1. Introduction
Alcohol consumption is a pervasive risk factor across myriad pathologies, with 60% of women and 80% of men in developed countries having consumed alcohol at least once in their lifetime [
1]. Over 200 million people in the United States have consumed alcohol at least once, and over 170 million have consumed alcohol in the past year, while 16 million people have had heavy alcohol consumption in the past month [
2]. The risk of developing an alcohol use disorder (AUD) in the last year was approximately 10% [
1]. Furthermore, over 2.4 million deaths across the world are attributed to alcohol use [
3], making alcohol use the eighth leading risk factor for deaths globally [
4].
Although the scope of this study was to investigate the effects of alcohol on the heart, alcohol and its metabolites detrimentally affect multiple organs and organ systems. Previous work has characterized the damage alcohol does to the body [
5,
6,
7,
8], but despite the risk alcohol poses to the cardiovascular system, there is a lack of research on alcohol-induced cardiomyopathy (ACM), particularly in preclinical models aimed at intervention.
The heart is at risk of alcohol-induced damage, both in acute and chronic consumption. Many studies have investigated the hormetic “J-curve” association between various cardiovascular pathologies and alcohol consumption, but there are studies that have shown that even in acute settings, alcohol consumption can be damaging to the heart. The risk of arrhythmias, particularly atrial fibrillation, increases after as little as one drink per day [
9]. High doses of alcohol increase heart rate and blood pressure 13 h after consumption [
10], and there is a linear association between systolic blood pressure and any amount of alcohol consumption. Further, in the first 24 h after alcohol consumption, there is an increased risk of myocardial infarction and hemorrhagic and ischemic stroke [
11]. Chronic alcohol consumption has been linked to other pathological cardiovascular states. AUD can lead to ischemic heart disease, hypertension [
12,
13], cerebrovascular events [
14], persistent arrhythmias (atrial fibrillation being the most common [
15]), both systolic and diastolic dysfunction [
16,
17], cardiomyopathy, and heart failure [
18]. ACM is the leading cause of non-ischemic dilated cardiomyopathy [
3], marked by significant fibrosis and both diastolic and systolic dysfunction [
19], eventually leading to heart failure. Current treatment protocols rely on treating the symptoms of heart failure and the patient ceasing alcohol use [
20]. Much of the current preclinical research on alcohol and ACM focuses on the mechanisms of alcohol-induced damage [
21], with very limited research carried out to characterize the effects of abstinence. There has been some research on AUD recovery and alcohol abstinence in human populations, though much of this is focused on the neurocognitive and psychological aspects of addiction and treatment.
There is significant potential to recover from alcohol-induced damage with adherence to abstinence. Thomes recently published a review on the regenerative capacity of the human body to repair and overcome alcohol damage after abstinence; while this review focuses primarily on liver and GI tract recovery, evidence of bone and heart recovery is also discussed [
22]. Interestingly, there is potential to recover function even after years of heavy alcohol use. One case study examined a patient with systolic heart failure, who presented with delirium tremens (severe alcohol withdrawal) and an ejection fraction (EF) of 20% with elevated cardiac troponin T. Abstinence from alcohol for one month led to an increase in EF to above 60% (a typical EF in a healthy person) [
23]. A recent study used echocardiography to assess morphological and functional changes in the hearts of active heavy-drinking patients; after 6 months of abstinence mean E/e’ ratio improved and posterior wall thickness increased [
24]. However, other than a few accounts of post-abstinence recovery in humans, there are limited data on cardiac recovery from alcohol-induced dysfunction. To our knowledge, this recovery process has not been studied in animal models of ACM.
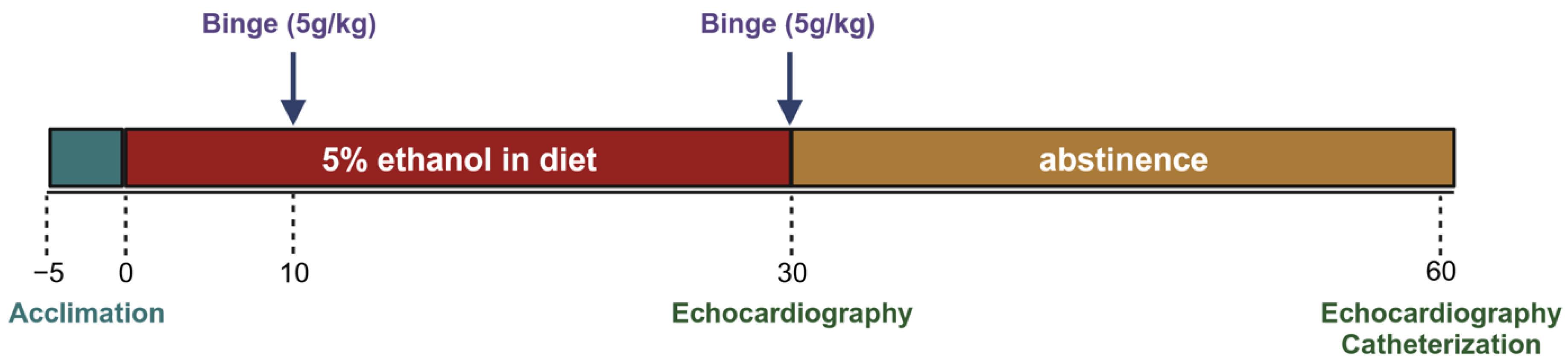
Here, using a mouse model of ACM, we assess the effects of 30 days of abstinence following established cardiac dysfunction. We performed echocardiography to monitor changes in left ventricular wall dimensions, left ventricular catheterization to assess cardiac systolic and diastolic function, histological staining and quantification of collagen, and analysis of mRNA expression of both fibrotic and inflammatory markers.
2. Materials and Methods
2.1. Study Design and Timeline
Mice were acclimated to the control liquid diet for five days before being randomly assigned to 2 groups. The experimental group received a 5% ethanol liquid diet and the control group received an isocaloric diet without ethanol. Both groups received a binge gavage dose at days 10 and 30 (5 g/kg dose of a 31.5% ethanol solution and a 9 g/kg dose of a maltose–dextrin solution). At least 12 h after the gavage, echocardiography was performed to assess morphologic changes. After day 30, a subset of each group was euthanized for terminal cardiac functional measures and tissue collection. Remaining mice in the experimental group were switched from the alcohol diet back to the control diet for an additional 30 days (these were the abstinence mice), while the control group continued to receive the control diet. On day 60, echocardiography was performed again. Terminal left ventricular catheterization was performed the subsequent day to assess functional changes (
Figure 1).
