Heavy Metals in Follicular Fluid Affect the Ultrastructure of the Human Mature Cumulus-Oocyte Complex
Abstract
Highlights
- For the first time, ultrastructural alterations were detected using transmission electron microscopy in human cumulus-oocyte complexes (COCs) sampled from follicles with Pb and Cd levels in the FF of infertile women subjected to assisted reproductive technologies.
- The intrafollicular presence of these metals could be responsible for morphological alterations in some cell organelles, which may lead to altered maturation and quality of oocytes, impairment of energetic metabolism, cellular dysfunction, and apoptosis of CCs.
- Since blood Cd levels were above the current reference values established by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA), whereas blood Pb levels were below the ATSDR reference values, we believe that these alterations could be due especially to Cd, even if we cannot exclude a possible additional effect of Pb.
- Cd levels in FF may influence the oocyte directly and indirectly (CC-mediated), affecting its quality, as well as fertilization, early embryonic development, and pregnancy.
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Sample Collection
2.3.1. FF and COCs Sampling
2.3.2. Cd Detection Using Atomic Absorption Spectrometry
2.3.3. Statistical Analyses
2.4. Electron Microscopy
3. Results
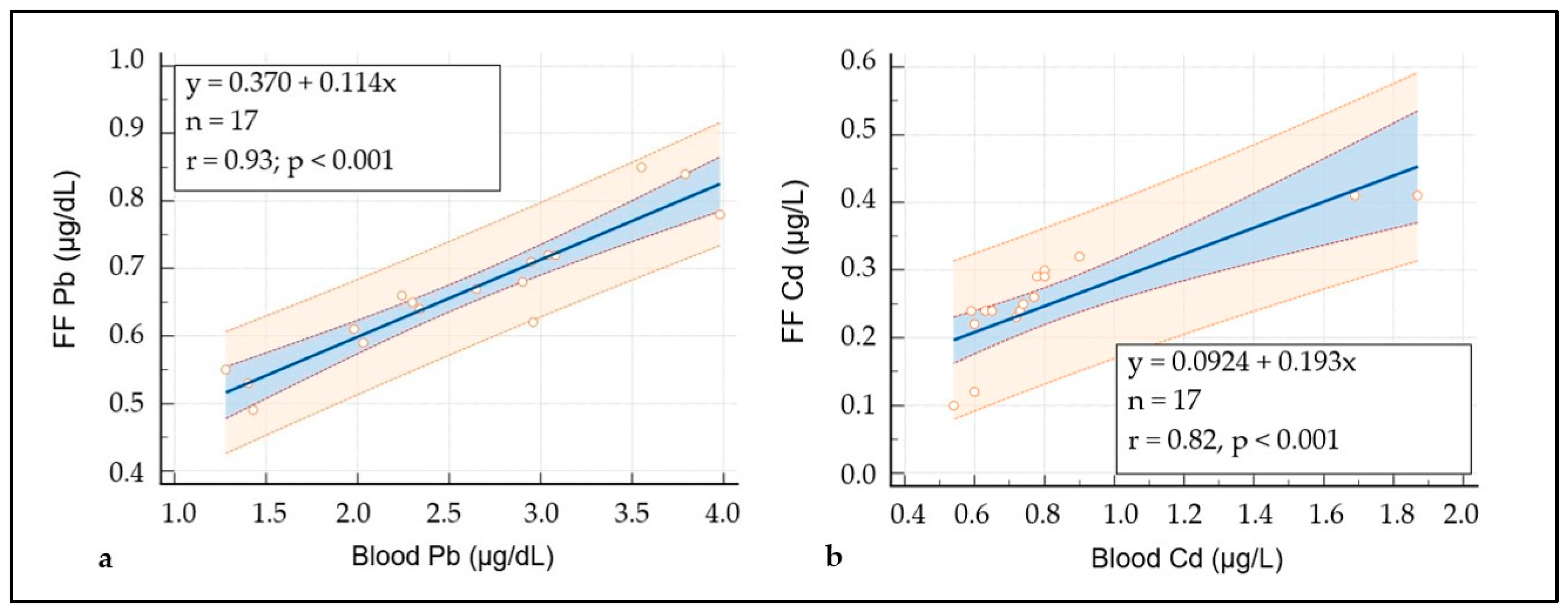
3.1. Characteristics of the Study Population and Heavy Metal’s Measurements
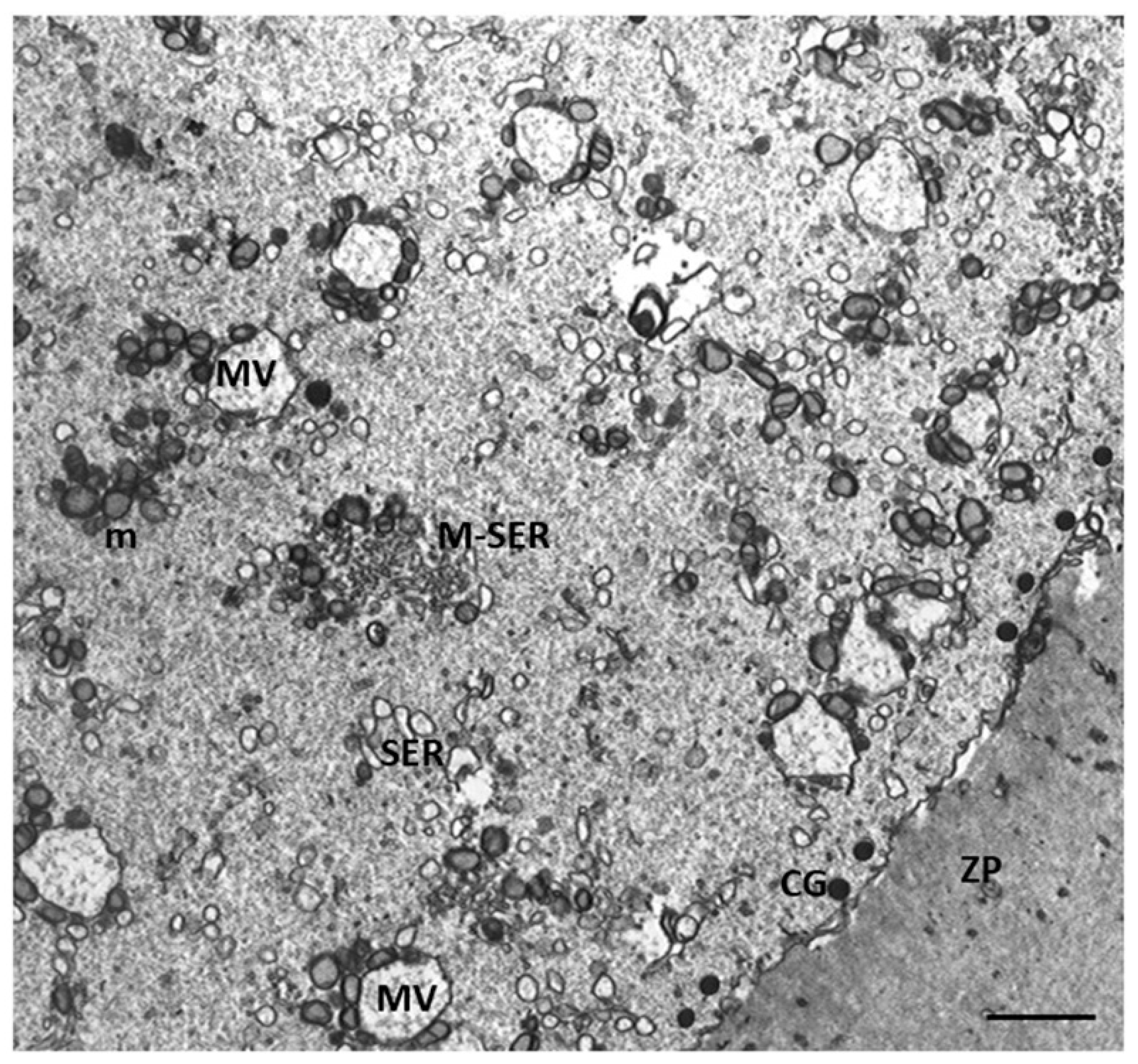
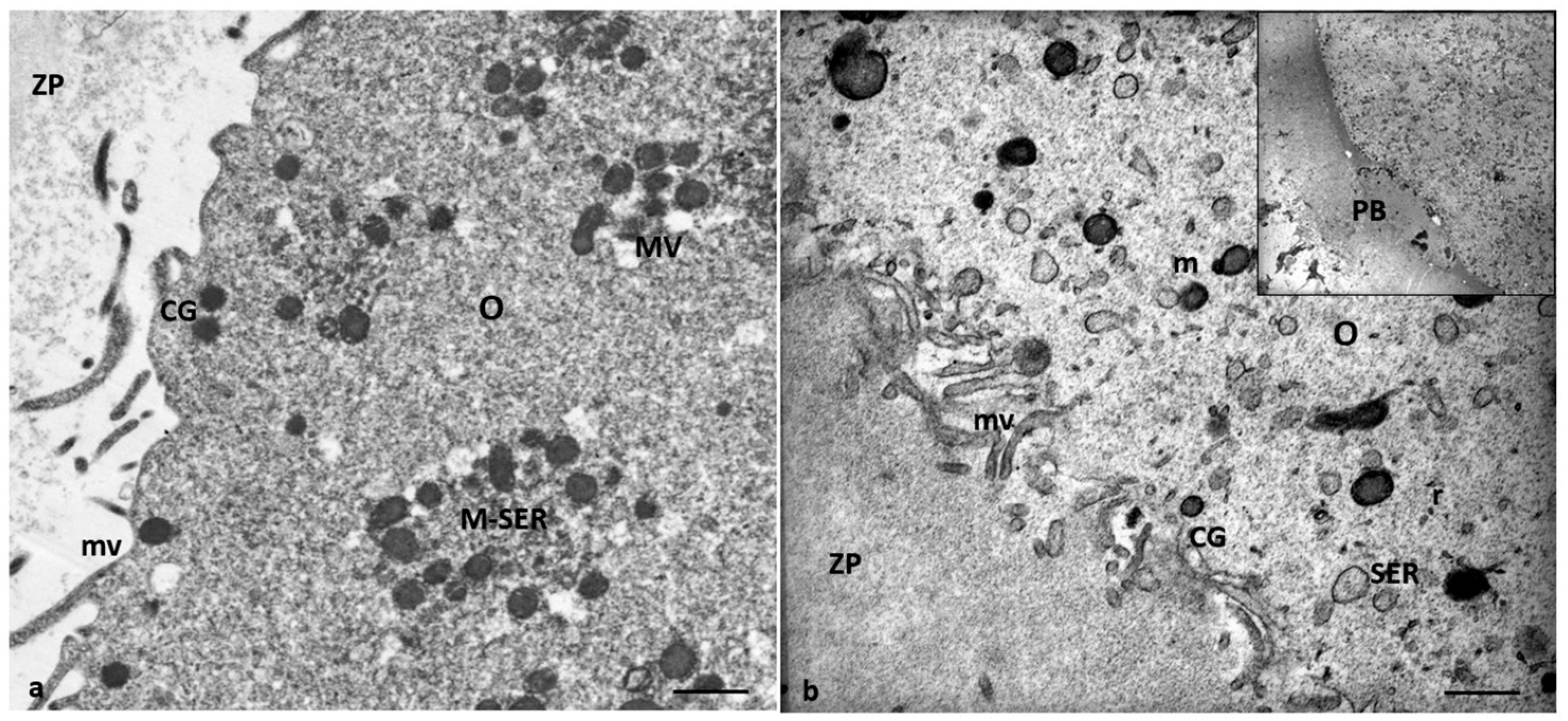
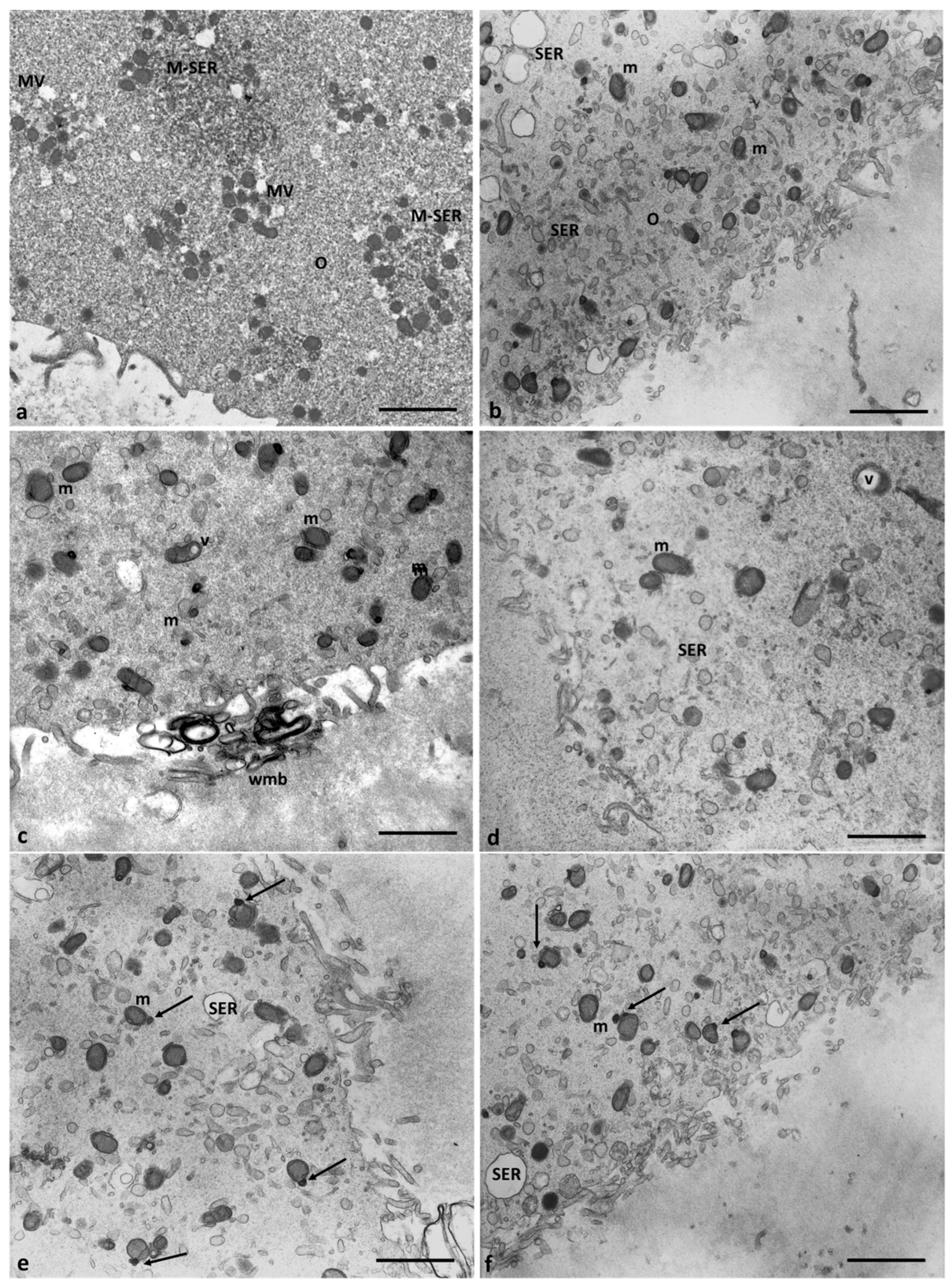
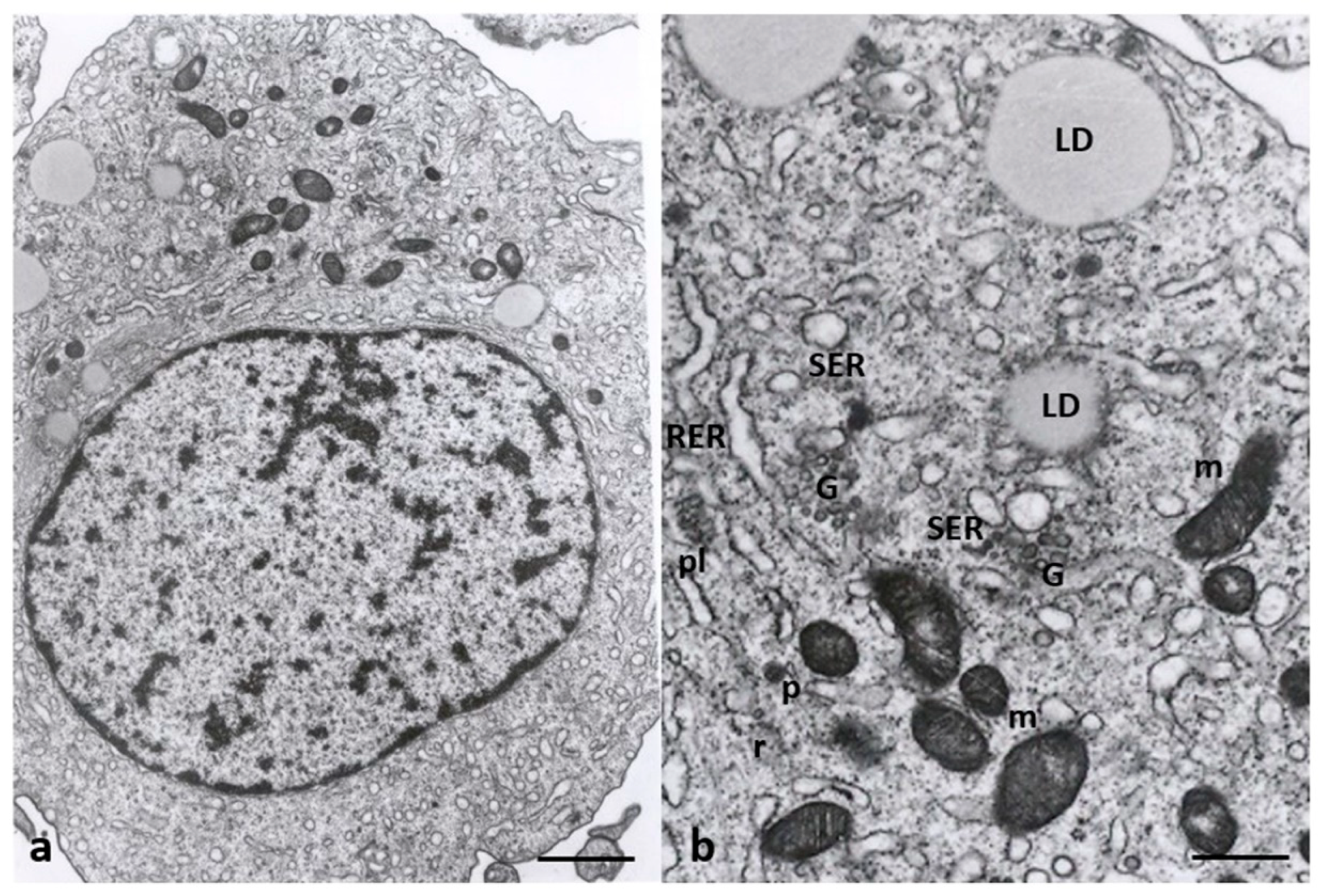
3.2. Electron Microscopy
4. Discussion
4.1. Oocyte Ultrastructural Changes That Are Heavy Metals Dependent
4.2. Cumulus Cells Ultrastructural Changes That Are Heavy Metals Dependent
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 3, 167–176. [Google Scholar] [CrossRef]
- Caserta, D.; Maranghi, L.; Mantovani, A.; Marci, R.; Maranghi, F.; Moscarini, M. Impact of endocrine disruptor chemicals in gynaecology. Hum. Reprod. Update 2008, 14, 59–72. [Google Scholar] [CrossRef]
- Caserta, D.; Mantovani, A.; Marci, R.; Fazi, A.; Ciardo, F.; La Rocca, C.; Maranghi, F.; Moscarini, M. Environment and women’s reproductive health. Hum. Reprod. Update 2011, 17, 418–433. [Google Scholar] [CrossRef]
- Caserta, D.; De Marco, M.P.; Besharat, A.R.; Costanzi, F. Endocrine Disruptors and Endometrial Cancer: Molecular Mechanisms of Action and Clinical Implications, a Systematic Review. Int. J. Mol. Sci. 2022, 23, 2956. [Google Scholar] [CrossRef]
