NAP (Davunetide): The Neuroprotective ADNP Drug Candidate Penetrates Cell Nuclei Explaining Pleiotropic Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. CRISPR/Cas9 DNA-Editing System
2.3. Antibodies
2.4. Immunocytochemistry
2.5. Live Cell Imaging
2.6. Cellular Fractionations
2.7. Microtubule Intoxication
2.8. RNA Extraction and Quantitative Real-Time PCR
2.9. Western Analysis
2.10. Experimental Timeline
2.11. In Silico Modeling
2.12. Statistical Analysis
3. Results
3.1. Exogenously Added NAP Is Present in and around the Nucleus
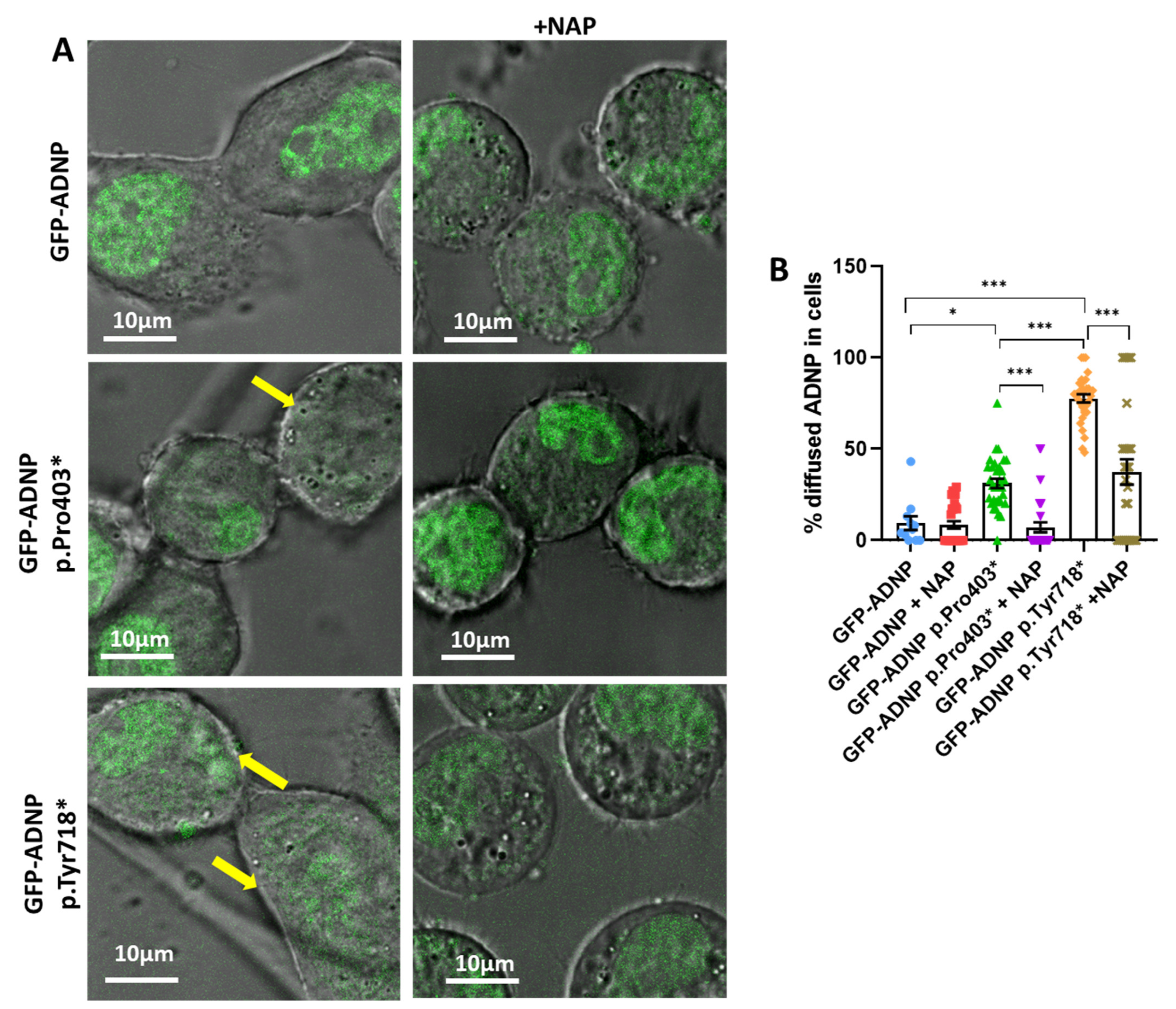
3.2. Immediate Correction of Mutant-Induced Undiscernible Nuclear Envelopes by NAP
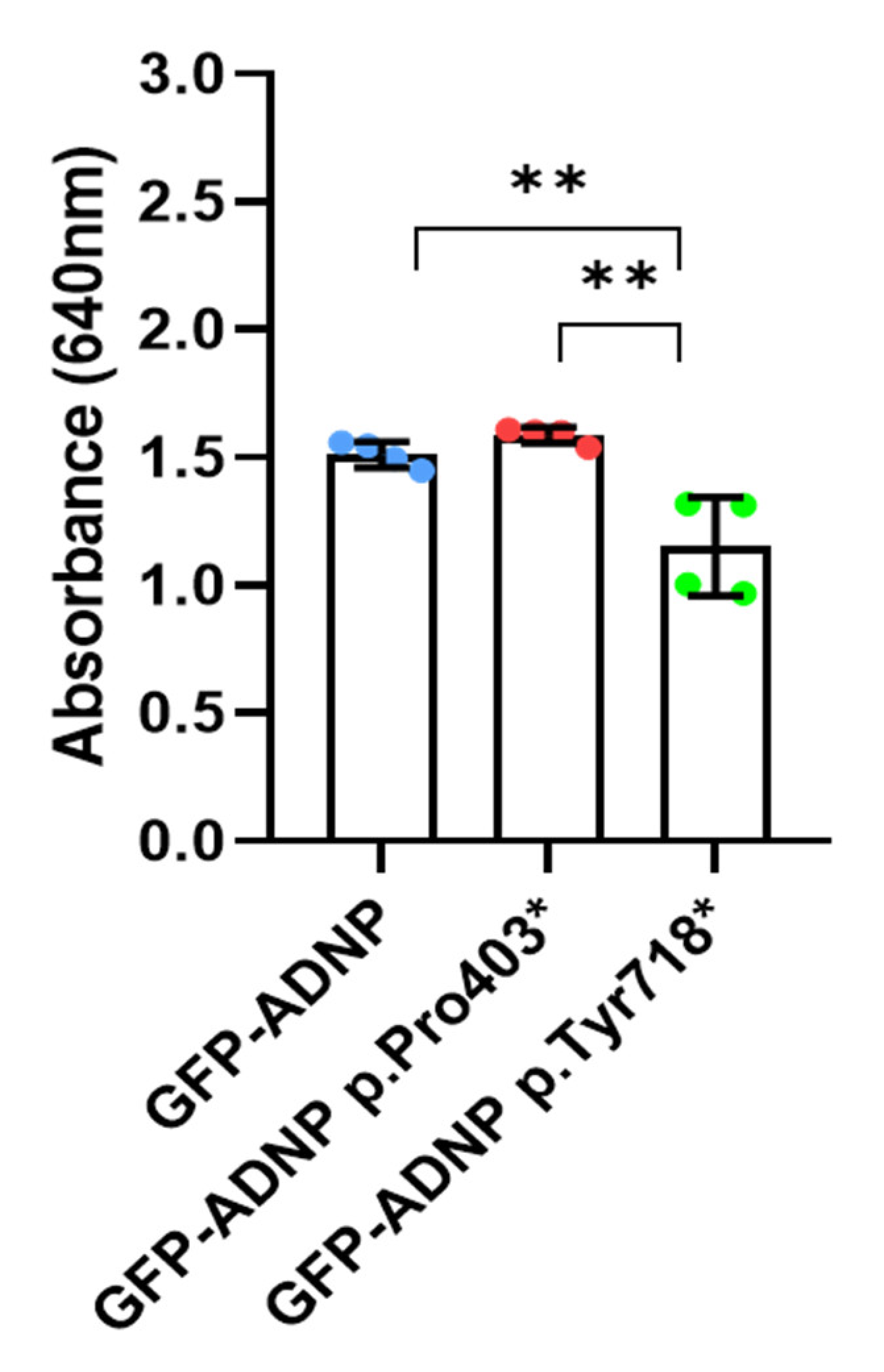
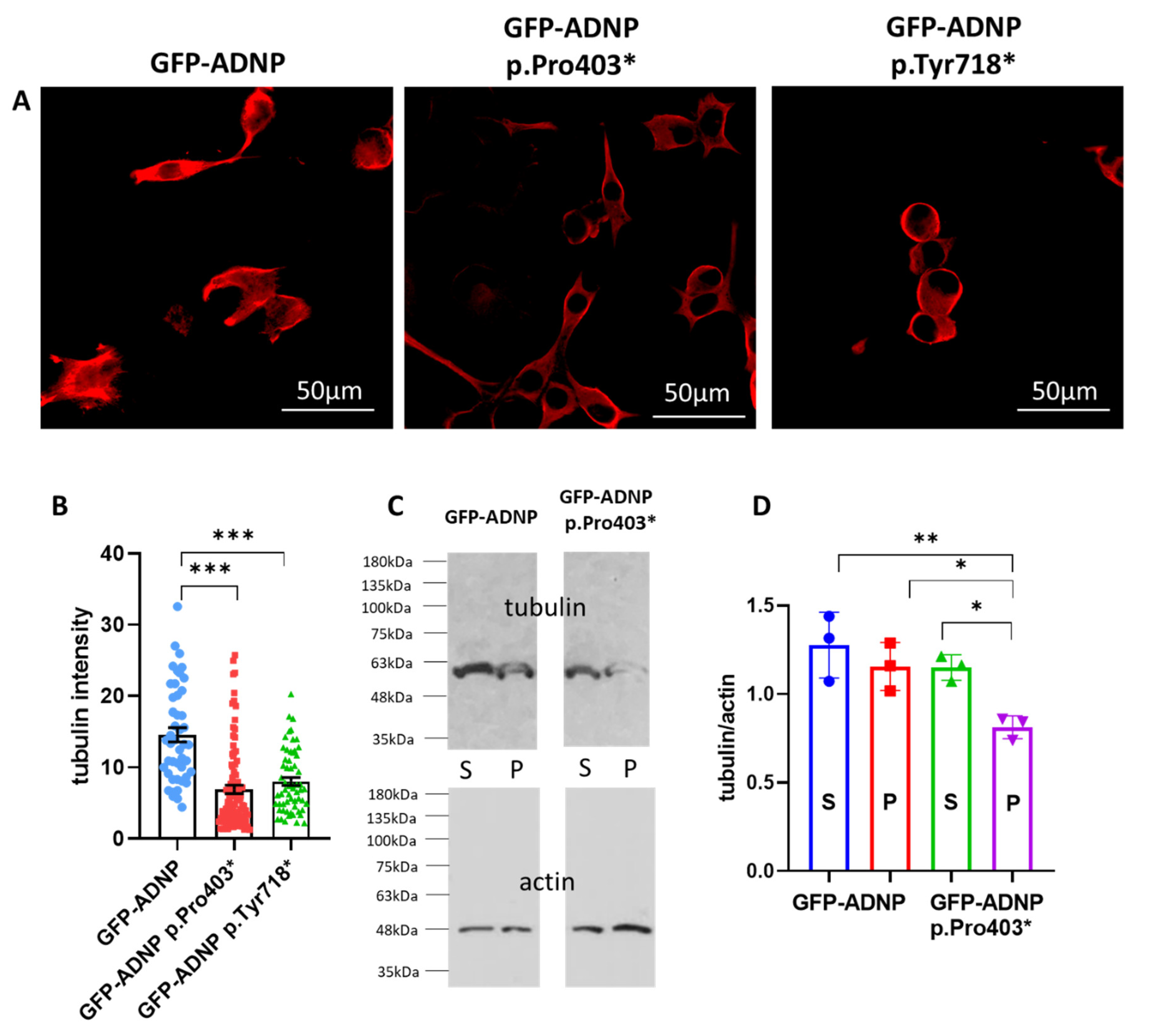
3.3. Tubulin/Microtubules Decrease as a Consequence of ADNP Mutations
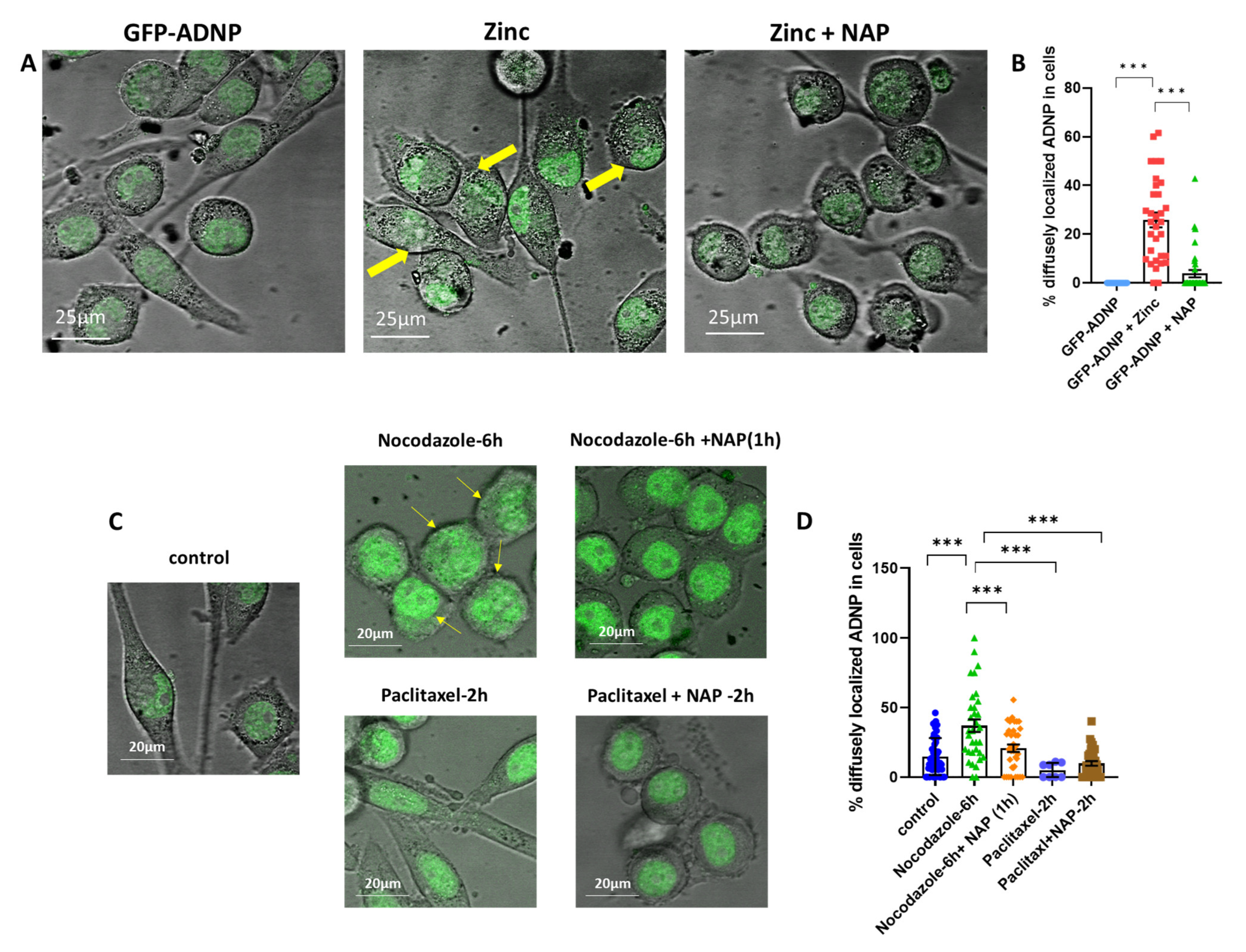
