Regulatory TR3-56 Cells in the Complex Panorama of Immune Activation and Regulation
Abstract
1. Introduction
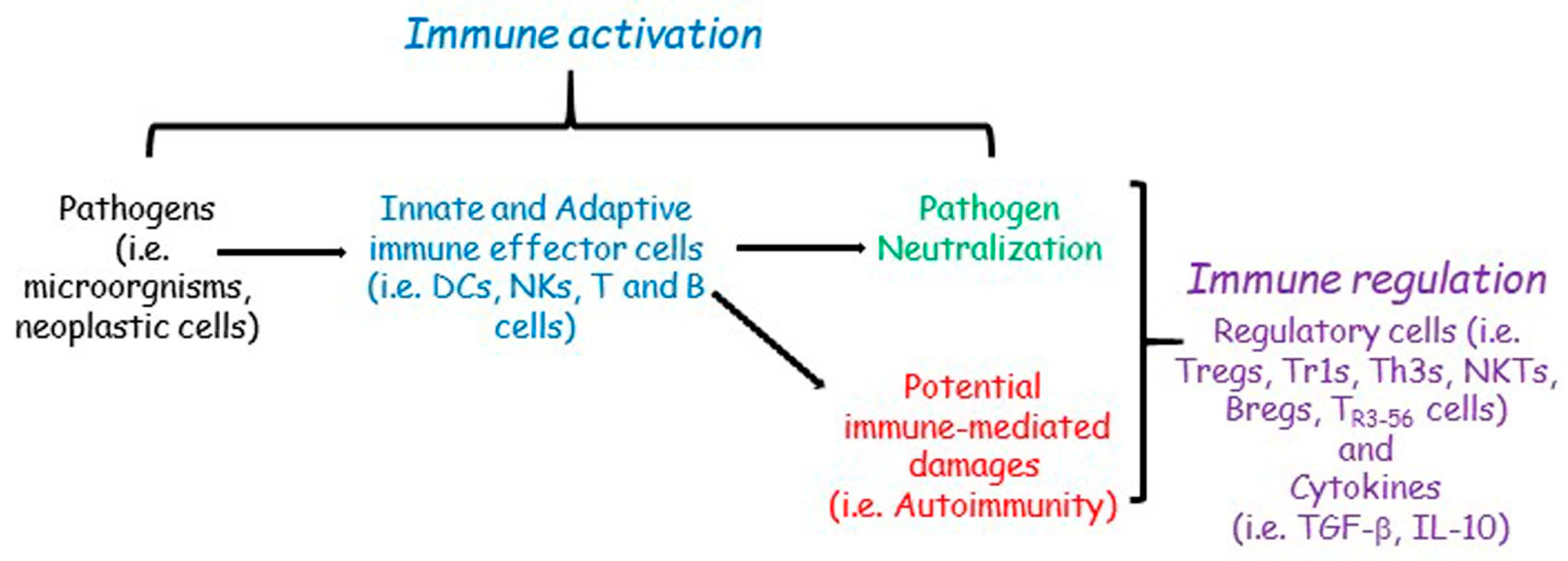
2. The Immune Regulation
2.1. The Interplay between Immune Activation and Regulation
2.2. The Main Features of Immune Regulation: Aspects, Molecules, and Cells
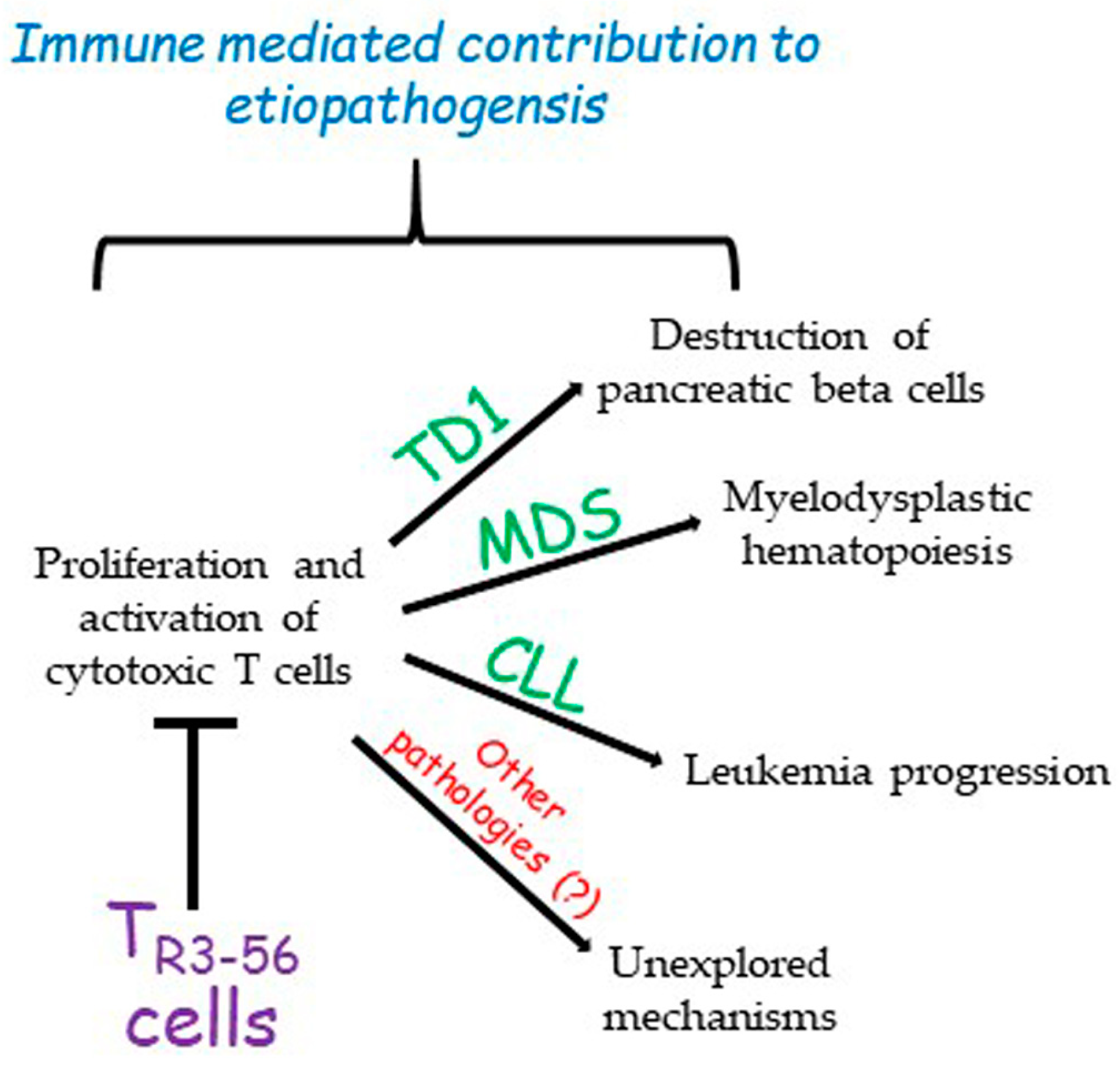
3. A New Cell Candidate for Immune Regulation: The TR3-56
4. Previous Observations on CD3+CD56+ Co-Expressing T Cells in Cancer Immune Surveillance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Delves, P.J.; Roitt, I.M. The immune system. First of two parts. N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E. Infectious diseases and the immune system. Sci. Am. 1993, 269, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Malissen, B. Innate and adaptive immunity: Specificities and signaling hierarchies revisited. Nat. Immunol. 2005, 6, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2015, 109, 11–14. [Google Scholar] [CrossRef]
- Riera Romo, M.; Pérez-Martínez, D.; Castillo Ferrer, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Freud, A.G.; Caligiuri, M.A. Human natural killer cell development. Immunol. Rev. 2006, 214, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Bak, S.H.; Park, T.; Kim, J.W.; Yoon, S.R.; Jung, H.; Noh, J.Y. Understanding NK cell biology for harnessing NK cell therapies: Targeting cancer and beyond. Front. Immunol. 2023, 14, 1192907. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Schlitzer, A.; Placek, K.; Joosten, L.A.B.; Schultze, J.L. Innate and Adaptive Immune Memory: An Evolutionary Continuum in the Host’s Response to Pathogens. Cell Host Microbe 2019, 25, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Cronkite, D.A.; Strutt, T.M. The Regulation of Inflammation by Innate and Adaptive Lymphocytes. J. Immunol. Res. 2018, 2018, 1467538. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Innate lymphoid cell and adaptive immune cell cross-talk: A talk meant not to forget. J. Leukoc. Biol. 2020, 108, 397–417. [Google Scholar] [CrossRef]
- Zinkernagel, R.M. On differences between immunity and immunological memory. Curr. Opin. Immunol. 2002, 14, 523–536. [Google Scholar] [CrossRef]
- Lau, C.M.; Sun, J.C. The widening spectrum of immunological memory. Curr. Opin. Immunol. 2018, 54, 42–49. [Google Scholar] [CrossRef]
- Cyster, J.G.; Allen, C.D.C. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Zielinski, C.E. T helper cell subsets: Diversification of the field. Eur. J. Immunol. 2023, e2250218. [Google Scholar] [CrossRef]
- Barry, M.; Bleackley, R. Cytotoxic T lymphocytes: All roads lead to death. Nat. Rev. Immunol. 2002, 2, 401–409. [Google Scholar] [CrossRef]
