Morphofunctional Investigation in a Transgenic Mouse Model of Alzheimer’s Disease: Non-Reactive Astrocytes Are Involved in Aβ Load and Reactive Astrocytes in Plaque Build-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. TgCRND8 Mice
2.2. Immunofluorescence Staining
2.2.1. Iba1 + GFAP + Aβ Triple Labeling Immunostaining
2.2.2. Iba1 + CD68 Double Labeling Immunostaining
2.2.3. GFAP Single Labeling Immunostaining
2.2.4. Cx43/MAP2 + GFAP Double Labeling Immunostaining
2.3. Confocal Microscopy
2.3.1. Marker Expression Analyses
2.3.2. Tracing Analysis of Astrocyte Processes
2.3.3. Machine Learning Analyses
Autofluorescent Deposits in the CA1 Stratum Pyramidale
Cx43 Clusters in CA1 Stratum Radiatum (Glia Limitans, Perivascular and Peri-Plaque Gliosis)
Dendrite Fragmentation in CA1 Stratum Radiatum (Peri-Plaque Gliosis)
2.4. Statistical Analysis
3. Results
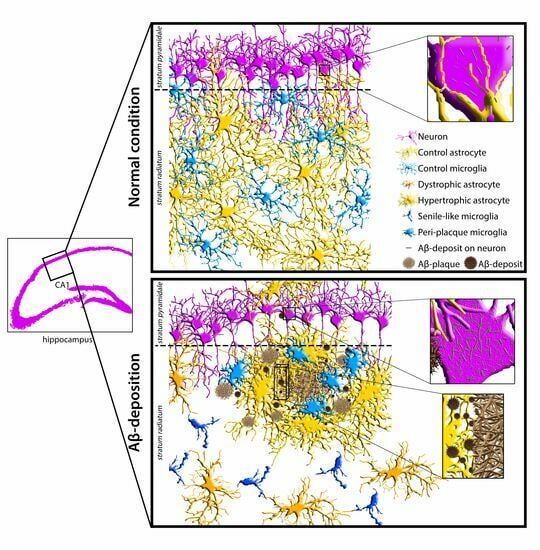
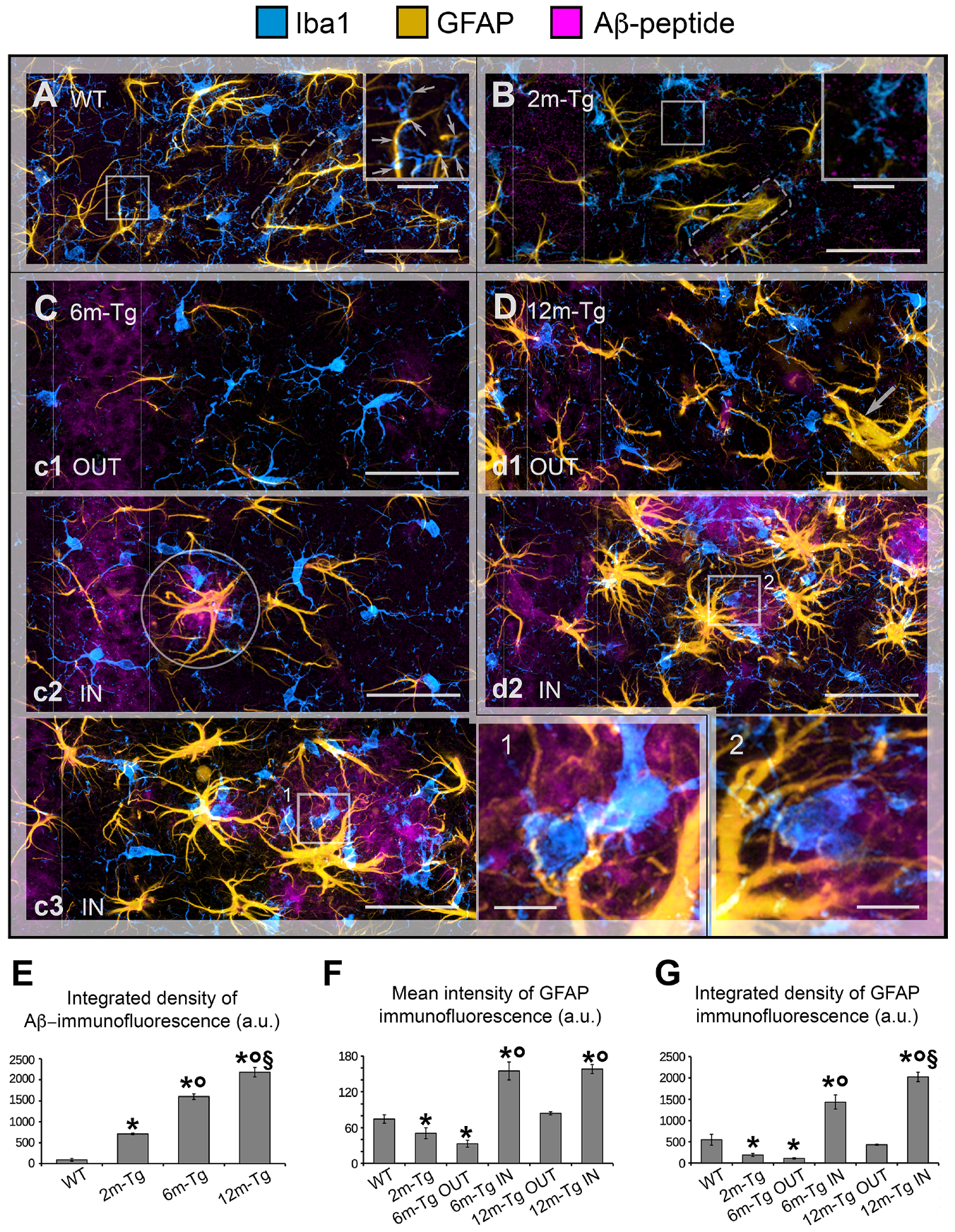
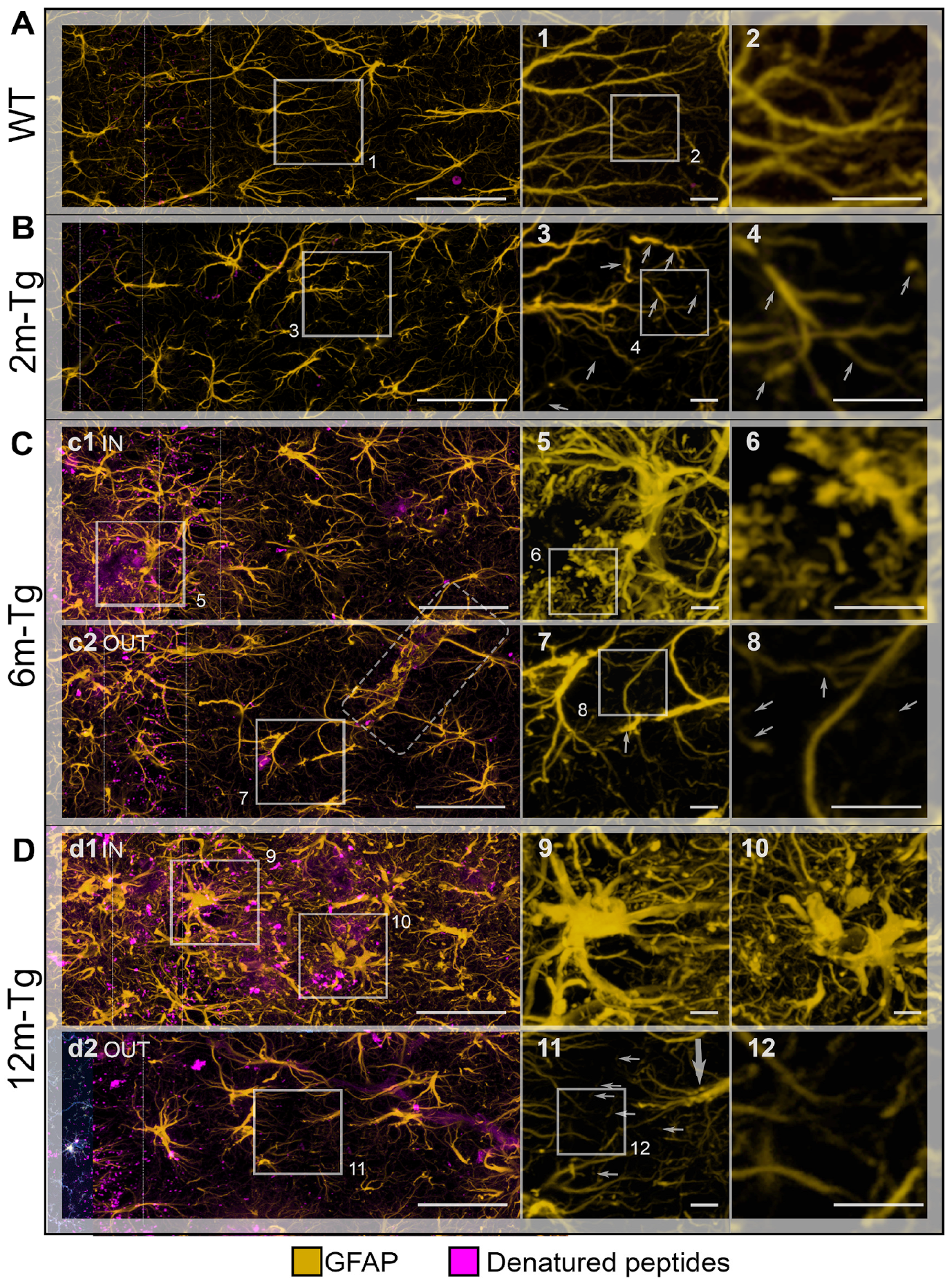
3.1. Analysis of Astrocyte (GFAP) and Microglia (Iba1) Morphology and Localization, and of GFAP and Aβ-Peptide Expression
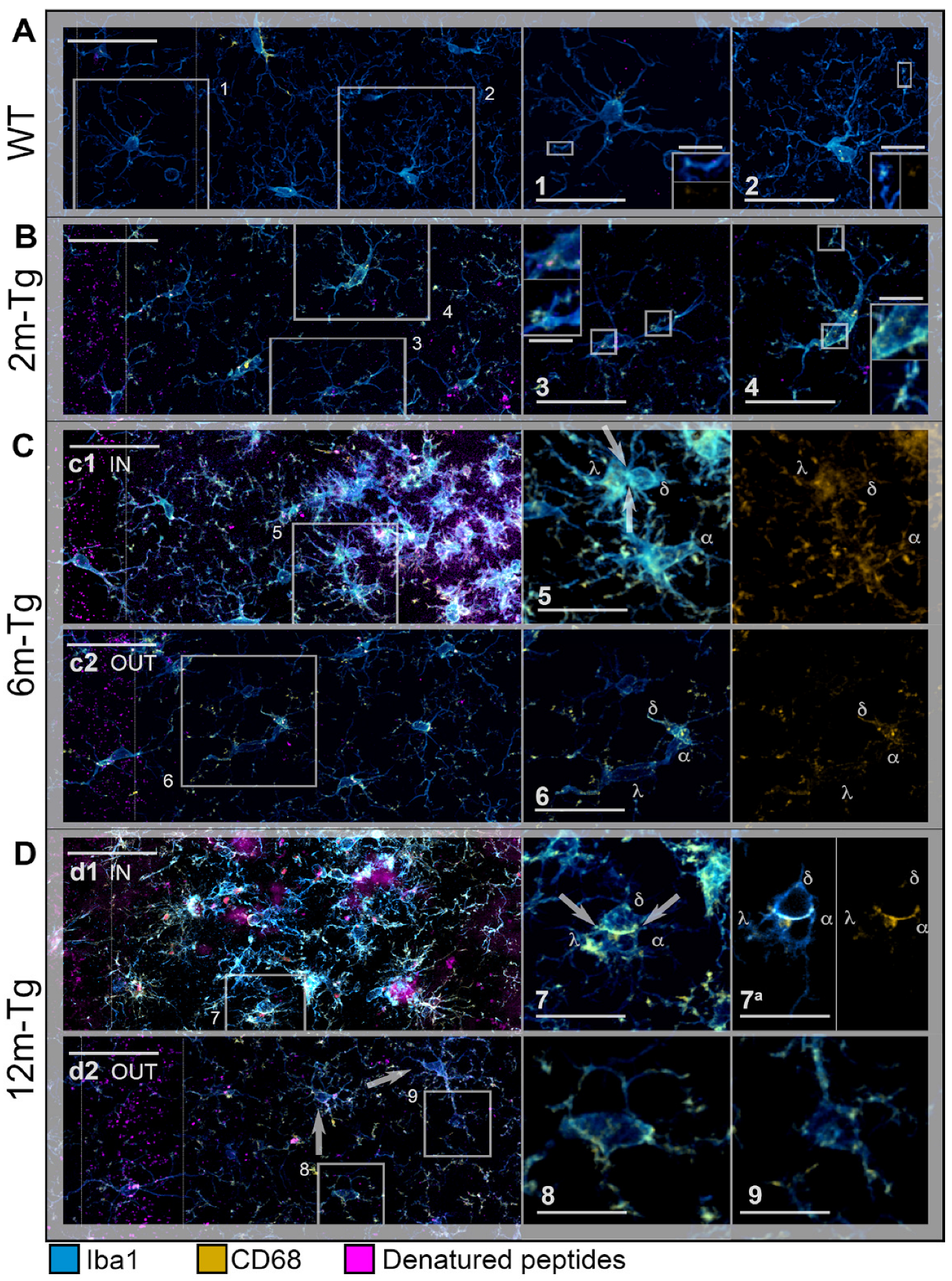
3.2. Analysis of Microglial Iba1 and CD68 Expression and In Situ Localization
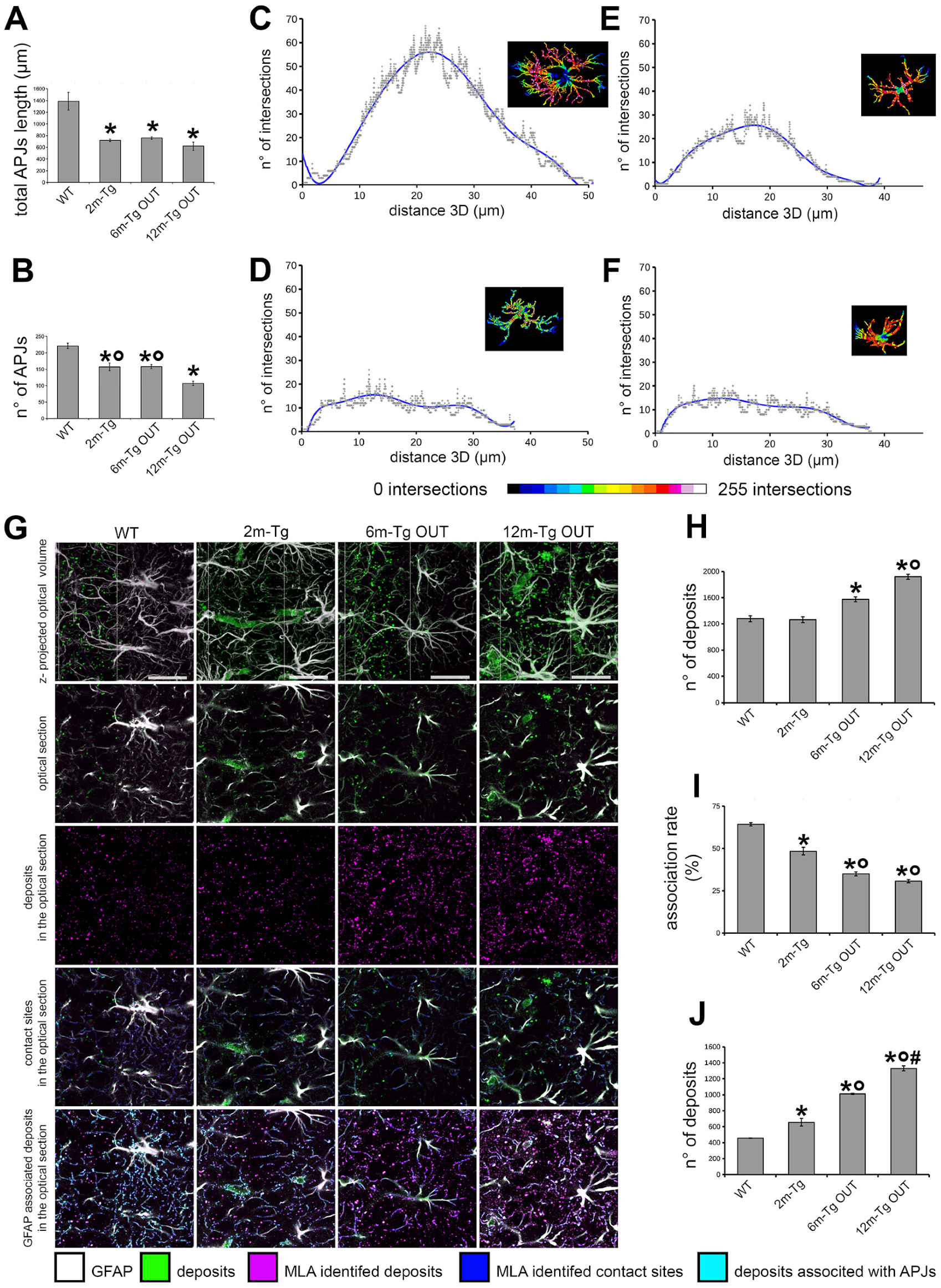
3.3. Morphological and Morphometric Analyses of GFAP+ APJs in the Stratum Radiatum and MLA Assessment of Their Interactions with Lipofuscin/Aβ–Deposits in the Stratum Pyramidale
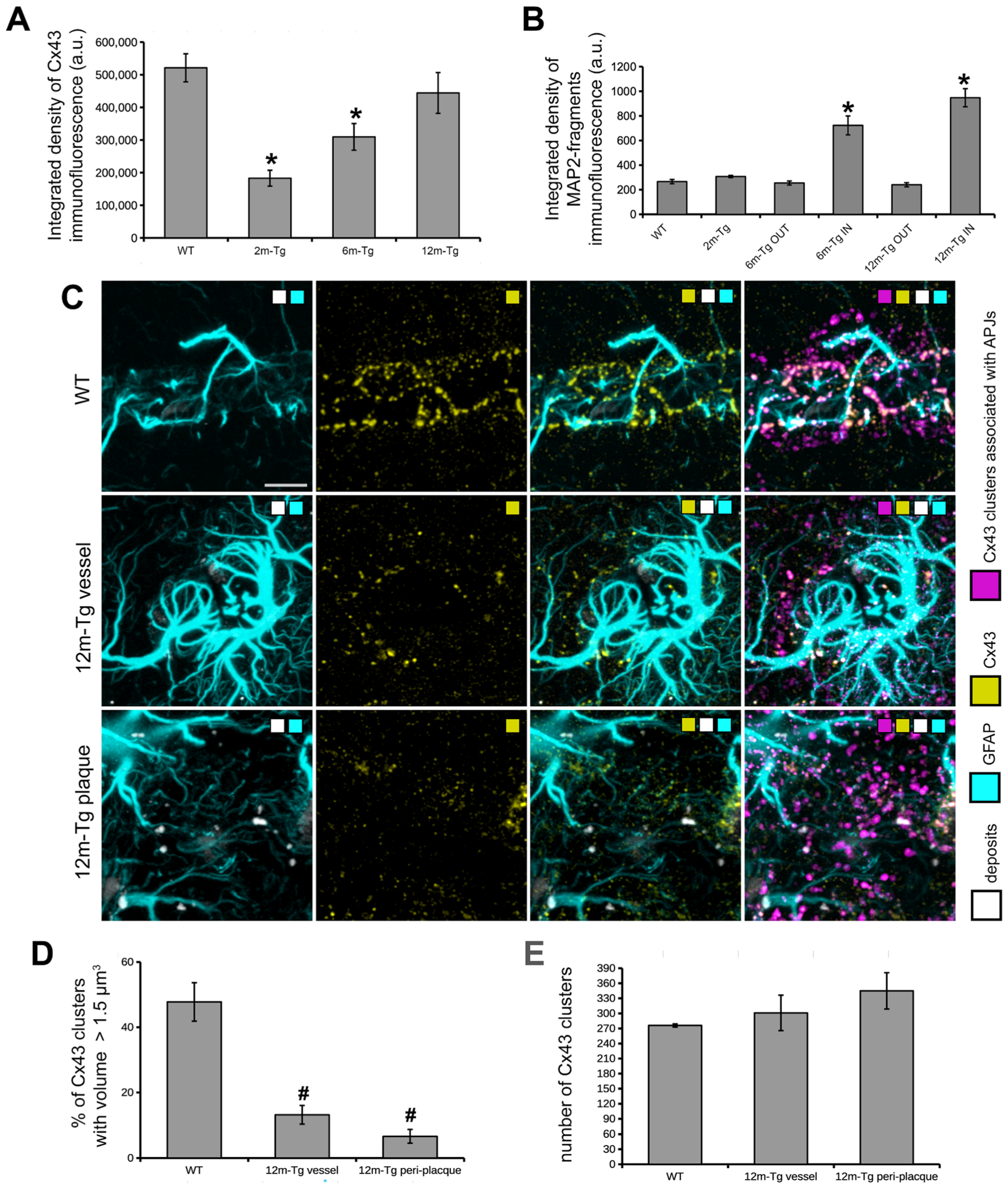
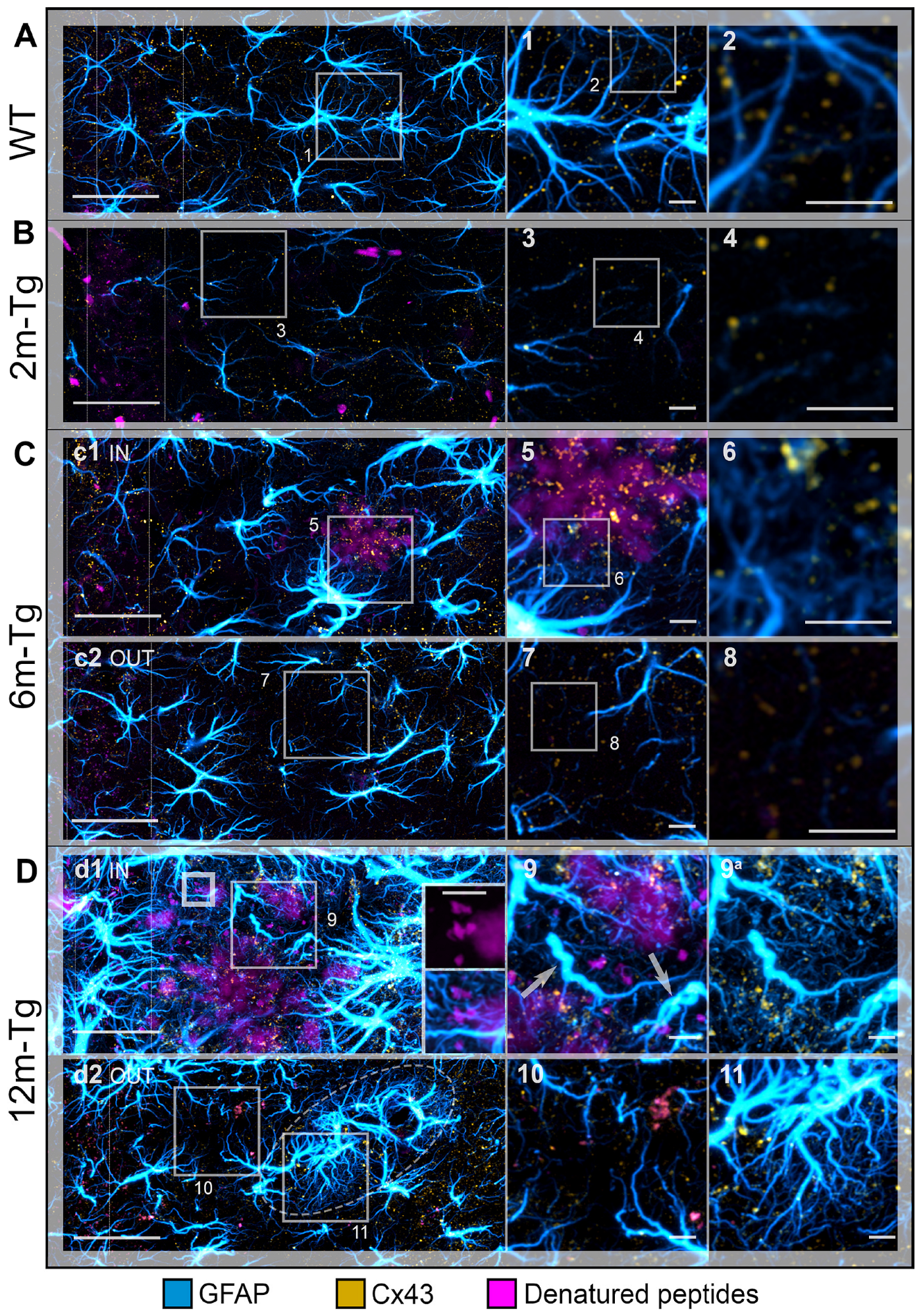
3.4. Analysis of the In Situ Expression and Localization of GFAP and Cx43 and the Morphometry and Distribution of Cx43 Clusters
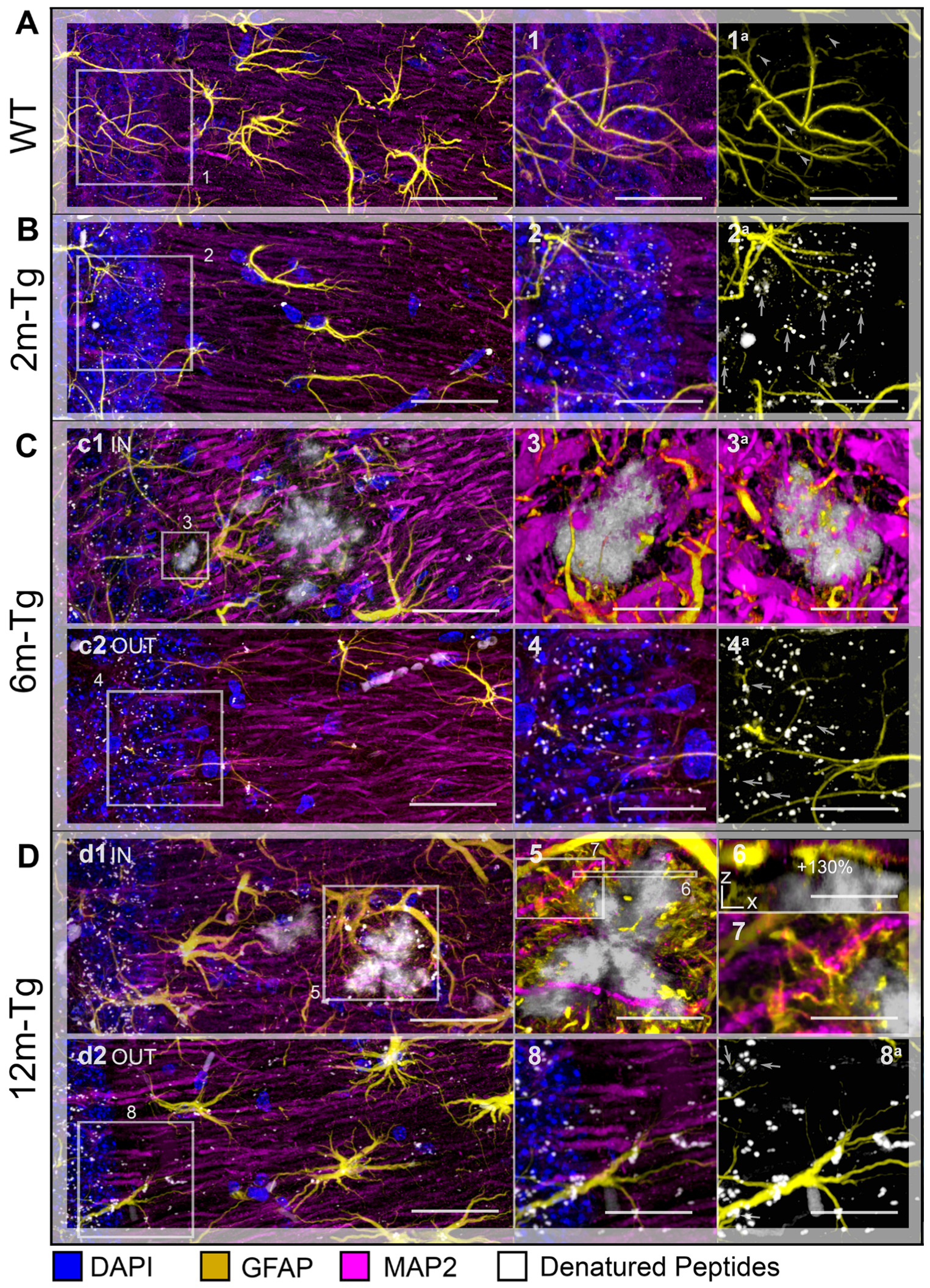
3.5. Analysis of the Expression and Localization of GFAP of Astrocytes and MAP2 of Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGeer, P.L.; Itagaki, S.; Tago, H.; McGeer, E.G. Reactive Microglia in Patients with Senile Dementia of the Alzheimer Type Are Positive for the Histocompatibility Glycoprotein HLA-DR. Neurosci. Lett. 1987, 79, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Inflammaging: Disturbed Interplay between Autophagy and Inflammasomes. Aging 2012, 4, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Ma, L.; Kaarela, T.; Li, Z. Neuroimmune Crosstalk in the Central Nervous System and Its Significance for Neurological Diseases. J. Neuroinflamm. 2012, 9, 594. [Google Scholar] [CrossRef]
- Hanisch, U.-K.; Kettenmann, H. Microglia: Active Sensor and Versatile Effector Cells in the Normal and Pathologic Brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Shen, Q.; Xu, P.; Luo, J.J.; Tang, Y. Phagocytosis of Microglia in the Central Nervous System Diseases. Mol. Neurobiol. 2014, 49, 1422–1434. [Google Scholar] [CrossRef]