2.2. Animals
Adult, male C57BL6/J mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) at 9–10 weeks old. Mice were given a week to acclimate to the animal care facilities at Louisiana State University Health Sciences Center (LSUHSC), before starting the liquid diet; these facilities are humidity and temperature controlled, and animals were monitored daily. The temperature was kept above 23 °C to prevent hypothermia, as alcohol causes the core body temperature to fall. Before the liquid diet was given, animals were fed a standard chow diet (Teklab Extruded Rodent Diet 2019S) and had ad libitum access to water. Mice were kept on a traditional 12 h/12 h light–dark cycle (6:00 a.m.–6:00 p.m. in light). All experiments and procedures were approved by the LSUHSC Institutional Animal Care and Use Committee (Protocol No. 3859).
2.3. Liquid Diet
The liquid diet was purchased from Bio-Serv, Flemington, NJ, USA (Products: F1258SP (Ethanol Shake and Pour Diet) and F1259SP (Control Shake and Pour Diet)) and administered in feeder tubes also purchased from Bio-Serv (Product Nos. 9019 and 9015). The control and ethanol diets were isocaloric diets, with ethanol accounting for 35% of calories in the ethanol diet. The diets were prepared as outlined in Bertola et al.; this chronic + binge model produces blood alcohol levels of ~180 mg/dL after 10 days on the 5% ethanol liquid diet and ~400 mg/dL after ethanol binges [
25]. Administration of the diet followed a modified version of the chronic + binge model. In our diet protocol, mice were given 5 days to acclimate to the liquid diet, where all animals were given the control liquid diet. After this period of acclimation, mice were randomly split into experimental and control groups. Experimental groups received a 5% ethanol liquid diet, while control animals continued receiving the control diet. Mice were pair-fed. The diet was administered fresh daily, within 2 h of the dark cycle. The ethanol diet was continued for 30 days to induce cardiomyopathy; then, a subgroup of mice was switched to the control diet (abstinence group) for an additional 30 days (~65 days total on liquid diets).
2.4. Gavage
Binge dosing by gavage was performed on days 10 and 30. Gavage was administered in the light portion of the light–dark cycle. Control mice received a maltose–dextrin solution (9 g/kg of body weight), while ethanol mice received a 31.5% (v/v) ethanol solution (5 g/kg of body weight). After gavage, mice were placed on a heated pad for an hour and then returned to their original cage location in the animal housing facility.
2.5. Echocardiography
To examine differences in left ventricular wall dimensions, echocardiography was performed after day 30 and day 60. Echocardiography was started a minimum of 12 h after ethanol gavage to avoid any cardio-depressive effects attributed to acute alcohol intoxication. A Vevo 3100 Imaging System and a 30 MHz probe from VisualSonics (Toronto, ON, Canada) were used. Isoflurane (1–2%) was used to immobilize the mice for imaging. Mice were kept on a 37 °C heated pad throughout the duration of the recordings, and the total time under anesthesia and obtaining measurements was under 15 min. Short and long axis views were obtained in B-mode and M-mode. Analysis was performed on M-mode images, using VisualSonics software (Vevo Lab 5.7.1). All measurements were made over a minimum of three cardiac cycles, and the averages were used for further analysis.
2.6. Left Ventricular Catheterization
After echocardiographic measurements were obtained, mice underwent terminal open chest left ventricular (LV) pressure-volume catheterization. LV catheterization was performed to assess systolic and diastolic function. Isoflurane at 3–4% was used for induction before mice were intubated; after intubation, anesthesia was maintained with 2–3% isoflurane. Mice were kept on a heated pad throughout the procedure. First, the skin on the anterior of the chest was dissected, revealing the abdominal muscles and the linea alba. An incision was made along the linea alba, starting inferior to the zyphoid process and through the sternum. Bleeding was minimal and controlled using both cauterization and bulldog clips as needed. After the pericardium was transected, the heart was revealed, and a 25 G needle was used to puncture the apex of the LV. A pressure-volume transducer (SPR-839; Millar, Houston, TX, USA) was inserted into the LV, through the hole created by the needle. The positioning of the transducer was achieved via observation of the pressure-volume loops that were plotted in real time. Anesthesia was closely monitored, and a heat lamp was used to aid core body temperature. For steady-state measurements, the transducer was kept in the left ventricle over several cardiac cycles. For load-independent measures, occlusion of the inferior vena cava was performed using blunted forceps covered with silastic tubing. Occlusion of the IVC reduces venous return (preload), which decreases the filling volume of the LV. The resulting PV loops are used to determine load-independent measures of cardiac function. E(es) is the slope of the end-systolic pressure-volume relationship, while preload-recruitable stroke work (PRSW) is the slope of stroke work versus end-diastolic volume. Both are measures of load-independent contractility. After occlusion data were collected, approximately 0.1 mL of 3% hypertonic saline was injected into the jugular vein for determination of parallel conductance. Volume calibration was performed using blood-filled cuvettes (Millar, Product No. 910–1049). LV pressures and volumes were recorded and analyzed using Labchart 8 cardiac axis pressure-volume loop software (Ver. 8.1.24).
2.7. Heart Collection
After catheterization and recordings were obtained, hearts were removed and placed in cold saline. They were gently blotted with gauze, and the total heart weight was recorded. Under a microscope, hearts were dissected. Atria were removed, and the right ventricle (RV) was separated from the LV and interventricular septum (IVS). RV weights were recorded, as were LV and septal weights. Then, the IVS was removed from the LV. The RVs and LVs with separated IVSs were snap frozen in cryotubes using liquid nitrogen. Tibias were obtained for normalization of weights to tibial lengths.
