The Role of Glutathione and Sulfhydryl Groups in Cadmium Uptake by Cultures of the Rainbow Trout RTG-2 Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cd Uptake
2.3. Cd Uptake under Different Intracellular Sulfhydryl Conditions
2.3.1. Effect of a Sulfhydryl Group Blocker on Cd Uptake
2.3.2. Effect of Depletion of Cellular GSH on Cd Uptake
2.4. Determination of Total GSH, Total Cysteine and GSSG
2.5. Statistical Analysis
3. Results
3.1. Analysis of Cd Concentrations in Exposure and Washing Media
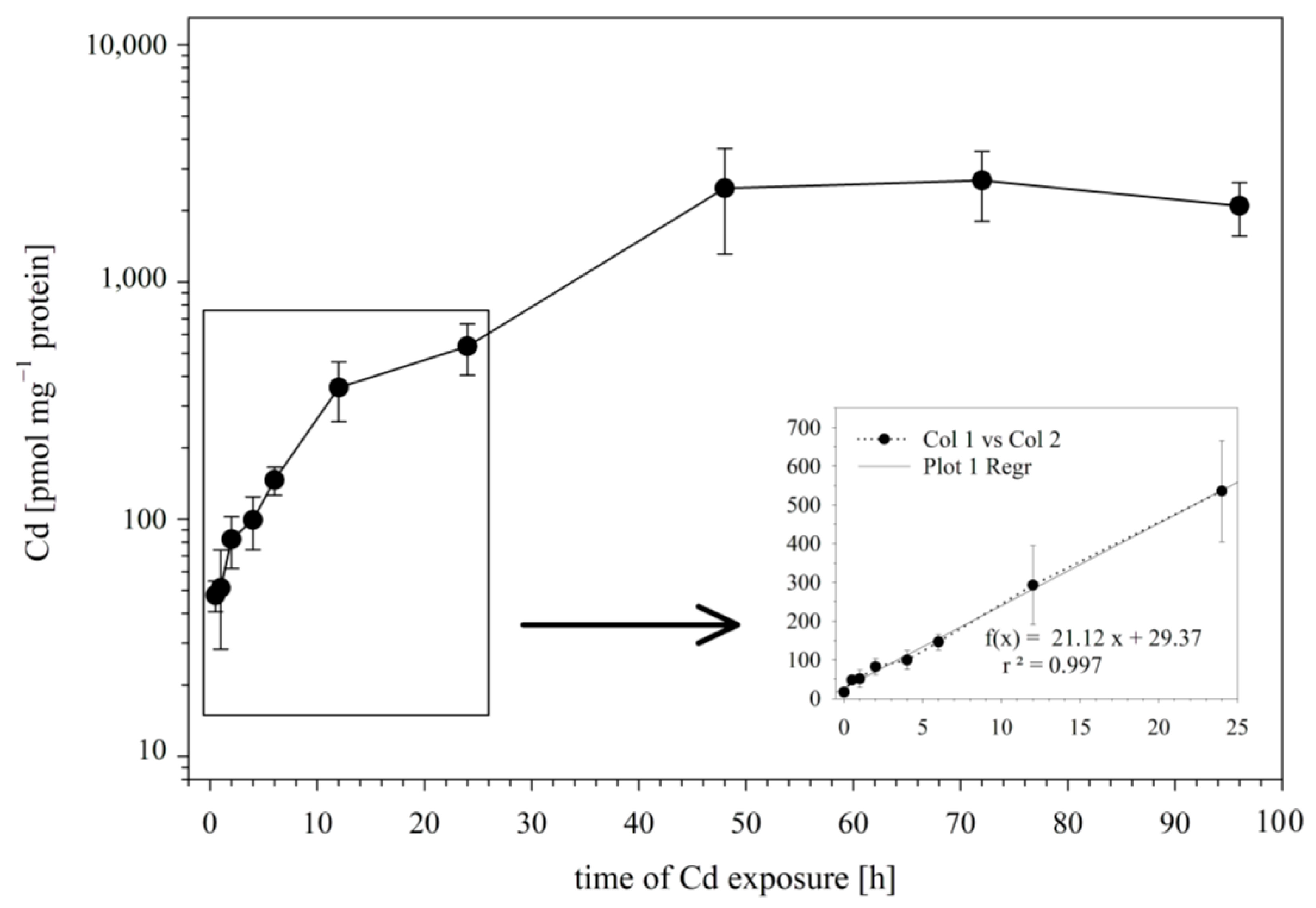
3.2. Time Course of Cadmium Uptake
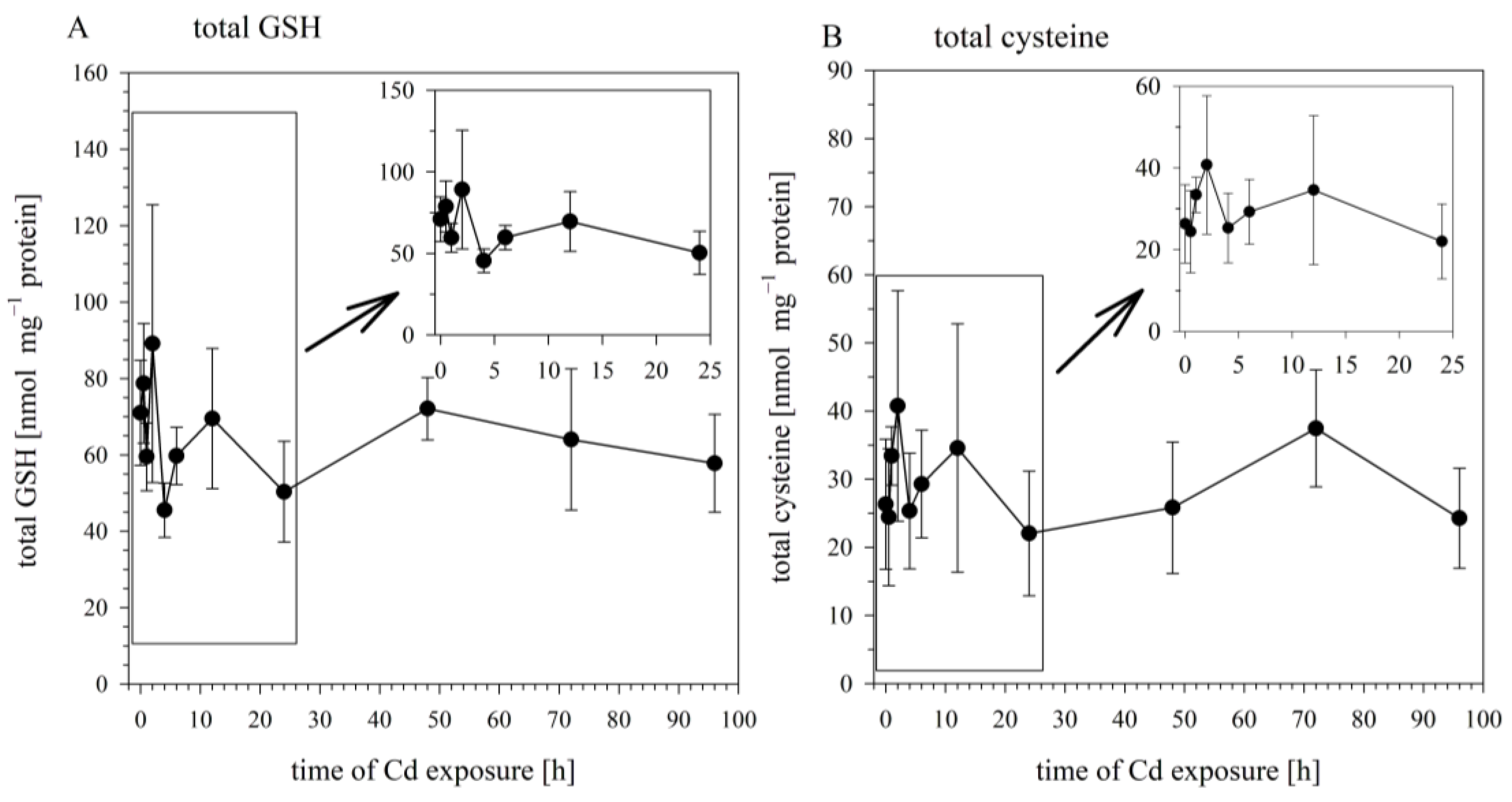
3.3. Time Course of Cd Exposure Effects on Cellular Sulfhydryl Levels
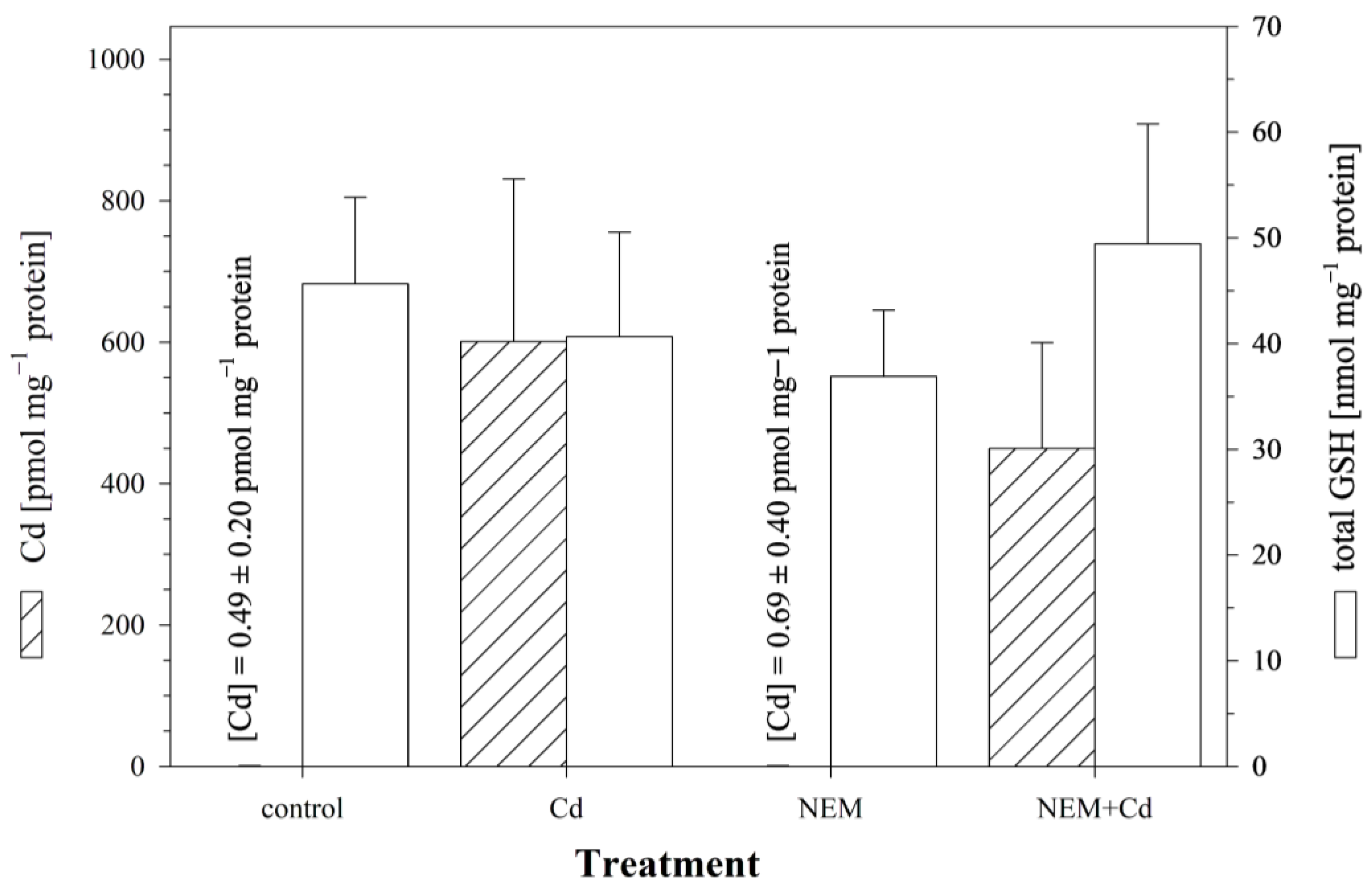
3.4. Effect of Blocking Cellular Sulfhydryl Groups on Cellular Cd Accumulation: NEM Experiments
3.5. Effect of Manipulating Cellular GSH Levels on Cellular Cd Accumulation: BSO Experiments
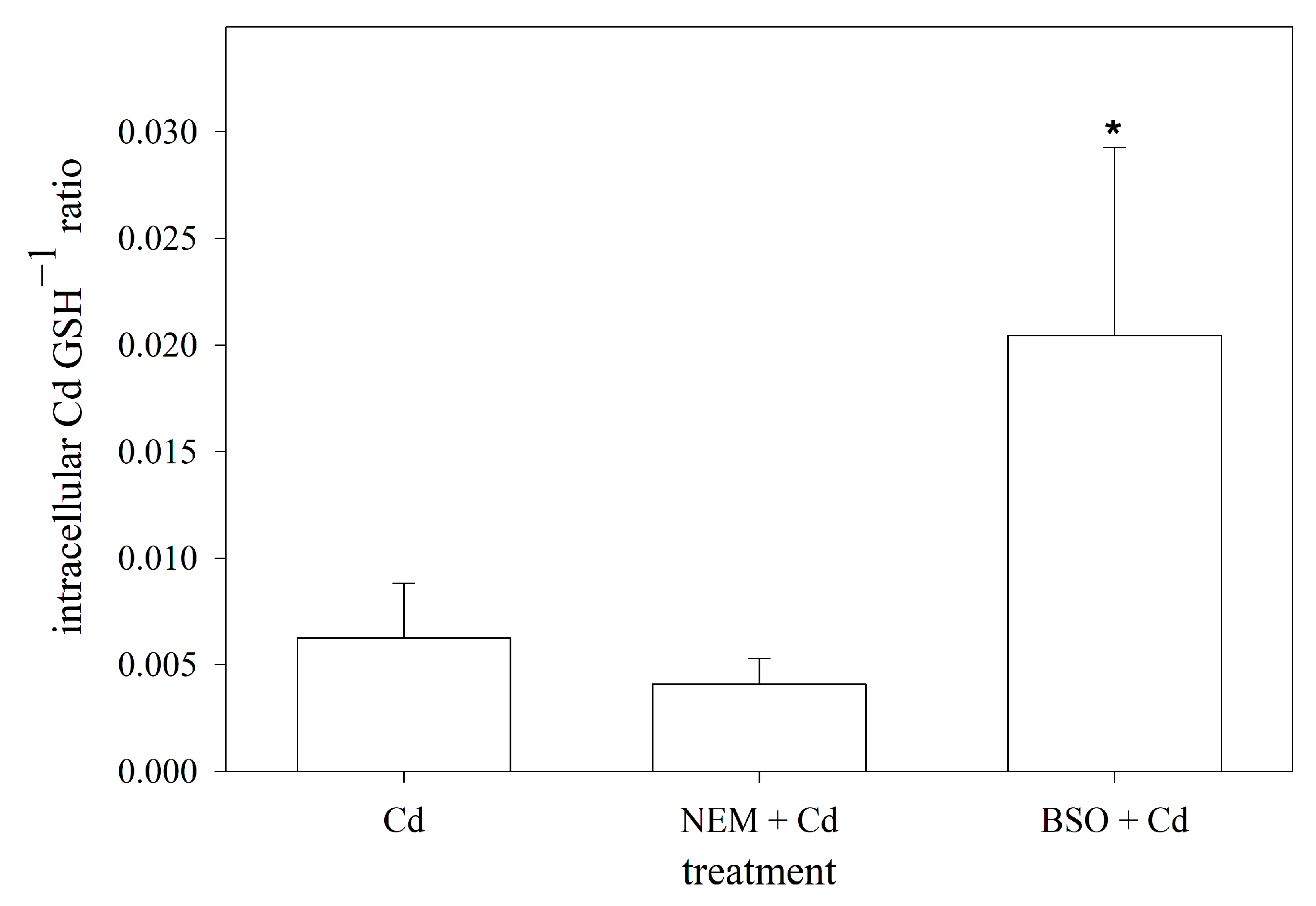
3.6. Intracellular Molar Ratio of Cd to GSH in RTG-2 Cells with Differently Modulated Sulfhydryl Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Srivastav, A.L.; Ranjan, M. Inorganic Water Pollutants. In Inorganic Pollutants in Water; Devi, P., Singh, P., Kansal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. ISBN 9780128189658. [Google Scholar]
- Cao, L.; Huang, W.; Shan, X.; Ye, Z.; Dou, S. Tissue-Specific Accumulation of Cadmium and Its Effects on Antioxidative Responses in Japanese Flounder Juveniles. Environ. Toxicol. Pharmacol. 2012, 33, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Choi, Y.J.; Kim, J.H. Toxic Effects of Waterborne Cadmium Exposure on Hematological Parameters, Oxidative Stress, Neurotoxicity, and Heat Shock Protein 70 in Juvenile Olive Flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2022, 122, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Jo, A.-H.; Choi, C.-Y.; Kim, J.-H. Review of Cadmium Toxicity Effects on Fish: Oxidative Stress and Immune Responses. Environ. Res. 2023, 236, 116600. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q.; Li, Y.; Bi, L.; Jin, L.; Peng, R. Toxic Effects of Cadmium on Fish. Toxics 2022, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- McGeer, J.C.; Niyogi, S.; Scott Smith, D. Cadmium. In Fish Physiology; Wood, C.M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 31B, pp. 125–184. [Google Scholar]
- da Silva, A.O.F.; Martinez, C.B.R. Acute Effects of Cadmium on Osmoregulation of the Freshwater Teleost Prochilodus lineatus: Enzymes Activity and Plasma Ions. Aquat. Toxicol. 2014, 156, 161–168. [Google Scholar] [CrossRef]
- Das, S.; Kar, I.; Patra, A.K. Cadmium Induced Bioaccumulation, Histopathology, Gene Regulation in Fish and Its Amelioration—A Review. J. Trace Elem. Med. Biol. 2023, 79, 127202. [Google Scholar] [CrossRef]
- Đukić-Ćosić, D.; Baralić, K.; Javorac, D.; Djordjevic, A.B.; Bulat, Z. An Overview of Molecular Mechanisms in Cadmium Toxicity. Curr. Opin. Toxicol. 2020, 19, 56–62. [Google Scholar] [CrossRef]
- Niyogi, S.; Wood, C.M. Biotic Ligand Model, a Flexible Tool for Developing Site-Specific Water Quality Guidelines for Metals. Environ. Sci. Technol. 2004, 38, 6177–6192. [Google Scholar] [CrossRef]
- Oldham, D.; Black, T.; Stewart, T.J.; Minghetti, M. Role of the Luminal Composition on Intestinal Metal Toxicity, Bioavailability and Bioreactivity: An in Vitro Approach Based on the Cell Line RTgutGC. Aquat. Toxicol. 2023, 256, 106411. [Google Scholar] [CrossRef]
- Anni, I.S.A.; Zebral, Y.D.; Afonso, S.B.; Jorge, M.B.; Moreno Abril, S.I.; Bianchini, A. Life-Time Exposure to Waterborne Copper II: Patterns of Tissue Accumulation and Gene Expression of the Metal-Transport Proteins Ctr1 and Atp7b in the Killifish Poecilia vivipara. Chemosphere 2019, 223, 257–262. [Google Scholar] [CrossRef]
- Hogstrand, C. Zinc. In Fish Physiology; Wood, C.M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 31A, pp. 135–200. [Google Scholar]
