The Major Hypotheses of Alzheimer’s Disease: Related Nanotechnology-Based Approaches for Its Diagnosis and Treatment
Abstract
:1. Introduction
2. Amyloid Cascade Hypothesis
2.1. Description of the Amyloid Cascade Hypothesis and Its Implications for Alzheimer’s Disease
2.2. Discussion of Current Diagnostic and Therapeutic Approaches Related to the Amyloid Cascade Hypothesis
- One of the commonly applied techniques to monitor Aβ is positron emission tomography (PET), which uses specific radiotracers that bind to the peptide [20,21,22]. It allows us to visualize the brain and see the accumulation of Aβ. Examples of well-known radiotracers for PET imaging are Pittsburgh Compound B (PiB), florbetapir, flutemetamol, and florbetaben, which binds specifically to Aβ [23,24]. Aβ PET imaging is not only used for diagnostic purposes but can also help in clinical trials for evaluating the efficacy of potential treatments for AD. The radiotracers can help identify patients who are appropriate for clinical trials of drugs targeting Aβ, such as amyloid-lowering agents.
- Another diagnostic tool that helps to evaluate Aβ levels in the brain is cerebrospinal fluid (CSF) analysis [25,26]. CSF is obtained through a lumbar puncture, and Aβ levels can be measured using enzyme-linked immunosorbent assays (ELISAs). Low concentrations of Aβ in the CSF have been shown to be associated with an increased risk of AD. This test is invasive but can provide valuable diagnostic information.
- Magnetic resonance imaging (MRI) is a non-invasive imaging technique that uses a magnetic field and radio waves to produce detailed images of the brain [27,28,29]. While MRI cannot directly measure Aβ, it can be used to detect changes in brain structure and function that may be associated with AD, including the shrinkage of the hippocampus, which is an area of the brain important for memory. Additionally, changes in brain activity in response to different stimuli might be monitored using functional MRI (fMRI) [30]. Studies have suggested that Aβ accumulation in the brain can affect brain activity and connectivity, which may be detectable using MRI and fMRI.
- Researchers are currently investigating whether blood tests could be truly valid to detect Aβ levels in the brain [34,35]. One approach involves measuring concentrations of Aβ in blood plasma, while another approach involves measuring the amount of Aβ oligomers, which are thought to be particularly toxic to neurons. This method could potentially provide a less invasive and more affordable diagnostic tool than, for example, PET imaging.
- In the last few years, it has been investigated whether Aβ accumulation in the retina of the eye or tears could be used as a biomarker for AD [36,37,38,39]. It has been shown that retinal imaging performed via optical coherence tomography (OCT) and fluorescence lifetime imaging ophthalmoscopy (FLIO) can indeed be used to detect changes in the retina that may be associated with AD.
- One important diagnostic method is also gene testing. Certain genetic mutations are known to increase the risk of developing AD, including mutations in the genes that encode Aβ and the presenilin proteins [40,41]. Gene testing can be used to identify individuals who carry these mutations and may be at increased risk of developing AD [42].
- Another tool used during each diagnostic analysis is neuropsychological test which helps to evaluate general cognitive function in patients with AD [43]. This test may include assessments of memory, language, attention, and executive function. However, while it does not directly measure Aβ, it can provide important information about the severity and progression of the disease.
- Finally, because no single diagnostic method for AD is completely accurate, researchers are exploring the use of multimodal biomarker approaches that combine several diagnostic evaluations. For example, combining PET imaging of Aβ with CSF analysis and neuropsychological testing may provide a more precise examination of AD [46,47]. Additionally, artificial intelligence (AI) algorithms are being developed to analyze data from multiple diagnostic tools to improve their efficiency. For example, machine learning algorithms can be trained to analyze brain imaging data, such as PET or MRI, to detect patterns of Aβ accumulation that may be associated with the disease [48,49].
- Potential for reducing the accumulation of Aβ and improving brain cognitive function is also shown by nutritional interventions and a complementary daily diet with certain nutrients, such as omega-3 fatty acids, antioxidants, and B vitamins. Nutritional interventions can work through various mechanisms, such as reducing inflammation and oxidative stress in the brain [61,62].
- A less common, and one of the most invasive, approaches involves stem cell therapies. They intend to replace damaged or dysfunctional cells in the brain with healthy cells. Researchers are exploring the use of stem cell therapies to replace or repair brain cells affected by AD, including those involved in the production and clearance of Aβ peptides [63,64].
- Lifestyle modifications, such as regular exercise, a healthy diet, and cognitive stimulation, have been also shown to reduce the risk of AD and may help to prevent or slow down the accumulation of Aβ protein in the brain.
- Approaches which are not exactly specified for Aβ peptides but are very important in the field of treatment of AD are brain stimulation techniques, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). They are being explored as potential treatments for AD [65,66]. These techniques aim at improving cognitive function through activating different areas of the brain, including those involved in learning and memory.
- Similarly, specific drugs (cognitive enhancers) aim at improving cognitive function in AD patients through targeting neurotransmitter systems involved in learning and memory, such as acetylcholine and glutamate [67]. While not directly targeting Aβ, cognitive enhancers may help to mitigate the decline associated with the accumulation of peptides in the brain.
2.3. Overview of Nanotechnology-Based Approaches Related to the Amyloid Cascade Hypothesis
- Nanoparticle-based delivery of small molecule drugs [78,79]. Specific drugs that target different stages of the amyloid cascade have been developed, but their efficacy is limited by poor brain penetration and off-target effects. Nanoparticles can be produced to improve brain targeting by these drugs, bypass BBB, and minimize their side effects. Researchers have developed nanoparticles as nanocarriers that can specifically bind to Aβ plaques and deliver drugs to clear them. For example, liposomes can be used to encapsulate drugs, deliver them to specific regions of the brain, and break down Aβ [80].
- Nanoparticle-based imaging agents, i.e., nanoparticles which serve as contrast agents in imaging techniques such as MRI or computed tomography (CT) scans [83]. They can detect Aβ deposits in the brain, making them visible in MRI or CT scans [84]. This can enable the early detection and monitoring of AD and facilitate the development of further therapies. Additionally, nanoparticles help to visualize peptides in the brain using imaging probes such as quantum dots that can selectively bind to Aβ peptides and allow their detection and visualization in vivo [70].
- Nanoparticle-based gene therapy for promoting Aβ clearance [58,85,86]. Nanoparticles can be designed to encapsulate therapeutic genes and deliver them directly to the brain. Once in the brain, the nanoparticles release the genes, which can then be taken up by brain cells. They promote the expression of proteins that are involved in the clearance of Aβ peptides.
- Nanosensors for early detection and monitoring. Another nanotechnology-based approach involves sensors for the early detection and monitoring of Aβ peptides in the brain and body fluids [87,88,89,90]. Nanosensors allow for the detection of Aβ at very small concentrations and for the recognition of specific proteins or various biomarkers that are associated with the formation of Aβ plaques. One approach to develop nanosensors for AD involves the use of nanomaterials such as graphene oxide, carbon nanotubes, or gold nanoparticles. These materials can be functionalized with specific antibodies or aptamers that can selectively bind to Aβ, allowing for its detection and quantification in biological samples. Another approach involves the use of nanoscale transistors and other electronic devices that can detect changes in electrical conductivity or other physical properties in response to binding to Aβ. These devices can be integrated into microfluidic systems. Nanotechnology-based sensors also offer the potential for real-time monitoring, for example, through using nanoscale probes that can detect changes in fluorescence or other optical properties in response to Aβ peptide aggregation.
- Nanoscale ultrasound. Nanoscale ultrasound is an example of an efficient method for breaking up Aβ aggregates [91,92]. The ultrasound-mediated elimination of Aβ plaques uses low-frequency ultrasound to induce vibrations in the brain tissue, disrupting the structure of Aβ aggregates and facilitating their clearance by the body’s immune system.
- DNA origami. Another option is DNA origami for creating nanoscale scaffolds and Aβ clearance [93,94,95]. DNA origami can be designed to produce nanoscale structures that can mimic the natural clearance mechanisms in the brain, such as the action of enzymes that break down Aβ peptides. Through attaching enzymes or other clearance-promoting molecules to the DNA origami scaffold, it may be possible to enhance the clearance of Aβ peptides from the brain.
- Stem cell therapy. Stem cell therapy apply nanotechnology to enhance the survival and integration of transplanted stem cells in the brain [96]. It involves the delivery and targeting of stem cells to specific areas of the brain affected by AD. For example, stem cells can be combined with nanoscale technologies, loaded on or encapsulated within nanoparticles, which can then be engineered to target specific cell types or regions of the brain. One potential approach is to use stem cells to produce and release factors that can promote the clearance of Aβ peptides from the brain. For instance, stem cells can be genetically engineered to produce enzymes that can break down Aβ peptides or cytokines that can stimulate immune cells to clear Aβ peptides from the brain. Another approach is to use stem cells to regenerate damaged or lost neurons in the brain. Stem cells can differentiate into various types of brain cells, including neurons, and can potentially replace damaged or lost neurons in the brain.
- Combinational therapy. Additionally, nanotechnology-based approaches are being investigated in combination with other therapies, such as gene therapy and stem cell therapy, to enhance their effectiveness. For example, nanoparticles can be used to deliver gene therapy to the brain to promote the production of factors that can clear Aβ or promote neural regeneration.
2.4. Summary of Key Findings on Nanotechnology-Based Approaches and Future Directions for Research
- Nanoparticles have been developed as contrast agents for imaging Aβ plaques in vivo, allowing for the earlier detection of AD.
- Nanosensors capable of detecting Aβ peptides in biological fluids and brain tissue allow for the real-time monitoring of their aggregation.
- Nanocarriers such as liposomes, dendrimers, and solid lipid nanoparticles have been investigated as delivery systems for Aβ-targeting drugs, including antibodies and small molecules.
- Nanotechnology-based approaches have been used to engineer molecules that can inhibit the aggregation of Aβ peptides, potentially slowing or preventing the formation of Aβ plaques.
- Metal nanoparticles have been explored as a potential therapeutic strategy to promote the clearance of Aβ plaques from the brain.
- The use of nanotechnology-based approaches for combination therapies targeting both Aβ and tau protein pathology in AD is an active area of research.
- Graphene oxide nanoparticles can be used to inhibit Aβ aggregation and reduce Aβ-induced cytotoxicity.
- It has been demonstrated that multifunctional nanocarriers play a potential role in simultaneously targeting Aβ plaques, inhibiting Aβ aggregation, and delivering anti-inflammatory drugs.
3. Tau Protein Hyperphosphorylation
3.1. Description of the Tau Protein Hyperphosphorylation Hypothesis and Its Implications for Alzheimer’s Disease
3.2. Discussion of Current Diagnostic and Therapeutic Approaches Related to the Tau Protein Hyperphosphorylation Hypothesis
- In some of studies, it has been found that a combination of tau and Aβ biomarkers can improve the accuracy of AD diagnosis, even in its early stages [102].
- Furthermore, advances in PET imaging using radiotracers that bind to tau protein have allowed for the visualization of its aggregates in vivo, providing valuable insights into disease progression and enabling earlier diagnosis [103,104,105]. A recent study also demonstrated the utility of tau–PET imaging in tracking the spread of tau pathology in the brain and predicting cognitive decline in AD patients [106,107].
- CSF biomarkers, such as tau and phosphorylated tau, have also been used as promising diagnostic tools [108,109]. CSF tau levels have shown to correlate with the degree of the pathology in the brain and can help differentiate tauopathies from non-tauopathies [110,111]. However, despite the high sensitivity of these measurements, CSF collection is invasive and not well-tolerated by some patients, and the interpretation of the protein concentrations is affected by various factors such as age, sex, and comorbidities.
- On the other hand, blood-based biomarkers for tau pathology have been explored as a less invasive and more convenient diagnostic approach [112,113]. Recent studies have shown that plasma tau levels are elevated in patients with AD and can help distinguish between AD and non-AD dementia [114,115,116]. For instance, researchers reported that higher levels of plasma tau were associated with greater cognitive decline and increased risk of mild cognitive impairment or dementia [115]. Additionally, it was found that plasma tau biomarkers had high accuracy in predicting Aβ positivity and discriminating individuals with AD from those without AD [116]. These findings suggest that plasma tau levels may be a useful biomarker for the early diagnosis and differentiation of AD. However, the accuracy and reliability of blood-based tau biomarkers are still being evaluated, and further validation is needed before their widespread use in clinical practice.
- In addition to these diagnostic approaches, efforts are also underway to develop novel biomarkers and imaging techniques to improve the early diagnosis and monitoring of tau hyperphosphorylation. For example, researchers are exploring the use of retinal imaging and other non-invasive biomarkers to detect early signs of tau pathology in AD [117,118].
- Another commonly used approach is the application of tau aggregation inhibitors. They are small molecules that target tau accumulation and prevent the formation of toxic aggregates. Few compounds have been identified that can inhibit tau aggregation, and preclinical studies have shown promising results [123,124]. Clinical trials of tau-targeted therapies are currently ongoing, with several promising candidates in the pipeline [125,126,127,128].
- Another potential solution also involves neuroprotective agents, or compounds that intend to protect neurons from tau-mediated toxicity. Several analytes have been identified that can protect against tau toxicity, including antioxidants, anti-inflammatory agents, and compounds that enhance autophagy [129].
- A promising technique, still in development, is gene therapy. The technique focuses on modifying the expression of genes involved in the tau pathway to reduce the formation of pathological tau protein. Several gene therapies are in preclinical development, targeting genes such as tau, glycogen synthase kinase 3 beta (GSK-3β), and microRNAs [101].
- Researchers also provide evidence for the high molecular diversity of tau contributing to the clinical heterogeneity of AD, which highlights the importance of personalized treatment approaches [130].
3.3. Overview of Nanotechnology-Based Approaches Related to the Tau Protein Hyperphosphorylation Hypothesis
- The nanoparticle-based delivery of tau-targeting therapeutics [78,132], or nanoparticles that can be specially designed to encapsulate and transport drugs that target tau pathology. For example, nanoparticles have been developed to deliver small molecule inhibitors of tau aggregation, such as methylene blue and curcumin, to the brain [133,134,135,136]. This approach could provide more targeted and effective delivery, minimizing off-target effects and increasing drug efficacy [137,138,139,140,141].
- Immunotherapy is another method that has shown promise in targeting tau pathology. Nanoparticles can be engineered to deliver antibodies that specifically target tau protein aggregates and stimulate an immune response against them. This could potentially provide a more targeted and effective way to deliver antibodies to the brain. Additionally, researchers highlight the advantages of immunotherapy over traditional drug therapies, including a better ability to target specific protein aggregates and elicit a sustained immune response, providing longer-term therapeutic effects [142,143].
- Nanoparticle-based imaging agents, or nanoparticles that can be used to detect and visualize tau pathology in the brain. Researchers are exploring the use of various types of nanoparticles, including quantum dots, gold, and iron oxide nanoparticles, for this purpose. [144,145,146] They can be designed to specifically bind to tau protein aggregates, allowing for their detection using imaging techniques such as MRI and PET scans. This approach could potentially aid in the early detection and diagnosis of AD, allowing for earlier intervention and treatment [147,148,149].
- Nanotechnology-based biosensors are another promising approach for the detection and monitoring of tau protein in biological fluids such as CSF or blood [150,151,152]. These biosensors can be designed to detect specific tau protein isoforms, including phosphorylated tau, which are known to be associated with AD. There are two main types of nanotechnology-based biosensors that are being investigated for tau detection: optical biosensors and electrochemical biosensors. Optical biosensors rely on the detection of light signals to measure changes in the target molecule, while electrochemical biosensors detect changes in electrical current or potential. Optical biosensors typically use fluorescent or luminescent nanoparticles, such as quantum dots or gold nanoparticles, as the detection platform. These nanoparticles are functionalized with tau-specific antibodies or aptamers, which bind to the target tau protein in biological fluids. When they bind, it causes a change in the fluorescence or luminescence of the nanoparticle, which can be measured and quantified.
- Gene-based therapies. Additionally, several gene-based therapies have been proposed for the treatment of tau pathology in AD, including gene therapy, RNA interference (RNAi), and CRISPR-Cas gene editing [153,154,155]. These therapies aim at targeting the underlying genetic mechanisms involved in the abnormal accumulation and hyperphosphorylation of tau protein. Gene therapy involves the delivery of specific genes that can regulate the expression of tau and other proteins involved in AD pathology. RNAi is another approach that uses small RNA molecules to selectively silence the expression of specific genes, including tau. CRISPR-Cas gene editing is a more recent development that involves manipulating the genome to correct genetic mutations or remove disease-causing genes.
3.4. Summary of Key Findings on Nanotechnology-Based Approaches and Future Directions for Research
- The potential of using nanoparticles to deliver drugs and other therapeutic agents directly to the brain. Through encapsulating drugs for tau pathology, they can be protected from degradation and cleared more slowly from the body, allowing for sustained release and longer-lasting effects.
- Nanoparticles can also be used as imaging agents to detect and monitor the accumulation of tau protein in the brain.