- Ghorbani, M.R.; Ghanavati, N.; Babaenejad, T.; Nazarpour, A.; Payandeh, K. Assessment of the potential ecological and human health risks of heavy metals in Ahvaz oil field, Iran. PLoS ONE 2020, 15, e242703. [Google Scholar] [CrossRef]
- Bloom, M.S.; Fujimoto, V.Y.; Steuerwald, A.J.; Cheng, G.; Browne, R.W.; Parsons, P.J. Background exposure to toxic metals in women adversely influences pregnancy during in vitro fertilization (IVF). Reprod. Toxicol. 2012, 34, 471–481. [Google Scholar] [CrossRef]
- Shen, L.; Liang, C.; Li, D.; Zhang, Z.; Wang, X.; Jiang, T.; Sua, X.; Yina, T.; Zoua, W.; Wang, X.; et al. The association between exposure to multiple toxic metals and the risk of endometriosis: Evidence from the results of blood and follicular fluid. Sci. Total Environ. 2023, 855, 158882. [Google Scholar] [CrossRef]
- La Llave León, O.; Pacheco, M.S. Effects of Lead on Reproductive Health. In Lead Chemistry; License CC BY 3.0; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Zhao, L.-L.; Ru, Y.-f.; Liu, M.; Tang, J.-n.; Zheng, J.-f.; Wu, B.; Gu, Y.; Shi, H.-j. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS ONE 2017, 12, e0186727. [Google Scholar] [CrossRef]
- Caserta, D.; Costanzi, F.; De Marco, M.P.; Di Benedetto, L.; Matteucci, E.; Assorgi, C.; Pacilli, M.C.; Besharat, A.R.; Bellati, F.; Ruscito, I. Effects of Endocrine-Disrupting Chemicals on Endometrial Receptivity and Embryo Implantation: A Systematic Review of 34 Mouse Model Studies. Int. J. Environ. Res. Public Health 2021, 25, 6840. [Google Scholar] [CrossRef]
- Dong, F.; Lia, J.; Leia, W.-L.; Wang, F.; Wanga, Y.; Ouyanga, Y.-C.; Houa, Y.; Wanga, Z.-B.; Schattenc, H.; Suna, Q.Y. Chronic cadmium exposure causes oocyte meiotic arrest by disrupting spindle assembly checkpoint and maturation promoting factor. Reprod. Toxicol. 2020, 96, 141–149. [Google Scholar] [CrossRef]
- Zhu, J.-Q.; Liu, Y.; Zhang, J.-H.; Liu, Y.-F.; Cao, J.-Q.; Huang, Z.-T.; Yuan, Y.; Bian, J.-C.; Liu, Z.-P. Cadmium Exposure of Female Mice Impairs the Meiotic Maturation of Oocytes and Subsequent Embryonic Development. Toxicol. Sci. 2018, 164, 289–299. [Google Scholar] [CrossRef]
- Tolunay, H.E.; Yavuz Emre Sukur, Y.E.; Ozkavukcu, S.; Seval, M.M.; Ates, C.; Turksoy, V.A.; Ecemis, T.; Atabekoglu, C.M.; Ozmen, B.; Berker, B.; et al. Heavy metal and trace element concentrations in blood and follicular fluid affect ART outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 73–77. [Google Scholar] [CrossRef]
