Thermosensation and Temperature Preference: From Molecules to Neuronal Circuits in Drosophila
Abstract
1. Introduction
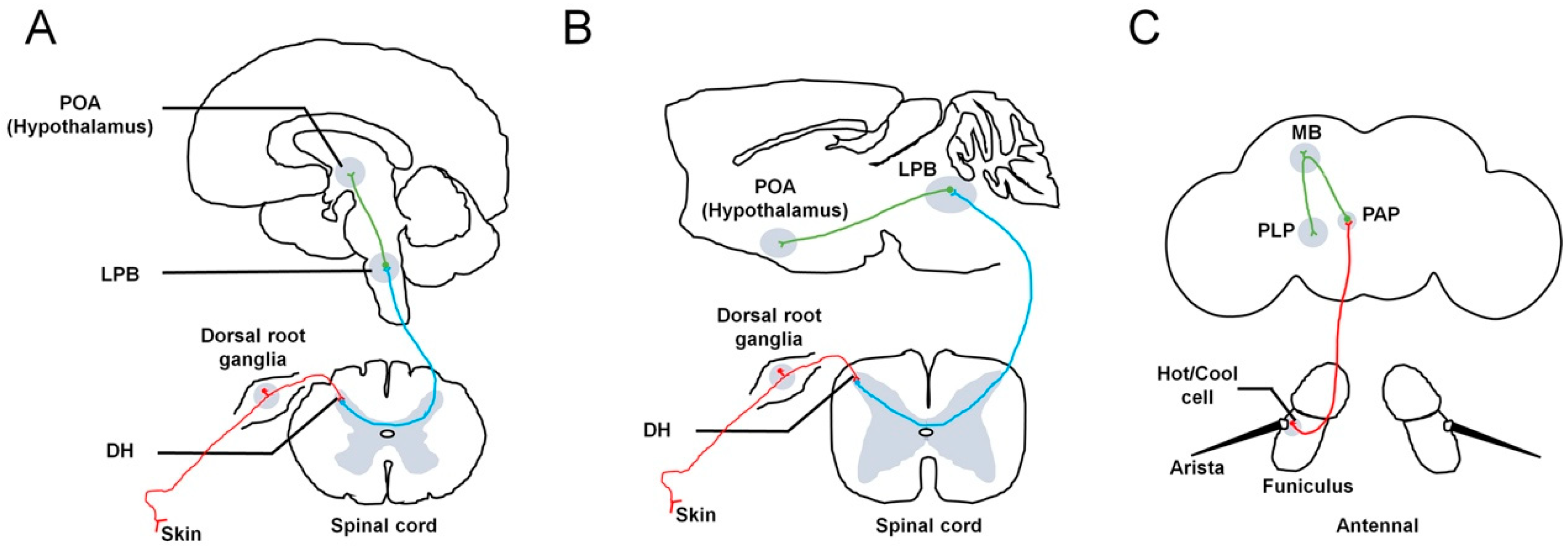
2. Thermosensation in Drosophila
2.1. The Molecular Basis of Thermosensation
2.2. The Neuronal Basis of Thermosensation
3. Temperature-Related Behavior in Drosophila
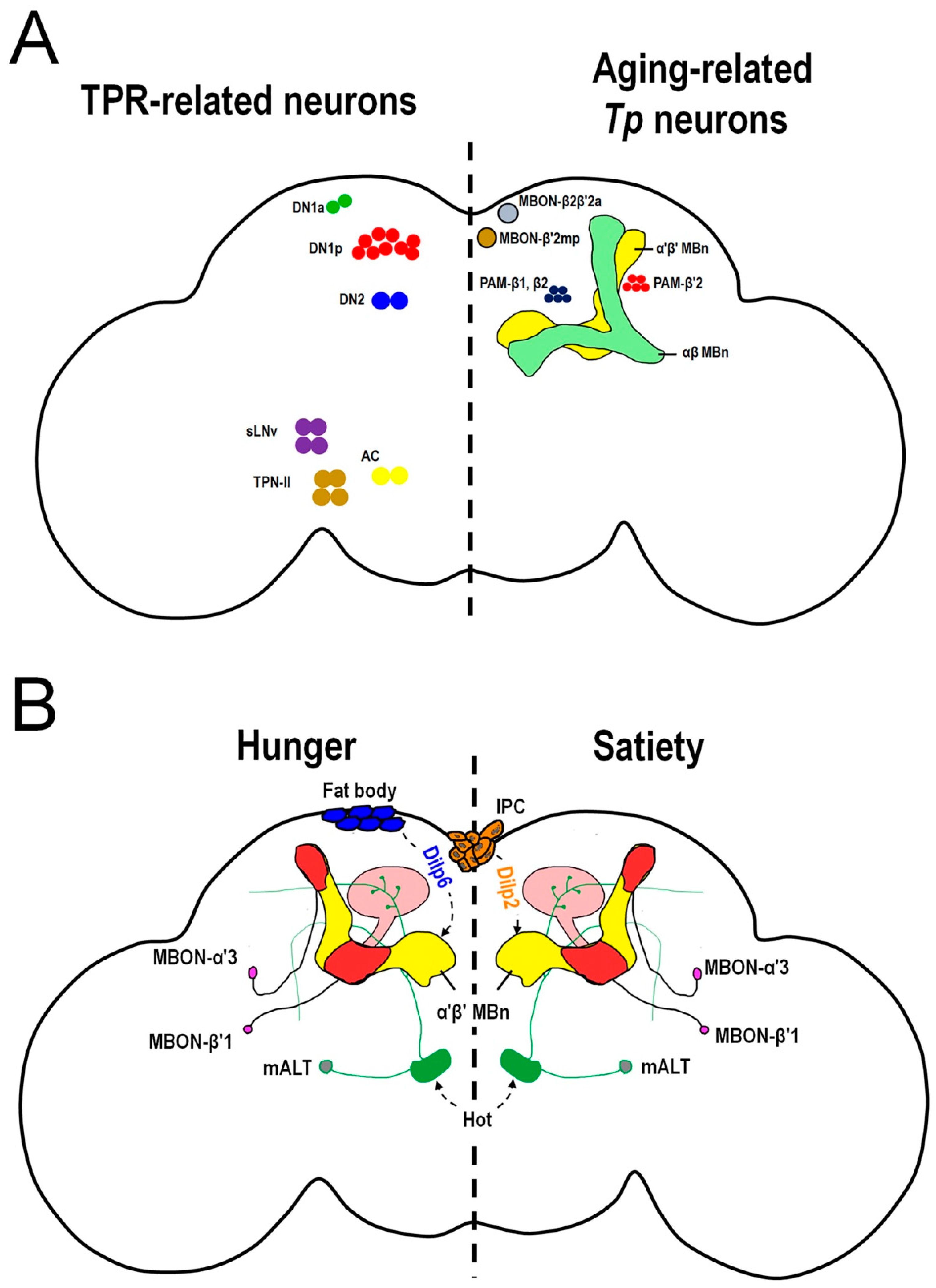
3.1. Temperature Preference in Aged Drosophila
3.2. Temperature Preference Rhythm
3.3. Feeding State-Dependent Temperature Preference
3.4. Thermal Nociception Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, C.L. Fundamental Concepts of Human Thermoregulation and Adaptation to Heat: A Review in the Context of Global Warming. Int. J. Environ. Res. Public. Health 2020, 17, 7795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Molecular sensors and modulators of thermoreception. Channels 2015, 9, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.N.; Gagnon, D.; Laitano, O.; Crandall, C.G. Human temperature regulation under heat stress in health, disease, and injury. Physiol. Rev. 2022, 102, 1907–1989. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Madden, C.J.; Morrison, S.F. Central nervous system circuits that control body temperature. Neurosci. Lett. 2019, 696, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.F. The Hot-Blooded Insects—Strategies and Mechanisms of Thermoregulation—Heinrich, B. Science 1993, 260, 1155–1156. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, B.; Garrity, P.A. Temperature sensation in Drosophila. Curr. Opin. Neurobiol. 2015, 34, 8–13. [Google Scholar] [CrossRef]
- Garrity, P.A.; Goodman, M.B.; Samuel, A.D.; Sengupta, P. Running hot and cold: Behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes. Dev. 2010, 24, 2365–2382. [Google Scholar] [CrossRef]
- Huey, R.B.; Hertz, P.E.; Sinervo, B. Behavioral drive versus behavioral inertia in evolution: A null model approach. Am. Nat. 2003, 161, 357–366. [Google Scholar] [CrossRef]
- Stevenson, R.D. The Relative Importance of Behavioral and Physiological Adjustments Controlling Body Temperature in Terrestrial Ectotherms. Am. Nat. 1985, 126, 362–386. [Google Scholar] [CrossRef]
- Clapham, D.E.; Runnels, L.W.; Strubing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Voets, T.; Peters, J. TRP channels in disease. Sci. STKE 2005, 2005, re8. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Sweet, T.B.; Clapham, D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Appendino, G.; Owsianik, G. The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflug. Arch. 2012, 464, 425–458. [Google Scholar] [CrossRef] [PubMed]
- Li, H. TRP Channel Classification. In Transient Receptor Potential Canonical Channels and Brain Diseases; Springer: Dordrecht, The Netherlands, 2017; Volume 976, pp. 1–8. [Google Scholar] [CrossRef]
- Bamps, D.; Vriens, J.; de Hoon, J.; Voets, T. TRP Channel Cooperation for Nociception: Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 655–677. [Google Scholar] [CrossRef]
- He, J.; Li, B.; Han, S.; Zhang, Y.; Liu, K.; Yi, S.; Liu, Y.; Xiu, M. Drosophila as a Model to Study the Mechanism of Nociception. Front. Physiol. 2022, 13, 854124. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef]
- Gees, M.; Owsianik, G.; Nilius, B.; Voets, T. TRP channels. Compr. Physiol. 2012, 2, 563–608. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Guler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.P.; Ooi, L.; et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef]
- Jordt, S.E.; McKemy, D.D.; Julius, D. Lessons from peppers and peppermint: The molecular logic of thermosensation. Curr. Opin. Neurobiol. 2003, 13, 487–492. [Google Scholar] [CrossRef]
- Lee, H.; Iida, T.; Mizuno, A.; Suzuki, M.; Caterina, M.J. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci. 2005, 25, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J., Jr.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.Y.; Glazer, J.M.; Corey, D.P.; Rice, F.L.; Stucky, C.L. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. 2009, 29, 4808–4819. [Google Scholar] [CrossRef] [PubMed]
- Gallio, M.; Ofstad, T.A.; Macpherson, L.J.; Wang, J.W.; Zuker, C.S. The coding of temperature in the Drosophila brain. Cell 2011, 144, 614–624. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S.; et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef]
- Song, K.; Wang, H.; Kamm, G.B.; Pohle, J.; Reis, F.C.; Heppenstall, P.; Wende, H.; Siemens, J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016, 353, 1393–1398. [Google Scholar] [CrossRef]
- Tan, C.H.; McNaughton, P.A. The TRPM2 ion channel is required for sensitivity to warmth. Nature 2016, 536, 460–463. [Google Scholar] [CrossRef]