3.4. Zinc- and Nocodazole-Induced Microtubule Dysfunction Enhances Cytoplasmic/Nuclear Aberrant Morphology, Which Is Corrected by NAP Treatment
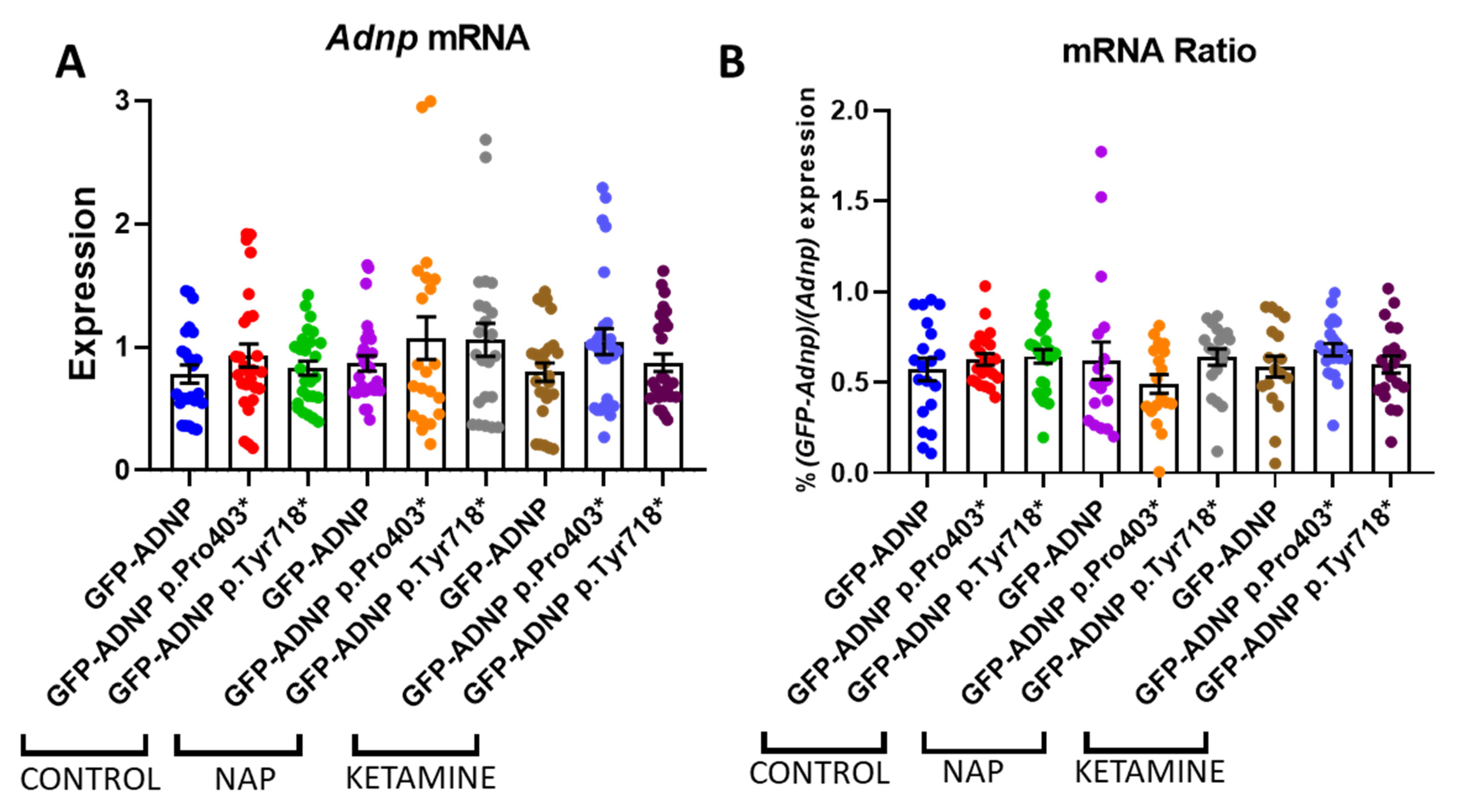
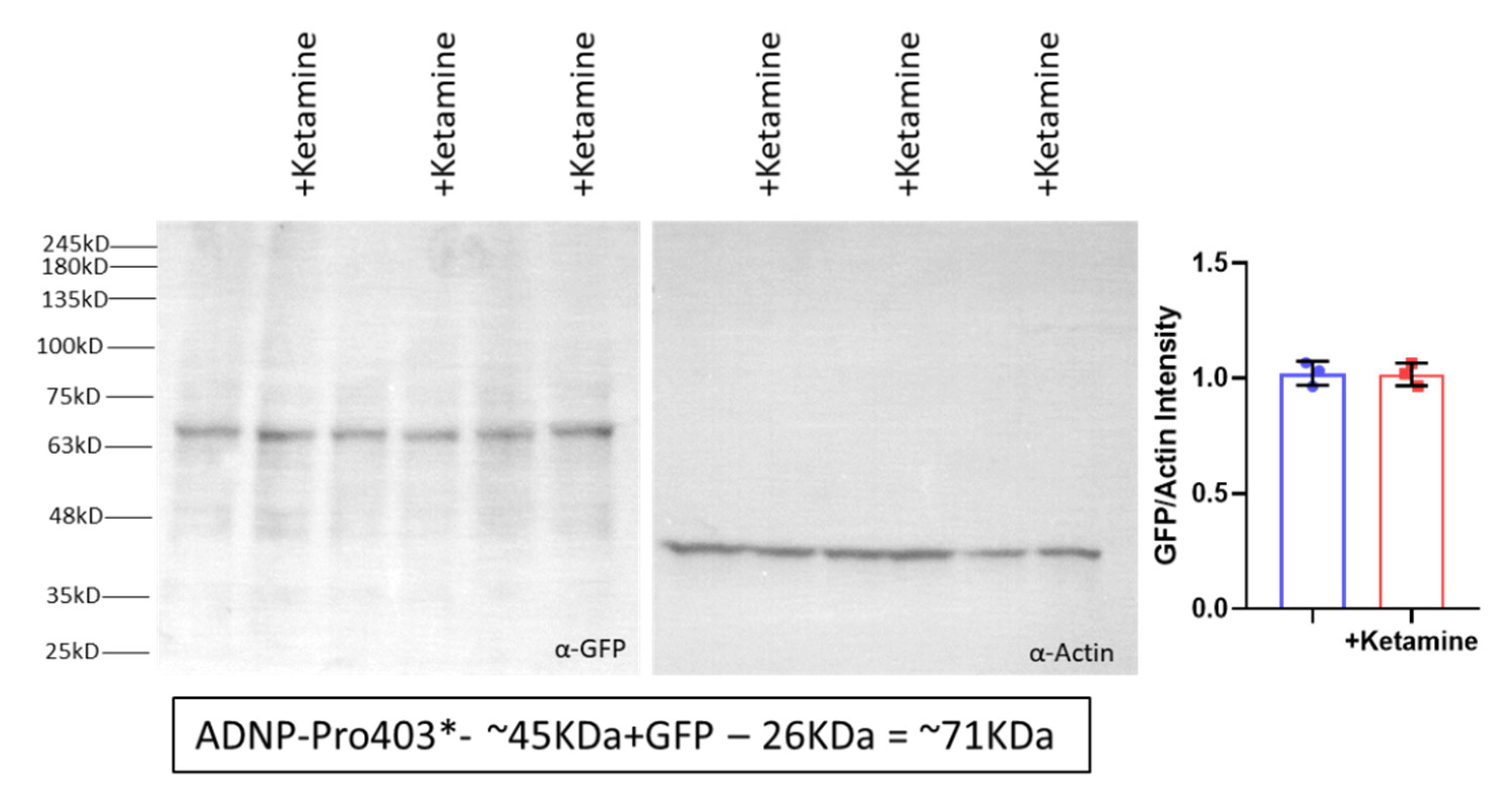
3.5. No Effect on ADNP Expression
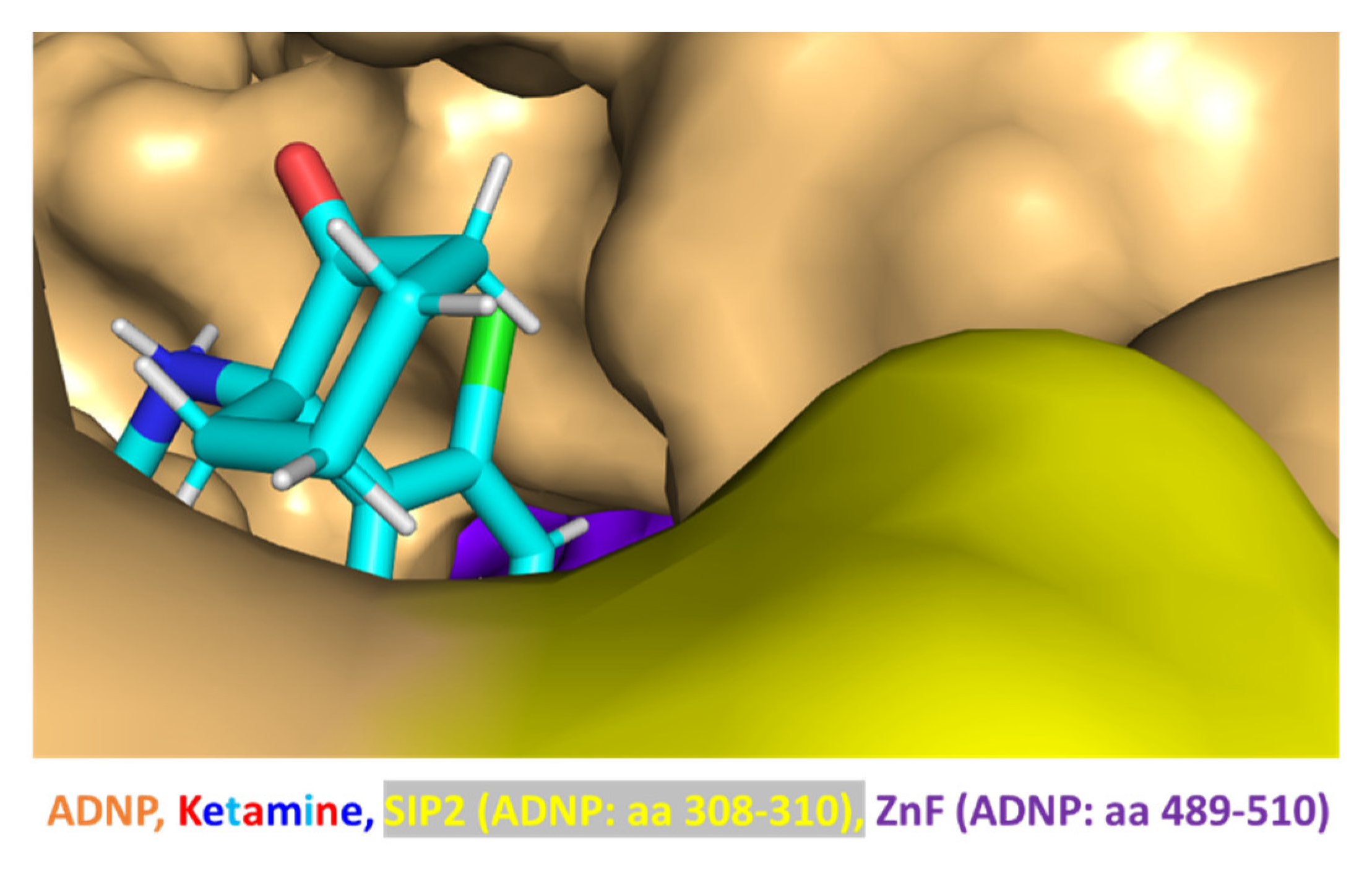
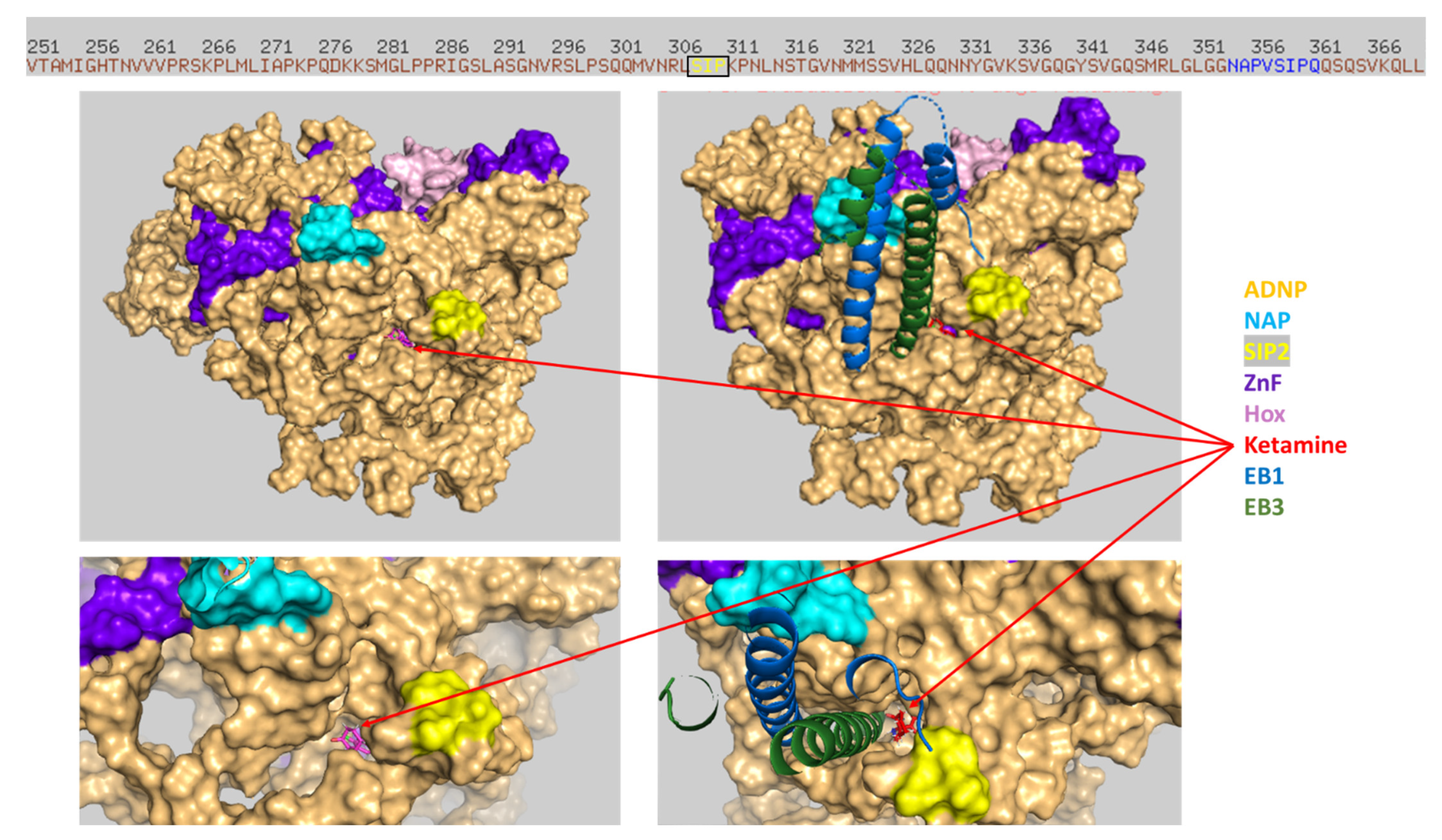
3.6. Ketamine Neither Increases Adnp mRNA Expression nor Protects against Mutation-Associated Aberrant ADNP Nuclear/Cytoplasmic Distribution While Interacting with ADNP at a SIP-Microtubule-Binding Motif
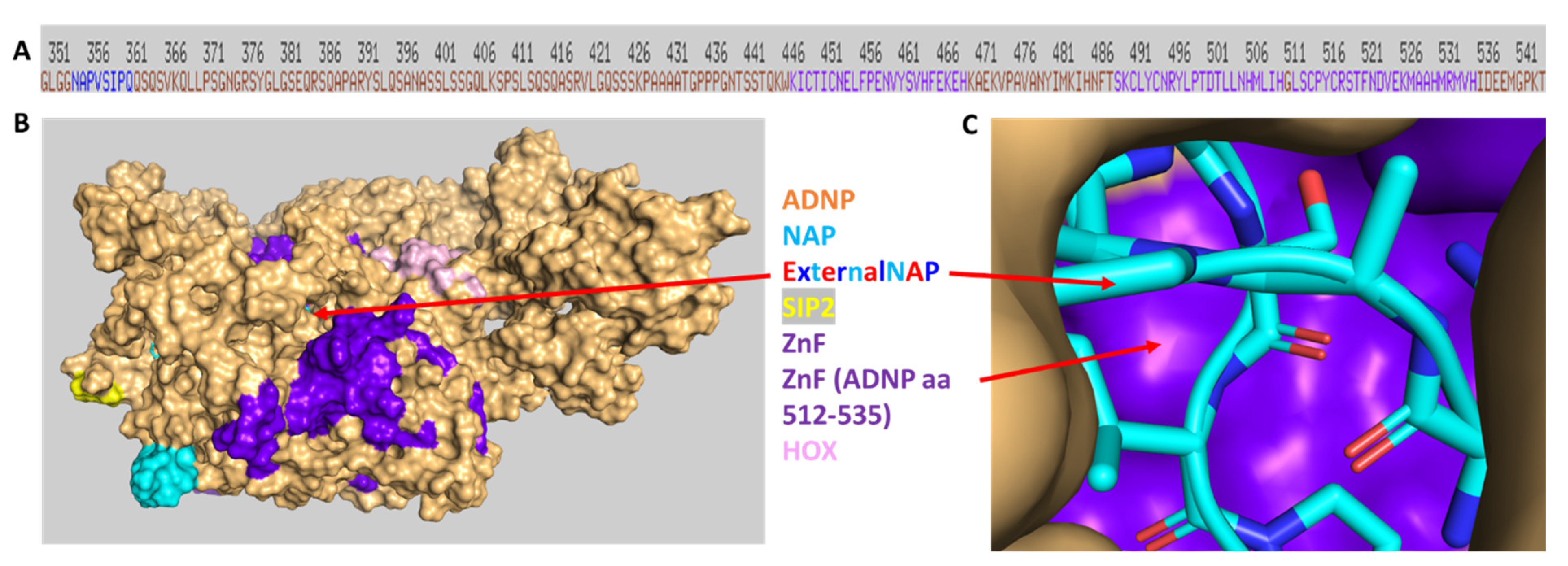
3.7. Discovery of NAP Interactions with ADNP Zinc Finger-Binding Motifs
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassan, M.; Zamostiano, R.; Davidson, A.; Pinhasov, A.; Giladi, E.; Perl, O.; Bassan, H.; Blat, C.; Gibney, G.; Glazner, G.; et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 1999, 72, 1283–1293. [Google Scholar] [CrossRef]