- Mohr, M.F.E.; Faris, F.; Bakry, S.; Hozyen, H.; Elshaer, F.M. Effect of Heavy Metals Levels in Follicular Fluid on ICSI Outcome. Egypt. Acad. J. Biol. Sci. 2020, 12, 87–95. [Google Scholar]
- Rodríguez-Díaz, R.; Blanes-Zamora, R.; Paz-Montelongo, S.; Gómez-Rodríguez, J.; Rodríguez Fiestas, S.; González-Weller, D.; Gutiérrez, A.J.; Rubio, C.; Hardisson, A.; Niebla-Canelo, D.; et al. The Influence of Follicular Fluid Metals on Assisted Reproduction Outcome. Biol. Trace Elem. Res. 2023, 201, 5069–5082. [Google Scholar] [CrossRef]
- Levay, P.E.; Huyser, C.; Fourie, F.L.R.; Rossouw, D.J. The Detection of Blood Contamination in Human Follicular Fluid. J. Assist. Reprod. Genet. 1997, 14, 212–217. [Google Scholar] [CrossRef]
- Butt, C.D.; Blooma, M.S.; McGoughd, A.; Lenhartd, N.; Wong, R.; Mok-Lind, E.; Parsonsa, P.J.; Galushaa, A.L.; Yucelg, R.M.; Feingolda, B.J.; et al. Variability of essential and non-essential trace elements in the follicular fluid of women undergoing in vitro fertilization (IVF). Ecotoxicol. Environ. Saf. 2021, 209, 111733. [Google Scholar] [CrossRef]
- Miglietta, S.; Cristiano, L.; Espinola, M.S.B.; Masiello, M.G.; Micara, G.; Battaglione, E.; Linari, A.; Palmerini, M.G.; Familiari, G.; Aragona, C.; et al. Effects of Simulated Microgravity In Vitro on Human Metaphase II Oocytes: An Electron Microscopy-Based Study. Cells 2023, 12, 1346. [Google Scholar] [CrossRef]
- Arabatzis, T. The discovery of the zeeman effect: A case study of the interplay Between theory and experiment. Stud. Hist. Phil. Sci. 1992, 23, 365–388. [Google Scholar] [CrossRef]
- Obaid Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Lead; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2020. [Google Scholar]
- WHO. Guideline for Clinical Management of Exposure to Lead: Executive Summary; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Alimonti, A.; Bocca, B.; Mattei, D.; Pino, A. Biomonitoraggio della popolazione italiana per l’esposizione ai metalli: Valori di riferimento 1990–2009. Rapp. Istisan 2010, 10, 22. [Google Scholar]
- Nottola, S.A.; Familiari, G.; Micara, G.; Aragona, C.; Motta, P.M. The ultrastructure of human cumulus-corona cells at the time of fertilization and early embryogenesis. A scanning and transmission electron microscopic study in an in vitro fertilization program. Arch. Histol. Cytol. 1991, 54, 145–161. [Google Scholar] [CrossRef]
- Motta, P.M.; Nottola, S.A.; Pereda, J.; Croxatto, H.B.; Familiari, G. Ultrastructure of human cumulus oophorus: A transmission electron microscopic study on oviductal oocytes and fertilized eggs. Hum. Reprod. 1995, 10, 2361–2367. [Google Scholar] [CrossRef]