- Cosens, D.J.; Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Minke, B.; Wu, C.; Pak, W.L. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 1975, 258, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.A.; Montell, C. Drosophila TRP channels and animal behavior. Life Sci. 2013, 92, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Bellemer, A. Thermotaxis, circadian rhythms, and TRP channels in Drosophila. Temperature 2015, 2, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Hamada, F.N.; Rosenzweig, M.; Kang, K.; Pulver, S.R.; Ghezzi, A.; Jegla, T.J.; Garrity, P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nature 2008, 454, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Pulver, S.R.; Panzano, V.C.; Chang, E.C.; Griffith, L.C.; Theobald, D.L.; Garrity, P.A. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010, 464, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, Y.; Akitake, B.; Woodward, O.M.; Guggino, W.B.; Montell, C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 8440–8445. [Google Scholar] [CrossRef] [PubMed]
- Tracey, W.D., Jr.; Wilson, R.I.; Laurent, G.; Benzer, S. Painless, a Drosophila gene essential for nociception. Cell 2003, 113, 261–273. [Google Scholar] [CrossRef]
- Sakai, T.; Watanabe, K.; Ohashi, H.; Sato, S.; Inami, S.; Shimada, N.; Kitamoto, T. Insulin-producing cells regulate the sexual receptivity through the painless TRP channel in Drosophila virgin females. PLoS ONE 2014, 9, e88175. [Google Scholar] [CrossRef]
- Sakai, T.; Sato, S.; Ishimoto, H.; Kitamoto, T. Significance of the centrally expressed TRP channel painless in Drosophila courtship memory. Learn. Mem. 2012, 20, 34–40. [Google Scholar] [CrossRef]
- Wang, K.; Guo, Y.; Wang, F.; Wang, Z. Drosophila TRPA channel painless inhibits male-male courtship behavior through modulating olfactory sensation. PLoS ONE 2011, 6, e25890. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Cang, C.L.; Liu, X.F.; Peng, Y.Q.; Ye, Y.Z.; Zhao, Z.Q.; Guo, A.K. Thermal nociception in adult Drosophila: Behavioral characterization and the role of the painless gene. Genes Brain Behav. 2006, 5, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, Y.; Lee, J.; Bang, S.; Hyun, S.; Kang, J.; Hong, S.T.; Bae, E.; Kaang, B.K.; Kim, J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat. Genet. 2005, 37, 305–310. [Google Scholar] [CrossRef]
- Yao, C.A.; Ignell, R.; Carlson, J.R. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 2005, 25, 8359–8367. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, O.; Benzer, S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc. Natl. Acad. Sci. USA 1996, 93, 6079–6084. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Wang, R.; Yin, C.; Dong, Q.; Hing, H.; Kim, C.; Welsh, M.J. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature 2007, 450, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Homberg, U.; Christensen, T.A.; Hildebrand, J.G. Structure and function of the deutocerebrum in insects. Annu. Rev. Entomol. 1989, 34, 477–501. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Brennan, K.M.; Tayler, T.D.; Phelps, P.O.; Patapoutian, A.; Garrity, P.A. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005, 19, 419–424. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Kang, K.; Garrity, P.A. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2008, 105, 14668–14673. [Google Scholar] [CrossRef]
- Xiao, R.; Xu, X.Z.S. Temperature Sensation: From Molecular Thermosensors to Neural Circuits and Coding Principles. Annu. Rev. Physiol. 2021, 83, 205–230. [Google Scholar] [CrossRef]
- Li, K.; Gong, Z. Feeling Hot and Cold: Thermal Sensation in Drosophila. Neurosci. Bull. 2017, 33, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Platt, M.D.; Lagnese, C.M.; Leslie, J.R.; Hamada, F.N. Temperature integration at the AC thermosensory neurons in Drosophila. J. Neurosci. 2013, 33, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.J.; Bruford, E.A.; Caterina, M.J.; Clapham, D.E.; Harteneck, C.; Heller, S.; Julius, D.; et al. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 2002, 9, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.H.; Lin, Y.C.; Chen, S.F.; Lee, P.S.; Fu, T.F.; Wu, T.; Wu, C.L. Independent insulin signaling modulators govern hot avoidance under different feeding states. PLoS Biol. 2023, 21, e3002332. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.W.; Wu, C.L.; Chang, S.W.; Liu, T.H.; Lai, J.S.; Fu, T.F.; Fu, C.C.; Chiang, A.S. Parallel circuits control temperature preference in Drosophila during ageing. Nat. Commun. 2015, 6, 7775. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.L.; Kwon, Y.; Adegbola, A.A.; Luo, J.; Chess, A.; Montell, C. Function of rhodopsin in temperature discrimination in Drosophila. Science 2011, 331, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Bronk, P.; Chang, E.C.; Lowell, A.M.; Flam, J.O.; Panzano, V.C.; Theobald, D.L.; Griffith, L.C.; Garrity, P.A. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 2013, 500, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Silbering, A.F.; Benton, R. Ionotropic and metabotropic mechanisms in chemoreception: ‘Chance or design’? EMBO Rep. 2010, 11, 173–179. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Stocker, R.F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007, 30, 505–533. [Google Scholar] [CrossRef]
