Wnt/β-Catenin-Signaling Modulates Megakaryopoiesis at the Megakaryocyte-Erythrocyte Progenitor Stage in the Hematopoietic System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Transgene Induction
2.2. Organ Dissection, Cryopreservation and Paraffin Embedding
2.3. Histology and Immunofluorescence
Preparation of Cryosections
2.4. Bone Decalcification
2.5. Complete Blood Cell (CBC) Analysis
2.6. Immunofluorescence Staining (IFS)
2.7. Immunohistochemistry Staining (IHC)
2.8. Image Acquisition and Processing
2.9. Preparation of Blood and BM Samples for FACS Analysis
Detection of Blood Cell Types
2.10. Preparation of BM Samples for FACS Analysis
2.11. Preparation of Spleen Samples for FACS Analysis
2.12. Detection of GFP+MKPs/MKs
2.13. Detection of HSCs and Progenitor Cells in the BM
2.14. RNA Isolation and RT-PCR and qRT-PCR Analyses
2.15. Bone Marrow Transplantation (BMT)
2.16. Detection of MKPs in BMT Animals
2.17. CFU—Assay Analysis
2.18. Colony Identification and Scoring
2.19. Statistics
2.20. Graphics and Artwork
3. Results
3.1. PDGFb-iCreERT2-Mediated Wnt/β-Catenin Pathway Activation in Mice Led to a Lethal Phenotype, Erythrocyte Reduction and Increased Megakaryopoiesis
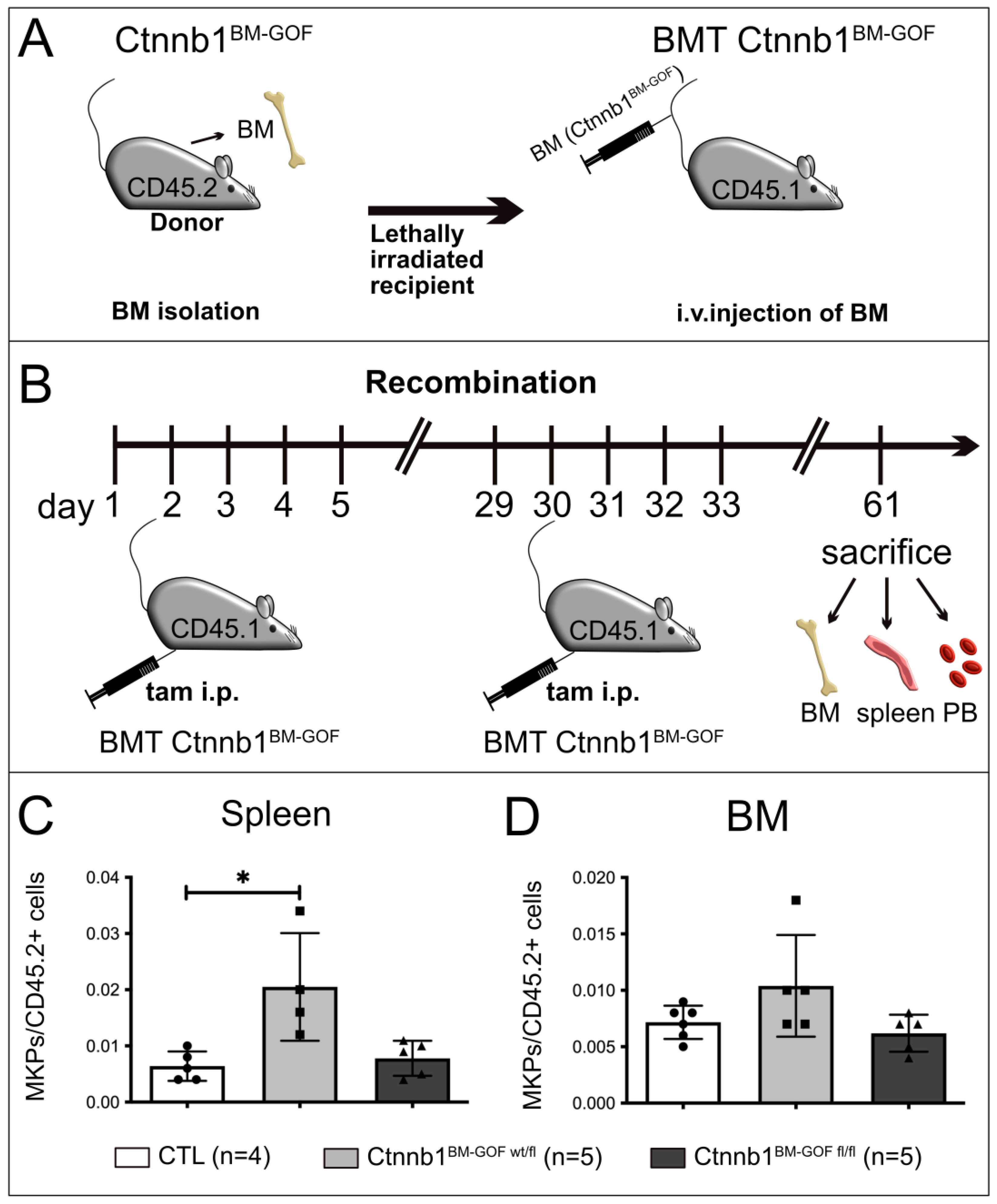
3.2. Bone Marrow Transplantation (BMT) from Ctnnb1BM-GOF Mice into Lethally Irradiated Controls Corroborated Increased Megakaryopoiesis in Mutant BM Cells
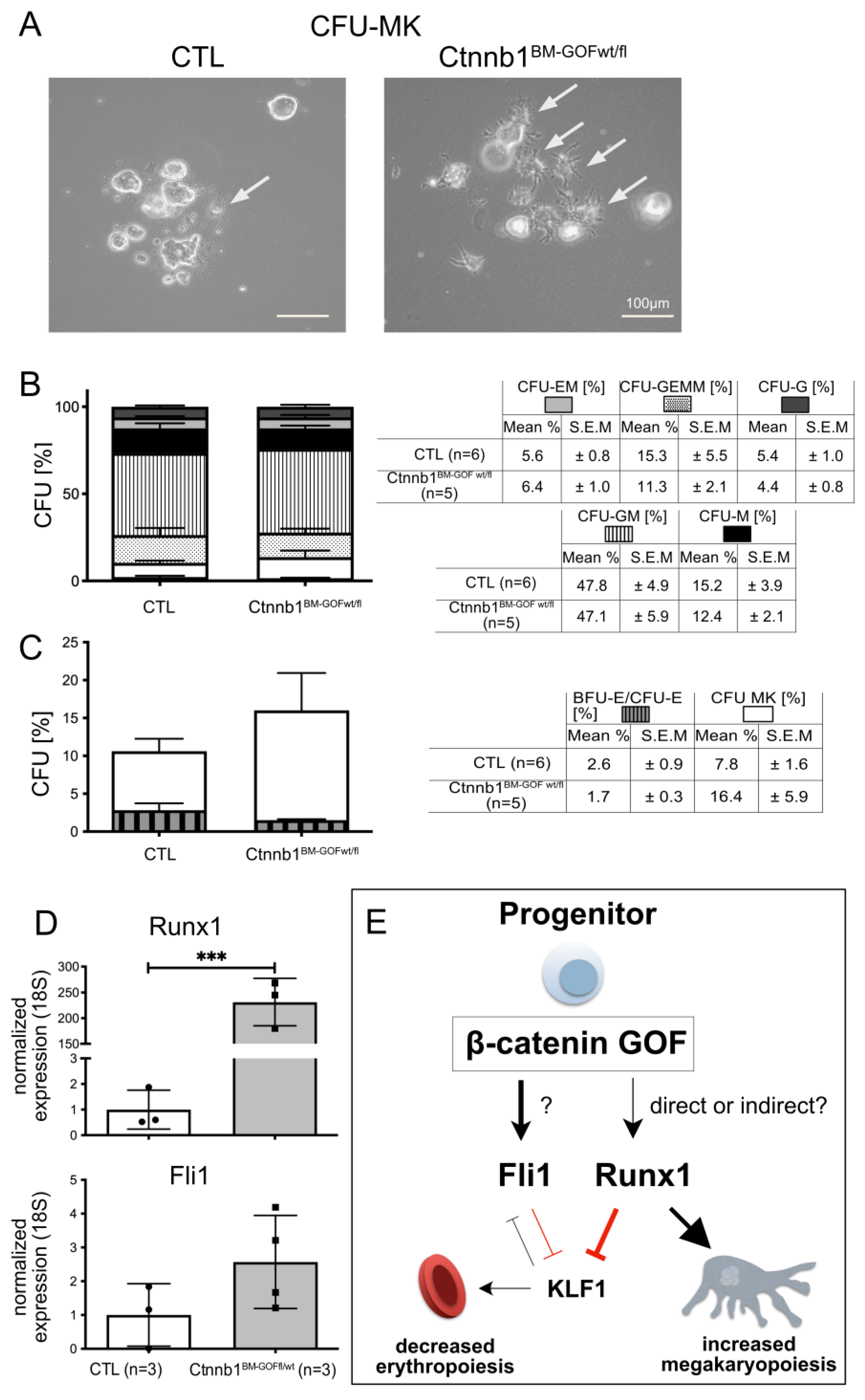
3.3. Activating Wnt/β-Catenin Signaling in Ctnnb1BM-GOF Cells In Vitro Leads to Enhanced Megakaryopoiesis and Decreased Erythropoiesis
3.4. PDGFb-iCreERT2-Mediated Wnt/β-Catenin Pathway Activation in MKPs Drives Megakaryopoiesis by Regulating Key Transcriptions Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Challen, G.A.; Boles, N.; Lin, K.K.; Goodell, M.A. Mouse Hematopoietic Stem Cell Identification and Analysis. Cytom. Part A 2009, 75A, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A Clonogenic Common Myeloid Progenitor That Gives Rise to All Myeloid Lineages. Nature 2000, 404, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Weissman, I.L.; Akashi, K. Identification of Clonogenic Common Lymphoid Progenitors in Mouse Bone Marrow. Cell 1997, 91, 661–672. [Google Scholar] [CrossRef]