- Pigman, E.A.; Blanchard, J.; Laird, H.E. A Study of Cadmium Transport Pathways Using the Caco-2 Cell Model. Toxicol. Appl. Pharmacol. 1997, 142, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N. Transport of Toxic Metals by Molecular Mimicry. Environ. Health Perspect. 2002, 110, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Martineau, C.; Jumarie, C.; Moreau, R. Characterization of Cadmium Uptake and Cytotoxicity in Human Osteoblast-like MG-63 Cells. Toxicol. Appl. Pharmacol. 2008, 231, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Ohba, K. Involvement of Metal Transporters in the Intestinal Uptake of Cadmium. J. Toxicol. Sci. 2020, 45, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, Z.A.; Blazka, M.E.; Endo, T. Metal Transport in Cells: Cadmium Uptake by Rat Hepatocytes and Renal Cortical Epithelial Cells. Environ. Health Perspect. 1995, 103, 73–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thévenod, F.; Fels, J.; Lee, W.-K.; Zarbock, R. Channels, Transporters and Receptors for Cadmium and Cadmium Complexes in Eukaryotic Cells: Myths and Facts. BioMetals 2019, 32, 469–489. [Google Scholar] [CrossRef]
- Blazka, M.E.; Shaikh, Z.A. Differences in Cadmium and Mercury Uptakes by Hepatocytes: Role of Calcium Channels. Toxicol. Appl. Pharmacol. 1991, 110, 355–363. [Google Scholar] [CrossRef]
- Hinkle, P.M.; Kinsella, P.A.; Osterhoudt, K.C. Cadmium Uptake and Toxicity via Voltage-Sensitive Calcium Channels. J. Biol. Chem. 1987, 262, 16333–16337. [Google Scholar] [CrossRef]
- Klinck, J.S.; Green, W.W.; Mirza, R.S.; Nadella, S.R.; Chowdhury, M.J.; Wood, C.M.; Pyle, G.G. Branchial Cadmium and Copper Binding and Intestinal Cadmium Uptake in Wild Yellow Perch (Perca flavescens) from Clean and Metal-Contaminated Lakes. Aquat. Toxicol. 2007, 84, 198–207. [Google Scholar] [CrossRef]
- Verbost, P.M.; Van Rooij, J.; Flik, G.; Lock, R.A.C.; Bonga, S.E.W. The Movement of Cadmium through Freshwater Trout Branchial Epithelium and Its Interference with Calcium Transport. J. Exp. Biol. 1989, 145, 185–197. [Google Scholar] [CrossRef]
