Cornerstone Cellular Pathways for Metabolic Disorders and Diabetes Mellitus: Non-Coding RNAs, Wnt Signaling, and AMPK
Abstract
:1. The Impact of Metabolic Disorders in the Global Population
2. Lifespan, Aging, Obesity, and Socioeconomic Status Can Impact Metabolic Disorders
3. The Need for Clinical Innovation for the Treatment of Metabolic Disease and Diabetes Mellitus
4. Cellular Mechanisms of Oxidative Stress, Energy Metabolism, and Programmed Cell Death with Metabolic Disorders
5. Non-Coding RNAs, MicroRNAs, and Circular RNAs in Metabolic Disorders
6. Wnt Signaling and WISP1 Oversight in Diabetes Mellitus and Metabolic Disorders
7. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- International Diabetes Federation. Diabetes. In IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Maiese, K. Innovative therapeutic strategies for cardiovascular disease. EXCLI J. 2023, 22, 690–715. [Google Scholar] [CrossRef] [PubMed]
- Barcena, M.L.; Tonini, G.; Haritonow, N.; Breiter, P.; Milting, H.; Baczko, I.; Muller-Werdan, U.; Ladilov, Y.; Regitz-Zagrosek, V. Sex and age differences in AMPK phosphorylation, mitochondrial homeostasis, and inflammation in hearts from inflammatory cardiomyopathy patients. Aging Cell 2023, 22, e13894. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.E.; Khosla, S.; Baur, J.A.; de Cabo, R.; Musi, N. Drugs Targeting Mechanisms of Aging to Delay Age-Related Disease and Promote Healthspan: Proceedings of a National Institute on Aging Workshop. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78 (Suppl. S1), 53–60. [Google Scholar] [CrossRef] [PubMed]
- Raghuvanshi, D.S.; Chakole, S.; Kumar, M. Relationship Between Vitamins and Diabetes. Cureus 2023, 15, e36815. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, J.; Zhou, M. A comprehensive meta-analysis on the association between vitamin C intake and gestational diabetes mellitus: Insights and novel perspectives. Medicine 2023, 102, e34740. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. New Insights for nicotinamide: Metabolic disease, autophagy, and mTOR. Front. Biosci. (Landmark Ed.) 2020, 25, 1925–1973. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Cellular Metabolism: A Fundamental Component of Degeneration in the Nervous System. Biomolecules 2023, 13, 816. [Google Scholar] [CrossRef]
- Jiang, W.; Ding, K.; Yue, R.; Lei, M. Therapeutic effects of icariin and icariside II on diabetes mellitus and its complications. In Critical Reviews in Food Science and Nutrition; Taylor Francis Group: Abingdon, UK, 2023; pp. 1–26. [Google Scholar] [CrossRef]
- Raut, S.K.; Khullar, M. Oxidative stress in metabolic diseases: Current scenario and therapeutic relevance. Mol. Cell. Biochem. 2023, 478, 185–196. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; pp. 1–83. [Google Scholar]
- World Health Organization. Description of the global burden of NCDs, their risk factors and determinants. Glob. Status Rep. Noncommun. Dis. 2010, 2011, 1–176. [Google Scholar]
- Mahmoudi, N.; Kiasalari, Z.; Rahmani, T.; Sanaierad, A.; Afshin-Majd, S.; Naderi, G.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin Attenuates Cognitive Impairment in Streptozotocin-Induced Diabetic Rats: Underlying Mechanisms. Neuropsychobiology 2021, 80, 25–35. [Google Scholar] [CrossRef]
- Maiese, K. Prospects and Perspectives for WISP1 (CCN4) in Diabetes Mellitus. Curr. Neurovasc. Res. 2020, 17, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Min, A.Y.; Yoo, J.M.; Sok, D.E.; Kim, M.R. Mulberry Fruit Prevents Diabetes and Diabetic Dementia by Regulation of Blood Glucose through Upregulation of Antioxidative Activities and CREB/BDNF Pathway in Alloxan-Induced Diabetic Mice. Oxid. Med. Cell. Longev. 2020, 2020, 1298691. [Google Scholar] [CrossRef] [PubMed]
- Swain, O.; Romano, S.K.; Miryala, R.; Tsai, J.; Parikh, V.; Umanah, G.K.E. SARS-CoV-2 Neuronal Invasion and Complications: Potential Mechanisms and Therapeutic Approaches. J. Neurosci. 2021, 41, 5338–5349. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, J.; Li, X.R.; Yu, Y.; Luo, X.; Zheng, X.; Cheng, Y.; Yu, P.Q.; Liu, Y. The mTOR/NF-kappaB Pathway Mediates Neuroinflammation and Synaptic Plasticity in Diabetic Encephalopathy. Mol. Neurobiol. 2021, 58, 3848–3862. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Ota, T.; Mizukoshi, E.; Nakamura, H.; Yamamoto, Y.; Kikuchi, M.; Yamashita, T.; Kaneko, S. Intake of omega-6 Polyunsaturated Fatty Acid-Rich Vegetable Oils and Risk of Lifestyle Diseases. Adv. Nutr. 2020, 11, 1489–1509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, Y.; Zhang, J.; Zhao, Y.; Zhang, Y.; Fu, J. Astragaloside IV supplementation attenuates cognitive impairment by inhibiting neuroinflammation and oxidative stress in type 2 diabetic mice. Front. Aging Neurosci. 2022, 14, 1004557. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yang, S.J. Supplementation with Nicotinamide Riboside Reduces Brain Inflammation and Improves Cognitive Function in Diabetic Mice. Int. J. Mol. Sci. 2019, 20, 4196. [Google Scholar] [CrossRef]
- Prattichizzo, F.; De Nigris, V.; Spiga, R.; Mancuso, E.; La Sala, L.; Antonicelli, R.; Testa, R.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Inflammageing and metaflammation: The yin and yang of type 2 diabetes. Ageing Res. Rev. 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Su, M.; Naderi, K.; Samson, N.; Youssef, I.; Fulop, L.; Bozso, Z.; Laroche, S.; Delatour, B.; Davis, S. Mechanisms Associated with Type 2 Diabetes as a Risk Factor for Alzheimer-Related Pathology. Mol. Neurobiol. 2019, 56, 5815–5834. [Google Scholar] [CrossRef]
- Bahorik, A.; Bobrow, K.; Hoang, T.; Yaffe, K. Increased risk of dementia in older female US veterans with alcohol use disorder. Addiction 2021, 116, 2049–2055. [Google Scholar] [CrossRef]
- Ciardullo, S.; Muraca, E.; Bianconi, E.; Cannistraci, R.; Perra, S.; Zerbini, F.; Perseghin, G. Diabetes Mellitus is Associated With Higher Serum Neurofilament Light Chain Levels in the General US Population. J. Clin. Endocrinol. Metab. 2023, 108, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Engin, A. Alzheimer’s Disease and Protein Kinases. Adv. Exp. Med. Biol. 2021, 1275, 285–321. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.F.; Liu, C.K.; Lee, C.T.; Yu, L.E.; Wang, J.Y. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci. Rep. 2019, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Cognitive impairment with diabetes mellitus and metabolic disease: Innovative insights with the mechanistic target of rapamycin and circadian clock gene pathways. Expert. Rev. Clin. Pharmacol. 2020, 13, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Dysregulation of metabolic flexibility: The impact of mTOR on autophagy in neurodegenerative disease. Int. Rev. Neurobiol. 2020, 155, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Stolk, R.P.; van Harskamp, F.; Pols, H.A.; Hofman, A.; Breteler, M.M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999, 53, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Y.; Qin, L.F.; Xu, Z.G.; Gao, X.R.; Liu, C.B.; Xu, G.T.; Chen, Y.Z. Comprehensive Bibliometric Analysis of Stem Cell Research in Alzheimer’s Disease from 2004 to 2022. Dement. Geriatr. Cogn. Disord. 2023, 52, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Arildsen, L.; Andersen, J.V.; Waagepetersen, H.S.; Nissen, J.B.D.; Sheykhzade, M. Hypermetabolism and impaired endothelium-dependent vasodilation in mesenteric arteries of type 2 diabetes mellitus db/db mice. Diabetes Vasc. Dis. Res. Off. J. Int. Soc. Diabetes Vasc. Dis. 2019, 16, 539–548. [Google Scholar] [CrossRef]
- Bayaraa, O.; Inman, C.K.; Thomas, S.A.; Al Jallaf, F.; Alshaikh, M.; Idaghdour, Y.; Ashall, L. Hyperglycemic conditions induce rapid cell dysfunction-promoting transcriptional alterations in human aortic endothelial cells. Sci. Rep. 2022, 12, 20912. [Google Scholar] [CrossRef]
- Feng, J.; Wang, H.; Jing, Z.; Wang, Y.; Cheng, Y.; Wang, W.; Sun, W. Role of Magnesium in Type 2 Diabetes Mellitus. Biol. Trace Elem. Res. 2020, 196, 74–85. [Google Scholar] [CrossRef]
- Januszewski, A.S.; Watson, C.J.; O’Neill, V.; McDonald, K.; Ledwidge, M.; Robson, T.; Jenkins, A.J.; Keech, A.C.; McClements, L. FKBPL is associated with metabolic parameters and is a novel determinant of cardiovascular disease. Sci. Rep. 2020, 10, 21655. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, J.; Wu, Y.; Xi, W.; Wei, Y.; Yuan, Z.; Zhuo, X. Zinc supplementation protects against diabetic endothelial dysfunction via GTP cyclohydrolase 1 restoration. Biochem. Biophys. Res. Commun. 2020, 521, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Mitter, S.K.; Yan, Y.; Busik, J.V.; Grant, M.B.; Boulton, M.E. Diurnal Rhythmicity of Autophagy Is Impaired in the Diabetic Retina. Cells 2020, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-de la Torre, R.; Garcia-Fontana, C.; Gonzalez-Salvatierra, S.; Andujar-Vera, F.; Martinez-Heredia, L.; Garcia-Fontana, B.; Munoz-Torres, M. The Contribution of Wnt Signaling to Vascular Complications in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 6995. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, D.; Tsubota, K.; Kurihara, T. PPARalpha Agonist Oral Therapy in Diabetic Retinopathy. Biomedicines 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Burillo, J.; Marques, P.; Jimenez, B.; Gonzalez-Blanco, C.; Benito, M.; Guillen, C. Insulin Resistance and Diabetes Mellitus in Alzheimer’s Disease. Cells 2021, 10, 1236. [Google Scholar] [CrossRef]
- Geng, K.; Ma, X.; Jiang, Z.; Huang, W.; Gao, C.; Pu, Y.; Luo, L.; Xu, Y.; Xu, Y. Innate Immunity in Diabetic Wound Healing: Focus on the Mastermind Hidden in Chronic Inflammatory. Front. Pharmacol. 2021, 12, 653940. [Google Scholar] [CrossRef]
- Maiese, K. Nicotinamide: Oversight of Metabolic Dysfunction Through SIRT1, mTOR, and Clock Genes. Curr. Neurovasc. Res. 2020, 17, 765–783. [Google Scholar] [CrossRef]
- Mocayar Maron, F.J.; Ferder, L.; Reiter, R.J.; Manucha, W. Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J. Steroid Biochem. Mol. Biol. 2020, 199, 105595. [Google Scholar] [CrossRef]
- Rotllan, N.; Camacho, M.; Tondo, M.; Diarte-Anazco, E.M.G.; Canyelles, M.; Mendez-Lara, K.A.; Benitez, S.; Alonso, N.; Mauricio, D.; Escola-Gil, J.C.; et al. Therapeutic Potential of Emerging NAD+-Increasing Strategies for Cardiovascular Diseases. Antioxidants 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Wasserfurth, P.; Nebl, J.; Ruhling, M.R.; Shammas, H.; Bednarczyk, J.; Koehler, K.; Bosslau, T.K.; Kruger, K.; Hahn, A.; Das, A.M. Impact of Dietary Modifications on Plasma Sirtuins 1, 3 and 5 in Older Overweight Individuals Undergoing 12-Weeks of Circuit Training. Nutrients 2021, 13, 3824. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Suo, H.; Song, J. Protective role of mitoquinone against impaired mitochondrial homeostasis in metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2021, 61, 3857–3875. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, C.; Zhu, S.Y.; Zou, H.C.; Xu, C.Y.; Chen, Y.X. Overexpression of HOTAIR attenuates Pi-induced vascular calcification by inhibiting Wnt/beta-catenin through regulating miR-126/Klotho/SIRT1 axis. Mol. Cell. Biochem. 2021, 476, 3551–3561. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, R.; Wu, X.; Chen, Y.; Ji, W.; Wang, J.; Zhang, Y.; Xia, Y.; Tang, Y.; Yuan, J. The Wnt Signaling Pathway in Diabetic Nephropathy. Front. Cell Dev. Biol. 2021, 9, 701547. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. Novel directions for diabetes mellitus drug discovery. Expert. Opin. Drug Discov. 2013, 8, 35–48. [Google Scholar] [CrossRef] [PubMed]
- McCoin, C.S.; Franczak, E.; Deng, F.; Pei, D.; Ding, W.X.; Thyfault, J.P. Acute exercise rapidly activates hepatic mitophagic flux. J. Appl. Physiol. 2022, 132, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.M.O.; Agostini, L.D.C.; Lima, W.G.; Camini, F.C.; Costa, D.C. Silymarin Attenuates Hepatic and Pancreatic Redox Imbalance Independent of Glycemic Regulation in the Alloxan-induced Diabetic Rat Model. Biomed. Environ. Sci. 2020, 33, 690–700. [Google Scholar] [CrossRef]
- Peng, S.; Li, W.; Hou, N.; Huang, N. A Review of FoxO1-Regulated Metabolic Diseases and Related Drug Discoveries. Cells 2020, 9, 184. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, S.; Zhang, X.; Chen, L. Targeting PRAS40: A novel therapeutic strategy for human diseases. J. Drug Target. 2021, 29, 703–715. [Google Scholar] [CrossRef]
- Dutta, R.K.; Jun, J.; Du, K.; Diehl, A.M. Hedgehog Signaling: Implications in Liver Pathophysiology. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: New York, NY, USA, 2023. [Google Scholar]
- Fan, X.; Zhao, Z.; Wang, D.; Xiao, J. Glycogen synthase kinase-3 as a key regulator of cognitive function. Acta Biochim. Biophys. Sin. 2020, 52, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Jiao, R.; Wang, P.; Zhu, Y.; Zhao, J.; De Jager, P.; Bennett, D.A.; Jin, L.; Xiong, M. Shared Causal Paths underlying Alzheimer’s dementia and Type 2 Diabetes. Sci. Rep. 2020, 10, 4107. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Tundis, R.; Ullah, H.; Aschner, M.; Belwal, T.; Mirzaei, H.; Akkol, E.K. Flavonoids targeting NRF2 in neurodegenerative disorders. Food Chem. Toxicol. 2020, 146, 111817. [Google Scholar] [CrossRef] [PubMed]
- Sonsalla, M.M.; Lamming, D.W. Geroprotective interventions in the 3xTg mouse model of Alzheimer’s disease. Geroscience 2023, 45, 1343–1381. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. The Metabolic Basis for Nervous System Dysfunction in Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease. Curr. Neurovasc. Res. 2023, 20, 314–333. [Google Scholar] [CrossRef] [PubMed]
- Palleria, C.; Leporini, C.; Maida, F.; Succurro, E.; De Sarro, G.; Arturi, F.; Russo, E. Potential effects of current drug therapies on cognitive impairment in patients with type 2 diabetes. Front. Neuroendocr. 2016, 42, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, Y.; Zhang, Y.; Wang, W.; Ye, C. Mutant Huntingtin Impairs Pancreatic beta-cells by Recruiting IRS-2 and Disturbing the PI3K/AKT/FoxO1 Signaling Pathway in Huntington’s Disease. J. Mol. Neurosci. 2021, 71, 2646–2658. [Google Scholar] [CrossRef]
- Fischer, F.; Grigolon, G.; Benner, C.; Ristow, M. Evolutionarily conserved transcription factors as regulators of longevity and targets for geroprotection. Physiol. Rev. 2022, 102, 1449–1494. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Q.; Gao, W.; Li, B.Y.; Zeng, C.; Xia, Z.; Zhao, B. Melatonin ameliorates cerebral ischemia-reperfusion injury in diabetic mice by enhancing autophagy via the SIRT1-BMAL1 pathway. FASEB J. 2021, 35, e22040. [Google Scholar] [CrossRef]
- Chiareli, R.A.; Carvalho, G.A.; Marques, B.L.; Mota, L.S.; Oliveira-Lima, O.C.; Gomes, R.M.; Birbrair, A.; Gomez, R.S.; Simao, F.; Klempin, F.; et al. The Role of Astrocytes in the Neurorepair Process. Front. Cell Dev. Biol. 2021, 9, 665795. [Google Scholar] [CrossRef]
- El-Marasy, S.A.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M. Neuroprotective effect of vildagliptin against cerebral ischemia in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.R.; Lomba, G.S.B.; Goncalves-de-Albuquerque, C.F.; Burth, P. Irisin, Exercise, and COVID-19. Front. Endocrinol. 2022, 13, 879066. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Beckman, K.B.; Mehta, T.; Karger, A.B.; Odde, D.J.; Tignanelli, C.J.; Buse, J.B.; Johnson, D.M.; Watson, R.H.B.; Daniel, J.J.; et al. Metformin reduces SARS-CoV-2 in a Phase 3 Randomized Placebo Controlled Clinical Trial. medRxiv 2023. [Google Scholar] [CrossRef]
