Vitamin D Influences the Activity of Mast Cells in Allergic Manifestations and Potentiates Their Effector Functions against Pathogens
Abstract
1. Vitamin D and Immunomodulation
2. Vitamin D and IgE-Mediated MC Activation
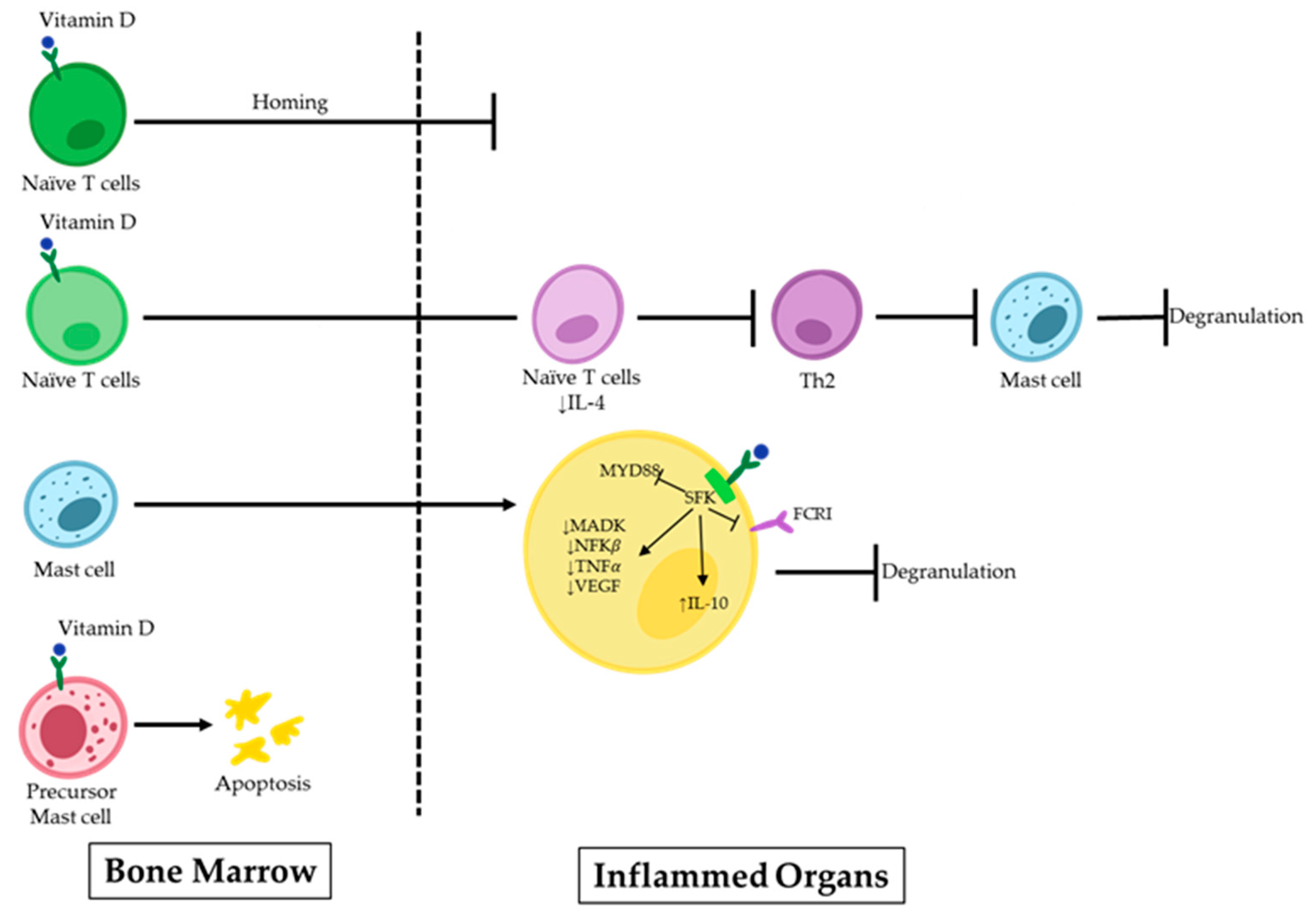
3. Vitamin D and MCs in Allergy
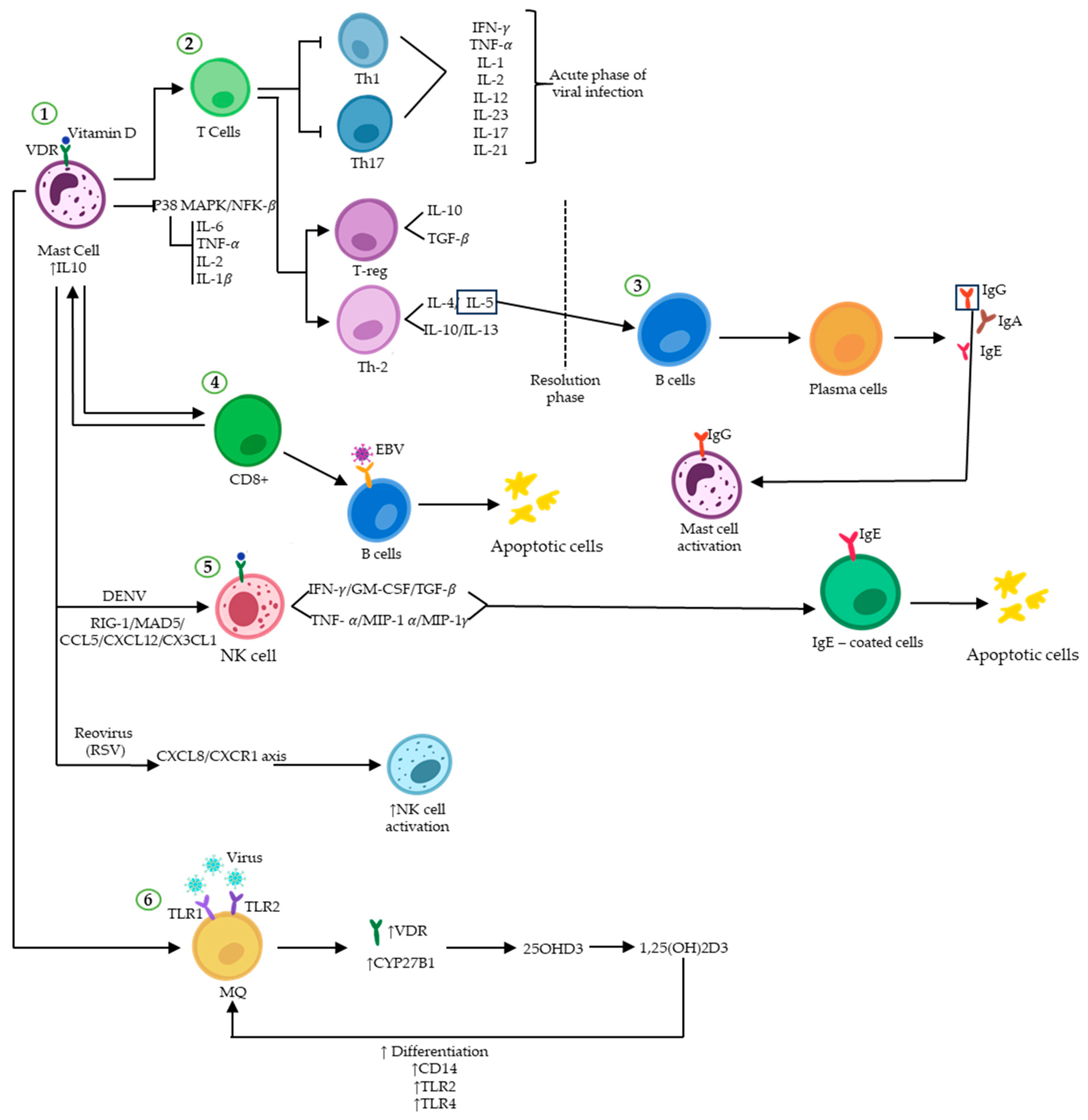
4. Vitamin D and Host MCs Defence against Pathogens
5. Vitamin D Affects MCs in Fighting Viral Infection and Viral Diseases
6. Controversy in Roles That Vitamin D May Play in Viral Infection
7. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhattoa, H.P.; Konstantynowicz, J.; Laszcz, N.; Wojcik, M.; Pludowski, P. Vitamin D: Musculoskeletal health. Rev. Endocr. Metab. Disord. 2017, 18, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Theoharides, T.C.; Pandolfi, F. Impact of Vitamin D on mast cell activity, immunity and inflammation. J. Food Nutr. Res. 2016, 4, 33–39. [Google Scholar]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 1, 123–133. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 2011, 13, 21–29. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Arora, J. Two lineages of immune cells that differentially express the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2023, 228, 106253. [Google Scholar] [CrossRef]
- Arora, J.; Wang, J.; Weaver, V.; Zhang, Y.; Cantorna, M.T. Novel insight into the role of the vitamin D receptor in the development and function of the immune system. J. Steroid Biochem. Mol. Biol. 2022, 219, 106084. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, W.; Ma, C.; Zhao, Y.; Xiong, R.; Wang, H.; Chen, W.; Zheng, S.G. Immunomodulatory Function of Vitamin D and Its Role in Autoimmune Thyroid Disease. Front. Immunol. 2021, 12, 574967. [Google Scholar] [CrossRef]
- Fernandez, G.J.; Ramírez-Mejía, J.M.; Urcuqui-Inchima, S. Vitamin D boosts immune response of macrophages through a regulatory network of microRNAs and mRNAs. J. Nutr. Biochem. 2022, 109, 109105. [Google Scholar] [CrossRef]
- Yu, C.; Fedoric, B.; Anderson, P.H.; Lopez, A.F.; Grimbaldeston, M.A. Vitamin D3 signalling to mast cells: A new regulatory axis. Int. J. Biochem. Cell Biol. 2011, 43, 41–46. [Google Scholar] [CrossRef]
- Soto, J.R.; Anthias, C.; Madrigal, A.; Snowden, J.A. Insights Into the Role of Vitamin D as a Biomarker in Stem Cell Transplantation. Front. Immunol. 2020, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Goleva, E.; Searing, D.A.; Jackson, L.P.; Richers, B.N.; Leung, D.Y. Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. J. Allergy Clin. Immunol. 2012, 129, 1243–1251. [Google Scholar] [CrossRef][Green Version]
- Di Rosa, M.; Malaguarnera, G.; De Gregorio, C.; Palumbo, M.; Nunnari, G.; Malaguarnera, L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell. Immunol. 2012, 280, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Lin, Y.-D.; Arora, J.; Bora, S.; Tian, Y.; Nichols, R.G.; Patterson, A.D. Vitamin D Regulates the Microbiota to Control the Numbers of RORγt/FoxP3+ Regulatory T Cells in the Colon. Front. Immunol. 2019, 10, 1772. [Google Scholar] [CrossRef] [PubMed]
- Farias, A.S.; Spagnol, G.S.; Bordeaux-Rego, P.; Oliveira, C.O.; Fontana, A.G.; de Paula, R.F.; Santos, M.P.; Pradella, F.; Moraes, A.S.; Oliveira, E.C.; et al. Vitamin D3 induces IDO+ tolerogenic DCs and enhances Treg, reducing the severity of EAE. CNS Neurosci. Ther. 2013, 19, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, I.; Pawliczak, R. The Active Metabolite of Vitamin D3 as a Potential Immunomodulator. Scand. J. Immunol. 2016, 83, 83–91. [Google Scholar] [CrossRef]
- White, J.H. Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity. Nutrients 2022, 14, 284. [Google Scholar] [CrossRef]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Fabbri, A.; Infante, M.; Ricordi, C. Editorial―Vitamin D status: A key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4048–4052. [Google Scholar]
- Bychinin, M.V.; Klypa, T.V.; Mandel, I.A.; Yusubalieva, G.M.; Baklaushev, V.P.; Kolyshkina, N.A.; Troitsky, A.V. Effect of vitamin D3 supplementation on cellular immunity and inflammatory markers in COVID-19 patients admitted to the ICU. Sci. Rep. 2022, 12, 18604. [Google Scholar] [CrossRef]
- Shefler, I.; Salamon, P.; Mekori, Y.A. Extracellular vesicles as mediators of mast cell cross talk with immune cells: Possible druggable targets. J. Allergy Clin. Immunol. 2023, 152, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis-Dimitriou, K.; Kotrba, J.; Voss, M.; Dudeck, J.; Dudeck, A. Mast Cell Functions Linking Innate Sensing to Adaptive Immunity. Cells 2020, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- Valeri, V.; Tonon, S.; Vibhushan, S.; Gulino, A.; Belmonte, B.; Adori, M.; Karlsson Hedestam, G.B.; Gautier, G.; Tripodo, C.; Blank, U.; et al. Mast cells crosstalk with B cells in the gut and sustain IgA response in the inflamed intestine. Eur. J. Immunol. 2021, 51, 445–458. [Google Scholar] [CrossRef]