- Grover, P.; Goel, P.N.; Greene, M.I. Regulatory T Cells: Regulation of Identity and Function. Front. Immunol. 2021, 12, 750542. [Google Scholar] [CrossRef]
- Chang, J.; Wherry, E.; Goldrath, A. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014, 15, 1104–1115. [Google Scholar] [CrossRef]
- Pelanda, R.; Piccirillo, C.A. Tolerance, immune regulation, and autoimmunity: Cells and cytokines that make a difference. Curr. Opin. Immunol. 2008, 20, 629–631. [Google Scholar] [CrossRef][Green Version]
- Devenish, L.P.; Mhlanga, M.M.; Negishi, Y. Immune Regulation in Time and Space: The Role of Local- and Long-Range Genomic Interactions in Regulating Immune Responses. Front. Immunol. 2021, 12, 662565. [Google Scholar] [CrossRef]
- Sakaguchi, S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004, 22, 531–562. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef]
- Davidson, A.; Diamond, B. Autoimmune diseases. N. Engl. J. Med. 2001, 345, 340–350. [Google Scholar] [CrossRef]
- McInnes, I.B.; Gravallese, E.M. Immune-mediated inflammatory disease therapeutics: Past, present and future. Nat. Rev. Immunol. 2021, 21, 680–686. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumors: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef]
- Matzinger, P. The evolution of the danger theory. Interview by Lauren Constable, Commissioning Editor. Expert Rev. Clin. Immunol. 2012, 8, 311–317. [Google Scholar] [CrossRef]
- Kivity, S.; Agmon-Levin, N.; Blank, M.; Shoenfeld, Y. Infections and autoimmunity-friends or foes? Trends Immunol. 2009, 30, 409–414. [Google Scholar] [CrossRef]
- Sundaresan, B.; Shirafkan, F.; Ripperger, K.; Rattay, K. The Role of Viral Infections in the Onset of Autoimmune Diseases. Viruses 2023, 15, 782. [Google Scholar] [CrossRef]
- Rijkers, G.T.; Riccardi, C.; Kroese, F.G.M. Editorial: Defects in Regulation: How, Where and When the Immune System Can Go Wrong. Front. Immunol. 2021, 12, 746418. [Google Scholar] [CrossRef]
- Van Parijs, L.; Abbas, A.K. Homeostasis and self-tolerance in the immune system: Turning lymphocytes off. Science 1998, 280, 243–248. [Google Scholar] [CrossRef]
- Chovatiya, R.; Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell. 2014, 54, 281–288. [Google Scholar] [CrossRef]
- Huntington, N.D.; Gray, D.H. Immune homeostasis in health and disease. Immunol. Cell Biol. 2018, 96, 451–452. [Google Scholar] [CrossRef]
- Laurent, P.; Jolivel, V.; Manicki, P.; Chiu, L.; Contin-Bordes, C.; Truchetet, M.E.; Pradeu, T. Immune-Mediated Repair: A Matter of Plasticity. Front. Immunol. 2017, 8, 454. [Google Scholar] [CrossRef]
