1. Introduction
Diarrhea can arise from intestinal malabsorption, active secretion or leak flux as a result of barrier defects, as well as motility disorders of the intestine [
1]. Chronic manifestation can be the result of a substantial number of different diagnoses, including infections such as lambliasis, amoebiasis or strongyloidiasis, as well as AIDS-related infections. Additionally, it can also be a consequence of immune- or autoimmune-related disorders, such as inflammatory bowel diseases (IBD), including microscopic colitis, neuroendocrine etiology, e.g., carcinoid syndrome, gastrinoma, VIPoma, or irritable bowel syndrome (IBS) [
2].
IBS is a chronic functional disorder of the gastrointestinal tract with a prevalence of 10–20% [
3]. It is characterized by abdominal pain and changes in bowel movements (diarrhea and/or constipation). According to the patient’s reported primary bowel habit, there are four different forms of IBS: IBS-D with predominantly diarrhea, IBS-C with predominantly constipation, IBS-M with mixed bowel habits and unclassified IBS-U [
4,
5].
While in IBS-C and IBS-M, obstipation is predominant—which is interrupted in IBS-M by solving diarrheal phases—IBS-D is characterized by ongoing diarrheal states. This diagnosis in particular requires the exclusion of somatic diseases by means of laboratory tests, imaging and microbiological testing [
6]. Often, this follows acute gastrointestinal infections with bacterial pathogens or
Giardia lamblia persisting in the long term even after successful antibiotic therapy. Despite being common throughout the world’s population [
7], the pathogenesis of IBS-D is still not completely understood. However, the efficacy for a therapeutic strategy will benefit from an increasing understanding of the underlying pathomechanisms.
One aspect of intestinal dysfunction in IBS is an impaired barrier function, for which a central role in pathophysiology is discussed [
8]. A previous study showed that microRNA-16 modulates epithelial tight junctions (TJ) via TLR4/NF-κB pathway in IBS-D [
9]. Structural changes in cellular TJ proteins have also been described by others to be associated with IBS. Cheng et al. showed a downregulation of claudin-1 in IBS-D patients, while it was increased in IBS-C patients [
10]. The expression of claudin-2 was shown to be significantly elevated in the ileum of patients diagnosed with diarrhea-predominant irritable bowel syndrome, while its expression was not altered in the rectal mucosa of patients diagnosed with IBS-D [
11].
TJ strands are formed by a multi-protein complex consisting of claudins, junction adhesion molecules (JAMs) and tight-junction-associated MARVEL proteins (TAMPs) [
12]. These highly regulated components play a crucial role in the closure of intercellular gaps, effectively controlling the passage of ions and molecules of either size across the epithelium. Furthermore, TJs maintain the epithelial cell polarity by separating the apical and basolateral plasma membrane compartments, restricting the lateral membrane diffusion of membrane proteins (fence function). Occludin, tricellulin and the claudin family of TJ proteins, with 27 distinct claudins identified in mammals [
13], are functionally important TJ protein components with either a barrier- or channel-forming activity. The majority of claudins are recognized for their role in tightening cellular junctions, such as claudin-1, claudin-3, claudin-4, claudin-5 and claudin-8. Conversely, claudin-2, claudin 10a and 10b, claudin-12 and claudin-15 are known to operate as ion channels [
14,
15,
16,
17,
18], which could contribute to diarrhea via a pore pathway, resulting in a leak flux mechanism when overexpressed.
Furthermore, it has been proposed that intestinal barrier dysfunction could be linked to visceral hypersensitivity in IBS patients [
19]. The activation of mast cells and the movement of mast cells into the vicinity of epithelial cells or sensory nerves in the intestinal mucosa lead to an interaction between the tissues and the immune cells, which results in barrier dysfunction and visceral hypersensitivity via the release of mediators, such as prostaglandin E2, histamine, tryptase and cytokines [
20]. Epithelial barrier disruption is frequently paralleled by hypersensitivity in IBS, worsening abdominal pain in this disorder [
20]. A previous clinical study found that IBS-D and post-infectious IBS patients have an increased small intestinal permeability for 339 Da
51Cr-EDTA compared with the controls [
21]. This brings the phenomena of an acute infectious barrier impairment and the prolongation of this barrier defect along the
leaky gut concept together with the onset of IBS.
However, it has now also become clear that microbial factors are very important in the biology of IBS. This includes differences in the microbiota in the two main types of IBS, namely IBS-D and IBS-C. IBS-D showed an overgrowth of small intestinal bacteria, which can be identified by a positive hydrogen breath test, while IBS-C had higher levels of methanogenic archaea [
22].
However, little is now known so far about the IBS-D subgroup’s functional changes in the large intestinal mucosa responsible for diarrhea. In particular, no direct measurements were performed in humans regarding the intestinal barrier function. Furthermore, the pattern of TJ protein expression in IBS-D has not yet been fully characterized. In order to better characterize IBS-D, we examined (i) ion permeability, as measured with impedance spectroscopy, (ii) paracellular macromolecule permeability in colonic biopsies, (iii) the expression pattern of TJ proteins and (iv) the subcellular localization of barrier-relevant TJ proteins. In addition, RNA sequencing (RNA-seq) using bioinformatics pathway analysis was conducted to clarify the signaling pathways, which are implicated in IBS-D.
2. Materials and Methods
2.1. Study Design
We included seven patients with IBS-D (four female, three male) and ten controls (five female, five male) in the observation study. In order to diagnose IBS-D, the Rome III criteria were used. The criteria include abdominal pain or discomfort, which has occurred at least three days per month for the past three months and is linked to two or more criteria: (i) improvement with defecation, (ii) onset associated with a change in stool frequency or (iii) onset associated with a change in stool form.
None of the IBS-D patients were taking any medication or showed any intestinal lesions. The average age was 35 ± 4 years (controls: 48 ± 3 years). A colonoscopy with biopsy sampling was conducted for each patient and was also performed on control subjects, for example for prevention of colon cancer. All biopsies were taken 25–35 cm ab ano. The sigmoid colon biopsies were placed into miniaturized Ussing chambers (exposed tissue area: 0.049 cm2) for functional measurements, and in parallel, biopsies were fixed for molecular analysis. It took approximately 30 min from the time the biopsy was taken until it was mounted in the Ussing chamber.
