Immune Cells Release MicroRNA-155 Enriched Extracellular Vesicles That Promote HIV-1 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Infection of Peripheral Blood Mononuclear Cells
2.2. Peripheral Blood Mononuclear Cells Stimulation
2.3. Infection Peripheral Blood Lymphocytes
2.4. CD4 T Lymphocytes
2.5. Flow Cytometry
2.6. Purification of PBMC-derived EVs
2.7. Production of miR-155-Enriched EVs
2.8. Virus Preparation and Titration
2.9. ELISA Calprotectin
2.10. ELISA IL-8
2.11. Dynamic Light Scattering (DLS) analysis
2.12. RNA Extraction
2.13. DNA Extraction
2.14. MicroRNA Quantification by RT-qPCR
2.15. mRNA RT-qPCR
2.16. HIV-1 RNA Quantification
2.17. HIV DNA Standard Curve Production
2.18. Quantification of HIV DNA
2.19. Statistical Analysis
3. Results
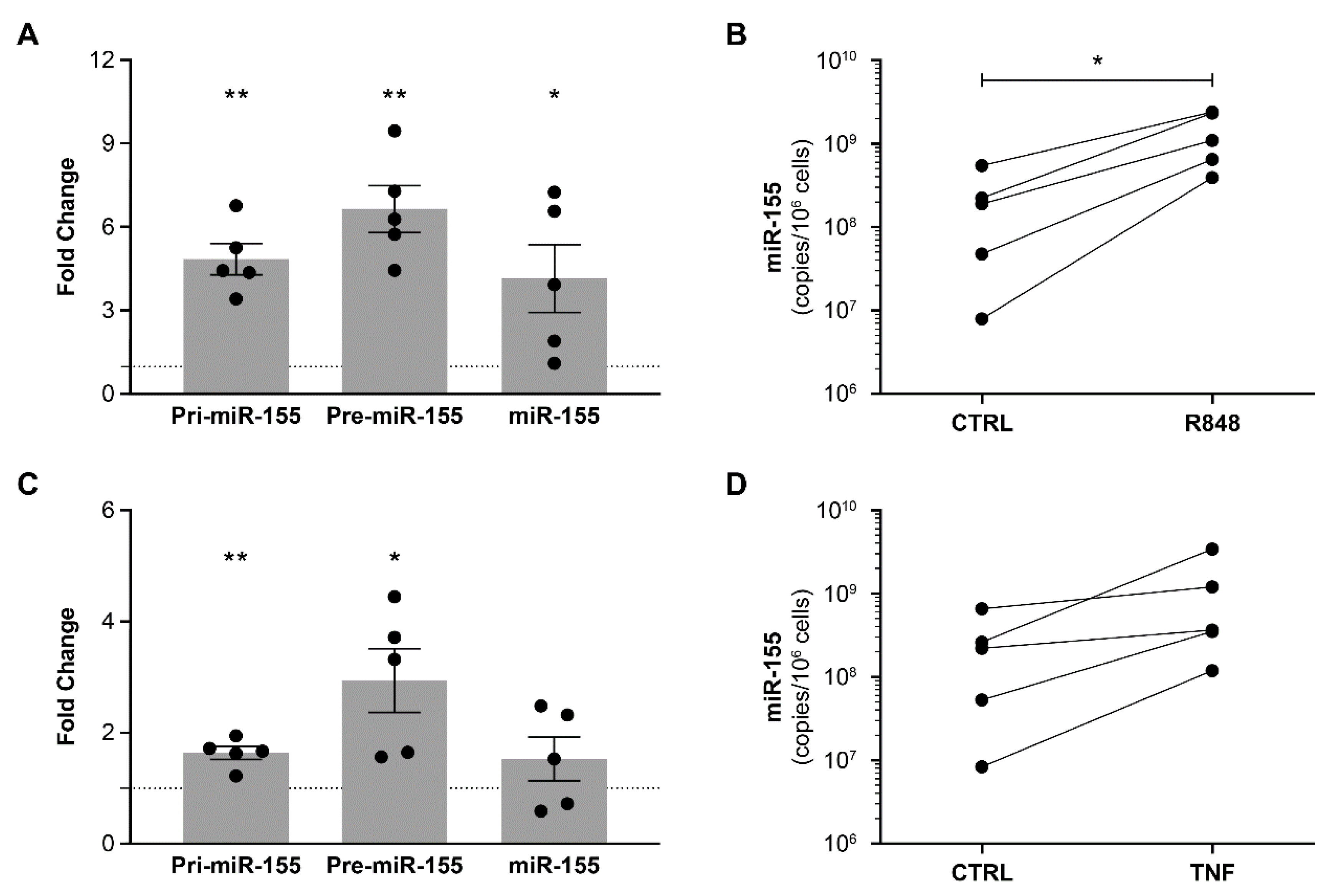
3.1. HIV and Other Inflammatory Factors Induce miR-155 Expression in PBMCs
3.2. MiR-155 Overexpressing PBMCs Release miR-155 Enriched EVs
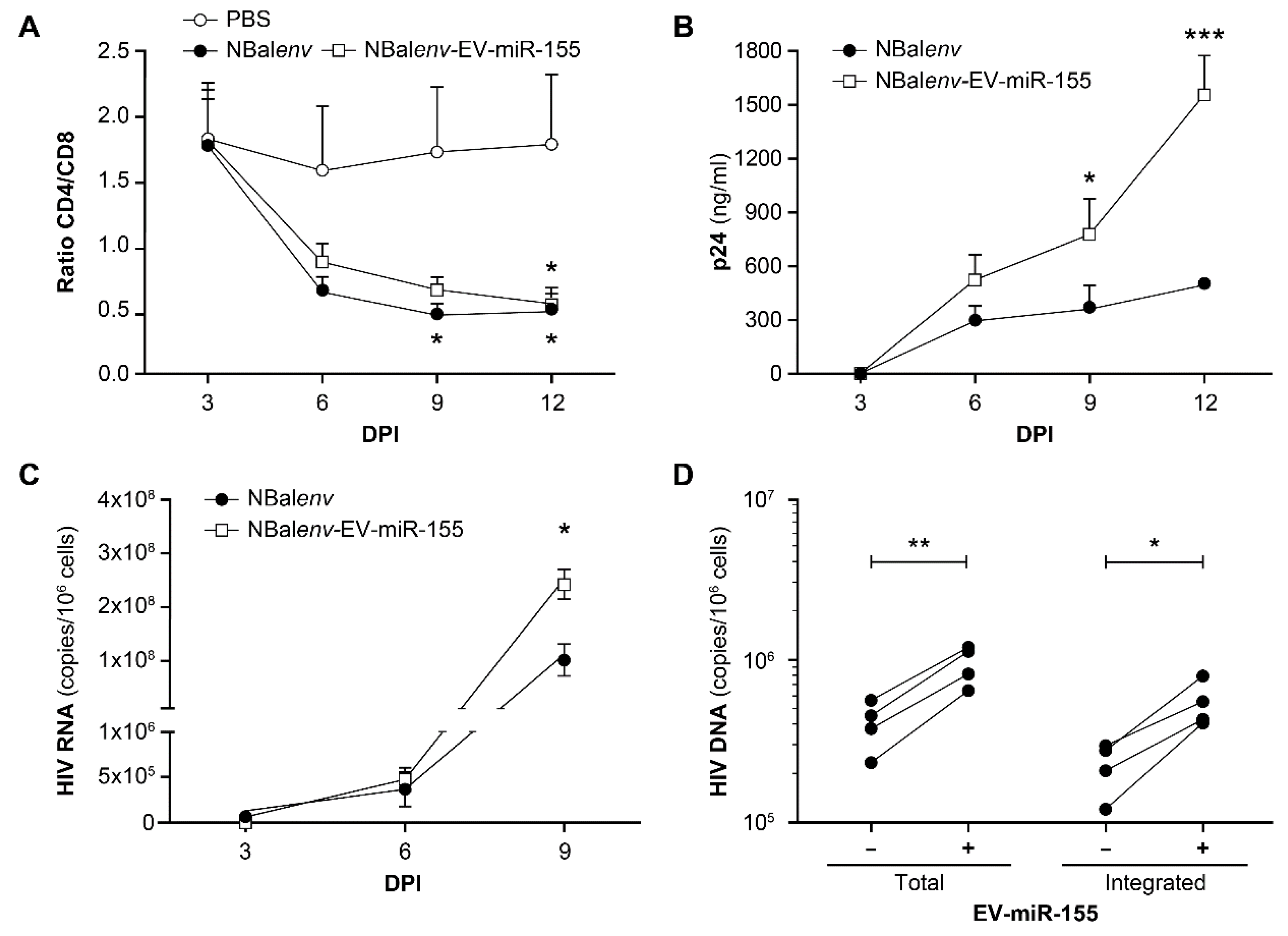
3.3. MiR-155-Enriched EVs Affect the Course of HIV-1 Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikhailova, A.; Valle-Casuso, J.C.; Sáez-Cirión, A. Cellular Determinants of HIV Persistence on Antiretroviral Therapy. In HIV Vaccines and Cure; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1075, pp. 213–239. [Google Scholar] [CrossRef]
- Hubert, A.; Subra, C.; Jenabian, M.-A.; Labrecque, P.-F.T.; Tremblay, C.; Laffont, B.; Provost, P.; Routy, J.-P.; Gilbert, C. Elevated abundance, size, and MicroRNA content of plasma extracellular vesicles in viremic HIV-1+, size, and MicroRNA content of plasma extracellular vesicles in viremic HIV-1+ patients: Correlations with known markers of disease progression. J. Acquir. Immune Defic. Syndr. 2015, 70, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Dillon, S.T.; Reeves, R.K.; Manickam, C.; Morgello, S.; Kirk, G.D.; Mehta, S.H.; Gabuzda, D. Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Konadu, K.A.; Chu, J.; Huang, M.B.; Amancha, P.K.; Armstrong, W.S.; Powell, M.D.; Villinger, F.; Bond, V.C. Association of Cytokines with Exosomes in the Plasma of HIV-1–Seropositive Individuals. J. Infect. Dis. 2014, 211, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Bazié, W.W.; Boucher, J.; Vitry, J.; Goyer, B.; Routy, J.P.; Tremblay, C.; Trottier, S.; Jenabian, M.-A.; Provost, P.; Alary, M.; et al. Plasma Extracellular Vesicle Subtypes May be Useful as Potential Biomarkers of Immune Activation in People with HIV. Pathog. Immun. 2021, 6, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Iordanskiy, S.; Das, R.; Van Duyne, R.; Santos, S.; Jaworski, E.; Guendel, I.; Sampey, G.; Dalby, E.; Iglesias-Ussel, M.; et al. Exosomes Derived from HIV-1-infected Cells Contain Trans-activation Response Element RNA. J. Biol. Chem. 2013, 288, 20014–20033. [Google Scholar] [CrossRef]