- Margraf, A.; Perretti, M. Immune Cell Plasticity in Inflammation: Insights into Description and Regulation of Immune Cell Phenotypes. Cells 2022, 11, 1824. [Google Scholar] [CrossRef]
- Tuano, K.S.; Seth, N.; Chinen, J. Secondary immunodeficiencies: An overview. Ann. Allergy Asthma Immunol. 2021, 127, 617–626. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Schwartz, R.H. Historical overview of immunological tolerance. Cold Spring Harb. Perspect. Biol. 2012, 4, a006908. [Google Scholar] [CrossRef]
- Burnet, F.M. A modification of Jerne’s theory of antibody production using the concept of clonal selection. CA Cancer J. Clin. 1976, 26, 119–121. [Google Scholar] [CrossRef]
- Tiegs, S.L.; Russell, D.M.; Nemazee, D. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 1993, 177, 1009–1020. [Google Scholar] [CrossRef]
- Kappler, J.W.; Roehm, N.; Marrack, P. T cell tolerance by clonal elimination in the thymus. Cell 1987, 49, 273–280. [Google Scholar] [CrossRef]
- Surh, C.D.; Sprent, J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature 1994, 372, 100–103. [Google Scholar] [CrossRef]
- Nishizuka, Y.; Sakakura, T. Thymus and reproduction: Sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science 1969, 166, 753–755. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Azimnasab-Sorkhabi, P.; Soltani-Asl, M.; Kfoury Junior, J.R. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) as an undetermined tool in tumor cells. Hum. Cell. 2023, 36, 1225–1232. [Google Scholar] [CrossRef]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune checkpoint therapy-current perspectives and future directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef]
- Galgani, M.; De Rosa, V.; La Cava, A.; Matarese, G. Role of Metabolism in the Immunobiology of Regulatory T Cells. J. Immunol. 2016, 197, 2567–2675. [Google Scholar] [CrossRef] [PubMed]
- Panduro, M.; Benoist, C.; Mathis, D. Tissue Tregs. Annu. Rev. Immunol. 2016, 34, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Corthay, A. How do regulatory T cells work? Scand. J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Giganti, G.; Atif, M.; Mohseni, Y.; Mastronicola, D.; Grageda, N.; Povoleri, G.A.; Miyara, M.; Scottà, C. Treg cell therapy: How cell heterogeneity can make the difference. Eur. J. Immunol. 2021, 51, 39–55. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, S.I.; Pernis, B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science 1992, 256, 1213–1215. [Google Scholar] [CrossRef]
- Li, S.; Xie, Q.; Zeng, Y.; Zou, C.; Liu, X.; Wu, S.; Deng, H.; Xu, Y.; Li, X.C.; Dai, Z. A naturally occurring CD8(+)CD122(+) T-cell subset as a memory-like Treg family. Cell Mol. Immunol. 2014, 11, 326–331. [Google Scholar] [CrossRef]