2.8. Quantitative Real-Time PCR (qPCR)
LV samples were homogenized in FisherbrandTM Pre-Filled Bead Mill Tubes (1.4 mm ceramic beads, Cat. # 15-340-153) using Benchmark Scientific BeadBlasterTM. Total RNA was isolated using an RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany, Cat. # 74804) according to the manufacturer’s protocol. RNA concentration and quality were analyzed using a NanoDropTM 1000 (Thermo Scientific, Waltham, MA, USA). RNA (2 µg) was reverse transcribed with the High-Capacity cDNA Reverse Transcription Kit (Applied BiosystemsTM, Foster City, CA, USA, Cat. # 4368814) according to the manufacturer’s protocol with the following cycling conditions: 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 5 min. qPCR was performed using TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific, Cat. # 4444963) in a CFX Opus 96 Real-Time PCR System (BioRad, Hercules, CA, USA) for the following targets: interleukin 1 beta (IL-1β, assay Mm00434228_m1), interleukin 6 (IL-6, assay Mm00446191_m1), interleukin 10 (IL-10, assay Mm01288386_m1), La Ribonucleoprotein 6 (LARP6, assay Mm00470891_m1), collagen type 1 alpha-1 (COL1A1, assay Mm00801666_g1), and collagen type 3 alpha-1 (COL3A1, assay Mm00802300_m1). The cycling conditions for qPCR included polymerase activation (95 °C for 20 s) followed by 40 cycles of denaturing (95 °C for 1 s) and annealing/extending (60 °C for 20 s). Relative quantification was calculated using the comparative CT method. 18S ribosomal RNA (18S rRNA, assay Mm03928990_g1) was used as a reference gene to normalize gene expression data.
2.9. Histology
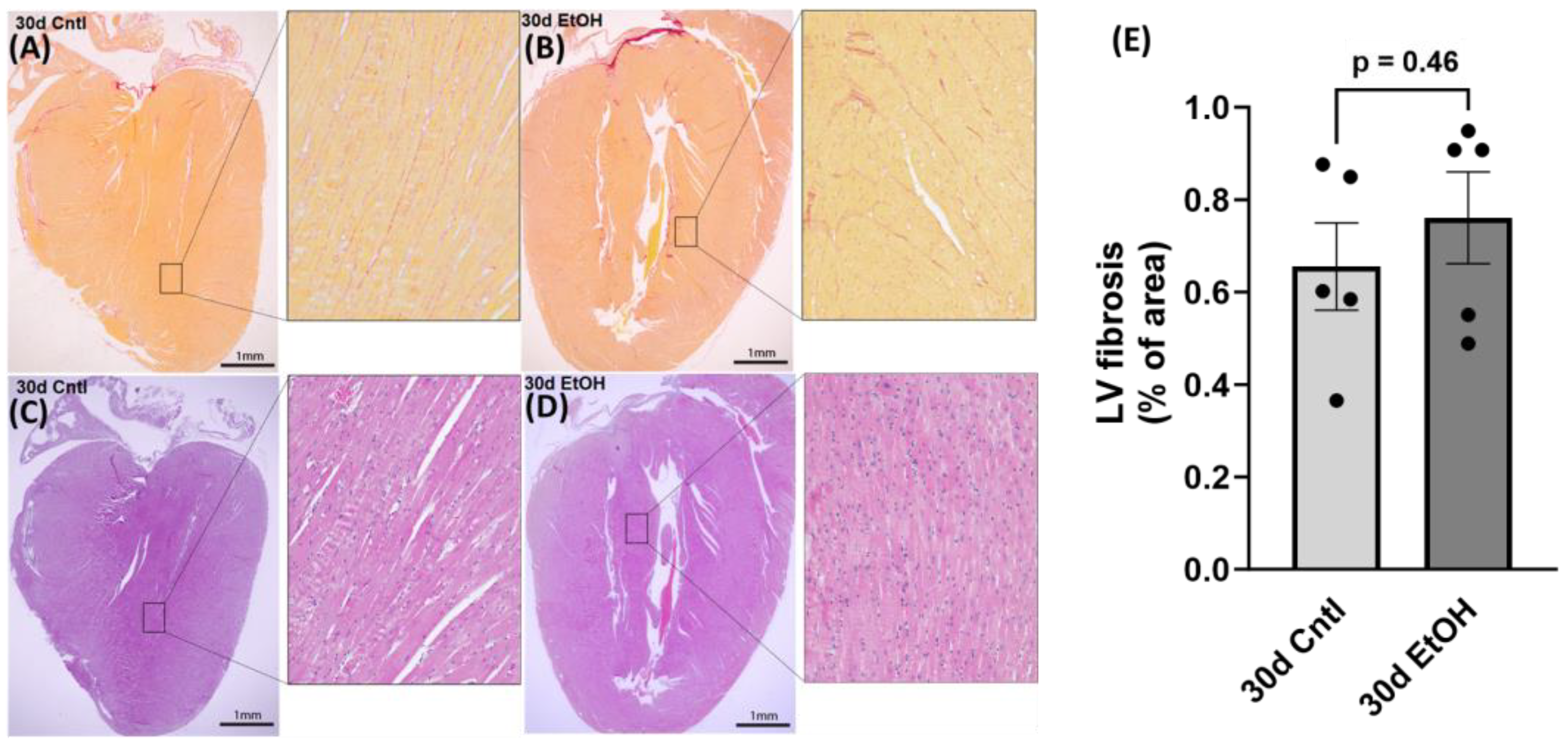
After routine formalin-fixed, paraffin-embedded specimen processing, 4 µm thick heart sections were prepared and stained with sirius red and hematoxylin–eosin (H&E) for the histological evaluation of cardiac fibrosis and morphology, respectively. For the quantitative analysis of sirius red staining, 8–10 random areas of the LV were selected from 5–6 different sections in each group using an Olympus BX-53 microscope (Olympus, Center Valley, PA, USA). Images were collected at 20× and subsequently analyzed using the NIH software ImageJ (
https://imagej.net/ij/, accessed on 11 October 2023), and sirius red coverage was expressed as a percentage of total imaged area. We performed histological assessment on samples from the 30-day timepoint to assess any changes due to chronic + binge ethanol.
2.10. Statistical Analysis
Student’s t test or a one-way ANOVA was used to assess significant differences in data collected, as appropriate. Findings were expressed as mean and standard error of the mean, with the exception of weights, which were expressed as mean and standard deviation. Statistical analysis and presentation were completed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). p < 0.05 was considered significant.