- Martino, N.A.; Marzanob, G.; Mangiacottia, M.; Miedicoa, O.; Sardanellic, A.M.; Gnonic, A.; Lacalandrae, G.M.; Chiaravallea, A.E.; Cianib, E.; Bogliolof, L.; et al. Exposure to cadmium during in vitro maturation at environmental nanomolar levels impairs oocyte fertilization through oxidative damage: A large animal model study. Reprod. Toxic. 2017, 69, 132–145. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef]
- Biagioli, M.; Pifferi, S.; Ragghianti, M.; Bucci, S.; Rizzuto, R.; Pinton, P. Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium 2008, 43, 184–195. [Google Scholar] [CrossRef]
- Kitamura, M.; Hiramatsu, N. The oxidative stress: Endoplasmic reticulum stress axis in cadmium toxicity. Biometals 2010, 23, 941–950. [Google Scholar] [CrossRef]
- Rajakumar, S.; Bhanupriya, N.; Ravi, C.; Nachiappan, V. Endoplasmic reticulum stress and calcium imbalance are involved in cadmium-induced lipid aberrancy in Saccharomyces cerevisiae. Cell Stress Chaperones 2016, 21, 895–906. [Google Scholar] [CrossRef]
- Wang, T.; Yuan, Y.; Zou, H.; Yang, J.; Zhao, S.; Ma, Y.; Wang, Y.; Bian, J.; Liu, X.; Gu, J.; et al. The ER stress regulator Bip mediates cadmium-induced autophagy and neuronal senescence. Sci. Rep. 2016, 6, 38091. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, S.; Zhang, J.; Xu, S.; Miao, Z. Cadmium induces endoplasmic reticulum stress-mediated apoptosis in pig pancreas via the increase of Th1 cells. Toxicology 2021, 457, 152790. [Google Scholar] [CrossRef]
- De Benedictis, M.; Gallo, A.; Migoni, D.; Papadia, P.; Roversi, P.; Santino, A. Cadmium treatment induces endoplasmic reticulum stress and unfolded protein response in Arabidopsis thaliana. Plant Physiol. Biochem. 2023, 196, 281–290. [Google Scholar] [CrossRef]
- Caserta, D.; Pegoraro, S.; Mallozzi, M.; Di Benedetto, L.; Colicino, E.; Lionetto, L.; Simmaco, M. Maternal exposure to endocrine disruptors and placental transmission: A pilot study. Gynecol. Endocrinol. 2018, 34, 1001–1004. [Google Scholar] [CrossRef]
- Burton, G.J.; Yung, H.W.; Murray, A.J. Mitochondrial—Endoplasmic reticulum interactions in the trophoblast: Stress and senescence. Placenta 2017, 52, 146–155. [Google Scholar] [CrossRef]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 2018, 69, 62–72. [Google Scholar] [CrossRef]
- Ragusa, A.; Matta, M.; Cristiano, L.; Matassa, R.; Battaglione, E.; Svelato, A.; De Luca, C.; D’Avino, S.; Gulotta, A.; Ciro, M.; et al. Deeply in Plasticenta: Presence of Microplastics in the Intracellular Compartment of Human Placentas. Int. J. Environ. Res. Public Health 2022, 19, 11593. [Google Scholar] [CrossRef]
