The Interleukin 6 Protein Level as well as a Genetic Variants, (rs1800795, rs1800797) Are Associated with Adverse Cardiovascular Outcomes within 10-Years Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. CV Patients
2.3. Interleukin 6 Analysis
2.4. Statistical Evaluation
3. Results
3.1. Clinical Characterisation of CV Patients
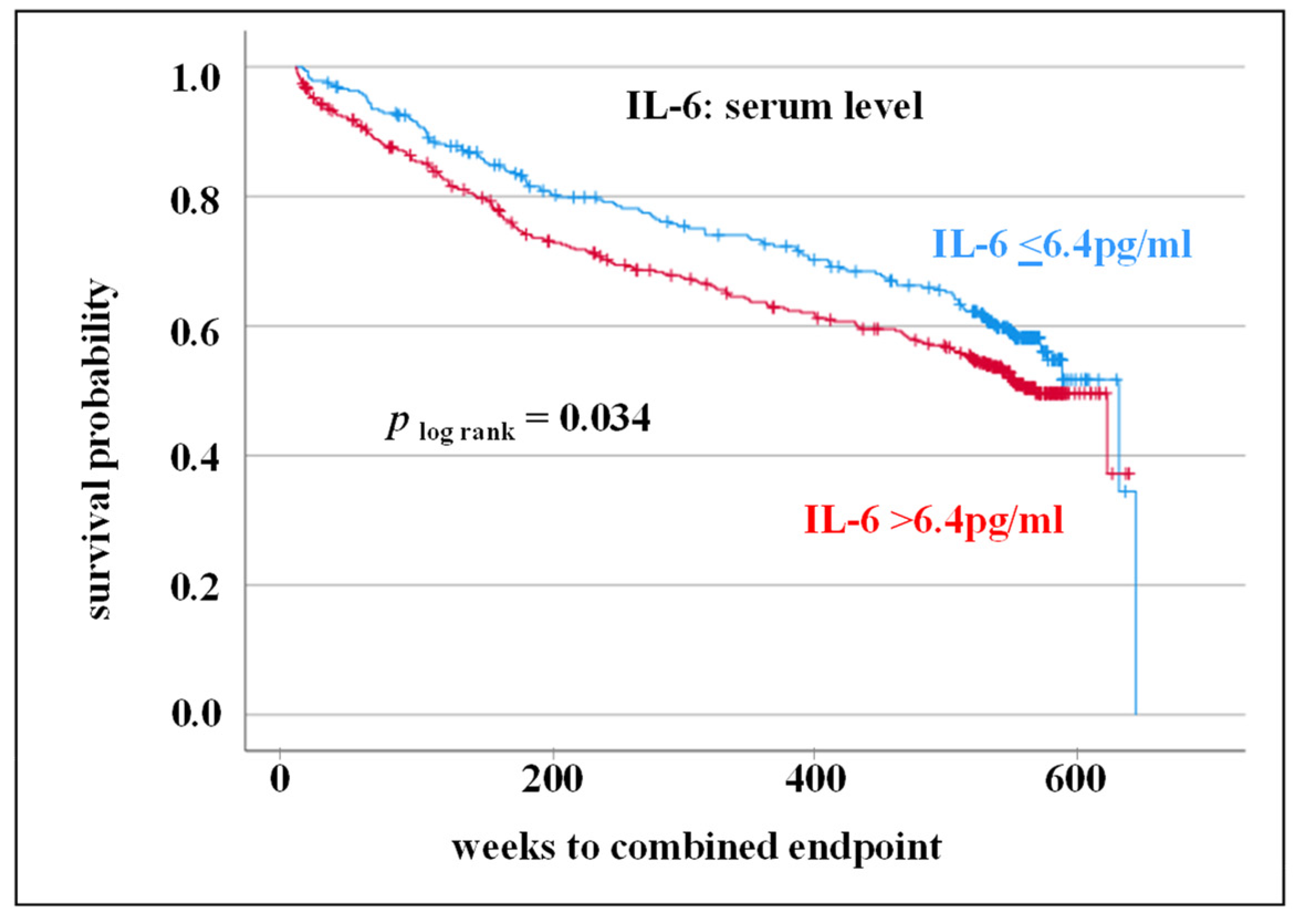
3.2. IL-6 Serum Level as Prognostic Markers for Adverse CV Outcome
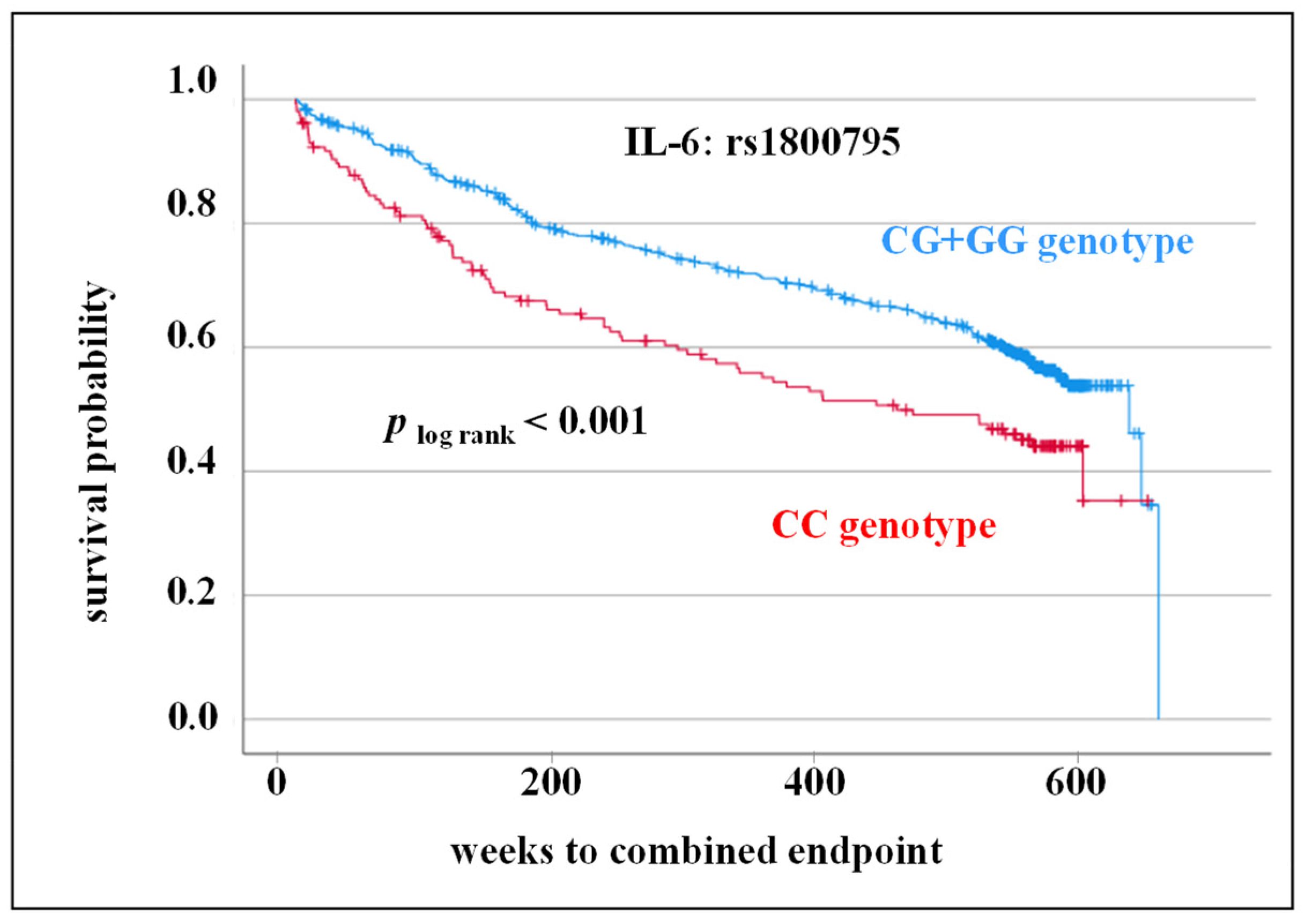
3.3. SNPs rs1800795, rs1800797 in IL-6 Gene as Prognostic Markers for Adverse CV Outcome
3.4. Adjustment of Cofactors in Multivariate Analysis
4. Discussion
4.1. Elevated IL-6 Serum Level as Risk Marker for Adverse CV Prognosis
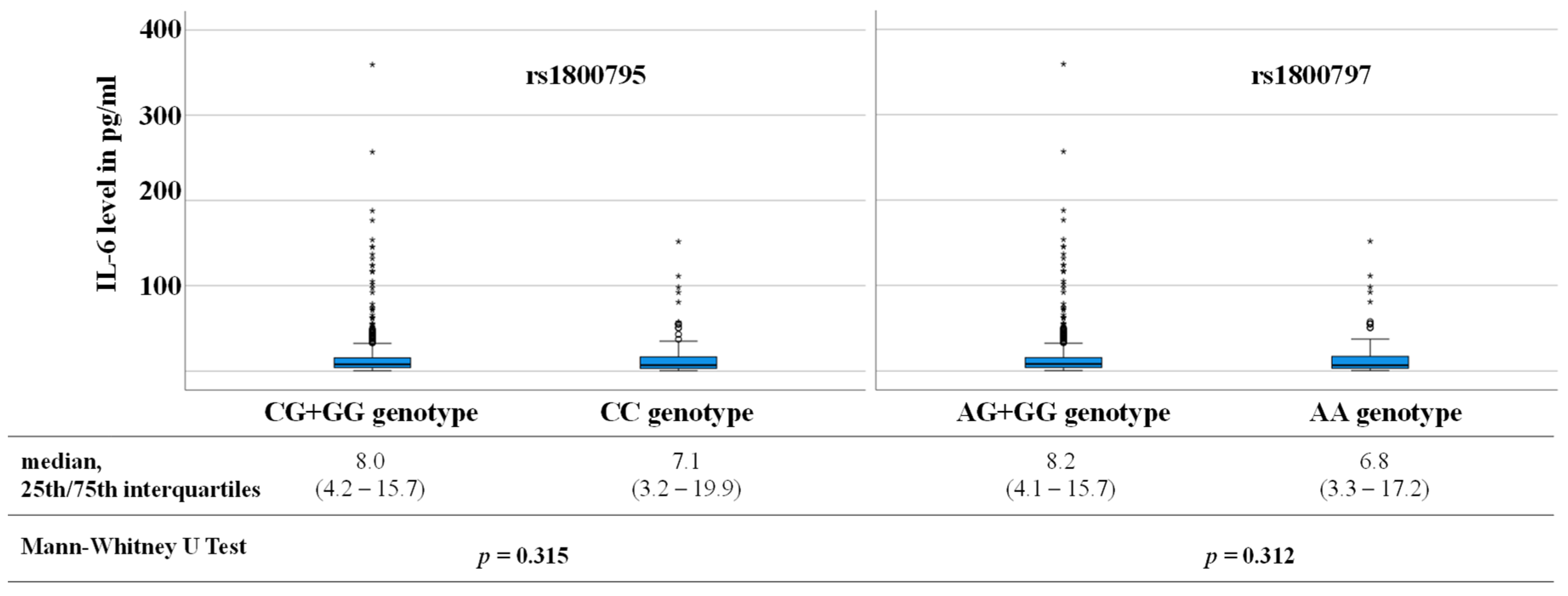
4.2. SNPs in IL-6 Gene as Prognostic Markers for Adverse CV Outcome
4.3. Interaction of Genetic Variants and IL-6 Serum Level
4.4. Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Libby, P.; Hansson, G.K. From focal lipid storage to systemic inflammation: JACC review topic of the week. J. Am. Coll. Cardiol. 2019, 74, 1594–1607. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Angelopoulos, A.; Papanikolaou, P.; Simantiris, S.; Oikonomou, E.K.; Vamvakaris, K.; Koumpoura, A.; Farmaki, M.; Trivella, M.; Vlachopoulos, C.; et al. Biomarkers of Vascular Inflammation for Cardiovascular Risk Prognostication: A Meta-Analysis. JACC Cardiovasc. Imaging 2022, 15, 460–471. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Anticytokine agents: Targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ. Res. 2019, 124, 437–450. [Google Scholar] [CrossRef]
