Porcine UL-16 Binding Protein 1 Is Not a Functional Ligand for the Human Natural Killer Cell Activating Receptor NKG2D
Abstract
1. Introduction
2. Materials and Methods
2.1. Maintenance of Immortalized Porcine Liver-Derived Endothelial Cells (ipLDEC)
2.2. CRISPR/Cas9-Mediated Disruption of the Porcine ULBP-1 Gene
2.3. Binding of pULBP-1 Deficient Cells to Human NKG2D-Fc Chimera Protein
2.4. Human NK Cell Activation in Response to Porcine Endothelial Cell Stimulation
2.5. Calcein-AM Release Assay
2.6. Statistical Analysis
3. Results
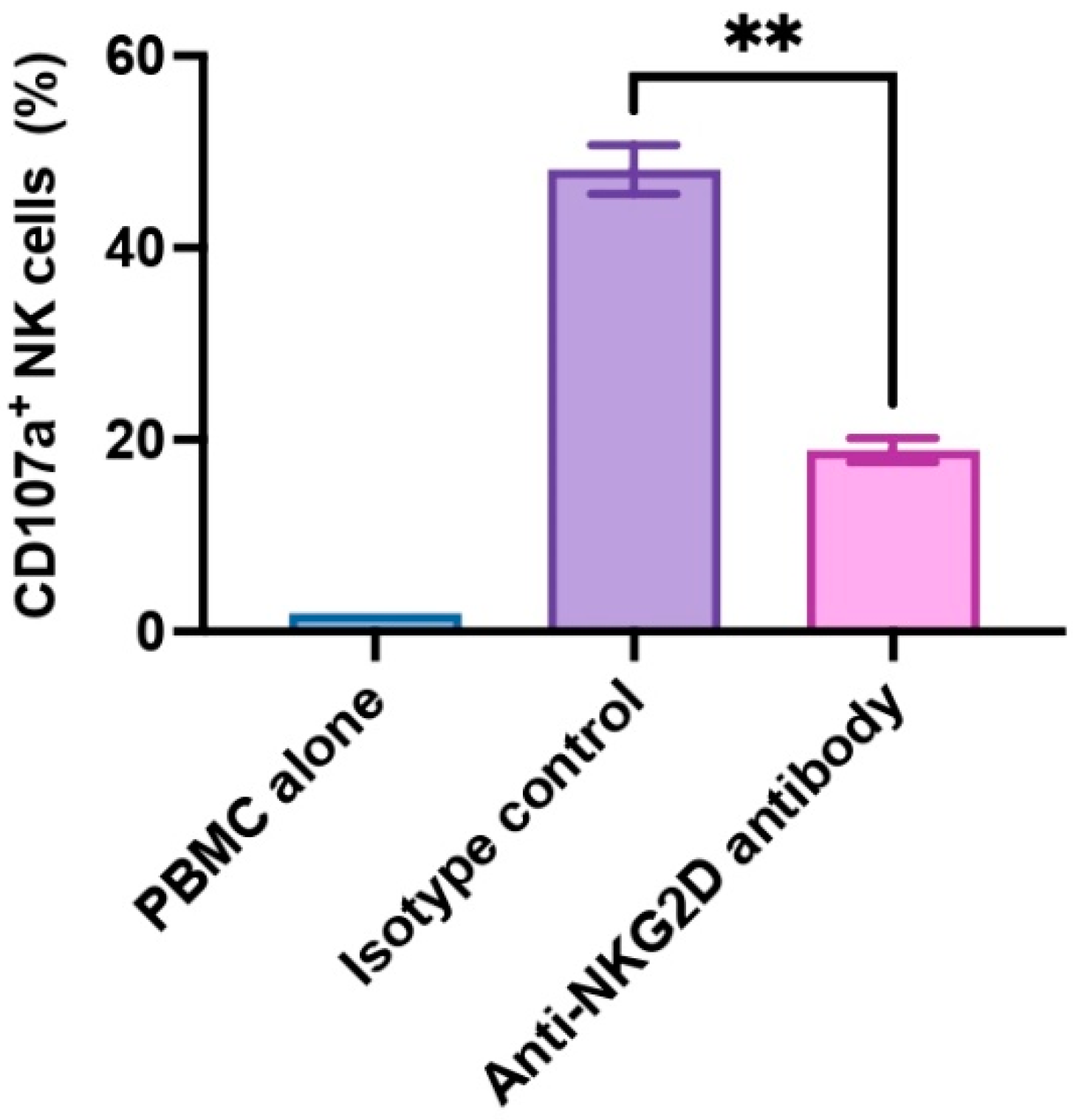
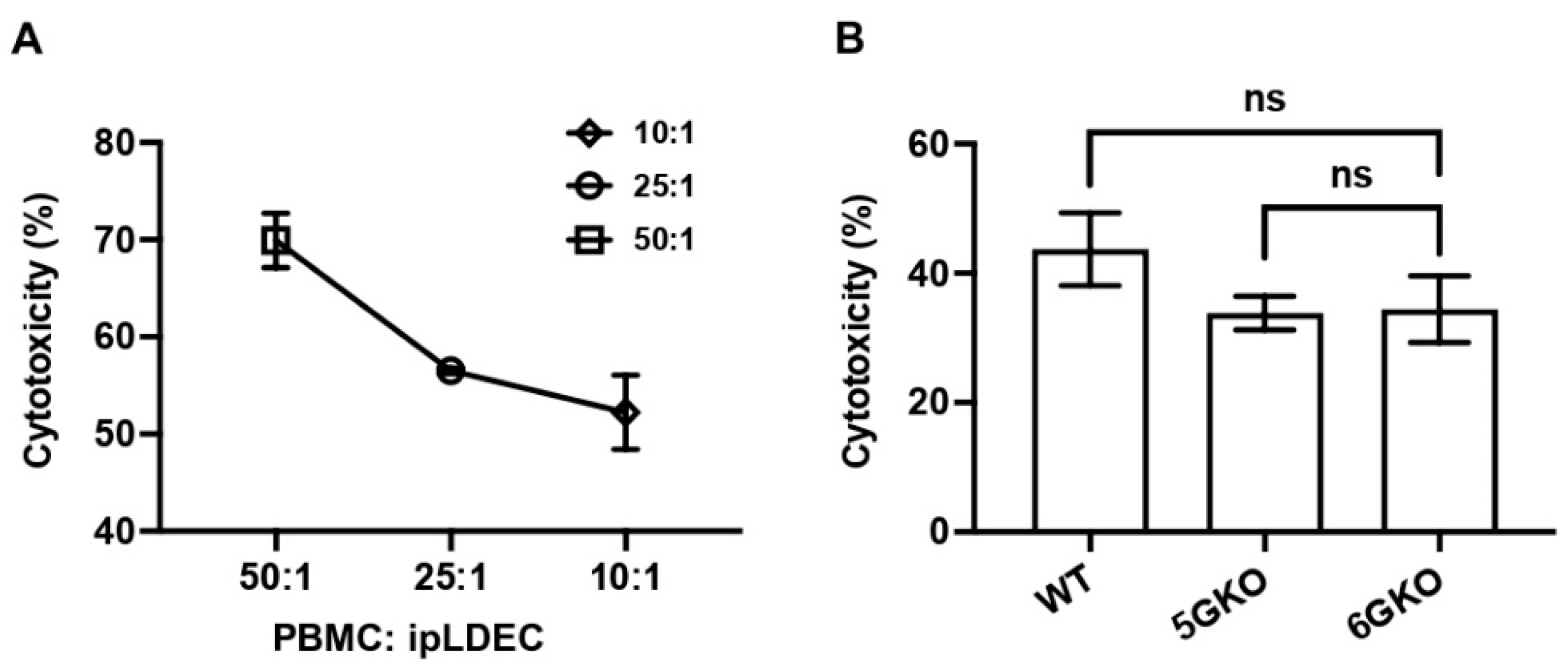
3.1. NKG2D Plays a Pivotal Role in Human NK Cell-to-Pig Endothelial Cell Xenogeneic Immune Response
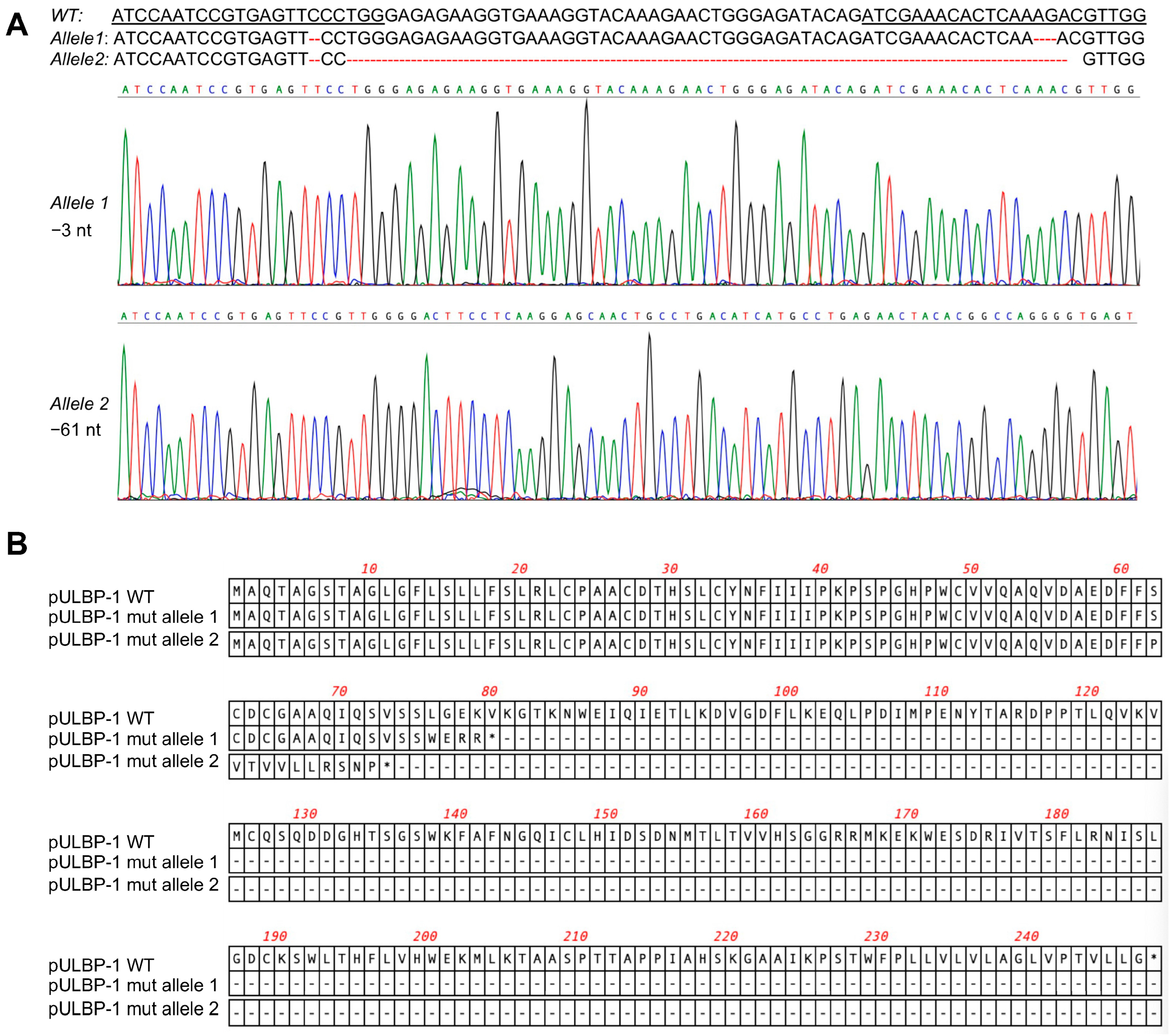
3.2. Disruption of pULBP-1 Gene in 5GKO Cells
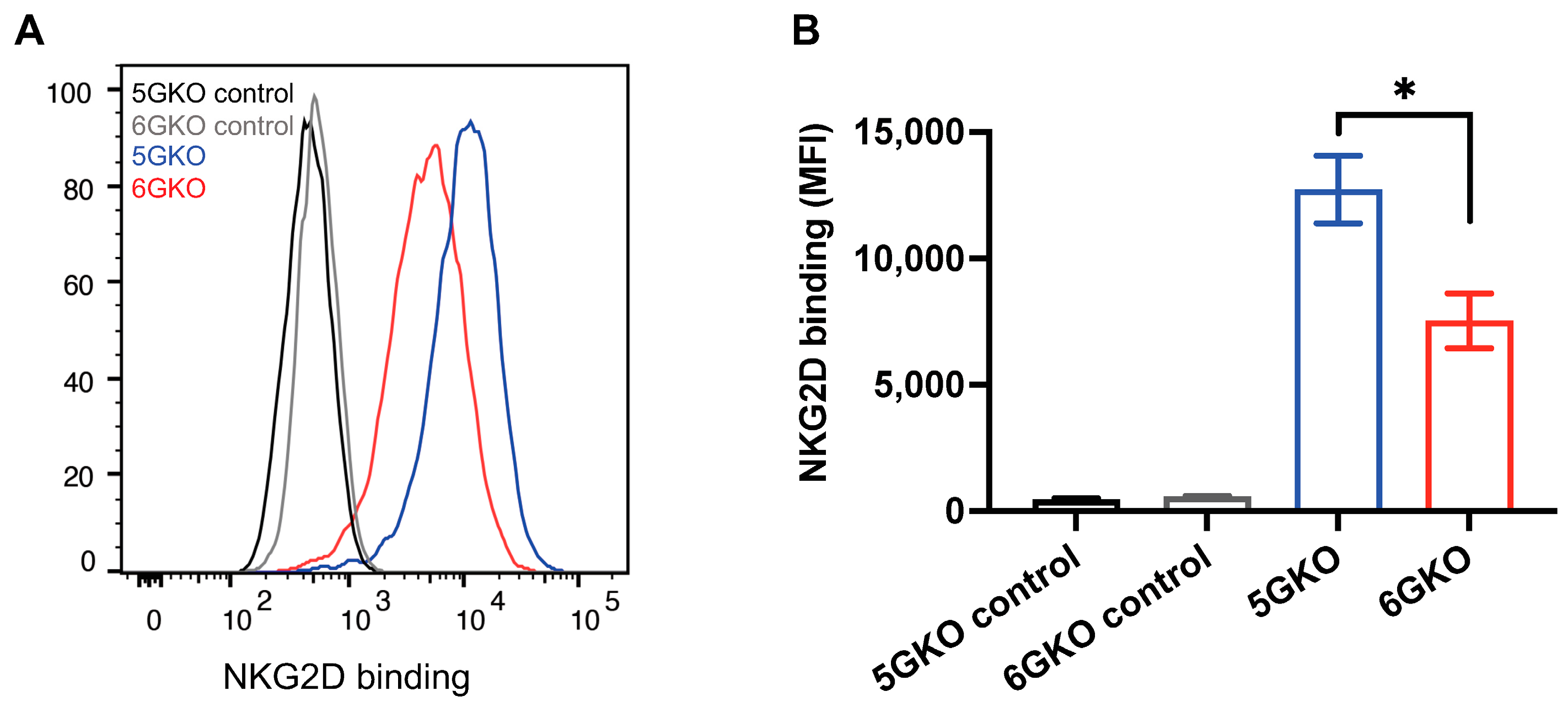
3.3. pULBP-1-Deficient Cells Exhibit a Reduced Binding Potential to NKG2D-Fc Chimera Protein
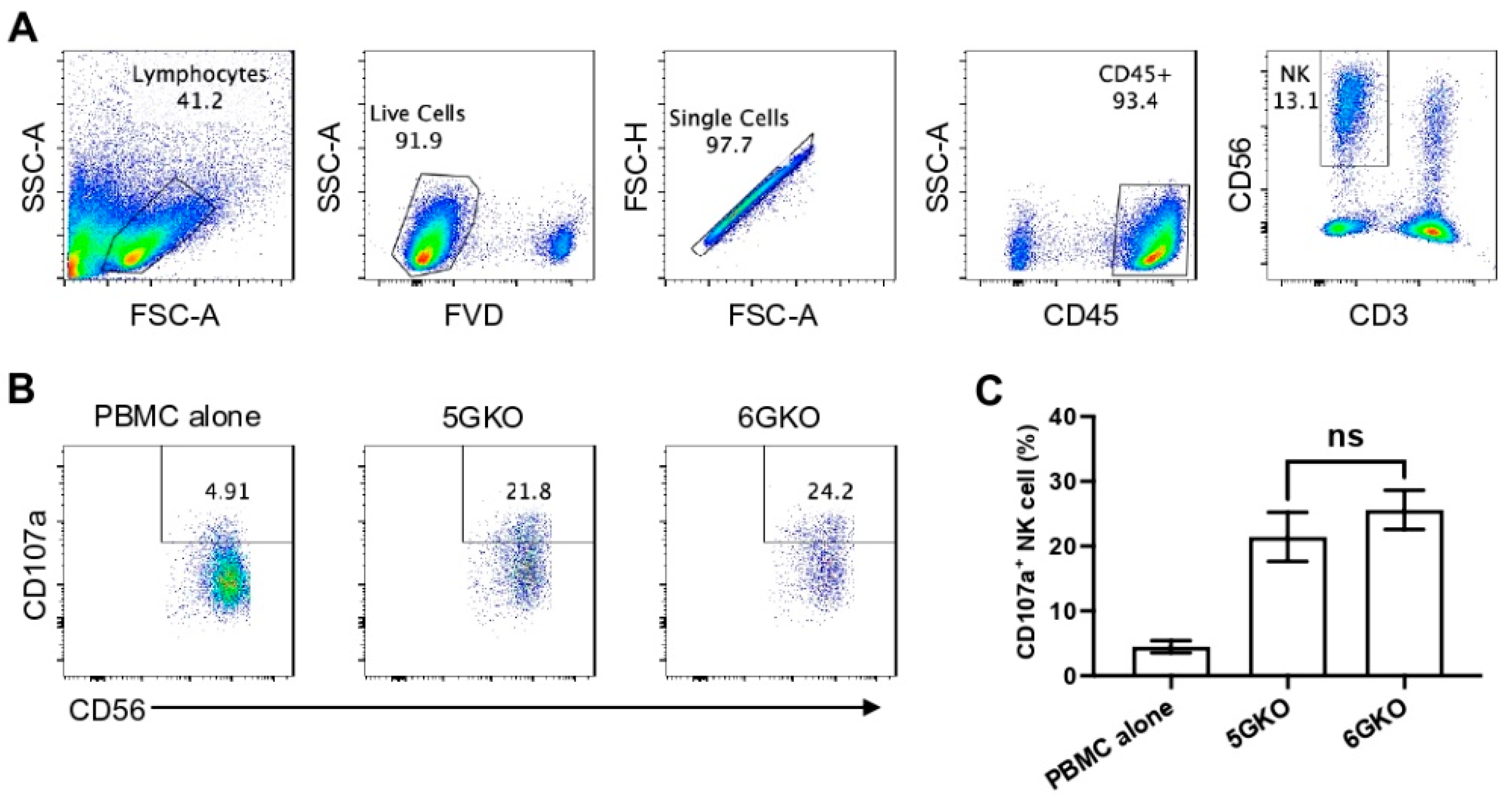
3.4. pULBP-1 Does Not Contribute to Xenogeneic Human NK Cell Activation
3.5. pULBP-1 on Porcine Cells Has No Effect on Human NK Cell-Mediated Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, D.K.; Ekser, B.; Ramsoondar, J.; Phelps, C.; Ayares, D. The role of genetically engineered pigs in xenotransplantation research. J. Pathol. 