- Thal, D.R. The Role of Astrocytes in Amyloid β-Protein Toxicity and Clearance. Exp. Neurol. 2012, 236, 1–5. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Liu, W.; Tang, Y.; Feng, J. Cross Talk between Activation of Microglia and Astrocytes in Pathological Conditions in the Central Nervous System. Life Sci. 2011, 89, 141–146. [Google Scholar] [CrossRef]
- Cerbai, F.; Lana, D.; Nosi, D.; Petkova-Kirova, P.; Zecchi, S.; Brothers, H.M.; Wenk, G.L.; Giovannini, M.G. The Neuron-Astrocyte-Microglia Triad in Normal Brain Ageing and in a Model of Neuroinflammation in the Rat Hippocampus. PLoS ONE 2012, 7, e45250. [Google Scholar] [CrossRef]
- Lana, D.; Ugolini, F.; Wenk, G.L.; Giovannini, M.G.; Zecchi-Orlandini, S.; Nosi, D. Microglial Distribution, Branching, and Clearance Activity in Aged Rat Hippocampus Are Affected by Astrocyte Meshwork Integrity: Evidence of a Novel Cell-cell Interglial Interaction. FASEB J. 2019, 33, 4007–4020. [Google Scholar] [CrossRef]
- Ugolini, F.; Lana, D.; Nardiello, P.; Nosi, D.; Pantano, D.; Casamenti, F.; Giovannini, M.G. Different Patterns of Neurodegeneration and Glia Activation in CA1 and CA3 Hippocampal Regions of TgCRND8 Mice. Front. Aging Neurosci. 2018, 10, 372. [Google Scholar] [CrossRef]
- Nosi, D.; Lana, D.; Giovannini, M.G.; Delfino, G.; Zecchi-Orlandini, S. Neuroinflammation: Integrated Nervous Tissue Response through Intercellular Interactions at the “Whole System” Scale. Cells 2021, 10, 1195. [Google Scholar] [CrossRef] [PubMed]
- Giunta, B.; Fernandez, F.; Nikolic, W.V.; Obregon, D.; Rrapo, E.; Town, T.; Tan, J. Inflammaging as a Prodrome to Alzheimer’s Disease. J. Neuroinflamm. 2008, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Deleidi, M.; Jäggle, M.; Rubino, G. Immune Aging, Dysmetabolism, and Inflammation in Neurological Diseases. Front. Neurosci. 2015, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Neniskyte, U.; Brown, G.C. Primary Phagocytosis of Neurons by Inflamed Microglia: Potential Roles in Neurodegeneration. Front. Pharmacol. 2012, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Vilalta, A.; Brown, G.C. Neurophagy, the Phagocytosis of Live Neurons and Synapses by Glia, Contributes to Brain Development and Disease. FEBS J. 2018, 285, 3566–3575. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Neher, J.J. Microglial Phagocytosis of Live Neurons. Nat. Rev. Neurosci. 