- Vasques-Nóvoa, F.; Pedro Ferreira, J.; Marques, P.; Sergio Neves, J.; Vale, C.; Ribeirinho-Soares, P.; Marques, J.; Martins, S.; Tiago Guimarães, J.; Barros, A.S.; et al. Interleukin-6, infection and cardiovascular outcomes in acute heart failure: Findings from the EDIFICA registry. Cytokine 2022, 160, 156053. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Deng, Z.; Li, J.; Ren, Z.; Liu, F. Meta-analysis of the relationship between interleukin-6 levels and the prognosis and severity of acute coronary syndrome. Clinics 2021, 76, e2690. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Devalaraja, M.; Baeres, F.M.M.; Engelmann, M.D.M.; Hovingh, G.K.; Ivkovic, M.; Lo, L.; Kling, D.; Pergola, P.; Raj, D.; et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021, 397, 2060–2069. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef]
- González-Castro, T.B.; Hernández-Díaz, Y.; Pérez-Hernández, N.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; López-Narvaez, M.L.; Blachman-Braun, R.; Posadas-Sánchez, R.; Vargas-Alarcón, G.; García-Flores, E.; et al. Interleukin 6 (rs1800795) gene polymorphism is associated with cardiovascular diseases: A meta-analysis of 74 studies with 86,229 subjects. EXCLI J. 2019, 18, 331–355. [Google Scholar] [CrossRef]
- Yin, Y.W.; Li, J.C.; Zhang, M.; Wang, J.Z.; Li, B.H.; Liu, Y.; Liao, S.Q.; Zhang, M.J.; Gao, C.Y.; Zhang, L.L. Influence of interleukin-6 gene -174G>C polymorphism on development of atherosclerosis: A meta-analysis of 50 studies involving 33,514 subjects. Gene 2013, 529, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Sukhinina, T.S.; Shakhnovich, R.M.; Barsova, R.M.; Matveeva, N.A.; Titov, B.V.; Sudomoina, M.A.; Favorova, O.O.; Ruda, M.I. Value of allele gene polymorphism of the inflammation system for prognosis of patients with myocardial infarction. Kardiologiia 2012, 52, 15–21. [Google Scholar] [PubMed]