- Mishra, S.; Srinivasan, S.; Ma, C.; Zhang, N. CD8+ Regulatory T Cell—A Mystery to Be Revealed. Front. Immunol. 2021, 12, 708874. [Google Scholar] [CrossRef]
- Groux, H.; O’Garra, A.; Bigler, M.; Rouleau, M.; Antonenko, S.; de Vries, J.E.; Roncarolo, M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997, 389, 737–742. [Google Scholar] [CrossRef]
- Battaglia, M.; Gregori, S.; Bacchetta, R.; Roncarolo, M.G. Tr1 cells: From discovery to their clinical application. Semin. Immunol. 2006, 18, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G.; Gregori, S.; Bacchetta, R.; Battaglia, M.; Gagliani, N. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity 2018, 49, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Terada, T.; Kitatani, K.; Kawata, R.; Nabe, T. Roles of type 1 regulatory T (Tr1) cells in allergen-specific immunotherapy. Front. Allergy 2022, 3, 981126. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L. Oral tolerance: Immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001, 3, 947–954. [Google Scholar] [CrossRef]
- Weiner, H.L. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 2001, 182, 207–214. [Google Scholar] [CrossRef]
- Horwitz, D.A.; Zheng, S.G.; Gray, J.D. The role of the combination of IL-2 and TGF-beta or IL-10 in the generation and function of CD4+ CD25+ and CD8+ regulatory T cell subsets. J. Leukoc. Biol. 2003, 74, 471–478. [Google Scholar] [CrossRef]
- Makino, Y.; Kanno, R.; Ito, T.; Higashino, K.; Taniguchi, M. Predominant expression of invariant V alpha 14+ TCR alpha chain in NK1.1+ T cell populations. Int. Immunol. 1995, 7, 1157–1161. [Google Scholar] [CrossRef]
- Hammond, K.J.; Pelikan, S.B.; Crowe, N.Y.; Randle-Barrett, E.; Nakayama, T.; Taniguchi, M.; Smyth, M.J.; van Driel, I.R.; Scollay, R.; Baxter, A.G.; et al. NKT cells are phenotypically and functionally diverse. Eur. J. Immunol. 1999, 29, 3768–3781. [Google Scholar] [CrossRef]
- Godfrey, D.I.; MacDonald, H.R.; Kronenberg, M.; Smyth, M.J.; Van Kaer, L. NKT cells: What’s in a name? Nat. Rev. Immunol. 2004, 4, 231–237. [Google Scholar] [CrossRef]
- Gadola, S.D.; Dulphy, N.; Salio, M.; Cerundolo, V. Valpha24-JalphaQ-independent, CD1d-restricted recognition of alpha-galactosylceramide by human CD4(+) and CD8alphabeta(+) T lymphocytes. J. Immunol. 2002, 168, 5514–5520. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.; Mattner, J. NKT Cells Contribute to the Control of Microbial Infections. Front. Cell Infect. Microbiol. 2021, 11, 718350. [Google Scholar] [CrossRef] [PubMed]