- Kuvardina, O.N.; Herglotz, J.; Kolodziej, S.; Kohrs, N.; Herkt, S.; Wojcik, B.; Oellerich, T.; Corso, J.; Behrens, K.; Kumar, A.; et al. RUNX1 Represses the Erythroid Gene Expression Program during Megakaryocytic Differentiation. Blood 2015, 125, 3570–3579. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Herkt, S.; Wang, L.; Feld, C.; Wesely, J.; Kuvardina, O.N.; Meyer, A.; Oellerich, T.; Häupl, B.; Seifried, E.; et al. PRMT6 Activates Cyclin D1 Expression in Conjunction with the Transcription Factor LEF1. Oncogenesis 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.H.M.; Ailles, L.E.; Dylla, S.J.; Muijtjens, M.; Jones, C.; Zehnder, J.L.; Gotlib, J.; Li, K.; Manz, M.G.; Keating, A.; et al. Granulocyte–Macrophage Progenitors as Candidate Leukemic Stem Cells in Blast-Crisis CML. N. Engl. J. Med. 2004, 351, 657–667. [Google Scholar] [CrossRef]
- Abrahamsson, A.E.; Geron, I.; Gotlib, J.; Dao, K.-H.T.; Barroga, C.F.; Newton, I.G.; Giles, F.J.; Durocher, J.; Creusot, R.S.; Karimi, M.; et al. Glycogen Synthase Kinase 3β Missplicing Contributes to Leukemia Stem Cell Generation. Proc. Natl. Acad. Sci. USA 2009, 106, 3925–3929. [Google Scholar] [CrossRef]
- Wang, Y.; Krivtsov, A.V.; Sinha, A.U.; North, T.E.; Goessling, W.; Feng, Z.; Zon, L.I.; Armstrong, S.A. The Wnt/β-Catenin Pathway Is Required for the Development of Leukemia Stem Cells in AML. Science 2010, 327, 1650–1653. [Google Scholar] [CrossRef]
- Geduk, A.; Atesoglu, E.B.; Tarkun, P.; Mehtap, O.; Hacihanefioglu, A.; Demirsoy, E.T.; Baydemir, C. The Role of β-Catenin in Bcr/Abl Negative Myeloproliferative Neoplasms: An Immunohistochemical Study. Clin. Lymphoma Myeloma Leuk. 2015, 15, 785–789. [Google Scholar] [CrossRef]
- Heil, J.; Olsavszky, V.; Busch, K.; Klapproth, K.; de la Torre, C.; Sticht, C.; Sandorski, K.; Hoffmann, J.; Schönhaber, H.; Zierow, J.; et al. Bone Marrow Sinusoidal Endothelium Controls Terminal Erythroid Differentiation and Reticulocyte Maturation. Nat. Commun. 2021, 12, 6963. [Google Scholar] [CrossRef]
- Macaulay, I.C.; Thon, J.N.; Tijssen, M.R.; Steele, B.M.; Macdonald, B.T.; Meade, G.; Burns, P.; Rendon, A.; Salunkhe, V.; Murphy, R.P.; et al. Canonical Wnt Signaling in Megakaryocytes Regulates Proplatelet Formation. Blood 2013, 121, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Scheller, M.; Huelsken, J.; Rosenbauer, F.; Taketo, M.M.; Birchmeier, W.; Tenen, D.G.; Leutz, A. Hematopoietic Stem Cell and Multilineage Defects Generated by Constitutive Beta-Catenin Activation. Nat. Immunol. 