2016, 238, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yang, B.; Wang, R.; Qin, C. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2019, 10, 3060. [Google Scholar] [CrossRef] [PubMed]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically Modified Porcine-to-Human Cardiac Xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.M.; Singh, A.K.; Scobie, L.; Goerlich, C.E.; Grazioli, A.; Saharia, K.; Crossan, C.; Burke, A.; Drachenberg, C.; Oguz, C.; et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: A case report. Lancet 2023, 402, 397–410. [Google Scholar] [CrossRef]
- Yazdani, S.; Callemeyn, J.; Gazut, S.; Lerut, E.; de Loor, H.; Wevers, M.; Heylen, L.; Saison, C.; Koenig, A.; Thaunat, O.; et al. Natural killer cell infiltration is discriminative for antibody-mediated rejection and predicts outcome after kidney transplantation. Kidney Int. 2019, 95, 188–198. [Google Scholar] [CrossRef]
- Quan, D.; Bravery, C.; Chavez, G.; Richards, A.; Cruz, G.; Copeman, L.; Atkinson, C.; Holmes, B.; Davies, H.; Cozzi, E.; et al. Identification, detection, and in vitro characterization of cynomolgus monkey natural killer cells in delayed xenograft rejection of hDAF transgenic porcine renal xenografts. Transplant. Proc. 2000, 32, 936–937. [Google Scholar] [CrossRef]
- Bancroft, G.J. The role of natural killer cells in innate resistance to infection. Curr. Opin. Immunol. 1993, 5, 503–510. [Google Scholar] [CrossRef]