- Fadini, G.P.; Morieri, M.L.; Longato, E.; Avogaro, A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Investig. 2020, 43, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Heer, C.D.; Sanderson, D.J.; Voth, L.S.; Alhammad, Y.M.O.; Schmidt, M.S.; Trammell, S.A.J.; Perlman, S.; Cohen, M.S.; Fehr, A.R.; Brenner, C. Coronavirus infection and PARP expression dysregulate the NAD metabolome: An actionable component of innate immunity. J. Biol. Chem. 2020, 295, 17986–17996. [Google Scholar] [CrossRef] [PubMed]
- Lally, M.A.; Tsoukas, P.; Halladay, C.W.; O’Neill, E.; Gravenstein, S.; Rudolph, J.L. Metformin is Associated with Decreased 30-Day Mortality Among Nursing Home Residents Infected with SARS-CoV2. J. Am. Med. Dir. Assoc. 2021, 22, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. The Mechanistic Target of Rapamycin (mTOR): Novel Considerations as an Antiviral Treatment. Curr. Neurovasc. Res. 2020, 17, 332–337. [Google Scholar] [CrossRef]
- Miller, R.; Wentzel, A.R.; Richards, G.A. COVID-19: NAD(+) deficiency may predispose the aged, obese and type2 diabetics to mortality through its effect on SIRT1 activity. Med. Hypotheses 2020, 144, 110044. [Google Scholar] [CrossRef]
- Pinchera, B.; Scotto, R.; Buonomo, A.R.; Zappulo, E.; Stagnaro, F.; Gallicchio, A.; Viceconte, G.; Sardanelli, A.; Mercinelli, S.; Villari, R.; et al. Diabetes and COVID-19: The potential role of mTOR. Diabetes Res. Clin. Pr. 2022, 186, 109813. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, Y.; Liang, Y.; Huang, H.; Xu, Y.; Zhong, C. Circular RNAs in Vascular Functions and Diseases. Adv. Exp. Med. Biol. 2018, 1087, 287–297. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, H.; Yu, P.; Qian, T.; Xu, X. Protective or Harmful: The Dual Roles of Autophagy in Diabetic Retinopathy. Front. Med. 2021, 8, 644121. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural Regen. Res. 2015, 10, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.F.; Li, N.; Tang, Y.L.; Yan, C.Q.; Shi, Z.; Yi, S.N.; Zhou, H.L.; Liao, D.F.; OuYang, X.P. Nicotinate-curcumin ameliorates cognitive impairment in diabetic rats by rescuing autophagic flux in CA1 hippocampus. CNS Neurosci. Ther. 2019, 25, 430–441. [Google Scholar] [CrossRef]
- Hu, R.; Zhu, X.; Yuan, M.; Ho, K.H.; Kaverina, I.; Gu, G. Microtubules and Galphao-signaling modulate the preferential secretion of young insulin secretory granules in islet beta cells via independent pathways. PLoS ONE 2021, 16, e0241939. [Google Scholar] [CrossRef]
- Kita, A.; Saito, Y.; Miura, N.; Miyajima, M.; Yamamoto, S.; Sato, T.; Yotsuyanagi, T.; Fujimiya, M.; Chikenji, T.S. Altered regulation of mesenchymal cell senescence in adipose tissue promotes pathological changes associated with diabetic wound healing. Commun. Biol. 2022, 5, 310. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2015, 2015, 875961. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Nicotinamide as a Foundation for Treating Neurodegenerative Disease and Metabolic Disorders. Curr. Neurovasc. Res. 2021, 18, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. FoxO proteins in the nervous system. Anal. Cell. Pathol. (Amst.) 2015, 2015, 569392. [Google Scholar] [CrossRef]
- O’Donnell, B.T.; Monjure, T.A.; Al-Ghadban, S.; Ives, C.J.; L’Ecuyer, M.P.; Rhee, C.; Romero-Lopez, M.; Li, Z.; Goodman, S.B.; Lin, H.; et al. Aberrant Expression of COX-2 and FOXG1 in Infrapatellar Fat Pad-Derived ASCs from Pre-Diabetic Donors. Cells 2022, 11, 2367. [Google Scholar] [CrossRef]
- Gutierrez-Pliego, L.E.; Martinez-Carrillo, B.E.; Resendiz-Albor, A.A.; Valdes-Ramos, R. Effect on Adipose Tissue of Diabetic Mice Supplemented with n-3 Fatty Acids Extracted from Microalgae. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 728–735. [Google Scholar] [CrossRef]
- Kahmini, F.R.; Ghaleh, H.D.; Shahgaldi, S. Sirtuins: Subtle Regulators Involved in Convoluted Mechanisms of Pregnancy. Cell. Physiol. Biochem. 2022, 56, 644–662. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Vaziri, N.D.; Swentek, L.; Takasu, C.; Vo, K.; Stamos, M.J.; Ricordi, C.; Ichii, H. Prevention of Autoimmune Diabetes in NOD Mice by Dimethyl Fumarate. Antioxid. (Basel Switz.) 2021, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C. OutFOXOing disease and disability: The therapeutic potential of targeting FoxO proteins. Trends Mol. Med. 2008, 14, 219–227. [Google Scholar] [CrossRef]
- Rashidi, S.; Mansouri, R.; Ali-Hassanzadeh, M.; Mojtahedi, Z.; Shafiei, R.; Savardashtaki, A.; Hamidizadeh, N.; Karimazar, M.; Nguewa, P.; Manzano-Roman, R. The host mTOR pathway and parasitic diseases pathogenesis. Parasitol. Res. 2021, 120, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Yu, T.Y.; Jiang, F.X.; Wang, W. Functional maturation of immature beta cells: A roadblock for stem cell therapy for type 1 diabetes. World J. Stem Cells 2021, 13, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Jiang, W.; Zhou, L. Islet Autoantibodies in the Patients with Sjogren’s Syndrome and Thyroid Disease and Risk of Progression to Latent Autoimmune Diabetes in Adults: A Case Series. Diabetes Metab. Syndr. Obes. 2021, 14, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Hajibabaie, F.; Abedpoor, N.; Safavi, K.; Taghian, F. Natural remedies medicine derived from flaxseed (secoisolariciresinol diglucoside, lignans, and alpha-linolenic acid) improve network targeting efficiency of diabetic heart conditions based on computational chemistry techniques and pharmacophore modeling. J. Food Biochem. 2022, 46, e14480. [Google Scholar] [CrossRef]
- Liu, J.J.; Shentu, L.M.; Ma, N.; Wang, L.Y.; Zhang, G.M.; Sun, Y.; Wang, Y.; Li, J.; Mu, Y.L. Inhibition of NF-kappaB and Wnt/beta-catenin/GSK3beta Signaling Pathways Ameliorates Cardiomyocyte Hypertrophy and Fibrosis in Streptozotocin (STZ)-induced Type 1 Diabetic Rats. Curr. Med. Sci. 2020, 40, 35–47. [Google Scholar] [CrossRef]
- Pabel, S.; Hamdani, N.; Luedde, M.; Sossalla, S. SGLT2 Inhibitors and Their Mode of Action in Heart Failure-Has the Mystery Been Unravelled? Curr. Heart Fail. Rep. 2021, 18, 315–328. [Google Scholar] [CrossRef]
- Tong, J.; Lai, Y.; Yao, Y.A.; Wang, X.J.; Shi, Y.S.; Hou, H.J.; Gu, J.Y.; Chen, F.; Liu, X.B. Qiliqiangxin Rescues Mouse Cardiac Function by Regulating AGTR1/TRPV1-Mediated Autophagy in STZ-Induced Diabetes Mellitus. Cell. Physiol. Biochem. 2018, 47, 1365–1376. [Google Scholar] [CrossRef]
- Xue, P.; Zhao, J.; Zheng, A.; Li, L.; Chen, H.; Tu, W.; Zhang, N.; Yu, Z.; Wang, Q.; Gu, M. Chrysophanol alleviates myocardial injury in diabetic db/db mice by regulating the SIRT1/HMGB1/NF-kappaB signaling pathway. Exp. Ther. Med. 2019, 18, 4406–4412. [Google Scholar] [CrossRef] [PubMed]
- Centers for Medicare and Medicaid Services. National Health Expenditure Projections 2018–2027. 2019. Available online: www.cms.gov (accessed on 7 September 2023).
- Hill, J.H.; Solt, C.; Foster, M.T. Obesity associated disease risk: The role of inherent differences and location of adipose depots. Horm. Mol. Biol. Clin. Investig. 2018, 33, 20180012. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wei, X.; Ma, H.; Fan, L.; Chen, W.D. The complex role of Wnt ligands in type 2 diabetes mellitus and related complications. J. Cell. Mol. Med. 2021, 25, 6479–6495. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Zang, G.; Wang, Y.; Sun, Z.; Li, Y.; Lu, C.; Wang, Z. Differences of Angiogenesis Factors in Tumor and Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2021, 14, 3375–3388. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M. circRNAs Signature as Potential Diagnostic and Prognostic Biomarker for Diabetes Mellitus and Related Cardiovascular Complications. Cells 2020, 9, 659. [Google Scholar] [CrossRef]
- Zarneshan, S.N.; Fakhri, S.; Farzaei, M.H.; Khan, H.; Saso, L. Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem. Toxicol. 2020, 145, 111714. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.I.; Eastman, R.C. Early detection of undiagnosed diabetes mellitus: A US perspective. Diabetes Metab. Res. Rev. 2000, 16, 230–236. [Google Scholar] [CrossRef]
- Maiese, K. Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural Regen. Res. 2016, 11, 372–385. [Google Scholar] [CrossRef]
- Lathe, R.; St Clair, D. Programmed ageing: Decline of stem cell renewal, immunosenescence, and Alzheimer’s disease. Biol. Rev. Camb. Philos. Soc. 2023, 98, 1424–1458. [Google Scholar] [CrossRef]
- Maiese, K. Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer. Curr. Neurovasc. Res. 2017, 14, 299–304. [Google Scholar] [CrossRef]
- Maiese, K. The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): Oversight for neurodegenerative disorders. Biochem. Soc. Trans. 2018, 46, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Ministrini, S.; Puspitasari, Y.M.; Beer, G.; Liberale, L.; Montecucco, F.; Camici, G.G. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Front. Physiol. 2021, 12, 733696. [Google Scholar] [CrossRef] [PubMed]
- Odnokoz, O.; Nakatsuka, K.; Wright, C.; Castellanos, J.; Klichko, V.I.; Kretzschmar, D.; Orr, W.C.; Radyuk, S.N. Mitochondrial Redox Signaling Is Critical to the Normal Functioning of the Neuronal System. Front. Cell Dev. Biol. 2021, 9, 613036. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, H.; Wang, B.; Zhang, Y.; Zheng, X.; Shao, B.; Zhuge, Q.; Jin, K. Key Signaling Pathways in Aging and Potential Interventions for Healthy Aging. Cells 2021, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics. National Center for Health Statistics. National Vital Statisitcs System. In National Center for Health Statistics Fact Sheet; National Center for Health Statistics: Hyattsville, MD, USA, 2019; pp. 1–2. [Google Scholar]
- Maiese, K. Targeting the core of neurodegeneration: FoxO, mTOR, and SIRT1. Neural Regen. Res. 2021, 16, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, O.; Strilbytska, O.; Piskovatska, V.; Storey, K.B.; Koliada, A.; Vaiserman, A. The role of the TOR pathway in mediating the link between nutrition and longevity. Mech. Ageing Dev. 2017, 164, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Cogger, V.C.; Solon-Biet, S.M.; Waern, R.V.; Gokarn, R.; Pulpitel, T.; Cabo, R.; Mattson, M.P.; Raubenheimer, D.; Simpson, S.J.; et al. Nutritional strategies to optimise cognitive function in the aging brain. Ageing Res. Rev. 2016, 31, 80–92. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shimomura, N.; Oura, K.; Hasegawa, Y. Nacre Extract from Pearl Oyster Shell Prevents D-Galactose-Induced Brain and Skin Aging. Mar Biotechnol (NY) 2023, 25, 503–518. [Google Scholar] [CrossRef]
- Maiese, K.; Li, F.; Chong, Z.Z.; Shang, Y.C. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol. Ther. 2008, 118, 58–81. [Google Scholar] [CrossRef]
- Prattichizzo, F.; De Nigris, V.; La Sala, L.; Procopio, A.D.; Olivieri, F.; Ceriello, A. “Inflammaging” as a Druggable Target: A Senescence-Associated Secretory Phenotype-Centered View of Type 2 Diabetes. Oxid. Med. Cell. Longev. 2016, 2016, 1810327. [Google Scholar] [CrossRef]
- Cardoso, S.; Lopez, I.P.; Pineiro-Hermida, S.; Pichel, J.G.; Moreira, P.I. IGF1R Deficiency Modulates Brain Signaling Pathways and Disturbs Mitochondria and Redox Homeostasis. Biomedicines 2021, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Ferrara-Romeo, I.; Martinez, P.; Saraswati, S.; Whittemore, K.; Grana-Castro, O.; Thelma Poluha, L.; Serrano, R.; Hernandez-Encinas, E.; Blanco-Aparicio, C.; Maria Flores, J.; et al. The mTOR pathway is necessary for survival of mice with short telomeres. Nat. Commun. 2020, 11, 1168. [Google Scholar] [CrossRef] [PubMed]
- Kuan, X.Y.; Fauzi, N.S.A.; Ng, K.Y.; Bakhtiar, A. Exploring the Causal Relationship Between Telomere Biology and Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 4169–4183. [Google Scholar] [CrossRef] [PubMed]
- Topiwala, A.; Nichols, T.E.; Williams, L.Z.J.; Robinson, E.C.; Alfaro-Almagro, F.; Taschler, B.; Wang, C.; Nelson, C.P.; Miller, K.L.; Codd, V.; et al. Telomere length and brain imaging phenotypes in UK Biobank. PLoS ONE 2023, 18, e0282363. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.Y.; Webster, C.; Kumari, S.; Gallacher, J.E.J.; Sarkar, C. The associations of socioeconomic status with incident dementia and Alzheimer’s disease are modified by leucocyte telomere length: A population-based cohort study. Sci. Rep. 2023, 13, 6163. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. The Implications of Telomere Length: Advanced Aging, Cell Senescence, MRI Phenotypes, Stem Cells and Alzheimer’s Disease. Curr. Neurovasc. Res. 2023, 20, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kim, H.W.; Matsu-ura, K.; Wang, Y.G.; Xu, M.; Ashraf, M. Abrogation of Age-Induced MicroRNA-195 Rejuvenates the Senescent Mesenchymal Stem Cells by Reactivating Telomerase. Stem Cells 2016, 34, 148–159. [Google Scholar] [CrossRef]
- Begum, M.K.; Konja, D.; Singh, S.; Chlopicki, S.; Wang, Y. Endothelial SIRT1 as a Target for the Prevention of Arterial Aging: Promises and Challenges. J. Cardiovasc. Pharmacol. 2021, 78, S63–S77. [Google Scholar] [CrossRef]
- Cai, J.; Qi, H.; Yao, K.; Yao, Y.; Jing, D.; Liao, W.; Zhao, Z. Non-Coding RNAs Steering the Senescence-Related Progress, Properties, and Application of Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 650431. [Google Scholar] [CrossRef]
- Dorvash, M.; Farahmandnia, M.; Tavassoly, I. A Systems Biology Roadmap to Decode mTOR Control System in Cancer. Interdiscip. Sci. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Kowalska, M.; Piekut, T.; Prendecki, M.; Sodel, A.; Kozubski, W.; Dorszewska, J. Mitochondrial and Nuclear DNA Oxidative Damage in Physiological and Pathological Aging. DNA Cell Biol. 2020, 39, 1410–1420. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Luo, B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cell. Mol. Life Sci. 2020, 77, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Driving neural regeneration through the mammalian target of rapamycin. Neural Regen. Res. 2014, 9, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Stem cell guidance through the mechanistic target of rapamycin. World J. Stem Cells 2015, 7, 999–1009. [Google Scholar] [PubMed]
- Maiese, K. Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br. J. Clin. Pharmacol. 2016, 82, 1245–1266. [Google Scholar] [CrossRef] [PubMed]
- Rapaka, D.; Bitra, V.R.; Challa, S.R.; Adiukwu, P.C. mTOR signaling as a molecular target for the alleviation of Alzheimer’s disease pathogenesis. Neurochem. Int. 2022, 155, 105311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Z.; Deng, Y.J.; Xie, Q.Q.; Ren, E.H.; Ma, Z.J.; He, X.G.; Gao, Y.C.; Kang, X.W. Sirtuins and intervertebral disc degeneration: Roles in inflammation, oxidative stress, and mitochondrial function. Clin. Chim. Acta 2020, 508, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, H.; Wang, Q.; Chen, S.; Wang, R.; Wang, Z.; Yang, C.; Chen, A.; Zhao, J.; Zhou, Z.; et al. Sirt1 overexpression improves senescence-associated pulmonary fibrosis induced by vitamin D deficiency through downregulating IL-11 transcription. Aging Cell 2022, 21, e13680. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. From causes of aging to death from COVID-19. Aging (Albany NY) 2020, 12, 10004–10021. [Google Scholar] [CrossRef]
- Maiese, K. The bright side of reactive oxygen species: Lifespan extension without cellular demise. J. Transl. Sci. 2016, 2, 185–187. [Google Scholar] [CrossRef]
- Watroba, M.; Szukiewicz, D. Sirtuins at the Service of Healthy Longevity. Front. Physiol. 2021, 12, 724506. [Google Scholar] [CrossRef]