2014, 15, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Del Mar Fernández-Arjona, M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ohmori, K.; Fujiwara, M. Microglial and Astroglial Reactions to Inflammatory Lesions of Experimental Autoimmune Encephalomyelitis in the Rat Central Nervous System. J. Neuroimmunol. 1992, 37, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Wheeler, M.A.; Clark, I.C.; Tjon, E.C.; Li, Z.; Zandee, S.E.J.; Couturier, C.P.; Watson, B.R.; Scalisi, G.; Alkwai, S.; Rothhammer, V.; et al. MAFG-Driven Astrocytes Promote CNS Inflammation. Nature 2020, 578, 593–599. [Google Scholar] [CrossRef]
- Ma, B.; Buckalew, R.; Du, Y.; Kiyoshi, C.M.; Alford, C.C.; Wang, W.; McTigue, D.M.; Enyeart, J.J.; Terman, D.; Zhou, M. Gap Junction Coupling Confers Isopotentiality on Astrocyte Syncytium: Electrical Coupling of Astrocytes in a Syncytium. Glia 2016, 64, 214–226. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. Fluid Transport in the Brain. Physiol. Rev. 2022, 102, 1025–1151. [Google Scholar] [CrossRef]
- Fróes, M.M.; Correia, A.H.P.; Garcia-Abreu, J.; Spray, D.C.; Campos de Carvalho, A.C.; Neto, V.M. Gap-Junctional Coupling between Neurons and Astrocytes in Primary Central Nervous System Cultures. Proc. Natl. Acad. Sci. USA 1999, 96, 7541–7546. [Google Scholar] [CrossRef]
- Mercatelli, R.; Lana, D.; Bucciantini, M.; Giovannini, M.G.; Cerbai, F.; Quercioli, F.; Zecchi-Orlandini, S.; Delfino, G.; Wenk, G.L.; Nosi, D. Clasmatodendrosis and Β-amyloidosis in Aging Hippocampus. FASEB J. 2016, 30, 1480–1491. [Google Scholar] [CrossRef]
- Hulse, R.E.; Winterfield, J.; Kunkler, P.E.; Kraig, R.P. Astrocytic Clasmatodendrosis in Hippocampal Organ Culture. Glia 2001, 33, 169–179. [Google Scholar] [CrossRef]
- Perez-Nievas, B.G.; Serrano-Pozo, A. Deciphering the Astrocyte Reaction in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Asahina, M.; Hattori, T. Selective Distribution of Matrix Metalloproteinase-3 (MMP-3) in Alzheimer’s Disease Brain. Acta Neuropathol. 2000, 99, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.C.; Baig, S.; Kehoe, P.G.; Love, S. Endothelin-Converting Enzyme-2 Is Increased in Alzheimer’s Disease and Up-Regulated by Aβ. Am. J. Pathol. 2009, 175, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.M.; Veerhuis, R.; Holmqvist, B.; Janciauskiene, S. Binding and Uptake of Aβ1-42 by Primary Human Astrocytes In Vitro. Glia 2009, 57, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; O’Connor, T.; Vassar, R. The Contribution of Activated Astrocytes to Aβ Production: Implications for Alzheimer’s Disease Pathogenesis. J. Neuroinflamm. 2011, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Bero, A.W.; Cirrito, J.R.; Xiao, Q.; Hu, X.; Wang, Y.; Gonzales, E.; Holtzman, D.M.; Lee, J.-M. Characterizing the Appearance and Growth of Amyloid Plaques in APP/PS1 Mice. J. Neurosci. 2009, 29, 10706–10714. [Google Scholar] [CrossRef]