- Nanoparticles can be designed to bind specifically to tau protein aggregates and other biomarkers of AD, allowing for the earlier detection and monitoring of disease progression.
- Research has also demonstrated the feasibility of using nanotechnology-based techniques for gene therapy and other emerging technologies, such as CRISPR-Cas gene editing, to target the underlying genetic causes of AD. Through delivering genes or RNA molecules that regulate the expression of specific proteins, including tau, or using CRISPR-Cas gene editing to modify or delete disease-causing genes, these approaches could potentially provide a more targeted and personalized treatment for AD.
- Nanoparticles can also be designed to address the heterogeneity and complexity of AD pathology through targeting multiple molecular mechanisms and pathways simultaneously. For example, nanoparticles can be designed to simultaneously target tau protein aggregates and Aβ peptides.
- In addition, the possibility of using nanotechnology-based methods to deliver multiple therapeutic agents simultaneously has also been shown. Through encapsulating different drugs or therapeutic agents in the same nanoparticle, they can be delivered simultaneously to the brain and work synergistically to slow disease progression.
4. Mitochondrial Cascade Hypothesis
4.1. Description of the Mitochondrial Cascade Hypothesis and Its Implications for Alzheimer’s Disease
4.2. Discussion of Current Diagnostic and Therapeutic Approaches Related to the Mitochondrial Cascade Hypothesis
- One of the most promising biomarkers is oxidative stress, which is a result of the imbalance between the production of ROS and the cell’s ability to detoxify them [162]. Increased oxidative stress has been observed in both human AD brains and animal models of the disease, supporting the role of mitochondrial dysfunction in AD pathology. Biomarkers of oxidative stress that have been proposed include lipid peroxidation and protein carbonylation [163,164].
- Another potential indication of mitochondrial dysfunction is mtDNA damage. MtDNA is a small circular DNA molecule that is present in mitochondria and encodes several genes that are critical for mitochondrial function. Because mitochondrial dysfunction is a key feature of AD, it is thought that mtDNA mutations and damage may contribute to the development and progression of the disease. Some studies have reported that mtDNA damage is higher in AD patients, and that mutations may be associated with an increased risk of developing the disease. However, further research is needed to fully understand the potential of mtDNA as a diagnostic biomarker for AD [165].
- An approach also being investigated is the use of exosomes as biomarkers for AD [166,167]. Exosomes are small vesicles that are released by cells and contain a variety of proteins, lipids, and nucleic acids. Because exosomes can be isolated from biological fluids such as blood and CSF, they may provide a less invasive and more accessible diagnostic tool for AD. Some studies have reported that exosomes derived from AD patients contain markers of mitochondrial dysfunction, such as increased levels of oxidative stress and altered expression of mitochondrial proteins [168].
- In addition, imaging techniques including PET and MRI can provide important information on mitochondrial activity, such as the rate of glucose metabolism, one of the indicators of mitochondrial function [163].
- Mitochondrial-targeted therapies aim at improving mitochondrial function and reducing accumulation of ROS through targeting specific mitochondrial components, such as the electron transport chain or mPTP. Several compounds that specifically target mitochondria have been investigated as potential treatments for AD [169,170]. These include mitochondria-targeted antioxidants, such as MitoQ, coenzyme Q10, alpha-lipoic acid, and vitamin E, and mitochondria-targeted SS peptides [171,172].
- The application of compounds that stimulate the production of new mitochondria, such as resveratrol, metformin and nicotinamide riboside, helps to compensate for the dysfunction of existing mitochondria. These activators may help improve mitochondrial function, increasing mitochondrial biogenesis or enhancing the production of ATP, and reduce the accumulation of damaged mitochondria [173].
- Mitochondrial fission/fusion modulators. The balance between mitochondrial fission and fusion is critical for maintaining mitochondrial health. This process can affect mitochondrial morphology and function [174]. Compounds that modulate these processes include mitochondrial division inhibitor 1 (Mdivi-1), which inhibits mitochondrial fission, and mitofusins (Mfn1 and Mfn2), which promote mitochondrial fusion [175,176].
- Anti-inflammatory therapies aim to reduce inflammation and oxidative stress in the brain through targeting immune cells and inflammatory pathways [177].
- Exercise and lifestyle interventions that promote regular exercise and healthy lifestyle habits such as a balanced diet, good sleep, and stress reduction have been shown to improve mitochondrial function and reduce the risk of AD [178].
- Another therapeutic approach related to the mitochondrial cascade hypothesis for AD is the use of mitophagy modulators. Mitophagy is a process through which damaged mitochondria are removed and recycled, promoting mitochondrial quality control. Dysregulation of mitophagy has been implicated in the pathogenesis of AD, and compounds that modulate this process may have therapeutic potential. For example, rapamycin and trehalose have been shown to induce mitophagy and reduce the accumulation of damaged mitochondria in AD models [179].
- A possible therapeutic approach might also be the use of stem cell therapies to replace damaged or dysfunctional neurons and restore mitochondrial function. Recent studies have shown promising results using induced pluripotent stem cells (iPSCs) to generate new neurons and improve cognitive function in AD models [180].
4.3. Overview of Nanotechnology-Based Approaches Related to the Mitochondrial Cascade Hypothesis
- Mitochondria-targeted antioxidants. This approach involves the use of mitochondria-targeted antioxidants, such as coenzyme Q10, encapsulated in nanoparticles for targeted delivery to the mitochondria. These nanoparticles can be designed to specifically target damaged mitochondria in the brain, reducing oxidative stress and promoting mitochondrial function [181].
- Conjugated nanoparticles. Nanoparticles can be designed to overcome the BBB through exploiting various mechanisms, such as receptor-mediated transcytosis or adsorptive-mediated transcytosis, which allow nanoparticles to cross the BBB and reach the brain [182]. One example of this approach is the use of liposomes to deliver mitochondria-targeted compounds to the brain [183]. Liposomes can be functionalized with various targeting ligands, such as transferrin or apolipoprotein E, which can enhance their uptake by brain cells and improve their efficacy in treating mitochondrial dysfunction in AD. Another example is the use of gold nanoparticles, which can be coated with a layer of polyethylene glycol (PEG) to improve their biocompatibility and stability in the body. Gold nanoparticles can be conjugated with various mitochondrial-targeting molecules, such as tri-phenyl-phosphonium (TPP) or ubiquinone, to enhance their uptake by mitochondria and improve their therapeutic efficacy.
- Gene therapy. Another approach is the use of nanoparticles to deliver small interfering RNA (siRNA) or antisense oligonucleotides (ASOs) to target genes involved in mitochondrial dysfunction [184]. For example, siRNA targeting amyloid precursor protein (APP) has been delivered using nanoparticles to reduce APP expression and improve mitochondrial function in AD models [185].
- Nanoparticles in drug delivery. Nanoparticles have also been used to deliver drugs targeting the mPTP, a key regulator of mitochondrial function and cell death. For example, a mitochondria-targeted derivative of cyclosporin A (CsA), a known inhibitor of the mPTP, has been encapsulated in nanoparticles and shown to improve mitochondrial function and reduce cell death [186].
- Targeting mitochondrial membrane potential. Nanostructures can be designed to target the mitochondrial membrane potential, which is a key regulator of mitochondrial function and has been found to be altered in AD. For example, cationic nanoparticles have been used to deliver peptides that target the mitochondrial membrane potential, leading to improved mitochondrial function and reduced oxidative stress [187].
- Imaging and diagnosis. In addition to drug delivery, nanotechnology-based approaches can also be used for imaging and diagnosis of mitochondrial dysfunction in AD. For example, quantum dots have been used to track mitochondrial dynamics and function. Another example is magnetic nanoparticles for MRI of mitochondrial function in the brain. Through targeting these nanoparticles specifically to the mitochondria, MRI can provide a non-invasive method for detecting and monitoring mitochondrial dysfunction in AD [188].
- Nanosensors. Another approach is the use of nanosensors to detect and monitor mitochondrial dysfunction in AD. Nanosensors are highly sensitive and selective devices that can detect small changes in molecular signals, such as ROS or mitochondrial membrane potential, two important indicators of mitochondrial dysfunction. Nanosensors can be designed to target specific subcellular compartments, such as mitochondria, and provide real-time monitoring of its activity.
4.4. Summary of Key Findings on Nanotechnology-Based Approaches and Future Directions for Research
- Nanoparticle-mediated delivery of therapeutic agents. Nanoparticles have been used to deliver therapeutic agents to the mitochondria, allowing for the targeted treatment of mitochondrial dysfunction.
- Mitochondria-targeting nanoparticles. Nanoparticles can be designed to specifically target the mitochondria, allowing for the precise delivery of therapeutic agents to these organelles. These nanoparticles can be engineered to selectively accumulate in the mitochondria due to their unique properties, such as size, surface charge, and surface chemistry.
- Nanosensors for monitoring mitochondrial function. Nanosensors can be used to monitor mitochondrial function in real time. For example, fluorescent nanosensors have been developed to monitor mitochondrial pH changes in response to oxidative stress.
- Nanoparticles for imaging mitochondria. Nanoparticles can also be used for imaging mitochondria in living cells. For example, quantum dot nanoparticles have been used to visualize the morphology and dynamics of mitochondria in real time.
5. Cholinergic Hypothesis
5.1. Description of the Cholinergic Hypothesis and Its Implications for Alzheimer’s Disease
5.2. Discussion of Current Diagnostic and Therapeutic Approaches Related to the Cholinergic Hypothesis
- For instance, neuropsychological testing is often used to diagnose AD and examine cognitive impairment. Tests that specifically assess cholinergic function, such as the evaluation of attention and memory, may be helpful to support the hypothesis that AD is related to a deficiency in cholinergic neurotransmission [190].
- Another useful diagnostic tool is PET imaging. It measures the levels of acetylcholine in the brain [191]. One commonly applied PET tracer for this purpose is [11C]methyl-4-piperidinyl propionate ([11C]PMP), which binds to the enzyme responsible for acetylcholine synthesis. PET imaging is also commonly used to estimate the density of cholinergic receptors in the brain.
- Additionally, the concentration of acetylcholine in the brain can be analyzed based on the signal from CSF biomarkers [192]. For example, the CSF levels of acetylcholinesterase are decreased in patients with AD.
- Apart from CSF, blood biomarkers related to cholinergic dysfunction, such as acetyltransferase and acetylcholinesterase, have been identified. To control their different amounts is crucial for diagnosing and monitoring of AD.
- Neuroimaging techniques, such as MRI, can be used to detect brain atrophy in AD. Brain atrophy in certain regions, such as the hippocampus, is believed to be associated with cholinergic dysfunction and cognitive decline [193].
- Functional MRI is another neuroimaging technique that measures changes in the brain blood flow in response to different tasks or stimuli. fMRI can be used to analyze brain activity related to cholinergic processes such as cognitive functions [194].
- Electroencephalography (EEG) and magnetoencephalography (MEG) are non-invasive techniques used to measure the electrical activity in the brain [195]. These techniques can be used to assess brain function related to cholinergic activity, such as attention and memory processing.
- Another non-invasive technique is transcranial magnetic stimulation (TMS). This technique uses magnetic fields to stimulate specific areas of the brain [196]. TMS can be used to assess the brain to investigate its cholinergic activity and has also been shown to improve cognitive processes in AD patients.
- Additionally, genetic testing has recently been used to identify mutations in genes related to AD, such as the presenilin-1 and presenilin-2 genes [197]. These genes play a role in the production of Aβ peptides, which accumulate in the brains of Alzheimer’s patients and are thought to contribute to cholinergic dysfunction.
- Eye tracking can be used to assess visual attention [198]. Studies have shown that patients with AD have impaired eye movements during visual tasks, which may be related to cholinergic dysfunction.
- Different from the other methods, post-mortem examination of the brain can provide definitive confirmation of AD and its association with cholinergic dysfunction [199]. Histological examination of brain tissue can reveal the presence of Aβ plaques and neurofibrillary tangles, as well as changes in cholinergic markers, such as choline acetyltransferase and acetylcholinesterase.
- The common method currently applied involves cholinesterase inhibitors [200]. These drugs work through preventing the breakdown of acetylcholine, thereby increasing its availability in the brain. Some examples of cholinesterase inhibitors frequently used for the treatment of AD include donepezil, galantamine, and rivastigmine.
- Another approach proposes nicotinic receptor agonists [201]. Nicotine is a natural agonist of nicotinic acetylcholine receptors. Drugs that activate these receptors are being investigated as potential treatments for AD. For example, the drug varenicline, which is used to aid smoking cessation, is being studied for its potential cognitive-enhancing effects in the disease.
- One therapy for AD also considers using different ACh precursors such as choline [202]. They are compounds that the body can use to make acetylcholine and can be found in foods such as eggs and liver.
- Some researchers are investigating the use of stem cells to restore cholinergic function in the brain [203]. For example, neural stem cells can be engineered to produce acetylcholine and are then transplanted into the brain to restore cholinergic function.
- Another potential therapeutic strategy applies dual-acting compounds. These are drugs that combine cholinesterase inhibition with other mechanisms of action that may also be beneficial in AD [204]. For example, the drug memantine is an N-methyl-D-aspartate (NMDA) receptor antagonist that is often used in combination with cholinesterase inhibitors for the treatment of moderate to severe AD.
- In addition to drugs, there are also non-pharmacological interventions that may be beneficial in AD. For example, cognitive stimulation programs that involve activities such as puzzles, games, and reminiscence therapy have been shown to improve cognitive function in people with the disease. It is thought that these types of interventions may enhance cholinergic function through promoting the release of acetylcholine in the brain.
- Researchers are also investigating the use of gene therapies to restore cholinergic function in the brain. For example, gene therapy approaches that involve the delivery of genes encoding cholinergic enzymes or receptors to the brain are being studied as potential treatments for AD.
5.3. Overview of Nanotechnology-Based Approaches Related to the Cholinergic Hypothesis
- Targeted drug delivery entails the use of nanoparticles to deliver drugs directly to the brain [205]. For example, nanoparticles can be engineered to encapsulate drugs that enhance cholinergic neurotransmission, such as acetylcholinesterase inhibitors [206]. This can be achieved through engineering the surface of nanoparticles with ligands that can recognize and bind to cholinergic receptors. Once bound, nanoparticles can release drugs or other therapeutic agents that can modulate cholinergic neurotransmission [207]. These nanoparticles can be designed to target specific regions of the brain, such as the hippocampus, where cholinergic neurons are particularly affected in AD.
- Nanosensors. Another nanotechnology-based approach is to develop nanosensors that can detect cholinergic neurotransmission in real time [208]. For example, researchers are exploring the use of nanowire sensors that can measure the levels of acetylcholine in the brain. These sensors can be implanted providing continuous monitoring of cholinergic neurotransmission, which can help researchers better understand the dynamics of this neurotransmitter system in health and disease [209].
- Nanowire electrodes. The development of nanoscale devices that can selectively stimulate cholinergic neurons in vivo also has an important impact. For example, researchers have designed brain-implantable nanoelectronic devices (nanowire electrodes) that can simulate the activity of cholinergic neurons using light or electrical signals. These devices can be controlled externally and provide a targeted approach to enhance cholinergic neurotransmission in the brain. The approach has been shown to improve cognitive function in animal models of AD [210,211].
- Scaffolds. Nanotechnology also involves developing scaffolds for the tissue engineering of cholinergic neurons [212]. Tissue engineering is based on creating three-dimensional structures that can support the growth and function of cells or tissues. Researchers are developing nanoscale scaffolds that can mimic the structure and function of the extracellular matrix in the brain and promote the differentiation and growth of cholinergic neurons. These scaffolds can be used to replace or repair damaged or lost cholinergic neurons in AD.
- Biomarkers. The development of nanotechnology-based diagnostic tools that can detect biomarkers associated with cholinergic dysfunction has also been reported. For example, researchers have developed gold nanoparticles that can detect levels of acetylcholinesterase in blood samples, which may be a biomarker for AD. These diagnostic tools can be used to detect early signs of cholinergic dysfunction and monitor the progression of the disease.
- Non-invasive stimulation. Finally, this nano-based strategy is used to develop novel therapies that can modulate cholinergic neurotransmission in a targeted and controlled manner. For example, researchers are exploring the use of nanoscale magnetic particles that can selectively activate cholinergic neurons in the brain using magnetic fields [213]. This approach can provide a non-invasive and targeted way to enhance cholinergic neurotransmission in AD.
5.4. Summary of Key Findings on Nanotechnology-Based Approaches and Future Directions for Research
- Nanoparticles can be designed to specifically target cholinergic neurons and to deliver cholinergic agents directly to the brain, enhancing their effectiveness and minimizing side effects.
- Nanoparticles can be used to deliver gene therapy for cholinergic neurons, which can promote their growth and survival or replace damaged neurons.
- Nanobased approaches show promise in improving cognitive function in animal models of AD through increasing acetylcholine levels in the brain.
- Various types of nanostructures, including liposomes, dendrimers, and nanogels, have been explored for their potential in cholinergic-based therapies for AD.
- Nano-based approaches can be used to overcome the limitations of traditional drug delivery methods, such as poor solubility, low bioavailability, and short half-life.