- Sun, C.; Bai, S.; Liang, Y.; Liu, D.; Liao, J.; Chen, Y.; Zhao, X.; Wu, B.; Huang, D.; Chen, M.; et al. The role of Sirtuin 1 and its activators in age-related lung disease. Biomed. Pharmacother. 2023, 162, 114573. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.B.; Santos, W.C. The mTOR pathway as a target for SARS-CoV-2: Rapamycin as a possible alternative pharmacological therapeutic for COVID-19. Act. Farma Ter. 2020, 18, 102–108. [Google Scholar]
- Braidy, N.; Liu, Y. NAD+ therapy in age-related degenerative disorders: A benefit/risk analysis. Exp. Gerontol. 2020, 132, 110831. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, Y.; Bijonowski, B.M.; Tsai, A.C.; Fu, Q.; Logan, T.M.; Ma, T.; Li, Y. NAD(+)/NADH redox alterations reconfigure metabolism and rejuvenate senescent human mesenchymal stem cells in vitro. Commun. Biol. 2020, 3, 774. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, Y.; Niringiyumukiza, J.D.; Su, P.; Xiang, W. Circular RNA involvement in aging: An emerging player with great potential. Mech. Ageing Dev. 2019, 178, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Li, C.; Xie, X.; Zhan, K.B.; Yang, S.Q.; Tang, Y.Y.; Zou, W.; Zhang, P.; Tang, X.Q. Hydrogen Sulfide Inhibits Homocysteine-Induced Neuronal Senescence by Up-Regulation of SIRT1. Int. J. Med. Sci. 2020, 17, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, S. Signaling pathways and effectors of aging. Front. Biosci. (Landmark Ed.) 2021, 26, 50–96. [Google Scholar] [CrossRef]
- Maiese, K. The Impact of Aging and Oxidative Stress in Metabolic and Nervous System Disorders: Programmed Cell Death and Molecular Signal Transduction Crosstalk. Front. Immunol. 2023, 14, 1273570. [Google Scholar]
- du Toit, W.L.; Kruger, R.; Gafane-Matemane, L.F.; Schutte, A.E.; Louw, R.; Mels, C.M.C. Markers of arterial stiffness and urinary metabolomics in young adults with early cardiovascular risk: The African-PREDICT study. Metabolomics 2023, 19, 28. [Google Scholar] [CrossRef]
- Holowko-Ziolek, J.; Cieszczyk, P.; Bilinski, J.; Basak, G.W.; Stachowska, E. What Model of Nutrition Can Be Recommended to People Ending Their Professional Sports Career? An Analysis of the Mediterranean Diet and the CRON Diet in the Context of Former Athletes. Nutrients 2020, 12, 3604. [Google Scholar] [CrossRef]
- Kalam, F.; James, D.L.; Li, Y.R.; Coleman, M.F.; Kiesel, V.A.; Cespedes Feliciano, E.M.; Hursting, S.D.; Sears, D.D.; Kleckner, A.S. Intermittent fasting interventions to leverage metabolic and circadian mechanisms for cancer treatment and supportive care outcomes. J. Natl. Cancer Inst. Monogr. 2023, 2023, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Klimontov, V.V.; Bulumbaeva, D.M.; Fazullina, O.N.; Lykov, A.P.; Bgatova, N.P.; Orlov, N.B.; Konenkov, V.I.; Pfeiffer, A.F.H.; Pivovarova-Ramich, O.; Rudovich, N. Circulating Wnt1-inducible signaling pathway protein-1 (WISP-1/CCN4) is a novel biomarker of adiposity in subjects with type 2 diabetes. J. Cell Commun. Signal 2020, 14, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, J.; Yang, L.; Wang, N.; Liu, Y.; Wei, X.; Gao, M.; Wang, Y.; Ma, Y.; Wen, D. Association of WISP1/CCN4 with Risk of Overweight and Gestational Diabetes Mellitus in Chinese Pregnant Women. Dis. Markers 2020, 2020, 4934206. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.T. Capsaicin-the major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Picking a bone with WISP1 (CCN4): New strategies against degenerative joint disease. J. Transl. Sci. 2016, 1, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Philips, A.M.; Khan, N. Amino acid sensing pathway: A major check point in the pathogenesis of obesity and COVID-19. Obes. Rev. 2021, 22, e13221. [Google Scholar] [CrossRef] [PubMed]
- Pinel, A.; Rigaudière, J.P.; Jouve, C.; Montaurier, C.; Jousse, C.; Lhomme, M.; Morio, B.; Capel, F. Transgenerational supplementation with eicosapentaenoic acid reduced the metabolic consequences on the whole body and skeletal muscle in mice receiving an obesogenic diet. Eur. J. Nutr. 2021, 60, 3143–3157. [Google Scholar] [CrossRef]
- Quesada, I.; de Paola, M.; Torres-Palazzolo, C.; Camargo, A.; Ferder, L.; Manucha, W.; Castro, C. Effect of Garlic’s Active Constituents in Inflammation, Obesity and Cardiovascular Disease. Curr. Hypertens. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Su, H.; Wang, W.J.; Zheng, G.D.; Yin, Z.P.; Li, J.E.; Chen, L.L.; Zhang, Q.F. The anti-obesity and gut microbiota modulating effects of taxifolin in C57BL/6J mice fed with a high-fat diet. J. Sci. Food Agric. 2022, 102, 1598–1608. [Google Scholar] [CrossRef]
- Ye, Q.; Fu, J.F. Paediatric type 2 diabetes in China-Pandemic, progression, and potential solutions. Pediatr. Diabetes 2018, 19, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, Q.; Sun, Y. FoxO1 Regulates Neuropeptide Y and Pro-opiomelanocortin in the Hypothalamus of Rat Offspring Small for Gestational Age. Reprod. Sci. 2022, 29, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Miao, H.; Song, Z.; Li, Y.; Xia, N.; Zhang, Z.; Zhang, H. Metformin alleviates the cognitive impairment induced by benzo[a]pyrene via glucolipid metabolism regulated by FTO/FoxO6 pathway in mice. Environ. Sci. Pollut. Res. Int. 2023, 30, 69192–69204. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Z.Y.; Ma, W.J.; Wang, Q.Z.; Liang, H.; Ma, A.G. B Vitamins Supplementation Can Improve Cognitive Functions and May Relate to the Enhancement of Transketolase Activity in A Rat Model of Cognitive Impairment Associated with High-fat Diets. Curr. Med. Sci. 2021, 41, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Cernea, M.; Tang, W.; Guan, H.; Yang, K. Wisp1 mediates Bmp3-stimulated mesenchymal stem cell proliferation. J. Mol. Endocrinol. 2016, 56, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gan, L.; Zhang, T.; Ren, Q.; Sun, C. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J. Pineal Res. 2018, 64, 12455. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.; Wardelmann, K.; Kleinridders, A. Untangling the effect of insulin action on brain mitochondria and metabolism. J. Neuroendocr. 2021, 33, e12932. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Ciesielska, K.; Gajewska, M. Fatty Acids as Potent Modulators of Autophagy Activity in White Adipose Tissue. Biomolecules 2023, 13, 255. [Google Scholar] [CrossRef]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health-A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- Stojanovic, D.; Stojanovic, M.; Milenkovic, J.; Velickov, A.; Ignjatovic, A.; Milojkovic, M. The Multi-Faceted Nature of Renalase for Mitochondrial Dysfunction Improvement in Cardiac Disease. Cells 2023, 12, 1607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Meng, X.; Jiang, H.; Ge, H.; Qian, K.; Zheng, Y.; Park, Y.; Wang, J. Restoration of energy homeostasis under oxidative stress: Duo synergistic AMPK pathways regulating arginine kinases. PLoS Genet. 2023, 19, e1010843. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Chen, W.; Wang, B.; Gao, C.; Liu, X.; Song, Y.; Qi, H.; Liu, H.; Wu, T.; Wang, R.; et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis. Redox Biol. 2023, 63, 102760. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Ingraham, N.E.; Murray, T.A.; Marmor, S.; Hovertsen, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Observational Study of Metformin and Risk of Mortality in Patients Hospitalized with COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Ong, A.N.; Tan, C.C.; Canete, M.T.; Lim, B.A.; Robles, J. Association Between Metformin Use and Mortality among Patients with Type 2 Diabetes Mellitus Hospitalized for COVID-19 Infection. J. ASEAN Fed. Endocr. Soc. 2021, 36, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Cognitive Impairment in Multiple Sclerosis. Bioengineering 2023, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Birnie, M.T.; Claydon, M.D.B.; Troy, O.; Flynn, B.P.; Yoshimura, M.; Kershaw, Y.M.; Zhao, Z.; Demski-Allen, R.C.R.; Barker, G.R.I.; Warburton, E.C.; et al. Circadian regulation of hippocampal function is disrupted with corticosteroid treatment. Proc. Natl. Acad. Sci. USA 2023, 120, e2211996120. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Wang, H.; Gao, Y.; Nie, K.; Tang, Y.; Su, H.; Hu, M.; Gong, J.; Fang, K.; et al. Diosgenin protects against podocyte injury in early phase of diabetic nephropathy through regulating SIRT6. Phytomed. Int. J. Phytother. Phytopharm. 2022, 104, 154276. [Google Scholar] [CrossRef]
- Karamzad, N.; Faraji, E.; Adeli, S.; Sullman, M.J.M.; Pourghassem Gargari, B. The effect of menaquinone-7 supplementation on dp-ucMGP, PIVKAII, inflammatory markers, and body composition in type 2 diabetes patients: A randomized clinical trial. Nutr. Diabetes 2022, 12, 15. [Google Scholar] [CrossRef]
- Beegum, F.; Anuranjana, P.V.; George, K.T.; Divya, K.P.; Begum, F.; Krishnadas, N.; Shenoy, R.R. Sirtuins as therapeutic targets for improving delayed wound healing in diabetes. J. Drug Target. 2022, 30, 911–926. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Jin, M.; Han, S.D.; Chon, G.R.; Kim, I.H.; Kim, S.; Kim, S.Y.; Choi, S.B.; Noh, Y.H. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp. Mol. Med. 2014, 46, e111. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Curr. Neurovasc. Res. 2015, 12, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Slezakova, D.; Kadlic, P.; Jezberova, M.; Bolekova, V.; Valkovic, P.; Minar, M. Brain volume loss in multiple sclerosis is independent of disease activity and might be prevented by early disease-modifying therapy. Neurol. Neurochir. Pol. 2023, 57, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, R.; Naderi, R.; Sheervalilou, R.; Alipour, M.R. Swimming training by affecting the pancreatic Sirtuin1 (SIRT1) and oxidative stress, improves insulin sensitivity in diabetic male rats. Horm. Mol. Biol. Clin. Investig. 2019, 40, 20190011. [Google Scholar] [CrossRef] [PubMed]
- Gkogkolou, P.; Sarna, M.; Sarna, T.; Paus, R.; Luger, T.A.; Bohm, M. Protection of glucotoxicity by a tripeptide derivative of alpha-melanocyte-stimulating hormone in human epidermal keratinocytes. Br. J. Dermatol. 2019, 180, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Liu, T.; Sun, Y.; Liu, X.; Xiong, R.; Li, H.; Li, Z.; Zhang, Z.; Tian, Z.; Tian, Y. Sonodynamic therapy inhibits palmitate-induced beta cell dysfunction via PINK1/Parkin-dependent mitophagy. Cell Death Dis. 2019, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Jalgaonkar, M.P.; Parmar, U.M.; Kulkarni, Y.A.; Oza, M.J. SIRT1-FOXOs activity regulates diabetic complications. Pharmacol. Res. 2022, 175, 106014. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed. Pharmacother. 2008, 62, 218–232. [Google Scholar] [CrossRef]
- Zuo, J.; Zhang, Z.; Luo, M.; Zhou, L.; Nice, E.C.; Zhang, W.; Wang, C.; Huang, C. Redox signaling at the crossroads of human health and disease. MedComm 2022, 3, e127. [Google Scholar] [CrossRef]
- Inoue, M.; Tanida, T.; Kondo, T.; Takenaka, S.; Nakajima, T. Oxygen-glucose deprivation-induced glial cell reactivity in the rat primary neuron-glia co-culture. J. Vet. Med. Sci. 2023, 85, 799–808. [Google Scholar] [CrossRef]
- Barinaga, M. Is nitric oxide the “retrograde messenger”? Science 1991, 254, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qi, H.; Ma, Y.; Deng, Y.; Liu, S.; Jie, Y.; Jing, J.; He, J.; Zhang, X.; Wheatley, L.; et al. A flexible and physically transient electrochemical sensor for real-time wireless nitric oxide monitoring. Nat. Commun. 2020, 11, 3207. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, Y.; Wang, X.X.; Truong, D.; Wu, Y.C. The Critical Role of SIRT1 in Parkinson’s Disease: Mechanism and Therapeutic Considerations. Aging Dis. 2020, 11, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Lee, I.; Jin, S.A.; Kim, S.; Nagar, H.; Choi, S.J.; Jeon, B.H.; Kim, C.S. SIRT1 Activation Attenuates the Cardiac Dysfunction Induced by Endothelial Cell-Specific Deletion of CRIF1. Biomedicines 2021, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, H.; Naziroglu, M.; Ovey, I.S.; Cig, B.; Akpinar, O. The neuroprotective action of dexmedetomidine on apoptosis, calcium entry and oxidative stress in cerebral ischemia-induced rats: Contribution of TRPM2 and TRPV1 channels. Sci. Rep. 2016, 6, 37196. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Li, F.; Maiese, K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005, 75, 207–246. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Lin, S.H.; Maiese, K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J. Cereb. Blood Flow. Metab. 2004, 24, 728–743. [Google Scholar] [CrossRef]
- Dabrowska-Bouta, B.; Struzynska, L.; Sidoryk-Wegrzynowicz, M.; Sulkowski, G. Memantine Modulates Oxidative Stress in the Rat Brain following Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2021, 22, 11330. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Wang, Y.; Tang, S.; Hoyer, D.; Schneider, E.K.; Velkov, T.; Xiao, X. Rapamycin Confers Neuroprotection against Colistin-Induced Oxidative Stress, Mitochondria Dysfunction, and Apoptosis through the Activation of Autophagy and mTOR/Akt/CREB Signaling Pathways. ACS Chem. Neurosci. 2018, 9, 824–837. [Google Scholar] [CrossRef]
- Dechandt, C.R.P.; Ferrari, G.D.; Dos Santos, J.R.; de Oliveira, J.A.C.; da Silva-Jr, R.M.P.; Cunha, A.O.S.; Garcia-Cairasco, N.; Alberici, L.C. Energy Metabolism and Redox State in Brains of Wistar Audiogenic Rats, a Genetic Model of Epilepsy. Front. Neurol. 2019, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Milkovic, L.; Zarkovic, N.; Zarkovic, K. Oxidative stress and regeneration. Free Radic. Biol. Med. 2022, 181, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, X.; Fan, Z.; Sun, G.; Han, Y.; Zhang, D.; Xu, L.; Wang, M.; Wang, X.; Zhang, S.; et al. Wnt Signaling Activates TP53-Induced Glycolysis and Apoptosis Regulator and Protects Against Cisplatin-Induced Spiral Ganglion Neuron Damage in the Mouse Cochlea. Antioxid. Redox Signal 2019, 30, 1389–1410. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.; Vieira, M.; Delerue-Matos, C.; Grosso, C.; Soares, C. Biological Potential, Gastrointestinal Digestion, Absorption, and Bioavailability of Algae-Derived Compounds with Neuroprotective Activity: A Comprehensive Review. Mar. Drugs 2022, 20, 362. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Yuan, W.; Wu, H.; Yin, X.; Xuan, H. Bioactive components and mechanisms of Chinese poplar propolis alleviates oxidized low-density lipoprotein-induced endothelial cells injury. BMC Complement. Altern. Med. 2018, 18, 142. [Google Scholar] [CrossRef]
- Csiszar, A.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Kiss, T.; Farkas, E.; Baur, J.A.; Ungvari, Z. Role of endothelial NAD(+) deficiency in age-related vascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1253–H1266. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Warming Up to New Possibilities with the Capsaicin Receptor TRPV1: mTOR, AMPK, and Erythropoietin. Curr. Neurovasc. Res. 2017, 14, 184–189. [Google Scholar] [CrossRef]