- Thorne, N.; Chromey, C.; Bray, S.; Amrein, H. Taste perception and coding in Drosophila. Curr. Biol. 2004, 14, 1065–1079. [Google Scholar] [CrossRef]
- Kang, K.; Panzano, V.C.; Chang, E.C.; Ni, L.; Dainis, A.M.; Jenkins, A.M.; Regna, K.; Muskavitch, M.A.; Garrity, P.A. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 2011, 481, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, V.; Story, G.M.; Peier, A.M.; Petrus, M.J.; Lee, V.M.; Hwang, S.W.; Patapoutian, A.; Jegla, T. Opposite thermosensor in fruitfly and mouse. Nature 2003, 423, 822–823. [Google Scholar] [CrossRef] [PubMed]
- Croset, V.; Rytz, R.; Cummins, S.F.; Budd, A.; Brawand, D.; Kaessmann, H.; Gibson, T.J.; Benton, R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010, 6, e1001064. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Klein, M.; Svec, K.V.; Budelli, G.; Chang, E.C.; Ferrer, A.J.; Benton, R.; Samuel, A.D.; Garrity, P.A. The Ionotropic Receptors IR21a and IR25a mediate cool sensing in Drosophila. eLife 2016, 5, e13254. [Google Scholar] [CrossRef] [PubMed]
- Silbering, A.F.; Rytz, R.; Grosjean, Y.; Abuin, L.; Ramdya, P.; Jefferis, G.S.; Benton, R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 2011, 31, 13357–13375. [Google Scholar] [CrossRef] [PubMed]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Rytz, R.; Croset, V.; Benton, R. Ionotropic receptors (IRs): Chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 2013, 43, 888–897. [Google Scholar] [CrossRef]
- Abuin, L.; Bargeton, B.; Ulbrich, M.H.; Isacoff, E.Y.; Kellenberger, S.; Benton, R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron 2011, 69, 44–60. [Google Scholar] [CrossRef]
- Klein, M.; Afonso, B.; Vonner, A.J.; Hernandez-Nunez, L.; Berck, M.; Tabone, C.J.; Kane, E.A.; Pieribone, V.A.; Nitabach, M.N.; Cardona, A.; et al. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. Proc. Natl. Acad. Sci. USA 2015, 112, E220–E229. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Dahanukar, A.; Weiss, L.A.; Carlson, J.R. Molecular and cellular organization of the taste system in the Drosophila larva. J. Neurosci. 2011, 31, 15300–15309. [Google Scholar] [CrossRef]
- Liu, L.; Yermolaieva, O.; Johnson, W.A.; Abboud, F.M.; Welsh, M.J. Identification and function of thermosensory neurons in Drosophila larvae. Nat. Neurosci. 2003, 6, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.F. The organization of the chemosensory system in Drosophila melanogaster: A review. Cell Tissue Res. 1994, 275, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.E.; Wang, G.; Garrity, P.A.; Huey, R.B. Review: Thermal preference in Drosophila. J. Therm. Biol. 2009, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Asahina, K.; Louis, M.; Piccinotti, S.; Vosshall, L.B. A circuit supporting concentration-invariant odor perception in Drosophila. J. Biol. 2009, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Budelli, G.; Ni, L.; Berciu, C.; van Giesen, L.; Knecht, Z.A.; Chang, E.C.; Kaminski, B.; Silbering, A.F.; Samuel, A.; Klein, M.; et al. Ionotropic Receptors Specify the Morphogenesis of Phasic Sensors Controlling Rapid Thermal Preference in Drosophila. Neuron 2019, 101, 738–747.e733. [Google Scholar] [CrossRef] [PubMed]
- Schepers, R.J.; Ringkamp, M. Thermoreceptors and thermosensitive afferents. Neurosci. Biobehav. Rev. 2009, 33, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Mazor, O.; Wilson, R.I. Thermosensory processing in the Drosophila brain. Nature 2015, 519, 353–357. [Google Scholar] [CrossRef]
- Aso, Y.; Grubel, K.; Busch, S.; Friedrich, A.B.; Siwanowicz, I.; Tanimoto, H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 2009, 23, 156–172. [Google Scholar] [CrossRef]
- Mao, Z.; Davis, R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front. Neural Circuits 2009, 3, 5. [Google Scholar] [CrossRef]
- Aso, Y.; Hattori, D.; Yu, Y.; Johnston, R.M.; Iyer, N.A.; Ngo, T.T.; Dionne, H.; Abbott, L.F.; Axel, R.; Tanimoto, H.; et al. The neuronal architecture of the mushroom body provides a logic for associative learning. eLife 2014, 3, e04577. [Google Scholar] [CrossRef]