2006, 7, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Claxton, S.; Kostourou, V.; Jadeja, S.; Chambon, P.; Hodivala-Dilke, K.; Fruttiger, M. Efficient, Inducible Cre-recombinase Activation in Vascular Endothelium. Genesis 2008, 46, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakayama, M.; Pitulescu, M.E.; Schmidt, T.S.; Bochenek, M.L.; Sakakibara, A.; Adams, S.; Davy, A.; Deutsch, U.; Lüthi, U.; et al. Ephrin-B2 Controls VEGF-Induced Angiogenesis and Lymphangiogenesis. Nature 2010, 465, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Tamai, Y.; Ishikawa, T.; Sauer, B.; Takaku, K.; Oshima, M.; Taketo, M.M. Intestinal Polyposis in Mice with a Dominant Stable Mutation of the Beta-Catenin Gene. EMBO J. 1999, 18, 5931–5942. [Google Scholar] [CrossRef] [PubMed]
- Pitulescu, M.E.; Schmidt, I.; Benedito, R.; Adams, R.H. Inducible Gene Targeting in the Neonatal Vasculature and Analysis of Retinal Angiogenesis in Mice. Nat. Protoc. 2010, 5, 1518–1534. [Google Scholar] [CrossRef] [PubMed]
- Guérit, S.; Fidan, E.; Macas, J.; Czupalla, C.J.; Figueiredo, R.; Vijikumar, A.; Yalcin, B.H.; Thom, S.; Winter, P.; Gerhardt, H.; et al. Astrocyte-Derived Wnt Growth Factors Are Required for Endothelial Blood-Brain Barrier Maintenance. Prog. Neurobiol. 2021, 199, 101937. [Google Scholar] [CrossRef]
- Benz, F.; Wichitnaowarat, V.; Lehmann, M.; Germano, R.F.; Mihova, D.; Macas, J.; Adams, R.H.; Taketo, M.M.; Plate, K.H.; Guérit, S.; et al. Low Wnt/β-Catenin Signaling Determines Leaky Vessels in the Subfornical Organ and Affects Water Homeostasis in Mice. eLife 2019, 8, 204. [Google Scholar] [CrossRef]
- Robin, C.; Ottersbach, K.; Boisset, J.-C.; Oziemlak, A.; Dzierzak, E. CD41 Is Developmentally Regulated and Differentially Expressed on Mouse Hematopoietic Stem Cells. Blood 2011, 117, 5088–5091. [Google Scholar] [CrossRef]
- Nakagawa, A.; Naito, A.T.; Sumida, T.; Nomura, S.; Shibamoto, M.; Higo, T.; Okada, K.; Sakai, T.; Hashimoto, A.; Kuramoto, Y.; et al. Activation of Endothelial β-Catenin Signaling Induces Heart Failure. Sci. Rep. 2016, 6, 25009. [Google Scholar] [CrossRef]
- Itkin, T.; Gur-Cohen, S.; Spencer, J.A.; Schajnovitz, A.; Ramasamy, S.K.; Kusumbe, A.P.; Ledergor, G.; Jung, Y.; Milo, I.; Poulos, M.G.; et al. Distinct Bone Marrow Blood Vessels Differentially Regulate Haematopoiesis. Nature 2016, 532, 323–328. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Adams, R.H. Osteoclast Progenitors Promote Bone Vascularization and Osteogenesis. Nat. Med. 2014, 20, 1238–1240. [Google Scholar] [CrossRef]