- Meng, J.; Chen, Y.; Wang, J.; Qiu, J.; Chang, C.; Bi, F.; Wu, X.; Liu, W. EGCG protects vascular endothelial cells from oxidative stress-induced damage by targeting the autophagy-dependent PI3K-AKT-mTOR pathway. Ann. Transl. Med. 2020, 8, 200. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, X.; Lv, S.; Sun, M.; Guo, H.; Zhai, Y.; Wang, Z.; Dai, P.; Zheng, L.; Ye, M.; et al. Salidroside attenuates oxidized low-density lipoprotein-induced endothelial cell injury via promotion of the AMPK/SIRT1 pathway. Int. J. Mol. Med. 2019, 43, 2279–2290. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Jiang, Y.; Liu, M.; Zhong, W.; Ge, Z.; Sun, Z.; Shen, X. Relationship between cognitive dysfunction and the promoter methylation of PER1 and CRY1 in patients with cerebral small vessel disease. Front. Aging Neurosci. 2023, 15, 1174541. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.L.; Santos, G.G.L.; Espirito-Santo, R.F.; Silva, G.S.A.; Evangelista, A.F.; Silva, D.N.; Soares, M.B.P.; Villarreal, C.F. Reestablishment of Redox Homeostasis in the Nociceptive Primary Afferent as a Mechanism of Antinociception Promoted by Mesenchymal Stem/Stromal Cells in Oxaliplatin-Induced Chronic Peripheral Neuropathy. Stem Cells Int. 2021, 2021, 8815206. [Google Scholar] [CrossRef] [PubMed]
- Oyefeso, F.A.; Muotri, A.R.; Wilson, C.G.; Pecaut, M.J. Brain organoids: A promising model to assess oxidative stress-induced central nervous system damage. Dev. Neurobiol. 2021, 81, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Jeyaraman, M.; Jeyaraman, N.; Rajendran, R.L.; Gangadaran, P. Where Do We Stand in Stem Cell Therapy for the Management of Diabetes Mellitus?-A Scientometric Research Trend Analysis from 1990 to 2020. Bioengineering 2021, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- BinMowyna, M.N.; AlFaris, N.A. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm. Biol. 2021, 59, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Cui, Z.; Zhang, R.; Zhao, K.; Wang, L.; Yao, J.; Liu, S.; Cai, C.; Cao, Y. The Effects of Rumen-Protected Choline and Rumen-Protected Nicotinamide on Liver Transcriptomics in Periparturient Dairy Cows. Metabolites 2023, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef]
- Martini, S.; Austin, T.; Aceti, A.; Faldella, G.; Corvaglia, L. Free radicals and neonatal encephalopathy: Mechanisms of injury, biomarkers, and antioxidant treatment perspectives. Pediatr. Res. 2020, 87, 823–833. [Google Scholar] [CrossRef]
- Ran, D.; Hong, W.; Yan, W.; Mengdie, W. Properties and molecular mechanisms underlying geniposide-mediated therapeutic effects in chronic inflammatory diseases. J. Ethnopharmacol. 2021, 273, 113958. [Google Scholar] [CrossRef]
- Xu, J.X.; Fang, K.; Gao, X.R.; Liu, S.; Ge, J.F. Resveratrol Protects SH-SY5Y Cells Against Oleic Acid-Induced Glucolipid Metabolic Dysfunction and Cell Injuries Via the Wnt/beta-Catenin Signalling Pathway. Neurochem. Res. 2021, 46, 2936–2947. [Google Scholar] [CrossRef]
- Hasbal, N.B.; Turgut, D.; Gok Oguz, E.; Ulu, S.; Gungor, O. Effect of Calcineurin Inhibitors and Mammalian Target of Rapamycin Inhibitors on the Course of COVID-19 in Kidney Transplant Recipients. Ann. Transpl. 2021, 26, e929279. [Google Scholar] [CrossRef] [PubMed]
- Temiz-Resitoglu, M.; Guden, D.S.; Senol, S.P.; Vezir, O.; Sucu, N.; Kibar, D.; Yilmaz, S.N.; Tunctan, B.; Malik, K.U.; Sahan-Firat, S. Pharmacological Inhibition of Mammalian Target of Rapamycin Attenuates Deoxycorticosterone Acetate Salt-Induced Hypertension and Related Pathophysiology: Regulation of Oxidative Stress, Inflammation, and Cardiovascular Hypertrophy in Male Rats. J. Cardiovasc. Pharmacol. 2022, 79, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Ye, W.; Liu, J.; Zhou, L.; Song, Y. The Emerging Key Role of Klotho in the Hypothalamus-Pituitary-Ovarian Axis. Reprod. Sci. 2021, 28, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Li, H.Y.; Jin, J.; Jin, J.Z.; Zhang, L.Y.; Xuan, M.Y.; Jin, X.M.; Jiang, Y.J.; Zheng, H.L.; Jin, Y.S.; et al. L-carnitine treatment attenuates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. Korean J. Intern. Med. 2021, 36, S180–S195. [Google Scholar] [CrossRef] [PubMed]
- Vaamonde-Garcia, C.; Lopez-Armada, M.J. Role of mitochondrial dysfunction on rheumatic diseases. Biochem. Pharmacol. 2019, 165, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ren, K.; Jiang, C.; Wang, L.; Yao, Q. Regulation of cartilage damage caused by lack of Klotho with thioredoxin/peroxiredoxin (Trx/Prx) system and succedent NLRP3 activation in osteoarthritis mice. Am. J. Transl. Res. 2019, 11, 7338–7350. [Google Scholar] [PubMed]
- Frantzidis, C.A.; Kontana, E.; Karkala, A.; Nigdelis, V.; Karagianni, M.; Nday, C.M.; Ganapathy, K.; Kourtidou-Papadeli, C. Current trends and future perspectives of space neuroscience towards preparation for interplanetary missions. Neurol. India 2019, 67, S182–S187. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.K.; Andersson, K.E. Regenerative pharmacology: Recent developments and future perspectives. Regen. Med. 2016, 11, 859–870. [Google Scholar] [CrossRef]
- Liu, C.; Zhong, C.; Chen, R.; Zhou, X.; Wu, J.; Han, J.; Li, X.; Zhang, Y.; Gao, Q.; Xiao, M.; et al. Higher dietary vitamin C intake is associated with a lower risk of gestational diabetes mellitus: A longitudinal cohort study. Clin. Nutr. 2020, 39, 198–203. [Google Scholar] [CrossRef]
- Nikooyeh, B.; Zahedirad, M.; Kalayi, A.; Shariatzadeh, N.; Hollis, B.W.; Neyestani, T.R. Improvement of vitamin D status through consumption of either fortified food products or supplement pills increased hemoglobin concentration in adult subjects: Analysis of pooled data from two randomized clinical trials. Nutr. Health 2023, 29, 567–574. [Google Scholar] [CrossRef]
- Orkaby, A.R.; Dushkes, R.; Ward, R.; Djousse, L.; Buring, J.E.; Lee, I.M.; Cook, N.R.; LeBoff, M.S.; Okereke, O.I.; Copeland, T.; et al. Effect of Vitamin D3 and Omega-3 Fatty Acid Supplementation on Risk of Frailty: An Ancillary Study of a Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2231206. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.S.; Rao, K.R.; Manjunath, M.; Saiswaroop, R.; Patnana, D.P.; Phalguna, K.S.; Choudhary, B.; Sivaramakrishnan, V. Vitamin B(6,) B(12) and folate modulate deregulated pathways and protein aggregation in yeast model of Huntington disease. 3 Biotech 2023, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xie, J.; Ma, L.; Hao, Z.; Zhang, W.; Li, L. Vitamin D Receptor Activation Targets ROS-Mediated Crosstalk Between Autophagy and Apoptosis in Hepatocytes in Cholestasic Mice. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Doroftei, B.; Ilie, O.D.; Cojocariu, R.O.; Ciobica, A.; Maftei, R.; Grab, D.; Anton, E.; McKenna, J.; Dhunna, N.; Simionescu, G. Minireview Exploring the Biological Cycle of Vitamin B3 and Its Influence on Oxidative Stress: Further Molecular and Clinical Aspects. Molecules 2020, 25, 3323. [Google Scholar] [CrossRef] [PubMed]
- Jahan, R.; Yousaf, M.; Khan, H.; Shah, S.A.; Khan, A.A.; Bibi, N.; Javed, F.; Ijaz, M.; Ali, A.; Wei, D.Q. Zinc Ortho Methyl Carbonodithioate Improved Pre and Post-Synapse Memory Impairment via SIRT1/p-JNK Pathway against Scopolamine in Adult Mice. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2023, 18, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Li, D.; Wang, C.; Guo, S. Ferroptosis and central nervous system demyelinating diseases. J. Neurochem. 2023, 165, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, G.; Li, Y.; Kong, K.; Li, X.; Kan, T.; Yang, F.; Wang, L.; Wang, X. Forkhead box O3 attenuates osteoarthritis by suppressing ferroptosis through inactivation of NF-kappaB/MAPK signaling. J. Orthop. Transl. 2023, 39, 147–162. [Google Scholar] [CrossRef]
- Ieraci, A.; Herrera, D.G. Nicotinamide Inhibits Ethanol-Induced Caspase-3 and PARP-1 Over-activation and Subsequent Neurodegeneration in the Developing Mouse Cerebellum. Cerebellum 2018, 17, 326–335. [Google Scholar] [CrossRef]
- Jobst, M.; Kiss, E.; Gerner, C.; Marko, D.; Del Favero, G. Activation of autophagy triggers mitochondrial loss and changes acetylation profile relevant for mechanotransduction in bladder cancer cells. Arch. Toxicol. 2023, 97, 217–233. [Google Scholar] [CrossRef]
- Kumar, A.; Ou, Y. From bench to behaviour: The role of lifestyle factors on intraocular pressure, neuroprotection, and disease progression in glaucoma. Clin. Exp. Ophthalmol. 2023, 51, 380–394. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.; Wang, Y.; Yang, S.; Luo, J.; Fang, F.; Liao, J.; Wen, W.; Cui, H.; Shang, H. Qing-Wen-Jie-Re Mixture Ameliorates Poly (I:C)-Induced Viral Pneumonia Through Regulating the Inflammatory Response and Serum Metabolism. Front. Pharmacol. 2022, 13, 891851. [Google Scholar] [CrossRef]
- Lin, Y.; Gong, T.; Ma, Q.; Jing, M.; Zheng, T.; Yan, J.; Chen, J.; Pan, Y.; Sun, Q.; Zhou, X.; et al. Nicotinamide could reduce growth and cariogenic virulence of Streptococcus mutans. J. Oral. Microbiol. 2022, 14, 2056291. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z. Nicotinamide: Necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol. Sci. 2003, 24, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Nejabati, H.R.; Samadi, N.; Shahnazi, V.; Mihanfar, A.; Fattahi, A.; Latifi, Z.; Bahrami-Asl, Z.; Roshangar, L.; Nouri, M. Nicotinamide and its metabolite N1-Methylnicotinamide alleviate endocrine and metabolic abnormalities in adipose and ovarian tissues in rat model of Polycystic Ovary Syndrome. Chem. Biol. Interact. 2020, 324, 109093. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.H.; Chao, L.C.; Huang, S.Y.; Lin, H.W.; Lee, A.H.; Chen, Y.Y.; Lee, E.J. Nicotinamide Deteriorates Post-Stroke Immunodepression Following Cerebral Ischemia-Reperfusion Injury in Mice. Biomedicines 2023, 11, 2145. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Liu, K.J.; Zhang, F.Y.; Xiang, B. Nicotinamide mitigates radiation injury in submandibular gland by protecting mitochondrial structure and functions. J. Oral. Pathol. Med. Off. Publ. Int. Assoc. Oral. Pathol. Am. Acad. Oral. Pathol. 2022, 51, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, W.; Duan, H.; Li, Z.; Jia, Y.; Zhang, S.; Wang, X.; Zhou, Q.; Shi, W. NAD(+) precursors protect corneal endothelial cells from UVB-induced apoptosis. Am. J. Physiol. Cell Physiol. 2020, 318, C796–C805. [Google Scholar] [CrossRef] [PubMed]
- Abo-Shady, A.M.; Gheda, S.F.; Ismail, G.A.; Cotas, J.; Pereira, L.; Abdel-Karim, O.H. Antioxidant and Antidiabetic Activity of Algae. Life 2023, 13, 460. [Google Scholar] [CrossRef] [PubMed]
- AlSaleh, A.; Shahid, M.; Farid, E.; Bindayna, K. The Effect of Ascorbic Acid and Nicotinamide on Panton-Valentine Leukocidin Cytotoxicity: An Ex Vivo Study. Toxins 2023, 15, 38. [Google Scholar] [CrossRef]
- Amini, J.; Sanchooli, N.; Milajerdi, M.H.; Baeeri, M.; Haddadi, M.; Sanadgol, N. The interplay between tauopathy and aging through interruption of UPR/Nrf2/autophagy crosstalk in the Alzheimer’s disease transgenic experimental models. In The International Journal of Neuroscience; Taylor Francis Group: Abingdon, UK, 2023; pp. 1–19. [Google Scholar] [CrossRef]
- Fernandes, J.; Uppal, K.; Liu, K.H.; Hu, X.; Orr, M.; Tran, V.; Go, Y.M.; Jones, D.P. Antagonistic Interactions in Mitochondria ROS Signaling Responses to Manganese. Antioxidants 2023, 12, 804. [Google Scholar] [CrossRef]
- Tong, Z.; Chu, G.; Wan, C.; Wang, Q.; Yang, J.; Meng, Z.; Du, L.; Yang, J.; Ma, H. Multiple Metabolites Derived from Mushrooms and Their Beneficial Effect on Alzheimer’s Diseases. Nutrients 2023, 15, 2758. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Hussain, A.; Asif, M.; Nawaz, F.; Rasool, M. Natural Products as Bioactive Agents in the Prevention of Dementia. CNS Neurol. Disord. Drug Targets 2023, 22, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Zhao, X.; Guo, Q.; Yang, R.; Zhang, C.; Huang, Y.; Ma, L.; Zhao, S. Effect of PPARgamma on oxidative stress in diabetes-related dry eye. Exp. Eye Res. 2023, 231, 109498. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Redox Biology of Melatonin: Discriminating Between Circadian and Noncircadian Functions. Antioxid. Redox Signal 2022, 37, 704–725. [Google Scholar] [CrossRef] [PubMed]
- Sabzali, M.; Eidi, A.; Khaksari, M.; Khastar, H. Anti-inflammatory, Antioxidant, and Antiapoptotic Action of Metformin Attenuates Ethanol Neurotoxicity in the Animal Model of Fetal Alcohol Spectrum Disorders. Neurotox. Res. 2022, 40, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lobos, R.; Lespay-Rebolledo, C.; Tapia-Bustos, A.; Palacios, E.; Vio, V.; Bustamante, D.; Morales, P.; Herrera-Marschitz, M. Vulnerability to a Metabolic Challenge Following Perinatal Asphyxia Evaluated by Organotypic Cultures: Neonatal Nicotinamide Treatment. Neurotox. Res. 2017, 32, 426–443. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Lin, S.H.; Li, F.; Maiese, K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through AKT, BAD, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr. Neurovasc. Res. 2005, 2, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Lin, S.H.; Maiese, K. Nicotinamide modulates mitochondrial membrane potential and cysteine protease activity during cerebral vascular endothelial cell injury. J. Vasc. Res. 2002, 39, 131–147. [Google Scholar] [CrossRef]
- Itzhaki, O.; Greenberg, E.; Shalmon, B.; Kubi, A.; Treves, A.J.; Shapira-Frommer, R.; Avivi, C.; Ortenberg, R.; Ben-Ami, E.; Schachter, J.; et al. Nicotinamide inhibits vasculogenic mimicry, an alternative vascularization pathway observed in highly aggressive melanoma. PLoS ONE 2013, 8, e57160. [Google Scholar] [CrossRef]
- Mikhed, Y.; Daiber, A.; Steven, S. Mitochondrial Oxidative Stress, Mitochondrial DNA Damage and Their Role in Age-Related Vascular Dysfunction. Int. J. Mol. Sci. 2015, 16, 15918–15953. [Google Scholar] [CrossRef]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine pathway, NAD(+) synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp. Gerontol. 2020, 132, 110841. [Google Scholar] [CrossRef] [PubMed]
- Klimova, N.; Kristian, T. Multi-targeted Effect of Nicotinamide Mononucleotide on Brain Bioenergetic Metabolism. Neurochem. Res. 2019, 44, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.A.; Brookes, P.S. Cellular Compartmentation and the Redox/Nonredox Functions of NAD. Antioxid. Redox Signal 2019, 31, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.F.; Wang, L.; Liu, W.Y. Nicotinamide pretreatment alleviates mitochondrial stress and protects hypoxic myocardial cells via AMPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Osorio Alves, J.; Matta Pereira, L.; Cabral Coutinho do Rego Monteiro, I.; Pontes Dos Santos, L.H.; Soares Marreiros Ferraz, A.; Carneiro Loureiro, A.C.; Calado Lima, C.; Leal-Cardoso, J.H.; Pires Carvalho, D.; Soares Fortunato, R.; et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants 2020, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.; Jiang, C.; Fang, Z.; Zhang, Z.; Lin, X.; Sun, L.; Jiang, W. Nicotinamide induces mitochondrial-mediated apoptosis through oxidative stress in human cervical cancer HeLa cells. Life Sci. 2017, 181, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Naia, L.; Rosenstock, T.R.; Oliveira, A.M.; Oliveira-Sousa, S.I.; Caldeira, G.L.; Carmo, C.; Laco, M.N.; Hayden, M.R.; Oliveira, C.R.; Rego, A.C. Comparative Mitochondrial-Based Protective Effects of Resveratrol and Nicotinamide in Huntington’s Disease Models. Mol. Neurobiol. 2017, 54, 5385–5399. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Woodfield, K.Y.; Connern, C.P. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J. Biol. Chem. 1997, 272, 3346–3354. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Ramezani Ali Akbari, F.; Fathi Moghadam, H. Effect of C-peptide Alone or in Combination with Nicotinamide on Glucose and Insulin Levels in Streptozotocin-Nicotinamide-Induced Type 2 Diabetic Mice. Malays. J. Med. Sci. MJMS 2014, 21, 12–17. [Google Scholar]