- Pinhasov, A.; Mandel, S.; Torchinsky, A.; Giladi, E.; Pittel, Z.; Goldsweig, A.M.; Servoss, S.J.; Brenneman, D.E.; Gozes, I. Activity-dependent neuroprotective protein: A novel gene essential for brain formation. Dev. Brain Res. 2003, 144, 83–90. [Google Scholar] [CrossRef]
- Ivashko-Pachima, Y.; Ganaiem, M.; Ben-Horin-Hazak, I.; Lobyntseva, A.; Bellaiche, N.; Fischer, I.; Levy, G.; Sragovich, S.; Karmon, G.; Giladi, E.; et al. SH3- and actin-binding domains connect ADNP and SHANK3, revealing a fundamental shared mechanism underlying autism. Mol. Psychiatry 2022, 27, 3316–3327. [Google Scholar] [CrossRef]
- Hadar, A.; Kapitansky, O.; Ganaiem, M.; Sragovich, S.; Lobyntseva, A.; Giladi, E.; Yeheskel, A.; Avitan, A.; Vatine, G.D.; Gurwitz, D.; et al. Introducing ADNP and SIRT1 as new partners regulating microtubules and histone methylation. Mol. Psychiatry 2021, 26, 6550–6561. [Google Scholar] [CrossRef]
- Zamostiano, R.; Pinhasov, A.; Gelber, E.; Steingart, R.A.; Seroussi, E.; Giladi, E.; Bassan, M.; Wollman, Y.; Eyre, H.J.; Mulley, J.C.; et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J. Biol. Chem. 2001, 276, 708–714. [Google Scholar] [CrossRef]
- Yan, Q.; Wulfridge, P.; Doherty, J.; Fernandez-Luna, J.L.; Real, P.J.; Tang, H.Y.; Sarma, K. Proximity labeling identifies a repertoire of site-specific R-loop modulators. Nat. Commun. 2022, 13, 53. [Google Scholar] [CrossRef]
- Mandel, S.; Gozes, I. Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J. Biol. Chem. 2007, 282, 34448–34456. [Google Scholar] [CrossRef]