2.2. Ethics
This study complied with the Helsinki Declaration regarding the use of human subjects. Each patient provided written informed consent for the study, which was authorized by the institutional review board of “The Ethics Committee of the Charité” under the approval number EA4/015/13.
2.3. Ussing Chamber Measurements
The colon biopsies were affixed into miniaturized Ussing chambers, which had an area of 0.049 cm
2. The colon mucosa’s total transepithelial electrical resistance (R
t = TER) comprises two distinct components: the epithelial resistance (R
epi) and the subepithelial resistance (R
sub). These components can be distinguished by using impedance spectroscopy [
23]. The experimental setup involved the utilization of a programmed frequency response analyzer (Model 402, Beran Instruments, Devon, UK) and an electrochemical interface (Model 1286, Solartron Schlumberger, Farnborough, UK) to apply alternating currents within the frequency range of 1 Hz to 65 kHz. The impedance was derived from the voltage responses. The electrical resistance of the bath was used for correction. The complex impedance values were presented in a Nyquist diagram subsequent to the adjustment of the resistance of the bathing solution and the frequency response of the measuring equipment at each frequency.
The total transepithelial resistance Rt was determined by identifying the point of intersection between the semicircle and the x-axis at a frequency of 0. Similarly, the Rsub was determined by identifying the point of intersection between the semicircle and the x-axis at an infinitely high frequency. The Repi was calculated by subtracting the Rsub from the Rt.
The bathing solution for Ussing chamber studies and the atmospheric conditions were the same as in a similar study described previously [
24].
Tissue vitality was assessed subsequent to each Ussing experiment. In order to achieve the intended objective, the activation of electrogenic chloride secretion was stimulated by prostaglandin E2 (PGE2, 10−6 mol/L), theophylline (10−2 mol/L) and carbachol (10−4 mol/L) (all Sigma-Aldrich, St. Louis, MO, USA).
2.4. Electrogenic Sodium Absorption
Aldosterone (3×10
−9 mol/L, Sigma-Aldrich, St. Louis, MO, USA) was administered to human biopsies during the Ussing chamber experiments to simulate steroid stimulation as present in diarrheal conditions. The activity of the epithelial sodium channel (ENaC) was assessed eight hours following stimulation. This was accomplished by introducing the ENaC blocker amiloride (10
−4 mol/L, Sigma-Aldrich, St. Louis, MO, USA) into the mucosal compartment. The subsequent decrease in short-circuit current (I
SC) was attributed to ENaC-dependent sodium absorption, as previously documented [
25].
2.5. Electrogenic Anion Secretion
The ISC of human colon biopsies was measured in parallel Ussing experiments in order to quantify basal rheogenic anion secretion. At the end of the experiment, bumetanide (Sigma-Aldrich, St. Louis, MO, USA) was added with a concentration of 10−5 mol/L to the serosal side in order to quantify electrogenic chloride secretion as the bumetanide-sensitive ISC.
2.6. Permeability Analysis
The functional measurement of macromolecule permeability was performed as described previously [
24]. Briefly, tracer flux measurements conducted under voltage-clamped conditions in Ussing chambers were used to determine the permeability for the paracellular flux marker fluorescein. A volume of 100 µmol/L fluorescein (Sigma-Aldrich, Darmstadt, Germany) was added to the apical side. The basolateral samples were obtained at time intervals of 15, 30, 45 and 60 min.
In parallel experiments, 0.4 mmol/L FITC-dextran 4 kDa was applied apically, while unlabeled 4 kDa dextran was added on the basal side of the tissue (both Sigma-Aldrich, Darmstadt, Germany). Fluorescence analysis was conducted in duplicate using a spectrophotometer plate reader at a wavelength of 535 nm (Tecan Infinite M200, Tecan, Männedorf, Switzerland). The fluorescence measurements were calibrated using predetermined dilutions. The permeability of fluorescein or FITC-dextran was determined using the formula P = J/Δc, with P = permeability (cm/s), J = flux (mol∙h−1∙cm−2) and c = concentration (mol/L).
2.7. Protein Expression
Colonic samples were subjected to Western blot analysis. Protein extraction and homogenization were performed by utilizing a lysis solution containing the following components: 20 mmol/L Tris (pH 7.4), 5 mmol/L MgCl2, 1 mmol/L EDTA, 0.3 mmol/L EGTA and a complete protease inhibitor combination (Roche AG, Basel, Switzerland). The extraction process involved passing the mixture through a 27.5 G needle. The insoluble material was removed from the extract by centrifuging it at 200× g for 5 min at 4 °C, and then, the supernatant was centrifuged at 43,000× g for 30 min at 4 °C.
Western blot analysis was performed as described previously [
24]. Briefly, the pellet (membrane fraction) was reconstituted in a lysate buffer. The process of separating 20 µg of protein was carried out using polyacrylamide gel electrophoresis, followed by transferring the separated proteins onto a PDVF membrane (Perkin Elmer, located in Rodgau, Germany). The generation of blots involved the use of primary rabbit (rb) polyclonal IgG antibodies against claudin-1, -2, -3, -4, -5, -8 and occludin (rb claudin-1 #519000, rb claudin-2 #516100, rb claudin-3 #341700, m claudin-4 #329400, rb claudin-5 #341600, rb claudin-8 #710222, rb occludin #711500; all Invitrogen, Karlsruhe, Germany) and the primary mouse monoclonal IgG antibodies against β-actin (Sigma-Aldrich, St. Louis, MO, USA).
The quantification of protein expression was performed using densitometry with luminous imaging (LAS-1000, Fuji Film, Tokyo, Japan). The AIDA software (Raytest, version 3.2.1, Berlin, Germany) was used for data analysis.
2.8. Analysis of Subcellular Tight Junction Distribution
Immunofluorescence microscopy is a technique for accurate visualization of the structures within tissues and cells by using antibodies. In this work, immunofluorescence was used to examine the subcellular localization of TJ proteins in the sigmoid colon, as described previously [
24].