- Yun, H.D.; Goel, Y.; Gupta, K. Crosstalk of Mast Cells and Natural Killer Cells with Neurons in Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2023, 24, 12543. [Google Scholar] [CrossRef] [PubMed]
- Symowski, C.; Voehringer, D. Interactions between Innate Lymphoid Cells and Cells of the Innate and Adaptive Immune System. Front. Immunol. 2017, 8, 1422. [Google Scholar] [CrossRef]
- Elishmereni, M.; Nissimbenefraim, A.; Bachelet, I.; Levischaffer, F. Mast Cell-Eosinophil Cross-Talk Regulates Function and Viability of Both Cells in Vitro. J. Allergy Clin. Immunol. 2008, 121, S110. [Google Scholar] [CrossRef]
- Coleman, J.; Brooks, B.; Eastmond, N.; Koranteng, R. Cross-Talk between Mast Cells and Macrophages Involving Interferon-γ and Nitric Oxide. Int. Arch. Allergy Immunol. 2001, 124, 160–162. [Google Scholar] [CrossRef]
- Carroll-Portillo, A.; Cannon, J.L.; Riet, J.T.; Holmes, A.; Kawakami, Y.; Kawakami, T.; Cambi, A.; Lidke, D.S. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J. Cell Biol. 2015, 210, 851–864. [Google Scholar] [CrossRef]
- Baroni, E.; Biffi, M.; Benigni, F.; Monno, A.; Carlucci, D.; Carmeliet, G.; Bouillon, R.; D’ambrosio, D. VDR-dependent regulation of mast cell maturation mediated by 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 2006, 81, 250–262. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Li, X.-X.; Qiu, S.-Q.; Yu, Y.; Li, M.-G.; Yang, L.-T.; Li, L.-J.; Wang, S.; Zheng, P.-Y.; Yang, P.-C. Vitamin D contributes to mast cell stabilization. Allergy 2017, 72, 1184–1192. [Google Scholar] [CrossRef]
- Yip, K.-H.; Kolesnikoff, N.; Yu, C.; Hauschild, N.; Taing, H.; Biggs, L.; Goltzman, D.; Gregory, P.A.; Anderson, P.H.; Samuel, M.S.; et al. Mechanisms of vitamin D3 metabolite repression of IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014, 133, 1356–1364.e14. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.C.; Temple, R.M.; Obar, J.J. Mast Cells and Influenza A Virus: Association with Allergic Responses and Beyond. Front. Immunol. 2015, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.G.; Oshansky, C.M.; Bajracharya, R.; Tang, L.; Sun, Y.; Wong, S.S.; Webby, R.; Thomas, P.G.; Hurwitz, J.L. Retinol binding protein and vitamin D associations with serum antibody isotypes, serum influenza virus-specific neutralizing activities and airway cytokine profiles. Clin. Exp. Immunol. 2015, 183, 239–247. [Google Scholar] [CrossRef]
- Mendoza, R.P.; Fudge, D.H.; Brown, J.M. Cellular Energetics of Mast Cell Development and Activation. Cells 2021, 10, 524. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Lieberman, P.; Garvey, L.H. Mast Cells and Anaphylaxis. Curr. Allergy Asthma Rep. 2016, 16, 20. [Google Scholar] [CrossRef]
- Yip, K.H.; Chao, J.; Coolen, C.; Pant, H.; Kral, A.; Smith, W.; Schwarz, Q.; Grimbaldeston, M.A.; Pitson, S.; Lopez, A.F.; et al. IgE receptor of mast cells signals mediator release and inflammation via adaptor protein 14-3-3ζ. J. Allergy Clin. Immunol. 2023, 152, 725–735. [Google Scholar] [CrossRef]
- Chang, T.W.; Shiung, Y.-Y. Anti-IgE as a mast cell–stabilizing therapeutic agent. J. Allergy Clin. Immunol. 2006, 117, 1203–1212. [Google Scholar] [CrossRef]
- Puxeddu, I.; Ribatti, D.; Crivellato, E.; Levi-Schaffer, F. Mast cells and eosinophils: A novel link between inflammation and angiogenesis in allergic diseases. J. Allergy Clin. Immunol. 2005, 116, 531–536. [Google Scholar] [CrossRef]
- Reber, L.; Da Silva, C.A.; Frossard, N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur. J. Pharmacol. 2006, 533, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Allegra, A.; Tonacci, A.; Musolino, C.; Ricciardi, L.; Gangemi, S. Mast Cells and Vitamin D Status: A Clinical and Biological Link in the Onset of Allergy and Bone Diseases. Biomedicines 2022, 10, 1877. [Google Scholar] [CrossRef] [PubMed]
- Kakavas, S.; Karayiannis, D.; Mastora, Z. The Complex Interplay between Immunonutrition, Mast Cells, and Histamine Signaling in COVID-19. Nutrients 2021, 13, 3458. [Google Scholar] [CrossRef] [PubMed]
- Muehleisen, B.; Gallo, R.L. Vitamin D in allergic disease: Shedding light on a complex problem. J. Allergy Clin. Immunol. 2013, 131, 324–329. [Google Scholar] [CrossRef]
- Jones, A.P.; Palmer, D.; Zhang, G.; Prescott, S.L. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics 2012, 130, 1128–1135. [Google Scholar] [CrossRef]
- Topilski, I.; Flaishon, L.; Naveh, Y.; Harmelin, A.; Levo, Y.; Shachar, I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur. J. Immunol. 2004, 34, 1068–1076. [Google Scholar] [CrossRef]
- Benigni, F.; Baroni, E.; Zecevic, M.; Zvara, P.; Streng, T.; Hedlund, P.; Colli, E.; D’Ambrosio, D.; Andersson, K.-E. Oral treatment with a vitamin D3 analogue (BXL628) has anti-inflammatory effects in rodent model of interstitial cystitis. BJU Int. 2006, 97, 617–624. [Google Scholar] [CrossRef]