- Karam, C.S.; Jones, S.K.; Javitch, J.A. Come Fly with Me: An overview of dopamine receptors in Drosophila melanogaster. Basic. Clin. Pharmacol. Toxicol. 2020, 126 (Suppl. 6), 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tomchik, S.M. Dopaminergic neurons encode a distributed, asymmetric representation of temperature in Drosophila. J. Neurosci. 2013, 33, 2166a–2176a. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Hamada, F.N. Drosophila Temperature Preference Rhythms: An Innovative Model to Understand Body Temperature Rhythms. Int. J. Mol. Sci. 2019, 20, 1988. [Google Scholar] [CrossRef] [PubMed]
- Khazaeli, A.A.; Van Voorhies, W.; Curtsinger, J.W. Longevity and metabolism in Drosophila melanogaster: Genetic correlations between life span and age-specific metabolic rate in populations artificially selected for long life. Genetics 2005, 169, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Berrigan, D.; Partridge, L. Influence of temperature and activity on the metabolic rate of adult Drosophila melanogaster. Comp. Biochem. Physiol. A Physiol. 1997, 118, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Umezaki, Y.; Hayley, S.E.; Chu, M.L.; Seo, H.W.; Shah, P.; Hamada, F.N. Feeding-State-Dependent Modulation of Temperature Preference Requires Insulin Signaling in Drosophila Warm-Sensing Neurons. Curr. Biol. 2018, 28, 779–787.e773. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.H.; Ohba, S. Temperature preferences of eleven Drosophila species from Japan—The relationship between preferred temperature and some ecological character istics in their natural habitats. Zool. Sci. 1984, 1, 631–640. [Google Scholar]
- Yoshii, T.; Sakamoto, M.; Tomioka, K. A temperature-dependent timing mechanism is involved in the circadian system that drives locomotor rhythms in the fruit fly Drosophila melanogaster. Zoolog Sci. 2002, 19, 841–850. [Google Scholar] [CrossRef]
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 2020, 21, 88–101. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Tumer, N.; Larochelle, J.S. Tyrosine hydroxylase expression in rat adrenal medulla: Influence of age and cold. Pharmacol. Biochem. Behav. 1995, 51, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Algeri, S.; Calderini, G.; Lomuscio, G.; Vantini, G.; Toffano, G.; Ponzio, F. Changes with age in rat central monoaminergic system responses to cold stress. Neurobiol. Aging 1982, 3, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Sokabe, T.; Chen, H.C.; Luo, J.; Montell, C. A Switch in Thermal Preference in Drosophila Larvae Depends on Multiple Rhodopsins. Cell Rep. 2016, 17, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.J.; Wilbourne, J.T.; Omelchenko, A.A.; Yoon, J.; Ni, L. Ionotropic Receptor-dependent cool cells control the transition of temperature preference in Drosophila larvae. PLoS Genet. 2021, 17, e1009499. [Google Scholar] [CrossRef] [PubMed]
- Molon, M.; Dampc, J.; Kula-Maximenko, M.; Zebrowski, J.; Molon, A.; Dobler, R.; Durak, R.; Skoczowski, A. Effects of Temperature on Lifespan of Drosophila melanogaster from Different Genetic Backgrounds: Links between Metabolic Rate and Longevity. Insects 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Salerian, A.J.; Saleri, N.G. Cooler biologically compatible core body temperatures may prolong longevity and combat neurodegenerative disorders. Med. Hypotheses 2006, 66, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.C.; Waner, J.I.; Vieira, E.F.; Taylor, S.R.; Driver, H.S.; Mitchell, D. Sleep and 24 hour body temperatures: A comparison in young men, naturally cycling women and women taking hormonal contraceptives. J. Physiol. 2001, 530, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Minors, D.S.; Waterhouse, J.M. Effects upon circadian rhythmicity of an alteration to the sleep-wake cycle: Problems of assessment resulting from measurement in the presence of sleep and analysis in terms of a single shifted component. J. Biol. Rhythms 1988, 3, 23–40. [Google Scholar] [CrossRef]
- Refinetti, R. Circadian rhythmicity of body temperature and metabolism. Temperature 2020, 7, 321–362. [Google Scholar] [CrossRef]
- Gradisar, M.; Lack, L. Relationships between the circadian rhythms of finger temperature, core temperature, sleep latency, and subjective sleepiness. J. Biol. Rhythm. 2004, 19, 157–163. [Google Scholar] [CrossRef]
- Sarabia, J.A.; Rol, M.A.; Mendiola, P.; Madrid, J.A. Circadian rhythm of wrist temperature in normal-living subjects A candidate of new index of the circadian system. Physiol. Behav. 2008, 95, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Mormont, M.C.; Langouet, A.M.; Claustrat, B.; Bogdan, A.; Marion, S.; Waterhouse, J.; Touitou, Y.; Levi, F. Marker rhythms of circadian system function: A study of patients with metastatic colorectal cancer and good performance status. Chronobiol. Int. 2002, 19, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.W. More than a marker: Interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol. Int. 2000, 17, 313–354. [Google Scholar] [CrossRef]