- McEwen-Smith, R.M.; Salio, M.; Cerundolo, V. The regulatory role of invariant NKT cells in tumor immunity. Cancer Immunol. Res. 2015, 3, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Kriegsmann, K.; Kriegsmann, M.; von Bergwelt-Baildon, M.; Cremer, M.; Witzens-Harig, M. NKT cells—New players in CAR cell immunotherapy? Eur. J. Haematol. 2018, 101, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L.; Wu, L. Therapeutic Potential of Invariant Natural Killer T Cells in Autoimmunity. Front. Immunol. 2018, 9, 519. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Catalán, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef]
- Dasgupta, S.; Dasgupta, S.; Bandyopadhyay, M. Regulatory B cells in infection, inflammation, and autoimmunity. Cell Immunol. 2020, 352, 104076. [Google Scholar] [CrossRef]
- Quatrini, L.; Della Chiesa, M.; Sivori, S.; Mingari, M.C.; Pende, D.; Moretta, L. Human NK cells, their receptors and function. Eur. J. Immunol. 2021, 51, 1566–1579. [Google Scholar] [CrossRef]
- Sivori, S.; Della Chiesa, M.; Carlomagno, S.; Quatrini, L.; Munari, E.; Vacca, P.; Tumino, N.; Mariotti, F.R.; Mingari, M.C.; Pende, D.; et al. Inhibitory Receptors and Checkpoints in Human NK Cells, Implications for the Immunotherapy of Cancer. Front. Immunol. 2020, 11, 2156. [Google Scholar] [CrossRef]
- Kucuksezer, U.C.; Aktas Cetin, E.; Esen, F.; Tahrali, I.; Akdeniz, N.; Gelmez, M.Y.; Deniz, G. The Role of Natural Killer Cells in Autoimmune Diseases. Front. Immunol. 2021, 12, 622306. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Qualls, A.E.; Marques-Fernandez, L.; Colucci, F. Biology and pathology of the uterine microenvironment and its natural killer cells. Cell Mol. Immunol. 2021, 18, 2101–2113. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, H.G.; Kärre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Terrazzano, G.; Ruggiero, G.; Zanzi, D.; Ottaiano, A.; Manzo, C.; Kärre, K.; Zappacosta, S. Recognition of autologous dendritic cells by human NK cells. Eur. J. Immunol. 1999, 29, 4022–4429. [Google Scholar] [CrossRef]
- Terrazzano, G.; Pisanti, S.; Grimaldi, S.; Sica, M.; Fontana, S.; Carbone, E.; Zappacosta, S.; Ruggiero, G. Interaction between natural killer and dendritic cells: The role of CD40, CD80 and major histocompatibility complex class i molecules in cytotoxicity induction and interferon-gamma production. Scand. J. Immunol. 2004, 59, 356–362. [Google Scholar] [CrossRef]
- Terrazzano, G.; Sica, M.; Gianfrani, C.; Mazzarella, G.; Maurano, F.; De Giulio, B.; de Saint-Mezard, S.; Zanzi, D.; Maiuri, L.; Londei, M.; et al. Gliadin regulates the NK-dendritic cell cross-talk by HLA-E surface stabilization. J. Immunol. 2007, 179, 372–381. [Google Scholar] [CrossRef]