- Pu, S.; Pan, Y.; Zhang, Q.; You, T.; Yue, T.; Zhang, Y.; Wang, M. Endoplasmic Reticulum Stress and Mitochondrial Stress in Drug-Induced Liver Injury. Molecules 2023, 28, 3160. [Google Scholar] [CrossRef]
- Xavier, V.J.; Martinou, J.C. RNA Granules in the Mitochondria and Their Organization under Mitochondrial Stresses. Int. J. Mol. Sci. 2021, 22, 9502. [Google Scholar] [CrossRef]
- Ghadially, F.N. Chapter 3: Mitochondria. In Ultrastructural Pathology of the Cell and Matrix, 3rd ed.; Butterworths: Oxford, UK, 1997; Volume 1, ISBN 0-407-01571-X. [Google Scholar]
- Abbott, A.L.; Ducibella, T. Calcium and the control of mammalian cortical granule exocytosis. Front. Biosci. 2001, 6, D792–D806. [Google Scholar] [CrossRef]
- Liu, M. The biology and dynamics of mammalian cortical granules. Reprod. Biol. Endocrinol. 2011, 9, 149. [Google Scholar] [CrossRef]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem. Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef]
- Tsai, P.-S.; Haeften, T.v.; Gadella, B.M. Preparation of the Cortical Reaction: Maturation-Dependent Migration of SNARE Proteins, Clathrin, and Complexin to the Porcine Oocyte’s Surface Blocks Membrane Traffic until Fertilization. Biol. Reprod. 2011, 84, 327–335. [Google Scholar] [CrossRef][Green Version]
- Familiari, G.; Heyn, R.; Relucenti, M.; Nottola, S.A.; Sathananthan, A.H. Ultrastructural dynamics of human reproduction, from ovulation to fertilization and early embryo development. Int. Rev. Cytol. 2006, 249, 53–141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, X.; Chen, Y.; Liu, X.; Sun, Y.; Xiong, B. Glutathione alleviates the cadmium exposure-caused porcine oocyte meiotic defects via eliminating the excessive ROS. Environ. Pollut. 2019, 255, 113194. [Google Scholar] [CrossRef] [PubMed]
- Nottola, S.A.; Coticchio, G.; De Santis, L.; Macchiarelli, G.; Maione, M.; Bianchi, S.; Iaccarino, M.; Flamigni, C.; Borini, A. Ultrastructure of human mature oocytes after slow cooling cryopreservation with ethylene glycol. Reprod. Biomed. Online 2008, 17, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Simoniello, P.; Filosa, S.; Scudiero, R.; Trinchella, F.; Motta, C.M. Cadmium Impairment of Reproduction in the Female Wall Lizard Podarcis sicula. Environ. Toxicol. 2013, 28, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zang, H. Cadmium toxicity: Effects on cytoskeleton, vesicular trafficking, and cell wall construction. Plant Signal. Behav. 2012, 7, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Song, X.; Chen, L.; Hu, D.; Hua, L.; Cui, Y.; Liu, J.; An, Z.; Yin, Z.; Ning, H. Cadmium induces actin cytoskeleton alterations and dysfunction in Neuro-2a cells. Environ. Toxicol. 2019, 34, 469–475. [Google Scholar] [CrossRef]