Briefly, sigmoid biopsies were subjected to fixation in a 1% paraformaldehyde solution for a duration of 60 min at room temperature. Subsequently, the samples were subjected to washing in PBS+Ca/Mg solution, followed by incubation in a 25 mmol/L glycine solution for a duration of five minutes. Following a second round of washing in PBS+Ca/Mg, the biopsies underwent a sequential dehydration process using an increasing sucrose series (10%, 20% and 30%) at room temperature for 60 min. In order to produce the cryoblocks, the biopsies underwent a freezing process using methylbutane frozen with liquid nitrogen. Following this, the biopsies were placed in TissueTek (Sakura Finetek, Europe B.V, Umkirch, Germany) and stored at a temperature of −80 °C. A cryostat (Leica CM 1950, Leica, Wetzlar, Germany) was used to prepare tissue sections of 5 μm thickness. Before they were stained, these were frozen on coated slides at −20 °C for 12–24 h. Permeabilization was achieved by adding 0.5% Triton-X to the tissue sections for 10 min. Subsequently, unspecific binding was prevented with a blocking solution for 60 min (1% Goat serum, 5% BSA, 0.05% Triton X-100).
Finally, the main antibody (tricellulin #700191, 1:100, Invitrogen, Karlsruhe, Germany) was added overnight at 4 °C. There were three washing steps after the first incubation step. Then, the fluorescent secondary antibodies Alexa Fluor-488 goat anti-rabbit (#A32731) and Alexa Fluor-594 goat anti-mouse (#A32742, 1:500, Invitrogen, Karlsruhe, Germany) were used for 120 min at 37 °C. DAPI (4′-d-diamidino-2-phenylidole dihydrochloride, 1:1000, Roche AG, Mannheim, Germany) was used to stain the nuclei. Afterward, Pro Tags Mount Fluor (Biocyc, Potsdam, Germany) was used to mount them on glass slides.
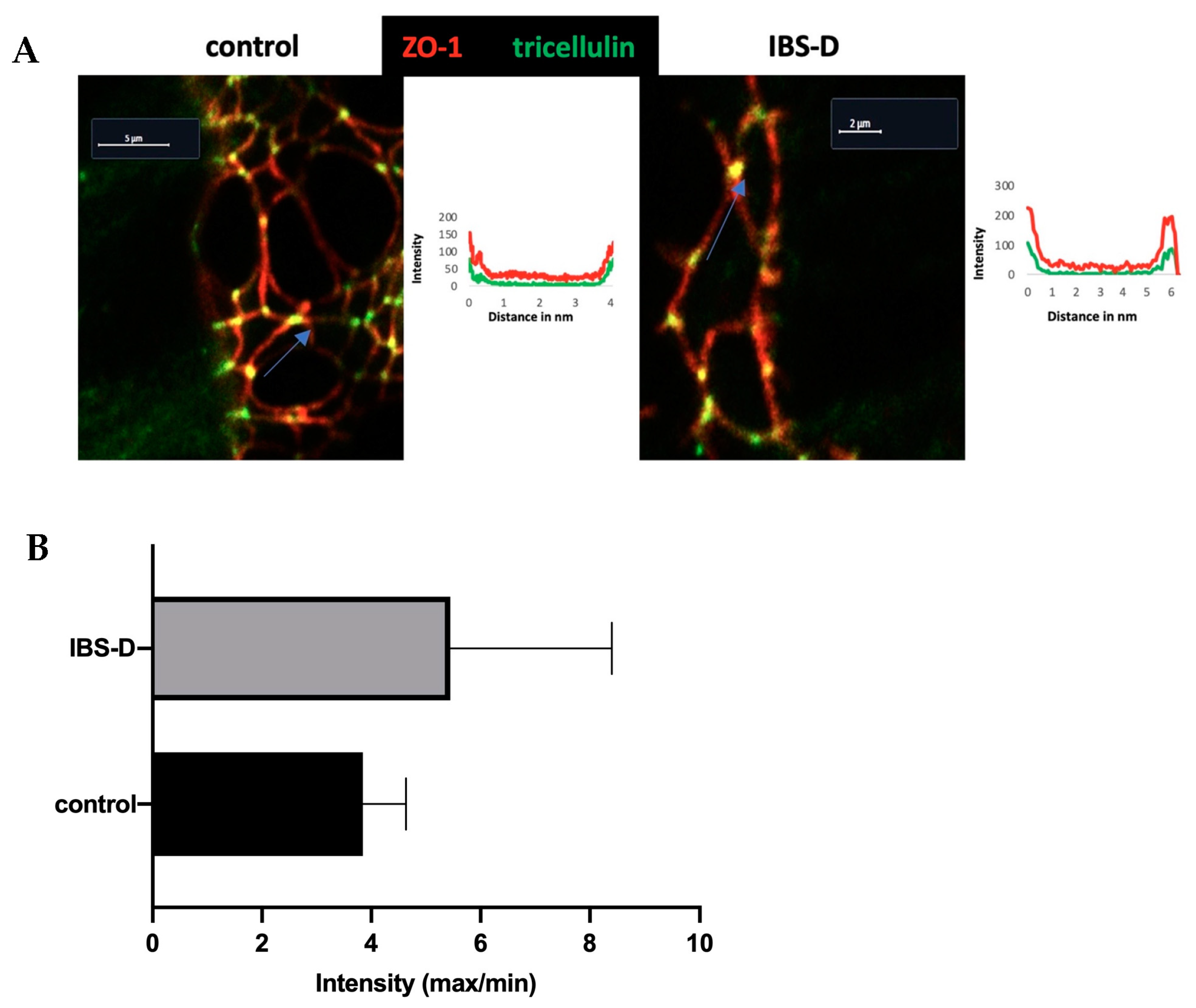
The subcellular distribution of the TJ proteins was determined by using confocal laser-scanning microscopy (Zeiss LSM 780, Zeiss, Jena, Germany) on fluorescence-labeled tissue sections. The secondary antibodies were utilized at excitation wavelengths of 594 nm (red), 488 nm (green) and 358 nm (blue for DAPI). The images were acquired with plan apochromat plan neofluor objectives with numerical apertures of 1.4 at magnifications of 40× and 63×. In order to visualize the primary localization of tricellulin, we performed an intensity plot analysis using the ZEN blue 2.5 lite software (Zeiss LSM ZEN software, version 2.5.75.0, Carl Zeiss Microscopy GmbH, Jena, Germany).