- Yin, D.; Zeng, H.; Ma, L.; Shen, J.; Xu, H.; Byrne, G.W.; Chong, A.S. Cutting Edge: NK cells mediate IgG1-dependent hyperacute rejection of xenografts. J. Immunol. 2004, 172, 7235–7238. [Google Scholar] [CrossRef]
- Forte, P.; Lilienfeld, B.G.; Baumann, B.C.; Seebach, J.D. Human NK cytotoxicity against porcine cells is triggered by NKp44 and NKG2D. J. Immunol. 2005, 175, 5463–5470. [Google Scholar] [CrossRef]
- Kim, T.J.; Kim, N.; Kim, E.O.; Choi, J.R.; Bluestone, J.A.; Lee, K.M. Suppression of human anti-porcine natural killer cell xenogeneic responses by combinations of monoclonal antibodies specific to CD2 and NKG2D and extracellular signal-regulated kinase kinase inhibitor. Immunology 2010, 130, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.C.; Goodman, J.; Sasaki, H.; Lowell, J.; Mohanakumar, T. Activation of natural killer cells and macrophages by porcine endothelial cells augments specific T-cell xenoresponse. Am. J. Transplant. 2002, 2, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.C.; Campos, E.F.; Saraiva Camara, N.O.; David, D.S.; Malheiros, D.M. Compartment-specific expression of natural killer cell markers in renal transplantation: Immune profile in acute rejection. Transpl. Int. 2016, 29, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Birmele, B.; Thibault, G.; Nivet, H.; Gruel, Y.; Bardos, P.; Lebranchu, Y. Human