- Krauchi, K.; Wirz-Justice, A. Circadian clues to sleep onset mechanisms. Neuropsychopharmacology 2001, 25, S92–S96. [Google Scholar] [CrossRef] [PubMed]
- Krauchi, K. The human sleep-wake cycle reconsidered from a thermoregulatory point of view. Physiol. Behav. 2007, 90, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Zeitzer, J.M.; Czeisler, C.A.; Dijk, D.J. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav. Brain Res. 2000, 115, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.; Waterhouse, J. Circadian aspects of body temperature regulation in exercise. J. Therm. Biol. 2009, 34, 161–170. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; van Doornen, L.J.P.; Buijs, R.M. Light and diurnal cycle affect human heart rate: Possible role for the circadian pacemaker. J. Biol. Rhythm. 1999, 14, 202–212. [Google Scholar] [CrossRef]
- Wakamura, T.; Tokura, H. Circadian rhythm of rectal temperature in humans under different ambient temperature cycles. J. Therm. Biol. 2002, 27, 439–447. [Google Scholar] [CrossRef]
- Waterhouse, J.; Edwards, B.; Mugarza, J.; Flemming, R.; Minors, D.; Calbraith, D.; Williams, G.; Atkinson, G.; Reilly, T. Purification of masked temperature data from humans: Some preliminary observations on a comparison of the use of an activity diary, wrist actimetry, and heart rate monitoring. Chronobiol. Int. 1999, 16, 461–475. [Google Scholar] [CrossRef]
- Krauchi, K.; Cajochen, C.; Wirz-Justice, A. Thermophysiologic aspects of the three-process-model of sleepiness regulation (vol 24, pg 287, 2005). Clin. Sport. Med. 2005, 24, 979. [Google Scholar] [CrossRef]
- Martinez-Nicolas, A.; Ortiz-Tudela, E.; Rol, M.A.; Madrid, J.A. Uncovering Different Masking Factors on Wrist Skin Temperature Rhythm in Free-Living Subjects. PLoS ONE 2013, 8, e61142. [Google Scholar] [CrossRef] [PubMed]
- Coiffard, B.; Diallo, A.B.; Mezouar, S.; Leone, M.; Mege, J.L. A Tangled Threesome: Circadian Rhythm, Body Temperature Variations, and the Immune System. Biology 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R. The circadian rhythm of body temperature. Front. Biosci. 2010, 15, 564–594. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian clock proteins and immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, A.M.; Turek, F.W. Neurobiology of Circadian Rhythm Regulation. Sleep. Med. Clin. 2015, 10, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.J.; Firth, B.T.; Belan, I. Circadian rhythms of locomotor activity and temperature selection in sleepy lizards, Tiliqua rugosa. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2007, 193, 695–701. [Google Scholar] [CrossRef]
- Kaneko, H.; Head, L.M.; Ling, J.; Tang, X.; Liu, Y.; Hardin, P.E.; Emery, P.; Hamada, F.N. Circadian rhythm of temperature preference and its neural control in Drosophila. Curr. Biol. 2012, 22, 1851–1857. [Google Scholar] [CrossRef]
- Goda, T.; Umezaki, Y.; Hamada, F.N. Molecular and Neural Mechanisms of Temperature Preference Rhythm in Drosophila melanogaster. J. Biol. Rhythms 2023, 38, 326–340. [Google Scholar] [CrossRef]
- Nitabach, M.N.; Taghert, P.H. Organization of the Drosophila circadian control circuit. Curr. Biol. 2008, 18, R84–R93. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Saez, L.; Young, M.W. PER-TIM interactions in living Drosophila cells: An interval timer for the circadian clock. Science 2006, 311, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Shafer, O.T.; Rosbash, M.; Truman, J.W. Sequential Nuclear Accumulation of the Clock Proteins Period and Timeless in the Pacemaker Neurons of Drosophila melanogaster. J. Neurosci. 2002, 22, 5946–5954. [Google Scholar] [CrossRef] [PubMed]
- Taghert, P.H.; Shafer, O.T. Mechanisms of clock output in the Drosophila circadian pacemaker system. J. Biol. Rhythms 2006, 21, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Vosshall, L.B.; Young, M.W. Circadian rhythms in Drosophila can be driven by period expression in a restricted group of central brain cells. Neuron 1995, 15, 345–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frisch, B.; Hardin, P.E.; Hamblen-Coyle, M.J.; Rosbash, M.; Hall, J.C. A promoterless period gene mediates behavioral rhythmicity and cyclical per expression in a restricted subset of the drosophila nervous system. Neuron 1994, 12, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Dushay, M.S.; Rosbash, M.; Hall, J.C. The disconnected visual system mutations in Drosophila melanogaster drastically disrupt circadian rhythms. J. Biol. Rhythms 1989, 4, 1–27. [Google Scholar] [CrossRef]