- Jacobson, K.B.; Turner, J.E. The Interaction of Cadmium and Certain Other Metal Ions with Proteins and Nucleic Acids. Toxicology 1980, 16, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Soleimani, M.; Girijashanker, K.; Reed, J.M.; He, L.; Dalton, T.P.; Nebert, D.W. Cd2+ versus Zn2+ Uptake by the ZIP8 HCO3−-Dependent Symporter: Kinetics, Electrogenicity and Trafficking. Biochem. Biophys. Res. Commun. 2008, 365, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N. Glutathione Mercaptides as Transport Forms of Metals. Adv. Pharmacol. 1994, 27, 271–298. [Google Scholar] [CrossRef] [PubMed]
- Limaye, D.A.; Shaikh, Z.A. Cytotoxicity of Cadmium and Characteristics of Its Transport in Cardiomyocytes. Toxicol. Appl. Pharmacol. 1999, 154, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Jumarie, C.; Fortin, C.; Houde, M.; Campbell, P.G.C.; Denizeau, F. Cadmium Uptake by Caco-2 Cells: Effects of Cd Complexation by Chloride, Glutathione, and Phytochelatins. Toxicol. Appl. Pharmacol. 2001, 170, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Meister, A. Potent and Specific Inhibition of Glutathione Synthesis by Buthionine Sulfoximine (S-n-Butyl Homocysteine Sulfoximine). J. Biol. Chem. 1979, 254, 7558–7560. [Google Scholar] [CrossRef]
- Kosower, N.S.; Kosower, E.M. The Glutathione Status of Cells. Int. Rev. Cytol. 1978, 54, 109–160. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and Its Role in Cellular Functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Chen, B.; Li, L.; Liu, L.; Cao, J. Effective Adsorption of Heavy Metal Ions in Water by Sulfhydryl Modified Nano Titanium Dioxide. Front. Chem. 2023, 10, 1072139c. [Google Scholar] [CrossRef]
- Gerson, R.J.; Shaikh, Z.A. Differences in the Uptake of Cadmium and Mercury by Rat Hepatocyte Primary Cultures: Role of a Sulfhydryl Carrier. Biochem. Pharmacol. 1984, 33, 199–203. [Google Scholar] [CrossRef]