- Dong, F.; Xiao, P.; Li, X.; Chang, P.; Zhang, W.; Wang, L. Cadmium triggers oxidative stress and mitochondrial injury mediated apoptosis in human extravillous trophoblast HTR-8/SVneo cells. Reprod. Toxicol. 2021, 101, 18–27. [Google Scholar] [CrossRef]
- Thompson, J.; Bannigan, J. Cadmium: Toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef]
- Yu, F.; Yan, L.; Sun, J.; Zhao, Y.; Yuan, Y.; Gu, J.; Bian, J.; Zou, H.; Liu, Z. Gap junction intercellular communication mediates cadmium-induced apoptosis in hepatocytes via the Fas/FasL pathway. Environ. Toxicol. 2022, 37, 2692–2702. [Google Scholar] [CrossRef]
- Lopez, W.; Ramachandran, J.; Alsamarah, A.; Luo, Y.; Harris, A.L.; Contreras, J.E. Mechanism of gating by calcium in connexin hemichannels. Proc. Natl. Acad. Sci. USA 2016, 113, E7986–E7995. [Google Scholar] [CrossRef]
- Hu, Z.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin. Int. J. Mol. Sci. 2020, 21, 8194. [Google Scholar] [CrossRef]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010, 139, 685–695. [Google Scholar] [CrossRef]
- Imanaka, S.; Shigetomi, H.; Kobayashi, H. Reprogramming of glucose metabolism of cumulus cells and oocytes and its therapeutic significance. Reprod. Sci. 2022, 29, 653–667. [Google Scholar] [CrossRef]
- Sabir, S.; Akasha, M.S.H.; Fiayyaz, F.; Saleemb, U.; Mehmoodb, M.H.; Rehmand, K. Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: Inserting the association into perspectives. Biomed. Pharmacother. 2019, 114, 108802. [Google Scholar] [CrossRef] [PubMed]
- Oluranti, O.L.; Agboola, E.A.; Fubara, N.E.; Ajayi, M.O.; Michael, O.S. Cadmium exposure induces cardiac glucometabolic dysregulation and lipid accumulation independent of pyruvate dehydrogenase activity. Ann. Med. 2021, 53, 1109–1118. [Google Scholar] [CrossRef]
- Shen, W.-J.; Azhar, S.; Kraemer, F.B. Lipid Droplets and Steroidogenic Cells. Exp. Cell Res. 2016, 340, 209–214. [Google Scholar] [CrossRef]
- Knazicka, Z.; Forgacs, Z.; Lukacova, J.; Roychoudhury, S.; Massanyi, P.; Lukac, N. Endocrine disruptive effects of cadmium on steroidogenesis: Human adrenocortical carcinoma cell line NCI-H295R as a cellular model for reproductive toxicity testing. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2015, 50, 348–356. [Google Scholar] [CrossRef]