4. Discussion
As one of the most widely used drugs, alcohol can be consumed excessively and lead to AUD, which can ultimately cause death due to both primary and secondary pathological challenges. While many of these challenges involve hepatic and neural health, the heart is also adversely affected by excessive alcohol consumption, and the effects and magnitude of these effects are dependent on the dose and frequency of alcohol use, as well as individual risk factors such as genetics [
26]. While some studies have shown that there may be slight benefits to drinking low amounts of alcohol [
27], various pathological issues arise at different levels of consumption. Less than 20 drinks a week is associated with a decreased risk for both ischemic and hemorrhagic stroke [
14], but consuming as little as 1.2 drinks a day significantly increases the risk of atrial fibrillation [
9]. Over time, heavy alcohol use can lead to ACM, which has a poor prognosis. If patients do not completely abstain from alcohol use, there is a 50% chance of death within four years [
3]. Despite this, human data and animal studies on the potential for restoration of cardiac function after developing ACM are limited. Before the diagnosis of ACM, there is an asymptomatic phase, but this asymptomatic phase can progress to systolic heart failure [
28], from which there is no known cure. The underlying pathophysiology and the progression of the asymptomatic phase of ACM to heart failure are still being studied. Several researchers have investigated the direct cardiotoxic effects of alcohol and its metabolites [
19], but the advancement to cardiac dysfunction has been difficult to describe, as the timeline and characteristics of the asymptomatic phase vary individually and may not warrant scrutiny. Furthermore, the exact amount of alcohol needed to reach symptomatic dysfunction is unknown. Diastolic dysfunction is present in at least 30% of patients with chronic alcohol consumption [
29], and ventricular dilation can be the result of cardiotoxicity and of heart failure. Dilation leads to an increased end-diastolic and end-systolic volume, which impairs both diastole and systole; progressive dilation can also lead to morphological pathologies such as mitral and tricuspid regurgitation [