- Research is ongoing to explore more the potential of non-pharmacological interventions, such as transcranial magnetic stimulation and cognitive training, to enhance cholinergic function in AD.
- Other nano-based approaches include the use of nanosensors for detecting biomarkers of AD and the development of nanocarriers for gene therapy.
6. Neuroinflammation Hypothesis
6.1. Description of the Neuroinflammation Hypothesis and Its Implications for Alzheimer’s Disease
6.2. Discussion of Current Diagnostic and Therapeutic Approaches Related to the Neuroinflammatory Hypothesis
- One commonly used diagnostic approach is PET imaging, which uses radioactive tracers to detect the presence of neuroinflammation markers in the brain and to track the progression of neuroinflammation over time [216]. PET imaging can detect changes in the levels of various biomarkers such as translocator protein (TSPO) and glial fibrillary acidic protein (GFAP), which are indicators of the condition. It can also provide valuable information about the dynamics of neuroinflammation and how it is related to disease progression [217,218].
- Recent advances in molecular biology and genomics are providing new opportunities for identifying novel biomarkers of neuroinflammation. For example, researchers are exploring the use of epigenetic markers, microRNAs, and other non-coding RNAs as potential indicators of neuroinflammation [219,220]. They may be more sensitive and specific than traditional biomarkers and give new insights into the mechanisms of the disease.
- In addition, MRI can be used to detect changes in the brain’s structure and function that are associated with neuroinflammation. For example, diffusion tensor imaging (DTI) can detect the changes in white matter integrity that are associated with the condition, and fMRI can be used to detect differences in brain activity during the process [222,223].
- Another approach for detecting neuroinflammation is the use of blood biomarkers, the substances that are released into the bloodstream in response to inflammation in the brain. They can be measured using a blood test, and their levels can indicate the presence and severity of the condition. Examples of blood biomarkers that are associated with neuroinflammation include C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) [224,225].
- Furthermore, advances in AI and machine learning techniques are allowing researchers to analyze large datasets of imaging and biomarker data to identify patterns and associations that may be missed by traditional methods [226,227]. This approach, known as radiomics, has the potential to improve the accuracy of neuroinflammation diagnosis, prediction of disease progression, and treatment response.
- Steroids such as prednisone are also important in the process [232]. However, the long-term use of steroids can also have side effects, such as weight gain and increased risk of infections.
- One drug-based therapeutic strategy involves modulating microglial activation or function [233]. Some drugs such as minocycline, which have shown to reduce microglial activation, are being studied for their potential therapeutic effects in neuroinflammatory diseases.
- A healthy diet, rich in anti-inflammatory supplements, and lifestyle can help reduce inflammation in the body and the brain [240]. Applying a diet rich in fruits, vegetables, and omega-3 fatty acids and engaging in regular exercise can help to decrease risk of the condition.
- Mind–body therapies such as meditation and yoga have been shown to reduce inflammation in the body and may have similar effects in the brain [241]. These therapies can also help reduce stress, which can contribute to inflammation.
- Precision medicine involves tailoring treatment to an individual’s specific genetic, environmental, and lifestyle factors [242,243]. With advances in technology such as genomic sequencing, precision medicine may help identify the underlying causes of neuroinflammation and guide personalized treatment approaches.
- Given the complex nature of neuroinflammation and its potential involvement in various neurological and psychiatric disorders, combination therapies may be more effective than single therapies alone. For example, a combination of anti-inflammatory drugs, immunomodulatory drugs, and lifestyle changes may be more effective in reducing inflammation and improving symptoms than any single therapy alone.
6.3. Overview of Nanotechnology-Based Approaches Related to the Neuroinflammatory Hypothesis
- Nanosensors. One potential application of nanotechnology in this field is the development of nanosensors that can detect biomarkers of inflammation in the brain [244]. These sensors can be designed to detect specific molecules, such as cytokines, that are indicative of the condition. This technology could allow for the earlier identification of neuroinflammatory processes and more accurate monitoring of disease progression.
- Nano-based targeted drug delivery. Nanoparticles have also been investigated as potential drug delivery vehicles for the treatment of neuroinflammatory conditions. They can be designed to target specific cells in the brain, such as microglia, which are key players in the inflammatory response [245]. Through delivering anti-inflammatory drugs directly to these cells, it may be possible to reduce inflammation in the brain while minimizing side effects.
- Gene therapy. In addition to drug delivery, nanoparticles can also be used to deliver genetic material to cells in the brain [246]. This approach, known as gene therapy, has the potential to target specific genes involved in the inflammatory response and modulate their expression. This technology is still in the early stages of development, but it has the potential to revolutionize the treatment of neuroinflammatory conditions.
- Nanoparticle-based imaging agents. Another area where nanotechnology-based approaches have shown promise in the context of neuroinflammation is in the development of imaging agents [247]. Specifically, nanoparticles can be designed to carry imaging agents, such as fluorescent dyes or contrast agents for MRI, to the site of inflammation in the brain. This technology can be used to visualize the extent and location of neuroinflammation, which is crucial for accurate diagnosis and monitoring disease progression.
- Nanoparticles can also be designed to penetrate the BBB [248]. This is particularly important for the treatment of neuroinflammatory conditions, as many anti-inflammatory drugs are unable to penetrate the BBB [249,250]. Through designing nanoparticles that can cross the BBB, it may be possible to deliver drugs directly to the brain and target inflammation more effectively.
- Nanotechnology-based approaches can also be used to develop implantable devices that can monitor and modulate neuroinflammation in real-time [251]. For example, nanoparticles can be incorporated into implantable devices to detect changes in cytokine levels, which can then trigger the release of anti-inflammatory drugs. These devices have the potential to provide continuous, personalized treatment for neuroinflammatory conditions.
- Another area where nanotechnology-based approaches can be used in the context of neuroinflammation is in the development of vaccines [252]. Specifically, nanoparticles can be designed to carry antigens and adjuvants to stimulate an immune response against inflammatory factors.
- Furthermore, nanotechnology-based approaches can be used to develop strategies to reduce inflammation and promote tissue repair [253,254,255]. For instance, nanoparticles can be designed to mimic extracellular matrix components, such as laminin, which can support nerve cell growth and regeneration. Additionally, nanoparticles can be functionalized with anti-inflammatory agents, growth factors, or other bioactive molecules to promote tissue repair and regeneration in the brain.
- Finally, nanotechnology-based approaches can also be used to improve the understanding of the mechanisms underlying neuroinflammation. For example, nanoparticles can be used to label and track immune cells, such as microglia, in the brain. This technology can provide insights into how these cells interact with others in the brain and how the interaction contributes to neuroinflammation and disease progression.
6.4. Summary of Key Findings on Nanotechnology-Based Approaches and Future Directions for Research
- Nanoparticles can be used to enhance the delivery of imaging agents to the brain, potentially improving the ability to detect and monitor neuroinflammatory changes in AD.
- Nano-based approaches can be used to develop gene therapies that target specific genes involved in the neuroinflammatory response in AD.
- Nanoparticles can be designed to specifically target activated microglia and astrocytes in the brain, which are key components of the neuroinflammatory response in AD. The targeted delivery of anti-inflammatory agents to these cells could potentially reduce neuroinflammation and improve cognitive function.
- Gold nanoparticles have been shown to have anti-inflammatory properties and could potentially be used as therapeutic agents to reduce neuroinflammation in AD.
- Iron oxide nanoparticles can be used as contrast agents in MRI imaging to detect neuroinflammatory changes in the brain.
- Nanoparticles can be used to deliver siRNA or other genetic material to brain cells, potentially allowing for the targeted knockdown of genes involved in the neuroinflammatory response in AD.
- Quantum dots can be used as fluorescent probes to detect and monitor the activity of microglia and astrocytes in the brain.
- Polymeric nanoparticles can be designed to encapsulate or conjugate with anti-inflammatory agents and target the brain to reduce neuroinflammation. The controlled targeted delivery of anti-inflammatory agents to the brain could potentially reduce systemic side effects and improve drug efficacy.
- Magnetic nanoparticles can be used to magnetically manipulate and sort activated microglia and astrocytes in the brain. This technology could potentially be used to isolate and study these cells, leading to a better understanding of the neuroinflammatory response in AD.
- Nanoparticles can be used to deliver growth factors or other neurotrophic agents to the brain, potentially promoting neuronal regeneration.
7. Conclusions and Discussions of the Potential Benefits and Limitations of Nanotechnology-Based Approaches for the Diagnosis and Treatment of Alzheimer’s Disease
- Early detection and diagnosis. One of the major advantages of nanotechnology-based approaches is their ability to detect Aβ peptides, hyperphosphorylated tau protein, cytokines associated with neuroinflammation, chemokines, and other biomarkers in the brain or body fluids such as CSF, blood, and urine. This can be performed even before clinical symptoms of the disease appear. Nanoparticles can be engineered to bind specifically to these biomarkers and generate signals that can be detected and quantified using imaging techniques such as MRI or PET. Early detection can provide an opportunity for rapid intervention and better therapy management.
- Targeted drug delivery involves nanoparticles that can be engineered (functionalized with peptides or antibodies) to target specific cells or regions of the brain affected by AD. They can carry drugs that can inhibit the production and accumulation of Aβ or hyperphosphorylated tau protein or promote their clearance. In the case of the neuroinflammation hypothesis, nanoparticles can be designed to target cells and tissues affected by inflammation, such as microglia and astrocytes. They can also enhance mitochondrial function or protect mitochondria from oxidative stress and dysfunction. For example, polyphenol-coated nanoparticles can scavenge free radicals and prevent mitochondrial damage in neurons. Moreover, nanoparticles loaded with mitochondria-targeting antioxidants may prevent their dysfunction and improve cognitive ability. Overall, targeted drug delivery can improve the efficacy of drug therapies while minimizing side effects.
- BBB penetration is an approach to use nanoparticles in order to overcome the BBB, a protective barrier that prevents the entry of many drugs and therapeutic agents into the brain. This can allow for the more effective delivery of drugs and minimally invasive treatment.
- Improved pharmacokinetics. This can be achieved through engineering nanocarriers to influence the pharmacokinetic properties of drugs, such as longer half-life, increased stability, and reduced clearance. This strategy can improve the efficacy and safety of AD therapies.
- Reduced toxicity. This approach uses biocompatible nanoparticles to reduce the toxicity of drugs, encapsulating and delivering them specifically to the affected areas of the brain and minimizing their dosages.
- Versatility. Apart from biocompatibility, nanoparticles can be produced with a wide range of sizes, shapes, and surface properties, making them versatile tools for developing new treatments for AD.
- Enhanced imaging. Nanoparticles are also used as contrast agents to enhance imaging techniques such as MRI, PET, and CT scans. These imaging techniques can provide more accurate and detailed information about, e.g., the extent of Aβ accumulation, visualizing inflammation and disease progression in the brain.
- Multi-modal imaging. Nanoparticles may potentially offer multi-modal imaging capabilities, allowing for the simultaneous detection of multiple biomarkers and disease features. This can improve the accuracy and reliability of AD diagnosis and monitoring.
- Monitoring treatment efficiency. Nanotechnology-based approaches can enable real-time monitoring of the treatment efficiency for AD through detecting changes in, e.g., hyperphosphorylated tau protein levels. This can help to optimize therapy and improve patient outcomes.
- Non-invasive treatment. Some nanotechnology-based strategies such as magnetic nanoparticles or ultrasound can be used to treat AD without invasive procedures. For example, magnetic nanoparticles can be used to induce hyperthermia in Aβ deposits, which can break down Aβ peptides and reduce their toxicity. Non-invasive therapies also include the delivery of nanoparticles via, e.g., internasal or oral administration, reducing the need for invasive treatment.
- Biomarker discovery. Nano-based methods can help identify new biomarkers that can be used to diagnose AD at an early stage. For example, nanoparticles can be used to capture and analyze peptides or other biomolecules in biological fluids such as blood or CSF.
- Disease prevention. Nanotechnology-based approaches can potentially be used to prevent AD through targeting the early stages of Aβ accumulation in the brain. For example, nanoparticles can be designed to capture and remove Aβ peptides from the brain before they form aggregates.
- Personalized medicine. Nanotechnology-based approaches can potentially provide personalized medicine through tailoring treatments to the specific needs of individual patients based on their unique genetic and molecular profiles. For example, nanoparticles can be designed to carry drugs that target specific genetic mutations or molecular pathways associated with the production of Aβ. They also can target specific forms of hyperphosphorylated tau protein that are present in some patients but not others.
- Combination therapy. Nanotechnology-based approaches can be used in combination with other therapies such as immunotherapy or gene therapy to provide a more comprehensive treatment for AD. For example, nanoparticles can be engineered to carry drugs that enhance those therapies or to deliver multiple drugs simultaneously. This can increase the effectiveness of treatments and reduce the overall dose of each drug.
- Remote monitoring. Nanotechnology-based approaches can potentially provide remote monitoring of AD progression and treatment response. For example, nanoparticles can be engineered to emit signals that can be detected using wearable devices, allowing for the real-time monitoring of Aβ clearance in the brain.
- Improved safety. Nanotechnology-based approaches can potentially improve the safety of AD treatments through minimizing off-target effects and reducing the toxicity of drugs. For example, nanoparticles can be designed to specifically target Aβ peptides, minimizing the risk of damage to healthy cells in the brain.
- Improved brain function. Nanoparticle-based therapies may have the potential to not only slow down the progression of AD but also improve brain function through restoring, e.g., the cholinergic system and enhancing memory and cognitive function.
- Cost-effective treatment. Nanotechnology-based approaches can potentially provide cost-effective treatments for AD through reducing the amount of drug required for effective treatment and minimizing the need for invasive procedures.
- Improved understanding. Nanotechnology-based approaches can be used to gain a deeper understanding of the mechanism of AD, leading to the development of new therapeutic targets and strategies.
- Safety concerns. One of the major challenges with using nanoparticles for treatment is ensuring their safety. Nanoparticles can interact with cells and tissues in unexpected ways and may cause toxicity or immune responses. It is important to thoroughly test the safety of nanoparticles before they can be used in humans.
- Manufacturing and quality control. Another challenge with nanotechnology-based approaches is manufacturing and quality control. Nanoparticles need to be manufactured to strict specifications, and quality control measures must be put in place to ensure consistency and safety. Scaling up the production of nanoparticles can also be difficult, and there may be batch-to-batch variability.
- Targeting specific cells and molecules. Another challenge is ensuring that nanoparticles can target specific cells affected by AD and molecules such as Aβ peptides. The BBB can make it difficult for nanoparticles to enter the brain, and targeting specific compounds can be challenging.
- Efficacy. Even if nanoparticles can be targeted to specific cells, it is not yet clear whether they will be effective in treating AD. More research is needed to determine the optimal properties and dosing of nanoparticles for the treatment.
- Regulatory approval. Any nanoparticles for AD treatment will need to go through regulatory approval before they can be used in humans. This process can be lengthy and expensive, and there is no guarantee that nanoparticles will be approved for clinical use.
- Cost. The cost of developing and producing nanoparticles may be high. This could limit access to treatment for some patients and may also limit the commercial viability of nanoparticle-based therapies.
- Clearance mechanisms. One potential limitation of using nanoparticles is that the clearance mechanisms for these particles in the brain are not yet fully understood. It is possible that nanoparticles may accumulate in the brain or other organs over time and cause toxicity or interfere with other body functions.
- Variability in patient response. Another limitation is that patient responses to nanoparticles may vary due to differences in genetics, disease progression, or other factors. This could make it difficult to predict how well nanoparticles will work in different patients.
- Limited treatment window. There may be a limited window of opportunity for using nanoparticles to treat AD, as the disease may progress to a point where it is no longer reversible or treatable. This means that nanoparticles may need to be used in combination with other treatments or in earlier stages of the disease to be effective.
- Long-term effects. It is not yet clear what the long-term effects of using nanoparticles may be. It is possible that they could cause unintended side effects or have other long-term effects that are not yet understood.
- Accurate detection. Another challenge of nanotechnology-based approaches is the possible limitation of specific biomarker detection, such as Aβ peptide or tau protein. These biomarkers may not be present in significant amounts until later stages of the disease and may not accurately reflect the extent of the neurons’ degradation.
- Technical challenges: The design and production of nanoparticles with specific properties, such as size, shape, and surface chemistry, can be technically challenging. This can limit the scalability of nanotechnology-based approaches for treating AD.
- Variable effectiveness: The effectiveness of nanotechnology-based approaches can vary depending on the type and severity of AD, as well as individual patient factors. This can limit their overall efficacy and utility for treating AD.
- Difficulty targeting specific cells. Even with targeted drug delivery, it can be challenging to ensure that nanoparticles are taken up by specific cells, such as activated microglia and astrocytes. This can limit the efficacy of treatment and lead to off-target effects.
- Ethical considerations. The use of nanotechnology-based approaches for treating AD raises ethical considerations, such as the potential for unintended consequences and the need to ensure equitable access to treatment.
- Patient acceptance. Some patients may be hesitant to undergo treatment with nanotechnology-based approaches due to concerns about safety, efficacy, or the use of unfamiliar technologies.