- Kraft, A.W.; Hu, X.; Yoon, H.; Yan, P.; Xiao, Q.; Wang, Y.; Gil, S.C.; Brown, J.; Wilhelmsson, U.; Restivo, J.L.; et al. Attenuating Astrocyte Activation Accelerates Plaque Pathogenesis in APP/PS1 Mice. FASEB J. 2013, 27, 187–198. [Google Scholar] [CrossRef]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Münch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C.; et al. Neurotoxic Reactive Astrocytes Induce Cell Death via Saturated Lipids. Nature 2021, 599, 102–107. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, M.; Gratuze, M.; Bao, X.; Shi, Y.; Andhey, P.S.; Manis, M.; Schroeder, C.; Yin, Z.; Madore, C.; et al. Selective Removal of Astrocytic APOE4 Strongly Protects against Tau-Mediated Neurodegeneration and Decreases Synaptic Phagocytosis by Microglia. Neuron 2021, 109, 1657–1674.e7. [Google Scholar] [CrossRef]
- Wang, H.; Kulas, J.A.; Wang, C.; Holtzman, D.M.; Ferris, H.A.; Hansen, S.B. Regulation of Beta-Amyloid Production in Neurons by Astrocyte-Derived Cholesterol. Proc. Natl. Acad. Sci. USA 2021, 118, e2102191118. [Google Scholar] [CrossRef]
- Pardo, A.C.; Wong, V.; Benson, L.M.; Dykes, M.; Tanaka, K.; Rothstein, J.D.; Maragakis, N.J. Loss of the Astrocyte Glutamate Transporter GLT1 Modifies Disease in SOD1G93A Mice. Exp. Neurol. 2006, 201, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Augusto, E.; Oliveira, C.R.; Agostinho, P. Amyloid-Beta Peptide Decreases Glutamate Uptake in Cultured Astrocytes: Involvement of Oxidative Stress and Mitogen-Activated Protein Kinase Cascades. Neuroscience 2008, 156, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Ao, Y.; Faas, G.C.; Nwaobi, S.E.; Xu, J.; Haustein, M.D.; Anderson, M.A.; Mody, I.; Olsen, M.L.; Sofroniew, M.V.; et al. Astrocyte Kir4.1 Ion Channel Deficits Contribute to Neuronal Dysfunction in Huntington’s Disease Model Mice. Nat. Neurosci. 2014, 17, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Chazalon, M.; Paredes-Rodriguez, E.; Morin, S.; Martinez, A.; Cristóvão-Ferreira, S.; Vaz, S.; Sebastiao, A.; Panatier, A.; Boué-Grabot, E.; Miguelez, C.; et al. GAT-3 Dysfunction Generates Tonic Inhibition in External Globus Pallidus Neurons in Parkinsonian Rodents. Cell Rep. 2018, 23, 1678–1690. [Google Scholar] [CrossRef]
- Chao, C.-C.; Gutiérrez-Vázquez, C.; Rothhammer, V.; Mayo, L.; Wheeler, M.A.; Tjon, E.C.; Zandee, S.E.J.; Blain, M.; de Lima, K.A.; Takenaka, M.C.; et al. Metabolic Control of Astrocyte Pathogenic Activity via CPLA2-MAVS. Cell 2019, 179, 1483–1498.e22. [Google Scholar] [CrossRef]