30]. However, the order of events leading to ACM is unknown, and some clinical studies have even shown that depressed systolic function is present in asymptomatic patients with AUD [
31]. A recent paper by Mirijello et al. discusses the uncertainty surrounding the progression of ACM to a symptomatic phase, characterized by systolic failure, dilation, and fibrosis [
24].
Symptoms such as a decreased ejection fraction, dilation of the LV, and impairment of contractility [
32] can be managed pharmacologically, but abstinence is the most recommended method of halting disease advancement. Our protocol of alcohol administration in mice resulted in significant diastolic and systolic dysfunction over the course of 30 days, and variations of this model have been used to study myocardial oxidative stress, mitochondrial stress, and cardiac dysfunction and steatosis [
33]. To our knowledge, no studies have characterized functional recovery from ACM in a preclinical animal model, though in 1997, a clinical paper studying nine patients showed that remarkable increases in ejection fraction were possible with abstinence [
34]. Therefore, our primary research goal was to investigate the effects of abstinence from alcohol on cardiac morphology and function in mice with established ACM. Our hypothesis was that abstinence would lead to improvement in cardiac function.
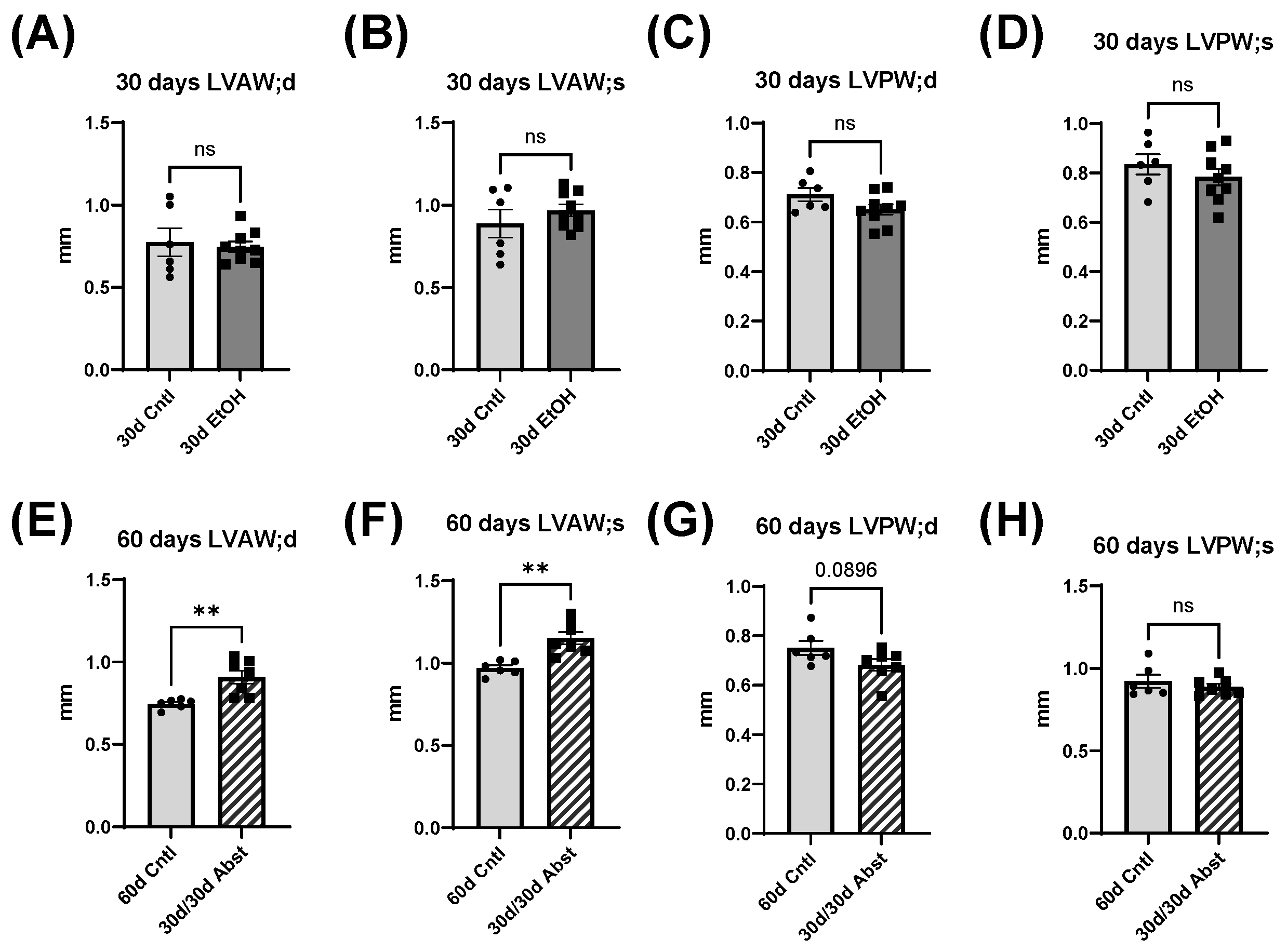
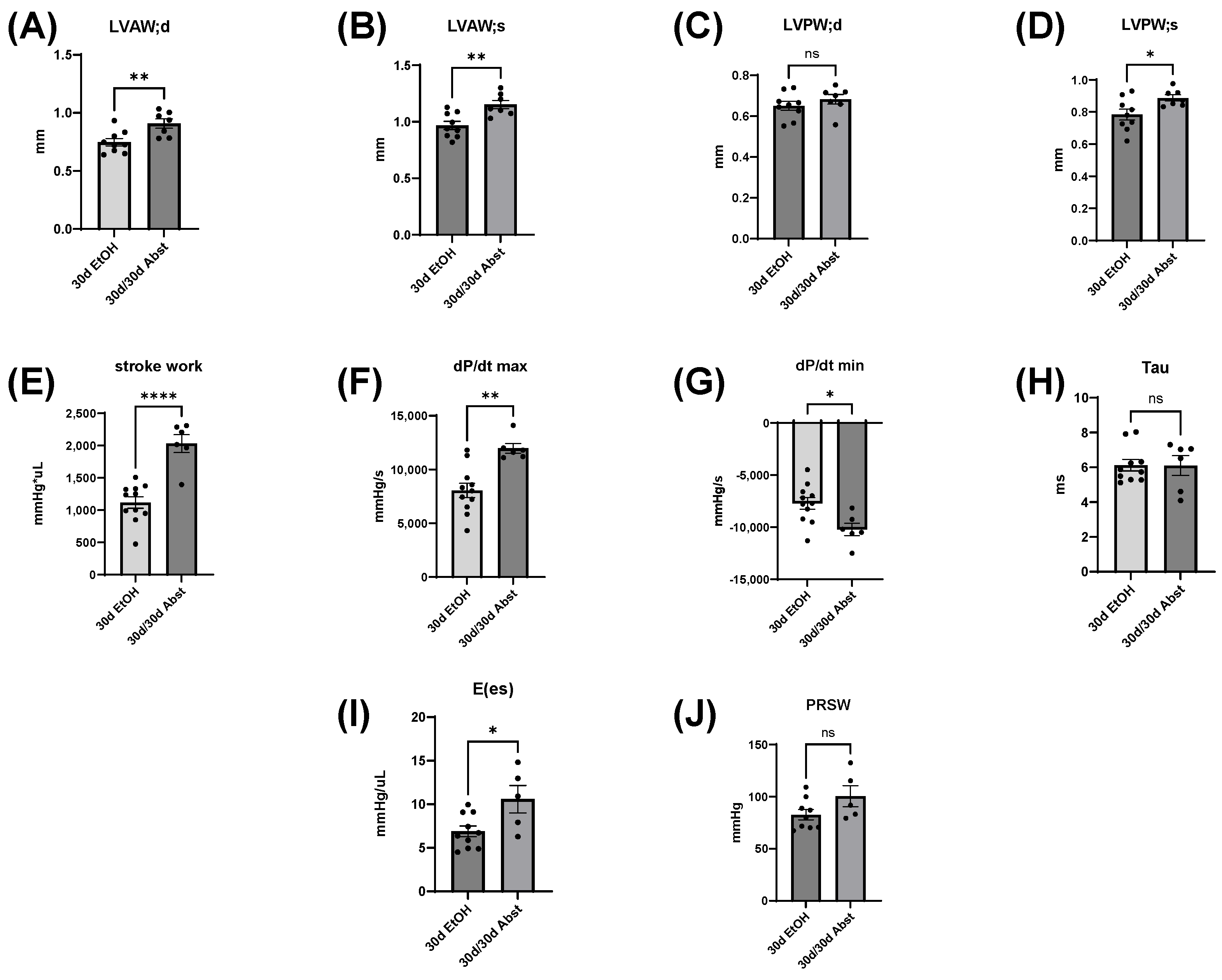
Our findings indicate that full functional recovery from alcohol-induced cardiac dysfunction is possible given a period of abstinence. Heart weights and echocardiography showed that significant cardiac dysfunction was present without overt morphological changes. Our chronic + binge model of alcohol consumption produced both systolic and diastolic dysfunction, evidenced by LV catheterization and pressure-volume loop analyses. Notably, the systolic dysfunction was apparent in load-independent measurements, indicating a reduction in cardiac contractility. After a period of abstinence, these mice made a remarkable recovery. LV catheterization showed that there was a recovery of diastolic function, as well as load-dependent and -independent systolic function. Echocardiography indicated that LV dimensions increased after abstinence, but this primarily occurred in the anterior wall.