- Compatibility with existing treatments. Nanotechnology-based approaches may not be compatible with existing treatments for AD, which can limit their utility as a standalone treatment or in combination with other treatments.
- Lack of long-term data. The long-term safety and efficacy of nanotechnology-based approaches for treating AD are not yet fully understood, and more research is needed to determine their effectiveness over time.
Author Contributions
Funding
Conflicts of Interest
References
- Bernell, S.; Howard, S.W. Use Your Words Carefully: What Is a Chronic Disease? Front. Public Health 2016, 4, 159. [Google Scholar] [CrossRef] [PubMed]
- Durães, F.; Pinto, M.; Sousa, E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, J.; James, B.; Johnson, T.; Reimer, J.; Solis, M.; Weuve, J.; Buckley, R.F.; Hohman, T.J. 2022 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Lashley, T.; Schott, J.M.; Weston, P.; Murray, C.E.; Wellington, H.; Keshavan, A.; Foti, S.C.; Foiani, M.; Toombs, J.; Rohrer, J.D.; et al. Molecular biomarkers of Alzheimer’s disease: Progress and prospects. Dis. Model. Mech. 2018, 11, dmm031781. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Mashal, Y.; Abdelhady, H.; Iyer, A.K. Comparison of Tau and Amyloid-Beta Targeted Immunotherapy Nanoparticles for Alzheimer’s Disease. Biomolecules 2022, 12, 1001. [Google Scholar] [CrossRef]
- Mullard, A. Alzheimer´s Drug Approval Could Affect Other Diseases. Nature 2021, 595, 162–163. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A Critical Appraisal of Amyloid-Beta Targeting Therapies for Alzheimer Disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef]
- Rampa, A.; Gobbi, S.; Belluti, F.; Bisi, A. Tackling Alzheimer’s Disease with Existing Drugs: A Promising Strategy for Bypassing Obstacles. Curr. Med. Chem. 2021, 28, 2305–2327. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta. Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Godoy, P.A.; Mennickent, D.; Cuchillo-Ibáñez, I.; Ramírez-Molina, O.; Silva-Grecchi, T.; Panes-Fernández, J.; Castro, P.; Sáez-Valero, J.; Fuentealba, J. Increased P2×2 Receptors Induced by Amyloid-β Peptide Participates in the Neurotoxicity in Alzheimer’s Disease. Biomed. Pharmacother. 2021, 142, 111968. [Google Scholar] [CrossRef] [PubMed]
- González-Sanmiguel, J.; Burgos, C.F.; Bascuñán, D.; Fernández-Pérez, E.J.; Riffo-Lepe, N.; Boopathi, S.; Fernández-Pérez, A.; Bobadilla-Azócar, C.; González, W.; Figueroa, M.; et al. Gabapentin Inhibits Multiple Steps in the Amyloid Beta Toxicity Cascade. ACS Chem. Neurosci. 2020, 11, 3064–3076. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Lynn, B.C.; Fister, S.; Bradley-Whitman, M.; Murphy, M.P.; Beckett, T.L.; Norris, C.M. A Novel Small Molecule Modulator of Amyloid Pathology. J. Alzheimer’s Dis. 2016, 53, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.C.; Simakova, O.; Jacobson, K.A.; Arispe, N.; Pollard, H.B. Small Molecule Blockers of the Alzheimer A Beta Calcium Channel Potently Protect Neurons from A Beta Cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 3348–3353. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, D.V.; Zaretskaia, M.V. Mini-Review: Amyloid Degradation Toxicity Hypothesis of Alzheimer’s Disease. Neurosci. Lett. 2021, 756, 135959. [Google Scholar] [CrossRef]
- Arispe, N.; Diaz, J.C.; Flora, M. Efficiency of Histidine-Associating Compounds for Blocking the Alzheimer’s A Beta Channel Activity and Cytotoxicity. Biophys. J. 2008, 95, 4879–4889. [Google Scholar] [CrossRef]
- Tong, C.K.B.; Wu, A.J.; Li, M.; Cheung, K.-H. Calcium signaling in Alzheimer’s disease & therapies. Biochim. Biophys. Acta (BBA) Bioenerg. 2018, 1865, 1745–1760. [Google Scholar] [CrossRef]
- Sepulveda, F.J.; Fierro, H.; Fernandez, E.; Castillo, C.; Peoples, R.W.; Opazo, C.; Aguayo, L.G. Nature of the Neurotoxic Membrane Actions of Amyloid-β on Hippocampal Neurons in Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 472–481. [Google Scholar] [CrossRef]
- Ausó, E.; Gómez-Vicente, V.; Esquiva, G. Biomarkers for Alzheimer’s Disease Early Diagnosis. J. Pers. Med. 2020, 10, 114. [Google Scholar] [CrossRef]
- Krishnadas, N.; Villemagne, V.L.; Doré, V.; Rowe, C.C. Advances in Brain Amyloid Imaging. Semin. Nucl. Med. 2021, 51, 241–252. [Google Scholar] [CrossRef]
- Sintini, I.; Whitwell, J.L. Update on neuroimaging in Alzheimer’s disease. Curr. Opin. Neurol. 2021, 34, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L. Alzheimer’s Disease Neuroimaging. Curr. Opin. Neurol. 2018, 31, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Sabbagh, M. Amyloid Imaging: Poised for Integration into Medical Practice. Neurotherapeutics 2016, 14, 54–61. [Google Scholar] [CrossRef]
- McCarter, S.J.; Lesnick, T.G.; Lowe, V.; Mielke, M.M.; Constantopoulos, E.; Rabinstein, A.A.; Przybelski, S.A.; Botha, H.; Jones, D.T.; Ramanan, V.K.; et al. Cerebral Amyloid Angiopathy Pathology and Its Association With Amyloid-Beta PET Signal. Neurology 2021, 97, E1799–E1808. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kim, S.J.; Hong, S.; Kim, Y. Diagnosis of Alzheimer’s disease utilizing amyloid and tau as fluid biomarkers. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Imbimbo, B.P.; Ippati, S.; Watling, M.; Imbimbo, C. Role of Monomeric Amyloid-Beta in Cognitive Performance in Alzheimer’s Disease: Insights from Clinical Trials with Secretase Inhibitors and Monoclonal Antibodies. Pharmacol. Res. 2023, 187, 106631. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeong, M.; Stiles, W.R.; Choi, H.S. Neuroimaging Modalities in Alzheimer’s Disease: Diagnosis and Clinical Features. Int. J. Mol. Sci. 2022, 23, 6079. [Google Scholar] [CrossRef]
- Spasov, S.; Passamonti, L.; Duggento, A.; Liò, P.; Toschi, N. A parameter-efficient deep learning approach to predict conversion from mild cognitive impairment to Alzheimer’s disease. NeuroImage 2019, 189, 276–287. [Google Scholar] [CrossRef]
- Martinez-Murcia, F.J.; Górriz, J.M.; Ramírez, J.; Illán, I.A.; Segovia, F.; Castillo-Barnes, D.; Salas-Gonzalez, D. Functional Brain Imaging Synthesis Based on Image Decomposition and Kernel Modeling: Application to Neurodegenerative Diseases. Front. Neurosci. 2017, 11, 65. [Google Scholar] [CrossRef]
- Li, W.; Lin, X.; Chen, X. Detecting Alzheimer’s disease Based on 4D fMRI: An exploration under deep learning framework. Neurocomputing 2020, 388, 280–287. [Google Scholar] [CrossRef]
- Maestu, F.; Cuesta, P.; Hasan, O.; Fernandez, A.; Funke, M.; Schulz, P.E. The Importance of the Validation of M/EEG With Current Biomarkers in Alzheimer’s Disease. Front. Hum. Neurosci. 2019, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.; Li, R.; Zhou, H.; Qing, K.; Liu, H.; Pan, H.; Lei, Y.; Fu, W.; Wang, X.; Xiao, X.; et al. Neural biomarker diagnosis and prediction to mild cognitive impairment and Alzheimer’s disease using EEG technology. Alzheimer’s Res. Ther. 2023, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Monllor, P.; Cervera-Ferri, A.; Lloret, M.A.; Esteve, D.; Lopez, B.; Leon, J.L.; Lloret, A. Electroencephalography as a Non-Invasive Biomarker of Alzheimer’s Disease: A Forgotten Candidate to Substitute CSF Molecules? Int. J. Mol. Sci. 2021, 22, 10889. [Google Scholar] [CrossRef] [PubMed]
- Altuna-Azkargorta, M.; Mendioroz-Iriarte, M. Blood Biomarkers in Alzheimer’s Disease. Neurologia 2021, 36, 704–710. [Google Scholar] [CrossRef]
- West, T.; Kirmess, K.M.; Meyer, M.R.; Holubasch, M.S.; Knapik, S.S.; Hu, Y.; Contois, J.H.; Jackson, E.N.; Harpstrite, S.E.; Bateman, R.J.; et al. A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: Findings from a multi cohort validity analysis. Mol. Neurodegener. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Tadokoro, K.; Yamashita, T.; Kimura, S.; Nomura, E.; Ohta, Y.; Omote, Y.; Takemoto, M.; Hishikawa, N.; Morihara, R.; Morizane, Y.; et al. Retinal Amyloid Imaging for Screening Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 83, 927–934. [Google Scholar] [CrossRef]
- Koronyo, Y.; Rentsendorj, A.; Mirzaei, N.; Regis, G.C.; Sheyn, J.; Shi, H.; Barron, E.; Cook-Wiens, G.; Rodriguez, A.R.; Medeiros, R.; et al. Retinal pathological features and proteome signatures of Alzheimer’s disease. Acta Neuropathol. 2023, 145, 409–438. [Google Scholar] [CrossRef]
- Gharbiya, M.; Visioli, G.; Trebbastoni, A.; Albanese, G.M.; Colardo, M.; D’antonio, F.; Segatto, M.; Lambiase, A. Beta-Amyloid Peptide in Tears: An Early Diagnostic Marker of Alzheimer’s Disease Correlated with Choroidal Thickness. Int. J. Mol. Sci. 2023, 24, 2590. [Google Scholar] [CrossRef]
- Xia, X.; Qin, Q.; Peng, Y.; Wang, M.; Yin, Y.; Tang, Y. Retinal Examinations Provides Early Warning of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 90, 1341–1357. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Ababei, D.C.; Rusu, R.N.; Bild, V.; Tamba, B.-I. Exploring the Involvement of the Amyloid Precursor Protein A673T Mutation against Amyloid Pathology and Alzheimer’s Disease in Relation to Therapeutic Editing Tools. Pharmaceutics 2022, 14, 1270. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, X.; Shi, J.; Liu, Z.; Peng, Y.; Wang, W.; Li, B.; Zhao, Y.; Xiao, J.; Huang, L.; et al. The Protective A673T Mutation of Amyloid Precursor Protein (APP) in Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 4038–4050. [Google Scholar] [CrossRef] [PubMed]
- Balmus, I.; Ciobica, A.; Gorgan, L. A populational review of the amyloid precursor protein gene mutations relevant to alzheimer’s disease. Eur. Psychiatry 2021, 64, S718. [Google Scholar] [CrossRef]
- Yun, G.; Kim, H.J.; Kim, H.G.; Lee, K.M.; Hong, I.K.; Kim, S.H.; Rhee, H.Y.; Jahng, G.H.; Yoon, S.S.; Park, K.C.; et al. Association Between Plasma Amyloid-Beta and Neuropsychological Performance in Patients With Cognitive Decline. Front. Aging Neurosci. 2021, 13, 736937. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Estrella, A.; Hakim, O.; Milazzo, P.; Patel, S.; Pintagro, C.; Li, D.; Zhao, R.; Vance, D.E.; Li, W.; et al. Mini-Mental State Examination and Montreal Cognitive Assessment as Tools for Following Cognitive Changes in Alzheimer’s Disease Neuroimaging Initiative Participants. J. Alzheimer’s Dis. 2022, 90, 263–270. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roqué-Figuls, M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Cosp, X.B.; Cullum, S. Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2021, 2021, CD010783. [Google Scholar] [CrossRef]
- Jeon, S.; Kang, J.M.; Seo, S.; Jeong, H.J.; Funck, T.; Lee, S.M.; Park, K.H.; Lee, Y.B.; Yeon, B.K.; Ldo, T.; et al. Topographical Heterogeneity of Alzheimer’s Disease Based on MR Imaging, Tau PET, and Amyloid PET. Front. Aging Neurosci. 2019, 11, 211. [Google Scholar] [CrossRef]
- Martínez-Torteya, A.; Treviño, V.; Tamez-Peña, J.G. Improved Diagnostic Multimodal Biomarkers for Alzheimer’s Disease and Mild Cognitive Impairment. BioMed Res. Int. 2015, 2015, 961314. [Google Scholar] [CrossRef]
- Initi, A.D.N.; Ezzati, A.; Harvey, D.J.; Habeck, C.; Golzar, A.; Qureshi, I.A.; Zammit, A.R.; Hyun, J.; Truelove-Hill, M.; Hall, C.B.; et al. Predicting Amyloid-Beta Levels in Amnestic Mild Cognitive Impairment Using Machine Learning Techniques. J. Alzheimers Dis. 2020, 73, 1211–1219. [Google Scholar] [CrossRef]
- Kim, N.H.; Yang, D.W.; Choi, S.H.; Kang, S.W. Machine Learning to Predict Brain Amyloid Pathology in Pre-dementia Alzheimer’s Disease Using QEEG Features and Genetic Algorithm Heuristic. Front. Comput. Neurosci. 2021, 15, 755499. [Google Scholar] [CrossRef]