- Polyzos, A.A.; Lee, D.Y.; Datta, R.; Hauser, M.; Budworth, H.; Holt, A.; Mihalik, S.; Goldschmidt, P.; Frankel, K.; Trego, K.; et al. Metabolic Reprogramming in Astrocytes Distinguishes Region-Specific Neuronal Susceptibility in Huntington Mice. Cell Metab. 2019, 29, 1258–1273.e11. [Google Scholar] [CrossRef]
- Chishti, M.A.; Yang, D.-S.; Janus, C.; Phinney, A.L.; Horne, P.; Pearson, J.; Strome, R.; Zuker, N.; Loukides, J.; French, J.; et al. Early-Onset Amyloid Deposition and Cognitive Deficits in Transgenic Mice Expressing a Double Mutant Form of Amyloid Precursor Protein 695*. J. Biol. Chem. 2001, 276, 21562–21570. [Google Scholar] [CrossRef]
- Bellucci, A.; Luccarini, I.; Scali, C.; Prosperi, C.; Giovannini, M.G.; Pepeu, G.; Casamenti, F. Cholinergic Dysfunction, Neuronal Damage and Axonal Loss in TgCRND8 Mice. Neurobiol. Dis. 2006, 23, 260–272. [Google Scholar] [CrossRef]
- Hamm, V.; Héraud, C.; Bott, J.-B.; Herbeaux, K.; Strittmatter, C.; Mathis, C.; Goutagny, R. Differential Contribution of APP Metabolites to Early Cognitive Deficits in a TgCRND8 Mouse Model of Alzheimer’s Disease. Sci. Adv. 2017, 3, e1601068. [Google Scholar] [CrossRef]
- Giovannini, M.G. Double-Label Confocal Microscopy of Phosphorylated Protein Kinases Involved in Long-Term Potentiation. In Methods in Enzymology; Iyengar, R., Hildebrandt, J.D., Eds.; G Protein Pathways; Academic Press: Cambridge, MA, USA, 2002; Volume 345, pp. 426–436. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Arshadi, C.; Günther, U.; Eddison, M.; Harrington, K.I.S.; Ferreira, T.A. SNT: A Unifying Toolbox for Quantification of Neuronal Anatomy. Nat. Methods 2021, 18, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.A.; Blackman, A.V.; Oyrer, J.; Jayabal, S.; Chung, A.J.; Watt, A.J.; Sjöström, P.J.; van Meyel, D.J. Neuronal Morphometry Directly from Bitmap Images. Nat. Methods 2014, 11, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Olabarria, M.; Noristani, H.N.; Verkhratsky, A.; Rodríguez, J.J. Concomitant Astroglial Atrophy and Astrogliosis in a Triple Transgenic Animal Model of Alzheimer’s Disease. Glia 2010, 58, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Dorman, L.C.; Pan, S.; Vainchtein, I.D.; Han, R.T.; Nakao-Inoue, H.; Taloma, S.E.; Barron, J.J.; Molofsky, A.B.; Kheirbek, M.A.; et al. Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell 2020, 182, 388–403.e15. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Rodrigues, J.J.; Pivoriunas, A.; Zorec, R.; Semyanov, A. Astroglial Atrophy in Alzheimer’s Disease. Pflügers Arch. Eur. J. Physiol. 2019, 471, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.I.; Li, W.; Hertzberg, E.L.; Marotta, C.A. Elevated Connexin43 Immunoreactivity at Sites of Amyloid Plaques in Alzheimer’s Disease. Brain Res. 1996, 717, 173–178. [Google Scholar] [CrossRef]