- Espinosa, Z.J.R.; Huerta, L.J.G.; Ortega-Martell, J.A. Stabilization of the mast cell by vitamin D. Alerg. Asma Inmunol. Pediatr. 2019, 28, 96–101. [Google Scholar] [CrossRef]
- Zhao, J.-W.; Ping, J.-D.; Wang, Y.-F.; Liu, X.-N.; Li, N.; Hu, Z.-L.; Ming, L. Vitamin D suppress the production of vascular endothelial growth factor in mast cell by inhibiting PI3K/Akt/p38 MAPK/HIF-1α pathway in chronic spontaneous urticaria. Clin. Immunol. 2020, 215, 108444. [Google Scholar] [CrossRef]
- Galli, S.J.; Grimbaldeston, M.; Tsai, M. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef]
- Metz, M.; Lammel, V.; Gibbs, B.F.; Maurer, M. Inflammatory murine skin responses to UV-B light are partially dependent on endothelin-1 and mast cells. Am. J. Pathol. 2006, 169, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Biggs, L.; Yu, C.; Fedoric, B.; Lopez, A.F.; Galli, S.J.; Grimbaldeston, M.A. Evidence that vitamin D3 promotes mast cell–dependent reduction of chronic UVB-induced skin pathology in mice. J. Exp. Med. 2010, 207, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Piliponsky, A.M.; Acharya, M.; Shubin, N.J. Mast Cells in Viral, Bacterial, and Fungal Infection Immunity. Int. J. Mol. Sci. 2019, 20, 2851. [Google Scholar] [CrossRef]
- Carlos, D.; Frantz, F.G.; Souza-Júnior, D.A.; Jamur, M.C.; Oliver, C.; Ramos, S.G.; Quesniaux, V.F.; Ryffel, B.; Silva, C.L.; Bozza, M.T.; et al. TLR2-dependent mast cell activation contributes to the control of Mycobacterium tuberculosis infection. Microbes Infect. 2009, 11, 770–778. [Google Scholar] [CrossRef]
- Youssef, D.A.; Miller, C.W.; El-Abbassi, A.M.; Cutchins, D.C.; Cutchins, C.; Grant, W.B.; Peiris, A.N. Antimicrobial implications of vitamin D. Dermato-Endocrinology 2011, 3, 220–229. [Google Scholar] [CrossRef]
- Lopes, J.P.; Stylianou, M.; Nilsson, G.; Urban, C.F. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Sci. Rep. 2015, 5, 12287. [Google Scholar] [CrossRef] [PubMed]
- Renga, G.; Moretti, S.; Oikonomou, V.; Borghi, M.; Zelante, T.; Paolicelli, G.; Costantini, C.; De Zuani, M.; Villella, V.R.; Raia, V.; et al. IL-9 and Mast Cells Are Key Players of Candida albicans Commensalism and Pathogenesis in the Gut. Cell Rep. 2018, 23, 1767–1778. [Google Scholar] [CrossRef]
- Khoo, A.L.; Chai, L.; Koenen, H.; Joosten, I.; Netea, M.; van der Ven, A. Translating the role of vitamin D3 in infectious diseases. Crit. Rev. Microbiol. 2011, 38, 122–135. [Google Scholar] [CrossRef]
- Teymoori-Rad, M.; Shokri, F.; Salimi, V.; Marashi, S.M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019, 29, e2032. [Google Scholar] [CrossRef]
- Siddiqui, M.; Manansala, J.S.; Abdulrahman, H.A.; Nasrallah, G.K.; Smatti, M.K.; Younes, N.; Althani, A.A.; Yassine, H.M. Immune Modulatory Effects of Vitamin D on Viral Infections. Nutrients 2020, 12, 2879. [Google Scholar] [CrossRef]
- Danai, P.A.; Sinha, S.; Moss, M.; Haber, M.J.; Martin, G.S. Seasonal variation in the epidemiology of sepsis. Crit. Care Med. 2007, 35, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Variations in Vitamin D Production Could Possibly Explain the Seasonality of Childhood Respiratory Infections in Hawaii. Pediatr. Infect. Dis. J. 2008, 27, 853. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Yisak, H.; Ewunetei, A.; Kefale, B.; Mamuye, M.; Teshome, F.; Ambaw, B.; Yitbarek, G.Y. Effects of Vitamin D on COVID-19 Infection and Prognosis: A Systematic Review. Risk Manag. Healthc. Policy 2021, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, A.; Amirhakimi, G.H. The rachitic lung. Pulmonary findings in 30 infants and children with malnutritional rickets. Clin. Pediatr. 1977, 16, 36–38. [Google Scholar] [CrossRef]
- Spirig, R.; Potapova, I.; Shaw-Boden, J.; Tsui, J.; Rieben, R.; Shaw, S.G. TLR2 and TLR4 agonists induce production of the vasoactive peptide endothelin-1 by human dendritic cells. Mol. Immunol. 2009, 46, 3178–3182. [Google Scholar] [CrossRef]
- Arababadi, M.K.; Nosratabadi, R.; Asadikaram, G. Vitamin D and toll like receptors. Life Sci. 2018, 203, 105–111. [Google Scholar] [CrossRef]
- Reins, R.Y.; Baidouri, H.; McDermott, A.M. Vitamin D Activation and Function in Human Corneal Epithelial Cells During TLR-Induced Inflammation. Investig. Opthalmology Vis. Sci. 2015, 56, 7715–7727. [Google Scholar] [CrossRef]
- Choi, B.; Lee, E.-S.; Sohn, S. Vitamin D3 ameliorates herpes simplex virus-induced Behçet’s disease-like inflammation in a mouse model through down-regulation of Toll-like receptors. Clin. Exp. Rheumatol. 2011, 29 (Suppl. S67), S13–S19. [Google Scholar]
- Karimi, N.; Morovati, S.; Chan, L.; Napoleoni, C.; Mehrani, Y.; Bridle, B.W.; Karimi, K. Mast Cell Tryptase and Implications for SARS-CoV-2 Pathogenesis. BioMed 2021, 1, 136–149. [Google Scholar] [CrossRef]
- Story, M.J. Essential sufficiency of zinc, ω-3 polyunsaturated fatty acids, vitamin D and magnesium for prevention and treatment of COVID-19, diabetes, cardiovascular diseases, lung diseases and cancer. Biochimie 2021, 187, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Baylink, D.J.; Chen, C.-S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020, 18, 322. [Google Scholar] [CrossRef]