- Poljsak, B.; Milisav, I. NAD+ as the Link Between Oxidative Stress, Inflammation, Caloric Restriction, Exercise, DNA Repair, Longevity, and Health Span. Rejuvenation Res. 2016, 19, 406–415. [Google Scholar] [CrossRef]
- Rehman, I.U.; Khan, A.; Ahmad, R.; Choe, K.; Park, H.Y.; Lee, H.J.; Atiq, A.; Park, J.; Hahm, J.R.; Kim, M.O. Neuroprotective Effects of Nicotinamide against MPTP-Induced Parkinson’s Disease in Mice: Impact on Oxidative Stress, Neuroinflammation, Nrf2/HO-1 and TLR4 Signaling Pathways. Biomedicines 2022, 10, 2929. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhao, Y.; Wang, Y.; Xie, R.; Tong, Y.; Sauer, J.D.; Gong, S. NAD(H)-loaded nanoparticles for efficient sepsis therapy via modulating immune and vascular homeostasis. Nat. Nanotechnol. 2022, 17, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, Q.; Sun, Y.; Chen, J. Nicotinamide protects against skeletal muscle atrophy in streptozotocin-induced diabetic mice. Arch. Physiol. Biochem. 2019, 125, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, Y.; Li, N.; Li, P.; Su, J.; Wang, Z.; Wang, T.; Yang, Z.; Yang, Y.; Chen, H.; et al. Muscle metabolomics analysis reveals potential biomarkers of exercise-dependent improvement of the diaphragm function in chronic obstructive pulmonary disease. Int. J. Mol. Med. 2020, 45, 1644–1660. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Y.I.; Mahmoud, A.A. Role of nicotinamide (vitamin B3) in acetaminophen-induced changes in rat liver: Nicotinamide effect in acetaminophen-damged liver. Exp. Toxicol. Pathol. 2016, 68, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sun, H.; Yan, J.; Kang, C.; Wu, J.; Yang, B. Enterohemorrhagic Escherichia coli senses microbiota-derived nicotinamide to increase its virulence and colonization in the large intestine. Cell Rep. 2023, 42, 112638. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. Targeting disease through novel pathways of apoptosis and autophagy. Expert. Opin. Ther. Targets 2012, 16, 1203–1214. [Google Scholar] [CrossRef]
- Guo, T.; Chen, M.; Liu, J.; Wei, Z.; Yuan, J.; Wu, W.; Wu, Z.; Lai, Y.; Zhao, Z.; Chen, H.; et al. Neuropilin-1 promotes mitochondrial structural repair and functional recovery in rats with cerebral ischemia. J. Transl. Med. 2023, 21, 297. [Google Scholar] [CrossRef]
- Hou, J.; Chong, Z.Z.; Shang, Y.C.; Maiese, K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr. Neurovasc. Res. 2010, 7, 95–112. [Google Scholar] [CrossRef]
- Shang, Y.C.; Chong, Z.Z.; Hou, J.; Maiese, K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal 2010, 22, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Mello, E.O.; Souza, S.B.; Monteiro, R.M.; Ramos, A.C.; Carvalho, A.O.; Rodrigues, R.; Okorokov, L.A.; Gomes, V.M. Programmed cell death in yeast by thionin-like peptide from Capsicum annuum fruits involving activation of caspases and extracellular H(+) flux. Biosci. Rep. 2018, 38, BSR20180119. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Catrinescu, M.M.; Binan, L.; Costantino, S.; Levin, L.A. Axonal Degeneration in Retinal Ganglion Cells Is Associated with a Membrane Polarity-Sensitive Redox Process. J. Neurosci. 2017, 37, 3824–3839. [Google Scholar] [CrossRef] [PubMed]
- Viola, G.; Bortolozzi, R.; Hamel, E.; Moro, S.; Brun, P.; Castagliuolo, I.; Ferlin, M.G.; Basso, G. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem. Pharmacol. 2012, 83, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.J.; Fossum, S.L.; Fimbel, S.M.; Montgomery, J.E.; Hyde, D.R. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp. Eye Res. 2010, 91, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.C.; Chong, Z.Z.; Hou, J.; Maiese, K. FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr. Neurovasc. Res. 2009, 6, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sun, C.; Lei, M.; Li, G.; Yi, L.; Luo, F.; Li, Y.; Ding, L.; Liu, Z.; Li, S.; et al. Activation of Wnt/beta-catenin pathway by exogenous Wnt1 protects SH-SY5Y cells against 6-hydroxydopamine toxicity. J. Mol. Neurosci. 2013, 49, 105–115. [Google Scholar] [CrossRef]
- Hou, J.; Wang, S.; Shang, Y.C.; Chong, Z.Z.; Maiese, K. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr. Neurovasc. Res. 2011, 8, 220–235. [Google Scholar] [CrossRef]
- Kim, S.; Kang, I.H.; Nam, J.B.; Cho, Y.; Chung, D.Y.; Kim, S.H.; Kim, J.S.; Cho, Y.D.; Hong, E.K.; Sohn, N.W.; et al. Ameliorating the effect of astragaloside IV on learning and memory deficit after chronic cerebral hypoperfusion in rats. Molecules 2015, 20, 1904–1921. [Google Scholar] [CrossRef]
- Xin, Y.J.; Yuan, B.; Yu, B.; Wang, Y.Q.; Wu, J.J.; Zhou, W.H.; Qiu, Z. Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress. Sci. Rep. 2015, 5, 7645. [Google Scholar] [CrossRef]
- Yu, T.; Li, L.; Chen, T.; Liu, Z.; Liu, H.; Li, Z. Erythropoietin attenuates advanced glycation endproducts-induced toxicity of Schwann cells in vitro. Neurochem. Res. 2015, 40, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Microglia: Formidable Players in Alzheimer’s Disease and Other Neurodegenerative Disorders. Curr. Neurovasc. Res. 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Kang, J.Q.; Maiese, K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation 2002, 106, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Yousafzai, N.A.; Jin, H.; Ullah, M.; Wang, X. Recent advances of SIRT1 and implications in chemotherapeutics resistance in cancer. Am. J. Cancer Res. 2021, 11, 5233–5248. [Google Scholar]
- Pang, Y.; Qin, M.; Hu, P.; Ji, K.; Xiao, R.; Sun, N.; Pan, X.; Zhang, X. Resveratrol protects retinal ganglion cells against ischemia induced damage by increasing Opa1 expression. Int. J. Mol. Med. 2020, 46, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Weiyao, J.; Chenghong, S.; Limei, L.; Xinghua, Z.; Bo, Y.; Xiaozheng, D.; Haidong, W. Rheumatoid arthritis and mitochondrial homeostasis: The crossroads of metabolism and immunity. Front. Med. 2022, 9, 1017650. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Vincent, A.M. Critical temporal modulation of neuronal programmed cell injury. Cell. Mol. Neurobiol. 2000, 20, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Xu, Y.; Li, S.; Li, N.; Zhang, S.; Zhu, H. Cornin protects against cerebral ischemia/reperfusion injury by preventing autophagy via the PI3K/Akt/mTOR pathway. BMC Pharmacol. Toxicol. 2022, 23, 82. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, M.; Tian, J.; Gao, M.; Liu, M.; Fu, X.; Jin, T.; Pan, J.; Chen, F.; An, F. WNT1-inducible signalling pathway protein 1 stabilizes atherosclerotic plaques in apolipoprotein-E-deficient mice via the focal adhesion kinase/mitogen-activated extracellular signal-regulated kinase/extracellular signal-regulated kinase pathway. J. Hypertens. 2022, 40, 1666–1681. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.; Li, P.; Li, L. A novel adipokine WISP1 attenuates lipopolysaccharide-induced cell injury in 3T3-L1 adipocytes by regulating the PI3K/Akt pathway. Obes. Res. Clin. Pr. 2022, 16, 122–129. [Google Scholar] [CrossRef]
- Mansour, R.M.; El Sayed, N.S.; Ahmed, M.A.E.; El-Sahar, A.E. Addressing Peroxisome Proliferator-Activated Receptor-gamma in 3-Nitropropionic Acid-Induced Striatal Neurotoxicity in Rats. Mol. Neurobiol. 2022, 59, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr. Neurovasc. Res. 2014, 11, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, A.; Choobineh, S.; Gaeini, A.; Soori, R. Interaction of exercise training with taurine attenuates infarct size and cardiac dysfunction via Akt-Foxo3a-Caspase-8 signaling pathway. Amino Acids 2023, 55, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Alloza, I.; Salegi, A.; Mena, J.; Navarro, R.T.; Martin, C.; Aspichueta, P.; Salazar, L.M.; Carpio, J.U.; Cagigal, P.D.; Vega, R.; et al. BIRC6 Is Associated with Vulnerability of Carotid Atherosclerotic Plaque. Int. J. Mol. Sci. 2020, 21, 9387. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wang, D.; Wu, C. Protective effect of liquiritin on coronary heart disease through regulating the proliferation of human vascular smooth muscle cells via upregulation of sirtuin1. Bioengineered 2022, 13, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Cheema, P.S.; Nandi, D.; Nag, A. Exploring the therapeutic potential of forkhead box O for outfoxing COVID-19. Open Biol. 2021, 11, 210069. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.; Niknam, Z.; Mohammadi Amirabad, L.; Amiri-Dashatan, N.; Koushki, M.; Nemati, M.; Danesh Pouya, F.; Rezaei-Tavirani, M.; Rasmi, Y.; Tayebi, L. Molecular pathways involved in COVID-19 and potential pathway-based therapeutic targets. Biomed. Pharmacother. 2022, 145, 112420. [Google Scholar] [CrossRef]
- Chen, S.; Li, B. MiR-128-3p Post-Transcriptionally Inhibits WISP1 to Suppress Apoptosis and Inflammation in Human Articular Chondrocytes via the PI3K/AKT/NF-kappaB Signaling Pathway. Cell Transpl. 2020, 29, 963689720939131. [Google Scholar] [CrossRef]
- Maiese, K.; Li, F.; Chong, Z.Z. New avenues of exploration for erythropoietin. JAMA 2005, 293, 90–95. [Google Scholar] [CrossRef]
- Cai, D.; Hong, S.; Yang, J.; San, P. The Effects of microRNA-515-5p on the Toll-Like Receptor 4 (TLR4)/JNK Signaling Pathway and WNT1-Inducible-Signaling Pathway Protein 1 (WISP-1) Expression in Rheumatoid Arthritis Fibroblast-Like Synovial (RAFLS) Cells Following Treatment with Receptor Activator of Nuclear Factor-kappa-B Ligand (RANKL). Med. Sci. Monit. 2020, 26, e920611. [Google Scholar] [CrossRef]
- Dehghanian, F.; Soltani, Z.; Khaksari, M. Can Mesenchymal Stem Cells Act Multipotential in Traumatic Brain Injury? J. Mol. Neurosci. 2020, 70, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Lu, L.; Liang, Y.; Peng, D.; Aschner, M.; Jiang, Y. Signal transduction associated with lead-induced neurological disorders: A review. Food Chem. Toxicol. 2021, 150, 112063. [Google Scholar] [CrossRef] [PubMed]
- Farid, H.A.; Sayed, R.H.; El-Shamarka, M.E.; Abdel-Salam, O.M.E.; El Sayed, N.S. PI3K/AKT signaling activation by roflumilast ameliorates rotenone-induced Parkinson’s disease in rats. In Inflammopharmacology; Spring: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Feng, H.; Xue, M.; Deng, H.; Cheng, S.; Hu, Y.; Zhou, C. Ginsenoside and Its Therapeutic Potential for Cognitive Impairment. Biomolecules 2022, 12, 1310. [Google Scholar] [CrossRef] [PubMed]
- Govindappa, P.K.; Elfar, J.C. Erythropoietin promotes M2 macrophage phagocytosis of Schwann cells in peripheral nerve injury. Cell Death Dis. 2022, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.W.; Huang, H.T.; Ma, J.; Zuo, Y.; Huang, D.; He, L.L.; Wan, Z.M.; Chen, C.; Yang, F.F.; You, Y.W. Circular RNA-0007059 protects cell viability and reduces inflammation in a nephritis cell model by inhibiting microRNA-1278/SHP-1/STAT3 signaling. Mol. Med. 2021, 27, 113. [Google Scholar] [CrossRef] [PubMed]
- Hajializadeh, Z.; Khaksari, M. The protective effects of 17-beta estradiol and SIRT1 against cardiac hypertrophy: A review. Heart Fail. Rev. 2022, 27, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Ciccarelli, G.; Baroni, M.G.; Cavallo, M.G. Sick fat: The good and the bad of old and new circulating markers of adipose tissue inflammation. J. Endocrinol. Investig. 2019, 42, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Ren, L. Circular RNA PIP5K1A act as microRNA-552-3p sponge to regulates inflammation, oxidative damage in glucolipotoxicity-induced pancreatic INS-1 beta-cells via Janus kinase 1. Bioengineered 2022, 13, 5724–5736. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Wu, C.; Li, D.; Wu, Y.; Ye, L.; Ye, L.; Chen, X.; Li, P.; Yuan, Y.; et al. Acidic fibroblast growth factor attenuates type 2 diabetes-induced demyelination via suppressing oxidative stress damage. Cell Death Dis. 2021, 12, 107. [Google Scholar] [CrossRef]
- Pan, Y.R.; Song, J.Y.; Fan, B.; Wang, Y.; Che, L.; Zhang, S.M.; Chang, Y.X.; He, C.; Li, G.Y. mTOR may interact with PARP-1 to regulate visible light-induced parthanatos in photoreceptors. Cell Commun. Signal 2020, 18, 27. [Google Scholar] [CrossRef]
- Sappington, R.M.; Sidorova, T.; Ward, N.J.; Chakravarthy, R.; Ho, K.W.; Calkins, D.J. Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress. Channels (Austin Tex.) 2015, 9, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, C.; Yao, A.; Qu, Y.; Qin, L.; Xiong, Z.; Zhang, J.; Wang, W. Intranasal administration of erythropoietin rescues the photoreceptors in degenerative retina: A noninvasive method to deliver drugs to the eye. Drug Deliv. 2019, 26, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Ma, J.; Xu, G.; Sun, Z. SHP-1 knockdown suppresses mitochondrial biogenesis and aggravates mitochondria-dependent apoptosis induced by all trans retinal through the STING/AMPK pathways. Mol. Med. 2022, 28, 125. [Google Scholar] [CrossRef]
- El-Beltagy, A.; Saleh, A.M.B.; Attaallah, A.; Gahnem, R.A. Therapeutic role of Azadirachta indica leaves ethanolic extract against diabetic nephropathy in rats neonatally induced by streptozotocin. Ultrastruct. Pathol. 2021, 45, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Shang, Y.C.; Maiese, K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr. Neurovasc. Res. 2007, 4, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Khan, A.; Rehman, I.U.; Lee, H.J.; Khan, I.; Kim, M.O. Lupeol Treatment Attenuates Activation of Glial Cells and Oxidative-Stress-Mediated Neuropathology in Mouse Model of Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 6086. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Beiram, R.; Azimullah, S.; Mf, N.M.; Ojha, S.K.; Adem, A.; Jalal, F.Y. Valeric Acid Protects Dopaminergic Neurons by Suppressing Oxidative Stress, Neuroinflammation and Modulating Autophagy Pathways. Int. J. Mol. Sci. 2020, 21, 7670. [Google Scholar] [CrossRef]
- Lei, Q.; Wu, T.; Wu, J.; Hu, X.; Guan, Y.; Wang, Y.; Yan, J.; Shi, G. Roles of alpha-synuclein in gastrointestinal microbiome dysbiosis-related Parkinson’s disease progression (Review). Mol. Med. Rep. 2021, 24, 734. [Google Scholar] [CrossRef]
- Zhang, W.B.; Huang, Y.; Guo, X.R.; Zhang, M.Q.; Yuan, X.S.; Zu, H.B. DHCR24 reverses Alzheimer’s disease-related pathology and cognitive impairment via increasing hippocampal cholesterol levels in 5xFAD mice. Acta Neuropathol. Commun. 2023, 11, 102. [Google Scholar] [CrossRef]
- Amidfar, M.; Garcez, M.L.; Kim, Y.K. The shared molecular mechanisms underlying aging of the brain, major depressive disorder, and Alzheimer’s disease: The role of circadian rhythm disturbances. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 123, 110721. [Google Scholar] [CrossRef]
- Liang, H.; Liu, Q. The role of non-coding RNA in lupus nephritis. Hum. Cell 2023, 36, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Scrimieri, R.; Locatelli, L.; Cazzaniga, A.; Cazzola, R.; Malucelli, E.; Sorrentino, A.; Iotti, S.; Maier, J.A. Ultrastructural features mirror metabolic derangement in human endothelial cells exposed to high glucose. Sci. Rep. 2023, 13, 15133. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.K.; Khan, R.; Mikhael, M.; Balez, R.; David, M.A.; Mahns, D.; Hardy, J.; Tayebi, M. Therapeutic anti-amyloid beta antibodies cause neuronal disturbances. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 2479–2496. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Li, S.G.; Zhang, H.W.; Hua, F.W.; Sun, G.Z.; Huang, Z. MiRNA-411 attenuates inflammatory damage and apoptosis following spinal cord injury. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, L.; Chen, Q.; Huang, Y.; Chen, X.; Qiao, D. The Role of Non-coding RNAs in Methamphetamine-Induced Neurotoxicity. Cell. Mol. Neurobiol. 2023, 43, 2415–2436. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. MicroRNAs and SIRT1: A Strategy for Stem Cell Renewal and Clinical Development? J. Transl. Sci. 2015, 1, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Disease onset and aging in the world of circular RNAs. J. Transl. Sci. 2016, 2, 327–329. [Google Scholar] [CrossRef]