- Ruggiero, G.; Sica, M.; Luciano, L.; Savoia, F.; Cosentini, E.; Alfinito, F.; Terrazzano, G. A case of myelodysplastic syndrome associated with CD14(+)CD56(+) monocytosis, expansion of NK lymphocytes and defect of HLA-E expression. Leuk. Res. 2009, 33, 181–185. [Google Scholar] [CrossRef]
- Pedroza-Pacheco, I.; Madrigal, A.; Saudemont, A. Interaction between natural killer cells and regulatory T cells: Perspectives for immunotherapy. Cell Mol. Immunol. 2013, 10, 222–229. [Google Scholar] [CrossRef]
- Bozward, A.G.; Warricker, F.; Oo, Y.H.; Khakoo, S.I. Natural Killer Cells and Regulatory T Cells Cross Talk in Hepatocellular Carcinoma: Exploring Therapeutic Options for the Next Decade. Front. Immunol. 2021, 12, 643310. [Google Scholar] [CrossRef]
- Saito, H.; Kranz, D.M.; Takagaki, Y.; Hayday, A.C.; Eisen, H.N.; Tonegawa, S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature 1984, 312, 36–40. [Google Scholar] [CrossRef]
- Ribot, J.C.; Lopes, N.; Silva-Santos, B. γδ T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 2021, 21, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Mensurado, S.; Blanco-Domínguez, R.; Silva-Santos, B. The emerging roles of γδ T cells in cancer immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, H.; Yang, X.; Hu, H.; Liu, P.; Liu, H. Crosstalk between dendritic cells and regulatory T cells: Protective effect and therapeutic potential in multiple sclerosis. Front. Immunol. 2022, 13, 970508. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Hawiger, D.; Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003, 21, 685–711. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target Ther. 2023, 8, 207–242. [Google Scholar] [CrossRef]
- Mantovani, A. The growing diversity and spectrum of action of myeloid-derived suppressor cells. Eur. J. Immunol. 2010, 40, 3317–3320. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Lehuen, A.; Diana, J.; Zaccone, P.; Cooke, A. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 2010, 10, 501–513. [Google Scholar] [CrossRef]
- Ochsner, S.A.; Pillich, R.T.; Rawool, D.; Grethe, J.S.; McKenna, N.J. Transcriptional regulatory networks of circulating immune cells in type 1 diabetes: A community knowledgebase. iScience 2022, 25, 104581. [Google Scholar] [CrossRef]
- Terrazzano, G.; Bruzzaniti, S.; Rubino, V.; Santopaolo, M.; Palatucci, A.T.; Giovazzino, A.; La Rocca, C.; de Candia, P.; Puca, A.; Perna, F.; et al. T1D progression is associated with loss of CD3+CD56+ regulatory T cells that control CD8+ T cell effector functions. Nat. Metab. 2020, 2, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.; Rubino, V.; Palatucci, A.T.; Giovazzino, A.; Carriero, F.; Cerciello, G.; Pane, F.; Ruggiero, G.; Terrazzano, G. Bone marrow CD3+CD56+ regulatory T lymphocytes (TR3-56 cells) are inversely associated with activation and expansion of bone marrow cytotoxic T cells in IPSS-R very-low/low risk MDS patients. Eur. J. Haematol. 2022, 109, 398–405. [Google Scholar] [CrossRef]