- Soderberg, L.; Johannesson, M.; Nygren, P.; Laudon, H.; Eriksson, F.; Osswald, G.; Moller, C.; Lannfelt, L. Lecanemab, Aducanumab, and Gantenerumab-Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics 2023, 20, 195–206. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer disease and aducanumab: Adjusting our approach. Nat. Rev. Neurol. 2019, 15, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.B.E.; Pirela, J.P.T.; Fortin, J.S. Revisiting Small Molecule Inhibitors of Amyloid-Beta Aggregation. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Li, G.; Yang, W.Y.; Li, W.H.; Luo, Y.Y.; Lim, Y.J.; Li, Y.; Paul, A.; Segal, D.; Hong, L.; Li, Y.M. Rational Design of a Cocktail of Inhibitors against A Beta Aggregation. Chem. Eur. J. 2020, 26, 3499–3503. [Google Scholar] [CrossRef] [PubMed]
- McDade, E.; Voytyuk, I.; Aisen, P.; Bateman, R.J.; Carrillo, M.C.; De Strooper, B.; Haass, C.; Reiman, E.M.; Sperling, R.; Tariot, P.N.; et al. The case for low-level BACE1 inhibition for the prevention of Alzheimer disease. Nat. Rev. Neurol. 2021, 17, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.E.; Loss, L.B.; Marcolongo-Pereira, C.; Rossoni, J.V.; Barcelos, R.M.; Chiarelli-Neto, O.; Silva, B.S.D.; Ambrosio, R.P.; Castro, F.; Teixeira, S.F.; et al. Advances in Alzheimer’s Disease’s Pharmacological Treatment. Front. Pharmacol. 2023, 14, 1101452. [Google Scholar] [CrossRef]
- Mantile, F.; Prisco, A. Vaccination against Beta-Amyloid as a Strategy for the Prevention of Alzheimer’s Disease. Biology 2020, 9, 425. [Google Scholar] [CrossRef]
- Lennon, M.J.; Rigney, G.; Raymont, V.; Sachdev, P. Genetic Therapies for Alzheimer’s Disease: A Scoping Review. J. Alzheimer’s Dis. 2021, 84, 491–504. [Google Scholar] [CrossRef]
- Park, K.W.; Wood, C.A.; Li, J.; Taylor, B.C.; Oh, S.; Young, N.L.; Jankowsky, J.L. Gene Therapy Using A Beta Variants for Amyloid Reduction. Mol. Ther. 2021, 29, 2294–2307. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Lotfinia, M.; Abdollahpour-Alitappeh, M.; Hatami, B.; Zali, M.R.; Karimipoor, M. Adeno-associated virus as a gene therapy vector: Strategies to neutralize the neutralizing antibodies. Clin. Exp. Med. 2019, 19, 289–298. [Google Scholar] [CrossRef]
- Cremonini, A.L.; Caffa, I.; Cea, M.; Nencioni, A.; Odetti, P.; Monacelli, F. Nutrients in the Prevention of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 9874159. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, O.; Dobrzyńska, M.; Drzymała-Czyż, S.; Przysławski, J. Diet in the Prevention of Alzheimer’s Disease: Current Knowledge and Future Research Requirements. Nutrients 2022, 14, 4564. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-Q.; Zeng, L.-L.; Zhang, F.-Q.; Jiang, J.-L.; Zhang, J.-T.; Niu, H. Current status and future prospects of stem cell therapy in Alzheimer’s disease. Neural Regen. Res. 2020, 15, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Han, F.B.; Bi, J.Z.; Qiao, L.Y.; Arancio, O. Stem Cell Therapy for Alzheimer’s Disease. In Stem Cell-Based Therapy for Neurodegenerative Diseases; Han, F., Lu, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1266, pp. 39–55. ISBN 0065-2598. [Google Scholar]
- Gonsalvez, I.; Baror, R.; Fried, P.; Santarnecchi, E.; Pascual-Leone, A. Therapeutic Noninvasive Brain Stimulation in Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 362–376. [Google Scholar] [CrossRef]
- Holczer, A.; Németh, V.L.; Vékony, T.; Vécsei, L.; Klivényi, P.; Must, A. Non-invasive Brain Stimulation in Alzheimer’s Disease and Mild Cognitive Impairment—A State-of-the-Art Review on Methodological Characteristics and Stimulation Parameters. Front. Hum. Neurosci. 2020, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Soobiah, C.; Tadrous, M.; Knowles, S.; Blondal, E.; Ashoor, H.M.; Ghassemi, M.; Khan, P.A.; Ho, J.; Tricco, A.C.; Straus, S.E. Variability in the validity and reliability of outcome measures identified in a systematic review to assess treatment efficacy of cognitive enhancers for Alzheimer’s Dementia. PLoS ONE 2019, 14, e0215225. [Google Scholar] [CrossRef]
- Huang, Y.L.; Chang, Y.; Liu, L.; Wang, J.X. Nanomaterials for Modulating the Aggregation of Beta-Amyloid Peptides. Molecules 2021, 26, 4301. [Google Scholar] [CrossRef]
- John, T.; Gladytz, A.; Kubeil, C.; Martin, L.L.; Risselada, H.J.; Abel, B. Impact of nanoparticles on amyloid peptide and protein aggregation: A review with a focus on gold nanoparticles. Nanoscale 2018, 10, 20894–20913. [Google Scholar] [CrossRef]
- Liu, Y.B.; Xu, L.P.; Dai, W.H.; Dong, H.F.; Wen, Y.Q.; Zhang, X.J. Graphene Quantum Dots for the Inhibition of Beta Amyloid Aggregation. Nanoscale 2015, 7, 19060–19065. [Google Scholar] [CrossRef]
- Carradori, D.; Balducci, C.; Re, F.; Brambilla, D.; Le Droumaguet, B.; Flores, O.; Gaudin, A.; Mura, S.; Forloni, G.; Ordoñez-Gutierrez, L.; et al. Antibody-functionalized polymer nanoparticle leading to memory recovery in Alzheimer’s disease-like transgenic mouse model. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 609–618. [Google Scholar] [CrossRef]
- Sun, Z.T.; Ma, C.; Li, G.J.; Zheng, X.Y.; Hao, Y.T.; Yang, Y.; Wang, X. Application of Antibody Fragments Against A Beta With Emphasis on Combined Application With Nanoparticles in Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 654611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Zhang, X.Y.; Li, Y.; Zhong, M.L.; Zhao, P.; Guo, C.; Xu, H.; Wang, T.; Gao, H.L. Brain Targeting and A Beta Binding Bifunctional Nanoparticles Inhibit Amyloid Protein Aggregation in APP/PS1 Transgenic Mice. ACS Chem. Neurosci. 2021, 12, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Yu, X.; Li, L.Y.; Zheng, J. Inhibition of Amyloid-Beta Aggregation in Alzheimer’s Disease. Curr. Pharm. Des. 2014, 20, 1223–1243. [Google Scholar] [CrossRef] [PubMed]
- Rupali; Joseph, B.; Thomas, S.; Sen, N.; Paschold, A.; Binder, W.H.; Kumar, S. Bioinspired Synthetic Polymers-Based Inhibitors of Alzheimer’s Amyloid-Beta Peptide Aggregation. Polym. Chem. 2023, 14, 392–411. [Google Scholar] [CrossRef]
- Puentes-Diaz, N.; Chaparro, D.; Morales-Morales, D.; Flores-Gaspar, A.; Ali-Torres, J. Role of Metal Cations of Copper, Iron, and Aluminum and Multifunctional Ligands in Alzheimer’s Disease: Experimental and Computational Insights. ACS Omega 2023, 8, 4508–4526. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, H.; Zheng, W.; Xu, H. Current understanding of the interactions between metal ions and Apolipoprotein E in Alzheimer’s disease. Neurobiol. Dis. 2022, 172, 105824. [Google Scholar] [CrossRef]
- Poudel, P.; Park, S. Recent Advances in the Treatment of Alzheimer’s Disease Using Nanoparticle-Based Drug Delivery Systems. Pharmaceutics 2022, 14, 835. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for Drug Delivery to the Central Nervous System. Nanomaterials 2019, 9, 371. [Google Scholar] [CrossRef]
- Zakharova, L.; Gaynanova, G.; Vasilieva, E.; Vasileva, L.; Pavlov, R.; Kashapov, R.; Petrov, K.; Sinyashin, O. Recent nanoscale carriers for therapy of Alzheimer’s disease: Current strategies and perspectives. Curr. Med. Chem. 2023, 30, 3743–3774. [Google Scholar] [CrossRef]
- Jung, M.; Lee, S.; Park, S.; Hong, J.; Kim, C.; Cho, I.; Sohn, H.S.; Kim, K.; Park, I.W.; Yoon, S.; et al. A Therapeutic Nanovaccine that Generates Anti-Amyloid Antibodies and Amyloid-specific Regulatory T Cells for Alzheimer’s Disease. Adv. Mater. 2022, 35, e2207719. [Google Scholar] [CrossRef]
- Hanif, S.; Muhammad, P.; Chesworth, R.; Rehman, F.U.; Qian, R.-J.; Zheng, M.; Shi, B.-Y. Nanomedicine-based immunotherapy for central nervous system disorders. Acta. Pharmacol. Sin. 2020, 41, 936–953. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Contrast Agents Delivery: An Up-to-Date Review of Nanodiagnostics in Neuroimaging. Nanomaterials 2019, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Porqueras, D.S.D. Alzheimer’s disease: The use of contrast agents for magnetic resonance imaging to detect amyloid beta peptide inside the brain. Coord. Chem. Rev. 2016, 327–328, 27–34. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, R. The application of nanotechnology in treatment of Alzheimer’s disease. Front. Bioeng. Biotechnol. 2022, 10, 1042986. [Google Scholar] [CrossRef] [PubMed]
- O’connor, D.M.; Boulis, N.M. Gene therapy for neurodegenerative diseases. Trends Mol. Med. 2015, 21, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, P.; Morais, S.; Pereira, M.C. Nanomaterials towards Biosensing of Alzheimer’s Disease Biomarkers. Nanomaterials 2019, 9, 1663. [Google Scholar] [CrossRef]
- Kaushik, A.; Jayant, R.D.; Tiwari, S.; Vashist, A.; Nair, M. Nano-biosensors to detect beta-amyloid for Alzheimer’s disease management. Biosens. Bioelectron. 2016, 80, 273–287. [Google Scholar] [CrossRef]
- Li, M.J.; Yu, Q.J.; Zheng, M.E.; Jiang, R.R.; Zhu, H.D.; Guo, H.L.; Sun, H.M.; Liu, M.X. A Label-Free Electrochemical Immunosensor Based on Au-BSN-RGO for Highly-Sensitive Detection of Beta-Amyloid 1–42. Nanoscale 2023, 15, 4063–4070. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kim, E.-S.; Mishra, S.; Ganbold, E.; Seong, R.-S.; Kim, Y.M.; Jahng, G.-H.; Rhee, H.Y.; Han, H.-S.; Kim, D.H.; et al. Ultrasensitive probeless capacitive biosensor for amyloid beta (Aβ1-42) detection in human plasma using interdigitated electrodes. Biosens. Bioelectron. 2022, 212, 114365. [Google Scholar] [CrossRef]
- Poon, C.T.; Shah, K.; Lin, C.T.; Tse, R.; Kim, K.K.; Mooney, S.; Aubert, I.; Stefanovic, B.; Hynynen, K. Time Course of Focused Ultrasound Effects on Beta-Amyloid Plaque Pathology in the TgCRND8 Mouse Model of Alzheimer’s Disease. Sci. Rep. 2018, 8, 14061. [Google Scholar] [CrossRef]
- Park, S.H.; Baik, K.; Jeon, S.; Chang, W.S.; Ye, B.S.; Chang, J.W. Extensive frontal focused ultrasound mediated blood–brain barrier opening for the treatment of Alzheimer’s disease: A proof-of-concept study. Transl. Neurodegener. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Udomprasert, A.; Bongiovanni, M.N.; Sha, R.; Sherman, W.B.; Wang, T.; Arora, P.S.; Canary, J.W.; Gras, S.L.; Seeman, N.C. Amyloid fibrils nucleated and organized by DNA origami constructions. Nat. Nanotechnol. 2014, 9, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Knappe, G.A.; Wamhoff, E.-C.; Bathe, M. Functionalizing DNA origami to investigate and interact with biological systems. Nat. Rev. Mater. 2022, 8, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Li, F.; Zou, Z.; Saeed, M.; Xu, Z.; Yu, H. Bio-inspired amyloid polypeptides: From self-assembly to nanostructure design and biotechnological applications. Appl. Mater. Today 2021, 22, 100966. [Google Scholar] [CrossRef]
- Wang, S.M.; Lee, C.U.; Lim, H.K. Stem Cell Therapies for Alzheimer’s Disease: Is It Time? Curr. Opin. Psychiatry 2019, 32, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.; Ashraf, G.M.; Mamun, A.; Mathew, B. Toxic tau: Structural origins of tau aggregation in Alzheimer’s disease. Neural Regen. Res. 2020, 15, 1417–1420. [Google Scholar] [CrossRef]
- Almansoub, H.A.; Tang, H.; Wu, Y.; Wang, D.-Q.; Mahaman, Y.A.R.; Wei, N.; Almansob, Y.A.M.; He, W.; Liu, D. Tau Abnormalities and the Potential Therapy in Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 67, 13–33. [Google Scholar] [CrossRef]
- Ondrejcak, T.; Klyubin, I.; Corbett, G.T.; Fraser, G.; Hong, W.; Mably, A.J.; Gardener, M.; Hammersley, J.; Perkinton, M.S.; Billinton, A.; et al. Cellular Prion Protein Mediates the Disruption of Hippocampal Synaptic Plasticity by Soluble Tau In Vivo. J. Neurosci. 2018, 38, 10595–10606. [Google Scholar] [CrossRef]