- Cibelli, A.; Stout, R.; Timmermann, A.; de Menezes, L.; Guo, P.; Maass, K.; Seifert, G.; Steinhäuser, C.; Spray, D.C.; Scemes, E. Cx43 Carboxyl Terminal Domain Determines AQP4 and Cx30 Endfoot Organization and Blood Brain Barrier Permeability. Sci. Rep. 2021, 11, 24334. [Google Scholar] [CrossRef]
- Kwon, M.J.; Shin, H.Y.; Cui, Y.; Kim, H.; Thi, A.H.L.; Choi, J.Y.; Kim, E.Y.; Hwang, D.H.; Kim, B.G. CCL2 Mediates Neuron–Macrophage Interactions to Drive Proregenerative Macrophage Activation Following Preconditioning Injury. J. Neurosci. 2015, 35, 15934–15947. [Google Scholar] [CrossRef]
- Finneran, D.J.; Nash, K.R. Neuroinflammation and Fractalkine Signaling in Alzheimer’s Disease. J. Neuroinflamm. 2019, 16, 30. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Shaikh, M.F.; Piperi, C. Fractalkine (CX3CL1) Signaling and Neuroinflammation in Parkinson’s Disease: Potential Clinical and Therapeutic Implications. Pharmacol. Res. 2020, 158, 104930. [Google Scholar] [CrossRef]
- Herman, F.J.; Pasinetti, G.M. Principles of Inflammasome Priming and Inhibition: Implications for Psychiatric Disorders. Brain Behav. Immun. 2018, 73, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Fiebich, B.L.; Akter, S.; Akundi, R.S. The Two-Hit Hypothesis for Neuroinflammation: Role of Exogenous ATP in Modulating Inflammation in the Brain. Front. Cell. Neurosci. 2014, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Culmsee, C.; Michels, S.; Scheu, S.; Arolt, V.; Dannlowski, U.; Alferink, J. Mitochondria, Microglia, and the Immune System-How Are They Linked in Affective Disorders? Front. Psychiatry 2018, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kui, L.; Demetrios, T.; Gong, X.; Tang, M. A Glimmer of Hope: Maintain Mitochondrial Homeostasis to Mitigate Alzheimer’s Disease. Aging Dis. 2020, 11, 1260–1275. [Google Scholar] [CrossRef]
- Stoyanov, S.; Sun, W.; Düsedau, H.P.; Cangalaya, C.; Choi, I.; Mirzapourdelavar, H.; Baidoe-Ansah, D.; Kaushik, R.; Neumann, J.; Dunay, I.R.; et al. Attenuation of the Extracellular Matrix Restores Microglial Activity during the Early Stage of Amyloidosis. Glia 2021, 69, 182–200. [Google Scholar] [CrossRef]
- Floden, A.M.; Combs, C.K. Microglia Demonstrate Age-Dependent Interaction with Beta-Amyloid Fibrils. J. Alzheimers Dis. 2011, 25, 279–293. [Google Scholar] [CrossRef]