- Maryon, E.B.; Molloy, S.A.; Kaplan, J.H. Cellular Glutathione Plays a Key Role in Copper Uptake Mediated by Human Copper Transporter 1. Am. J. Physiol.-Cell Physiol. 2013, 304, C768–C779. [Google Scholar] [CrossRef] [PubMed]
- McArdle, H.J.; Gross, S.M.; Danks, D.M. Uptake of Copper by Mouse Hepatocytes. J. Cell Physiol. 1988, 136, 373–378. [Google Scholar] [CrossRef]
- Seibert, H.; Gulden, M.; Voss, J.-U. Comparative Cell Toxicology: The Basis for in Vitro Toxicity Testing. Altern. Lab. Anim. 1994, 22, 168–174. [Google Scholar] [CrossRef]
- Walum, E.; Stenberg, K.; Jenssen, D. Understanding Cell Toxicology—Principles and Practice; Horwood, E., Ed.; Ellis Horwood Limited: New York, NY, USA, 1990. [Google Scholar]
- Ballatori, N.; Villalobos, A.R. Defining the Molecular and Cellular Basis of Toxicity Using Comparative Models. Toxicol. Appl. Pharmacol. 2002, 183, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Bols, N.C.; Dayeh, V.R.; Lee, L.E.J.; Schirmer, K. Use of Fish Cell Lines in the Toxicology and Ecotoxicology of Fish. Piscine Cell Lines in Environmental Toxicology. In Biochemistry and Molecular Biology of Fishes; Moon, T.W., Mommsen, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 6, pp. 43–84. [Google Scholar]
- Segner, H. Fish Cell Lines as a Tool in Aquatic Toxicology. In Fish Ecotoxicology; Braunbeck, T., Hinton, D.E., Streit, B., Eds.; Birkhäuser Basel: Basel, Switzerland, 1998; pp. 1–38. ISBN 978-3-0348-8853-0. [Google Scholar]
- Babich, H.; Borenfreund, E. Cytotoxicity and Genotoxicity Assays with Cultured Fish Cells: A Review. Toxicol. Vitr. 1991, 5, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Castaño, A.; Bols, N.; Braunbeck, T.; Dierickx, P.; Halder, M.; Isomaa, B.; Kawahara, K.; Lee, L.E.J.; Mothersill, C.; Pärt, P.; et al. The Use of Fish Cells in Ecotoxicology: The Report and Recommendations of ECVAM Workshop 47. Altern. Lab. Anim. 2003, 31, 317–351. [Google Scholar] [CrossRef]
- Goswami, M.; Yashwanth, B.S.; Trudeau, V.; Lakra, W.S. Role and Relevance of Fish Cell Lines in Advanced in Vitro Research. Mol. Biol. Rep. 2022, 49, 2393–2411. [Google Scholar] [CrossRef]
