi, D.A.; Woodcock, S.R.; Golin-Bisello, F.; Cole, M.P.; et al. Conjugated linoleic acid is a preferential substrate for fatty acid nitration. J. Biol. Chem. 2012, 287, 44071–44082. [Google Scholar] [CrossRef] [PubMed]
- Vitturi, D.A.; Minarrieta, L.; Salvatore, S.R.; Postlethwait, E.M.; Fazzari, M.; Ferrer-Sueta, G.; Lancaster, J.R.; Freeman, B.A.; Schopfer, F.J. Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride. Nat. Chem. Biol. 2015, 11, 504–510. [Google Scholar] [CrossRef]
- Villacorta, L.; Minarrieta, L.; Salvatore, S.R.; Khoo, N.K.; Rom, O.; Gao, Z.; Berman, R.C.; Jobbagy, S.; Li, L.; Woodcock, S.R.; et al. In situ generation, metabolism and immunomodulatory signaling actions of nitro-conjugated linoleic acid in a murine model of inflammation. Redox Biol. 2018, 15, 522–531. [Google Scholar] [CrossRef]
- Tsikas, D.; Zoerner, A.A.; Mitschke, A.; Gutzki, F.M. Nitro-fatty acids occur in human plasma in the picomolar range: A targeted nitro-lipidomics GC-MS/MS study. Lipids 2009, 44, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.R.; Rowart, P.; Schopfer, F.J. Mass spectrometry-based study defines the human urine nitrolipidome. Free Radic. Biol. Med. 2021, 162, 327–337. [Google Scholar] [CrossRef]
- Rudolph, V.; Rudolph, T.K.; Schopfer, F.J.; Bonacci, G.; Woodcock, S.R.; Cole, M.P.; Baker, P.R.S.; Ramani, R.; Freeman, B.A. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc. Res. 2010, 85, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Koenitzer, J.R.; Bonacci, G.; Woodcock, S.R.; Chen, C.S.; Cantu-Medellin, N.; Kelley, E.E.; Schopfer, F.J. Fatty acid nitroalkenes induce resistance to ischemic cardiac injury by modulating mitochondrial respiration at complex II. Redox. Biol. 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Turell, L.; Vitturi, D.A.; Coitiño, E.L.; Lebrato, L.; Möller, M.N.; Sagasti, C.; Salvatore, S.R.; Woodcock, S.R.; Alvarez, B.; Schopfer, F.J. The Chemical Basis of Thiol Addition to Nitro-conjugated Linoleic Acid, a Protective Cell-signaling Lipid. J. Biol. Chem. 2017, 292, 1145–1159. [Google Scholar] [CrossRef]
- Trostchansky, A.; Bonilla, L.; González-Perilli, L.; Rubbo, H. Nitro-fatty acids: Formation, redox signaling, and therapeutic potential. Antioxid. Redox Signal. 2013, 19, 1257–1265. [Google Scholar] [CrossRef]
- Diaz-Amarilla, P.; Miquel, E.; Trostchansky, A.; Trias, E.; Ferreira, A.M.; Freeman, B.A.; Cassina, P.; Barbeito, L.; Vargas, M.R.; Rubbo, H. Electrophilic nitro-fatty acids prevent astrocyte-mediated toxicity to motor neurons in a cell model of familial amyotrophic lateral sclerosis via nuclear factor erythroid 2-related factor activation. Free Radic. Biol. Med. 2016, 95, 112–120. [Google Scholar] [CrossRef]
- Nakamura, S.; Noguchi, T.; Inoue, Y.; Sakurai, S.; Nishinaka, A.; Hida, Y.; Masuda, T.; Nakagami, Y.; Horai, N.; Tsusaki, H.; et al. Nrf2 Activator RS9 Suppresses Pathological Ocular Angiogenesis and Hyperpermeability. Invest. Ophthalmol. Vis. Sci. 2019, 60, 1943–1952. [Google Scholar] [CrossRef]
- Huang, Z.; Kin Ng, T.; Chen, W.; Sun, X.; Huang, D.; Zheng, D.; Yi, J.; Xu, Y.; Zhuang, X.; Chen, S. Nattokinase Attenuates Retinal Neovascularization Via Modulation of Nrf2/HO-1 and Glial Activation. Investig. Ophthalmol. Vis. Sci. 2021, 62, 25. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gong, J.; Xu, Z.; Duh, E.J. Nrf2 promotes reparative angiogenesis through regulation of NADPH oxidase-2 in oxygen-induced retinopathy. Free Radic. Biol. Med. 2016, 99, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wei, Y.; Gong, J.; Cho, H.; Park, J.K.; Sung, E.R.; Huang, H.; Wu, L.; Eberhart, C.; Handa, J.T.; et al. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia 2014, 57, 204–213. [Google Scholar] [CrossRef]
