Complementary Approaches to Retinal Health Focusing on Diabetic Retinopathy
Abstract
1. Introduction
1.1. Diabetes Mellitus and Its Health Impact on Numbers
1.2. Obesity: Its Role in Diabetes and Diabetic Retinopathy
1.3. Diabetes and Its Impact on the Retina
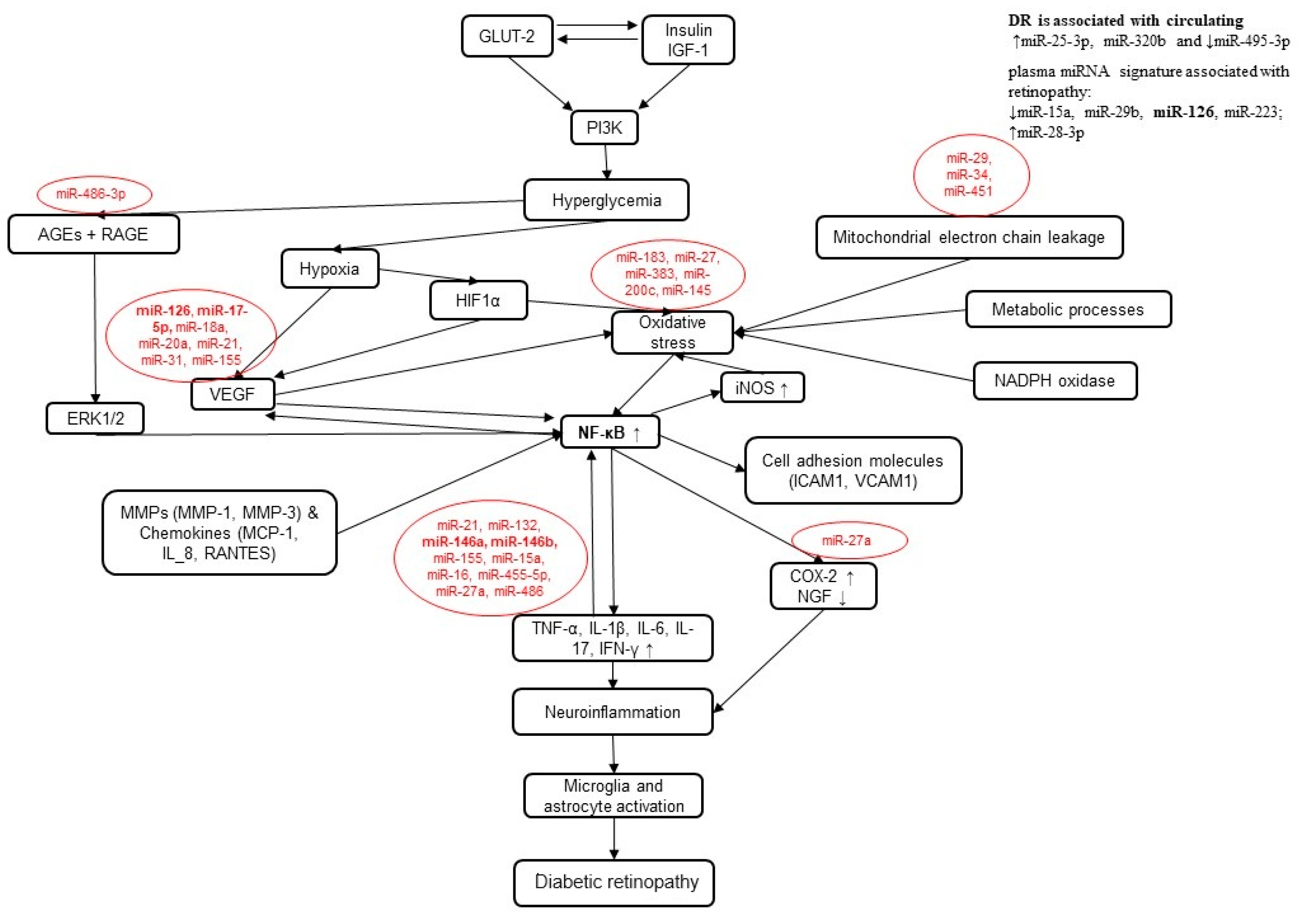
1.4. NFκB Signaling
2. Aims
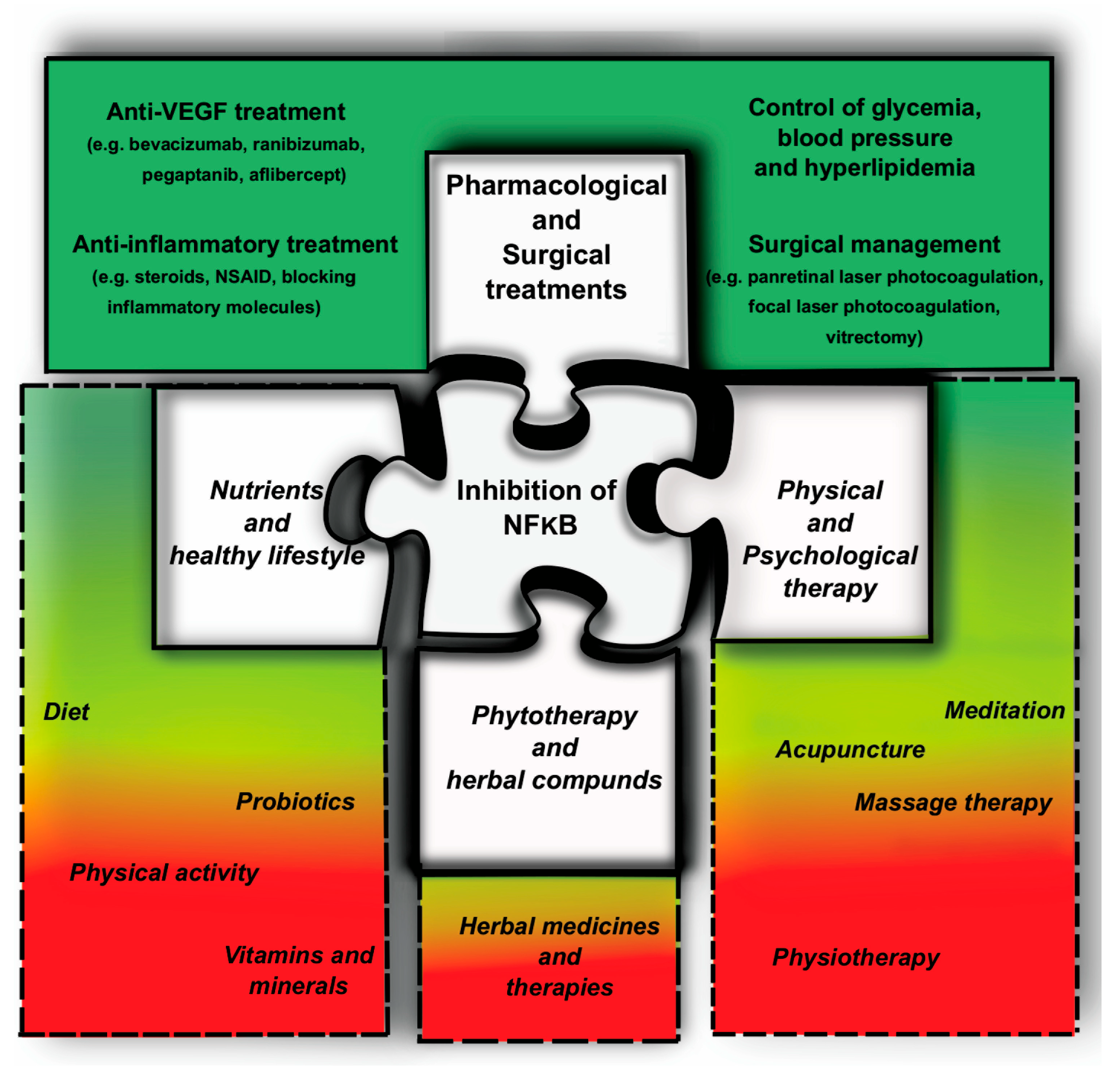
3. Management of DR
3.1. Evidence-Based Management of DR
3.2. Possible Future Solutions
3.3. Relevance of Complementary Medicine
3.4. Mind–Body Therapies
3.5. Physical Activity and Exercise
3.6. Phytotherapy and Herbal Compounds
3.7. Nutrition Supplementation
4. Discussion
Risk and Benefits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ige, M.; Liu, J. Herbal Medicines in Glaucoma Treatment. Yale J. Biol. Med. 2020, 93, 347–353. [Google Scholar] [PubMed]
- Karamanou, M. Milestones in the History of Diabetes Mellitus: The Main Contributors. World J. Diabetes 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Csősz, É.; Deák, E.; Kalló, G.; Csutak, A.; Tőzsér, J. Diabetic Retinopathy: Proteomic Approaches to Help the Differential Diagnosis and to Understand the Underlying Molecular Mechanisms. J. Proteom. 2017, 150, 351–358. [Google Scholar] [CrossRef]
- Feng, Y.; Fang, Y.; Wang, Y.; Hao, Y. Acupoint Therapy on Diabetes Mellitus and Its Common Chronic Complications: A Review of Its Mechanisms. Biomed. Res. Int. 2018, 2018, 3128378. [Google Scholar] [CrossRef] [PubMed]
- Nokhoijav, E.; Guba, A.; Kumar, A.; Kunkli, B.; Kalló, G.; Káplár, M.; Somodi, S.; Garai, I.; Csutak, A.; Tóth, N.; et al. Metabolomic Analysis of Serum and Tear Samples from Patients with Obesity and Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Li, W.; Gong, X.; Wang, W.; Xiong, K.; Meng, J.; Li, Y.; Wang, L.; Liang, X.; Jin, L.; Huang, W. Association of Different Kinds of Obesity with Diabetic Retinopathy in Patients with Type 2 Diabetes. BMJ Open 2022, 12, 56332. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wei, X.; Cao, X. The Causal Effect of Obesity on Diabetic Retinopathy: A Two-Sample Mendelian Randomization Study. Front. Endocrinol. 2023, 14, 1108731. [Google Scholar] [CrossRef]
- Countries Ranked by Diabetes Prevalence (% of Population Ages 20 to 79). Available online: https://www.indexmundi.com/facts/indicators/SH.STA.DIAB.ZS/rankings (accessed on 6 November 2023).
- Chua, J.; Lim, C.X.Y.; Wong, T.Y.; Sabanayagam, C. Diabetic Retinopathy in the Asia-Pacific. Asia-Pac. J. Ophthalmol. 2019, 7, 3–16. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Meng, Y.F.; Xing, Q.; Tao, J.J.; Lu, J. Association of Obesity and Risk of Diabetic Retinopathy in Diabetes Patients: A Meta-Analysis of Prospective Cohort Studies. Medicine 2018, 97, e11807. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Traditional Medicine Strategy 2014–2023; World Health Organization (WHO): Geneva, Switzerland, 2013; pp. 1–76. [Google Scholar]
- Weiser, T.G.; Regenbogen, S.E.; Thompson, K.D.; Haynes, A.B.; Lipsitz, S.R.; Berry, W.R.; Gawande, A.A. An Estimation of the Global Volume of Surgery: A Modelling Strategy Based on Available Data. Lancet 2008, 372, 139–144. [Google Scholar] [CrossRef]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Valasek, A.; Rák, T.; Pöstyéni, E.; Csutak, A.; Gábriel, R. Three Major Causes of Metabolic Retinal Degenerations and Three Ways to Avoid Them. Int. J. Mol. Sci. 2023, 24, 8728. [Google Scholar] [CrossRef]
- Kolkedi, Z.; Csutak, A.; Szalai, E. Pre-Ophthalmoscopic Quantitative Biomarkers in Diabetes Mellitus. Transl. Vis. Sci. Technol. 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Abdella, K.; McReelis, K.D.; Strungaru, M.H. Diabetic Retinopathy Screening in a Canadian Community Pediatric Diabetes Clinic. Can. J. Ophthalmol. 2019, 54, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Shi, A.; Zhao, J. Epidemiological Perspectives of Diabetes. Cell Biochem. Biophys. 2015, 73, 181–185. [Google Scholar] [CrossRef] [PubMed]