Body weight, heart weight, and tibial length provided important information on the recovery of the abstained mice. First, their hearts increased in weight, particularly the LV, and this increase in size brought them to the same heart weights as mice that were on the control diet. Abstinence restored cardiac function back to a control level, and in some measures, exceeded control levels. A recent study showed that cardiac growth can be compromised by alcohol consumption in adolescent Wistar rats [
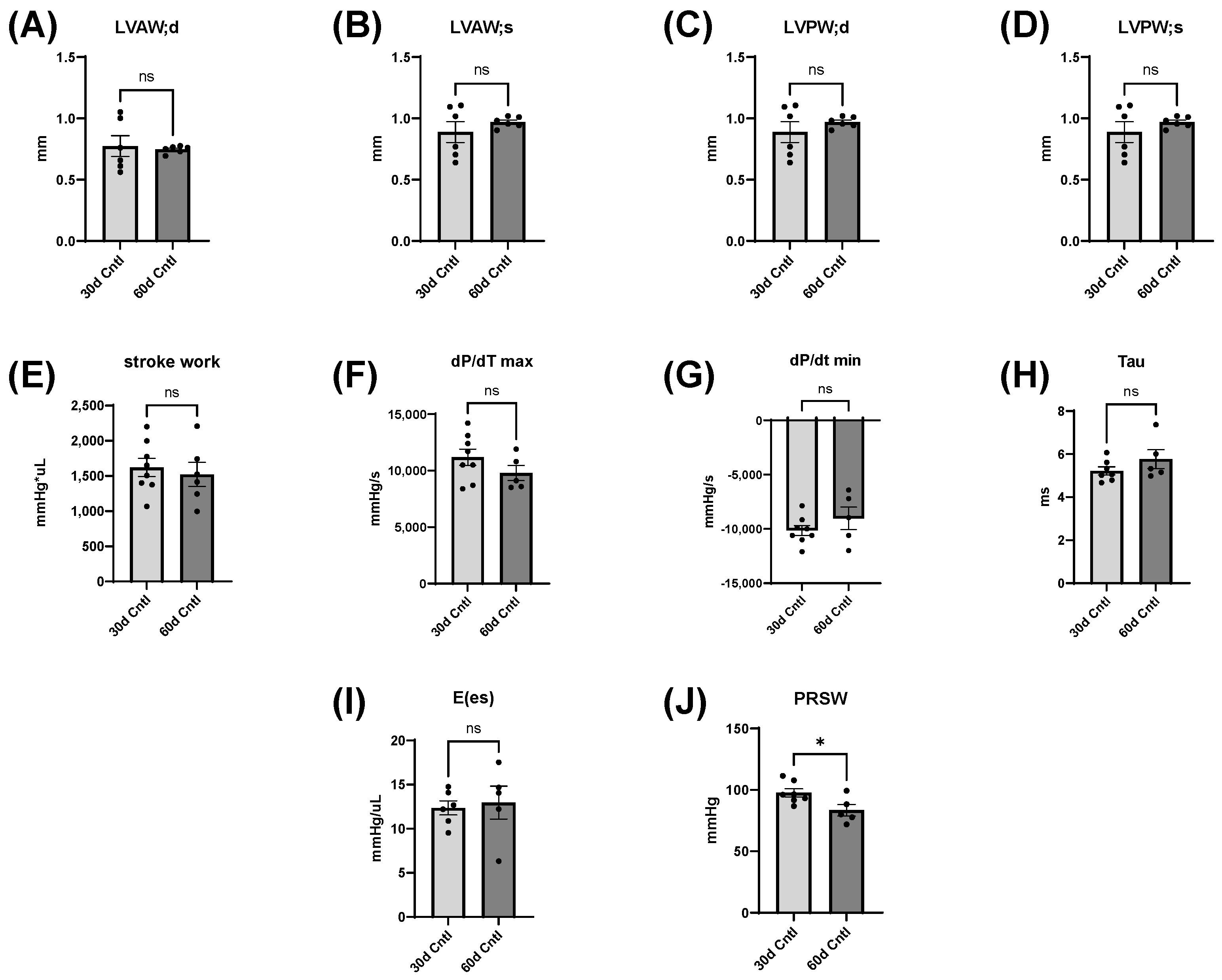
35], which is consistent with our findings, though our mice were already in early adulthood. It is possible that alcohol consumption delayed the growth of their hearts, which rebounded after abstinence. There were no significant differences in body weight except for between the 30- and 60-day control mice and between 30-day ethanol mice and 60-day control mice. It is reasonable to expect these changes, as mice around this age should gain weight from 30 to 60 days.
The heart can hypertrophy in different patterns to adapt to increased stress and demand. Our laboratory has previously used surgical approaches to induce pressure and volume overload and studied the resulting cardiac responses and pathological states [
36,
37,
38]. We found that while alcohol can activate cardiac fibroblasts (and cause differentiation to cardiac myofibroblasts), which is consistent with findings by Law and Carver [
39], chronic ethanol exposure also reduces adaptive responses of the heart to both pressure and volume overload [
37,
38]. Furthermore, alcohol causes accelerated eccentric remodeling in response to chronic volume overload [
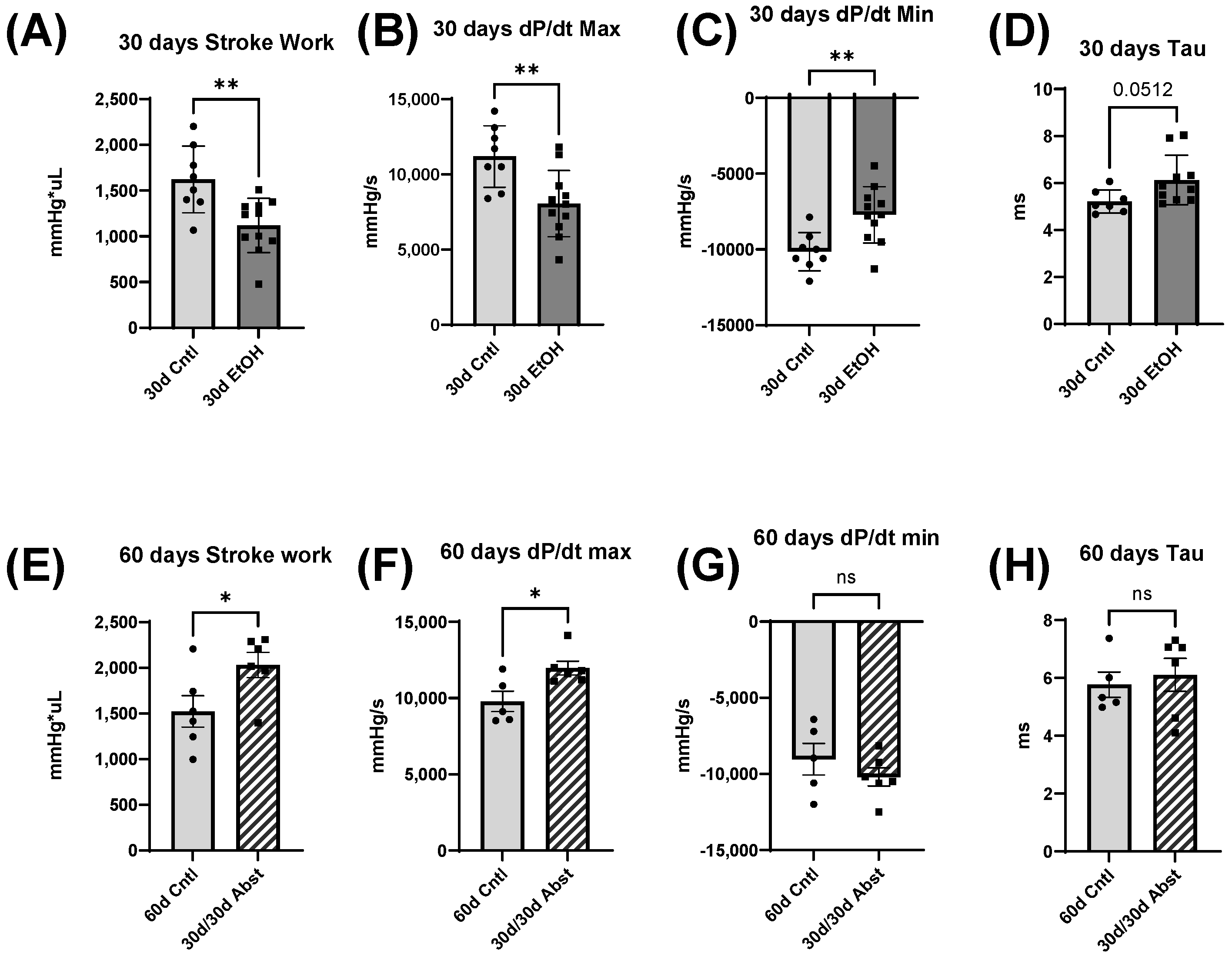
38]. Our model of chronic + binge alcohol consumption produced diastolic dysfunction as determined by LV catheterization, but echocardiography did not show a significant decrease or increase in LV dimensions at the 30-day timepoint. What was found, however, was that abstained mice exhibited increased LVAW thickness in both systole and diastole compared to control mice. Both the LVAW and the LVPW increased in size during abstinence compared to their size at 30 days. The increase in LVAW and LVPW can potentially be explained by abstinence removing the impairment that alcohol had on cardiac growth. Pressure-volume loop analysis is the gold standard for measuring in vivo cardiac function, as it is highly sensitive and yields an abundance of data regarding both load-dependent and -independent ventricular function. Stroke work is crucial in the context of systolic function and is the work needed to eject blood from the LV through the aortic valve. This measure describes mechanical efficiency. Stroke work was significantly lower in ethanol-fed mice versus the control mice, indicating that alcohol reduced contractility and systolic function. This effect of alcohol was completely reversed after the 30-day period of abstinence.