- Yeung, J.; Esposito, M.T.; Gandillet, A.; Zeisig, B.B.; Griessinger, E.; Bonnet, D.; So, C.W.E. β-Catenin Mediates the Establishment and Drug Resistance of MLL Leukemic Stem Cells. Cancer Cell 2010, 18, 606–618. [Google Scholar] [CrossRef]
- Gandhirajan, R.K.; Staib, P.A.; Minke, K.; Gehrke, I.; Plickert, G.; Schlösser, A.; Schmitt, E.K.; Hallek, M.; Kreuzer, K.-A. Small Molecule Inhibitors of Wnt/Beta-Catenin/Lef-1 Signaling Induces Apoptosis in Chronic Lymphocytic Leukemia Cells in Vitro and in Vivo. Neoplasia 2010, 12, 326–335. [Google Scholar] [CrossRef]
- Nakeff, A.; Maat, B. Separation of Megakaryocytes From Mouse Bone Marrow by Velocity Sedimentation. Blood 1974, 43, 591–595. [Google Scholar] [CrossRef]
- Bartley, T.D.; Bogenberger, J.; Hunt, P.; Li, Y.-S.; Lu, H.S.; Martin, F.; Chang, M.-S.; Samal, B.; Nichol, J.L.; Swift, S.; et al. Identification and Cloning of a Megakaryocyte Growth and Development Factor That Is a Ligand for the Cytokine Receptor MpI. Cell 1994, 77, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Machlus, K.R.; Italiano, J.E. The Incredible Journey: From Megakaryocyte Development to Platelet formationMegakaryocyte Development and Platelet Formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.; Charo, I.; Parise, L.; Fitzgerald, L. The Platelet Membrane Glycoprotein IIb-IIIa Complex. Blood 1988, 71, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.; Ralph, S.; Tan, A. Cell-Lineage Antigens of the Stem Cell-Megakaryocyte-Platelet Lineage Are Associated with the Platelet IIb-IIIa Glycoprotein Complex. Blood 1985, 66, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Debili, N.; Robin, C.; Schiavon, V.; Letestu, R.; Pflumio, F.; Mitjavila-Garcia, M.-T.; Coulombel, L.; Vainchenker, W. Different Expression of CD41 on Human Lymphoid and Myeloid Progenitors from Adults and Neonates. Blood 2001, 97, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Leahy, M.; Berridge, M. Expression of Antigens of the Platelet Glycoprotein IIb/IIIa Complex on Human Hematopoietic Stem Cells. Blood 1986, 68, 762–769. [Google Scholar] [CrossRef]
- Weksberg, D.C.; Chambers, S.M.; Boles, N.C.; Goodell, M.A. CD150− Side Population Cells Represent a Functionally Distinct Population of Long-Term Hematopoietic Stem Cells. Blood 2008, 111, 2444–2451. [Google Scholar] [CrossRef] [PubMed]