- Mandel, S.; Rechavi, G.; Gozes, I. Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev. Biol. 2007, 303, 814–824. [Google Scholar] [CrossRef]
- Amram, N.; Hacohen-Kleiman, G.; Sragovich, S.; Malishkevich, A.; Katz, J.; Touloumi, O.; Lagoudaki, R.; Grigoriadis, N.C.; Giladi, E.; Yeheskel, A.; et al. Sexual divergence in microtubule function: The novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol. Psychiatry 2016, 21, 1467–1476. [Google Scholar] [CrossRef]
- Karmon, G.; Sragovich, S.; Hacohen-Kleiman, G.; Ben-Horin-Hazak, I.; Kasparek, P.; Schuster, B.; Sedlacek, R.; Pasmanik-Chor, M.; Theotokis, P.; Touloumi, O.; et al. Novel ADNP Syndrome Mice Reveal Dramatic Sex-Specific Peripheral Gene Expression With Brain Synaptic and Tau Pathologies. Biol. Psychiatry 2022, 92, 81–95. [Google Scholar] [CrossRef]
- Gozes, I.; Van Dijck, A.; Hacohen-Kleiman, G.; Grigg, I.; Karmon, G.; Giladi, E.; Eger, M.; Gabet, Y.; Pasmanik-Chor, M.; Cappuyns, E.; et al. Premature primary tooth eruption in cognitive/motor-delayed ADNP-mutated children. Transl. Psychiatry 2017, 7, e1043. [Google Scholar] [CrossRef] [PubMed]
- Grigg, I.; Ivashko-Pachima, Y.; Hait, T.A.; Korenkova, V.; Touloumi, O.; Lagoudaki, R.; Van Dijck, A.; Marusic, Z.; Anicic, M.; Vukovic, J.; et al. Tauopathy in the young autistic brain: Novel biomarker and therapeutic target. Transl. Psychiatry 2020, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Kaaij, L.J.T.; Mohn, F.; van der Weide, R.H.; de Wit, E.; Buhler, M. The ChAHP Complex Counteracts Chromatin Looping at CTCF Sites that Emerged from SINE Expansions in Mouse. Cell 2019, 178, 1437–1451.e14. [Google Scholar] [CrossRef] [PubMed]