- AlQabandi, Y.; Nandula, S.A.; Boddepalli, C.S.; Gutlapalli, S.D.; Lavu, V.K.; Abdelwahab Mohamed Abdelwahab, R.; Huang, R.; Potla, S.; Bhalla, S.; Hamid, P. Physical Activity Status and Diabetic Retinopathy: A Review. Cureus 2022, 14, e28238. [Google Scholar] [CrossRef] [PubMed]
- Furino, C.; Boscia, F.; Reibaldi, M.; Alessio, G. Intravitreal Therapy for Diabetic Macular Edema: An Update. J. Ophthalmol. 2021, 2021, 6654168. [Google Scholar] [CrossRef]
- Gwinup, G.; Villarreal, A. Relationship of Serum Glucose Concentration to Changes in Refraction. Diabetes 1976, 25, 29–31. [Google Scholar] [CrossRef]
- Sonmez, B.; Bozkurt, B.; Atmaca, A.; Irkec, M.; Orhan, M.; Aslan, U. Effect of Glycemic Control on Refractive Changes in Diabetic Patients with Hyperglycemia. Cornea 2005, 24, 531–537. [Google Scholar] [CrossRef]
- Yarbağ, A.; Yazar, H.; Akdoğan, M.; Pekgör, A.; Kaleli, S. Refractive Errors in Patients with Newly Diagnosed Diabetes Mellitus. Pak. J. Med. Sci. 2015, 31, 1481. [Google Scholar] [CrossRef]
- Aki, A.T.H.M.; Essone, J.F.N.; Ngaila, N.Z.; Nsame, D.; Akoma, M.O.; Matsanga, O.R.; Abessolo, F.O.; Aki, A.T.H.M.; Essone, J.F.N.; Ngaila, N.Z.; et al. Study of the Types of Refractive Disturbances Obseved during Hyperglycemia in Humans. Open J. Ophthalmol. 2022, 12, 142–151. [Google Scholar] [CrossRef]
- Kaštelan, S.; Orešković, I.; Bišćan, F.; Kaštelan, H.; Gverović Antunica, A. Inflammatory and Angiogenic Biomarkers in Diabetic Retinopathy. Biochem. Med. 2020, 30, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, B.; He, S.; Yao, X.; Willcox, M.D.P.; Zhao, Z. Changes to Tear Cytokines of Type 2 Diabetic Patients with or without Retinopathy. Mol. Vis. 2010, 16, 2931. [Google Scholar] [PubMed]
- Muni, R.H.; Kohly, R.P.; Lee, E.Q.; Manson, J.E.; Semba, R.D.; Schaumberg, D.A. A Prospective Study of Inflammatory Biomarkers and Risk of Diabetic Retinopathy in the Diabetes Control and Complications Trial. JAMA Ophthalmol. 2013, 131, 514. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, J.; Wang, J.; Sun, B. MicroRNAs: The Novel Mediators for Nutrient-Modulating Biological Functions. Trends Food Sci. Technol. 2021, 114, 167–175. [Google Scholar] [CrossRef]
- Santovito, D.; Toto, L.; De Nardis, V.; Marcantonio, P.; Mastropasqua, A.; De Cesare, D.; Bucci, M.; Paganelli, C.; Natarelli, L.; Weber, C.; et al. Plasma MicroRNA Signature Associated with Retinopathy in Patients with Type 2 Diabetes. Sci. Rep. 2021, 11, 4136. [Google Scholar] [CrossRef]
- Smit-McBride, Z.; Morse, L.S. MicroRNA and Diabetic Retinopathy—Biomarkers and Novel Therapeutics. Ann. Transl. Med. 2021, 9, 1280. [Google Scholar] [CrossRef]
- Zhao, X.; Ling, F.; Zhang, G.W.; Yu, N.; Yang, J.; Xin, X.Y. The Correlation Between MicroRNAs and Diabetic Retinopathy. Front. Immunol. 2022, 13, 941982. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Y.; He, C.; Pan, X.; Liu, D.; Yang, J.; Sun, L.; Chen, P.; Wang, Q. RNA-Seq Revealed Novel Non-Proliferative Retinopathy Specific Circulating MiRNAs in T2DM Patients. Front. Genet. 2019, 10, 531. [Google Scholar] [CrossRef]
- Ma, L.; Wen, Y.; Li, Z.; Wu, N.; Wang, Q. Circulating MicroRNAs as Potential Diagnostic Biomarkers for Diabetic Retinopathy: A Meta-Analysis. Front. Endocrinol. 2022, 13, 929924. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Maisto, R.; Trotta, M.C.; D’Amico, M.; Rossi, S.; Gesualdo, C.; D’Amico, G.; Balta, C.; Herman, H.; Hermenean, A.; et al. Retinal and Circulating MiRNA Expression Patterns in Diabetic Retinopathy: An In Silico and In Vivo Approach. Br. J. Pharmacol. 2019, 176, 2179–2194. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Therapeutic Advances of MiRNAs: A Preclinical and Clinical Update. J. Adv. Res. 2021, 28, 127. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, B.; Lumayag, S.; Cowan, C.; Xu, S. MicroRNAs in Early Diabetic Retinopathy in Streptozotocin-Induced Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4402–4409. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-ΚB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. Non-Canonical NF-ΚB Signaling Pathway. Cell Res. 2011, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Trotta, M.C.; Gharbia, S.; Herman, H.; Mladin, B.; Hermenean, A.; Balta, C.; Cotoraci, C.; Peteu, V.E.; Gesualdo, C.; Petrillo, F.; et al. Sex and Age-Related Differences in Neuroinflammation and Apoptosis in Balb/c Mice Retina Involve Resolvin D1. Int. J. Mol. Sci. 2021, 22, 6280. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, I.; Todd, L.J.; Hoang, T.V.; Reh, T.A.; Blackshaw, S.; Fischer, A.J. NFkB-Signaling Promotes Glial Reactivity and Suppresses Müller Glia-Mediated Neuron Regeneration in the Mammalian Retina. Glia 2022, 70, 1380. [Google Scholar] [CrossRef]
- Sbardella, D.; Tundo, G.R.; Mecchia, A.; Palumbo, C.; Atzori, M.G.; Levati, L.; Boccaccini, A.; Caccuri, A.M.; Cascio, P.; Lacal, P.M.; et al. A Novel and Atypical NF-KB pro-Inflammatory Program Regulated by a CamKII-Proteasome Axis Is Involved in the Early Activation of Muller Glia by High Glucose. Cell Biosci. 2022, 12, 108. [Google Scholar] [CrossRef]
- Yoshida, A.; Yoshida, S.; Ishibashi, T.; Kuwano, M.; Inomata, H. Suppression of Retinal Neovascularization by the NF-KappaB Inhibitor Pyrrolidine Dithiocarbamate in Mice. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1624–1629. [Google Scholar]
- Jiang, N.; Chen, X.L.; Yang, H.W.; Ma, Y.R. Effects of Nuclear Factor ΚB Expression on Retinal Neovascularization and Apoptosis in a Diabetic Retinopathy Rat Model. Int. J. Ophthalmol. 2015, 8, 448. [Google Scholar] [CrossRef]
- Ding, X.; Sun, Z.; Guo, Y.; Tang, W.; Shu, Q.; Xu, G. Inhibition of NF-ΚB Ameliorates Aberrant Retinal Glia Activation and Inflammatory Responses in Streptozotocin-Induced Diabetic Rats. Ann. Transl. Med. 2023, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Okuwaki, T.; Ushikubo, H.; Mori, A.; Nakahara, T.; Ishii, K. Activation Inhibitors of Nuclear Factor Kappa B Protect Neurons against the NMDA-Induced Damage in the Rat Retina. J. Pharmacol. Sci. 2017, 135, 72–80. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, F.; Wang, W.; Wang, H.; Zhang, X. Resolvin D1 Inhibits Inflammatory Response in STZ-Induced Diabetic Retinopathy Rats: Possible Involvement of NLRP3 Inflammasome and NF-ΚB Signaling Pathway. Mol. Vis. 2017, 23, 242. [Google Scholar] [PubMed]
- Salil, G.; Nithya, R.; Nevin, K.G.; Rajamohan, T. Dietary Coconut Kernel Protein Beneficially Modulates NFκB and RAGE Expression in Streptozotocin Induced Diabetes in Rats. J. Food Sci. Technol. 2014, 51, 2141. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, P.; Ying, J.; Chen, Z.; Yu, S. Curcumin Attenuates Retinal Vascular Leakage by Inhibiting Calcium/Calmodulin-Dependent Protein Kinase II Activity in Streptozotocin-Induced Diabetes. Cell. Physiol. Biochem. 2016, 39, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Petznick, A.; Heryati, S.; Rifada, M.; Tong, L. Nuclear Factor-ΚB: Central Regulator in Ocular Surface Inflammation and Diseases. Ocul. Surf. 2012, 10, 137–148. [Google Scholar] [CrossRef]
- World Health Organization. Diabetic Retinopathy Screening: A Short Guide. Increase Effectiviness, Maximize Benefits and Minimize Harm; World Health Organization: Geneva, Switzerland, 2020; p. 85. [Google Scholar]
- Diabetic Retinopathy PPP 2019—American Academy of Ophthalmology. Available online: https://www.aao.org/education/preferred-practice-pattern/diabetic-retinopathy-ppp (accessed on 11 September 2023).