lymphocyte adhesion to xenogeneic porcine endothelial cells: Modulation by human TNF-alpha and involvement of VLA-4 and LFA-1. Transpl. Immunol. 1996, 4, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Walsh, J.R.; Lopez, K.; Isidan, A.; Zhang, W.; Chen, A.M.; Goggins, W.C.; Higgins, N.G.; Liu, J.; Brutkiewicz, R.R.; et al. Genetic engineering of porcine endothelial cell lines for evaluation of human-to-pig xenoreactive immune responses. Sci. Rep. 2021, 11, 13131. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Oettinger, H.F.; Sachs, D.H.; Edge, A.S. Analysis of polymorphism in porcine MHC class I genes: Alterations in signals recognized by human cytotoxic lymphocytes. J. Immunol. 1997, 159, 2318–2326. [Google Scholar] [CrossRef]
- Seebach, J.D.; Comrack, C.; Germana, S.; LeGuern, C.; Sachs, D.H.; DerSimonian, H. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J. Immunol. 1997, 159, 3655–3661. [Google Scholar] [CrossRef]
- Cosman, D.; Mullberg, J.; Sutherland, C.L.; Chin, W.; Armitage, R.; Fanslow, W.; Kubin, M.; Chalupny, N.J. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001, 14, 123–133. [Google Scholar] [CrossRef]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef]