- Chiabrando, G.; Bonacci, G.; Sanchez, C.; Ramos, A.; Zalazar, F.; Vides, M.A. A procedure for human pregnancy zone protein (and human alpha 2-macroglobulin) purification using hydrophobic interaction chromatography on phenyl-sepharose CL-4B column. Protein. Expr. Purif. 1997, 9, 399–406. [Google Scholar] [CrossRef]
- Chiabrando, G.A.; Sánchez, M.C.; Skornicka, E.L.; Koo, P.H. Low-density lipoprotein receptor-related protein mediates in PC12 cell cultures the inhibition of nerve growth factor-promoted neurite outgrowth by pregnancy zone protein and alpha2-macroglobulin. J. Neurosci. Res. 2002, 70, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Vaglienti, M.V.; Subirada, P.V.; Barcelona, P.F.; Bonacci, G.; Sanchez, M.C. Quantification of Reactive Oxygen Species Using 2’,7’-Dichlorofluorescein Diacetate Probe and Flow-Cytometry in Müller Glial Cells. J. Vis. Exp. 2022, 13, 183. [Google Scholar] [CrossRef]
- Ridano, M.E.; Racca, A.C.; Flores-Martín, J.; Camolotto, S.A.; de Potas, G.M.; Genti-Raimondi, S.; Panzetta-Dutari, G.M. Chlorpyrifos modifies the expression of genes involved in human placental function. Reprod. Toxicol. 2012, 33, 331–338. [Google Scholar] [CrossRef]
- Subirada, P.V.; Vaglienti, M.V.; Joray, M.B.; Paz, M.C.; Barcelona, P.F.; Sánchez, M.C. Rapamycin and Resveratrol Modulate the Gliotic and Pro-Angiogenic Response in Müller Glial Cells Under Hypoxia. Front. Cell Dev. Biol. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutova, I.; Kleinman, H.K. In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010, 5, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Kansanen, E.; Jyrkkänen, H.K.; Volger, O.L.; Leinonen, H.; Kivelä, A.M.; Häkkinen, S.K.; Woodcock, S.R.; Schopfer, F.J.; Horrevoets, A.J.; Ylä-Herttuala, S.; et al. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: Identification of heat shock response as the major pathway activated by nitro-oleic acid. J. Biol. Chem. 2009, 284, 33233–33241. [Google Scholar] [CrossRef]
- Vazquez, M.M.; Gutierrez, M.v.; Salvatore, S.R.; Puiatti, M.; Dato, V.A.; Chiabrando, G.A.; Freeman, B.A.; Schopfer, F.J.; Bonacci, G. Nitro-oleic acid, a ligand of CD36, reduces cholesterol accumulation by modulating oxidized-LDL uptake and cholesterol efflux in RAW264.7 macrophages. Redox Biol. 2020, 36, 101591. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.C.; Holm, M.; Austeng, D.; Morken, T.S.; Zhou, T.E.; Beaudry-Richard, A.; Sierra, E.M.; Dammann, O.; Chemtob, S. Retinopathy of prematurity: Inflammation, choroidal degeneration, and novel promising therapeutic strategies. J. Neuroinflammation 2017, 14, 1–4. [Google Scholar] [CrossRef]
- Yamane, K.; Minamoto, A.; Yamashita, H.; Takamura, H.; Miyamoto-Myoken, Y.; Yoshizato, K.; Nabetani, T.; Tsugita, A.; Mishima, H.K. Proteome analysis of human vitreous proteins. Mol. Cell Proteomics. 2003, 2, 1177–1187. [Google Scholar] [CrossRef]
- Ridano, M.E.; Subirada, P.v.; Paz, M.C.; Lorenc, V.E.; Stupirski, J.C.; Gramajo, A.L.; Luna, J.D.; Croci, D.O.; Rabinovich, G.A.; Sánchez, M.C. Galectin-1 expression imprints a neurovascular phenotype in proliferative retinopathies and delineates responses to anti-VEGF. Oncotarget 2017, 8, 32505–32522. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015, 49, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Tah, V.; Orlans, H.O.; Hyer, J.; Casswell, E.; Din, N.; Sri Shanmuganathan, V.; Ramskold, L.; Pasu, S. Anti-VEGF Therapy and the Retina: An Update. J. Ophthalmol. 2015, 2015, 627674. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Aguilar, E.; Jacobson, R.; Trauger, S.A.; Friedlander, J.; Siuzdak, G.; Friedlander, M. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia 2010, 58, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Lemtalsi, T.; Toque, H.; Xu, Z.; Fulton, D.; Caldwell, R.; Caldwell, R. () NOX2-Induced Activation of Arginase and Diabetes-Induced Retinal Endothelial Cell Senescence. Antioxidants 2017, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gross, S.; Chatterjee, A.; Wang, Y.; Lin, J.; Hammes, H.P. Transcription of Inflammatory Cytokine TNFα is Upregulated in Retinal Angiogenesis under Hyperoxia. Cell Physiol. Biochem. 2016, 39, 573–583. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Bartoli, M.; El-Remessy, A.B.; Platt, D.H.; Matragoon, S.; Behzadian, M.A.; Caldwell, R.W.; Caldwell, R.B. Inhibition of NAD(P)H Oxidase Activity Blocks Vascular Endothelial Growth Factor Overexpression and Neovascularization during Ischemic Retinopathy. Am. J. Pathol. 2005, 167, 599–607. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.X.; Hartnett, M.E. Signaling pathways triggered by oxidative stress that mediate features of severe retinopathy of prematurity. JAMA Ophthalmol. 2013, 131, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Graziosi, A.; Perrotta, M.; Russo, D.; Gasparroni, G.; D’Egidio, C.; Marinelli, B.; di Marzio, G.; Falconio, G.; Mastropasqua, L.; Li Volti, G.; et al. Oxidative Stress Markers and the Retinopathy of Prematurity. J. Clin. Med. 2020, 9, 2711. [Google Scholar] [CrossRef]
- Ogihara, T.; Mino, M. Vitamin E and preterm infants. Free Radic. Biol. Med. 2022, 180, 13–32. [Google Scholar] [CrossRef]
- Khoo, N.K.H.; Li, L.; Salvatore, S.R.; Schopfer, F.J.; Freeman, B.A. Electrophilic fatty acid nitroalkenes regulate Nrf2 and NF-κB signaling:A medicinal chemistry investigation of structure-function relationships. Sci. Rep. 2018, 8, 2295. [Google Scholar] [CrossRef]
- Arlt, A.; Sebens, S.; Krebs, S.; Geismann, C.; Grossmann, M.; Kruse, M.L.; Schreiber, S.; Schäfer, H. Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene 2013, 32, 4825–4835. [Google Scholar] [CrossRef]
- Wright, M.M.; Kim, J.; Hock, T.D.; Leitinger, N.; Freeman, B.A.; Agarwal, A. Human haem oxygenase-1 induction by nitro-linoleic acid is mediated by cAMP, AP-1 and E-box response element interactions. Biochem. J. 2009, 422, 353–361. [Google Scholar] [CrossRef]
- Tan, S.M.; Deliyanti, D.; Figgett, W.A.; Talia, D.M.; de Haan, J.B.; Wilkinson-Berka, J.L. Ebselen by modulating oxidative stress improves hypoxia-induced macroglial Müller cell and vascular injury in the retina. Exp. Eye Res. 2015, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Fu, Z.J.; Lo, A.C.Y. Hypoxia-induced oxidative stress in ischemic retinopathy. Oxid. Med. Cell Longev. 2012, 2012, 426769. [Google Scholar] [CrossRef]
- Rudolph, T.K.; Rudolph, V.; Edreira, M.M.; Cole, M.P.; Bonacci, G.; Schopfer, F.J.; Woodcock, S.R.; Franek, A.; Pekarova, M.; Khoo, N.K.H.; et al. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Luna, J.D.; Barcelona, P.F.; Gramajo, A.L.; Juarez, P.C.; Riera, C.M.; Chiabrando, G.A. Effect of retinal laser photocoagulation on the activity of metalloproteinases and the alpha(2)-macroglobulin proteolytic state in the vitreous of eyes with proliferative diabetic retinopathy. Exp. Eye Res. 2007, 85, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Baydas, G.; Nedzvetskii, V.S.; Tuzcu, M.; Yasar, A.; Kirichenko, S.V. Increase of glial fibrillary acidic protein and S-100B in hippocampus and cortex of diabetic rats: Effects of vitamin E. Eur. J. Pharmacol. 2003, 462, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ho, G.; Zhang, Y.; Zhuo, L. In vivo imaging of retinal gliosis: A platform for diagnosis of PD and Screening of anti-PD compounds. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2010, 2010, 3049–3052. [Google Scholar]