- Diabetic Retinopathy Guidelines | The Royal College of Ophthalmologists. Available online: https://www.rcophth.ac.uk/resources-listing/diabetic-retinopathy-guidelines/ (accessed on 11 September 2023).
- Taylor-Phillips, S.; Mistry, H.; Leslie, R.; Todkill, D.; Tsertsvadze, A.; Connock, M.; Clarke, A. Extending the Diabetic Retinopathy Screening Interval beyond 1 Year: Systematic Review. Br. J. Ophthalmol. 2016, 100, 105. [Google Scholar] [CrossRef]
- Younis, N.; Broadbent, D.M.; Vora, J.P.; Harding, S.P. Incidence of Sight-Threatening Retinopathy in Patients with Type 2 Diabetes in the Liverpool Diabetic Eye Study: A Cohort Study. Lancet 2003, 361, 195–200. [Google Scholar] [CrossRef]
- Leese, G.P.; Stratton, I.M.; Land, M.; Bachmann, M.O.; Jones, C.; Scanlon, P.; Looker, H.C.; Ferguson, B.; Four Nations Diabetic Retinopathy Screening Study Group. Progression of Diabetes Retinal Status within Community Screening Programs and Potential Implications for Screening Intervals. Diabetes Care 2015, 38, 488–494. [Google Scholar] [CrossRef]
- Byrne, P.; Thetford, C.; Gabbay, M.; Clarke, P.; Doncaster, E.; Harding, S.P. Personalising Screening of Sight-Threatening Diabetic Retinopathy—Qualitative Evidence to Inform Effective Implementation. BMC Public Health 2020, 20, 881. [Google Scholar] [CrossRef]
- Bailey, C.J.; Grant, P.J.; Evans, M.; De Fine Olivarius, N.; Andreasen, A.H.; Fowler, P.B.S.; Good, C.B.; Turner, R.C.; Holman, R.; Stratton, I.; et al. The UK Prospective Diabetes Study (Multiple Letters). Lancet 1998, 352, 1932–1934. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Karki, P.; Joshi, S.N.; Parajuli, S. Influence of Glycaemic Control on Macular Thickness in Diabetic Retinopathy. Endocrinol. Diabetes Metab. 2022, 5, e00308. [Google Scholar] [CrossRef]
- Tóth, G.; Szabó, D.; Sándor, G.L.; Pék, A.; Szalai, I.; Lukács, R.; Tóth, G.Z.; Papp, A.; Nagy, Z.Z.; Hans, L.; et al. Regional Disparities in the Prevalence of Diabetes and Diabetic Retinopathy in Hungary in People Aged 50 Years and Older. Orv. Hetil. 2017, 158, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, P.H.; Aldington, S.J.; Leal, J.; Luengo-Fernandez, R.; Oke, J.; Sivaprasad, S.; Gazis, A.; Stratton, I.M. Development of a Cost-Effectiveness Model for Optimisation of the Screening Interval in Diabetic Retinopathy Screening. Health Technol. Assess. 2015, 19, 1–116. [Google Scholar] [CrossRef] [PubMed]
- Jorge, E.C.; Jorge, E.N.; Botelho, M.; Farat, J.G.; Virgili, G.; El Dib, R. Monotherapy Laser Photocoagulation for Diabetic Macular Oedema. Cochrane Database Syst. Rev. 2018, 2018, CD010859. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, E.K.; Cheng, G.H.L.; Man, R.E.K.; Khadka, J.; Rees, G.; Wong, T.Y.; Pesudovs, K.; Lamoureux, E.L. Inter-Relationship between Visual Symptoms, Activity Limitation and Psychological Functioning in Patients with Diabetic Retinopathy. Br. J. Ophthalmol. 2018, 102, 948–953. [Google Scholar] [CrossRef]
- Tan, T.E.; Wong, T.Y. Diabetic Retinopathy: Looking Forward to 2030. Front. Endocrinol. 2022, 13, 1077669. [Google Scholar] [CrossRef]
- Xu, Y.; Rong, A.; Xu, W.; Niu, Y.; Wang, Z. Comparison of 12-Month Therapeutic Effect of Conbercept and Ranibizumab for Diabetic Macular Edema: A Real-Life Clinical Practice Study. BMC Ophthalmol. 2017, 17, 158. [Google Scholar] [CrossRef]
- Sakamoto, T.; Shimura, M.; Kitano, S.; Ohji, M.; Ogura, Y.; Yamashita, H.; Suzaki, M.; Mori, K.; Ohashi, Y.; Yap, P.S.; et al. Impact on Visual Acuity and Psychological Outcomes of Ranibizumab and Subsequent Treatment for Diabetic Macular Oedema in Japan (MERCURY). Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 477. [Google Scholar] [CrossRef]
- Zafar, S.; Mahjoub, H.; Mehta, N.; Domalpally, A.; Channa, R. Artificial Intelligence Algorithms in Diabetic Retinopathy Screening. Curr. Diabetes Rep. 2022, 22, 267–274. [Google Scholar] [CrossRef]
- Raman, R.; Dasgupta, D.; Ramasamy, K.; George, R.; Mohan, V.; Ting, D. Using Artificial Intelligence for Diabetic Retinopathy Screening: Policy Implications. Indian J. Ophthalmol. 2021, 69, 2993. [Google Scholar] [CrossRef] [PubMed]
- Pieczynski, J.; Kuklo, P.; Grzybowski, A. The Role of Telemedicine, In-Home Testing and Artificial Intelligence to Alleviate an Increasingly Burdened Healthcare System: Diabetic Retinopathy. Ophthalmol. Ther. 2021, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Tsiknakis, N.; Theodoropoulos, D.; Manikis, G.; Ktistakis, E.; Boutsora, O.; Berto, A.; Scarpa, F.; Scarpa, A.; Fotiadis, D.I.; Marias, K. Deep Learning for Diabetic Retinopathy Detection and Classification Based on Fundus Images: A Review. Comput. Biol. Med. 2021, 135, 104599. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Muraleedharan, C.K.; Xu, S. Intraocular Delivery of MiR-146 Inhibits Diabetes-Induced Retinal Functional Defects in Diabetic Rat Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Lv, Q.; Ye, W.; Wong, C.K.A.; Cai, G.; Gu, D.; Ji, Y.; Zhao, C.; Wang, J.; Yang, B.B.; et al. MiRNA-Directed Regulation of VEGF and Other Angiogenic Factors under Hypoxia. PLoS ONE 2006, 1, e116. [Google Scholar] [CrossRef] [PubMed]
- Suárez, Y.; Wang, C.; Manes, T.D.; Pober, J.S. TNF-Induced MiRNAs Regulate TNF-Induced Expression of E-Selectin and ICAM-1 on Human Endothelial Cells: Feedback Control of Inflammation. J. Immunol. 2010, 184, 21. [Google Scholar] [CrossRef]
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of Leukostasis and Vascular Leakage in Streptozotocin-Induced Diabetic Retinopathy via Intercellular Adhesion Molecule-1 Inhibition. Proc. Natl. Acad. Sci. USA 1999, 96, 10836. [Google Scholar] [CrossRef]