- Hyun, S.; Lee, Y.; Hong, S.-T.; Bang, S.; Paik, D.; Kang, J.; Shin, J.; Lee, J.; Jeon, K.; Hwang, S.; et al. Drosophila GPCR Han Is a Receptor for the Circadian Clock Neuropeptide PDF. Neuron 2005, 48, 267–278. [Google Scholar] [CrossRef]
- Taghert, P.H.; Nitabach, M.N. Peptide neuromodulation in invertebrate model systems. Neuron 2012, 76, 82–97. [Google Scholar] [CrossRef]
- Chen, S.C.; Tang, X.; Goda, T.; Umezaki, Y.; Riley, A.C.; Sekiguchi, M.; Yoshii, T.; Hamada, F.N. Dorsal clock networks drive temperature preference rhythms in Drosophila. Cell Rep. 2022, 39, 110668. [Google Scholar] [CrossRef]
- Alpert, M.H.; Frank, D.D.; Kaspi, E.; Flourakis, M.; Zaharieva, E.E.; Allada, R.; Para, A.; Gallio, M. A Circuit Encoding Absolute Cold Temperature in Drosophila. Curr. Biol. 2020, 30, 2275–2288.e2275. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Tang, X.; Umezaki, Y.; Chu, M.L.; Kunst, M.; Nitabach, M.N.N.; Hamada, F.N. Drosophila DH31 Neuropeptide and PDF Receptor Regulate Night-Onset Temperature Preference. J. Neurosci. 2016, 36, 11739–11754. [Google Scholar] [CrossRef] [PubMed]
- Mertens, I.; Vandingenen, A.; Johnson, E.C.; Shafer, O.T.; Li, W.; Trigg, J.S.; De Loof, A.; Schoofs, L.; Taghert, P.H. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron 2005, 48, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Roessingh, S.; Hayley, S.E.; Chu, M.L.; Tanaka, N.K.; Wolfgang, W.; Song, S.; Stanewsky, R.; Hamada, F.N. The role of PDF neurons in setting the preferred temperature before dawn in Drosophila. eLife 2017, 6, e23206. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; BroŽEk, J.; Henschel, A.; Mickelsen, O.; Taylor, H.L.; Simonson, E.; Skinner, A.S.; Wells, S.M.; Drummond, J.C.; Wilder, R.M.; et al. The Biology of Human Starvation; University of Minnesota Press: Minneapolis, MN, USA, 1950; Volume I. [Google Scholar]
- Piccione, G.; Caola, G.; Refinetti, R. Circadian modulation of starvation-induced hypothermia in sheep and goats. Chronobiol. Int. 2002, 19, 531–541. [Google Scholar] [CrossRef] [PubMed]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hung, C.C.; Randall, D.J. The comparative physiology of food deprivation: From feast to famine. Annu. Rev. Physiol. 2006, 68, 223–251. [Google Scholar] [CrossRef]
- Bicego, K.C.; Barros, R.C.H.; Branco, L.G.S. Physiology of temperature regulation: Comparative aspects. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 616–639. [Google Scholar] [CrossRef]
- Young, V.R.; Scrimshaw, N.S. The physiology of starvation. Sci. Am. 1971, 225, 14–21. [Google Scholar] [CrossRef]
- Owen, O.E.; Smalley, K.J.; D’Alessio, D.A.; Mozzoli, M.A.; Dawson, E.K. Protein, fat, and carbohydrate requirements during starvation: Anaplerosis and cataplerosis. Am. J. Clin. Nutr. 1998, 68, 12–34. [Google Scholar] [CrossRef]
- Owen, O.E.; Reichard, G.A., Jr.; Patel, M.S.; Boden, G. Energy metabolism in feasting and fasting. Adv. Exp. Med. Biol. 1979, 111, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.W.; Foster, D.O. Starvation-induced changes in metabolic rate, blood flow, and regional energy expenditure in rats. Can. J. Physiol. Pharmacol. 1986, 64, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Cherel, Y.; Robin, J.P.; Heitz, A.; Calgari, C.; Le Maho, Y. Relationships between lipid availability and protein utilization during prolonged fasting. J. Comp. Physiol. B 1992, 162, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.N.; Lowell, B.; Belur, E.; Ruderman, N.B. Sites of protein conservation and loss during starvation: Influence of adiposity. Am. J. Physiol. 1984, 246, E383–E390. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K. How Rats Economize: Energy Loss in Starvation. Physiol. Zool. 1977, 50, 331–362. [Google Scholar] [CrossRef]
- Desautels, M. Mitochondrial thermogenin content is unchanged during atrophy of BAT of fasting mice. Am. J. Physiol. 1985, 249, E99–E106. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, I.; Schreiber, R.; Prokesch, A. Regulation of thermogenic adipocytes during fasting and cold. Mol. Cell Endocrinol. 2020, 512, 110869. [Google Scholar] [CrossRef]
- Nassel, D.R.; Kubrak, O.I.; Liu, Y.T.; Luo, J.N.; Lushchak, O.V. Factors that regulate insulin producing cells and their output in Drosophila. Front. Physiol. 2013, 4, 252. [Google Scholar] [CrossRef]
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001, 11, 213–221. [Google Scholar] [CrossRef]
- Cao, C.; Brown, M.R. Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res. 2001, 304, 317–321. [Google Scholar] [CrossRef]