The measurement of tricellulin intensity was conducted in the tricellular tight junction (tTJ) and compared with the intensity of tricellulin in the bicellular tight junction (bTJ) located 2 µm away from the measurement location of three cells in the tTJ. The measurements were performed on 3 controls in comparison to 3 patients with IBS-D. Measurements were taken 3 times in each of the 3–6 sections for each patient.
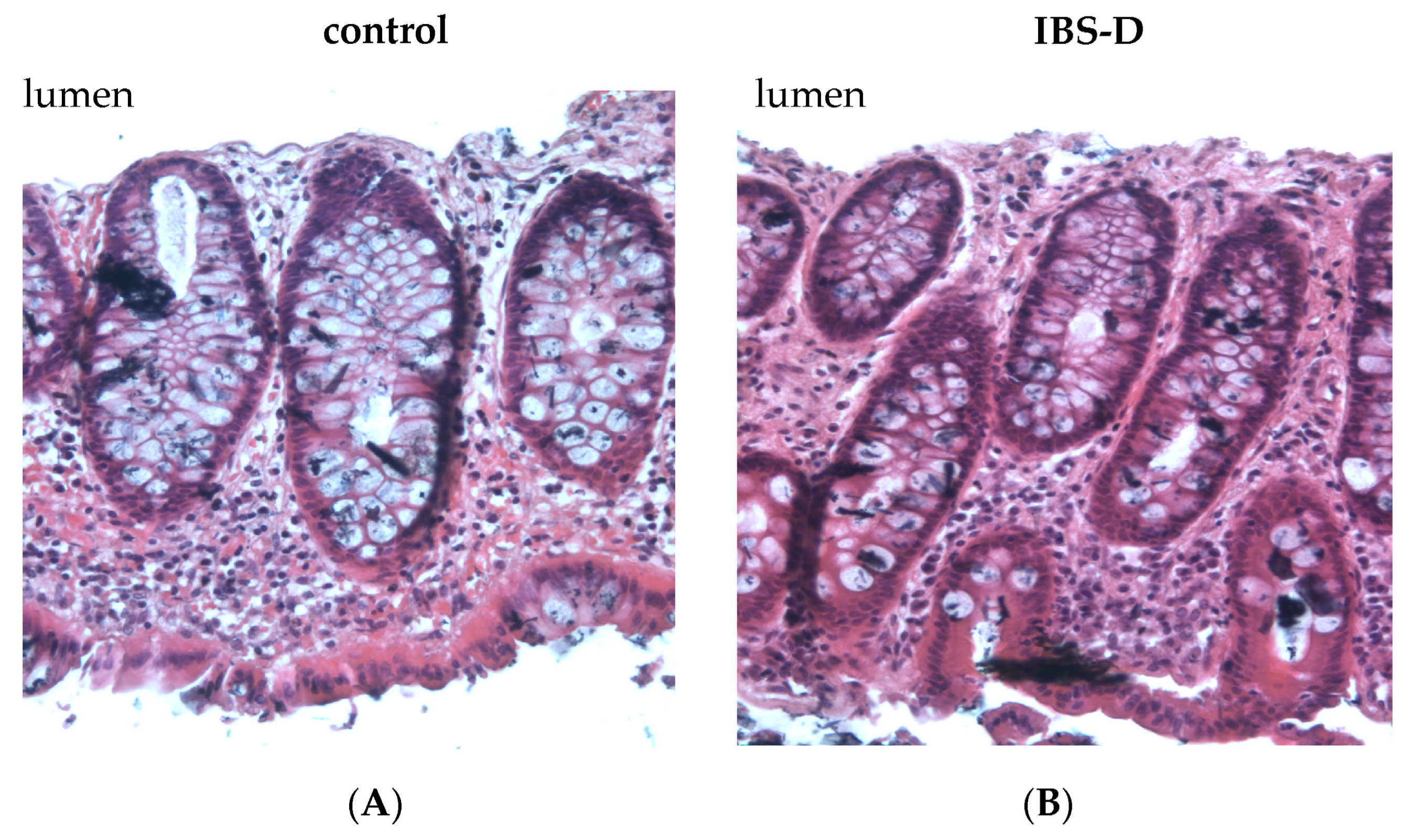
2.9. Morphometry of the Mucosal Surface Area
The calculation of mucosal surface area was performed in both individuals with IBS-D and control subjects, as the transport and barrier characteristics are known to be influenced by the mucosal architecture.
In this study, the tissues were promptly fixed with 4% formalin immediately following the Ussing experiments. Subsequently, the tissues were embedded in paraffin using the same degree of stretch as in the electrophysiological experiments.
H&E sections (hematoxylin and eosin) were prepared and examined with light microscopy in low-power fields (10× magnification). Image J was used to perform morphometry on digitally stored light micrographs. The inner crypt diameter in cross-sections of the tissues, crypt length and crypt density were all measured by morphometry.
2.10. TUNEL Assay
Apoptotic cells can be specifically stained by the terminal transferase dUTP nick end labeling (TUNEL) assay. Thew staining for apoptosis was performed as described previously. Briefly, biopsies fixed in 4% paraformaldehyde underwent morphological assessments. Paraffin-embedded specimens were serially sectioned. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL, In Situ Cell Death Detection Kit- Fluorescein, Roche, Mannheim, Germany) was used to label DNA fragments.
Highly condensed, highly fluorescent and multi-segmented nuclei were considered apoptotic. The apoptosis rate is equal to the percentage of apoptotic epithelial cells out of all epithelial cells visible in the field of view (~150 epithelial cells/field of view). The apoptosis rate was determined for each patient as a mean value from at least five different visual fields.
2.11. RNA Expression Analysis
We analyzed differential gene expression using RNA-seq followed by bioinformatics-based pathway analysis, as described previously [
24]. Biopsies of the sigmoid colon were processed with the mirVana RNA isolation reagent (Ambion, Life Technologies, Carlsbad, CA, USA) to isolate total RNA. RNA sequencing was performed using the TrueSeq Stranded Total RNA technique on an Illumina NovaSeq 6000 sequencing system with RNA quality values of 80%. The RNA sequences acquired from RNA-seq were aligned with the human genome GRCh38 release 97 and organized using the STAR aligner version 2.7.1a in a two-pass mode. The frames for first-pass read mapping were determined using the coordinates from Ensembl Annotation Release 97. The gene-count tables, which include gene-read coverages, were generated using the Counts Function of the Bioconductor package Rsubread. The Bioconductor package DESeq2 was applied to assess the differential expression of genes between two states, quantifying log2-fold changes and their respective
p-values. The pathway analysis and upstream regulator analyses were conducted using the Ingenuity Pathway Analysis program (IPA, Qiagen Silicon Valley, Redwood, CA, USA).