- Wolf, K.; Quimby, M.C. Established Eurythermic Line of Fish Cells in Vitro. Science 1962, 135, 1065–1066. [Google Scholar] [CrossRef]
- Kling, P.G.; Olsson, P.E. Involvement of Differential Metallothionein Expression in Free Radical Sensitivity of RTG-2 and CHSE-214 Cells. Free Radic. Biol. Med. 2000, 28, 1628–1637. [Google Scholar] [CrossRef]
- Mayer, G.D.; Leach, A.; Kling, P.; Olsson, P.E.; Hogstrand, C. Activation of the Rainbow Trout Metallothionein-A Promoter by Silver and Zinc. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 134, 181–188. [Google Scholar] [CrossRef]
- Zafarullah, M.; Olsson, P.E.; Gedamu, L. Differential Regulation of Metallothionein Genes in Rainbow Trout Fibroblasts, RTG-2. Biochim. Et Biophys. Acta (BBA)—Gene Struct. Expr. 1990, 1049, 318–323. [Google Scholar] [CrossRef]
- Maracine, M.; Segner, H. Cytotoxicity of Metals in Isolated Fish Cells: Importance of the Cellular Glutathione Status. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 120, 83–88. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Borenfreund, E.; Puerner, J.A. A Simple Quantitative Procedure Using Monolayer Cultures for Cytotoxicity Assays (HTD/NR-90). J. Tissue Cult. Methods 1985, 9, 7–9. [Google Scholar] [CrossRef]
- Lange, A.; Ausseil, O.; Segner, H. Alterations of Tissue Glutathione Levels and Metallothionein MRNA in Rainbow Trout during Single and Combined Exposure to Cadmium and Zinc. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 131, 231–243. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, P.; Montigny, C.; Garrigos, M.; Champeil, P. Metal Binding to Ligands: Cadmium Complexes with Glutathione Revisited. Anal. Biochem. 2007, 371, 215–228. [Google Scholar] [CrossRef]