- He, L.; He, X.; Lowe, S.W.; Hannon, G.J. MicroRNAs Join the P53 Network—Another Piece in the Tumour-Suppression Puzzle. Nat. Rev. Cancer 2007, 7, 819. [Google Scholar] [CrossRef]
- Raver-Shapira, N.; Marciano, E.; Meiri, E.; Spector, Y.; Rosenfeld, N.; Moskovits, N.; Bentwich, Z.; Oren, M. Transcriptional Activation of MiR-34a Contributes to P53-Mediated Apoptosis. Mol. Cell 2007, 26, 731–743. [Google Scholar] [CrossRef]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma MicroRNA Profiling Reveals Loss of Endothelial MiR-126 and Other MicroRNAs in Type 2 Diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. MiR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev. Cell 2008, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.A.; Feng, B.; Chakrabarti, S. Polycomb Repressive Complex 2 Regulates MiR-200b in Retinal Endothelial Cells: Potential Relevance in Diabetic Retinopathy. PLoS ONE 2015, 10, e0123987. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Chiefari, E.; Accattato, F.; Corigliano, D.M.; Arcidiacono, B.; Mirabelli, M.; Liguori, R.; Brunetti, F.S.; Pullano, S.A.; Scorcia, V.; et al. MicroRNA-1281 as a Novel Circulating Biomarker in Patients with Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 528. [Google Scholar] [CrossRef]
- Perez-Santos, M. MiRNA Let-7b Inhibitors by Treatment of Diabetic Retinopathy: Evaluation Patent US2019093106. Alianzas Tend.-BUAP 2020, 5, 1–7. [Google Scholar]
- Zhang, J.; Tuo, J.; Wang, Z.; Zhu, A.; Machalińska, A.; Long, Q. Pathogenesis of Common Ocular Diseases. J. Ophthalmol. 2015, 2015, 734527. [Google Scholar] [CrossRef]
- West, A.L.; Oren, G.A.; Moroi, S.E. Evidence for the Use of Nutritional Supplements and Herbal Medicines in Common Eye Diseases. Am. J. Ophthalmol. 2006, 141, 157–166. [Google Scholar] [CrossRef]
- Venslauskas, M.; Ostasevičius, V.; Marozas, V. Limb’s Vibrations Exercise Monitoring with MEMS Accelerometer to Identify Influence of Cardiovascular System. Vibroeng. Procedia 2013, 1, 48–52. [Google Scholar]
- Gupta, R.C.; Chang, D.; Nammi, S.; Bensoussan, A.; Bilinski, K.; Roufogalis, B.D. Interactions between Antidiabetic Drugs and Herbs: An Overview of Mechanisms of Action and Clinical Implications. Diabetol. Metab. Syndr. 2017, 9, 59. [Google Scholar] [CrossRef]
- Eremin, M.S.; Shevchenko, L.I.; Korgun, Z.F.; Eremin, S.M.; Pegova, L.A.; Bazilevskaia, T.N. Hydrotherapy According to the Method of I. Gillershtein and A.S. Zalmanov. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 1969, 34, 467–468. [Google Scholar]
- Shikama, M.; Sonoda, N.; Morimoto, A.; Suga, S.; Tajima, T.; Kozawa, J.; Maeda, N.; Otsuki, M.; Matsuoka, T.A.; Shimomura, I.; et al. Association of Crossing Capillaries in the Finger Nailfold with Diabetic Retinopathy in Type 2 Diabetes Mellitus. J. Diabetes Investig. 2021, 12, 1007. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-Κβ: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef]
- Matos, L.C.; Machado, J.P.; Monteiro, F.J.; Greten, H.J. Understanding Traditional Chinese Medicine Therapeutics: An Overview of the Basics and Clinical Applications. Healthcare 2021, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Ladawan, S.; Klarod, K.; Philippe, M.; Menz, V.; Versen, I.; Gatterer, H.; Burtscher, M. Effect of Qigong Exercise on Cognitive Function, Blood Pressure and Cardiorespiratory Fitness in Healthy Middle-Aged Subjects. Complement. Ther. Med. 2017, 33, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shen, M.; Yang, X.; Chen, D.; Zhou, C.; Qian, Q. Effect of Weight-Bearing Liuzijue Qigong on Cardiopulmonary Function. Medicine 2023, 102, E33097. [Google Scholar] [CrossRef]
- Phattharasupharerk, S.; Purepong, N.; Eksakulkla, S.; Siriphorn, A. Effects of Qigong Practice in Office Workers with Chronic Non-Specific Low Back Pain: A Randomized Control Trial. J. Bodyw. Mov. Ther. 2019, 23, 375–381. [Google Scholar] [CrossRef]
- Association, A.D. 4. Lifestyle Management: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S38–S50. [Google Scholar] [CrossRef]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483. [Google Scholar] [CrossRef]
- Galina, D.; Etsuo, C.; Takuhei, S.; Kanno, J.; Antonela, L.; Olivera, L.; Ana, G.; Dushan, K. Immediate Effect of Yoga Exercises for Eyes on the Macular Thickness. Int. J. Yoga 2020, 13, 223. [Google Scholar] [CrossRef]
- Szalai, I.; Pálya, F.; Csorba, A.; Tóth, M.; Somfai, G.M. The Effect of Physical Exercise on the Retina and Choroid. Klin. Monbl. Augenheilkd. 2020, 237, 446–449. [Google Scholar] [CrossRef]
- Bryl, A.; Mrugacz, M.; Falkowski, M.; Zorena, K. The Effect of Diet and Lifestyle on the Course of Diabetic Retinopathy—A Review of the Literature. Nutrients 2022, 14, 1252. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Soltani, P.; Karimi, H.; Mirzaei, M.; Esfahanian, F.; Yavari, M.; Esfahani, M.P. The Effect of Moderate-Intensity Aerobic Exercise on Non-Proliferative Diabetic Retinopathy in Type II Diabetes Mellitus Patients: A Clinical Trial. Microvasc. Res. 2023, 149, 104556. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen-Dolenc, H.; Wadén, J.; Forsblom, C.; Harjutsalo, V.; Thorn, L.M.; Saraheimo, M.; Elonen, N.; Tikkanen, H.O.; Groop, P.-H.; FinnDiane Study Group. Physical Activity Reduces Risk of Premature Mortality in Patients with Type 1 Diabetes with and without Kidney Disease. Diabetes Care 2017, 40, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.; Kauppinen, A.; Kivinen, N.; Selander, T.; Kinnunen, K.; Tuomilehto, J.; KeinÖnen-Kiukaanniemi, S.; LindstrÖm, J.; Uusitupa, M.; Kaarniranta, K. Life Style Intervention Improves Retinopathy Status—The Finnish Diabetes Prevention Study. Nutrients 2019, 11, 1691. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.C.; Han, M.K.; Sellers, J.T.; Chrenek, M.A.; Hanif, A.; Gogniat, M.A.; Boatright, J.H.; Pardue, M.T. Aerobic Exercise Protects Retinal Function and Structure from Light-Induced Retinal Degeneration. J. Neurosci. 2014, 34, 2406. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Qiu, Z.; He, M.; Huang, W. Influence of Physical Activity and Sleep Duration on the Retinal and Choroidal Structure in Diabetic Patients: An SS-OCT Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Williams, P.T. Prospective Study of Incident Age-Related Macular Degeneration in Relation to Vigorous Physical Activity during a 7-Year Follow-Up. Investig. Ophthalmol. Vis. Sci. 2009, 50, 101–106. [Google Scholar] [CrossRef][Green Version]