The raw unprocessed sequencing data, in the form of Fastq files, and a raw data matrix table were deposited in the European Genome-Phenome Archive (EGA) database under record number EGAD50000000063 (
https://ega-archive.org/datasets/EGAD50000000063, accessed on 10 November 2021).
2.12. Statistics
For statistical analysis, the GraphPad Prism software version 10.0 (GraphPad Software Inc., San Diego, CA, USA) was used. Data are expressed as mean value and standard error of the mean (SEM). The p-values were calculated using Student’s t-test. p < 0.05 was considered significant.
4. Discussion
Given the high heterogeneity of patients with IBS, we aimed for the most precise definition possible for our study group. Therefore, we specifically included only individuals diagnosed with IBS-D. As one of the main unsolved questions in IBS research, the pathogenesis of diarrhea in the IBS-D subgroup is not clearly understood. Therefore, in Part A of our study, we analyzed colon biopsies from IBS-D patients maintained functionally intact under in vitro conditions to distinguish the three main mechanisms of diarrhea, namely malabsorption, induction of active secretion and leak flux diarrhea secondary to barrier dysfunction. Thus, this is the first study, which carried out a systematic investigation of all possible mechanisms of diarrhea in the colonic mucosa and analyzed the expression of the strand-forming TJ proteins expressed in the colon.
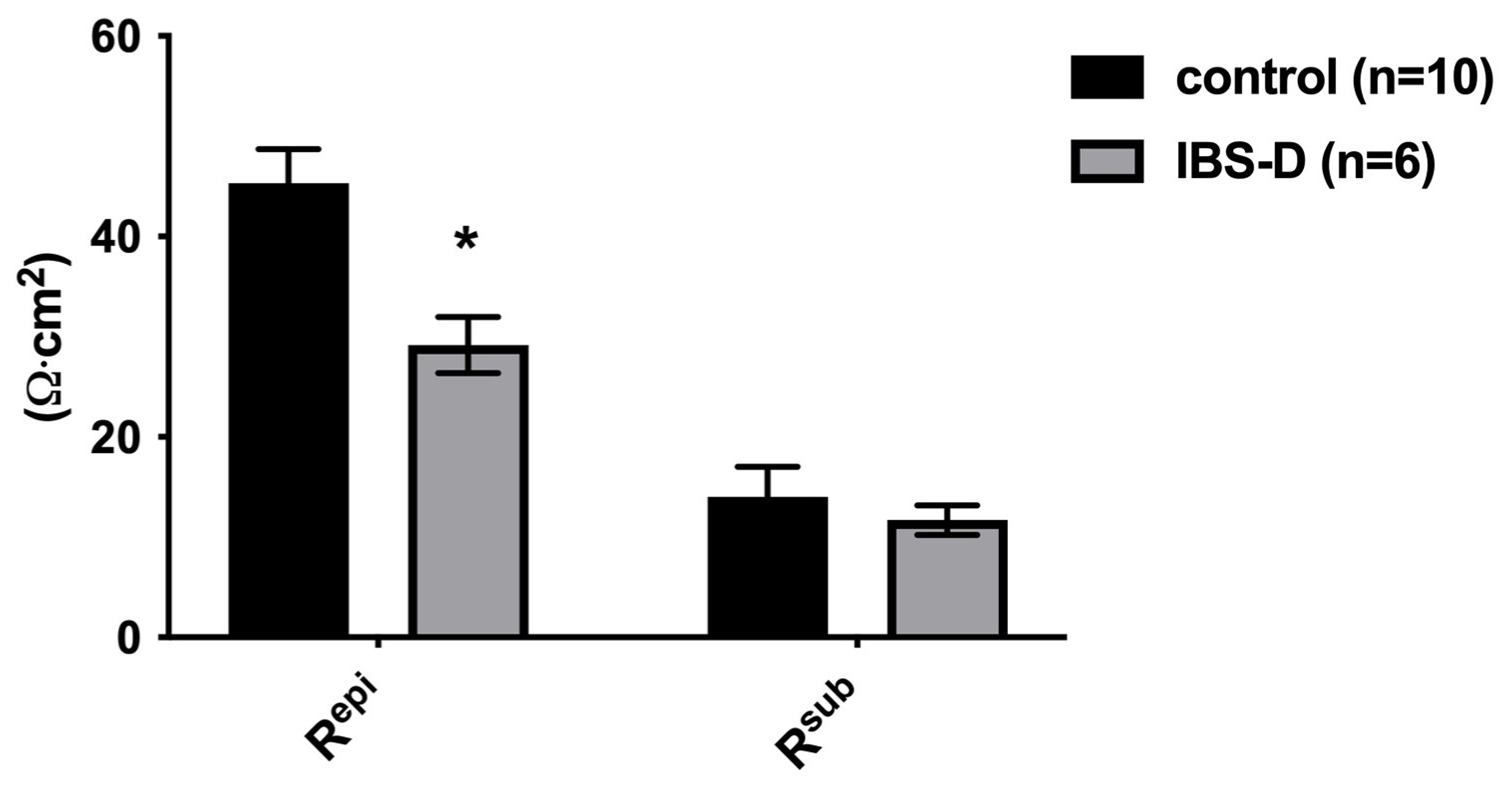
The first important result we found was evidence of a defect in the epithelial barrier in IBS-D, namely a reduced epithelial resistance in impedance spectroscopy. This reduction in epithelial resistance could be due to various factors, which may occur independently or in combination. These include changes in the structure of the TJs, in the epithelial apoptosis rate, altered mucosal architecture with altered mucosal surface geometry and the appearance of mucosal gross lesions, such as erosions. To determine the characteristics of the epithelial barrier defect in IBS-D, in Part B, we conducted a comprehensive experimental study on the structural aspects of the epithelial barrier in the large intestine.