- Nigam, D.; Shukla, G.S.; Agarwal, A.K. Glutathione Depletion and Oxidative Damage in Mitochondria Following Exposure to Cadmium in Rat Liver and Kidney. Toxicol. Lett. 1999, 106, 151–157. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Cherkasov, A.S.; Sokolova, I.M. Effects of Cadmium on Cellular Protein and Glutathione Synthesis and Expression of Stress Proteins in Eastern Oysters, Crassostrea virginica Gmelin. J. Exp. Biol. 2008, 211, 577–586. [Google Scholar] [CrossRef]
- Le Croizier, G.; Lacroix, C.; Artigaud, S.; Le Floch, S.; Munaron, J.M.; Raffray, J.; Penicaud, V.; Rouget, M.L.; Laë, R.; Tito De Morais, L. Metal Subcellular Partitioning Determines Excretion Pathways and Sensitivity to Cadmium Toxicity in Two Marine Fish Species. Chemosphere 2019, 217, 754–762. [Google Scholar] [CrossRef]
- Pearson, S.A.; Cowan, J.A. Glutathione-Coordinated Metal Complexes as Substrates for Cellular Transporters. Metallomics 2021, 13, mfab015. [Google Scholar] [CrossRef] [PubMed]
- Roesijadi, G.; Robinson, W. Metal Regulation in Aquatic Animals: Mechanisms of Uptake, Accumulation, and Release. In Aquatic Toxicology; Malins, D.C., Ostrander, G.K., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 1994; pp. 387–420. [Google Scholar]
- DelRaso, N.J.; Foy, B.D.; Gearhart, J.M.; Frazier, J.M. Cadmium Uptake Kinetics in Rat Hepatocytes: Correction for Albumin Binding. Toxicol. Sci. 2003, 72, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Aduayom, I.; Campbell, P.G.C.; Denizeau, F.; Jumarie, C. Different Transport Mechanisms for Cadmium and Mercury in Caco-2 Cells: Inhibition of Cd Uptake by Hg without Evidence for Reciprocal Effects. Toxicol. Appl. Pharmacol. 2003, 189, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.; Bucio, L.; Gutiérrez-Ruiz, M.C. Cadmium Uptake by a Human Hepatic Cell Line (WRL-68 Cells). Toxicology 1997, 120, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.H.; Ciriolo, M.R.; Peisach, J. The Role of Glutathione in Copper Metabolism and Toxicity. J. Biol. Chem. 1989, 264, 5598–5605. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.K.; Anderson, M.E.; Meister, A. Glutathione, a First Line of Defense against Cadmium Toxicity. FASEB J. 1987, 1, 220–223. [Google Scholar] [CrossRef]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The Mechanism of the Cadmium-Induced Toxicity and Cellular Response in the Liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef]