- Sarafian, T.A.; Montes, C.; Imura, T.; Qi, J.; Coppola, G.; Geschwind, D.H.; Sofroniew, M.v. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS ONE 2010, 5, e9532. [Google Scholar] [CrossRef]
- Wang, T.; Tsirukis, D.I.; Sun, Y. Targeting Neuroinflammation in Neovascular Retinal Diseases. Front Pharmacol. 2020, 11, 234. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Simon, A.; van der Meer, J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug. Discov. 2012, 11, 633–652. [Google Scholar]
- Nadeau-Vallée, M.; Quiniou, C.; Palacios, J.; Hou, X.; Erfani, A.; Madaan, A.; Sanchez, M.; Leimert, K.; Boudreault, A.; Duhamel, F.; et al. Novel Noncompetitive IL-1 Receptor-Biased Ligand Prevents Infection- and Inflammation-Induced Preterm Birth. J. Immunol. 2015, 195, 3402–3415. [Google Scholar] [CrossRef]
- Rivera, J.C.; Sitaras, N.; Noueihed, B.; Hamel, D.; Madaan, A.; Zhou, T.; Honoré, J.C.; Quiniou, C.; Joyal, J.S.; Hardy, P.; et al. Microglia and interleukin-1β in ischemic retinopathy elicit microvascular degeneration through neuronal semaphorin-3A. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Mantsounga, C.S.; Lee, C.; Neverson, J.; Sharma, S.; Healy, A.; Berus, J.M.; Parry, C.; Ceneri, N.M.; López-Giráldez, F.; Chun, H.J.; et al. Macrophage IL-1β promotes arteriogenesis by autocrine STAT3- and NF-κB-mediated transcription of pro-angiogenic VEGF-A. Cell Rep. 2022, 38, 110309. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kanai, H.; Sekiguchi, K.; Aihara, Y.; Yokoyama, T.; Arai, M.; Kanda, T.; Nagai, R.; Kurabayashi, M. Induction of VEGF gene transcription by IL-1 beta is mediated through stress-activated MAP kinases and Sp1 sites in cardiac myocytes. J Mol. Cell Cardiol. 2000, 32, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gong, J.; Yoshida, T.; Eberhart, C.G.; Xu, Z.; Kombairaju, P.; Sporn, M.B.; Handa, J.T.; Duh, E.J. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic. Biol. Med. 2011, 51, 216–224. [Google Scholar] [CrossRef]
- Wei, Y.; Gong, J.; Thimmulappa, R.K.; Kosmider, B.; Biswal, S.; Duh, E.J. Nrf2 acts cell-autonomously in endothelium to regulate tip cell formation and vascular branching. Proc. Natl. Acad. Sci. USA 2013, 110, E3910–E3918. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaglienti, M.V.; Subirada, P.V.; Joray, M.B.; Bonacci, G.; Sánchez, M.C. Protective Effect of NO2-OA on Oxidative Stress, Gliosis, and Pro-Angiogenic Response in Müller Glial Cells. Cells 2023, 12, 494. https://doi.org/10.3390/cells12030494
Vaglienti MV, Subirada PV, Joray MB, Bonacci G, Sánchez MC. Protective Effect of NO2-OA on Oxidative Stress, Gliosis, and Pro-Angiogenic Response in Müller Glial Cells. Cells. 2023; 12(3):494. https://doi.org/10.3390/cells12030494
Chicago/Turabian StyleVaglienti, María V., Paula V. Subirada, Mariana B. Joray, Gustavo Bonacci, and María C. Sánchez. 2023. "Protective Effect of NO2-OA on Oxidative Stress, Gliosis, and Pro-Angiogenic Response in Müller Glial Cells" Cells 12, no. 3: 494. https://doi.org/10.3390/cells12030494
APA StyleVaglienti, M. V., Subirada, P. V., Joray, M. B., Bonacci, G., & Sánchez, M. C. (2023). Protective Effect of NO2-OA on Oxidative Stress, Gliosis, and Pro-Angiogenic Response in Müller Glial Cells. Cells, 12(3), 494. https://doi.org/10.3390/cells12030494