- Kadiu, I.; Narayanasamy, P.; Dash, P.K.; Zhang, W.; Gendelman, H.E. Biochemical and Biologic Characterization of Exosomes and Microvesicles as Facilitators of HIV-1 Infection in Macrophages. J. Immunol. 2012, 189, 744–754. [Google Scholar] [CrossRef]
- Khatua, A.K.; Taylor, H.E.; Hildreth, J.E.K.; Popik, W. Exosomes Packaging APOBEC3G Confer Human Immunodeficiency Virus Resistance to Recipient Cells. J. Virol. 2009, 83, 512–521. [Google Scholar] [CrossRef]
- Näslund, T.I.; Paquin-Proulx, D.; Paredes, P.T.; Vallhov, H.; Sandberg, J.K.; Gabrielsson, S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. Aids 2014, 28, 171–180. [Google Scholar] [CrossRef]
- Raymond, A.; Campbell-Sims, T.; Khan, M.; Lang, M.; Huang, M.; Bond, V.; Powell, M. HIV Type 1 Nef Is Released from Infected Cells in CD45+ Microvesicles and Is Present in the Plasma of HIV-Infected Individuals. AIDS Res. Hum. Retrovir. 2011, 27, 167–178. [Google Scholar] [CrossRef]
- Cabezas, S.C.; Federico, M. Sequences within RNA coding for HIV-1 Gag p17 are efficiently targeted to exosomes. Cell. Microbiol. 2012, 15, 412–429. [Google Scholar] [CrossRef]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Arenaccio, C.; Chiozzini, C.; Columba-Cabezas, S.; Manfredi, F.; Affabris, E.; Baur, A.; Federico, M. Exosomes from Human Immunodeficiency Virus Type 1 (HIV-1)-Infected Cells License Quiescent CD4 + T Lymphocytes to Replicate HIV-1 through a Nef- and ADAM17-Dependent Mechanism. J. Virol. 2014, 88, 11529–11539. [Google Scholar] [CrossRef] [PubMed]
- Arakelyan, A.; Fitzgerald, W.; Zicari, S.; Vanpouille, C.; Margolis, L. Extracellular vesicles carry HIV Env and facilitate Hiv infection of human lymphoid tissue. Sci. Rep. 2017, 7, 1695. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of Novel Genes Coding for Small Expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef]
- Zendjabil, M.; Favard, S.; Tse, C.; Abbou, O.; Hainque, B. The microRNAs as biomarkers: What prospects? Comptes Rendus Biol. 2017, 340, 114–131. [Google Scholar] [CrossRef]
- Niaz, S. The AGO proteins: An overview. Biol. Chem. 2017, 399, 525–547. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The Role of miRNAs in Regulating Gene Expression Networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A Mammalian microRNA Expression Atlas Based on Small RNA Library Sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef]