- Liu, S.L.; Wang, C.; Jiang, T.; Tan, L.; Xing, A.; Yu, J.T. The Role of Cdk5 in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 4328–4342. [Google Scholar] [CrossRef]
- Toral-Rios, D.; Pichardo-Rojas, P.S.; Alonso-Vanegas, M.; Campos-Pena, V. GSK3 Beta and Tau Protein in Alzheimer’s Disease and Epilepsy. Front. Cell Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A.; et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2020, 324, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Smith, R.; Ohlsson, T.; Strandberg, O.; Mattsson, N.; Insel, P.S.; Palmqvist, S.; Hansson, O. Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology 2019, 92, e601–e612. [Google Scholar] [CrossRef] [PubMed]
- Schöll, M.; Lockhart, S.N.; Schonhaut, D.R.; O’neil, J.P.; Janabi, M.; Ossenkoppele, R.; Baker, S.L.; Vogel, J.W.; Faria, J.; Schwimmer, H.D.; et al. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron 2016, 89, 971–982. [Google Scholar] [CrossRef]
- James, O.G.; Doraiswamy, P.M.; Borges-Neto, S. PET Imaging of Tau Pathology in Alzheimer’s Disease and Tauopathies. Front. Neurol. 2015, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Scholl, M.; Strandberg, O.; Smith, R.; Palmqvist, S.; Insel, P.S.; Hgerstrom, D.; Ohlsson, T.; Zetterberg, H.; Jogi, J.; et al. 18F-AV-1451 and CSF T-Tau and P-Tau as Biomarkers in Alzheimer’s Disease. EMBO Mol. Med. 2017, 9, 1212–1223. [Google Scholar] [CrossRef]
- Petersen, G.C.; Roytman, M.; Chiang, G.C.; Li, Y.; Gordon, M.L.; Franceschi, A.M. Overview of tau PET molecular imaging. Curr. Opin. Neurol. 2022, 35, 230–239. [Google Scholar] [CrossRef]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Stomrud, E.; Lindberg, O.; Palmqvist, S.; Zetterberg, H.; Blennow, K.; Hansson, O. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 2018, 91, e867–e877. [Google Scholar] [CrossRef]
- Schöll, M.; Maass, A.; Mattsson, N.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Jagust, W. Biomarkers for tau pathology. Mol. Cell. Neurosci. 2018, 97, 18–33. [Google Scholar] [CrossRef]
- Zetterberg, H.; Bendlin, B.B. Biomarkers for Alzheimer’s disease—Preparing for a new era of disease-modifying therapies. Mol. Psychiatry 2020, 26, 296–308. [Google Scholar] [CrossRef]
- Mielke, M.M.; Hagen, C.E.; Xu, J.; Chai, X.; Vemuri, P.; Lowe, V.J.; Airey, D.C.; Knopman, D.S.; Roberts, R.O.; Machulda, M.M.; et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimer’s Dement. 2018, 14, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Niu, X.; Wang, Y.; Lv, S.; Zhou, X.; Yang, Z.; Peng, D. Plasma tau proteins for the diagnosis of mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. Front. Aging Neurosci. 2022, 14, 942629. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Hagen, C.E.; Wennberg, A.M.V.; Airey, D.C.; Savica, R.; Knopman, D.S.; Machulda, M.M.; Roberts, R.O.; Jack, C.R.; Petersen, R.C.; et al. Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in the Mayo Clinic Study on Aging. JAMA Neurol. 2017, 74, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Dore, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High Performance Plasma Amyloid-Beta Biomarkers for Alzheimer’s Disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- La Morgia, C.; Ross-Cisneros, F.N.; Koronyo, Y.; Hannibal, J.; Gallassi, R.; Cantalupo, G.; Sambati, L.; Pan, B.X.; Tozer, K.R.; Barboni, P.; et al. Melanopsin Retinal Ganglion Cell Loss in Alzheimer Disease. Ann. Neurol. 2016, 79, 90–109. [Google Scholar] [CrossRef]
- Mutlu, U.; Colijn, J.M.; Ikram, M.A.; Bonnemaijer, P.W.M.; Licher, S.; Wolters, F.J.; Tiemeier, H.; Koudstaal, P.J.; Klaver, C.C.W.; Ikram, M.K. Association of Retinal Neurodegeneration on Optical Coherence Tomography With Dementia A Population-Based Study. JAMA Neurol. 2018, 75, 1256–1263. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- West, T.; Hu, Y.; Verghese, P.B.; Bateman, R.J.; Braunstein, J.B.; Fogelman, I.; Budur, K.; Florian, H.; Mendonca, N.; Holtzman, D.M. Preclinical and Clinical Development of ABBV-8E12, a Humanized Anti-Tau Antibody, for Treatment of Alzheimer’s Disease and Other Tauopathies. J. Prev. Alzheimers Dis. 2017, 4, 236–241. [Google Scholar] [CrossRef]
- Kfoury, N.; Holmes, B.B.; Jiang, H.; Holtzman, D.M.; Diamond, M.I. Trans-cellular Propagation of Tau Aggregation by Fibrillar Species. J. Biol. Chem. 2012, 287, 19440–19451. [Google Scholar] [CrossRef]
- Wu, K.-M.; Zhang, Y.-R.; Huang, Y.-Y.; Dong, Q.; Tan, L.; Yu, J.-T. The role of the immune system in Alzheimer’s disease. Ageing Res. Rev. 2021, 70, 101409. [Google Scholar] [CrossRef] [PubMed]
- Yanamandra, K.; Patel, T.K.; Jiang, H.; Schindler, S.; Ulrich, J.D.; Boxer, A.L.; Miller, B.L.; Kerwin, D.R.; Gallardo, G.; Stewart, F.; et al. Anti-tau antibody administration increases plasma tau in transgenic mice and patients with tauopathy. Sci. Transl. Med. 2017, 9, eaal2029. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s Disease Drug Development Pipeline: 2021. Alzheimer’s Dement Transl. Res. Clin. Interv. 2022, 8, e12179. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef]
- Harrison, T.M.; La Joie, R.; Maass, A.; Baker, S.L.; Swinnerton, K.; Fenton, L.; Mellinger, T.J.; Edwards, L.; Pham, J.; Miller, B.L.; et al. Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Ann. Neurol. 2018, 85, 229–240. [Google Scholar] [CrossRef]
- Wessels, A.M.; Tariot, P.N.; Zimmer, J.A. Efficacy and Safety of Lanabecestat for Treatment of Early and Mild Alzheimer Disease: The AMARANTH and DAYBREAK-ALZ Randomized Clinical Trials. JAMA Neurol. 2020, 77, 199–209. [Google Scholar] [CrossRef]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021, 141, 709–724. [Google Scholar] [CrossRef]
- Medina, M. An Overview on the Clinical Development of Tau-Based Therapeutics. Int. J. Mol. Sci. 2018, 19, 1160. [Google Scholar] [CrossRef]
- Dujardin, S.; Commins, C.; Lathuiliere, A.; Beerepoot, P.; Fernandes, A.R.; Kamath, T.V.; De Los Santos, M.B.; Klickstein, N.; Corjuc, D.L.; Corjuc, B.T.; et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 2020, 26, 1256–1263. [Google Scholar] [CrossRef]
- Kaur, P.; Khera, A.; Alajangi, H.K.; Sharma, A.; Jaiswal, P.K.; Singh, G.; Barnwal, R.P. Role of Tau in Various Tauopathies, Treatment Approaches, and Emerging Role of Nanotechnology in Neurodegenerative Disorders. Mol. Neurobiol. 2022, 60, 1690–1720. [Google Scholar] [CrossRef]
- Altinoglu, G.; Adali, T. Alzheimer’s Disease Targeted Nano-Based Drug Delivery Systems. Curr. Drug Targets 2020, 21, 628–646. [Google Scholar] [CrossRef]
- Jinwal, U.K.; Groshev, A.; Zhang, J.; Grover, A.; Sutariya, V.B. Preparation and characterization of methylene blue nanoparticles for Alzheimer’s disease and other tauopathies. Curr. Drug Deliv. 2013, 11, 541–550. [Google Scholar] [CrossRef]
- Gao, C.H.; Chu, X.Y.; Gong, W.; Zheng, J.P.; Xie, X.Y.; Wang, Y.L.; Yang, M.Y.; Li, Z.P.; Gao, C.S.; Yang, Y. Neuron Tau-Targeting Biomimetic Nanoparticles for Curcumin Delivery to Delay Progression of Alzheimer’s Disease. J. Nanotechnol. 2020, 18, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, L.; Wu, X.; Deng, F.; Ma, R.; Liu, Y.; Huang, F.; Shi, L. Tau-Targeted Multifunctional Nanoinhibitor for Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2021, 13, 23328–23338. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.Q.; Wang, Y.; Yang, X.T.; Ling, G.X.; Zhang, P. Application of Curcumin Nanoformulations in Alzheimer’s Disease: Prevention, Diagnosis and Treatment. Nutr. Neurosci. 2023, 26, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, S.D.; Zhou, Y.Q.; Seven, E.; Lakshmana, M.K.; Kaushik, A.K.; Chand, H.S.; Leblanc, R.M. Nanoparticle-Mediated Approaches for Alzheimer’s Disease Pathogenesis, Diagnosis, and Therapeutics. J. Control. Release 2019, 314, 125–140. [Google Scholar] [CrossRef]
- Xu, L.; Ding, Y.; Ma, F.; Chen, Y.; Chen, G.; Zhu, L.; Long, J.; Ma, R.; Liu, Y.; Liu, J.; et al. Engineering a pathological tau-targeted nanochaperone for selective and synergetic inhibition of tau pathology in Alzheimer’s Disease. Nano Today 2022, 43, 101388. [Google Scholar] [CrossRef]
- Latina, V.; Giacovazzo, G.; Cordella, F.; Balzamino, B.O.; Micera, A.; Varano, M.; Marchetti, C.; Malerba, F.; Florio, R.; Ercole, B.B.; et al. Systemic delivery of a specific antibody targeting the pathological N-terminal truncated tau peptide reduces retinal degeneration in a mouse model of Alzheimer’s Disease. Acta Neuropathol. Commun. 2021, 9, 1–27. [Google Scholar] [CrossRef]
- Bilal, M.; Barani, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanomaterials for the treatment and diagnosis of Alzheimer’s disease: An overview. NanoImpact 2020, 20, 100251. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, M.; Zhou, D.; Zheng, T.; Zhang, H. The application of multifunctional nanomaterials in Alzheimer’s disease: A potential theranostics strategy. BioMedicine 2021, 137, 111360. [Google Scholar] [CrossRef]
- Hoque, M.; Samanta, A.; Alam, S.S.M.; Zughaibi, T.A.; Kamal, M.A.; Tabrez, S. Nanomedicine-Based Immunotherapy for Alzheimer’s Disease. Neurosci. Biobehav. Rev. 2023, 144, 104973. [Google Scholar] [CrossRef] [PubMed]
- Bittar, A.; Sengupta, U.; Kayed, R. Prospects for strain-specific immunotherapy in Alzheimer’s disease and tauopathies. NPJ Vaccines 2018, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, S.T.; Jo, M.G.; Choi, M.J.; Kim, M.O. A novel kit for early diagnosis of Alzheimer’s disease using a fluorescent nanoparticle imaging. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Selvan, S.T.; Ravichandar, R.; Ghosh, K.K.; Mohan, A.; Mahalakshmi, P.; Gulyás, B.; Padmanabhan, P. Coordination chemistry of ligands: Insights into the design of amyloid beta/tau-PET imaging probes and nanoparticles-based therapies for Alzheimer’s disease. Coord. Chem. Rev. 2020, 430, 213659. [Google Scholar] [CrossRef]
- Oyarzún, M.P.; Tapia-Arellano, A.; Cabrera, P.; Jara-Guajardo, P.; Kogan, M.J. Plasmonic Nanoparticles as Optical Sensing Probes for the Detection of Alzheimer’s Disease. Sensors 2021, 21, 2067. [Google Scholar] [CrossRef]
- Parekh, P.; Mu, Q.; Badachhape, A.; Bhavane, R.; Srivastava, M.; Devkota, L.; Sun, X.; Bhandari, P.; Eriksen, J.L.; Tanifum, E.; et al. A surrogate marker for very early-stage tau pathology is detectable by molecular magnetic resonance imaging. Theranostics 2022, 12, 5504–5521. [Google Scholar] [CrossRef]
- Ramesh, M.; Govindaraju, T. Multipronged diagnostic and therapeutic strategies for Alzheimer’s disease. Chem. Sci. 2022, 13, 13657–13689. [Google Scholar] [CrossRef]
- Zhou, J.; Jangili, P.; Son, S.; Ji, M.S.; Won, M.; Kim, J.S. Fluorescent Diagnostic Probes in Neurodegenerative Diseases. Adv. Mater. 2020, 32, 2001945. [Google Scholar] [CrossRef]
- Phan, L.M.T.; Hoang, T.X.; Vo, T.A.T.; Kim, J.Y.; Lee, S.-M.; Cho, W.W.; Kim, Y.H.; Choi, S.H.; Cho, S. Nanobiosensors for Non-Amyloidbeta-Tau Biomarkers as Advanced Reporters of Alzheimer’s Disease. Diagnostics 2020, 10, 913. [Google Scholar] [CrossRef]
- Phan, L.M.T.; Hoang, T.X.; Vo, T.A.T.; Pham, H.L.; Le, H.T.N.; Chinnadayyala, S.R.; Kim, J.Y.; Lee, S.-M.; Cho, W.W.; Kim, Y.H.; et al. Nanomaterial-based Optical and Electrochemical Biosensors for Amyloid beta and Tau: Potential for early diagnosis of Alzheimer’s Disease. Expert Rev. Mol. Diagn. 2021, 21, 175–193. [Google Scholar] [CrossRef]