- Cerwenka, A.; Bakker, A.B.; McClanahan, T.; Wagner, J.; Wu, J.; Phillips, J.H.; Lanier, L.L. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 2000, 12, 721–727. [Google Scholar] [CrossRef]
- Diefenbach, A.; Jamieson, A.M.; Liu, S.D.; Shastri, N.; Raulet, D.H. Ligands for the murine NKG2D receptor: Expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 2000, 1, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Borges, C.N.; Phanavanh, B.; Saraswati, S.; Dennis, R.A.; Crew, M.D. Molecular cloning and characterization of a porcine UL16 binding protein (ULBP)-like cDNA. Mol. Immunol. 2005, 42, 665–671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chardon, P.; Rogel-Gaillard, C.; Cattolico, L.; Duprat, S.; Vaiman, M.; Renard, C. Sequence of the swine major histocompatibility complex region containing all non-classical class I genes. Tissue Antigens 2001, 57, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, B.G.; Garcia-Borges, C.; Crew, M.D.; Seebach, J.D. Porcine UL16-binding protein 1 expressed on the surface of endothelial cells triggers human NK cytotoxicity through NKG2D. J. Immunol. 2006, 177, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.D.; Christiansen, D.; Winterhalter, A.; Brooks, A.; Gorrell, M.; Lilienfeld, B.G.; Seebach, J.D.; Sandrin, M.; Sharland, A. Porcine cells express more than one functional ligand for the human lymphocyte activating receptor NKG2D. Xenotransplantation 2008, 15, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Estrada, J.; Zhang, F.; Waghmare, S.K.; Mir, B. Isolation, characterization, and nuclear reprogramming of cell lines derived from porcine adult liver and fat. Cell Reprogram 2010, 12, 599–607. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Li, P.; Estrada, J.L.; Burlak, C.; Montgomery, J.; Butler, J.R.; Santos, R.M.; Wang, Z.Y.; Paris, L.L.; Blankenship, R.L.; Downey, S.M.; et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation 2015, 22, 20–31. [Google Scholar] [CrossRef]
- Cross-Najafi, A.A.; Farag, K.; Isidan, A.; Li, W.; Zhang, W.; Lin, Z.; Walsh, J.R.; Lopez, K.; Park, Y.; Higgins, N.G.; et al. Co-expression of HLA-E and HLA-G on genetically modified porcine endothelial cells attenuates human NK cell-mediated degranulation. Front. Immunol. 2023, 14, 1217809. [Google Scholar] [CrossRef]
- Neri, S.; Mariani, E.; Meneghetti, A.; Cattini, L.; Facchini, A. Calcein-acetyoxymethyl cytotoxicity assay: Standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin. Diagn. Lab. Immunol. 2001, 8, 1131–1135. [Google Scholar] [CrossRef]
- Kerdiles, Y.; Ugolini, S.; Vivier, E. T cell regulation of natural killer cells. J. Exp. Med. 2013, 210, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, B.G.; Schildknecht, A.; Imbach, L.L.; Mueller, N.J.; Schneider, M.K.; Seebach, J.D. Characterization