- Georgantas, R.W., III; Hildreth, R.; Morisot, S.; Alder, J.; Liu, C.G.; Heimfeld, S.; Calin, G.A.; Croce, C.M.; Civin, C.I. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: A circuit diagram of differentiation control. Proc. Natl. Acad. Sci. USA 2007, 104, 2750–2755. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interf. Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Chaudhuri, A.A.; Rao, D.S.; Baltimore, D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc. Natl. Acad. Sci. USA 2009, 106, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Guo, R.; Shi, Y.; Qi, F.; Guo, C.; Yang, L. miR-155 Regulated Inflammation Response by the SOCS1-STAT3-PDCD4 Axis in Atherogenesis. Mediat. Inflamm. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Cheng, L.; Höxtermann, S.; Xie, T.; Lu, X.; Wu, H.; Skaletz-Rorowski, A.; Brockmeyer, N.; Wu, N. MicroRNA-155 is a biomarker of T-cell activation and immune dysfunction in HIV-1-infected patients. HIV Med. 2016, 18, 354–362. [Google Scholar] [CrossRef]
- Jin, C.; Cheng, L.; Lu, X.; Xie, T.; Wu, H.; Wu, N. Elevated expression of miR-155 is associated with the differentiation of CD8+ T cells in patients with HIV-1. Mol. Med. Rep. 2017, 16, 1584–1589. [Google Scholar] [CrossRef]
- Bazié, W.W.; Boucher, J.; Goyer, B.; Traoré, I.T.; Kania, D.; Somé, D.Y.; Alary, M.; Gilbert, C. Plasma vesicular miR-155 as a biomarker of immune activation in antiretroviral treated people living with HIV. Front. Immunol. 2022, 13, 916599. [Google Scholar] [CrossRef]
- Bazié, W.W.; Boucher, J.; Traoré, I.T.; Kania, D.; Somé, D.Y.; Alary, M.; Gilbert, C. Vesicular MicroRNA as Potential Biomarkers of Viral Rebound. Cells 2022, 11, 859. [Google Scholar] [CrossRef]
- Subra, C. Dendritic Cells Pulsed with HIV-1 Release Exosomes that Promote Apoptosis in Cd4+ T Lymphocytes. J. Clin. Cell. Immunol. S 2013, 4, 7. [Google Scholar] [CrossRef]
- Ryckman, C.; Vandal, K.; Rouleau, P.; Talbot, M.; Tessier, P.A. Proinflammatory Activities of S100: Proteins S100A8, S100A9, and S100A8/A9 Induce Neutrophil Chemotaxis and Adhesion. J. Immunol. 2003, 170, 3233–3242. [Google Scholar] [CrossRef]
- Cantin, R.; Fortin, J.F.; Lamontagne, G.; Tremblay, M. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J. Virol. 1997, 71, 1922–1930. [Google Scholar] [CrossRef]
- Dornadula, G.; Zhang, H.; Shetty, S.; Pomerantz, R.J. HIV-1 Virions Produced from Replicating Peripheral Blood Lymphocytes Are More Infectious Than Those from Nonproliferating Macrophages Due to Higher Levels of Intravirion Reverse Transcripts: Implications for Pathogenesis and Transmission. Virology 1999, 253, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bounou, S.; Leclerc, J.E.; Tremblay, M.J. Presence of Host ICAM-1 in Laboratory and Clinical Strains of Human Immunodeficiency Virus Type 1 Increases Virus Infectivity and CD4 + -T-Cell Depletion in Human Lymphoid Tissue, a Major Site of Replication In Vivo. J. Virol. 2002, 76, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Tardif, M.R.; Chapeton-Montes, J.A.; Posvandzic, A.; Pagé, N.; Gilbert, C.; Tessier, P.A. Secretion of S100A8, S100A9, and S100A12 by Neutrophils Involves Reactive Oxygen Species and Potassium Efflux. J. Immunol. Res. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, C.; Gilbert, C.; de Médicis, R.; Lussier, A.; Vandal, K.; Tessier, P.A. Monosodium urate monohydrate crystals induce the release of the proinflammatory protein S100A8/A9 from neutrophils. J. Leukoc. Biol. 2004, 76, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA Using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb-prot5439. [Google Scholar] [CrossRef]
- Watson, S.; Mercier, S.; Bye, C.; Wilkinson, J.; Cunningham, A.L.; Harman, A.N. Determination of suitable housekeeping genes for normalisation of quantitative real time PCR analysis of cells infected with human immunodeficiency virus and herpes viruses. Virol. J. 2007, 4, 130. [Google Scholar] [CrossRef]
- Rouet, F.; Ekouevi, D.K.; Chaix, M.-L.; Burgard, M.; Inwoley, A.; Tony, T.D.; Danel, C.; Anglaret, X.; Leroy, V.; Msellati, P.; et al. Transfer and Evaluation of an Automated, Low-Cost Real-Time Reverse Transcription-PCR Test for Diagnosis and Monitoring of Human Immunodeficiency Virus Type 1 Infection in a West African Resource-Limited Setting. J. Clin. Microbiol. 2005, 43, 2709–2717. [Google Scholar] [CrossRef]
- Clouse, K.A.; Powell, D.; Washington, I.; Poli, G.; Strebel, K.; Farrar, W.; Barstad, P.; Kovacs, J.; Fauci, A.S.; Folks, T.M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J. Immunol. 1989, 142, 431–438. [Google Scholar] [CrossRef]
- Folks, T.M.; Clouse, K.A.; Justement, J.; Rabson, A.; Duh, E.; Kehrl, J.H.; Fauci, A.S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl. Acad. Sci. USA 1989, 86, 2365–2368. [Google Scholar] [CrossRef]
- Vandergeeten, C.; Fromentin, R.; Merlini, E.; Lawani, M.B.; DaFonseca, S.; Bakeman, W.; McNulty, A.; Ramgopal, M.; Michael, N.; Kim, J.H.; et al. Cross-Clade Ultrasensitive PCR-Based Assays to Measure HIV Persistence in Large-Cohort Studies. J. Virol. 2014, 88, 12385–12396. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Cao, Z.; Li, J.; Long, H.; Wu, Y.; Zhang, Z.; Sun, Y. R848 Is Involved in the Antibacterial Immune Response of Golden Pompano (Trachinotus ovatus) Through TLR7/8-MyD88-NF-κB-Signaling Pathway. Front. Immunol. 2021, 11, 617522. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.J.V.; Bishop, G.A. TLR7 and CD40 cooperate in IL-6 productionviaenhanced JNK and AP-1 activation. Eur. J. Immunol. 2008, 38, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, E.; Audigé, A.; Joller, H.; Speck, R.F. TLR7/8 Triggering Exerts Opposing Effects in Acute versus Latent HIV Infection. J. Immunol. 2006, 176, 2888–2895. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. TNF-α/NF-κB/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 2010, 102, 639–644. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Bernal, R.S.; Ruiz-Mateos, E.; Rosado, I.; Dominguez-Molina, B.; Alvarez-Rios, A.I.; Carrillo-Vico, A.; De La Rosa, R.; Delgado, J.; Munoz-Fernandez, M.A.; Leal, M.; et al. TNF- levels in HIV-infected patients after long-term suppressive cART persist as high as in elderly, HIV-uninfected subjects. J. Antimicrob. Chemother. 2014, 69, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Tumor-infiltrating monocytes/macrophages promote tumor invasion and migration by upregulating S100A8 and S100A9 expression in cancer cells. Oncogene 2016, 35, 5735–5745. [Google Scholar] [CrossRef]
- Lood, C.; Stenström, M.; Tydén, H.; Gullstrand, B.; Källberg, E.; Leanderson, T.; Truedsson, L.; Sturfelt, G.; Ivars, F.; Bengtsson, A.A. Protein synthesis of the pro-inflammatory S100A8/A9 complex in plasmacytoid dendritic cells and cell surface S100A8/A9 on leukocyte subpopulations in systemic lupus erythematosus. Arthritis Res. Ther. 2011, 13, R60. [Google Scholar] [CrossRef]
- Romas, L.M.; Hasselrot, K.; Aboud, L.G.; Birse, K.D.; Ball, T.B.; Broliden, K.; Burgener, A.D. A Comparative Proteomic Analysis of the Soluble Immune Factor Environment of Rectal and Oral Mucosa. PLoS ONE 2014, 9, e100820. [Google Scholar] [CrossRef]
- Månberg, A.; Bradley, F.; Qundos, U.; Guthrie, B.L.; Birse, K.; Noël-Romas, L.; Lindskog, C.; Bosire, R.; Kiarie, J.; Farquhar, C.; et al. A High-throughput Bead-based Affinity Assay Enables Analysis of Genital Protein Signatures in Women at Risk of HIV Infection. Mol. Cell. Proteom. 2019, 18, 461–476. [Google Scholar] [CrossRef]
- Arnold, K.B.; Burgener, A.; Birse, K.; Romas, L.; Dunphy, L.J.; Shahabi, K.; Abou, M.; Westmacott, G.R.; McCorrister, S.; Kwatampora, J.; et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. 2015, 9, 194–205. Available online: https://www.nature.com/articles/mi201551#supplementary-information (accessed on 10 September 2022). [CrossRef]
- Anderson, E.M.; Maldarelli, F. The role of integration and clonal expansion in HIV infection: Live long and prosper. Retrovirology 2018, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Butin-Israeli, V.; Bui, T.M.; Wiesolek, H.L.; Mascarenhas, L.; Lee, J.J.; Mehl, L.C.; Knutson, K.R.; Adam, S.A.; Goldman, R.D.; Beyder, A.; et al. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J. Clin. Investig. 2019, 129, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Provenzano, M.; Rossi, C.R.; Pilati, P.; Nitti, D.; Lise, M. Use of quantitative real-time PCR to determine immune cell density and cytokine gene profile in the tumor microenvironment. J. Immunol. Methods 2003, 280, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.-S.; Kim, J.-H.; Lee, D.-K.; Park, M.; Choi, S.; Park, W.; Kim, S.; Choi, Y.K.; Hwang, J.Y.; et al. Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia. Free. Radic. Biol. Med. 2017, 104, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.; Shah, K.M.; Coles, M.C.; Sharp, T.V.; Lagos, D. MicroRNA-155 induction via TNF-α and IFN-γ suppresses expression of programmed death ligand-1 (PD-L1) in human primary cells. J. Biol. Chem. 2017, 292, 20683–20693. [Google Scholar] [CrossRef]