- Ameri, M.; Shabaninejad, Z.; Movahedpour, A.; Sahebkar, A.; Mohammadi, S.; Hosseindoost, S.; Ebrahimi, M.S.; Savardashtaki, A.; Karimipour, M.; Mirzaei, H. Biosensors for detection of Tau protein as an Alzheimer’s disease marker. Int. J. Biol. Macromol. 2020, 162, 1100–1108. [Google Scholar] [CrossRef]
- Sun, J.; Roy, S. Gene-based therapies for neurodegenerative diseases. Nat. Neurosci. 2021, 24, 297–311. [Google Scholar] [CrossRef] [PubMed]
- VandeVrede, L.; Boxer, A.L.; Polydoro, M. Targeting tau: Clinical trials and novel therapeutic approaches. Neurosci. Lett. 2020, 731, 134919. [Google Scholar] [CrossRef] [PubMed]
- Martier, R.; Konstantinova, P. Gene Therapy for Neurodegenerative Diseases: Slowing Down the Ticking Clock. Front. Neurosci. 2020, 14, 580179. [Google Scholar] [CrossRef] [PubMed]
- Grueso, S.; Viejo-Sobera, R. Machine learning methods for predicting progression from mild cognitive impairment to Alzheimer’s disease dementia: A systematic review. Alzheimer’s Res. Ther. 2021, 13, 1–29. [Google Scholar] [CrossRef]
- Tanveer, M.; Richhariya, B.; Khan, R.U.; Rashid, A.H.; Khanna, P.; Prasad, M.; Lin, C.T. Machine Learning Techniques for the Diagnosis of Alzheimer’s Disease: A Review. ACM Trans. Multimedia Comput. Commun. Appl. 2020, 16, 1–35. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimer’s Dement. 2022, 19, 333–342. [Google Scholar] [CrossRef]
- Millichap, L.E.; Damiani, E.; Tiano, L.; Hargreaves, I.P. Targetable Pathways for Alleviating Mitochondrial Dysfunction in Neurodegeneration of Metabolic and Non-Metabolic Diseases. Int. J. Mol. Sci. 2021, 22, 11444. [Google Scholar] [CrossRef]
- Swerdlow, R.H. The Mitochondrial Hypothesis: Dysfunction, Bioenergetic Defects, and the Metabolic Link to Alzheimer’s Disease. Int. Rev. Neurobiol. 2020, 154, 207–233. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxidative Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Perluigi, M.; Sultana, R. Oxidative stress in Alzheimer’s disease brain: New insights from redox proteomics. Eur. J. Pharmacol. 2006, 545, 39–50. [Google Scholar] [CrossRef]
- Hubens, W.; Vallbona-Garcia, A.; de Coo, I.; van Tienen, F.; Webers, C.; Smeets, H.; Gorgels, T. Blood biomarkers for assessment of mitochondrial dysfunction: An expert review. Mitochondrion 2021, 62, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.S.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2015, 11, 600–607.e1. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.O.; Ahn, H.-S.; Dao, T.N.T.; Hong, J.; Shin, W.; Lim, Y.-M.; Chung, S.J.; Lee, J.-H.; Liu, H.; Koo, B.; et al. Magnetic transferrin nanoparticles (MTNs) assay as a novel isolation approach for exosomal biomarkers in neurological diseases. Biomater. Res. 2023, 27, 1–19. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front. Pharmacol. 2015, 6, 206. [Google Scholar] [CrossRef]
- Chang, P.-S.; Chou, H.-H.; Lai, T.-J.; Yen, C.-H.; Pan, J.-C.; Lin, P.-T. Investigation of coenzyme Q10 status, serum amyloid-β, and tau protein in patients with dementia. Front. Aging Neurosci. 2022, 14, 910289. [Google Scholar] [CrossRef]
- Shinn, L.J.; Lagalwar, S. Treating Neurodegenerative Disease with Antioxidants: Efficacy of the Bioactive Phenol Resveratrol and Mitochondrial-Targeted MitoQ and SkQ. Antioxidants 2021, 10, 573. [Google Scholar] [CrossRef]
- Chiao, Y.A.; Zhang, H.L.; Sweetwyne, M.; Whitson, J.; Ting, Y.S.; Basisty, N.; Pino, L.K.; Quarles, E.; Nguyen, N.H.; Campbell, M.D.; et al. Late-Life Restoration of Mitochondrial Function Reverses Cardiac Dysfunction in Old Mice. Elife 2020, 9, e55513. [Google Scholar] [CrossRef]
- Whitaker, R.M.; Corum, D.; Beeson, C.C.; Schnellmann, R.G. Mitochondrial Biogenesis as a Pharmacological Target: A New Approach to Acute and Chronic Diseases. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 229–249. [Google Scholar] [CrossRef]
- Yu, R.; Liu, T.; Jin, S.B.; Ankarcrona, M.; Lendahl, U.; Nister, M.; Zhao, J. MIEF1/2 Orchestrate Mitochondrial Dynamics through Direct Engagement with Both the Fission and Fusion Machineries. BMC Biol. 2021, 19, 229. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.Y.; Park, K.J.; Kim, W.-H.; Roh, G.S. A mitochondrial division inhibitor, Mdivi-1, inhibits mitochondrial fragmentation and attenuates kainic acid-induced hippocampal cell death. BMC Neurosci. 2016, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Zacharioudakis, E.; Gavathiotis, E. Mitochondrial dynamics proteins as emerging drug targets. Trends Pharmacol. Sci. 2022, 44, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Voos, W. Mitochondria as Potential Targets in Alzheimer Disease Therapy: An Update. Front. Pharmacol. 2019, 10, 902. [Google Scholar] [CrossRef]
- Massoud, F.; Belleville, S.; Bergman, H.; Kirk, J.; Chertkow, H.; Nasreddine, Z.; Joanette, Y.; Freedman, M. Mild Cognitive Impairment and Cognitive Impairment, No Dementia: Part B, Therapy. Alzheimers Dement. 2007, 3, 283–291. [Google Scholar] [CrossRef]
- Han, S.; Jeong, Y.Y.; Sheshadri, P.; Cai, Q. Mitophagy coordination with retrograde transport ensures the integrity of synaptic mitochondria. Autophagy 2020, 16, 1925–1927. [Google Scholar] [CrossRef]
- Hawkins, K.E.; Duchen, M.R. Modelling mitochondrial dysfunction in Alzheimer’s disease using human induced pluripotent stem cells. World J. Stem Cells 2019, 11, 236–253. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Li, C.; Yu, L.; Chang, Y.; Qu, M. Delivery of coenzyme Q10 with mitochondria-targeted nanocarrier attenuates renal ischemia-reperfusion injury in mice. Mater. Sci. Eng. C 2021, 131, 112536. [Google Scholar] [CrossRef]
- Thangudu, S.; Cheng, F.-Y.; Su, C.-H. Advancements in the Blood–Brain Barrier Penetrating Nanoplatforms for Brain Related Disease Diagnostics and Therapeutic Applications. Polymers 2020, 12, 3055. [Google Scholar] [CrossRef]
- Seo, M.-W.; Park, T.-E. Recent advances with liposomes as drug carriers for treatment of neurodegenerative diseases. Biomed. Eng. Lett. 2021, 11, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Grabowska-Pyrzewicz, W.; Want, A.; Leszek, J.; Wojda, U. Antisense oligonucleotides for Alzheimer’s disease therapy: From the mRNA to miRNA paradigm. EBioMedicine 2021, 74, 103691. [Google Scholar] [CrossRef] [PubMed]
- Shyam, R.; Ren, Y.; Lee, J.; E Braunstein, K.; Mao, H.-Q.; Wong, P.C. Intraventricular Delivery of siRNA Nanoparticles to the Central Nervous System. Mol. Ther.—Nucleic Acids 2015, 4, e242. [Google Scholar] [CrossRef] [PubMed]
- Buchke, S.; Sharma, M.; Bora, A.; Relekar, M.; Bhanu, P.; Kumar, J. Mitochondria-Targeted, Nanoparticle-Based Drug-Delivery Systems: Therapeutics for Mitochondrial Disorders. Life 2022, 12, 657. [Google Scholar] [CrossRef]
- Khan, T.; Waseem, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef]
- Ortiz, J.M.P.; Swerdlow, R.H. Mitochondrial dysfunction in Alzheimer’s disease: Role in pathogenesis and novel therapeutic opportunities. Br. J. Pharmacol. 2019, 176, 3489–3507. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Hochstetler, H.; Wang, S.; Walker, B.; Saykin, A.J. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for Assessment of Mild Cognitive Impairment in Llder Adults. BMC Geriatr. 2015, 15, 107. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Grothe, M.J.; Ray, N.J.; Müller, M.L.T.M.; Teipel, S.J. Recent Advances in Cholinergic Imaging and Cognitive Decline—Revisiting the Cholinergic Hypothesis of Dementia. Curr. Geriatr. Rep. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Karami, A.; Darreh-Shori, T.; Schultzberg, M.; Eriksdotter, M. CSF and Plasma Cholinergic Markers in Patients With Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 704583. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, G.; Gao, M.; Cai, W.; Ning, X. Large Margin and Local Structure Preservation Sparse Representation Classifier for Alzheimer’s Magnetic Resonance Imaging Classification. Front. Aging Neurosci. 2022, 14, 916020. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, D.; Rauch, A.; Logothetis, N.K.; Goense, J. Two distinct profiles of fMRI and neurophysiological activity elicited by acetylcholine in visual cortex. Proc. Natl. Acad. Sci. USA 2018, 115, E12073–E12082. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.L. EEG and MEG: Relevance to Neuroscience. Neuron 2013, 80, 1112–1128. [Google Scholar]
- Nardone, R.; Golaszewski, S.; Ladurner, G.; Tezzon, F.; Trinka, E. A Review of Transcranial Magnetic Stimulation in the in vivo Functional Evaluation of Central Cholinergic Circuits in Dementia. Dement. Geriatr. Cogn. Disord. 2011, 32, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.H.; Seelaar, H.; Melhem, S.; Rozemuller, A.J.; van Swieten, J.C. Genetic screening in early-onset Alzheimer’s disease identified three novel presenilin mutations. Neurobiol. Aging 2020, 86, 201.e9–201.e14. [Google Scholar] [CrossRef]
- Naicker, P.; Anoopkumar-Dukie, S.; Grant, G.D.; Neumann, D.L.; Kavanagh, J.J. Central cholinergic pathway involvement in the regulation of pupil diameter, blink rate and cognitive function. Neuroscience 2016, 334, 180–190. [Google Scholar] [CrossRef]
- Vallianatou, T.; Shariatgorji, M.; Nilsson, A.; Fridjonsdottir, E.; Källback, P.; Schintu, N.; Svenningsson, P.; Andrén, P.E. Molecular imaging identifies age-related attenuation of acetylcholine in retrosplenial cortex in response to acetylcholinesterase inhibition. Neuropsychopharmacology 2019, 44, 2091–2098. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase Inhibitors as Alzheimer’s Therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Hoskin, J.L.; Al-Hasan, Y.; Sabbagh, M.N. Nicotinic Acetylcholine Receptor Agonists for the Treatment of Alzheimer’s Dementia: An Update. Nicotine Tob. Res. 2018, 21, 370–376. [Google Scholar] [CrossRef]
- Velazquez, R.; Ferreira, E.; Knowles, S.; Fux, C.; Rodin, A.; Winslow, W.; Oddo, S. Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell 2019, 18, e13037. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Yang, L.-P.; Zhao, L. Stem cell therapy for Alzheimer’s disease. World J. Stem Cells 2020, 12, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Kabir, T.; Uddin, S.; Al Mamun, A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef]
- Garg, Y.; Kapoor, D.N.; Sharma, A.K.; Bhatia, A. Drug Delivery Systems and Strategies to Overcome the Barriers of Brain. Curr. Pharm. Des. 2022, 28, 619–641. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Surface-modified lipid nanocarriers for crossing the blood-brain barrier (BBB): A current overview of active targeting in brain diseases. Colloids Surf. B Biointerfaces 2023, 221, 112999. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Napolitano, F.; Montesarchio, D.; Sampaolo, S.; Melone, M.A.B. Nanoparticle-Guided Brain Drug Delivery: Expanding the Therapeutic Approach to Neurodegenerative Diseases. Pharmaceutics 2021, 13, 1897. [Google Scholar] [CrossRef]
- Chatard, C.; Meiller, A.; Marinesco, S. Microelectrode Biosensors for Invivo Analysis of Brain Interstitial Fluid. Electroanalysis 2018, 30, 977–998. [Google Scholar] [CrossRef]
- Ardakani, T.; Hosu, O.; Cristea, C.; Ardakani, M.; Marrazza, G. Latest Trends in Electrochemical Sensors for Neurotransmitters: A Review. Sensors 2019, 19, 2037. [Google Scholar] [CrossRef]
- Yang, X.; McGlynn, E.; Das, R.; Paşca, S.P.; Cui, B.; Heidari, H. Nanotechnology Enables Novel Modalities for Neuromodulation. Adv. Mater. 2021, 33, e2103208. [Google Scholar] [CrossRef]