- Maiese, K. Harnessing the Power of SIRT1 and Non-coding RNAs in Vascular Disease. Curr. Neurovasc. Res. 2017, 14, 82–88. [Google Scholar] [CrossRef]
- Yeger, H. CCN proteins: Opportunities for clinical studies-a personal perspective. J. Cell Commun. Signal 2023, 17, 333–352. [Google Scholar] [CrossRef]
- Sierra-Pagan, J.E.; Dsouza, N.; Das, S.; Larson, T.A.; Sorensen, J.R.; Ma, X.; Stan, P.; Wanberg, E.J.; Shi, X.; Garry, M.G.; et al. FOXK1 regulates Wnt signalling to promote cardiogenesis. Cardiovasc. Res. 2023, 119, 1728–1739. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, H.; Ding, C. The ameliorative effect of CangFu Daotan Decoction on polycystic ovary syndrome of rodent model is associated with m6A methylation and Wnt/beta-catenin pathway. Gynecol. Endocrinol. 2023, 39, 2181637. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, L.; Chen, W.; Cheng, J.; Zhao, X.; Zhang, Y.; Li, R.; Zhou, M.; Yao, Y.; Li, Y.; et al. Expression of EPO and related factors in the liver and kidney of plain and Tibetan sheep. Histol. Histopathol. 2023, 18592. [Google Scholar] [CrossRef]
- Hu, G.; Wang, T.; Ma, C. EPO activates PI3K-IKKalpha-CDK1 signaling pathway to promote the proliferation of Glial Cells under hypoxia environment. Genet. Mol. Biol. 2022, 45, e20210249. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Sun, X.; Zhan, C.; Li, Z.; Qiu, L.; Luo, R.; Liu, H.; Sun, X.; Li, R.; et al. Silk Fibroin/Collagen/Hydroxyapatite Scaffolds Obtained by 3D Printing Technology and Loaded with Recombinant Human Erythropoietin in the Reconstruction of Alveolar Bone Defects. ACS Biomater. Sci. Eng. 2022, 8, 5245–5256. [Google Scholar] [CrossRef] [PubMed]
- Senousy, M.A.; Hanafy, M.E.; Shehata, N.; Rizk, S.M. Erythropoietin and Bacillus Calmette-Guerin Vaccination Mitigate 3-Nitropropionic Acid-Induced Huntington-like Disease in Rats by Modulating the PI3K/Akt/mTOR/P70S6K Pathway and Enhancing the Autophagy. ACS Chem. Neurosci. 2022, 13, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Sergio, C.M.; Rolando, C.A. Erythropoietin regulates signaling pathways associated with neuroprotective events. Exp. Brain Res. 2022, 240, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Regeneration in the nervous system with erythropoietin. Front. Biosci. (Landmark Ed.) 2016, 21, 561–596. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. Erythropoietin: New directions for the nervous system. Int. J. Mol. Sci. 2012, 13, 11102–11129. [Google Scholar] [CrossRef]
- Kubat Oktem, E.; Aydin, B.; Yazar, M.; Arga, K.Y. Integrative Analysis of Motor Neuron and Microglial Transcriptomes from SOD1(G93A) Mice Models Uncover Potential Drug Treatments for ALS. J. Mol. Neurosci. 2022, 72, 2360–2376. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Chu, F.; Huang, J.; Yang, Z. Graphene oxide enhances beta-amyloid clearance by inducing autophagy of microglia and neurons. Chem. Biol. Interact. 2020, 325, 109126. [Google Scholar] [CrossRef]
- Samuels, J.D.; Lukens, J.R.; Price, R.J. Emerging roles for ITAM and ITIM receptor signaling in microglial biology and Alzheimer’s disease-related amyloidosis. J. Neurochem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Y.; Wang, L.; Zhan, H.; Luo, X.; Zeng, Y.; Wu, W.; Zhang, X.; Wang, F. TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/FoxO3a signaling pathway in Alzheimer’s disease mice. Aging (Albany NY) 2020, 12, 20862–20879. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Jia, L.; Liu, C.C.; Rong, Z.; Zhong, L.; Yang, L.; Chen, X.F.; Fryer, J.D.; Wang, X.; Zhang, Y.W.; et al. TREM2 Promotes Microglial Survival by Activating Wnt/beta-Catenin Pathway. J. Neurosci. 2017, 37, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M.; Maes, M.; Puri, B.K. Could Alzheimer’s Disease Originate in the Periphery and If So How So? Mol. Neurobiol. 2019, 56, 406–434. [Google Scholar] [CrossRef] [PubMed]
- Carobene, A.; Maiese, K.; Abou-Diwan, C.; Locatelli, M.; Serteser, M.; Coskun, A.; Unsal, I. Biological variation estimates for serum neurofilament light chain in healthy subjects. Clin. Chim. Acta 2023, 551, 117608. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zeng, Q.; Brooks, D.; Geisbrecht, E.R. A conserved STRIPAK complex is required for autophagy in muscle tissue. Mol. Biol. Cell 2023, 34, ar91. [Google Scholar] [CrossRef] [PubMed]
- Mastrapasqua, M.; Rossi, R.; De Cosmo, L.; Resta, A.; Errede, M.; Bizzoca, A.; Zampatti, S.; Resta, N.; Giardina, E.; Ruggieri, M.; et al. Autophagy increase in Merosin-Deficient Congenital Muscular Dystrophy type 1A. Eur. J. Transl. Myol. 2023, 33, 11501. [Google Scholar] [CrossRef]
- Thomas, S.D.; Jha, N.K.; Ojha, S.; Sadek, B. mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules 2023, 28, 1889. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, C.; Zhang, X.; Liu, J.; Liu, J.; Xia, Z. Advances in the mTOR signaling pathway and its inhibitor rapamycin in epilepsy. Brain Behav. 2023, 13, e2995. [Google Scholar] [CrossRef]
- Ali, N.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alnaaim, S.A.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E. Autophagy and autophagy signaling in Epilepsy: Possible role of autophagy activator. Mol. Med. 2023, 29, 142. [Google Scholar] [CrossRef]
- Maiese, K. Cognitive Impairment and Dementia: Gaining Insight through Circadian Clock Gene Pathways. Biomolecules 2021, 11, 1002. [Google Scholar] [CrossRef] [PubMed]
- Corti, O.; Blomgren, K.; Poletti, A.; Beart, P.M. Autophagy in neurodegeneration: New insights underpinning therapy for neurological diseases. J. Neurochem. 2020, 154, 354–371. [Google Scholar] [CrossRef]
- Eshraghi, M.; Ahmadi, M.; Afshar, S.; Lorzadeh, S.; Adlimoghaddam, A.; Rezvani Jalal, N.; West, R.; Dastghaib, S.; Igder, S.; Torshizi, S.R.N.; et al. Enhancing autophagy in Alzheimer’s disease through drug repositioning. Pharmacol. Ther. 2022, 237, 108171. [Google Scholar] [CrossRef]
- Maiese, K. Neurodegeneration, memory loss, and dementia: The impact of biological clocks and circadian rhythm. Front. Biosci. (Landmark Ed.) 2021, 26, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Solon-Biet, S.M.; Cogger, V.C.; Fontana, L.; Simpson, S.J.; Le Couteur, D.G.; Ribeiro, R.V. Aging, lifestyle and dementia. Neurobiol. Dis. 2019, 130, 104481. [Google Scholar] [CrossRef] [PubMed]
- Potthast, A.B.; Nebl, J.; Wasserfurth, P.; Haufe, S.; Eigendorf, J.; Hahn, A.; Das, A. Impact of Nutrition on Short-Term Exercise-Induced Sirtuin Regulation: Vegans Differ from Omnivores and Lacto-Ovo Vegetarians. Nutrients 2020, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, Z.; Marandi, S.M.; Alaei, H.; Esfarjani, F. Exercise-Induced Neuroprotection in the 6-Hydroxydopamine Parkinson’s Disease Model. Neurotox. Res. 2020, 38, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liang, J.; Wu, L.; Zhang, H.; Lv, J.; Chen, N. Exercise-Induced Autophagy Suppresses Sarcopenia Through Akt/mTOR and Akt/FoxO3a Signal Pathways and AMPK-Mediated Mitochondrial Quality Control. Front. Physiol. 2020, 11, 583478. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, J.; Chen, N. Do not neglect the role of circadian rhythm in muscle atrophy. Ageing Res. Rev. 2020, 63, 101155. [Google Scholar] [CrossRef]
- He, C.; Bassik, M.C.; Moresi, V.; Sun, K.; Wei, Y.; Zou, Z.; An, Z.; Loh, J.; Fisher, J.; Sun, Q.; et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 2012, 481, 511–515. [Google Scholar] [CrossRef]
- Liu, Y.; Palanivel, R.; Rai, E.; Park, M.; Gabor, T.V.; Scheid, M.P.; Xu, A.; Sweeney, G. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes 2015, 64, 36–48. [Google Scholar] [CrossRef]
- Dong, W.; Wang, R.; Ma, L.N.; Xu, B.L.; Zhang, J.S.; Zhao, Z.W.; Wang, Y.L.; Zhang, X. Influence of age-related learning and memory capacity of mice: Different effects of a high and low caloric diet. Aging Clin. Exp. Res. 2016, 28, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Caberlotto, L.; Nguyen, T.P.; Lauria, M.; Priami, C.; Rimondini, R.; Maioli, S.; Cedazo-Minguez, A.; Sita, G.; Morroni, F.; Corsi, M.; et al. Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci. Rep. 2019, 9, 3965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Stanojevic, V.; Brindamour, L.J.; Habener, J.F. GLP1-derived nonapeptide GLP1(28-36)amide protects pancreatic beta-cells from glucolipotoxicity. J. Endocrinol. 2012, 213, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.M.; Lim, H.; Hur, K.Y.; Quan, W.; Lee, H.Y.; Cheon, H.; Ryu, D.; Koo, S.H.; Kim, H.L.; Kim, J.; et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nat. Commun. 2014, 5, 4934. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Fu, R.; Duan, Z.; Lu, J.; Gao, J.; Tian, L.; Lv, Z.; Chen, Z.; Han, J.; Jia, L.; et al. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol. Res. Pract. 2016, 212, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Lindner, J.; Kumar, A.; Yuan, W.; Magnuson, M.A. Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes 2011, 60, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, F.H.; Zhu, X.M.; Lv, Z.M. Impact of diabetic hyperglycaemia and insulin therapy on autophagy and impairment in rat epididymis. Andrologia 2020, 52, e13889. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xiao, Y.H.; Geng, T.; Sun, C.; Gu, J.; Tang, K.F.; Liu, B.; Liu, Y.M.; Sun, F. Clusterin suppresses spermatogenic cell apoptosis to alleviate diabetes-induced testicular damage by inhibiting autophagy via the PI3K/AKT/mTOR axis. Biol. Cell 2021, 113, 14–27. [Google Scholar] [CrossRef]
- Hu, P.; Lai, D.; Lu, P.; Gao, J.; He, H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int. J. Mol. Med. 2012, 29, 613–618. [Google Scholar] [CrossRef]
- Lee, Y.; Hong, Y.; Lee, S.R.; Chang, K.T.; Hong, Y. Autophagy contributes to retardation of cardiac growth in diabetic rats. Lab. Anim. Res. 2012, 28, 99–107. [Google Scholar] [CrossRef]
- Martino, L.; Masini, M.; Novelli, M.; Beffy, P.; Bugliani, M.; Marselli, L.; Masiello, P.; Marchetti, P.; De Tata, V. Palmitate activates autophagy in INS-1E beta-cells and in isolated rat and human pancreatic islets. PLoS ONE 2012, 7, e36188. [Google Scholar] [CrossRef]
- Ka, M.; Smith, A.L.; Kim, W.Y. MTOR controls genesis and autophagy of GABAergic interneurons during brain development. Autophagy 2017, 13, 1348–1363. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Biswas, S.C. Tribbles Pseudokinase 3 Induces Both Apoptosis and Autophagy in Amyloid-beta-induced Neuronal Death. J. Biol. Chem. 2017, 292, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Du, J.; Jin, H.; Zhang, J.; Niu, M.; Qin, J. Recombinant Human Erythropoietin Protects Against Hippocampal Damage in Developing Rats with Seizures by Modulating Autophagy via the S6 Protein in a Time-Dependent Manner. Neurochem. Res. 2018, 43, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, J.; Li, B.; Ding, Z.; Cheng, W.; Gao, F.; Zhang, Y.; Xu, Y.; Zhang, S. Cornin protects SH-SY5Y cells against oxygen and glucose deprivation-induced autophagy through the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 2018, 17, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Hu, X.Y.; Chen, M.W.; Xiong, C.H.; Zhao, N.; Ge, Y.H.; Wang, H.; Gao, X.L.; Xu, N.J.; Zhao, L.X.; et al. p85S6K sustains synaptic GluA1 to ameliorate cognitive deficits in Alzheimer’s disease. Transl. Neurodegener. 2023, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Zhang, J.B.; Guo, Y.X.; Xia, T.S.; Xu, L.C.; Rahmand, K.; Wang, G.P.; Li, X.J.; Han, T.; Wang, N.N.; et al. Xanthohumol ameliorates memory impairment and reduces the deposition of beta-amyloid in APP/PS1 mice via regulating the mTOR/LC3II and Bax/Bcl-2 signalling pathways. J. Pharm. Pharmacol. 2021, 73, 1230–1239. [Google Scholar] [CrossRef]
- Maiese, K. Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann. Med. 2014, 46, 587–596. [Google Scholar] [CrossRef]
- Gao, X.; Li, S.; Cong, C.; Wang, Y.; Xu, L. A Network Pharmacology Approach to Estimate Potential Targets of the Active Ingredients of Epimedium for Alleviating Mild Cognitive Impairment and Treating Alzheimer’s Disease. Evid.-Based Complement. Altern. Med. Ecam 2021, 2021, 2302680. [Google Scholar] [CrossRef]
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Barone, E.; Butterfield, D.A. mTOR in Alzheimer disease and its earlier stages: Links to oxidative damage in the progression of this dementing disorder. Free Radic. Biol. Med. 2021, 169, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.T.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging 2019, 11, 3117–3137. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shen, H.; Nibona, E.; Wu, K.; Ke, X.; Al Hafiz, M.A.; Liang, X.; Zhong, X.; Zhou, Q.; Qi, C.; et al. Fundc1 is necessary for proper body axis formation during embryogenesis in zebrafish. Sci. Rep. 2019, 9, 18910. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Maiese, K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br. J. Pharmacol. 2007, 150, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. PRAS40 is an integral regulatory component of erythropoietin mTOR signaling and cytoprotection. PLoS ONE 2012, 7, e45456. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Wu, M.; Ali, F.; Guo, Z.; Fang, Y.; Zhou, Y.; Zhang, S.; Zhang, W.; Wen, Y.; Yu, M.; et al. Based on network pharmacology, gastrodin attenuates hypertension-induced vascular smooth muscle cell proliferation and PI3K/AKT pathway activation. Sci. Rep. 2023, 13, 12140. [Google Scholar] [CrossRef] [PubMed]
- Tramutola, A.; Lanzillotta, S.; Aceto, G.; Pagnotta, S.; Ruffolo, G.; Cifelli, P.; Marini, F.; Ripoli, C.; Palma, E.; Grassi, C.; et al. Intranasal Administration of KYCCSRK Peptide Rescues Brain Insulin Signaling Activation and Reduces Alzheimer’s Disease-like Neuropathology in a Mouse Model for Down Syndrome. Antioxidants 2023, 12, 111. [Google Scholar] [CrossRef]
- Fessel, J. Supplementary Pharmacotherapy for the Behavioral Abnormalities Caused by Stressors in Humans, Focused on Post-Traumatic Stress Disorder (PTSD). J. Clin. Med. 2023, 12, 1680. [Google Scholar] [CrossRef]
- Fessel, J. Cure of Alzheimer’s Dementia Requires Addressing All of the Affected Brain Cell Types. J. Clin. Med. 2023, 12, 2049. [Google Scholar] [CrossRef]
- Lee, H.J.; Koh, S.H.; Song, K.M.; Seol, I.J.; Park, H.K. The Akt/mTOR/p70S6K Pathway Is Involved in the Neuroprotective Effect of Erythropoietin on Hypoxic/Ischemic Brain Injury in a Neonatal Rat Model. Neonatology 2016, 110, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Silva, E.; Meuth, S.G.; Peixoto, C.A. The role of iron metabolism in the pathogenesis and treatment of multiple sclerosis. Front. Immunol. 2023, 14, 1137635. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Ferroptosis, Iron Metabolism, and Forkhead Transcription Factors (FoxOs). Curr. Neurovasc. Res. 2023, 20, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Cirotti, C.; Taddei, I.; Contadini, C.; Di Girolamo, C.; Pepe, G.; De Bardi, M.; Borsellino, G.; Helmer-Citterich, M.; Barilà, D. NRF2 connects Src tyrosine kinase to ferroptosis resistance in glioblastoma. Life Sci. Alliance 2024, 7, e202302205. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, Y.; Chen, J.; Zou, P.; Li, J. Transcriptional activation of ENPP2 by FoxO4 protects cardiomyocytes from doxorubicin-induced toxicity. Mol. Med. Rep. 2021, 24, 668. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Hurtado-Navarro, L.; Pappolla, A.; Villar, L.M.M.; Rio, J.; Montalban, X.; Pelegrin, P.; Comabella, M. Increased NLRP3 Inflammasome Activation and Pyroptosis in Patients With Multiple Sclerosis With Fingolimod Treatment Failure. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200100. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.T.; Lu, S.; Yang, Y.D.; Ning, W.Y.; Cai, Y.; Hu, X.M.; Zhang, Q.; Xiong, K. Research trends, hot spots and prospects for necroptosis in the field of neuroscience. Neural Regen. Res. 2021, 16, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar] [CrossRef]