- Kusaka, Y.; Sato, K.; Zhang, Q.; Morita, A.; Kasahara, T.; Yanagihara, Y. Association of natural killer cell activity with serum IgE. Int. Arch. Allergy Immunol. 1997, 112, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P. CD8+ T-Cell Deficiency, Epstein-Barr Virus Infection, Vitamin D Deficiency, and Steps to Autoimmunity: A Unifying Hypothesis. Autoimmune Dis. 2012, 2012, 189096. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Thewissen, M.; Peelen, E.; Menheere, P.; Tervaert, J.W.C.; Damoiseaux, J.; Hupperts, R. Vitamin D Status Is Positively Correlated with Regulatory T Cell Function in Patients with Multiple Sclerosis. PLoS ONE 2009, 4, e6635. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 80–90. [Google Scholar] [CrossRef]
- Røsjø, E.; Lossius, A.; Abdelmagid, N.; Lindstrøm, J.C.; Kampman, M.T.; Jørgensen, L.; Sundström, P.; Olsson, T.; Steffensen, L.H.; Torkildsen, Ø.; et al. Effect of high-dose vitamin D3 supplementation on antibody responses against Epstein–Barr virus in relapsing-remitting multiple sclerosis. Mult. Scler. J. 2016, 23, 395–402. [Google Scholar] [CrossRef]
- Arase, N.; Arase, H.; Hirano, S.; Yokosuka, T.; Sakurai, D.; Saito, T. IgE-Mediated Activation of NK Cells Through FcγRIII. J. Immunol. 2003, 170, 3054–3058. [Google Scholar] [CrossRef]
- Quesada, J.; Solana, R.; Martin, A.; Santamaria, M.; Serrano, I.; Martinez, M.; Aljama, P.; Peña, J. The effect of calcitriol on natural killer cell activity in hemodialyzed patients. J. Steroid Biochem. 1989, 34, 423–425. [Google Scholar] [CrossRef]
- John, A.L.S.; Rathore, A.P.S.; Yap, H.; Ng, M.-L.; Metcalfe, D.D.; Vasudevan, S.G.; Abraham, S.N. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. USA 2011, 108, 9190–9195. [Google Scholar] [CrossRef] [PubMed]
- Al-Afif, A.; Alyazidi, R.; Oldford, S.A.; Huang, Y.Y.; King, C.A.; Marr, N.; Haidl, I.D.; Anderson, R.; Marshall, J.S. Faculty Opinions recommendation of Respiratory syncytial virus infection of primary human mast cells induces the selective production of type I interferons, CXCL10, and CCL4. J. Allergy Clin. Immunol. 2019, 136, 1346–1354.e1. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Iwabuchi, K.; Someya, A.; Hirata, M.; Matsuda, H.; Ogawa, H.; Nagaoka, I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 2002, 106, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Portales-Cervantes, L.; Haidl, I.D.; Lee, P.W.; Marshall, J.S. Virus-Infected Human Mast Cells Enhance Natural Killer Cell Functions. J. Innate Immun. 2016, 9, 94–108. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Marshall, J.S.; Portales-Cervantes, L.; Leong, E. Mast Cell Responses to Viruses and Pathogen Products. Int. J. Mol. Sci. 2019, 20, 4241. [Google Scholar] [CrossRef]
- Rathore, A.P.; John, A.L.S. Protective and pathogenic roles for mast cells during viral infections. Curr. Opin. Immunol. 2020, 66, 74–81. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, M.P.; Kumar, R.S.; Ratho, R.K. 25-Hydroxyvitamin D3 and 1,25 Dihydroxyvitamin D3 as an Antiviral and Immunomodulator Against Herpes Simplex Virus-1 Infection in HeLa Cells. Viral Immunol. 2018, 31, 589–593. [Google Scholar] [CrossRef]
- Wang, T.T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef]
- Gallo, R.L.; Murakami, M.; Ohtake, T.; Zaiou, M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol. 2002, 110, 823–831. [Google Scholar] [CrossRef]
- Di Nardo, A.; Vitiello, A.; Gallo, R.L. Cutting Edge: Mast Cell Antimicrobial Activity Is Mediated by Expression of Cathelicidin Antimicrobial Peptide. Perspect. Surg. 2003, 170, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; MacLeod, D.T.; Di Nardo, A. Commensal Bacteria Lipoteichoic Acid Increases Skin Mast Cell Antimicrobial Activity against Vaccinia Viruses. Pediatrics 2012, 189, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Bąbolewska, E.; Brzezińska-Błaszczyk, E. Human-derived cathelicidin LL-37 directly activates mast cells to proinflammatory mediator synthesis and migratory response. Cell. Immunol. 2015, 293, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Grigat, J.; Soruri, A.; Forssmann, U.; Riggert, J.; Zwirner, J. Chemoattraction of macrophages, T lymphocytes, and mast cells is evolutionarily conserved within the human alpha-defensin family. J. Immunol. 2007, 179, 3958–3965. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Lee, D.; Bayir, A.K.; Ahn, H.; Ali, H. β-Defensins Activate Human Mast Cells via Mas-Related Gene X2. J. Immunol. 2013, 191, 345–352. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Guo, Q.; Price, R.; Ali, H. Mas-related Gene X2 (MrgX2) Is a Novel G Protein-coupled Receptor for the Antimicrobial Peptide LL-37 in Human Mast Cells: Resistance to receptor phosphorylation, desensitization, and internalization. J. Biol. Chem. 2011, 286, 44739–44749. [Google Scholar] [CrossRef]