- Máchal, J.; Pávková-Goldbergová, M.; Hlinomaz, O.; Groch, L.; Vašků, A. Patients with chronic three-vessel disease in a 15-year follow-up study: Genetic and non-genetic predictors of survival. Medicine 2014, 93, e278. [Google Scholar] [CrossRef]
- Reichert, S.; Schlitt, A.; Benten, A.C.; Hofmann, B.; Schaller, H.G.; Schulz, S. The interleukin 6 c.-174 CC genotype is a predictor for new cardiovascular events in patients with coronary heart disease within three years follow-up. Cytokine 2016, 83, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Reichert, S.; Schlitt, A.; Beschow, V.; Lutze, A.; Lischewski, S.; Seifert, T.; Dudakliewa, T.; Gawe, R.; Werdan, K.; Hofmann, B.; et al. Use of floss/interdental brushes is associated with lower risk for new cardiovascular events among patients with coronary heart disease. J. Periodontal. Res. 2015, 50, 180–188. [Google Scholar] [CrossRef]
- Edsfeldt, A.; Gonçalves, I.; Vigren, I.; Jovanović, A.; Engström, G.; Shore, A.C.; Natali, A.; Khan, F.; Nilsson, J. Circulating soluble IL-6 receptor associates with plaque inflammation but not with atherosclerosis severity and cardiovascular risk. Vascul. Pharmacol. 2023, 152, 107214. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M. Targeting Interleukin-1 and Interleukin-6: The Time Has Come to Aggressively Address Residual Inflammatory Risk. J. Am. Coll. Cardiol. 2020, 76, 1774–1776. [Google Scholar] [CrossRef]
- Smith, A.J.; Humphries, S.E. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 2009, 20, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Terry, C.F.; Loukaci, V.; Green, F.R. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J. Biol. Chem. 2000, 275, 18138–18144. [Google Scholar] [CrossRef] [PubMed]