- Migita, K.; Iwanaga, N.; Izumi, Y.; Kawahara, C.; Kumagai, K.; Nakamura, T.; Koga, T.; Kawakami, A. TNF-α-induced miR-155 regulates IL-6 signaling in rheumatoid synovial fibroblasts. BMC Res. Notes 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Cremer, T.J.I.; Fatehchand, K.; Shah, P.; Gillette, D.; Patel, H.; Marsh, R.L.; Besecker, B.Y.; Rajaram, M.V.S.; Cormet-Boyaka, E.; Kanneganti, T.-D.; et al. MiR-155 Induction by Microbes/Microbial Ligands Requires NF-κB-Dependent de novo Protein Synthesis. Front. Cell. Infect. Microbiol. 2012, 2, 73. [Google Scholar] [CrossRef]
- Fiume, G.; Vecchio, E.; De Laurentiis, A.; Trimboli, F.; Palmieri, C.; Pisano, A.; Falcone, C.; Pontoriero, M.; Rossi, A.; Scialdone, A.; et al. Human immunodeficiency virus-1 Tat activates NF-κB via physical interaction with IκB-α and p65. Nucleic Acids Res. 2011, 40, 3548–3562. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.-J.; Baltimore, D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- Kumar, V.; Palermo, R.; Talora, C.; Campese, A.F.; Checquolo, S.; Bellavia, D.; Tottone, L.; Testa, G.; Miele, E.; Indraccolo, S.; et al. Notch and NF-kB signaling pathways regulate miR-223/FBXW7 axis in T-cell acute lymphoblastic leukemia. Leukemia 2014, 28, 2324–2335. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Verhaar, M.C.; Chen, Y.; Vader, P.; Gremmels, H.; Posthuma, G.; Schiffelers, R.M.; Gucek, M.; Van Balkom, B.W.M. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles 2012, 1, 18396. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ruelas, D.S.; Chan, J.K.; Oh, E.; Heidersbach, A.J.; Hebbeler, A.M.; Chavez, L.; Verdin, E.; Rape, M.; Greene, W.C. MicroRNA-155 Reinforces HIV Latency. J. Biol. Chem. 2015, 290, 13736–13748. [Google Scholar] [CrossRef]
- Gokavi, J.; Sadawarte, S.; Shelke, A.; Kulkarni-Kale, U.; Thakar, M.; Saxena, V. Inhibition of miR-155 Promotes TGF-β Mediated Suppression of HIV Release in the Cervical Epithelial Cells. Viruses 2021, 13, 2266. [Google Scholar] [CrossRef] [PubMed]
- Albanese, M.; Chen, Y.-F.A.; Hüls, C.; Gärtner, K.; Tagawa, T.; Mejias-Perez, E.; Keppler, O.T.; Göbel, C.; Zeidler, R.; Shein, M.; et al. MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet. 2021, 17, e1009951. [Google Scholar] [CrossRef]
- Alexander, M.; Hu, R.; Runtsch, M.C.; Kagele, D.A.; Mosbruger, T.L.; Tolmachova, T.; Seabra, M.C.; Round, J.L.; Ward, D.M.; O’Connell, R.M. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat. Commun. 2015, 6, 7321. [Google Scholar] [CrossRef]