- Paksy, K.; Rajczy, K.; Forgacs, Z.; Lazar, L.; Bernard, A.; Gati, I.; Kaali, G.S. Effect of Cadmium on Morphology and Steroidogenesis of Cultured Human Ovarian Granulosa Cells. J. Appl. Toxicol. 1997, 17, 321–327. [Google Scholar] [CrossRef]
- Almeida, C.P.; Ferreira, M.C.F.; Silveira, C.O.; Campos, J.R.; Borges, I.T.; Baeta, P.G.; Silva, F.H.S.; Reis, F.M.M.; Del Puerto, H.L. Clinical correlation of apoptosis in human granulosa cells. A review. Cell Biol. Int. 2018, 42, 1276–1281. [Google Scholar] [CrossRef]
- Huang, R.-H.; Zhou, W.-H. Granulosa Cell Biomarkers to Predict Oocyte and Embryo Quality in Assisted Reproductive Technology. Reprod. Dev. Med. 2021, 5, 30–37. [Google Scholar] [CrossRef]
- Turathum, B.; Gao, E.-M.; Chian, R.-C. The Function of Cumulus Cells in Oocyte Growth and Maturation and in Subsequent Ovulation and Fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, S.; Huang, M.; Jiang, X.; Yang, M. Cadmium induces apoptosis of human granulosa cell line KGN via mitochondrial dysfunction-mediated pathways. Ecotoxicol. Environ. Saf. 2021, 220, 112341. [Google Scholar] [CrossRef] [PubMed]







| Patients | Age | Infertility Causes | ART Procedure | Collected Oocytes | TransferredEmbryos | Outcome |
|---|---|---|---|---|---|---|
| 1 | 34 | Male factor alone | ICSI | 6 | 2 | no pregnancy |
| 2 | 39 | unexplained | FIVET | 6 | 3 | Pregnancy & miscarriage |
| 3 | 32 | Male factor alone | ICSI | 8 | 3 | pregnancy |
| 4 | 39 | Blocked Fallopian tubes | FIVET | 9 | 3 | no pregnancy |
| 5 | 33 | Blocked Fallopian tubes | FIVET | 13 | 3 | no pregnancy |
| 6 | 32 | Blocked Fallopian tubes | FIVET | 11 | 3 | no pregnancy |
| 7 | 31 | Male factor alone | ICSI | 9 | 2 | no pregnancy |
| 8 | 39 | Male factor alone | ICSI | 10 | 3 | no pregnancy |
| 9 | 30 | Blocked Fallopian tubes | FIVET | 15 | 3 | no pregnancy |
| 10 | 25 | Blocked Fallopian tubes | FIVET | 12 | 3 | pregnancy |
| 11 | 29 | Male factor alone | ICSI | 5 | 2 | No pregnancy |
| 12 | 30 | Blocked Fallopian tubes | FIVET | 11 | 2 | No pregnancy |
| 13 | 37 | Blocked Fallopian tubes | FIVET | 7 | 2 | No pregnancy |
| 14 | 24 | Blocked Fallopian tubes | FIVET | 14 | 3 | pregnancy |
| 15 | 32 | Blocked Fallopian tubes | FIVET | 8 | 2 | no pregnancy |
| 16 | 32 | Male factor alone | ICSI | 7 | 3 | no pregnancy |
| 17 | 33 | Blocked Fallopian tubes | FIVET | 10 | 3 | pregnancy and miscarriage |
| Blood | Follicolar Fluid | |||||
|---|---|---|---|---|---|---|