- Zalups, R.K.; Barfuss, D.W.; Lash, L.H. Relationships between Alterations in Glutathione Metabolism and the Disposition of Inorganic Mercury in Rats: Effects of Biliary Ligation and Chemically Induced Modulation of Glutathione Status. Chem. Biol. Interact. 1999, 123, 171–195. [Google Scholar] [CrossRef]
- Ramya, D.; Thatheyus, A.J. Microscopic Investigations on the Biosorption of Heavy Metals by Bacterial Cells: A Review. Sci. Int. 2018, 6, 11–17. [Google Scholar] [CrossRef]
- L’Azou, B.; Passagne, I.; Mounicou, S.; Tréguer-Delapierre, M.; Puljalté, I.; Szpunar, J.; Lobinski, R.; Ohayon-Courtès, C. Comparative Cytotoxicity of Cadmium Forms (CdCl2, CdO, CdS Micro- and Nanoparticles) in Renal Cells. Toxicol. Res. 2014, 3, 32–41. [Google Scholar] [CrossRef]
- Segner, H. Response of Fed and Starved Roach, Rutilus rutilus, to Sublethal Copper Contamination. J. Fish. Biol. 1987, 30, 423–437. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, A.; Segner, H. The Role of Glutathione and Sulfhydryl Groups in Cadmium Uptake by Cultures of the Rainbow Trout RTG-2 Cell Line. Cells 2023, 12, 2720. https://doi.org/10.3390/cells12232720
Lange A, Segner H. The Role of Glutathione and Sulfhydryl Groups in Cadmium Uptake by Cultures of the Rainbow Trout RTG-2 Cell Line. Cells. 2023; 12(23):2720. https://doi.org/10.3390/cells12232720
Chicago/Turabian StyleLange, Anke, and Helmut Segner. 2023. "The Role of Glutathione and Sulfhydryl Groups in Cadmium Uptake by Cultures of the Rainbow Trout RTG-2 Cell Line" Cells 12, no. 23: 2720. https://doi.org/10.3390/cells12232720
APA StyleLange, A., & Segner, H. (2023). The Role of Glutathione and Sulfhydryl Groups in Cadmium Uptake by Cultures of the Rainbow Trout RTG-2 Cell Line. Cells, 12(23), 2720. https://doi.org/10.3390/cells12232720