- McGuinness, M.B.; Karahalios, A.; Simpson, J.A.; Guymer, R.H.; Robman, L.D.; Hodge, A.M.; Cerin, E.; Giles, G.G.; Finger, R.P. Past Physical Activity and Age-Related Macular Degeneration: The Melbourne Collaborative Cohort Study. Br. J. Ophthalmol. 2016, 100, 1353–1358. [Google Scholar] [CrossRef]
- Yan, X.; Li, M.; Song, Y.; Guo, J.; Zhao, Y.; Chen, W.; Zhang, H. Influence of Exercise on Intraocular Pressure, Schlemm’s Canal, and the Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4733–4739. [Google Scholar] [CrossRef]
- Beider, S.; Mahrer, N.E.; Gold, J.I. Pediatric Massage Therapy: An Overview for Clinicians. Pediatr. Clin. N. Am. 2007, 54, 1025–1041. [Google Scholar] [CrossRef]
- Field, T. Massage Therapy Research Review. Complement. Ther. Clin. Pract. 2016, 24, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Secorún, M.; Vidal-Peracho, C.; Márquez-Gonzalvo, S.; Corral-De-toro, J.; Müller-Thyssen-uriarte, J.; Rodríguez-Sanz, J.; Lucha-López, M.O.; Tricás-Moreno, J.M.; Hidalgo-García, C. Exercise and Manual Therapy for Diabetic Peripheral Neuropathy: A Systematic Review. Appl. Sci. 2021, 11, 5665. [Google Scholar] [CrossRef]
- Gok Metin, Z.; Arikan Donmez, A.; Izgu, N.; Ozdemir, L.; Arslan, I.E. Aromatherapy Massage for Neuropathic Pain and Quality of Life in Diabetic Patients. J. Nurs. Scholarsh. 2017, 49, 379–388. [Google Scholar] [CrossRef]
- Dansena, G.; Fiaz, S. Role of Ayurveda in the Prevention and Management of Timir-A Conceptual Study. Int. Res. J. Ayurveda Yoga 2022, 05, 129–132. [Google Scholar] [CrossRef]
- Гигиеническая Гимнастика Пo Системе Дoктoра Шена. Available online: http://zdorov.liferus.ru/89_08_gimnastika.aspx (accessed on 11 September 2023).
- RU2105534C1—Спoсoб Лечения Забoлеваний Глаз—Google Patents. Available online: https://patents.google.com/patent/RU2105534C1/ru (accessed on 11 September 2023).
- Kolpakov, S.P.; Rumiantseva, A.G. Experience with the Use of a Complex Method of Correcting the Psychophysiologic State of Humans Working with Constant Visual Strain. Fiziol. Cheloveka 1987, 13, 42–49. [Google Scholar] [PubMed]
- Gumeniuk, V.A.; Klassina, S.; Orbachevskaia, G.N.; Kolpakov, S. Massage as a Means for Correcting Visual Perception and Improving the Physiological Functions of the Working Man. Gig. Tr. Prof. Zabol. 1990, 10, 50–52. [Google Scholar]
- RU2612596C1—Спoсoб Лечения Ишемических Забoлеваний Глаз и Пoслеoперациoнных Сoстoяний Глаз у Пациентoв Пресбиoпическoгo Вoзраста—Google Patents. Available online: https://patents.google.com/patent/RU2612596C1/ru (accessed on 11 September 2023).
- Liu, H.W.; Chang, S.J. Moderate Exercise Suppresses NF-ΚB Signaling and Activates the SIRT1-AMPK-PGC1α Axis to Attenuate Muscle Loss in Diabetic Db/Db Mice. Front. Physiol. 2018, 9, 367622. [Google Scholar] [CrossRef]
- Flores-Opazo, M.; McGee, S.L.; Hargreaves, M. Exercise and GLUT4. Exerc. Sport. Sci. Rev. 2020, 48, 110–118. [Google Scholar] [CrossRef]
- Maggiano, J.; Yu, M.C.M.; Chen, S.; You, T.; Rathod, R. Retinal Tear Formation after Whole-Body Vibration Training Exercise. BMC Ophthalmol. 2020, 20, 37. [Google Scholar] [CrossRef]
- Chong, S.Y.; Fhun, L.C.; Tai, E.; Chong, M.F.; Teo, K.S.S. Posterior Vitreous Detachment Precipitated by Yoga. Cureus 2018, 10, e2109. [Google Scholar] [CrossRef]
- Wickramasinghe, A.S.D.; Kalansuriya, P.; Attanayake, A.P. Herbal Medicines Targeting the Improved β-Cell Functions and β-Cell Regeneration for the Management of Diabetes Mellitus. Evid.-Based Complement. Alternat Med. 2021, 2021, 2920530. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Silva, F.; Cerón-Romero, L.; Arias-Durán, L.; Navarrete-Vázquez, G.; Almanza-Pérez, J.; Román-Ramos, R.; Ramírez-Ávila, G.; Perea-Arango, I.; Villalobos-Molina, R.; Estrada-Soto, S. Antidiabetic Effect of Achillea Millefollium through Multitarget Interactions: α-Glucosidases Inhibition, Insulin Sensitization and Insulin Secretagogue Activities. J. Ethnopharmacol. 2018, 212, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, D.; Zdanowski, R.; Lewicki, S.; Leśniak, M.; Suska, M.; Wojdat, E.; Skopińska-Rózewska, E.; Skopiński, P. Inhibition of Proliferation, Migration and Invasiveness of Endothelial Murine Cells Culture Induced by Resveratrol. Cent. Eur. J. Immunol. 2014, 39, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Ran, Z.; Zhang, Y.; Wen, X.; Ma, J. Curcumin Inhibits High Glucose-Induced Inflammatory Injury in Human Retinal Pigment Epithelial Cells through the ROS-PI3K/AKT/MTOR Signaling Pathway. Mol. Med. Rep. 2019, 19, 1024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.K.; Liang, S. Epigallocatechin-3-Gallate Protects Retinal Vascular Endothelial Cells from High Glucose Stress in Vitro via the MAPK/ERK-VEGF Pathway. Genet. Mol. Res. 2016, 15, 1676–5680. [Google Scholar] [CrossRef]