dP/dt is the derivative of pressure (P) with respect to time (t); this is a measurement of the rate of change in pressure. dP/dt max refers to the maximum rate of pressure generated, while dP/dt min refers to the maximum rate of pressure decrease. The maximum pressure generated in a cardiac cycle occurs part way through the ejection phase, as the heart reaches its maximal contraction. Thus, dP/dt max can be thought of as a measurement of the rate at which peak contraction occurs during systole. The lowest pressure generated by the LV occurs at the end of isovolumetric relaxation; the chamber creates negative pressure by expanding while both the mitral and aortic valves are closed. Thus, dP/dt min is a measurement of the rate at which peak relaxation occurs during diastole. At 30 days, dP/dt max was significantly less in mice that had been consuming alcohol; this reflects a reduction in the ability of the heart to generate the necessary force to pump blood effectively. dP/dt min was also significantly reduced in these mice and reflects the reduced ability of the heart to relax efficiently. Both dP/dt min and max completely recovered after 30 days of abstinence.
Tau, another index of diastolic function, is the time constant of isovolumetric relaxation; it characterizes the exponential decline in pressure as the ventricles relax and blood starts to flow back into the ventricles from the atria. There was a trend of Tau increasing in the mice that had consumed ethanol for 30 days (p = 0.051). A larger Tau indicates an increased amount of time needed to fully relax during diastole. This increase in Tau was completely reversed by abstinence.
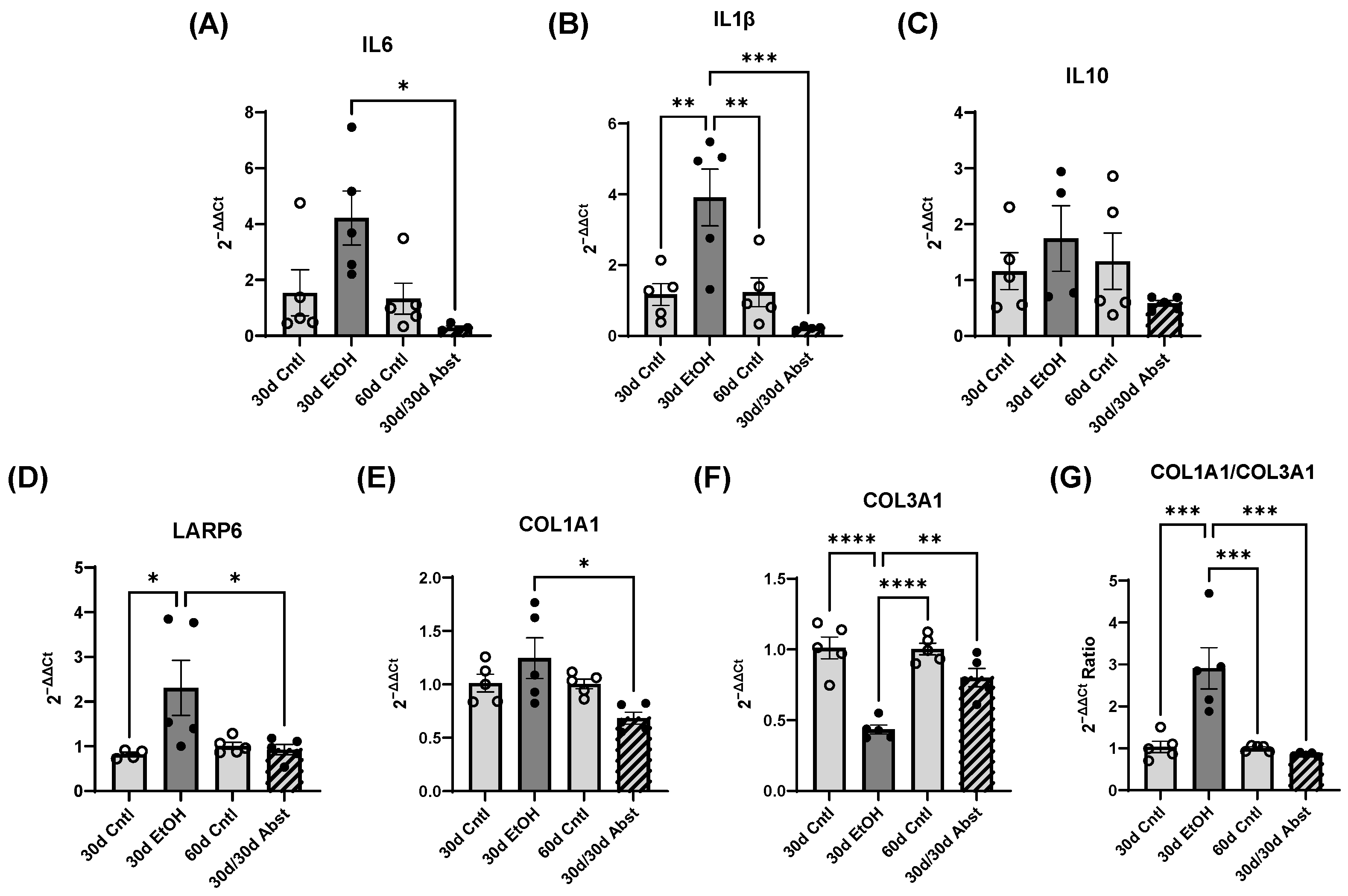
Inferior vena cava occlusion during pressure–volume catheterization is used to assess load-independent measures of contractility, as occlusion decreases the volume of preload and measurements can be assessed over multiple volumes. The end-systolic pressure-volume relationship is linear, and as preload decreases, the end-systolic pressure and the end-systolic volume will also decrease. The slope of this line, E(es), or the ventricular elastance, is a load-independent measurement of cardiac contractility. Typically, a steeper slope indicates more forceful ventricular contraction. At thirty days, mice consuming the ethanol diet had a significantly decreased E(es), illustrating an intrinsic reduction in cardiac contractility, not dependent on Frank–Starling forces. This dysfunction was completely reversed after abstinence. Preload-recruitable stroke work (PRSW) uses inferior vena cava occlusion to plot stroke work against end-diastolic volume and is another measure of intrinsic contractility; a steeper slope indicates greater contractility. PRSW was significantly lower in mice on the ethanol diet at 30 days compared to control mice at 30 days, which, similarly to E(es), indicates that these mice had decreased systolic function, independent of preload. This decrease in contractility was also completely reversed with abstinence, as the 30d/30d Abst mice did not have significantly different PRSW values compared to control mice at 60 days. After abstinence, we observed a reduction in both inflammatory and fibrotic biomarkers in the LV, evidenced by qPCR. Chronic ethanol exposure in C57BL6/J mice increases pro-inflammatory cytokines in several tissues, including the heart [
40], and clinically, IL6 and IL1β are known to be elevated with alcohol abuse [
41]. We observed an increase in these biomarkers after 30 days of ethanol exposure. After abstinence, we observed a reduction in IL6 and IL1β compared to the levels of these biomarkers after 30 days of alcohol exposure. This decrease brought the 30d/30d Abst mice to control levels of IL6 and IL1β, as there was no significant difference between 30d/30d Abst mice and 60d Cntl mice (as well as 30d Cntl mice). This reduction in inflammation after abstinence has also recently been found in the liver, in C57BL6/J mice fed the Leiber-DeCarli liquid diet with ethanol for 28 days [