of porcine UL16-binding protein 1 endothelial cell surface expression. Xenotransplantation 2008, 15, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Joanna, Z.; Magdalena, H.; Agnieszka, N.T.; Jacek, J.; Ryszard, S.; Zdzislaw, S.; Barbara, G.; Daniel, L. The production of UL16-binding protein 1 targeted pigs using CRISPR technology. 3 Biotech. 2018, 8, 70. [Google Scholar] [CrossRef]
- Forte, P.; Matter-Reissmann, U.B.; Strasser, M.; Schneider, M.K.; Seebach, J.D. Porcine aortic endothelial cells transfected with HLA-G are partially protected from xenogeneic human NK cytotoxicity. Hum. Immunol. 2000, 61, 1066–1073. [Google Scholar] [CrossRef]
- Weiss, E.H.; Lilienfeld, B.G.; Muller, S.; Muller, E.; Herbach, N.; Kessler, B.; Wanke, R.; Schwinzer, R.; Seebach, J.D.; Wolf, E.; et al. HLA-E/human beta2-microglobulin transgenic pigs: Protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation 2009, 87, 35–43. [Google Scholar] [CrossRef]
- Lilienfeld, B.G.; Crew, M.D.; Forte, P.; Baumann, B.C.; Seebach, J.D. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation 2007, 14, 126–134. [Google Scholar] [CrossRef]
- Lopez, K.J.; Cross-Najafi, A.A.; Farag, K.; Obando, B.; Thadasina, D.; Isidan, A.; Park, Y.; Zhang, W.; Ekser, B.; Li, P. Strategies to induce natural killer cell tolerance in xenotransplantation. Front. Immunol. 