- Von Bernhardi, R.; Eugenín-von Bernhardi, L.; Eugenín, J. Microglial Cell Dysregulation in Brain Aging and Neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef]
- Waller, R.; Baxter, L.; Fillingham, D.J.; Coelho, S.; Pozo, J.M.; Mozumder, M.; Frangi, A.F.; Ince, P.G.; Simpson, J.E.; Highley, J.R. Iba-1−/CD68+ Microglia Are a Prominent Feature of Age-Associated Deep Subcortical White Matter Lesions. PLoS ONE 2019, 14, e0210888. [Google Scholar] [CrossRef]
- Bennett, F.C.; Bennett, M.L.; Yaqoob, F.; Mulinyawe, S.B.; Grant, G.A.; Hayden Gephart, M.; Plowey, E.D.; Barres, B.A. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 2018, 98, 1170–1183.e8. [Google Scholar] [CrossRef]
- Van der Poel, M.; Ulas, T.; Mizee, M.R.; Hsiao, C.-C.; Miedema, S.S.M.; Adelia; Schuurman, K.G.; Helder, B.; Tas, S.W.; Schultze, J.L.; et al. Transcriptional Profiling of Human Microglia Reveals Grey–White Matter Heterogeneity and Multiple Sclerosis-Associated Changes. Nat. Commun. 2019, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.G.; Puvanachandra, N.; Horner, C.H.; Polito, A.; Ostenfeld, T.; Svendsen, C.N.; Mucke, L.; Johnson, M.H.; Sofroniew, M.V. Leukocyte Infiltration, Neuronal Degeneration, and Neurite Outgrowth after Ablation of Scar-Forming, Reactive Astrocytes in Adult Transgenic Mice. Neuron 1999, 23, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Cui, Y.; Wang, H.; Jiang, J.; Zhang, T.; Sun, S.; Zhou, Z.; Zhong, Y.; Xiao, B. Astrocytic Piezo1-Mediated Mechanotransduction Determines Adult Neurogenesis and Cognitive Functions. Neuron 2022, 110, 2984–2999.e8. [Google Scholar] [CrossRef]
- Moeendarbary, E.; Weber, I.P.; Sheridan, G.K.; Koser, D.E.; Soleman, S.; Haenzi, B.; Bradbury, E.J.; Fawcett, J.; Franze, K. The Soft Mechanical Signature of Glial Scars in the Central Nervous System. Nat. Commun. 2017, 8, 14787. [Google Scholar] [CrossRef] [PubMed]
- Haupt, C.; Witte, O.W.; Frahm, C. Up-Regulation of Connexin43 in the Glial Scar Following Photothrombotic Ischemic Injury. Mol. Cell. Neurosci. 2007, 35, 89–99. [Google Scholar] [CrossRef]

| Antibody | Specificity | Supplier, Source, Dilution |
|---|---|---|
| Iba1 Ionized calcium-binding adaptor molecule 1 | A calcium-binding protein with actin-bundling activity that participates in branching, membrane ruffling, and phagocytosis in reactive microglia | WAKO (Osaka, Japan) Code #016-20001 rabbit, 1:300 |
| CD68 Cluster of Differentiation 68 | This marker plays a role in phagocytic activity of tissue macrophages, both in intracellular lysosomal pathways and in extracellular cell–cell and cell-pathogen interactions. It mediates the homing of macrophage subsets to particular tissue sites | AbCam (Cambridge, UK) Code #Ab955 rabbit, 1:100 |