- Pronk, C.J.H.; Rossi, D.J.; Månsson, R.; Attema, J.L.; Norddahl, G.L.; Chan, C.K.F.; Sigvardsson, M.; Weissman, I.L.; Bryder, D. Elucidation of the Phenotypic, Functional, and Molecular Topography of a Myeloerythroid Progenitor Cell Hierarchy. Cell Stem Cell 2007, 1, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Crispino, J.D.; Weiss, M.J. Erythro-Megakaryocytic Transcription Factors Associated with Hereditary Anemia. Blood 2014, 123, 3080–3088. [Google Scholar] [CrossRef]
- Noetzli, L.J.; French, S.L.; Machlus, K.R. New Insights Into the Differentiation of Megakaryocytes From Hematopoietic Progenitors. Arterioscler. Thromb. Vasc. Biol. 2019, 196, ATVBAHA119312129. [Google Scholar] [CrossRef] [PubMed]
- Tijssen, M.R.; Ghevaert, C. Transcription Factors in Late Megakaryopoiesis and Related Platelet Disorders. J. Thromb. Haemostasis 2013, 11, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Mazzi, S.; Lordier, L.; Debili, N.; Raslova, H.; Vainchenker, W. Megakaryocyte and Polyploidization. Exp. Hematol. 2018, 57, 1–13. [Google Scholar] [CrossRef]
- Tijssen, M.R.; Cvejic, A.; Joshi, A.; Hannah, R.L.; Ferreira, R.; Forrai, A.; Bellissimo, D.C.; Oram, S.H.; Smethurst, P.A.; Wilson, N.K.; et al. Genome-Wide Analysis of Simultaneous GATA1/2, RUNX1, FLI1, and SCL Binding in Megakaryocytes Identifies Hematopoietic Regulators. Dev. Cell 2011, 20, 597–609. [Google Scholar] [CrossRef]
- Mancini, E.; Sanjuan-Pla, A.; Luciani, L.; Moore, S.; Grover, A.; Zay, A.; Rasmussen, K.D.; Luc, S.; Bilbao, D.; O’Carroll, D.; et al. FOG-1 and GATA-1 Act Sequentially to Specify Definitive Megakaryocytic and Erythroid Progenitors. EMBO J. 2012, 31, 351–365. [Google Scholar] [CrossRef]
- Lordier, L.; Bluteau, D.; Jalil, A.; Legrand, C.; Pan, J.; Rameau, P.; Jouni, D.; Bluteau, O.; Mercher, T.; Leon, C.; et al. RUNX1-Induced Silencing of Non-Muscle Myosin Heavy Chain IIB Contributes to Megakaryocyte Polyploidization. Nat. Commun. 2012, 3, 717. [Google Scholar] [CrossRef]
- Zang, C.; Luyten, A.; Chen, J.; Liu, X.S.; Shivdasani, R.A. NF-E2, FLI1 and RUNX1 Collaborate at Areas of Dynamic Chromatin to Activate Transcription in Mature Mouse Megakaryocytes. Sci. Rep. 2016, 6, 30255. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Soung, D.Y.; Schwarz, E.M.; O’Keefe, R.J.; Drissi, H. Wnt Induction of Chondrocyte Hypertrophy through the Runx2 Transcription Factor. J. Cell. Physiol. 