42]. Taken together, these data suggest that while chronic alcohol does lead to inflammation, resolution is possible with the cessation of alcohol intake. Chronic alcohol exposure increases collagen expression in some animal models [
43], and cardiac fibrosis is a hallmark of clinical ACM [
21]. Collagen I and III serve as structural support in the heart, but collagen I is more stiff, while collagen III is more elastic [
44]. We found significant diastolic dysfunction after 30 days of alcohol exposure. We also observed an increase in COL1A1 and a decrease in COL3A1 expression, which could indicate a stiffer heart. This ratio of COL1A1 to COL3A1 returned to normal after abstinence, which was consistent with our functional findings of restored diastolic function in those animals. We also found an increase in LARP6 in the 30d EtOH mice; LARP6 is a positive regulator of collagen expression [
45], and inhibition of LARP6 decreases hepatic fibrosis resulting from alcohol exposure [
46]. In 30d EtOH mice, LARP6 was increased relative to the control, but after abstinence, mRNA abundance decreased to control levels. While cardiac fibrosis is a key finding in clinical ACM, preclinical models do not accurately represent these changes in cardiac morphology. Despite finding a reduction in diastolic function and increased expression of COL1A1 after 30 days of alcohol, we did not find evidence of LV fibrosis in histological sections.
Alcohol and its effects have been studied in various animal models and ethanol intake paradigms, all with their own strengths and weaknesses, but fewer studies focus on the cardiac effects of alcohol. A hallmark of clinical ACM is significant cardiac fibrosis, which can arise from various pathogenic processes including microvascular ischemia, increased inflammation, neurohormonal activation, wall stress, and direct damage [
47]. Fibrosis is often a secondary concern in clinical ACM, as patients typically present with symptoms of heart failure and systolic dysfunction, which arise after fibrosis and diastolic dysfunction. Although fibrosis occurs prior to overt systolic failure, cardiac morphology, dimensions, and apparent fibrosis can aid in the staging of a patient’s risk. Few preclinical studies using alcohol administration have been able to mimic the extensive cardiac fibrosis present in clinical ACM, though one genetic mouse line with knocked-out metallothionine expression exhibited cardiac fibrosis with heavy alcohol exposure [
48]. Alcohol and its metabolites (acetaldehyde and reactive oxygen species (ROS)) are cardiotoxic and have direct toxic effects on the myocardium. Cardiomyocyte dysfunction could also occur through disruption of mitochondrial function by alcohol [
33,
49] or through activation of neurohormonal signaling, such as the renin–angiotensin–aldosterone system [
5,
50].
While the findings of this study provide valuable insights into the topic at hand, it is essential to acknowledge limitations that warrant consideration in interpreting the results. While we observed a functional recovery after abstinence in a preclinical model, humans consume alcohol for years, at varying amounts, and the point at which irreversible damage occurs is unknown [
51]. No animal models perfectly mimic clinical ACM, and it is particularly difficult to reproduce the extensive fibrosis typically present. The chronic + binge ACM mouse model does not lead to overt fibrosis but does produce robust and consistent cardiomyopathy with both diastolic and systolic functional depression as seen clinically. Another limitation of our study is the absence of a female cohort; these studies are currently underway, but further investigation is needed. While clinical AUDs affect males disproportionately more than females [
52], females, in several pathological measures, have worse health outcomes related to alcohol-induced damage [
53]. Given the limited existing data and knowledge on reversal of cardiac damage with abstinence, we chose to initially concentrate on a male cohort to establish a foundational understanding before potentially expanding our investigation to include female animals. It is not known if the chronic + binge models will exhibit sex differences in cardiac dysfunction or recovery after abstinence.