| Cx43 Connexin 43 | Structural protein of gap junctional connexons allowing ion and small molecule transit between neighboring cells. It is involved in the onset and maintenance of astroglial functional syncytium | Cell Signaling (Danvers, MA, USA) Code #3512 rabbit, 1:50 |
| GFAP Glial Fibrillary Acidic Protein | A class-III intermediate filament and astrocyte-specific marker expressed mainly in APJs | DakoCytomation (Glostrup, Denmark) Code #Z0334 mouse, 1:500 |
| Aβ Beta-amyloid | A 36–43 amino acid peptide, the main component of amyloid plaques found in the CNS of AD people and related animal models | Covance (Emeryville, CA, USA) Code #SIG-39320, mouse, 1:400 |
| MAP2 Microtubule-associated protein 2 | Neuron-specific proteins of the neuro-tubular cytoskeleton widely used to identify neuronal cells and trace their processes | Cell Signaling (Danvers, MA, USA) Code #4542 rabbit, 1:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lana, D.; Branca, J.J.V.; Delfino, G.; Giovannini, M.G.; Casamenti, F.; Nardiello, P.; Bucciantini, M.; Stefani, M.; Zach, P.; Zecchi-Orlandini, S.; et al. Morphofunctional Investigation in a Transgenic Mouse Model of Alzheimer’s Disease: Non-Reactive Astrocytes Are Involved in Aβ Load and Reactive Astrocytes in Plaque Build-Up. Cells 2023, 12, 2258. https://doi.org/10.3390/cells12182258
Lana D, Branca JJV, Delfino G, Giovannini MG, Casamenti F, Nardiello P, Bucciantini M, Stefani M, Zach P, Zecchi-Orlandini S, et al. Morphofunctional Investigation in a Transgenic Mouse Model of Alzheimer’s Disease: Non-Reactive Astrocytes Are Involved in Aβ Load and Reactive Astrocytes in Plaque Build-Up. Cells. 2023; 12(18):2258. https://doi.org/10.3390/cells12182258
Chicago/Turabian StyleLana, Daniele, Jacopo Junio Valerio Branca, Giovanni Delfino, Maria Grazia Giovannini, Fiorella Casamenti, Pamela Nardiello, Monica Bucciantini, Massimo Stefani, Petr Zach, Sandra Zecchi-Orlandini, and et al. 2023. "Morphofunctional Investigation in a Transgenic Mouse Model of Alzheimer’s Disease: Non-Reactive Astrocytes Are Involved in Aβ Load and Reactive Astrocytes in Plaque Build-Up" Cells 12, no. 18: 2258. https://doi.org/10.3390/cells12182258
APA StyleLana, D., Branca, J. J. V., Delfino, G., Giovannini, M. G., Casamenti, F., Nardiello, P., Bucciantini, M., Stefani, M., Zach, P., Zecchi-Orlandini, S., & Nosi, D. (2023). Morphofunctional Investigation in a Transgenic Mouse Model of Alzheimer’s Disease: Non-Reactive Astrocytes Are Involved in Aβ Load and Reactive Astrocytes in Plaque Build-Up. Cells, 12(18), 2258. https://doi.org/10.3390/cells12182258