- Rubino, V.; Leone, S.; Carriero, F.; Pane, F.; Ruggiero, G.; Terrazzano, G. The potential etiopathogenetic role and diagnostic utility of CD3+CD56+ regulatory T lymphocytes in Myelodysplastic Syndromes. Eur. J. Haematol. 2023, 110, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Giovazzino, A.; Leone, S.; Rubino, V.; Palatucci, A.T.; Cerciello, G.; Alfinito, F.; Pane, F.; Ruggiero, G.; Terrazzano, G. Reduced regulatory T cells (Treg) in bone marrow preferentially associate with the expansion of cytotoxic T lymphocytes in low risk MDS patients. Br. J. Haematol. 2019, 185, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Rubino, V.; Carriero, F.; Palatucci, A.T.; Giovazzino, A.; Leone, S.; Nicolella, V.; Calabrò, M.; Montanaro, R.; Brancaleone, V.; Pane, F.; et al. Adaptive and Innate Cytotoxic Effectors in Chronic Lymphocytic Leukaemia (CLL) Subjects with Stable Disease. Int. J. Mol Sci. 2023, 24, 9596. [Google Scholar] [CrossRef] [PubMed]
- Akbari, C.M.; Saouaf, R.; Barnhill, D.F.; Newman, P.A.; LoGerfo, F.W.; Veves, A. Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J. Vasc. Surg. 1998, 28, 687–694. [Google Scholar] [CrossRef]
- Williams, S.B.; Goldfine, A.B.; Timimi, F.K.; Ting, H.H.; Roddy, M.A.; Simonson, D.C.; Creager, M.A. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation 1998, 97, 1695–1701. [Google Scholar] [CrossRef]
- Mannucci, E.; Dicembrini, I.; Lauria, A.; Pozzilli, P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care 2013, 36 (Suppl. 2), 259–263. [Google Scholar] [CrossRef]
- Zdrazilova-Dubska, L.; Valik, D.; Budinska, E.; Frgala, T.; Bacikova, L.; Demlova, R. NKT-like cells are expanded in solid tumor patients. Klin. Onkol. 2012, 25 (Suppl. 2), 2S21–2S25. [Google Scholar]
- Wang, H.; Yang, D.; Xu, W.; Wang, Y.; Ruan, Z.; Zhao, T.; Han, J.; Wu, Y. Tumor-derived soluble MICs impair CD3(+)CD56(+) NKT-like cell cytotoxicity in cancer patients. Immunol. Lett. 2008, 120, 65–71. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, X.; Wang, Z.; Wang, J.; Sun, H.; Hu, Y. High circulating CD3+CD56+CD16+ natural killer-like T cell levels predict a better IVF treatment outcome. J. Reprod. Immunol. 2013, 97, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Shimizu, Y.; Tsuneyama, K.; Sugiyama, T. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2009, 21, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Atanackovic, D.; Nowottne, U.; Freier, E.; Weber, C.S.; Meyer, S.; Bartels, K.; Hildebrandt, Y.; Cao, Y.; Kröger, N.; Brunner-Weinzierl, M.C.; et al. Acute psychological stress increases peripheral blood CD3+CD56+ natural killer T cells in healthy men: Possible implications for the development and treatment of allergic and autoimmune disorders. Stress 2013, 16, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xing, C.; Dong, A.; Lin, X.; Lin, Y.; Zhu, B.; He, M.; Yao, R. Numbers and cytotoxicities of CD3+CD56+ T lymphocytes in peripheral blood of patients with acute myeloid leukemia and acute lymphocytic leukemia. Cancer Biol. Ther. 2013, 14, 916–921. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rohon, P.; Porkka, K.; Mustjoki, S. Immunoprofiling of patients with chronic myeloid leukemia at diagnosis and during tyrosine kinase inhibitor therapy. Eur. J. Haematol. 2010, 85, 387–398. [Google Scholar] [CrossRef]
- Almeida, J.S.; Couceiro, P.; López-Sejas, N.; Alves, V.; Růžičková, L.; Tarazona, R.; Solana, R.; Freitas-Tavares, P.; Santos-Rosa, M.; Rodrigues-Santos, P. NKT-Like (CD3+CD56+) Cells in Chronic Myeloid Leukemia Patients Treated with Tyrosine Kinase Inhibitors. Front. Immunol. 2019, 10, 2493. [Google Scholar] [CrossRef]
- Gibson, S.E.; Swerdlow, S.H.; Felgar, R.E. Natural killer cell subsets and natural killer-like T-cell populations in benign and neoplastic B-cell proliferations vary based on clinicopathologic features. Hum. Pathol. 2011, 42, 679–687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carriero, F.; Rubino, V.; Leone, S.; Montanaro, R.; Brancaleone, V.; Ruggiero, G.; Terrazzano, G. Regulatory TR3-56 Cells in the Complex Panorama of Immune Activation and Regulation. Cells 2023, 12, 2841. https://doi.org/10.3390/cells12242841
Carriero F, Rubino V, Leone S, Montanaro R, Brancaleone V, Ruggiero G, Terrazzano G. Regulatory TR3-56 Cells in the Complex Panorama of Immune Activation and Regulation. Cells. 2023; 12(24):2841. https://doi.org/10.3390/cells12242841
Chicago/Turabian StyleCarriero, Flavia, Valentina Rubino, Stefania Leone, Rosangela Montanaro, Vincenzo Brancaleone, Giuseppina Ruggiero, and Giuseppe Terrazzano. 2023. "Regulatory TR3-56 Cells in the Complex Panorama of Immune Activation and Regulation" Cells 12, no. 24: 2841. https://doi.org/10.3390/cells12242841
APA StyleCarriero, F., Rubino, V., Leone, S., Montanaro, R., Brancaleone, V., Ruggiero, G., & Terrazzano, G. (2023). Regulatory TR3-56 Cells in the Complex Panorama of Immune Activation and Regulation. Cells, 12(24), 2841. https://doi.org/10.3390/cells12242841