| Patients | Mean Pb (µg/dL) | Mean Cd (µg/L) | FF Specimen | Mean Pb (µg/dL) ± SD | Mean Cd (µg/L) ± SD | Groups |
| 1 | 3.79 | 1.87 | 3 | 0.84 ± 0.098 | 0. 41 ± 0.017 | 2 |
| 2 | 2.65 | 0.90 | 3 | 0.67 ± 0.035 | 0.32 ± 0.021 | 2 |
| 3 | 1.98 | 0.74 | 4 | 0.61 ± 0.093 | 0.25 ± 0.015 | 1 |
| 4 | 2.34 | 0.73 | 4 | 0.64 ± 0.087 | 0.24 ± 0.009 | 1 |
| 5 | 2.24 | 0.78 | 4 | 0.66 ± 0.065 | 0.29 ± 0.007 | 2 |
| 6 | 3.08 | 0.80 | 4 | 0.72 ± 0.046 | 0.30 ± 0.009 | 2 |
| 7 | 2.03 | 0.72 | 4 | 0.59 ± 0.088 | 0.23 ± 0.016 | 1 |
| 8 | 3.55 | 1.69 | 3 | 0.85 ± 0.060 | 0.41 ± 0.012 | 2 |
| 9 | 2.30 | 0.59 | 4 | 0.65 ± 0.094 | 0.24 ± 0.014 | 1 |
| 10 | 1.43 | 0.60 | 3 | 0.49 ± 0.020 | 0.12 ± 0.007 | 1 |
| 11 | 2.96 | 0.63 | 3 | 0.62 ± 0.030 | 0.24 ± 0.013 | 1 |
| 12 | 3.98 | 0.77 | 3 | 0.78 ± 0.020 | 0.26 ± 0.015 | 2 |
| 13 | 3.04 | 0.65 | 3 | 0.72 ± 0.070 | 0.24 ± 0.012 | 1 |
| 14 | 1.40 | 0.54 | 3 | 0.53 ± 0.046 | 0.10 ± 0.008 | 1 |
| 15 | 2.95 | 0.80 | 3 | 0.71 ± 0.081 | 0.29 ± 0.013 | 2 |
| 16 | 2.90 | 0.60 | 3 | 0.68 ± 0.040 | 0.22 ± 0.013 | 1 |
| 17 | 1.28 | 0.54 | 4 | 0.55 ± 0.084 | 0.10 ± 0.009 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miglietta, S.; Cristiano, L.; Battaglione, E.; Macchiarelli, G.; Nottola, S.A.; De Marco, M.P.; Costanzi, F.; Schimberni, M.; Colacurci, N.; Caserta, D.; et al. Heavy Metals in Follicular Fluid Affect the Ultrastructure of the Human Mature Cumulus-Oocyte Complex. Cells 2023, 12, 2577. https://doi.org/10.3390/cells12212577
Miglietta S, Cristiano L, Battaglione E, Macchiarelli G, Nottola SA, De Marco MP, Costanzi F, Schimberni M, Colacurci N, Caserta D, et al. Heavy Metals in Follicular Fluid Affect the Ultrastructure of the Human Mature Cumulus-Oocyte Complex. Cells. 2023; 12(21):2577. https://doi.org/10.3390/cells12212577
Chicago/Turabian StyleMiglietta, Selenia, Loredana Cristiano, Ezio Battaglione, Guido Macchiarelli, Stefania Annarita Nottola, Maria Paola De Marco, Flavia Costanzi, Mauro Schimberni, Nicola Colacurci, Donatella Caserta, and et al. 2023. "Heavy Metals in Follicular Fluid Affect the Ultrastructure of the Human Mature Cumulus-Oocyte Complex" Cells 12, no. 21: 2577. https://doi.org/10.3390/cells12212577
APA StyleMiglietta, S., Cristiano, L., Battaglione, E., Macchiarelli, G., Nottola, S. A., De Marco, M. P., Costanzi, F., Schimberni, M., Colacurci, N., Caserta, D., & Familiari, G. (2023). Heavy Metals in Follicular Fluid Affect the Ultrastructure of the Human Mature Cumulus-Oocyte Complex. Cells, 12(21), 2577. https://doi.org/10.3390/cells12212577