- Fishman, D.; Faulds, G.; Jeffery, R.; Mohamed-Ali, V.; Yudkin, J.S.; Humphries, S.; Woo, P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J. Clin. Investig. 1998, 102, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Noss, E.H.; Nguyen, H.N.; Chang, S.K.; Watts, G.F.; Brenner, M.B. Genetic polymorphism directs IL-6 expression in fibroblasts but not selected other cell types. Proc. Natl. Acad. Sci. USA 2015, 112, 14948–14953. [Google Scholar] [CrossRef]
- Phulukdaree, A.; Khan, S.; Ramkaran, P.; Govender, R.; Moodley, D.; Chuturgoon, A.A. The interleukin-6 -147 g/c polymorphism is associated with increased risk of coronary artery disease in young South African Indian men. Metab. Syndr. Relat. Disord. 2013, 11, 205–209. [Google Scholar] [CrossRef]
- Reichert, S.; Schlitt, A.; Benten, A.C.; Hofmann, B.; Schaller, H.G.; Schulz, S. Data on IL-6 c.-174 G>C genotype and allele frequencies in patients with coronary heart disease in dependence of cardiovascular outcome. Data Brief 2016, 8, 1295–1299. [Google Scholar] [CrossRef]



| Characteristics | All Patients (n = 942) | IL-6 ≤ 6.4 pg/mL (n = 422) | IL-6 > 6.4 pg/mL (n = 520) | p-Value |
|---|---|---|---|---|
| Demographical and anamnestic parameters | ||||
| Age, years (median; 25/75 IQR) | 69.1 (60.4/74.7) | 68.9 (60.0/74.2) | 69.3 (60.6/75.0) | 0.331 |
| Female gender (%) | 26.7 | 30.3 | 23.9 | 0.058 1 |
| Current smoking (%) | 10.6 | 11.1 | 10.3 | 0.790 1 |
| Body mass index, kg/m2 (median; 25/75 IQR) | 28.1 (25.3/30.7) | 28.1 (25.2/30.5) | 28.1 (25.4/30.7) | 0.560 |
| History of | ||||
| Diabetes mellitus (%) | 34.5 | 33.7 | 35.1 | 0.763 1 |
| Hypertension (%) | 87.6 | 88.5 | 86.9 | 0.567 1 |
| MI (%) | 38.9 | 39.3 | 38.7 | 0.916 1 |
| Stroke/TIA (%) | 12.5 | 13.3 | 11.9 | 0.652 1 |
| Peripheral artery disease (%) | 9.7 | 9.0 | 10.3 | 0.645 1 |