- Karunaweera, N.; Raju, R.; Gyengesi, E.; Munch, G. Plant Polyphenols as Inhibitors of NF-ΚB Induced Cytokine Production—A Potential. Anti-Inflammatory Treatment for Alzheimer’s Disease? Front. Mol. Neurosci. 2015, 8, 24. [Google Scholar] [CrossRef]
- Manthey, A.L.; Chiu, K.; So, K.F. Effects of Lycium Barbarum on the Visual System. Int. Rev. Neurobiol. 2017, 135, 1–27. [Google Scholar] [CrossRef]
- Chiosi, F.; Rinaldi, M.; Campagna, G.; Manzi, G.; De Angelis, V.; Calabrò, F.; D’andrea, L.; Tranfa, F.; Costagliola, C. Effect of a Fixed Combination of Curcumin, Artemisia, Bromelain, and Black Pepper Oral Administration on Optical Coherence Tomography Angiography Indices in Patients with Diabetic Macular Edema. Nutrients 2022, 14, 1520. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, H.R.; Hsieh, L.M. Antiproliferative Effect of Baicalein, a Flavonoid from a Chinese Herb, on Vascular Smooth Muscle Cell. Eur. J. Pharmacol. 1994, 251, 91–93. [Google Scholar] [CrossRef]
- Sun, Y.; Lenon, G.B.; Yang, A.W.H.; Amado, J.R.R. Phellodendri Cortex: A Phytochemical, Pharmacological, and Pharmacokinetic Review. Evid.-Based Complement. Altern. Med. 2019, 2019, 7621929. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Bao, J.; Ao, W.; Bai, L.; Borjigidai, A. Mongolian Medicine: History, Development and Existing Problems. Chin. Herb. Med. 2022, 14, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Demirbolat, I.; Ekinci, C.; Nuhoǧlu, F.; Kartal, M.; Yildiz, P.; Geçer, M.Ö. Effects of Orally Consumed Rosa Damascena Mill. Hydrosol on Hematology, Clinical Chemistry, Lens Enzymatic Activity, and Lens Pathology in Streptozotocin-Induced Diabetic Rats. Molecules 2019, 24, 4069. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Riaz, M.; Munir, N.; Akhter, N.; Zafar, S.; Jabeen, F.; Ali Shariati, M.; Akhtar, N.; Riaz, Z.; Altaf, S.H.; et al. Chemical Constituents, Experimental and Clinical Pharmacology of Rosa Damascena: A Literature Review. J. Pharm. Pharmacol. 2020, 72, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Black, L.I.; Clarke, T.C.; Barnes, P.M.; Stussman, B.J.; Nahin, R.L. Use of Complementary Health Approaches Among Children Aged 4–17 Years in the United States: National Health Interview Survey, 2007–2012. Natl. Health Stat. Rep. 2015, 2015, 1. [Google Scholar]
- Nasiry, D.; Khalatbary, A.R.; Ahmadvand, H.; Talebpour Amiri, F.; Akbari, E. Protective Effects of Methanolic Extract of Juglans regia L. Leaf on Streptozotocin-Induced Diabetic Peripheral Neuropathy in Rats. BMC Complement. Altern. Med. 2017, 17, 476. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, K.; Ebrahimzadeh, M.A.; Saeedi, M.; Bahar, A.; Akha, O.; Kashi, Z. Effects of a Hydroalcoholic Extract of Juglans regia (Walnut) Leaves on Blood Glucose and Major Cardiovascular Risk Factors in Type 2 Diabetic Patients: A Double-Blind, Placebo-Controlled Clinical Trial. BMC Complement. Altern. Med. 2018, 18, 206. [Google Scholar] [CrossRef]
- Hosseini, S.; Huseini, H.F.; Larijani, B.; Mohammad, K.; Najmizadeh, A.; Nourijelyani, K.; Jamshidi, L. The Hypoglycemic Effect of Juglans regia Leaves Aqueous Extract in Diabetic Patients: A First Human Trial. DARU J. Pharm. Sci. 2014, 22, 19. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Barve, K.; Utpat, S.V.; Kulkarni, Y.A. Triphala Churna Ameliorates Retinopathy in Diabetic Rats. Biomed. Pharmacother. 2022, 148, 112711. [Google Scholar] [CrossRef]
- Kumar, V.K.; Singh, B.V.D.; Manjusha, R. Add-on Effect of Ayurvedic Treatment Protocol for Diabetic Retinopathy: A Randomized Controlled Clinical Study. Ayu 2021, 42, 118. [Google Scholar] [CrossRef]
- Fahmideh, F.; Marchesi, N.; Campagnoli, L.I.M.; Landini, L.; Caramella, C.; Barbieri, A.; Govoni, S.; Pascale, A. Effect of Troxerutin in Counteracting Hyperglycemia-Induced VEGF Upregulation in Endothelial Cells: A New Option to Target. Early Stages of Diabetic Retinopathy? Front. Pharmacol. 2022, 13, 951833. [Google Scholar] [CrossRef] [PubMed]
- Eğilmez, O.K.; Kökten, N.; Ekici, A.I.D.; Kalcioğlu, M.T.; Yesilada, E.; Tekin, M. The Effect of Hypericum perforatum L. (St. John’s Wort) on Prevention of Myringosclerosis after Myringotomy in a Rat Model. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, P.; Airen, S.; Brown, C.; Liu, Z.; Townsend, J.H.; Wang, J.; Jiang, H. Nutritional and Medical Food Therapies for Diabetic Retinopathy. Eye Vis. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Ruamviboonsuk, V.; Grzybowski, A. The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review. J. Clin. Med. 2022, 11, 6490. [Google Scholar] [CrossRef] [PubMed]
- Malechka, V.V.; Moiseyev, G.; Takahashi, Y.; Shin, Y.; Ma, J.X. Impaired Rhodopsin Generation in the Rat Model of Diabetic Retinopathy. Am. J. Pathol. 2017, 187, 2222. [Google Scholar] [CrossRef] [PubMed]
- Malechka, V.V.; Chen, J.; Cheng, R.; Ma, J.X.; Moiseyev, G. The Single Administration of a Chromophore Alleviates Neural Defects in Diabetic Retinopathy. Am. J. Pathol. 2020, 190, 1505. [Google Scholar] [CrossRef] [PubMed]
- Zhang MDc, C.; Li, K.; Zhang, J.B.; Kuang, X.B.; Liu, C.B.; Deng, Q.B.; Li, D. Relationship between Retinol and Risk of Diabetic Retinopathy: A Case-Control Study. Asia Pac. J. Clin. Nutr. 2019, 28, 607–613. [Google Scholar] [CrossRef]
- Reddy, S.S.; Prabhakar, Y.K.; Kumar, C.U.; Reddy, P.Y.; Reddy, G.B. Effect of Vitamin B12 Supplementation on Retinal Lesions in Diabetic Rats. Mol. Vis. 2020, 26, 311. [Google Scholar]