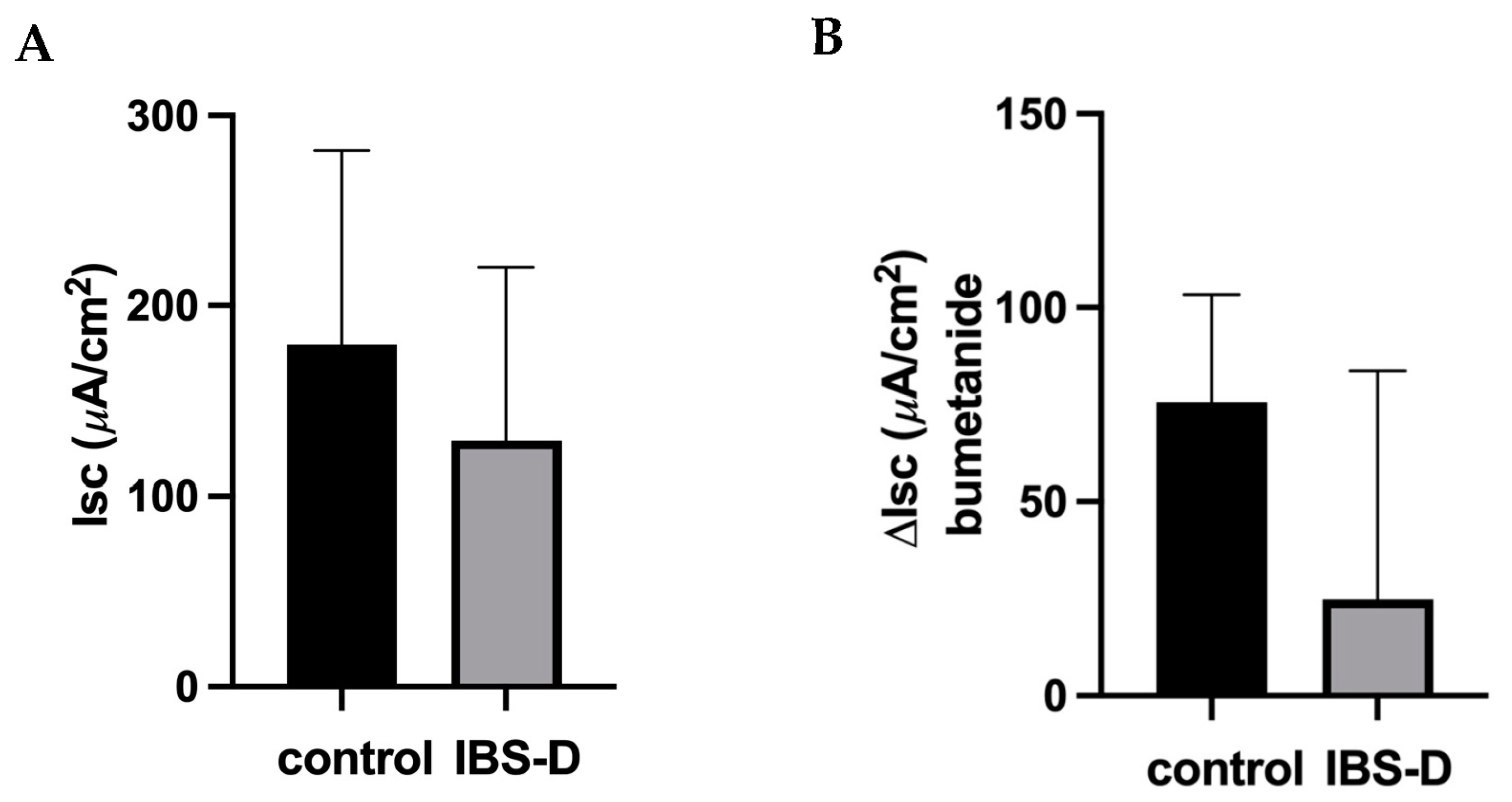
According to our results, diarrhea in IBS-D is not explained by the activation of active anion/chloride secretion, as neither the basal I
SC in IBS-D biopsy specimens nor the bumetanide-sensitive I
SC were altered. In support of this view, in our accompanying mRNA-seq analysis of these IBS-D biopsy samples, the expression of components of the active chloride secretion system (such as NKCC1, CLCA1 and CFTR) was not altered. In the past, we also checked the activation of chloride secretion in other groups of patients with different diseases using exactly the same measuring system in the same intestinal segment—the sigmoid colon. In another cohort of IBS-M patients, chloride secretion was found to be even reduced, consistent with the constipation phase of these patients [
24]. In contrast, we found that chloride secretion was activated in patients with collagenous colitis and diarrhea [
23], proving that our measuring system is capable of detecting active anion secretion states through I
SC measurements in miniaturized Ussing devices.
In addition to active ion secretion, diarrhea in IBS-D could also be due to a malabsorptive mechanism. In the distal colon and rectum, active sodium absorption via the ENaC is the main absorptive transport system. In one of our previous studies, we demonstrated that ENaC-dependent sodium absorption is significantly impaired in lymphocytic colitis [
25]. Therefore, we also tested this absorptive transport system in IBS-D. However, unlike in lymphocytic colitis, it was not reduced in IBS-D compared to controls. Unimpaired ENaC function in IBS-D is another important finding of our study, as impairment may promote diarrhea as a relevant pathomechanism in the large intestine, as it has been shown to be involved in patients with ulcerative colitis [
27] or campylobacteriosis [
28].