- Agier, J.; Brzezińska-Błaszczyk, E.; Żelechowska, P.; Wiktorska, M.; Pietrzak, J.; Różalska, S. Cathelicidin LL-37 Affects Surface and Intracellular Toll-Like Receptor Expression in Tissue Mast Cells. J. Immunol. Res. 2018, 2018, 7357162. [Google Scholar] [CrossRef]
- Yoshioka, M.; Fukuishi, N.; Kubo, Y.; Yamanobe, H.; Ohsaki, K.; Kawasoe, Y.; Murata, M.; Ishizumi, A.; Nishii, Y.; Matsui, N.; et al. Human Cathelicidin CAP18/LL-37 Changes Mast Cell Function toward Innate Immunity. Biol. Pharm. Bull. 2008, 31, 212–216. [Google Scholar] [CrossRef]
- Agier, J.; Różalska, S.; Wiktorska, M.; Żelechowska, P.; Pastwińska, J.; Brzezińska-Błaszczyk, E. The RLR/NLR expression and pro-inflammatory activity of tissue mast cells are regulated by cathelicidin LL-37 and defensin hBD-2. Sci. Rep. 2018, 8, 11750. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2020, 5, 10405. [Google Scholar] [CrossRef]
- Wang, T.-T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. Pediatrics 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Presta, I.; Donato, A.; Zaffino, P.; Spadea, M.F.; Mancuso, T.; Malara, N.; Chiefari, E.; Donato, G. Does a polarization state exist for mast cells in cancer? Med. Hypotheses 2019, 131, 109281. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E. HMGB1, IL-1α, IL-33 and S100 proteins: Dual-function alarmins. Cell Mol. Immunol. 2017, 14, 43–64. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Girard, J.-P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi-Tago, M.; Tago, K.; Hayakawa, M.; Tominaga, S.-I.; Ohshio, T.; Sonoda, Y.; Kasahara, T. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell. Signal. 2008, 20, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Kaieda, S.; Ameri, S.; Fishgal, N.; Dwyer, D.; Dellinger, A.; Kepley, C.L.; Gurish, M.F.; Nigrovic, P.A. IL-33/ST2 axis promotes mast cell survival via BCLXL. Proc. Natl. Acad. Sci. USA 2014, 111, 10281–10286. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdi, Z.; Smith, D.E.; Comeau, M.R.; Delespesse, G. Cutting Edge: The ST2 Ligand IL-33 Potently Activates and Drives Maturation of Human Mast Cells. J. Immunol. 2007, 179, 2051–2054. [Google Scholar] [CrossRef]
- Andrade, M.V.; Iwaki, S.; Ropert, C.; Gazzinelli, R.T.; Cunha-Melo, J.R.; Beaven, M.A. Amplification of cytokine production through synergistic activation of NFAT and AP-1 following stimulation of mast cells with antigen and IL-33. Eur. J. Immunol. 2011, 41, 760–772. [Google Scholar] [CrossRef]
- Saluja, R.; Khan, M.; Church, M.K.; Maurer, M. The role of IL-33 and mast cells in allergy and inflammation. Clin. Transl. Allergy 2015, 5, 33. [Google Scholar] [CrossRef]
- Cho, K.-A.; Suh, J.W.; Sohn, J.H.; Park, J.W.; Lee, H.; Kang, J.L.; Woo, S.-Y.; Cho, Y.J. IL-33 induces Th17-mediated airway inflammation via mast cells in ovalbumin-challenged mice. Am. J. Physiol. Cell. Mol. Physiol. 2012, 302, L429–L440. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Kordkhayli, M.; Ahangar-Parvin, R.; Azizi, S.V.; Nemati, M.; Shamsizadeh, A.; Khaksari, M.; Moazzeni, S.M.; Jafarzadeh, A. Vitamin D Modulates the Expression of IL-27 and IL-33 in the Central Nervous System in Experimental Autoimmune Encephalomyelitis (EAE). Iran. J. Immunol. 2015, 12, 35–49. [Google Scholar] [PubMed]
- Paplińska-Goryca, M.; Nejman-Gryz, P.; Proboszcz, M.; Krenke, R. The effect of 1,25-dihydroxyvitamin D3 on TSLP, IL-33 and IL-25 expression in respiratory epithelium. Eur. Cytokine Netw. 2016, 27, 54–62. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Ginaldi, L.; Sirufo, M.M.; Bassino, E.M.; De Pietro, F.; Pioggia, G.; Gangemi, S. IL-33/Vitamin D Crosstalk in Psoriasis-Associated Osteoporosis. Front. Immunol. 2021, 11, 604055. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, J.M.; Piotrowska, A.; Purzycka-Bohdan, D.; Olszewska, A.; Nowak, J.I.; Szczerkowska-Dobosz, A.; Nedoszytko, B.; Nowicki, R.J.; Żmijewski, M.A. The Effects of Vitamin D on the Expression of IL-33 and Its Receptor ST2 in Skin Cells; Potential Implication for Psoriasis. Int. J. Mol. Sci. 2021, 22, 12907. [Google Scholar] [CrossRef] [PubMed]
- Sallard, E.; Lescure, F.-X.; Yazdanpanah, Y.; Mentre, F.; Peiffer-Smadja, N. Type 1 interferons as a potential treatment against COVID-19. Antivir. Res. 2020, 178, 104791. [Google Scholar] [CrossRef]
- Sabbah, A.; Chang, T.H.; Harnack, R.; Frohlich, V.; Tominaga, K.; Dube, P.H.; Xiang, Y.; Bose, S. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009, 10, 1073–1080. [Google Scholar] [CrossRef]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Gois, P.H.F.; Ferreira, D.; Olenski, S.; Seguro, A.C. Vitamin D and Infectious Diseases: Simple Bystander or Contributing Factor? Nutrients 2017, 9, 651. [Google Scholar] [CrossRef]
- Watkins, R.R.; Lemonovich, T.L.; Salata, R.A. An update on the association of vitamin D deficiency with common infectious diseases. Can. J. Physiol. Pharmacol. 2015, 93, 363–368. [Google Scholar] [CrossRef]