2006, 208, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.A.; Menon, R.; Bailey, K.M.; Thomas, D.G.; Noord, R.A.V.; Tran, J.; Wang, H.; Qu, P.P.; Hoering, A.; Fearon, E.R.; et al. Activation of Wnt/β-Catenin in Ewing Sarcoma Cells Antagonizes EWS/ETS Function and Promotes Phenotypic Transition to More Metastatic Cell States. Cancer Res. 2016, 76, 5040–5053. [Google Scholar] [CrossRef] [PubMed]
- North, T.E.; Stacy, T.; Matheny, C.J.; Speck, N.A.; Bruijn, M.F.T.R.d. Runx1 Is Expressed in Adult Mouse Hematopoietic Stem Cells and Differentiating Myeloid and Lymphoid Cells, But Not in Maturing Erythroid Cells. Stem Cells 2004, 22, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.H.M.; Gotlib, J.; Durocher, J.A.; Chao, M.P.; Mariappan, M.R.; Lay, M.; Jones, C.; Zehnder, J.L.; Lilleberg, S.L.; Weissman, I.L. The JAK2 V617F Mutation Occurs in Hematopoietic Stem Cells in Polycythemia Vera and Predisposes toward Erythroid Differentiation. Proc. Natl. Acad. Sci. USA 2006, 103, 6224–6229. [Google Scholar] [CrossRef]
- Ohba, S.; Lanigan, T.M.; Roessler, B.J. Leptin Receptor JAK2/STAT3 Signaling Modulates Expression of Frizzled Receptors in Articular Chondrocytes. Osteoarthr. Cartil. 2010, 18, 1620–1629. [Google Scholar] [CrossRef]
- Lento, W.; Ito, T.; Zhao, C.; Harris, J.R.; Huang, W.; Jiang, C.; Owzar, K.; Piryani, S.; Racioppi, L.; Chao, N.; et al. Loss of β-Catenin Triggers Oxidative Stress and Impairs Hematopoietic Regeneration. Genes. Dev. 2014, 28, 995–1004. [Google Scholar] [CrossRef]
- Sardina, J.L.; López-Ruano, G.; Prieto-Bermejo, R.; Sánchez-Sánchez, B.; Pérez-Fernández, A.; Sánchez-Abarca, L.I.; Pérez-Simón, J.A.; Quintales, L.; Sánchez-Yagüe, J.; Llanillo, M.; et al. PTPN13 Regulates Cellular Signalling and β-Catenin Function during Megakaryocytic Differentiation. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2886–2899. [Google Scholar] [CrossRef]
- Paluru, P.; Hudock, K.M.; Cheng, X.; Mills, J.A.; Ying, L.; Galvão, A.M.; Lu, L.; Tiyaboonchai, A.; Sim, X.; Sullivan, S.K.; et al. The Negative Impact of Wnt Signaling on Megakaryocyte and Primitive Erythroid Progenitors Derived from Human Embryonic Stem Cells. Stem Cell Res. 2014, 12, 441–451. [Google Scholar] [CrossRef]