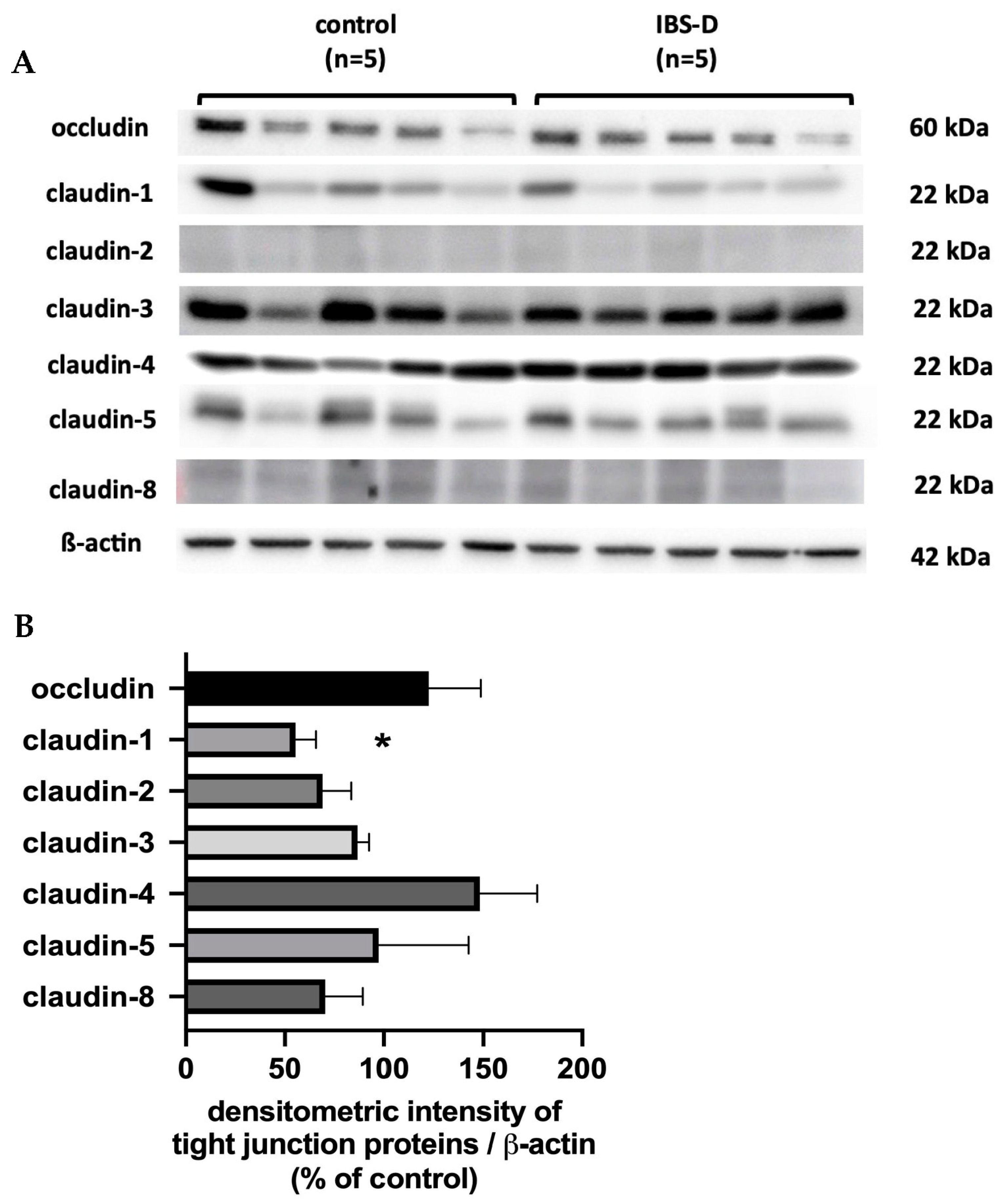
Since it turned out that neither the activation of active secretion nor malabsorption were involved in the diarrhea in IBS-D, a disturbed barrier function for electrolytes and water must be assumed to be the only and predominant pathomechanism of diarrhea. To investigate the structures responsible for this barrier dysfunction, we took a closer look at the epithelial TJs in IBS-D. For this purpose, strand-forming TJ proteins were examined. Based on the functional and expression data from the sigmoid colon, claudins -1, -2, -3, -4, -5 and -8, as well as occludin and tricellulin, are considered the key components of the TJ-determining epithelial barrier function in the colon. In our analysis, we found that only claudin-1 was decreased in IBS-D, while the other strand-forming claudins examined were not altered in their expression. However, the reduction in the barrier-forming TJ protein claudin-1 is certainly suitable to explain the decrease in epithelial barrier function in our analysis.
Martínez and co-workers, focusing on barrier properties in the small intestine, demonstrated increased protein expression of claudin-2, decreased occludin phosphorylation status and increased redistribution of occludin from the plasma membrane to the cytoplasm of enterocytes in IBS-D patients [
29]. Taken together, our results and the data of others seem to support the fundamental importance of claudin-1 in the pathogenesis of diarrhea in IBS-D.
To identify the possible regulatory influences on claudin-1 expression, claudin-1 mRNA levels were examined. However, an unchanged mRNA expression level rather suggests post-transcriptional mechanisms of regulation. A prominent candidate for this is increased expression of miRNAs targeting CLDN1, as identified by Zhou and co-workers for miRNA-29 [
30], or accelerated degradation through activation of proteases. The latter regulatory mechanism was proposed for occludin by Coëffier and co-workers, who showed increased proteasome-mediated degradation in IBS patients [
31].
The recognition and description of subtype-related pathological changes in IBS will make it possible in the future to individualize effective therapies depending on the subtype. For example, glutamine has been described to have a stimulatory effect on claudin-1 expression in the colon mucosa of patients with IBS-D [
32].