- Wu, J.; Gu, J.; Shen, L.; Fang, D.; Zou, X.; Cao, Y.; Wang, S.; Mao, L. Exosomal MicroRNA-155 Inhibits Enterovirus A71 Infection by Targeting PICALM. Int. J. Biol. Sci. 2019, 15, 2925–2935. [Google Scholar] [CrossRef]
- Hubert, A. Influence du miR-155 Vésiculaire sur la Pathogenèse Associée à L’infection par le Virus de L’immunodéficience Humaine de Type 1 (VIH-1). Ph.D. Thesis, Université Laval, Québec, QC, Canada, 2016. Available online: http://hdl.handle.net/20.500.11794/27317 (accessed on 29 August 2022).
- Vaillancourt, M.; Hubert, A.; Subra, C.; Boucher, J.; Bazié, W.; Vitry, J.; Berrazouane, S.; Routy, J.-P.; Trottier, S.; Tremblay, C.; et al. Velocity Gradient Separation Reveals a New Extracellular Vesicle Population Enriched in miR-155 and Mitochondrial DNA. Pathogens 2021, 10, 526. [Google Scholar] [CrossRef]
- Miller, R.C.; Schlaepfer, E.; Baenziger, S.; Crameri, R.; Zeller, S.; Byland, R.; Audigé, A.; Nadal, D.; Speck, R.F. HIV interferes with SOCS-1 and -3 expression levels driving immune activation. Eur. J. Immunol. 2011, 41, 1058–1069. [Google Scholar] [CrossRef]
- Yoshimura, A.; Ito, M.; Chikuma, S.; Akanuma, T.; Nakatsukasa, H. Negative Regulation of Cytokine Signaling in Immunity. Cold Spring Harb. Perspect. Biol. 2017, 10, a028571. [Google Scholar] [CrossRef]
- Biancotto, A.; Iglehart, S.J.; Vanpouille, C.; Condack, C.E.; Lisco, A.; Ruecker, E.; Hirsch, I.; Margolis, L.B.; Grivel, J.-C. HIV-1–induced activation of CD4+ T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood 2008, 111, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Jahantigh, M.; Wei, Y.; Noels, H.; Akhtar, S.; Zhou, Z.; Koenen, R.R.; Heyll, K.; Gremse, F.; Kiessling, F.; Grommes, J.; et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J. Clin. Investig. 2012, 122, 4190–4202. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Volinia, S.; Costinean, S.; Galasso, M.; Neinast, R.; Santhanam, R.; Parthun, M.R.; Perrotti, D.; Marcucci, G.; Garzon, R.; et al. miR-155 targets histone deacetylase 4 (HDAC4) and impairs transcriptional activity of B-cell lymphoma 6 (BCL6) in the Eµ-miR-155 transgenic mouse model. Proc. Natl. Acad. Sci. USA 2012, 109, 20047–20052. [Google Scholar] [CrossRef]
- Gomez, I.; Ward, B.; Souilhol, C.; Recarti, C.; Ariaans, M.; Johnston, J.; Burnett, A.; Mahmoud, M.; Luong, L.A.; West, L.; et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Y.; Yang, J.; Wang, Y.; Xu, J.; Fan, Y. MiR-155-5p modulates inflammatory phenotype of activated oral lichen-planus-associated-fibroblasts by targeting SOCS1. Mol. Biol. Rep. 2022, 49, 7783–7792. [Google Scholar] [CrossRef] [PubMed]
- Natekar, J.P.; Rothan, H.A.; Arora, K.; Strate, P.G.; Kumar, M. Cellular microRNA-155 Regulates Virus-Induced Inflammatory Response and Protects against Lethal West Nile Virus Infection. Viruses 2019, 12, 9. [Google Scholar] [CrossRef]