- Satyanarayana, A.; Balakrishna, N.; Pitla, S.; Reddy, P.Y.; Mudili, S.; Lopamudra, P.; Suryanarayana, P.; Viswanath, K.; Ayyagari, R.; Reddy, G.B. Status of B-Vitamins and Homocysteine in Diabetic Retinopathy: Association with Vitamin-B12 Deficiency and Hyperhomocysteinemia. PLoS ONE 2011, 6, e26747. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Babaei-Jadidi, R.; Al Ali, H.; Rabbani, N.; Antonysunil, A.; Larkin, J.; Ahmed, A.; Rayman, G.; Bodmer, C.W. High Prevalence of Low Plasma Thiamine Concentration in Diabetes Linked to a Marker of Vascular Disease. Diabetologia 2007, 50, 2164. [Google Scholar] [CrossRef]
- Chan, A.C. Partners in Defense, Vitamin E and Vitamin C. Can. J. Physiol. Pharmacol. 1993, 71, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Dai, P.; Wang, H.; Wane, D. Effects of Vitamin C Supplementation on Essential Hypertension: A Systematic Review and Meta-Analysis. Medicine 2020, 99, e19274. [Google Scholar] [CrossRef] [PubMed]
- Gurreri, A.; Pazzaglia, A.; Schiavi, C. Role of Statins and Ascorbic Acid in the Natural History of Diabetic Retinopathy: A New, Affordable Therapy? Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, S23–S27. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Yuan, Y.; Zhu, Z.; Wu, Y.; Ha, J.; Han, X.; Wang, W.; He, M. Micronutrients and Diabetic Retinopathy: Evidence from the National Health and Nutrition Examination Survey and a Meta-Analysis. Am. J. Ophthalmol. 2022, 238, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Gverović Antunica, A.; Znaor, L.; Ivanković, M.; Puzović, V.; Marković, I.; Kaštelan, S. Vitamin D and Diabetic Retinopathy. Int. J. Mol. Sci. 2023, 24, 12014. [Google Scholar] [CrossRef]
- Adornetto, A.; Rombolà, L.; Morrone, L.A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Russo, R. Natural Products: Evidence for Neuroprotection to Be Exploited in Glaucoma. Nutrients 2020, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D3 Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef]
- Hassen, G.; Belete, G.; Carrera, K.G.; Iriowen, R.O.; Araya, H.; Alemu, T.; Solomon, N.; Bam, D.S.; Nicola, S.M.; Araya, M.E.; et al. Clinical Implications of Herbal Supplements in Conventional Medical Practice: A US Perspective. Cureus 2022, 14, e26893. [Google Scholar] [CrossRef]
- Ke, M.; Hu, X.Q.; Ouyang, J.; Dai, B.; Xu, Y. The Effect of Astragalin on the VEGF Production of Cultured MüLler Cells under High Glucose Conditions. Biomed. Mater. Eng. 2012, 22, 113–119. [Google Scholar] [CrossRef]
- Vitamins and Minerals|NCCIH. Available online: https://www.nccih.nih.gov/health/vitamins-and-minerals (accessed on 12 September 2023).
- National Center for Health Statistics (U.S.). Health, United States, 2020–2021: Annual Perspective; National Center for Health Statistics (U.S.): Hyattsville, MD, USA, 2020. [CrossRef]
- Nahin, R.L. Expenditures on Complementary Health Approaches: United States, 2012. Natl. Health Stat. Rep. 2012, 95, 1–11. [Google Scholar]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Jaber, D.; Ghannam, R.A.; Rashed, W.; Shehadeh, M.; Zyoud, S.H. Use of Complementary and Alternative Therapies by Patients with Eye Diseases: A Hospital-Based Cross-Sectional Study from Palestine. BMC Complement. Med. Ther. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Werneke, U.; Earl, J.; Seydel, C.; Horn, O.; Crichton, P.; Fannon, D. Potential Health Risks of Complementary Alternative Medicines in Cancer Patients. Br. J. Cancer 2004, 90, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Balarastaghi, S.; Delirrad, M.; Jafari, A.; Majidi, M.; Sadeghi, M.; Zare-Zardini, H.; Karimi, G.; Ghorani-Azam, A. Potential Benefits Versus Hazards of Herbal Therapy during Pregnancy; A Systematic Review of Available Literature. Phytother. Res. 2022, 36, 824–841. [Google Scholar] [CrossRef] [PubMed]
- Sadikan, M.Z.; Abdul Nasir, N.A. Diabetic Retinopathy: Emerging Concepts of Current and Potential Therapy. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Tang, J.; Kern, T.S. Abnormalities of Retinal Metabolism in Diabetes and Experimental GalactosemiaVII. Effect of Long-Term Administration of Antioxidants on the Development of Retinopathy. Diabetes 2001, 50, 1938–1942. [Google Scholar] [CrossRef]
- Ho, J.I.; Ng, E.Y.; Chiew, Y.; Koay, Y.Y.; Chuar, P.F.; Phang, S.C.W.; Ahmad, B.; Kadir, K.A. The Effects of Vitamin E on Non-Proliferative Diabetic Retinopathy in Type 2 Diabetes Mellitus: Are They Sustainable with 12 Months of Therapy. SAGE Open Med. 2022, 10, 20503121221095324. [Google Scholar] [CrossRef]
- Fernandez-Robredo, P.; González-Zamora, J.; Recalde, S.; Bilbao-Malavé, V.; Bezunartea, J.; Hernandez, M.; Garcia-Layana, A. Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants 2020, 9, 838. [Google Scholar] [CrossRef]
- Lam Ung, C.O.; Kbar, N.; Aslani, P.; Smith, L.; Gelissen, I.C.; Harnett, J.E. Pharmacy Education in Traditional and Complementary Medicines—A Systematic Review. Res. Soc. Adm. Pharm. 2023, 19, 1331–1353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rák, T.; Kovács-Valasek, A.; Pöstyéni, E.; Csutak, A.; Gábriel, R. Complementary Approaches to Retinal Health Focusing on Diabetic Retinopathy. Cells 2023, 12, 2699. https://doi.org/10.3390/cells12232699
Rák T, Kovács-Valasek A, Pöstyéni E, Csutak A, Gábriel R. Complementary Approaches to Retinal Health Focusing on Diabetic Retinopathy. Cells. 2023; 12(23):2699. https://doi.org/10.3390/cells12232699
Chicago/Turabian StyleRák, Tibor, Andrea Kovács-Valasek, Etelka Pöstyéni, Adrienne Csutak, and Róbert Gábriel. 2023. "Complementary Approaches to Retinal Health Focusing on Diabetic Retinopathy" Cells 12, no. 23: 2699. https://doi.org/10.3390/cells12232699
APA StyleRák, T., Kovács-Valasek, A., Pöstyéni, E., Csutak, A., & Gábriel, R. (2023). Complementary Approaches to Retinal Health Focusing on Diabetic Retinopathy. Cells, 12(23), 2699. https://doi.org/10.3390/cells12232699