Contrary to the result of our recent study on IBS-M, which found that tricellulin was redistributed out of the tTJ as a structural correlate of the increased macromolecule permeability [
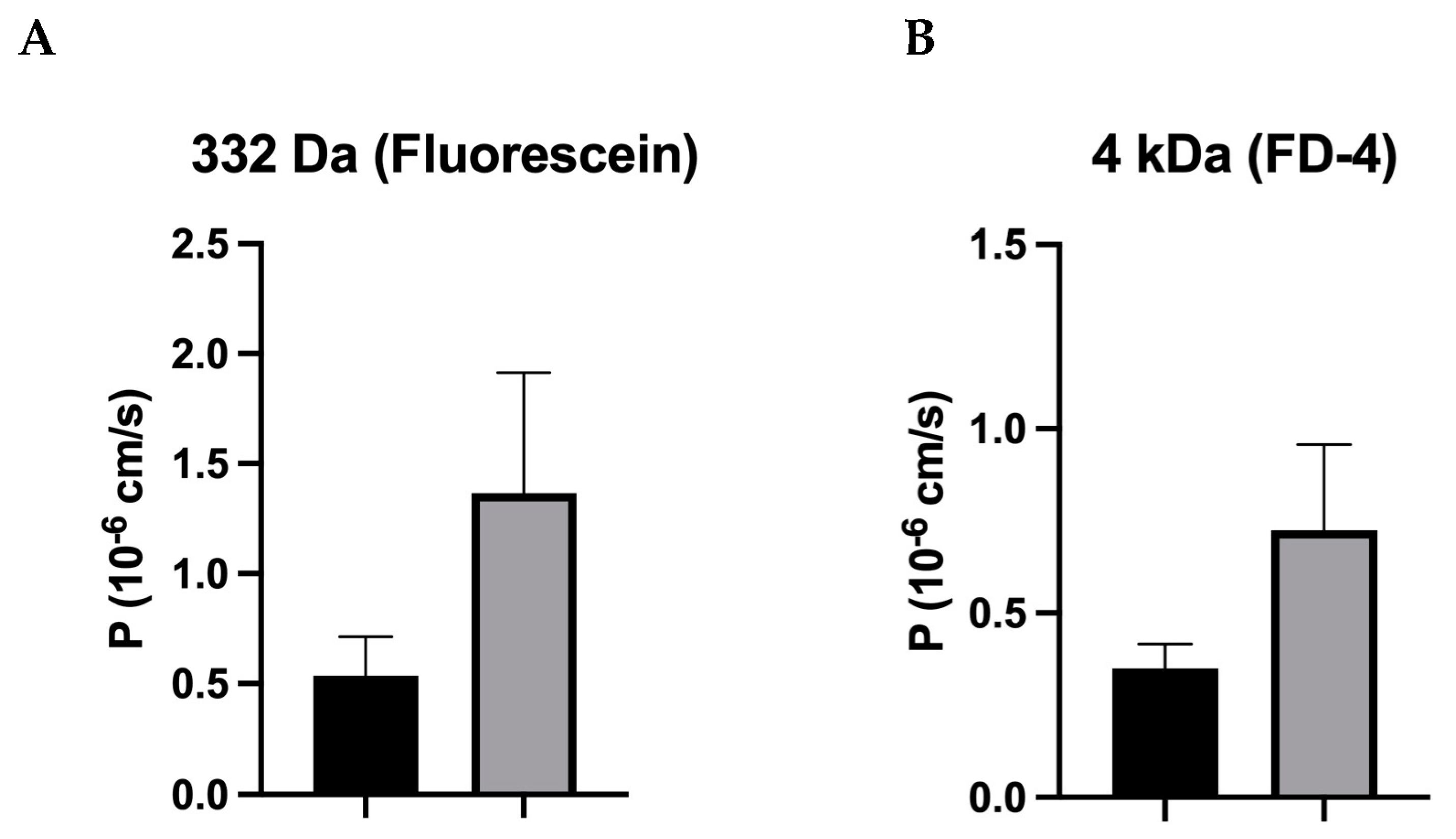
26], this was not observed in IBS-D. Furthermore, in our present study on IBS-D patients, the permeability of macromolecules only tended to be increased and did not reach statistical significance. The fact that there was only one trend without significance was mainly due to the large scattering of the 4 kDa dextran permeability data, which may be due to the heterogeneity in this IBS-D group. This means that at least some of the IBS-D patients could have increased paracellular macromolecular permeability and that other mechanisms of antigen uptake, such as transcytosis, must be assumed for the other patients (see below).
At least in our RNA-seq, we obtained evidence of persistent low-grade immune activation in the IBS-D mucosa. This means that an activating influence on the mucosal immune system can be assumed, which supports the assumption of a resulting impaired epithelial barrier in IBS-D patients, even if this activating influence is only weak and has a rather long-term functional effect. The trigger for such immune activation could be endotoxins, in particular lipopolysaccharides or lipo-oligosaccharides (LPS or LOS) [
28]. If this sub-inflammatory immune activation weakens the barrier integrity of the mucosa, this would lead to a vicious cycle of barrier loss and subepithelial immune activation (along the
leaky gut concept). This interpretation is also supported by the fact that the cytokine profile determined by Upstream regulator analysis in IBS-D included barrier-impairing cytokines and was similar to the cytokine profile previously observed in IBS-M [
24], in which a macromolecule permeability increase was measurable.
A particularly noteworthy finding in our present IBS-D study was increased claudin-4 mRNA level, which could be interpreted as a counter-regulatory signal and response to the downregulation of claudin-1 with the aim of maintaining barrier integrity. However, the role of claudin-4 in the pathogenesis of IBS-D is still unclear and requires further investigation. The identification of individual cytokines responsible for barrier dysfunction in IBS-D will enable new therapeutic options. In this way, Huang and co-workers were able to identify a herbal mixture called “QingHuaZhiXie”, which suppressed inflammatory factors and TLR4/MyD88/NF-κB pathway proteins, ultimately leading to increased protein levels of occludin and claudin-1 [
33].
In addition to the cytokines (TNF-α, IFN-γ) and LPS in IBS-D, which also play a dominant role in IBS-M, we found in our IPA analysis of RNA-seq data of IBS-D patients that IL-4 is also an upstream regulator. Interestingly, in this context, a recent study from our laboratory found increased transcytotic macromolecule uptake (measured as temperature-dependent horseradish peroxidase flux (HRP, 44 kDa) at 37 °C) in patients with post-infectious irritable bowel syndrome (PI-IBS), resulting in an immune activation [
34]. This transcytotic uptake was primarily IL-4-mediated [
34]. As already mentioned above, a
leaky gut component could also be observed in IBS-D, but only in some of the patients, associated with an increased macromolecule permeability to 4 kDa dextran. It is quite possible that in another subset of our IBS-D patients, epithelial transcytosis of antigens could have a functional significance instead. Moreover, the cytokine profiles found here—with cytokines, which influence the epithelium toward the
leaky gut, such as IL-4 or IL-13, which are similarly activated in infections such as
Campylobacter jejuni [
28]—may also lead to low-grade inflammatory smoldering in IBS as a consequence of previous infectious diarrheal episodes. However, these hypotheses have yet to be confirmed experimentally.
A critical limitation of our present work is that the biopsy samples were collected after an overnight fasting period. Therefore, we cannot exclude with certainty that the stimulation effects from foods, to which IBS patients may react more sensitive than control subjects, have already subsided and were therefore no longer detectable in our Ussing chamber experiment. Thus, we cannot rule out that such stimulatory influence on intestinal secretion is of importance in this context. To exclude this possibility with certainty, transport measurements with a very complex in vivo perfusion system would have to be carried out in these patients.