- Huang, H.; Yu, M.; Akie, T.E.; Moran, T.B.; Woo, A.J.; Tu, N.; Waldon, Z.; Lin, Y.Y.; Steen, H.; Cantor, A.B. Differentiation-Dependent Interactions between RUNX-1 and FLI-1 during Megakaryocyte Development. Mol. Cell. Biol. 2009, 29, 4103–4115. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Tefferi, A. The Complete Evaluation of Erythrocytosis: Congenital and Acquired. Leukemia 2009, 23, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Pardanani, A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Cortelazzo, S.; Finazzi, G.; Ruggeri, M.; Vestri, O.; Galli, M.; Rodeghiero, F.; Barbui, T. Hydroxyurea for Patients with Essential Thrombocythemia and a High Risk of Thrombosis. N. Engl. J. Med. 1995, 332, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Larrán, A.; Martínez-Avilés, L.; Hernández-Boluda, J.C.; Ferrer-Marín, F.; Antelo, M.L.; Burgaleta, C.; Mata, M.I.; Xicoy, B.; Martínez-Trillos, A.; Gómez-Casares, M.T.; et al. Busulfan in Patients with Polycythemia Vera or Essential Thrombocythemia Refractory or Intolerant to Hydroxyurea. Ann. Hematol. 2014, 93, 2037–2043. [Google Scholar] [CrossRef]
- Silver, R.T. Long-term Effects of the Treatment of Polycythemia Vera with Recombinant Interferon-α. Cancer 2006, 107, 451–458. [Google Scholar] [CrossRef]




| Antibody | Host | Company | Catalog # | Dilution | Method |
|---|---|---|---|---|---|
| ABs for Immunofluorescence and Immunohistochemistry | |||||
| GFP | Chicken | Abcam (Cambridge, UK) | ab13970 | 1:500 | PFA |
| CD41 | Rat | BD Biosciences (Heidelberg, Germany) | 553847 | 1:200 | PFA |
| Ki67 (SP6) | Rabbit | ThermoFisher Scientific (Darmstadt, Germany) | RM-9106S0 | 1:200 | PFA |
| Donkey α Rat 550 | Donkey | ThermoFisher Scientific (Darmstadt, Germany) | SA5-10027 | 1:200 | PFA |
| Goat α Chicken 488 | Goat | Abcam | Ab150169 | 1:200 | PFA |
| ABs for flow cytometry (FACS) | |||||
| CD3-PE/Cy7 | BioLegend (Amsterdam, The Netherlands) | 100220 | 1:70 | ||
| CD4- PerCP | BD Biosciences (Heidelberg, Germany) | 553052 | 1:50 | ||
| CD11b-v450 | BD Biosciences (Heidelberg, Germany) | 560456 | 1:50 | ||
| CD16/32-PE/Cy7 | BioLegend | 101318 | 1:75 | ||
| CD19-PE | BD Biosciences (Heidelberg, Germany) | 557399 | 1:50 | ||
| CD34-PE | BD Biosciences (Heidelberg, Germany) | 551387 | 1:75 | ||
| CD41- PE/Cy7 | BioLegend (Amsterdam The Netherlands) | 133916 | 1:50 | ||
| CD45.1-PerCP/Cy5.5 | BioLegend (Amsterdam The Netherlands) | 110727 | 1:70 | ||
| CD45.2-BV510 | BioLegend (Amsterdam The Netherlands) | 109837 | 1:70 | ||
| CD150-PE | BioLegend (Amsterdam The Netherlands) | 115904 | 1:50 | ||