- Berkan-Kawińska, A.; Koślińska-Berkan, E.; Piekarska, A. Original article The prevalence and severity of 25-(OH)-vitamin D insufficiency in HCV infected and in HBV infected patients: A prospective study. Clin. Exp. Hepatol. 2015, 1, 5–11. [Google Scholar] [CrossRef]
- Oliveira, K.S.; Buss, C.; Tovo, C.V. Is there an association between vitamin D and liver fibrosis in patients with chronic hepatitis C? Arq. Gastroenterol. 2017, 54, 57–59. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, M.; Zhao, J.; Ren, F.; Chen, Y.; Li, J.-F.; Zhang, J.-Y.; Qu, F.; Zhang, J.-L.; Duan, Z.-P.; et al. Serum vitamin D3 does not correlate with liver fibrosis in chronic hepatitis C. World J. Gastroenterol. 2015, 21, 11152–11159. [Google Scholar] [CrossRef]
- Esmat, G.; El Raziky, M.; Elsharkawy, A.; Sabry, D.; Hassany, M.; Ahmed, A.; Assem, N.; El Kassas, M.; Doss, W. Impact of Vitamin D Supplementation on Sustained Virological Response in Chronic Hepatitis C Genotype 4 Patients Treated by Pegylated Interferon/Ribavirin. J. Interf. Cytokine Res. 2015, 35, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Grammatikos, G.; Lange, C.; Susser, S.; Schwendy, S.; Dikopoulos, N.; Buggisch, P.; Encke, J.; Teuber, G.; Goeser, T.; Thimme, R.; et al. Vitamin D Levels Vary during Antiviral Treatment but Are Unable to Predict Treatment Outcome in HCV Genotype 1 Infected Patients. PLoS ONE 2014, 9, e87974. [Google Scholar] [CrossRef] [PubMed]
- Kitson, M.T.; Sarrazin, C.; Toniutto, P.; Eslick, G.D.; Roberts, S.K. Vitamin D level and sustained virologic response to interferon-based antiviral therapy in chronic hepatitis C: A systematic review and meta-analysis. J. Hepatol. 2014, 61, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Kitson, M.T.; Dore, G.J.; George, J.; Button, P.; McCaughan, G.W.; Crawford, D.H.; Sievert, W.; Weltman, M.D.; Cheng, W.S.; Roberts, S.K. Vitamin D status does not predict sustained virologic response or fibrosis stage in chronic hepatitis C genotype 1 infection. J. Hepatol. 2012, 58, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Lapidus, N.; Pol, S.; Serfaty, L.; Ratziu, V.; Asselah, T.; Thibault, V.; Souberbielle, J.C.; Carrat, F.; Cacoub, P. Vitamin D in addition to peg-interferon-alpha/ribavirin in chronic hepatitis C virus infection: ANRS-HC25-VITAVIC study. World J. Gastroenterol. 2015, 21, 5647–5653. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.J.; Fleckenstein, J.F.; Marion, T.N.; Madey, M.A.; Mahmoudi, T.M.; Schechtman, K.B. Vitamin D and the racial difference in the genotype 1 chronic hepatitis C treatment response. Am. J. Clin. Nutr. 2012, 96, 1025–1031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chan, H.L.-Y.; Elkhashab, M.; Trinh, H.; Tak, W.Y.; Ma, X.; Chuang, W.-L.; Kim, Y.J.; Martins, E.B.; Lin, L.; Dinh, P.; et al. Association of baseline vitamin D levels with clinical parameters and treatment outcomes in chronic hepatitis B. J. Hepatol. 2015, 63, 1086–1092. [Google Scholar] [CrossRef]
- Parfieniuk-Kowerda, A.; Świderska, M.; Rogalska, M.; Maciaszek, M.; Jaroszewicz, J.; Flisiak, R. Chronic hepatitis B virus infection is associated with decreased serum 25(OH)D concentration in non-cirrhotic patients. Clin. Exp. Hepatol. 2019, 5, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Tan, D.; Ning, Q.; Niu, J.; Bai, X.; Chen, S.; Cheng, J.; Yu, Y.; Wang, H.; Xu, M.; et al. Association of baseline vitamin D level with genetic determinants and virologic response in patients with chronic hepatitis B. Hepatol. Res. 2017, 48, E213–E221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Li, J.; Wang, J.H.; Habib, S.; Wei, W.; Sun, S.J.; Strobel, H.W.; Jia, J.D. Vitamin D serum level is associated with Child-Pugh score and metabolic enzyme imbalances, but not viral load in chronic hepatitis B patients. Medecine 2016, 95, e3926. [Google Scholar] [CrossRef]
- Farnik, H.; Bojunga, J.; Berger, A.; Allwinn, R.; Waidmann, O.; Kronenberger, B.; Keppler, O.T.; Zeuzem, S.; Sarrazin, C.; Lange, C.M. Low vitamin D serum concentration is associated with high levels of hepatitis B virus replication in chronically infected patients. Hepatology 2013, 58, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Gal-Tanamy, M.; Bachmetov, L.; Ravid, A.; Koren, R.; Erman, A.; Tur-Kaspa, R.; Zemel, R. Vitamin D: An innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology 2011, 54, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.A.; Jones, K.A.; Flores, R.; Singhania, A.; Woelk, C.H.; Schooley, R.T.; Wyles, D.L. Vitamin D Metabolites Inhibit Hepatitis C Virus and Modulate Cellular Gene Expression. J. Virol. Antivir. Res. 2014, 3. [Google Scholar] [CrossRef]
- Matsumura, T.; Kato, T.; Sugiyama, N.; Tasaka-Fujita, M.; Murayama, A.; Masaki, T.; Wakita, T.; Imawari, M. 25-hydroxyvitamin D3 suppresses hepatitis C virus production. Hepatology 2012, 56, 1231–1239. [Google Scholar] [CrossRef]