- Crespo, I.; Fernandez-Palanca, P.; San-Miguel, B.; Alvarez, M.; Gonzalez-Gallego, J.; Tunon, M.J. Melatonin modulates mitophagy, innate immunity and circadian clocks in a model of viral-induced fulminant hepatic failure. J. Cell. Mol. Med. 2020, 24, 7625–7636. [Google Scholar] [CrossRef]
- Park, M.H.; Gutierrez-Garcia, A.K.; Choudhury, M. Mono-(2-ethylhexyl) Phthalate Aggravates Inflammatory Response via Sirtuin Regulation and Inflammasome Activation in RAW 264.7 Cells. Chem. Res. Toxicol. 2019, 32, 935–942. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, C.; Jiang, N.; He, D.; Bai, Y.; Xin, Y. Rapamycin combined with MCC950 to treat multiple sclerosis in experimental autoimmune encephalomyelitis. J. Cell. Biochem. 2019, 120, 5160–5168. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A.; Hu, D.; Chereshnev, V. Problems of Pathogenesis and Pathogenetic Therapy of COVID-19 from the Perspective of the General Theory of Pathological Systems (General Pathological Processes). Int. J. Mol. Sci. 2021, 22, 7582. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Dai, S.; Sun, Y.; Yuan, Y.; Wang, L. MiR-128-3p Attenuates the Neurotoxicity in Rats Induced by Isoflurane Anesthesia. Neurotox. Res. 2022, 40, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, G.; Lakshmanan, A.P.; Samuel, S.M.; Triggle, C.R.; Ding, H. Molecular Interplay between microRNA-34a and Sirtuin1 in Hyperglycemia-Mediated Impaired Angiogenesis in Endothelial Cells: Effects of Metformin. J. Pharmacol. Exp. Ther. 2016, 356, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wu, Q.; Xie, Y.; Tan, J.; Ding, Y.; Bai, L. Hypoglycemic mechanisms of Ganoderma lucidum polysaccharides F31 in db/db mice via RNA-seq and iTRAQ. Food Funct. 2018, 9, 6495–6507. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Novel Treatment Strategies for the Nervous System: Circadian Clock Genes, Non-coding RNAs, and Forkhead Transcription Factors. Curr. Neurovasc. Res. 2018, 15, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.L.; Corno, C.; Zaffaroni, N.; Perego, P. Role of FoxO Proteins in Cellular Response to Antitumor Agents. Cancers 2019, 11, 90. [Google Scholar] [CrossRef]
- Li, N.; Yue, L.; Wang, J.; Wan, Z.; Bu, W. MicroRNA-24 alleviates isoflurane-induced neurotoxicity in rat hippocampus via attenuation of oxidative stress. Biochem. Cell Biol. 2020, 98, 208–218. [Google Scholar] [CrossRef]
- Tao, Y.; Gao, K.; Shen, B.; Zhang, K.; Zhang, Z.; Wang, C. MicroRNA-135b-5p Downregulation Causes Antidepressant Effects by Regulating SIRT1 Expression. Biochem. Genet. 2021, 59, 1582–1598. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Zhang, K.; Wan, Y.; Zhou, Y.; Yang, Z. miR-135a-5p inhibitor protects glial cells against apoptosis via targeting SIRT1 in epilepsy. Exp. Ther. Med. 2021, 21, 431. [Google Scholar] [CrossRef]
- Xie, L.; Huang, W.; Fang, Z.; Ding, F.; Zou, F.; Ma, X.; Tao, J.; Guo, J.; Xia, X.; Wang, H.; et al. CircERCC2 ameliorated intervertebral disc degeneration by regulating mitophagy and apoptosis through miR-182-5p/SIRT1 axis. Cell Death Dis. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.; Salazar, L.A. Autophagy and Polyphenols in Osteoarthritis: A Focus on Epigenetic Regulation. Int. J. Mol. Sci. 2021, 23, 421. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Chen, D.; Chen, N. Physical Activity Alleviates Cognitive Dysfunction of Alzheimer’s Disease through Regulating the mTOR Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 1591. [Google Scholar] [CrossRef] [PubMed]
- Prathipati, P.; Nandi, S.S.; Mishra, P.K. Stem Cell-Derived Exosomes, Autophagy, Extracellular Matrix Turnover, and miRNAs in Cardiac Regeneration during Stem Cell Therapy. Stem Cell Rev. Rep. 2017, 13, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Ramalinga, M.; Roy, A.; Srivastava, A.; Bhattarai, A.; Harish, V.; Suy, S.; Collins, S.; Kumar, D. MicroRNA-212 negatively regulates starvation induced autophagy in prostate cancer cells by inhibiting SIRT1 and is a modulator of angiogenesis and cellular senescence. Oncotarget 2015, 6, 34446–34457. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, W.; Chen, J.; Li, Y.; Xu, M.; Cai, Y. miR-122 and miR-199 synergistically promote autophagy in oral lichen planus by targeting the Akt/mTOR pathway. Int. J. Mol. Med. 2019, 43, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, J.; Tang, P.; Tu, N.; Wang, K.; Wu, G. Overexpression of miR-185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson’s disease. Mol. Med. Rep. 2018, 17, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Song, J.; Chen, Y.; Zhu, S.; Tu, W.; Ke, B.; Wu, L. LncRNA SNHG1 knockdown inhibits hyperglycemia induced ferroptosis via miR-16-5p/ACSL4 axis to alleviate diabetic nephropathy. J. Diabetes Investig. 2023, 14, 1056–1069. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, C.F.; Li, Z.F.; Huang, X.J.; Cai, S.Q.; Ye, S.Y.; Zhao, L.J.; Xiong, Y.; Chen, D.F.; Liu, H.L.; et al. Berberine blocks inflammasome activation and alleviates diabetic cardiomyopathy via the miR-18a-3p/Gsdmd pathway. Int. J. Mol. Med. 2023, 51, 49. [Google Scholar] [CrossRef]
- He, Z.; Zhao, Y.; Zhu, Y.; Wang, W.; Liu, X.; Lu, F. Interfering TUG1 Attenuates Cerebrovascular Endothelial Apoptosis and Inflammatory injury After Cerebral Ischemia/Reperfusion via TUG1/miR-410/FOXO3 ceRNA Axis. Neurotox. Res. 2022, 40, 1–13. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Wang, Z.; Zong, T.; Fu, X.; Aung, L.H.H.; Wang, K.; Wang, J.X.; Yu, T. Piwi-interacting RNAs (piRNAs) as potential biomarkers and therapeutic targets for cardiovascular diseases. Angiogenesis 2021, 24, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, X.; Zhan, X.; Sun, L.; Gao, J.; Cao, Y.; Qiu, H. Construction of competitive endogenous RNA network reveals regulatory role of long non-coding RNAs in type 2 diabetes mellitus. J. Cell. Mol. Med. 2017, 21, 3204–3213. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tan, L.; Wang, X. Circular HDAC9/microRNA-138/Sirtuin-1 Pathway Mediates Synaptic and Amyloid Precursor Protein Processing Deficits in Alzheimer’s Disease. Neurosci. Bull. 2019, 35, 877–888. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Jiang, Z. CircRNAs: A new perspective of biomarkers in the nervous system. Biomed. Pharmacother. 2020, 128, 110251. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.Q.; Lin, X.; Zhan, J.K.; Liu, Y.S. Roles and Functions of Exosomal Non-coding RNAs in Vascular Aging. Aging Dis. 2020, 11, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, P.; Clements, T.; Veno, M.; Abrahams, V.M.; Holder, B. Distinct non-coding RNA cargo of extracellular vesicles from M1 and M2 human primary macrophages. J. Extracell. Vesicles 2022, 11, e12293. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Guo, Y.; Lou, S. LncRNA ANCR Promotes Invasion and Migration of Gastric Cancer by Regulating FoxO1 Expression to Inhibit Macrophage M1 Polarization. Dig. Dis. Sci. 2020, 65, 2863–2872. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Q.; He, D.; Ling, Y.; Li, Y.; Li, J.; Zhang, H. Circular RNA: New star, new hope in cancer. BMC Cancer 2018, 18, 834. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.J. Glycometabolic rearrangements-aerobic glycolysis in pancreatic ductal adenocarcinoma (PDAC): Roles, regulatory networks, and therapeutic potential. Expert. Opin. Ther. Targets 2021, 25, 1077–1093. [Google Scholar] [CrossRef]
- Li, Q.; Kim, Y.R.; Vikram, A.; Kumar, S.; Kassan, M.; Gabani, M.; Lee, S.K.; Jacobs, J.S.; Irani, K. P66Shc-Induced MicroRNA-34a Causes Diabetic Endothelial Dysfunction by Downregulating Sirtuin1. Arter. Thromb. Vasc. Biol. 2016, 36, 2394–2403. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.C.; Song, S.M.; Wei, S.Y.; Li, J.S.; Zhao, S.L.; Li, B. The administration of erythropoietin attenuates kidney injury induced by ischemia/reperfusion with increased activation of Wnt/beta-catenin signaling. J. Formos. Med. Assoc. = Taiwan. Yi Zhi 2015, 114, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, W.; Liu, X.; Wang, Y.; Mai, H.; Huang, R. Circular RNA expression profiles of ovarian granulosa cells in advanced-age women explain new mechanisms of ovarian aging. Epigenomics 2022, 14, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Eyob, W.; George, A.K.; Homme, R.P.; Stanisic, D.; Sandhu, H.; Tyagi, S.C.; Singh, M. Regulation of the parental gene GRM4 by circGrm4 RNA transcript and glutamate-mediated neurovascular toxicity in eyes. Mol. Cell. Biochem. 2021, 476, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.B.; Song, K.J.; Chen, G.B.; Liu, R.; Jiang, Z.F.; He, Y.L. Circular RNA hsa_circ_0003204 promotes cervical cancer cell proliferation, migration, and invasion by regulating MAPK pathway. Cancer Biol. Ther. 2020, 21, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Schiano, C.; Benincasa, G.; Franzese, M.; Della Mura, N.; Pane, K.; Salvatore, M.; Napoli, C. Epigenetic-sensitive pathways in personalized therapy of major cardiovascular diseases. Pharmacol. Ther. 2020, 210, 107514. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, S.; Lei, Z.; Chen, G.; Huang, L.; Yang, F.; Lei, Y.; Liu, Y.; Yang, L.; Liu, W.; et al. Circular RNA profile in liver tissue of EpCAM knockout mice. Int. J. Mol. Med. 2019, 44, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Impacting dementia and cognitive loss with innovative strategies: Mechanistic target of rapamycin, clock genes, circular non-coding ribonucleic acids, and Rho/Rock. Neural Regen. Res. 2019, 14, 773–774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016, 7, 11215. [Google Scholar] [CrossRef]
- Cocquerelle, C.; Mascrez, B.; Hetuin, D.; Bailleul, B. Mis-splicing yields circular RNA molecules. FASEB J. 1993, 7, 155–160. [Google Scholar] [CrossRef]
- Hsu, M.T.; Coca-Prados, M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature 1979, 280, 339–340. [Google Scholar] [CrossRef]
- Maiese, K. Biomarkers for Parkinson’s Disease and Neurodegenerative Disorders: A Role for Non-coding RNAs. Curr. Neurovasc. Res. 2022, 19, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Carril, J.C.; Cacabelos, N.; Kazantsev, A.G.; Vostrov, A.V.; Corzo, L.; Cacabelos, P.; Goldgaber, D. Sirtuins in Alzheimer’s Disease: SIRT2-Related GenoPhenotypes and Implications for PharmacoEpiGenetics. Int. J. Mol. Sci. 2019, 20, 1249. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.R.; Qu, Y.J.; Hu, B.; An, H.M. Signal pathways in the treatment of Alzheimer’s disease with traditional Chinese medicine. Biomed. Pharmacother. 2022, 152, 113208. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ullah, R.; Rehman, S.U.; Shah, S.A.; Saeed, K.; Muhammad, T.; Park, H.Y.; Jo, M.H.; Choe, K.; Rutten, B.P.F.; et al. 17beta-Estradiol Modulates SIRT1 and Halts Oxidative Stress-Mediated Cognitive Impairment in a Male Aging Mouse Model. Cells 2019, 8, 928. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Gu, J.; Dai, W.; Jin, N.; Chu, D.; Huang, Q.; Liu, F.; Qian, W. Sirt1 enhances tau exon 10 inclusion and improves spatial memory of Htau mice. Aging (Albany NY) 2018, 10, 2498–2510. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.H.; Fathy, N.; Kortam, M.A.; Rabie, M.A.; Mohamed, A.F.; Kamel, A.S. Vildagliptin Attenuates Huntington’s Disease through Activation of GLP-1 Receptor/PI3K/Akt/BDNF Pathway in 3-Nitropropionic Acid Rat Model. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2020, 17, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guo, S.; Li, W.; Yu, P. The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells. Sci. Rep. 2015, 5, 12453. [Google Scholar] [CrossRef]
- Gaudreau, P.O.; Clairefond, S.; Class, C.A.; Boulay, P.L.; Chrobak, P.; Allard, B.; Azzi, F.; Pommey, S.; Do, K.A.; Saad, F.; et al. WISP1 is associated to advanced disease, EMT and an inflamed tumor microenvironment in multiple solid tumors. Oncoimmunology 2019, 8, e1581545. [Google Scholar] [CrossRef]
- Gonzalez, P.; Gonzalez-Fernandez, C.; Campos-Martin, Y.; Mollejo, M.; Carballosa-Gautam, M.; Marcillo, A.; Norenberg, M.; Rodriguez, F.J. Frizzled 1 and Wnt1 as new potential therapeutic targets in the traumatically injured spinal cord. Cell. Mol. Life Sci. 2020, 77, 4631–4662. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Gonzalez, P.; Gonzalez-Perez, F.; Rodriguez, F.J. Characterization of Ex Vivo and In Vitro Wnt Transcriptome Induced by Spinal Cord Injury in Rat Microglial Cells. Brain Sci. 2022, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- Knotek, T.; Janeckova, L.; Kriska, J.; Korinek, V.; Anderova, M. Glia and Neural Stem and Progenitor Cells of the Healthy and Ischemic Brain: The Workplace for the Wnt Signaling Pathway. Genes 2020, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Liu, T.; Lv, N.; Yuan, X.; Li, P. WISP1 induces ovarian cancer via the IGF1/alphavbeta3/Wnt axis. J. Ovarian Res. 2022, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, W.; Zhang, F.; Wang, J.; Li, X.; Li, S.; Qin, X.; Lu, Y. Association between WNT-1-inducible signaling pathway protein-1 (WISP1) genetic polymorphisms and the risk of gastric cancer in Guangxi Chinese. Cancer Cell Int. 2021, 21, 405. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. mTOR: On target for novel therapeutic strategies in the nervous system. Trends Mol. Med. 2013, 19, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Bapat, P.; Deogharkar, A.; Kazi, S.; Singh, S.K.V.; Gupta, T.; Jalali, R.; Sridhar, E.; Moiyadi, A.; Shetty, P.; et al. MiR-592 activates the mTOR kinase, ERK1/ERK2 kinase signaling and imparts neuronal differentiation signature characteristic of Group 4 medulloblastoma. Hum. Mol. Genet. 2021, 30, 2416–2428. [Google Scholar] [CrossRef]
- Sedighi, M.; Baluchnejadmojarad, T.; Afshin-Majd, S.; Amiri, M.; Aminzade, M.; Roghani, M. Anti-aging Klotho Protects SH-SY5Y Cells Against Amyloid beta1-42 Neurotoxicity: Involvement of Wnt1/pCREB/Nrf2/HO-1 Signaling. J. Mol. Neurosci. 2021, 71, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Serapide, M.F.; L’Episcopo, F.; Tirolo, C.; Testa, N.; Caniglia, S.; Giachino, C.; Marchetti, B. Boosting Antioxidant Self-defenses by Grafting Astrocytes Rejuvenates the Aged Microenvironment and Mitigates Nigrostriatal Toxicity in Parkinsonian Brain via an Nrf2-Driven Wnt/beta-Catenin Prosurvival Axis. Front. Aging Neurosci. 2020, 12, 24. [Google Scholar] [CrossRef]
- Xu, D.; Li, F.; Hou, K.; Gou, X.; Fang, W.; Li, Y. XQ-1H attenuates ischemic injury in PC12 cells via Wnt/beta-catenin signaling though inhibition of apoptosis and promotion of proliferation. Cell Biol. Int. 2020, 44, 2363–2369. [Google Scholar] [CrossRef]
- Gao, J.; Xu, H.; Rong, Z.; Chen, L. Wnt family member 1 (Wnt1) overexpression-induced M2 polarization of microglia alleviates inflammation-sensitized neonatal brain injuries. Bioengineered 2022, 13, 12409–12420. [Google Scholar] [CrossRef]