- Bai, H.; Kang, P.; Tatar, M. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell 2012, 11, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Geminard, C.; Rulifson, E.J.; Leopold, P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009, 10, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, S.R.; Pathak, H.; Rehman, N.; Fernandes, J.; Vishnu, S.; Varghese, J. Insulin signalling elicits hunger-induced feeding in Drosophila. Dev. Biol. 2020, 459, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, R.S. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol. Metab. 2002, 13, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.L.; Cline, H.T. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Semaniuk, U.; Piskovatska, V.; Strilbytska, O.; Strutynska, T.; Burdyliuk, N.; Vaiserman, A.; Bubalo, V.; Storey, K.B.; Lushchak, O. Drosophila insulin-like peptides: From expression to functions—A review. Entomol. Exp. Appl. 2021, 169, 195–208. [Google Scholar] [CrossRef]
- Shilo, B.Z. The regulation and functions of MAPK pathways in Drosophila. Methods 2014, 68, 151–159. [Google Scholar] [CrossRef]
- Wassarman, D.A.; Therrien, M.; Rubin, G.M. The Ras signaling pathway in Drosophila. Curr. Opin. Genet. Dev. 1995, 5, 44–50. [Google Scholar] [CrossRef]
- Lim, B.; Dsilva, C.J.; Levario, T.J.; Lu, H.; Schupbach, T.; Kevrekidis, I.G.; Shvartsman, S.Y. Dynamics of Inductive ERK Signaling in the Drosophila Embryo. Curr. Biol. 2015, 25, 1784–1790. [Google Scholar] [CrossRef]
- Lee, D.E.; Kim, S.J.; Zhuo, M. Comparison of behavioral responses to noxious cold and heat in mice. Brain Res. 1999, 845, 117–121. [Google Scholar] [CrossRef]
- Malafoglia, V.; Bryant, B.; Raffaeli, W.; Giordano, A.; Bellipanni, G. The zebrafish as a model for nociception studies. J. Cell Physiol. 2013, 228, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Malafoglia, V.; Colasanti, M.; Raffaeli, W.; Balciunas, D.; Giordano, A.; Bellipanni, G. Extreme thermal noxious stimuli induce pain responses in zebrafish larvae. J. Cell Physiol. 2014, 229, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Prober, D.A.; Zimmerman, S.; Myers, B.R.; McDermott, B.M., Jr.; Kim, S.H.; Caron, S.; Rihel, J.; Solnica-Krezel, L.; Julius, D.; Hudspeth, A.J.; et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J. Neurosci. 2008, 28, 10102–10110. [Google Scholar] [CrossRef] [PubMed]
- Wittenburg, N.; Baumeister, R. Thermal avoidance in Caenorhabditis elegans: An approach to the study of nociception. Proc. Natl. Acad. Sci. USA 1999, 96, 10477–10482. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.C.; Zhu, J.; Ohyama, T. Nociception in fruit fly larvae. Front. Pain Res. 2023, 4, 1076017. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.Y.; Zhong, L.; Xu, Y.; Johnson, T.; Zhang, F.; Deisseroth, K.; Tracey, W.D. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 2007, 17, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Tenedini, F.; Hoyer, N.; Kutschera, F.; Soba, P. Assaying Thermo-nociceptive Behavior in Drosophila Larvae. Bio Protoc. 2018, 8, e2737. [Google Scholar] [CrossRef]
- Oswald, M.; Rymarczyk, B.; Chatters, A.; Sweeney, S.T. A novel thermosensitive escape behavior in Drosophila larvae. Fly 2011, 5, 304–306. [Google Scholar] [CrossRef][Green Version]
- Neely, G.G.; Hess, A.; Costigan, M.; Keene, A.C.; Goulas, S.; Langeslag, M.; Griffin, R.S.; Belfer, I.; Dai, F.; Smith, S.B.; et al. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell 2010, 143, 628–638. [Google Scholar] [CrossRef]
- Aldrich, B.T.; Kasuya, J.; Faron, M.; Ishimoto, H.; Kitamoto, T. The amnesiac gene is involved in the regulation of thermal nociception in Drosophila melanogaster. J. Neurogenet. 2010, 24, 33–41. [Google Scholar] [CrossRef]
- Benzer, S. Behavioral mutants of Drosophila isolated by Countercurrent Distribution. Proc. Natl. Acad. Sci. USA 1967, 58, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Boonen, B.; Startek, J.B.; Milici, A.; Lopez-Requena, A.; Beelen, M.; Callaerts, P.; Talavera, K. Activation of Drosophila melanogaster TRPA1 Isoforms by Citronellal and Menthol. Int. J. Mol. Sci. 2021, 22, 997. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Shim, H.S.; Wang, X.; Montell, C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 2008, 11, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Neely, G.G.; Keene, A.C.; Duchek, P.; Chang, E.C.; Wang, Q.P.; Aksoy, Y.A.; Rosenzweig, M.; Costigan, M.; Woolf, C.J.; Garrity, P.A.; et al. TrpA1 regulates thermal nociception in Drosophila. PLoS ONE 2011, 6, e24343. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shen, W.L.; Montell, C. TRPA1 mediates sensation of the rate of temperature change in Drosophila larvae. Nat. Neurosci. 2017, 20, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.B. Sensation is painless. Trends Neurosci. 2003, 26, 643–645. [Google Scholar] [CrossRef]
- Sokabe, T.; Tominaga, M. A temperature-sensitive TRP ion channel, Painless, functions as a noxious heat sensor in fruit flies. Commun. Integr. Biol. 2009, 2, 170–173. [Google Scholar] [CrossRef]