2022, 13, 941880. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, K.J.; Spence, J.P.; Li, W.; Zhang, W.; Wei, B.; Cross-Najafi, A.A.; Butler, J.R.; Cooper, D.K.C.; Ekser, B.; Li, P. Porcine UL-16 Binding Protein 1 Is Not a Functional Ligand for the Human Natural Killer Cell Activating Receptor NKG2D. Cells 2023, 12, 2587. https://doi.org/10.3390/cells12222587
Lopez KJ, Spence JP, Li W, Zhang W, Wei B, Cross-Najafi AA, Butler JR, Cooper DKC, Ekser B, Li P. Porcine UL-16 Binding Protein 1 Is Not a Functional Ligand for the Human Natural Killer Cell Activating Receptor NKG2D. Cells. 2023; 12(22):2587. https://doi.org/10.3390/cells12222587
Chicago/Turabian StyleLopez, Kevin J., John Paul Spence, Wei Li, Wenjun Zhang, Barry Wei, Arthur A. Cross-Najafi, James R. Butler, David K. C. Cooper, Burcin Ekser, and Ping Li. 2023. "Porcine UL-16 Binding Protein 1 Is Not a Functional Ligand for the Human Natural Killer Cell Activating Receptor NKG2D" Cells 12, no. 22: 2587. https://doi.org/10.3390/cells12222587
APA StyleLopez, K. J., Spence, J. P., Li, W., Zhang, W., Wei, B., Cross-Najafi, A. A., Butler, J. R., Cooper, D. K. C., Ekser, B., & Li, P. (2023). Porcine UL-16 Binding Protein 1 Is Not a Functional Ligand for the Human Natural Killer Cell Activating Receptor NKG2D. Cells, 12(22), 2587. https://doi.org/10.3390/cells12222587