- Swaminathan, S.; Qiu, J.; Rupert, A.W.; Hu, Z.; Higgins, J.; Dewar, R.L.; Stevens, R.; Rehm, C.A.; Metcalf, J.A.; Sherman, B.T.; et al. Interleukin-15 (IL-15) Strongly Correlates with Increasing HIV-1 Viremia and Markers of Inflammation. PLoS ONE 2016, 11, e0167091. [Google Scholar] [CrossRef]
- Manganaro, L.; Hong, P.; Hernandez, M.M.; Argyle, D.; Mulder, L.C.F.; Potla, U.; Diaz-Griffero, F.; Lee, B.; Fernandez-Sesma, A.; Simon, V. IL-15 regulates susceptibility of CD4 + T cells to HIV infection. Proc. Natl. Acad. Sci. USA 2018, 115, E9659–E9667. [Google Scholar] [CrossRef]
- Ilangumaran, S.; Ramanathan, S.; La Rose, J.; Poussier, P.; Rottapel, R. Suppressor of Cytokine Signaling 1 Regulates IL-15 Receptor Signaling in CD8+CD44high Memory T Lymphocytes. J. Immunol. 2003, 171, 2435–2445. [Google Scholar] [CrossRef]
- Perera, P.-Y.; Lichy, J.H.; Waldmann, T.A.; Perera, L.P. The role of interleukin-15 in inflammation and immune responses to infection: Implications for its therapeutic use. Microbes Infect. 2012, 14, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Mueller, Y.M.; Bojczuk, P.M.; Halstead, E.S.; Kim, A.H.J.; Witek, J.; Altman, J.D.; Katsikis, P.D. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood 2003, 101, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Abad-Fernandez, M.; Tuyishime, M.; Pollara, J.J.; Ferrari, G.; Soriano-Sarabia, N.; Margolis, D.M. Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo. J. Virol. 2018, 92, e00235-18. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boucher, J.; Rousseau, A.; Boucher, C.; Subra, C.; Bazié, W.W.; Hubert, A.; Bourgeault, E.; Benmoussa, A.; Goyer, B.; Tessier, P.A.; et al. Immune Cells Release MicroRNA-155 Enriched Extracellular Vesicles That Promote HIV-1 Infection. Cells 2023, 12, 466. https://doi.org/10.3390/cells12030466
Boucher J, Rousseau A, Boucher C, Subra C, Bazié WW, Hubert A, Bourgeault E, Benmoussa A, Goyer B, Tessier PA, et al. Immune Cells Release MicroRNA-155 Enriched Extracellular Vesicles That Promote HIV-1 Infection. Cells. 2023; 12(3):466. https://doi.org/10.3390/cells12030466
Chicago/Turabian StyleBoucher, Julien, Alyssa Rousseau, Catherine Boucher, Caroline Subra, Wilfried W. Bazié, Audrey Hubert, Emma Bourgeault, Abderrahim Benmoussa, Benjamin Goyer, Philippe A. Tessier, and et al. 2023. "Immune Cells Release MicroRNA-155 Enriched Extracellular Vesicles That Promote HIV-1 Infection" Cells 12, no. 3: 466. https://doi.org/10.3390/cells12030466
APA StyleBoucher, J., Rousseau, A., Boucher, C., Subra, C., Bazié, W. W., Hubert, A., Bourgeault, E., Benmoussa, A., Goyer, B., Tessier, P. A., & Gilbert, C. (2023). Immune Cells Release MicroRNA-155 Enriched Extracellular Vesicles That Promote HIV-1 Infection. Cells, 12(3), 466. https://doi.org/10.3390/cells12030466