- Murayama, A.; Saitoh, H.; Takeuchi, A.; Yamada, N.; Matsumura, T.; Shiina, M.; Muramatsu, M.; Wakita, T.; Imawari, M.; Kato, T. Vitamin D derivatives inhibit hepatitis C virus production through the suppression of apolipoprotein. Antivir. Res. 2018, 160, 55–63. [Google Scholar] [CrossRef]
- Ravid, A.; Rapaport, N.; Issachar, A.; Erman, A.; Bachmetov, L.; Tur-Kaspa, R.; Zemel, R. 25-Hydroxyvitamin D Inhibits Hepatitis C Virus Production in Hepatocellular Carcinoma Cell Line by a Vitamin D Receptor-Independent Mechanism. Int. J. Mol. Sci. 2019, 20, 2367. [Google Scholar] [CrossRef]
- Greiller, C.L.; Suri, R.; Jolliffe, D.A.; Kebadze, T.; Hirsman, A.G.; Griffiths, C.J.; Johnston, S.L.; Martineau, A.R. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J. Steroid Biochem. Mol. Biol. 2018, 187, 152–159. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M.; Lovan, N.; Powers, L.; Gerke, A.; Hunninghake, G.W. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J. Immunol. 2010, 184, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, I.; Majak, P.; Jerzynska, J.; Podlecka, D.; Polańska, K.; Gromadzińska, J.; Wąsowicz, W.; Hanke, W. Cord serum 25-hydroxyvitamin D correlates with early childhood viral-induced wheezing. Respir. Med. 2015, 109, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Beigelman, A.; Castro, M.; Schweiger, T.L.; Wilson, B.S.; Zheng, J.; Yin-DeClue, H.; Sajol, G.; Giri, T.; Sierra, O.L.; Isaacson-Schmid, M.; et al. Vitamin D Levels Are Unrelated to the Severity of Respiratory Syncytial Virus Bronchiolitis Among Hospitalized Infants. J. Pediatr. Infect. Dis. Soc. 2014, 4, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Brockman-Schneider, R.A.; Pickles, R.J.; Gern, J.E. Effects of Vitamin D on Airway Epithelial Cell Morphology and Rhinovirus Replication. PLoS ONE 2014, 9, e86755. [Google Scholar] [CrossRef]
- Fitch, N.; Becker, A.B.; HayGlass, K.T. Vitamin D [1,25(OH)2D3] Differentially Regulates Human Innate Cytokine Responses to Bacterial versus Viral Pattern Recognition Receptor Stimuli. J. Immunol. 2016, 196, 2965–2972. [Google Scholar] [CrossRef]
- Lee, M.-D.; Lin, C.-H.; Lei, W.-T.; Chang, H.-Y.; Lee, H.-C.; Yeung, C.-Y.; Chiu, N.-C.; Chi, H.; Liu, J.-M.; Hsu, R.-J.; et al. Does Vitamin D Deficiency Affect the Immunogenic Responses to Influenza Vaccination? A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 409. [Google Scholar] [CrossRef]
- Campbell, G.R.; Spector, S.A. Vitamin D Inhibits Human Immunodeficiency Virus Type 1 and Mycobacterium tuberculosis Infection in Macrophages through the Induction of Autophagy. PLoS Pathog. 2012, 8, e1002689. [Google Scholar] [CrossRef]
- Aguilar-Jiménez, W.; Zapata, W.; Caruz, A.; Rugeles, M.T. High Transcript Levels of Vitamin D Receptor Are Correlated with Higher mRNA Expression of Human Beta Defensins and IL-10 in Mucosa of HIV-1-Exposed Seronegative Individuals. PLoS ONE 2013, 8, e82717. [Google Scholar] [CrossRef]
- Ezeamama, A.E.; Guwatudde, D.; Wang, M.; Bagenda, D.; Kyeyune, R.; Sudfeld, C.; Manabe, Y.C.; Fawzi, W.W. Vitamin-D deficiency impairs CD4+T-cell count recovery rate in HIV-positive adults on highly active antiretroviral therapy: A longitudinal study. Clin. Nutr. 2015, 35, 1110–1117. [Google Scholar] [CrossRef]
- Ashenafi, S.; Amogne, W.; Kassa, E.; Gebreselassie, N.; Bekele, A.; Aseffa, G.; Getachew, M.; Aseffa, A.; Worku, A.; Hammar, U.; et al. Daily Nutritional Supplementation with Vitamin D3 and Phenylbutyrate to Treatment-Naïve HIV Patients Tested in a Randomized Placebo-Controlled Trial. Nutrients 2019, 11, 133. [Google Scholar] [CrossRef]
- Bang, U.; Kolte, L.; Hitz, M.; Dam Nielsen, S.; Schierbeck, L.L.; Andersen, O.; Haugaard, S.B.; Mathiesen, L.; Benfield, T.; Jensen, J.E. Correlation of increases in 1,25-dihydroxyvitamin D during vitamin D therapy with activation of CD4+ T lymphocytes in HIV-1-infected males. HIV Clin. Trials 2012, 13, 162–170. [Google Scholar] [CrossRef]
- Eckard, A.R.; Judd, S.E.; Ziegler, T.R.; Camacho-Gonzalez, A.F.; Fitzpatrick, A.M.; Hadley, G.R.; Grossmann, R.E.; Seaton, L.; Seydafkan, S.; Mulligan, M.J.; et al. Risk Factors for Vitamin D Deficiency and Relationship with Cardiac Biomarkers, Inflammation and Immune Restoration in HIV-Infected Youth. Antivir. Ther. 2012, 17, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Erlandson, K.M.; Gudza, I.; Fiorillo, S.; Ndemera, B.; Schooley, R.T.; Gwanzura, L.; Borok, M.; Campbell, T.B. Relationship of vitamin D insufficiency to AIDS-associated Kaposi’s sarcoma outcomes: Retrospective analysis of a prospective clinical trial in Zimbabwe. Int. J. Infect. Dis. 2014, 24, 6–10. [Google Scholar] [CrossRef]
- Visser, M.E.; Durao, S.; Sinclair, D.; Irlam, J.H.; Siegfried, N. Micronutrient supplementation in adults with HIV infection. Cochrane Database Syst. Rev. 2017, 2017, CD003650. [Google Scholar] [CrossRef] [PubMed]