- Shou, J.; Ali-Osman, F.; Multani, A.S.; Pathak, S.; Fedi, P.; Srivenugopal, K.S. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene 2002, 21, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, W.; Yang, Y.; Li, Y.; Zhao, Y. WISP1 indicates poor prognosis and regulates cell proliferation and apoptosis in gastric cancer via targeting AKT/mTOR signaling pathway. Am. J. Transl. Res. 2020, 12, 7297–7311. [Google Scholar] [PubMed]
- Chong, Z.Z.; Li, F.; Maiese, K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell. Signal 2007, 19, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Shang, Y.C.; Hou, J.; Maiese, K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid. Med. Cell. Longev. 2010, 3, 153–165. [Google Scholar] [CrossRef]
- Aly, H.; Rohatgi, N.; Marshall, C.A.; Grossenheider, T.C.; Miyoshi, H.; Stappenbeck, T.S.; Matkovich, S.J.; McDaniel, M.L. A novel strategy to increase the proliferative potential of adult human beta-cells while maintaining their differentiated phenotype. PLoS ONE 2013, 8, e66131. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.C.; Chong, Z.Z.; Wang, S.; Maiese, K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr. Neurovasc. Res. 2011, 8, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.C.; Chong, Z.Z.; Wang, S.; Maiese, K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging 2012, 4, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, J.; Cheng, R.; Chen, Y.; Lee, K.; Hu, Y.; Yi, J.; Liu, Z.; Ma, J.X. Nitrosative stress plays an important role in Wnt pathway activation in diabetic retinopathy. Antioxid. Redox Signal 2013, 18, 1141–1153. [Google Scholar] [CrossRef]
- Danielyan, L.; Schafer, R.; Schulz, A.; Ladewig, T.; Lourhmati, A.; Buadze, M.; Schmitt, A.L.; Verleysdonk, S.; Kabisch, D.; Koeppen, K.; et al. Survival, neuron-like differentiation and functionality of mesenchymal stem cells in neurotoxic environment: The critical role of erythropoietin. Cell Death Differ. 2009, 16, 1599–1614. [Google Scholar] [CrossRef]
- Tanioka, M.; Park, W.K.; Shim, I.; Kim, K.; Choi, S.; Kim, U.J.; Lee, K.H.; Hong, S.K.; Lee, B.H. Neuroprotection from Excitotoxic Injury by Local Administration of Lipid Emulsion into the Brain of Rats. Int. J. Mol. Sci. 2020, 21, 2706. [Google Scholar] [CrossRef]
- Han, X.R.; Wen, X.; Wang, Y.J.; Wang, S.; Shen, M.; Zhang, Z.F.; Fan, S.H.; Shan, Q.; Wang, L.; Li, M.Q.; et al. MicroRNA-140-5p elevates cerebral protection of dexmedetomidine against hypoxic-ischaemic brain damage via the Wnt/beta-catenin signalling pathway. J. Cell. Mol. Med. 2018, 22, 3167–3182. [Google Scholar] [CrossRef]
- Tabatadze, N.; Tomas, C.; McGonigal, R.; Lin, B.; Schook, A.; Routtenberg, A. Wnt transmembrane signaling and long-term spatial memory. Hippocampus 2012, 22, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Bayod, S.; Felice, P.; Andres, P.; Rosa, P.; Camins, A.; Pallas, M.; Canudas, A.M. Downregulation of canonical Wnt signaling in hippocampus of SAMP8 mice. Neurobiol. Aging 2015, 36, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Hu, Y.; Ding, L.; Chen, Y.; Takahashi, Y.; Mott, R.; Ma, J.X. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes 2012, 61, 2948–2957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhou, K.K.; Lee, K.; Gao, G.; Lyons, T.J.; Kowluru, R.; Ma, J.X. The role of lipid peroxidation products and oxidative stress in activation of the canonical wingless-type MMTV integration site (WNT) pathway in a rat model of diabetic retinopathy. Diabetologia 2011, 54, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Hou, J.; Shang, Y.C.; Wang, S.; Maiese, K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3beta, and beta-catenin to foster vascular integrity during experimental diabetes. Curr. Neurovasc. Res. 2011, 8, 103–120. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Lu, Q.; Sherchan, P.; Huang, L.; Hu, X.; Zhang, J.H.; Dai, H.; Tang, J. Activation of Frizzled-7 attenuates blood-brain barrier disruption through Dvl/beta-catenin/WISP1 signaling pathway after intracerebral hemorrhage in mice. Fluids Barriers CNS 2021, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, C.; Guo, X.; Wen, Y.; Liu, L.; Liang, X.; Du, Y.; Zhang, L.; Ma, M.; Cheng, S.; et al. Integrative Analysis of Genome-Wide Association Studies and DNA Methylation Profile Identified Genetic Control Genes of DNA Methylation for Kashin-Beck Disease. Cartilage 2021, 13, 780S–788S. [Google Scholar] [CrossRef]
- Wang, S.; Chong, Z.Z.; Shang, Y.C.; Maiese, K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr. Neurovasc. Res. 2012, 9, 20–31. [Google Scholar] [CrossRef]
- Wang, S.; Chong, Z.Z.; Shang, Y.C.; Maiese, K. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr. Neurovasc. Res. 2013, 10, 54–69. [Google Scholar] [CrossRef]
- Shang, Y.C.; Chong, Z.Z.; Wang, S.; Maiese, K. Wnt1 inducible signaling pathway protein 1 (WISP1) targets PRAS40 to govern beta-amyloid apoptotic injury of microglia. Curr. Neurovasc. Res. 2012, 9, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.C.; Chong, Z.Z.; Wang, S.; Maiese, K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr. Neurovasc. Res. 2013, 10, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Capoccia, D.; De Gioannis, R.; Porzia, A.; Mainiero, F.; Di Martino, M.; Bertoccini, L.; De Bernardinis, M.; Leonetti, F.; et al. WISP1 Is a Marker of Systemic and Adipose Tissue Inflammation in Dysmetabolic Subjects With or Without Type 2 Diabetes. J. Endocr. Soc. 2017, 1, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Lee, J.E.; Shin, S.J.; Lee, Y.E.; Oh, S.H.; Park, J.Y.; Seong, J.K.; Park, J.S. Identification of differentially expressed mRNA during pancreas regeneration of rat by mRNA differential display. Biochem. Biophys. Res. Commun. 2002, 299, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, R.; Garcia-Alaman, A.; Esteban, Y.; Mir-Coll, J.; Serra-Navarro, B.; Fontcuberta-PiSunyer, M.; Broca, C.; Armanet, M.; Wojtusciszyn, A.; Kram, V.; et al. Wisp1 is a circulating factor that stimulates proliferation of adult mouse and human beta cells. Nat. Commun. 2020, 11, 5982. [Google Scholar] [CrossRef] [PubMed]
- Sahin Ersoy, G.; Altun Ensari, T.; Subas, S.; Giray, B.; Simsek, E.E.; Cevik, O. WISP1 is a novel adipokine linked to metabolic parameters in gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2017, 30, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.R.; Yan, X.Q.; Zhang, C.; Du, C.Q.; Long, W.J.; Zhan, D.; Ren, J.; Luo, X.P. Characterization of Wnt1-inducible Signaling Pathway Protein-1 in Obese Children and Adolescents. Curr. Med. Sci. 2018, 38, 868–874. [Google Scholar] [CrossRef]
- Murahovschi, V.; Pivovarova, O.; Ilkavets, I.; Dmitrieva, R.M.; Docke, S.; Keyhani-Nejad, F.; Gogebakan, O.; Osterhoff, M.; Kemper, M.; Hornemann, S.; et al. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes 2015, 64, 856–866. [Google Scholar] [CrossRef]
- Atef, M.M.; El-Sayed, N.M.; Ahmed, A.A.M.; Mostafa, Y.M. Donepezil improves neuropathy through activation of AMPK signalling pathway in streptozotocin-induced diabetic mice. Biochem. Pharmacol. 2019, 159, 1–10. [Google Scholar] [CrossRef]
- Gao, J.; Yao, M.; Chang, D.; Liu, J. mTOR (Mammalian Target of Rapamycin): Hitting the Bull’s Eye for Enhancing Neurogenesis After Cerebral Ischemia? Stroke 2023, 54, 279–285. [Google Scholar] [CrossRef]
- Kim, S.H.; Yu, H.S.; Huh, S.; Kang, U.G.; Kim, Y.S. Electroconvulsive seizure inhibits the mTOR signaling pathway via AMPK in the rat frontal cortex. Psychopharmacology 2022, 239, 443–454. [Google Scholar] [CrossRef]
- Hua, K.; Li, T.; He, Y.; Guan, A.; Chen, L.; Gao, Y.; Xu, Q.; Wang, H.; Luo, R.; Zhao, L.; et al. Resistin secreted by porcine alveolar macrophages leads to endothelial cell dysfunction during Haemophilus parasuis infection. Virulence 2023, 14, 2171636. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.T.; Huang, R.S.; Tsai, B.C.; Su, Y.C.; Chiu, P.L.; Chang, Y.M.; Padma, V.V.; Ho, T.J.; Yao, C.H.; Kuo, W.W.; et al. Folic Acid and Folinic Acid Protect Hearts of Aging Triple-transgenic Alzheimer’s Disease mice via IGF1R/PI3K/AKT and SIRT1/AMPK Pathways. In Neurotoxicity Research; Spring: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, J.; Zheng, F.; Jin, M.; Li, H. Glucagon-like peptide-1 analog-mediated protection against cholesterol-induced apoptosis via mammalian target of rapamycin activation in pancreatic betaTC-6 cells -1mTORbetaTC-6. J. Diabetes 2015, 7, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Li, L. The Molecular Mechanism of Glucagon-Like Peptide-1 Therapy in Alzheimer’s Disease, Based on a Mechanistic Target of Rapamycin Pathway. CNS Drugs 2017, 31, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.S.; Tsai, M.L.; Badmaev, V.; Jimenez, M.; Ho, C.T.; Pan, M.H. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARgamma and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J. Agric. Food Chem. 2012, 60, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, H.; Gao, J.; Liu, Y.; Li, J.; Wang, J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J. Mol. Cell. Cardiol. 2019, 136, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.B.; Sonowal, H.; Shukla, K.; Srivastava, S.K.; Ramana, K.V. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-alpha1/mTOR pathway. J. Mol. Endocrinol. 2019, 63, 11–25. [Google Scholar] [CrossRef]
- Tsai, C.F.; Kuo, Y.H.; Yeh, W.L.; Wu, C.Y.; Lin, H.Y.; Lai, S.W.; Liu, Y.S.; Wu, L.H.; Lu, J.K.; Lu, D.Y. Regulatory effects of caffeic acid phenethyl ester on neuroinflammation in microglial cells. Int. J. Mol. Sci. 2015, 16, 5572–5589. [Google Scholar] [CrossRef]
- Andreucci, M.; Fuiano, G.; Presta, P.; Lucisano, G.; Leone, F.; Fuiano, L.; Bisesti, V.; Esposito, P.; Russo, D.; Memoli, B.; et al. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009, 42, 554–561. [Google Scholar] [CrossRef]
- Chen, G.H.; Li, X.L.; Deng, Y.Q.; Zhou, F.M.; Zou, W.Q.; Jiang, W.X.; Shangguan, S.Q.; Lu, Z.N. The Molecular Mechanism of EPO Regulates the Angiogenesis after Cerebral Ischemia through AMPK-KLF2 Signaling Pathway. Crit. Rev. Eukaryot. Gene Expr. 2019, 29, 105–112. [Google Scholar] [CrossRef]
- Jang, W.; Kim, H.J.; Li, H.; Jo, K.D.; Lee, M.K.; Yang, H.O. The Neuroprotective Effect of Erythropoietin on Rotenone-Induced Neurotoxicity in SH-SY5Y Cells Through the Induction of Autophagy. Mol. Neurobiol. 2016, 53, 3812–3821. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Su, K.H.; Kou, Y.R.; Guo, B.C.; Lee, K.I.; Wei, J.; Lee, T.S. Role of transient receptor potential vanilloid 1 in regulating erythropoietin-induced activation of endothelial nitric oxide synthase. Acta Physiol (Oxf) 2017, 219, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Erythropoietin and mTOR: A “One-Two Punch” for Aging-Related Disorders Accompanied by Enhanced Life Expectancy. Curr. Neurovasc. Res. 2016, 13, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chong, Z.Z.; Shang, Y.C.; Maiese, K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr. Neurovasc. Res. 2012, 9, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Z.; Hou, X.; Sun, Y. LncRNA AFAP1-AS1 Promotes the Progression of Colorectal Cancer through miR-195-5p and WISP1. J. Oncol. 2021, 2021, 6242798. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Feng, Y.J.; Ji, R. High expression of WISP1 promotes metastasis and predicts poor prognosis in hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10445–10451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, S.H.; Hsu, P.W.; Chien, S.Y.; Wang, C.Q.; Su, C.M.; Dong, X.F.; Zhao, Y.M.; Tang, C.H. Impact of WNT1-inducible signaling pathway protein-1 (WISP-1) genetic polymorphisms and clinical aspects of breast cancer. Medicine 2019, 98, e17854. [Google Scholar] [CrossRef]
- Bitterman, K.J.; Anderson, R.M.; Cohen, H.Y.; Latorre-Esteves, M.; Sinclair, D.A. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J. Biol. Chem. 2002, 277, 45099–45107. [Google Scholar] [CrossRef]
- Bernardo-Bermejo, S.; Sanchez-Lopez, E.; Castro-Puyana, M.; Fernandez-Martinez, A.B.; Lucio-Cazana, F.J.; Marina, M.L. Exploring the Metabolic Differences between Cisplatin- and UV Light-Induced Apoptotic Bodies in HK-2 Cells by an Untargeted Metabolomics Approach. Int. J. Mol. Sci. 2023, 24, 7237. [Google Scholar] [CrossRef]
- Rani, S.; Dhar, S.B.; Khajuria, A.; Gupta, D.; Jaiswal, P.K.; Singla, N.; Kaur, M.; Singh, G.; Barnwal, R.P. Advanced Overview of Biomarkers and Techniques for Early Diagnosis of Alzheimer’s Disease. Cell. Mol. Neurobiol. 2023, 43, 2491–2523. [Google Scholar] [CrossRef]
- Hong, Y.; Flinkman, D.; Suomi, T.; Pietila, S.; James, P.; Coffey, E.; Elo, L.L. PhosPiR: An automated phosphoproteomic pipeline in R. Brief. Bioinform. 2022, 23, bbab510. [Google Scholar] [CrossRef] [PubMed]
- Mohebichamkhorami, F.; Niknam, Z.; Khoramjouy, M.; Heidarli, E.; Ghasemi, R.; Hosseinzadeh, S.; Mohseni, S.S.; Hajikarim-Hamedani, A.; Heidari, A.; Ghane, Y.; et al. Brain Homogenate of a Rat Model of Alzheimer’s Disease Modifies the Secretome of 3D Cultured Periodontal Ligament Stem Cells: A Potential Neuroregenerative Therapy. Iran. J. Pharm. Res. 2022, 21, e133668. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Gu, Q.; Li, Q.; Cui, Z. Nucleoporin 62-Like Protein is Required for the Development of Pharyngeal Arches through Regulation of Wnt/beta-Catenin Signaling and Apoptotic Homeostasis in Zebrafish. Cells 2019, 8, 1038. [Google Scholar] [CrossRef] [PubMed]
- Sanadgol, N.; Barati, M.; Houshmand, F.; Hassani, S.; Clarner, T.; Shahlaei, M.; Golab, F. Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of AMPK/Nrf2/mTOR signaling and maintenance of endogenous oligodendrogenesis during brain self-repairing period. Pharmacol. Rep. 2020, 72, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. The Oversight of Circadian Clock Genes for the Detection, Prevention, and Treatment of COVID-19 Infection. Curr. Neurovasc. Res. 2021, 18, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.S. Metformin Protects against Experimental Acrylamide Neuropathy in Rats. Drug Dev. Res. 2017, 78, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Amanollahi, M.; Jameie, M.; Heidari, A.; Rezaei, N. The Dialogue Between Neuroinflammation and Adult Neurogenesis: Mechanisms Involved and Alterations in Neurological Diseases. Mol. Neurobiol. 2023, 60, 923–959. [Google Scholar] [CrossRef]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brule, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J.; et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef]
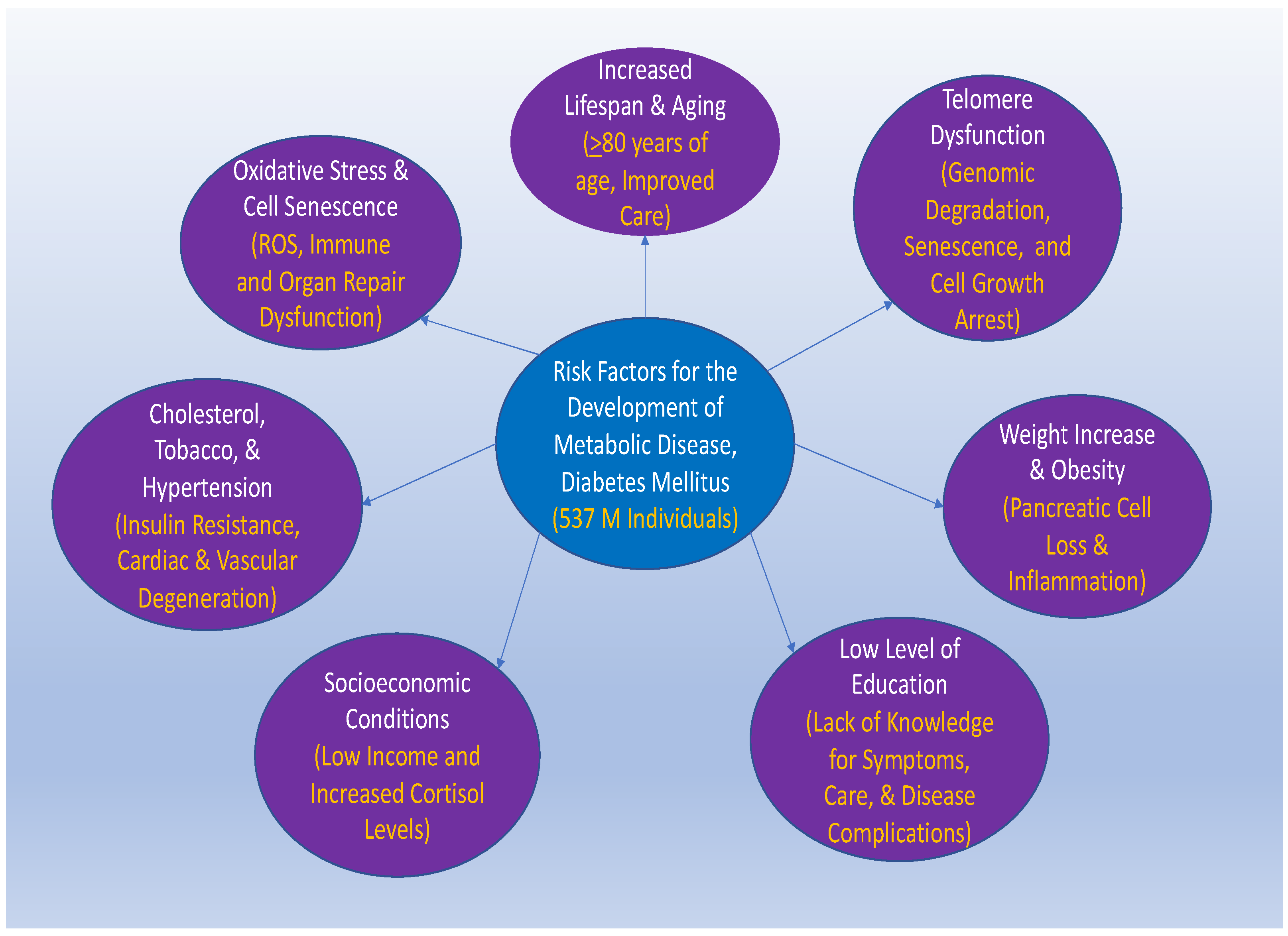
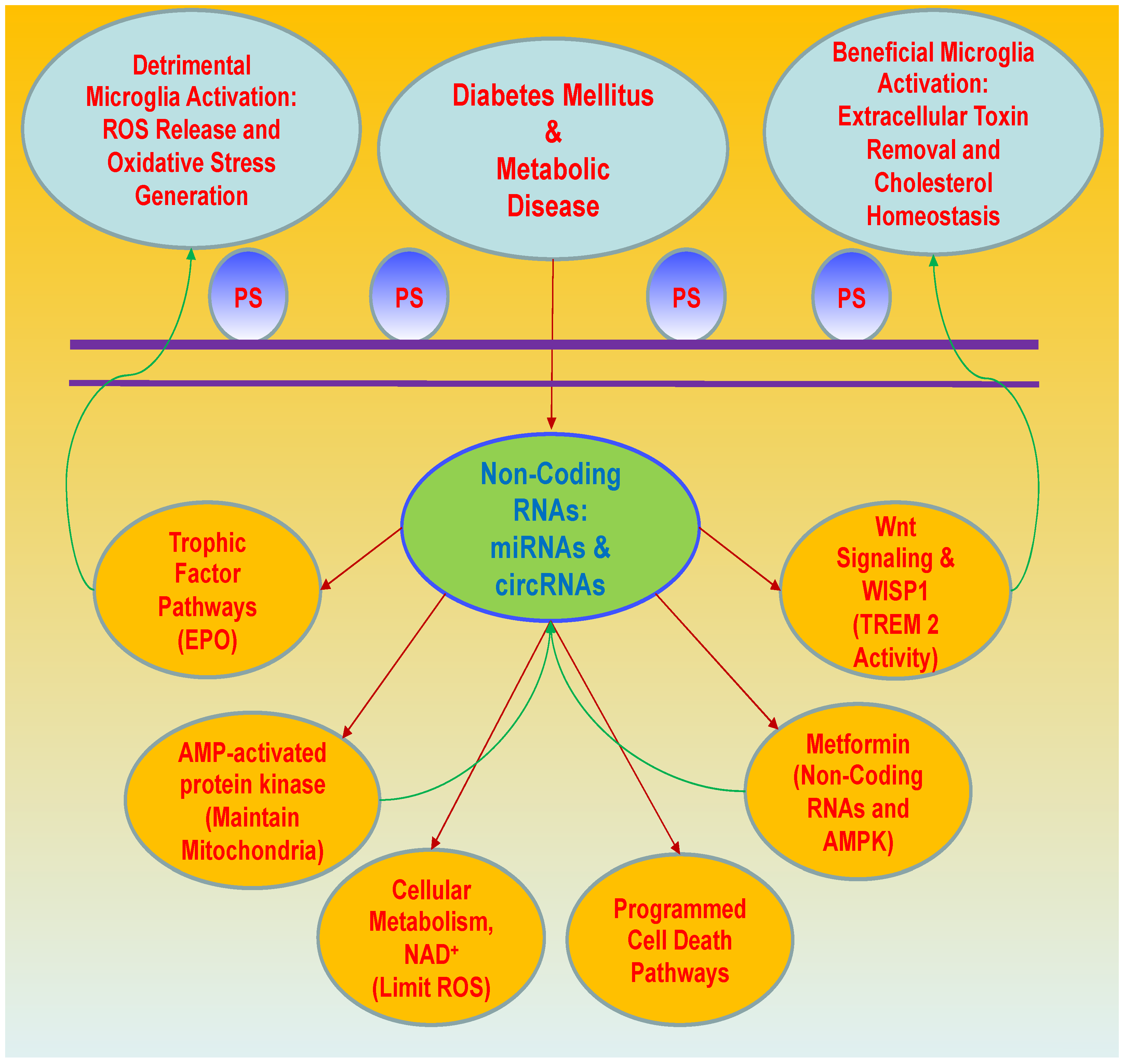
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiese, K. Cornerstone Cellular Pathways for Metabolic Disorders and Diabetes Mellitus: Non-Coding RNAs, Wnt Signaling, and AMPK. Cells 2023, 12, 2595. https://doi.org/10.3390/cells12222595
Maiese K. Cornerstone Cellular Pathways for Metabolic Disorders and Diabetes Mellitus: Non-Coding RNAs, Wnt Signaling, and AMPK. Cells. 2023; 12(22):2595. https://doi.org/10.3390/cells12222595
Chicago/Turabian StyleMaiese, Kenneth. 2023. "Cornerstone Cellular Pathways for Metabolic Disorders and Diabetes Mellitus: Non-Coding RNAs, Wnt Signaling, and AMPK" Cells 12, no. 22: 2595. https://doi.org/10.3390/cells12222595
APA StyleMaiese, K. (2023). Cornerstone Cellular Pathways for Metabolic Disorders and Diabetes Mellitus: Non-Coding RNAs, Wnt Signaling, and AMPK. Cells, 12(22), 2595. https://doi.org/10.3390/cells12222595