- Al-Anzi, B.; Tracey, W.D., Jr.; Benzer, S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr. Biol. 2006, 16, 1034–1040. [Google Scholar] [CrossRef]
- Xu, J.; Sornborger, A.T.; Lee, J.K.; Shen, P. Drosophila TRPA channel modulates sugar-stimulated neural excitation, avoidance and social response. Nat. Neurosci. 2008, 11, 676–682. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Thakur, D.; Mack, J.; Spina, A.; Montell, C. Alleviation of thermal nociception depends on heat-sensitive neurons and a TRP channel in the brain. Curr. Biol. 2023, 33, 2397–2406.e2396. [Google Scholar] [CrossRef]
- Sizemore, T.R.; Carlson, J.R. Pain: The agony and the AstC. Curr. Biol. 2023, 33, R695–R697. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Polgar, E.; Solinski, H.J.; Mishra, S.K.; Tseng, P.Y.; Iwagaki, N.; Boyle, K.A.; Dickie, A.C.; Kriegbaum, M.C.; Wildner, H.; et al. Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci. 2018, 21, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Birgul, N.; Weise, C.; Kreienkamp, H.J.; Richter, D. Reverse physiology in drosophila: Identification of a novel allatostatin-like neuropeptide and its cognate receptor structurally related to the mammalian somatostatin/galanin/opioid receptor family. EMBO J. 1999, 18, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Lenz, C.; Williamson, M.; Grimmelikhuijzen, C.J. Molecular cloning and genomic organization of a second probable allatostatin receptor from Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2000, 273, 571–577. [Google Scholar] [CrossRef]
- Kornberg, T.B.; Krasnow, M.A. The Drosophila genome sequence: Implications for biology and medicine. Science 2000, 287, 2218–2220. [Google Scholar] [CrossRef]

| Category | Gene | Hot/Cold | References |
|---|---|---|---|
| TRP family | |||
| TRPA homologs | dTrpA1 | Hot | [46,59,60] |
| painless | Hot | [49] | |
| pyrexia | Hot | [54] | |
| TRPP homologs | brv1, brv2, brv3 | Cold | [38] |
| Non TRP family | |||
| Rhodopsin | rh1 | Hot/Cold | [67] |
| Gustatory receptor | GR28B(D) | Hot | [68] |
| Ionotropic receptor | IR21a | Cold | [75] |
| Ionotropic receptor | IR25a | Cold | [75] |
| Category | Gene/Protein | Hot/Cold | Circuit | References |
|---|---|---|---|---|
| Drosophila larvae | ||||
| DOG | IR21a/IR25a | Cold | to antennal lobes | [75,80,82,84,85] |
| TOG | Cold | to antennal lobes | [75,80,82,84,85] | |
| lateral body wall | Hot/Cold | [82] | ||
| Drosophila adult | ||||
| hot cell (also named warmth cell) | GR28B(D) | Hot | [68] | |
| cool cell | brv1, IR21a, IR25a, IR93a | Cold | cool cell > fast/slow-cool-PNs | [38,86,87] |
| AC | dTrpA1 | Hot | [46] | |
| PPL1-γ1pedc | Cold | PPL1-γ1pedc > MBn | [93] | |
| PPL1-γ2α′1 | Cold | PPL1-γ2α′1 > MBn | [93] |
| Category | Gene/Protein | Neuron/Microcircuit | References |
|---|---|---|---|
| Aging-dependent temperature preference | |||
| early third-instar fly larvae | IR21a/IR25a/IR93a | DOCC | [105] |
| mid-third-instar fly larvae | rh1/Gq/PLC/TRPA1 | dTrpA1-positive neuron | [67] |
| late-third-instar fly larvae | rh5/rh6/Gq/PLC/TRPA1; IR21a/IR25a/IR93a | dTrpA1-positive neuron | [104] |
| adult fly | Dop1R1 | PAM-β1/β2 > αβ MBn > MBON-β2β′2a; PAM-β′2 > α′β′ MBn > MBON-β′2mp | [66] |
| Temperature preference rhythm | |||
| daytime | PER | DN2s > DN1ps; TPN-II > DN1a | [130,131,141] |
| night-onset | PDFR/DH31 | DN2 | [130,131,143] |
| predawn | PDF/5HT1b | AC > sLNvs > DN2 | [130,131,145] |
| Feeding-state dependent temperature preference | |||
| AC-based | TRPA1/Dilp6/PI3K | fat body - AC | [97] |
| MB-based | Dilp2/PI3K; Dilp6/Ras | IPC/fat body - α′β′ MBn - MBON-β′2/α′3; mALT | [65] |
| Thermal nociception behavior | |||
| fly larvae | dTrpA1; painless | [49,187] | |
| adult fly | dTrpA1; painless | Epi neuron | [53,186,189,192] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, M.-H.; Lin, Y.-C.; Wu, T.; Wu, C.-L. Thermosensation and Temperature Preference: From Molecules to Neuronal Circuits in Drosophila. Cells 2023, 12, 2792. https://doi.org/10.3390/cells12242792
Chiang M-H, Lin Y-C, Wu T, Wu C-L. Thermosensation and Temperature Preference: From Molecules to Neuronal Circuits in Drosophila. Cells. 2023; 12(24):2792. https://doi.org/10.3390/cells12242792
Chicago/Turabian StyleChiang, Meng-Hsuan, Yu-Chun Lin, Tony Wu, and Chia-Lin Wu. 2023. "Thermosensation and Temperature Preference: From Molecules to Neuronal Circuits in Drosophila" Cells 12, no. 24: 2792. https://doi.org/10.3390/cells12242792
APA StyleChiang, M.-H., Lin, Y.-C., Wu, T., & Wu, C.-L. (2023). Thermosensation and Temperature Preference: From Molecules to Neuronal Circuits in Drosophila. Cells, 12(24), 2792. https://doi.org/10.3390/cells12242792