- Belbasis, L.; Bellou, V.; Evangelou, E.; Ioannidis, J.P.A.; Tzoulaki, I. Environmental risk factors and multiple sclerosis: An umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015, 14, 263–273. [Google Scholar] [CrossRef]
- Salzer, J.; Nyström, M.; Hallmans, G.; Stenlund, H.; Wadell, G.; Sundström, P. Epstein-Barr virus antibodies and vitamin D in prospective multiple sclerosis biobank samples. Mult. Scler. J. 2013, 19, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Lünemann, J.D.; Tintoré, M.; Messmer, B.; Strowig, T.; Rovira, Á.; Perkal, H.; Caballero, E.; Münz, C.; Montalban, X.; Comabella, M. Elevated Epstein-Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann. Neurol. 2009, 67, 159–169. [Google Scholar] [CrossRef]
- Holmøy, T. Vitamin D status modulates the immune response to Epstein Barr virus: Synergistic effect of risk factors in multiple sclerosis. Med. Hypotheses 2008, 70, 66–69. [Google Scholar] [CrossRef]
- James, E.; Dobson, R.; Kuhle, J.; Baker, D.; Giovannoni, G.; Ramagopalan, S.V. The effect of vitamin D-related interventions on multiple sclerosis relapses: A meta-analysis. Mult. Scler. J. 2013, 19, 1571–1579. [Google Scholar] [CrossRef]
- Lee, C. Controversial Effects of Vitamin D and Related Genes on Viral Infections, Pathogenesis, and Treatment Outcomes. Nutrients 2020, 12, 962. [Google Scholar] [CrossRef]
- Wu, M.L.; Liu, F.L.; Sun, J.; Li, X.; He, X.Y.; Zheng, H.Y.; Zhou, Y.H.; Yan, Q.; Chen, L.; Yu, G.Y.; et al. SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury. Signal. Transduct. Target. Ther. 2021, 6, 428. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Grant, W.B.; Frias-Toral, E.; Sarno, G.; Vetrani, C.; Ceriani, F.; Garcia-Velasquez, E.; Contreras-Briceño, J.; Savastano, S.; et al. Vitamin D: A Role Also in Long COVID-19? Nutrients 2022, 14, 1625. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, F. Vitamin-D and COVID-19: Do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I.; Hebblewaite, D.; Brandt, W.E.; Ennis, F.A. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J. Virol. 1984, 52, 223–230. [Google Scholar] [CrossRef]
- King, C.A.; Anderson, R.; Marshall, J.S. Dengue Virus Selectively Induces Human Mast Cell Chemokine Production. J. Virol. 2002, 76, 8408–8419. [Google Scholar] [CrossRef]
- Sanchez, L.F.; Hotta, H.; Hotta, S.; Homma, M. Degranulation and Histamine Release from Murine Mast Cells Sensitized with Dengue Virus-Immune Sera. Microbiol. Immunol. 1986, 30, 753–759. [Google Scholar] [CrossRef]
- Mantri, C.K.; St John, A.L. Immune synapses between mast cells and γδ T cells limit viral infection. J. Clin. Investig. 2019, 129, 1094–1108. [Google Scholar] [CrossRef]
- Arboleda, J.F.; Urcuqui-Inchima, S. Vitamin D-Regulated MicroRNAs: Are They Protective Factors against Dengue Virus Infection? Adv. Virol. 2016, 2016, 1016840. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.C.; Hilmer, K.M.; Zickovich, J.M.; Obar, J.J. Inflammatory Response of Mast Cells during Influenza A Virus Infection Is Mediated by Active Infection and RIG-I Signaling. Pediatrics 2013, 190, 4676–4684. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, Y.; Han, D.; Zhang, G.; Cao, S.; Xie, J.; Xue, J.; Li, Y.; Meng, D.; Fan, X.; et al. Mast Cell-Induced Lung Injury in Mice Infected with H5N1 Influenza Virus. J. Virol. 2012, 86, 3347–3356. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral Activity and Increased Host Defense against Influenza Infection Elicited by the Human Cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehrani, Y.; Morovati, S.; Tieu, S.; Karimi, N.; Javadi, H.; Vanderkamp, S.; Sarmadi, S.; Tajik, T.; Kakish, J.E.; Bridle, B.W.; et al. Vitamin D Influences the Activity of Mast Cells in Allergic Manifestations and Potentiates Their Effector Functions against Pathogens. Cells 2023, 12, 2271. https://doi.org/10.3390/cells12182271
Mehrani Y, Morovati S, Tieu S, Karimi N, Javadi H, Vanderkamp S, Sarmadi S, Tajik T, Kakish JE, Bridle BW, et al. Vitamin D Influences the Activity of Mast Cells in Allergic Manifestations and Potentiates Their Effector Functions against Pathogens. Cells. 2023; 12(18):2271. https://doi.org/10.3390/cells12182271
Chicago/Turabian StyleMehrani, Yeganeh, Solmaz Morovati, Sophie Tieu, Negar Karimi, Helia Javadi, Sierra Vanderkamp, Soroush Sarmadi, Tahmineh Tajik, Julia E. Kakish, Byram W. Bridle, and et al. 2023. "Vitamin D Influences the Activity of Mast Cells in Allergic Manifestations and Potentiates Their Effector Functions against Pathogens" Cells 12, no. 18: 2271. https://doi.org/10.3390/cells12182271
APA StyleMehrani, Y., Morovati, S., Tieu, S., Karimi, N., Javadi, H., Vanderkamp, S., Sarmadi, S., Tajik, T., Kakish, J. E., Bridle, B. W., & Karimi, K. (2023). Vitamin D Influences the Activity of Mast Cells in Allergic Manifestations and Potentiates Their Effector Functions against Pathogens. Cells, 12(18), 2271. https://doi.org/10.3390/cells12182271