| Hyperlipoproteinemia (%) | 59.2 | 61.0 | 57.8 | 0.416 1 |
| Polymorphisms in IL-6 gene | ||||
| rs1800795 | ||||
| CC genotype (%) | 21.3 | 23.0 | 20.0 | |
| CG + GG genotype (%) | 78.7 | 77.0 | 80.0 | 0.365 1 |
| rs1800797 | ||||
| AA genotype (%) | 20.3 | 22.4 | 18.8 | |
| AG + GG genotype (%) | 79.7 | 77.6 | 81.3 | 0264 1 |
| All Patients (n = 742) | Without Combined Endpoint (n = 427) | With Combined Endpoint (n = 315) | p-Value | |
|---|---|---|---|---|
| IL-6 (pg/mL), (median; 25/75 IQR) | 7.8 (3.8/15.9) | 7.2 (3.4/13.9) | 8.7 (4.3/18.5) | 0.017 1 |
| All Patients (n = 742) | Without Combined Endpoint (n = 427) | With Combined Endpoint (n = 315) | p-Value | |
|---|---|---|---|---|
| rs1800795 | ||||
| CC genotype (%) | 21.2 | 17.9 | 25.6 | |
| CG + GG genotype (%) | 78.8 | 82.1 | 74.4 | 0.014 1 |
| rs1800797 | ||||
| AA genotype (%) | 20.2 | 17.4 | 24.1 | |
| AG + GG genotype (%) | 79.8 | 82.6 | 75.9 | 0.033 1 |
| Regression Coefficient | Standard Error | p-Value | Hazard Ratio | 95% CI | ||
|---|---|---|---|---|---|---|
| Upper | Lower | |||||
| (a) | ||||||
| CC genotype (rs1800795) | 0.545 | 0.131 | <0.001 | 1.72 | 1.33 | 2.23 |
| IL-6 level > 6.4 pg/mL | 0.251 | 0.117 | 0.032 | 1.29 | 1.02 | 1.62 |
| Age | 0.042 | 0.007 | <0.001 | 1.04 | 1.03 | 1.06 |
| Male gender | 0.329 | 0.135 | 0.015 | 1.39 | 1.07 | 1.81 |
| BMI | −0.014 | 0.014 | 0.346 | 0.99 | 0.96 | 1.02 |
| smoking | 0.308 | 0.218 | 0.156 | 1.36 | 0.89 | 2.08 |
| Diabetes mellitus | 0.552 | 0.122 | <0.001 | 1.74 | 1.37 | 2.21 |
| Hypertension | 0.143 | 0.192 | 0.454 | 1.15 | 0.79 | 1.68 |
| Hyperlipoproteinemia | −0.119 | 0.119 | 0.315 | 0.89 | 0.70 | 1.12 |
| (b) | ||||||
| AA genotype (rs1800797) | 0.484 | 0.134 | <0.001 | 1.62 | 1.25 | 2.11 |