- Luan, L.; Sullender, C.T.; Li, X.; Zhao, Z.; Zhu, H.; Wei, X.; Xie, C.; Dunn, A.K. Nanoelectronics enabled chronic multimodal neural platform in a mouse ischemic model. J. Neurosci. Methods 2018, 295, 68–76. [Google Scholar] [CrossRef]
- Tarricone, G.; Carmagnola, I.; Chiono, V. Tissue-Engineered Models of the Human Brain: State-of-the-Art Analysis and Challenges. J. Funct. Biomater. 2022, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, B.; Kulkarni, S.D.; Villar, P.S.; Smith, R.S.; Eberly, C.; Araneda, R.C.; Depireux, D.A.; Shapiro, B. Movement of magnetic nanoparticles in brain tissue: Mechanisms and impact on normal neuronal function. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Si, Z.-Z.; Zou, C.-J.; Mei, X.; Li, X.-F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.-X.; Wu, L. Targeting neuroinflammation in Alzheimer’s disease: From mechanisms to clinical applications. Neural Regen. Res. 2022, 18, 708–715. [Google Scholar] [CrossRef]
- Latta, C.; Brothers, H.; Wilcock, D. Neuroinflammation in Alzheimer’s disease; A source of heterogeneity and target for personalized therapy. Neuroscience 2014, 302, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Ji, B.; Kong, Y.; Qin, L.; Ren, W.; Guan, Y.; Ni, R. PET Imaging of Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2021, 12, 739130. [Google Scholar] [CrossRef] [PubMed]
- Gouilly, D.; Saint-Aubert, L.; Ribeiro, M.; Salabert, A.; Tauber, C.; Péran, P.; Arlicot, N.; Pariente, J.; Payoux, P. Neuroinflammation PET imaging of the translocator protein (TSPO) in Alzheimer’s disease: An update. Eur. J. Neurosci. 2022, 55, 1322–1343. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Kim, M.-J.; Coughlin, J.M.; Henter, I.D.; Owen, D.R.; Innis, R.B. PET imaging of neuroinflammation in neurological disorders. Lancet Neurol. 2020, 19, 940–950. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K.; Watkins, L.R.; Nelson, R.J.; Popovich, P.G. MicroRNAs: Roles in Regulating Neuroinflammation. Neurosci. 2017, 24, 221–245. [Google Scholar] [CrossRef]
- Giallongo, S.; Longhitano, L.; Denaro, S.; D’aprile, S.; Torrisi, F.; La Spina, E.; Giallongo, C.; Mannino, G.; Furno, D.L.; Zappalà, A.; et al. The Role of Epigenetics in Neuroinflammatory-Driven Diseases. Int. J. Mol. Sci. 2022, 23, 15218. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Ribeiro, F.C.; Santos, L.E.; Beckman, D.; Melo, H.M.; Sudo, F.K.; Drummond, C.; Assuncao, N.; Vanderborght, B.; Tovar-Moll, F.; et al. Cerebrospinal Fluid Neurotransmitters, Cytokines, and Chemokines in Alzheimer’s and Lewy Body Diseases. J. Alzheimers Dis. 2021, 82, 1067–1074. [Google Scholar] [CrossRef]
- Leng, F.; Hinz, R.; Gentleman, S.; Hampshire, A.; Dani, M.; Brooks, D.J.; Edison, P. Neuroinflammation is independently associated with brain network dysfunction in Alzheimer’s disease. Mol. Psychiatry 2022, 28, 1303–1311. [Google Scholar] [CrossRef]
- Kamagata, K.; Andica, C.; Kato, A.; Saito, Y.; Uchida, W.; Hatano, T.; Lukies, M.; Ogawa, T.; Takeshige-Amano, H.; Akashi, T.; et al. Diffusion Magnetic Resonance Imaging-Based Biomarkers for Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 5216. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Clouston, S.A.P.; Smith, D.M. Elevated C-Reactive Protein in Alzheimer’s Disease Without Depression in Older Adults: Findings From the Health and Retirement Study. J. Gerontol. Ser. A 2021, 77, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Verma, R.; Kumar, K.; Chawla, R. Role of pro-inflammatory cytokines in Alzheimer’s disease and neuroprotective effects of pegylated self-assembled nanoscaffolds. Curr. Res. Pharmacol. Drug Discov. 2023, 4, 100149. [Google Scholar] [CrossRef]
- Gaetani, L.; Bellomo, G.; Parnetti, L.; Blennow, K.; Zetterberg, H.; Di Filippo, M. Neuroinflammation and Alzheimer’s Disease: A Machine Learning Approach to CSF Proteomics. Cells 2021, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Jang, H.; Lee, H. Potential Applications of Artificial Intelligence in Clinical Trials for Alzheimer’s Disease. Life 2022, 12, 275. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Sun, Y.; Peng, G. Neuroinflammation as a Potential Therapeutic Target in Alzheimer’s Disease. Clin. Interv. Aging 2022, 17, 665–674. [Google Scholar] [CrossRef]
- Stopschinski, B.E.; Weideman, R.A.; McMahan, D.; Jacob, D.A.; Little, B.B.; Chiang, H.-S.; Calveras, N.S.; Stuve, O. Microglia as a cellular target of diclofenac therapy in Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2023, 16, 17562864231156674. [Google Scholar] [CrossRef]
- Kasindi, A.; Fuchs, D.-T.; Koronyo, Y.; Rentsendorj, A.; Black, K.L.; Koronyo-Hamaoui, M. Glatiramer Acetate Immunomodulation: Evidence of Neuroprotection and Cognitive Preservation. Cells 2022, 11, 1578. [Google Scholar] [CrossRef]
- Di Liberto, V.; Frinchi, M.; Nuzzo, D.; Scaduto, P.; Plescia, F.; Massenti, M.F.; Di Carlo, M.; Cannizzaro, C.; Cassata, G.; Cicero, L.; et al. Anti-Inflammatory and Cognitive Effects of Interferon-Beta 1a (IFN Beta 1a) in a Rat Model of Alzheimer’s Disease. Acta Physiol. 2019, 227, 80. [Google Scholar] [CrossRef]
- Yılmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef] [PubMed]
- Althafar, Z.M. Targeting Microglia in Alzheimer’s Disease: From Molecular Mechanisms to Potential Therapeutic Targets for Small Molecules. Molecules 2022, 27, 4124. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.d.A.; Pereira-Rufino, L.d.S.; Vissoto, T.C.S.; Kerkis, I.; Neves, A.d.C.; da Silva, M.C.P. The Immunomodulatory Potential Role of Mesenchymal Stem Cells in Diseases of the Central Nervous System. Neurodegener. Dis. 2022, 22, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Wang, K.; Zhang, L.; Bai, L. Stem cell therapy for Alzheimer’s disease: An overview of experimental models and reality. Anim. Model. Exp. Med. 2022, 5, 15–26. [Google Scholar] [CrossRef]
- Aftanas, L.I.; Gevorgyan, M.M.; Zhanaeva, S.Y.; Dzemidovich, S.S.; Kulikova, K.I.; Al’perina, E.L.; Danilenko, K.V.; Idova, G.V. Therapeutic Effects of Repetitive Transcranial Magnetic Stimulation (RTMS) on Neuroinflammation and Neuroplasticity in Patients with Parkinson’s Disease: A Placebo-Controlled Study. Bull. Exp. Biol. Med. 2018, 165, 195–199. [Google Scholar] [CrossRef]
- Menardi, A.; Dotti, L.; Ambrosini, E.; Vallesi, A. Transcranial magnetic stimulation treatment in Alzheimer’s disease: A meta-analysis of its efficacy as a function of protocol characteristics and degree of personalization. J. Neurol. 2022, 269, 5283–5301. [Google Scholar] [CrossRef]
- Currais, A.; Quehenberger, O.; M Armando, A.; Daugherty, D.; Maher, P.; Schubert, D. Amyloid Proteotoxicity Initiates an Inflammatory Response Blocked by Cannabinoids. NPJ Aging Mech. Dis. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Abate, G.; Uberti, D.; Tambaro, S. Potential and Limits of Cannabinoids in Alzheimer’s Disease Therapy. Biology 2021, 10, 542. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Ashford, J.W.; Mahoney, L.; Burkett, T. A Role for Complementary and Integrative Medicine in Alzheimer’s Disease Prevention. J. Alzheimer’s Dis. 2015, 48, 13–14. [Google Scholar] [CrossRef]
- Arafah, A.; Khatoon, S.; Rasool, I.; Khan, A.; Rather, M.A.; Abujabal, K.A.; Faqih, Y.A.H.; Rashid, H.; Rashid, S.M.; Ahmad, S.B.; et al. The Future of Precision Medicine in the Cure of Alzheimer’s Disease. Biomedicines 2023, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- APMI; Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nistico, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; et al. A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer’s Disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Soares-Dos-Reis, R.; Melo, P.N.; Relvas, J.B.; Guimarães, J.; Sá, M.J.; Cruz, A.P.; Pinto, I.M. Emerging Biosensing Technologies for Neuroinflammatory and Neurodegenerative Disease Diagnostics. Front. Mol. Neurosci. 2018, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.X.; Francis, N.L.; Calvelli, H.R.; Moghe, P.V. Microglia-Targeting Nanotherapeutics for Neurodegenerative Diseases. APL Bioengineering 2020, 4, 030902. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Okur, M.E.; Erim, P.D.; Caglar, E.S.; Ozgenc, E.; Gundogdu, E.; Koprulu, R.E.P.; Karantas, I.D.; Okur, N.U. Protein and Gene Delivery Systems for Neurodegenerative Disorders: Where Do We Stand Today? Pharmaceutics 2022, 14, 2425. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Valenzuela, A.; Petit, F.; Chow, S.; Leung, K.; Gorin, F.; Louie, A.Y.; Dhenain, M. In Vivo MRI of Functionalized Iron Oxide Nanoparticles for Brain Inflammation. Contrast Media Mol. Imaging 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Zhu, F.-D.; Hu, Y.-J.; Yu, L.; Zhou, X.-G.; Wu, J.-M.; Tang, Y.; Qin, D.-L.; Fan, Q.-Z.; Wu, A.-G. Nanoparticles: A Hope for the Treatment of Inflammation in CNS. Front. Pharmacol. 2021, 12, 683935. [Google Scholar] [CrossRef]
- Cerqueira, S.R.; Ayad, N.G.; Lee, J.K. Neuroinflammation Treatment via Targeted Delivery of Nanoparticles. Front. Cell. Neurosci. 2020, 14, 576037. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. BioMedicine 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Skousen, J.L.; Bridge, M.J.; Tresco, P.A. A strategy to passively reduce neuroinflammation surrounding devices implanted chronically in brain tissue by manipulating device surface permeability. Biomaterials 2015, 36, 33–43. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Advances in nanomedicines for diagnosis of central nervous system disorders. Biomaterials 2020, 269, 120492. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Lin, H.; Yan, Z.; Shi, J.; Fan, C. Functional nanomaterials in peripheral nerve regeneration: Scaffold design, chemical principles and microenvironmental remodeling. Mater. Today 2021, 51, 165–187. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Gherasim, O.; Gherasim, T.G.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, D.M. Nanomaterial-Based Approaches for Neural Regeneration. Pharmaceutics 2019, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Ao, Q. Nanoparticles in peripheral nerve regeneration: A mini review. J. Neurorestoratol. 2022, 10, 1–12. [Google Scholar] [CrossRef]
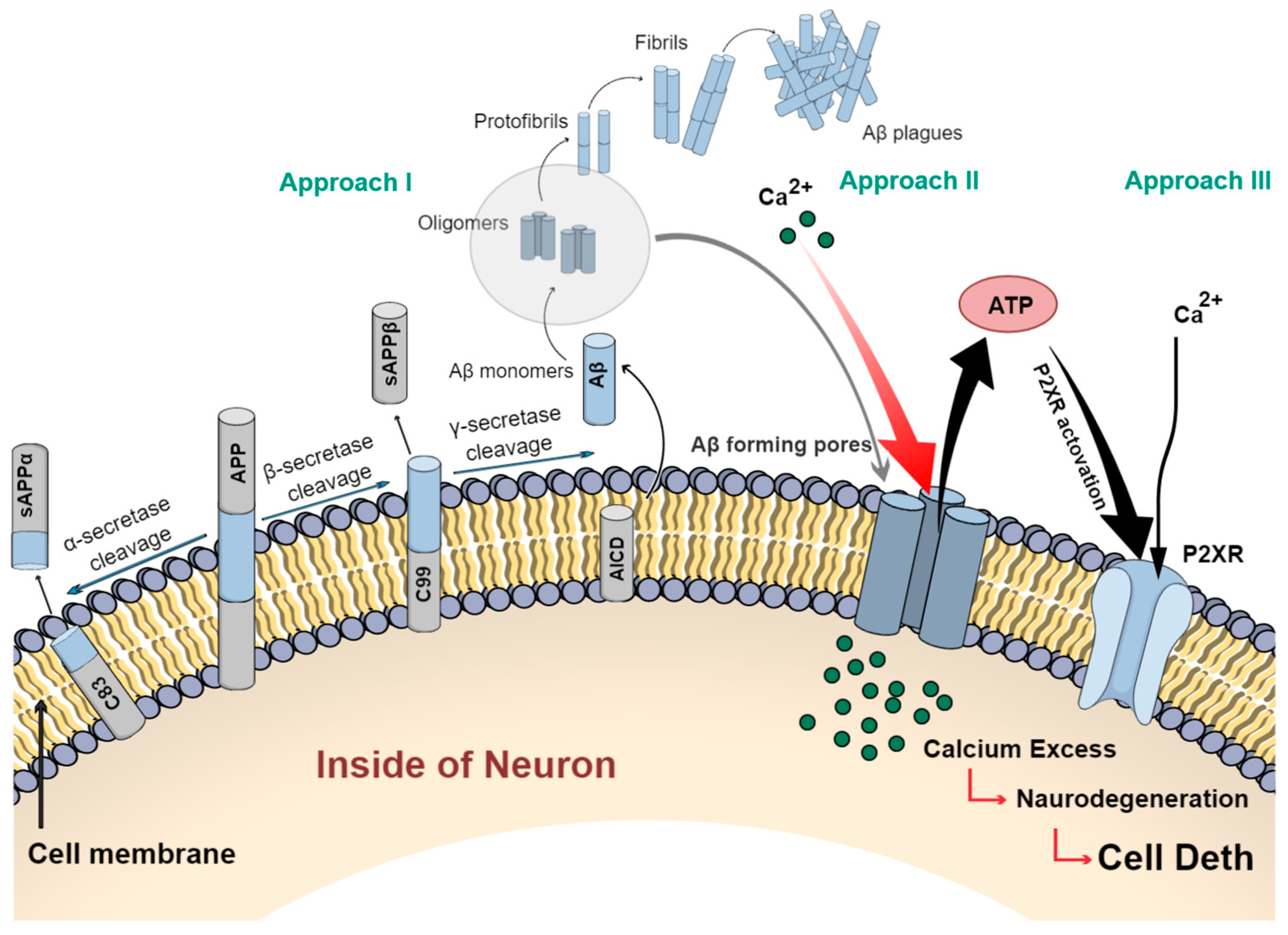
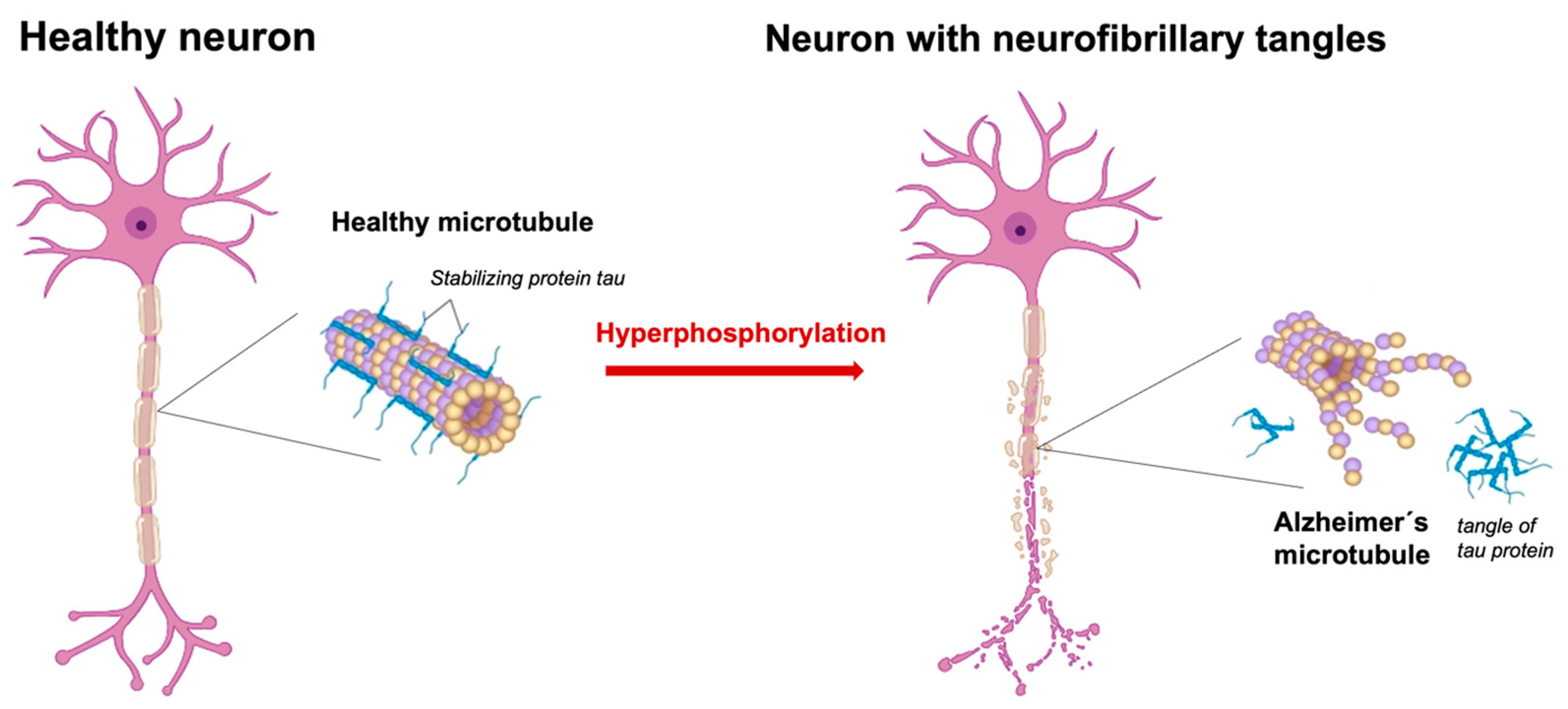
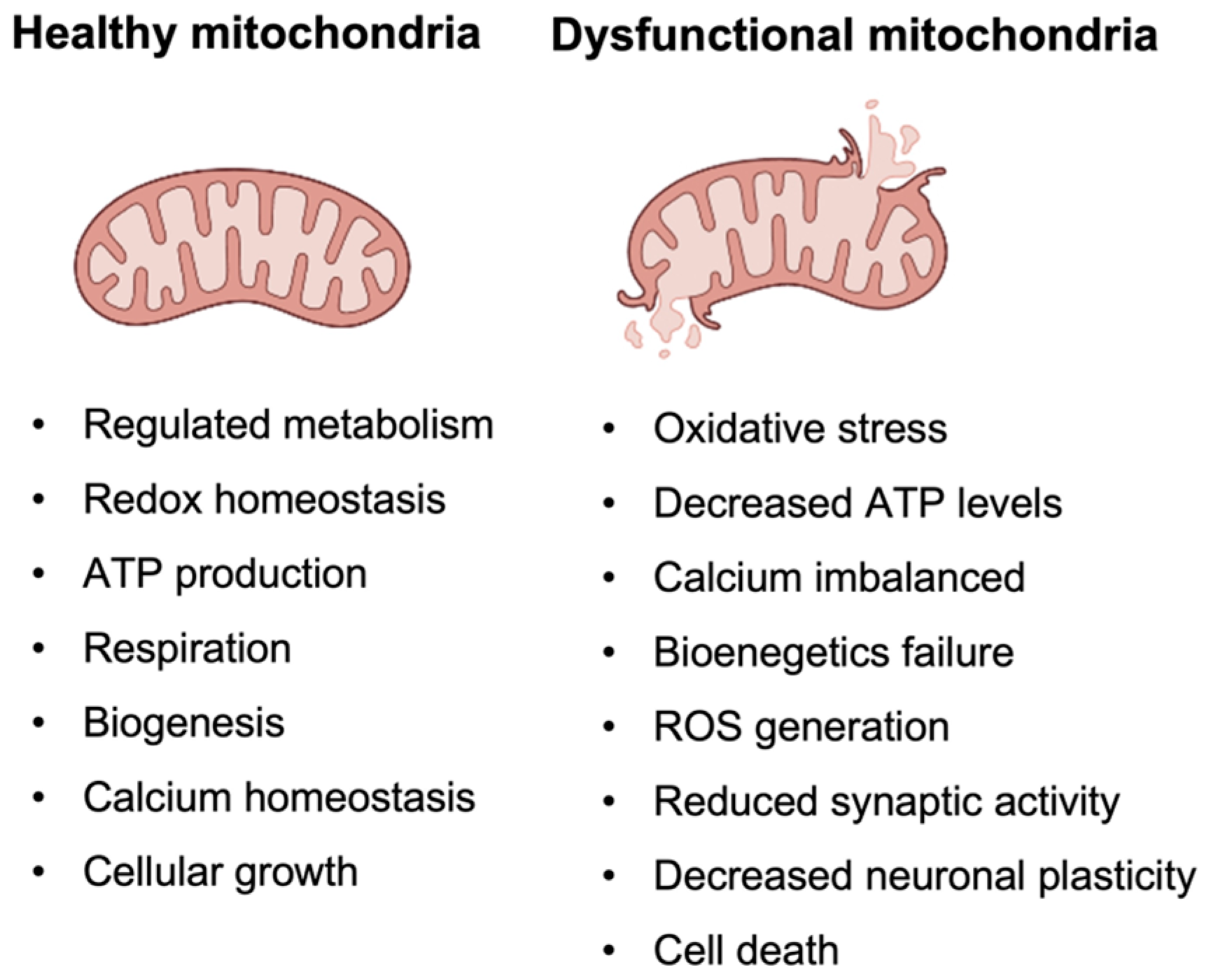
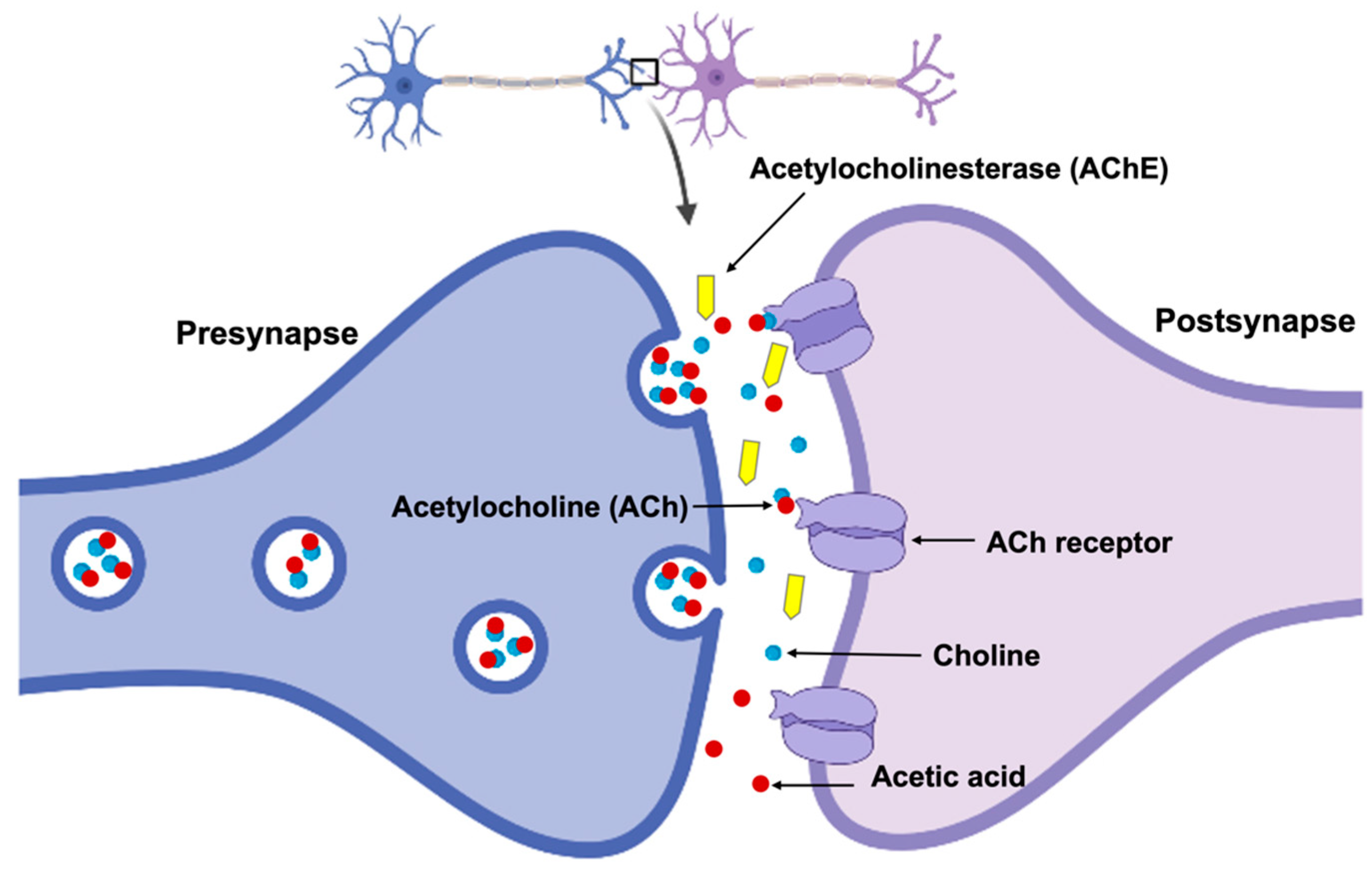
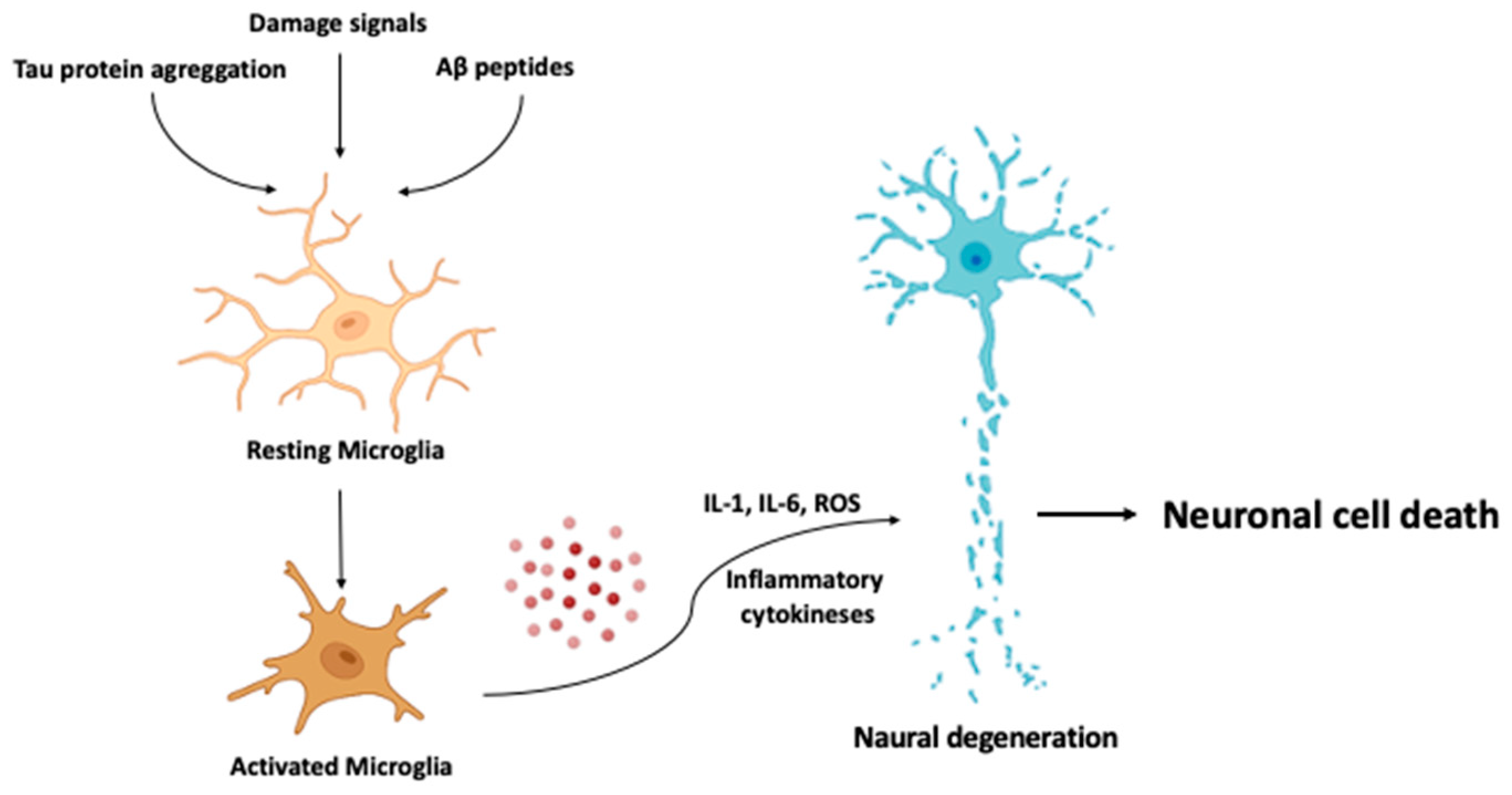
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres, C.; Heusser, B.; Garnham, A.; Moczko, E. The Major Hypotheses of Alzheimer’s Disease: Related Nanotechnology-Based Approaches for Its Diagnosis and Treatment. Cells 2023, 12, 2669. https://doi.org/10.3390/cells12232669
Cáceres C, Heusser B, Garnham A, Moczko E. The Major Hypotheses of Alzheimer’s Disease: Related Nanotechnology-Based Approaches for Its Diagnosis and Treatment. Cells. 2023; 12(23):2669. https://doi.org/10.3390/cells12232669
Chicago/Turabian StyleCáceres, César, Bernardita Heusser, Alexandra Garnham, and Ewa Moczko. 2023. "The Major Hypotheses of Alzheimer’s Disease: Related Nanotechnology-Based Approaches for Its Diagnosis and Treatment" Cells 12, no. 23: 2669. https://doi.org/10.3390/cells12232669
APA StyleCáceres, C., Heusser, B., Garnham, A., & Moczko, E. (2023). The Major Hypotheses of Alzheimer’s Disease: Related Nanotechnology-Based Approaches for Its Diagnosis and Treatment. Cells, 12(23), 2669. https://doi.org/10.3390/cells12232669