| c-Kit-APC | BD Biosciences (Heidelberg, Germany) | 553356 | 1:75 | ||
| F4/80-APC | eBioscience (Darmstadt, Germany) | 17-4801-82 | 1:50 | ||
| GFP-Alexa 488 | Life Technologies (Darmstadt, Germany) | A-21311 | 1:400 | PFA | |
| Lineage Panel: (TER-119, CD11b, Ly-6G/Ly-6C, CD3e, CD45R/B220)-Biotin | BioLegend (Amsterdam The Netherlands) | 133307 | 1:300 | ||
| Ly6G-FITC | BD Biosciences (Heidelberg, Germany) | 551460 | 1:50 | ||
| Sca-1-PB | BioLegend (Amsterdam The Netherlands) | 122520 | 1:75 | ||
| Streptavidin-PerCP/Cy5.5 | BioLegend (Amsterdam The Netherlands) | 405214 | 1:300 | ||
| Primer For | Sequence 5′-3′ Sense | Sequence 5′-3′ Antisense |
|---|---|---|
| RUNX1b | CCTCCGGTAGTAATAAAGGCTTC | CCGATTGAGTAAGGACCCTGAA |
| FLI1 | CCCTGCAGCCACATCCAACAG | GGAGGATGGGTGAGACGGGAC |
| Rplp0 | CTTTGGTCGCTCGCTCCTC | CTGACCGGGTTGGTTTTGAT |
| 18s | GTGTTTGACAACGGCAGCATT | TCTCCACAGACAATGCCAGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalcin, B.H.; Macas, J.; Wiercinska, E.; Harter, P.N.; Fawaz, M.; Schmachtel, T.; Ghiro, I.; Bieniek, E.; Kosanovic, D.; Thom, S.; et al. Wnt/β-Catenin-Signaling Modulates Megakaryopoiesis at the Megakaryocyte-Erythrocyte Progenitor Stage in the Hematopoietic System. Cells 2023, 12, 2765. https://doi.org/10.3390/cells12232765
Yalcin BH, Macas J, Wiercinska E, Harter PN, Fawaz M, Schmachtel T, Ghiro I, Bieniek E, Kosanovic D, Thom S, et al. Wnt/β-Catenin-Signaling Modulates Megakaryopoiesis at the Megakaryocyte-Erythrocyte Progenitor Stage in the Hematopoietic System. Cells. 2023; 12(23):2765. https://doi.org/10.3390/cells12232765
Chicago/Turabian StyleYalcin, Burak H., Jadranka Macas, Eliza Wiercinska, Patrick N. Harter, Malak Fawaz, Tessa Schmachtel, Ilaria Ghiro, Ewa Bieniek, Djuro Kosanovic, Sonja Thom, and et al. 2023. "Wnt/β-Catenin-Signaling Modulates Megakaryopoiesis at the Megakaryocyte-Erythrocyte Progenitor Stage in the Hematopoietic System" Cells 12, no. 23: 2765. https://doi.org/10.3390/cells12232765
APA StyleYalcin, B. H., Macas, J., Wiercinska, E., Harter, P. N., Fawaz, M., Schmachtel, T., Ghiro, I., Bieniek, E., Kosanovic, D., Thom, S., Fruttiger, M., Taketo, M. M., Schermuly, R. T., Rieger, M. A., Plate, K. H., Bonig, H., & Liebner, S. (2023). Wnt/β-Catenin-Signaling Modulates Megakaryopoiesis at the Megakaryocyte-Erythrocyte Progenitor Stage in the Hematopoietic System. Cells, 12(23), 2765. https://doi.org/10.3390/cells12232765