| IL-6 level > 6.4 pg/mL | 0.248 | 0.117 | 0.033 | 1.29 | 1.02 | 1.62 |
| Age | 0.042 | 0.007 | <0.001 | 1.04 | 1.03 | 1.06 |
| Male gender | 0.327 | 0.135 | 0.016 | 1.39 | 1.06 | 1.81 |
| BMI | −0.013 | 0.014 | 0.366 | 0.99 | 0.96 | 1.02 |
| smoking | 0.299 | 0.218 | 0.170 | 1.35 | 0.88 | 2.07 |
| Diabetes mellitus | 0.538 | 0.122 | <0.001 | 1.71 | 1.35 | 2.18 |
| Hypertension | 0.137 | 0.192 | 0.474 | 1.15 | 0.79 | 1.67 |
| Hyperlipoproteinemia | −0.110 | 0.119 | 0.355 | 0.90 | 0.71 | 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, S.; Rehm, S.; Schlitt, A.; Bitter, K.; Reichert, S. The Interleukin 6 Protein Level as well as a Genetic Variants, (rs1800795, rs1800797) Are Associated with Adverse Cardiovascular Outcomes within 10-Years Follow-Up. Cells 2023, 12, 2722. https://doi.org/10.3390/cells12232722
Schulz S, Rehm S, Schlitt A, Bitter K, Reichert S. The Interleukin 6 Protein Level as well as a Genetic Variants, (rs1800795, rs1800797) Are Associated with Adverse Cardiovascular Outcomes within 10-Years Follow-Up. Cells. 2023; 12(23):2722. https://doi.org/10.3390/cells12232722
Chicago/Turabian StyleSchulz, Susanne, Selina Rehm, Axel Schlitt, Kerstin Bitter, and Stefan Reichert. 2023. "The Interleukin 6 Protein Level as well as a Genetic Variants, (rs1800795, rs1800797) Are Associated with Adverse Cardiovascular Outcomes within 10-Years Follow-Up" Cells 12, no. 23: 2722. https://doi.org/10.3390/cells12232722
APA StyleSchulz, S., Rehm, S., Schlitt, A., Bitter, K., & Reichert, S. (2023). The Interleukin 6 Protein Level as well as a Genetic Variants, (rs1800795, rs1800797) Are Associated with Adverse Cardiovascular Outcomes within 10-Years Follow-Up. Cells, 12(23), 2722. https://doi.org/10.3390/cells12232722