- Markenscoff-Papadimitriou, E.; Binyameen, F.; Whalen, S.; Price, J.; Lim, K.; Ypsilanti, A.R.; Catta-Preta, R.; Pai, E.L.; Mu, X.; Xu, D.; et al. Autism risk gene POGZ promotes chromatin accessibility and expression of clustered synaptic genes. Cell Rep. 2021, 37, 110089. [Google Scholar] [CrossRef]
- Thorn, G.J.; Clarkson, C.T.; Rademacher, A.; Mamayusupova, H.; Schotta, G.; Rippe, K.; Teif, V.B. DNA sequence-dependent formation of heterochromatin nanodomains. Nat. Commun. 2022, 13, 1861. [Google Scholar] [CrossRef]
- Sun, X.; Peng, X.; Cao, Y.; Zhou, Y.; Sun, Y. ADNP promotes neural differentiation by modulating Wnt/beta-catenin signaling. Nat. Commun. 2020, 11, 2984. [Google Scholar] [CrossRef]
- Ferreira, A.C.F.; Szeto, A.C.H.; Clark, P.A.; Crisp, A.; Kozik, P.; Jolin, H.E.; McKenzie, A.N.J. Neuroprotective protein ADNP-dependent histone remodeling complex promotes T helper 2 immune cell differentiation. Immunity 2023, 56, 1468–1484.e7. [Google Scholar] [CrossRef]
- Cho, H.; Yoo, T.; Moon, H.; Kang, H.; Yang, Y.; Kang, M.; Yang, E.; Lee, D.; Hwang, D.; Kim, H.; et al. Adnp-mutant mice with cognitive inflexibility, CaMKIIalpha hyperactivity, and synaptic plasticity deficits. Mol. Psychiatry 2023. [Google Scholar] [CrossRef]
- Sharifi Tabar, M.; Giardina, C.; Feng, Y.; Francis, H.; Moghaddas Sani, H.; Low, J.K.K.; Mackay, J.P.; Bailey, C.G.; Rasko, J.E.J. Unique protein interaction networks define the chromatin remodelling module of the NuRD complex. FEBS J. 2022, 289, 199–214. [Google Scholar] [CrossRef]
- Timberlake, A.T.; McGee, S.; Allington, G.; Kiziltug, E.; Wolfe, E.M.; Stiegler, A.L.; Boggon, T.J.; Sanyoura, M.; Morrow, M.; Wenger, T.L.; et al. De novo variants implicate chromatin modification, transcriptional regulation, and retinoic acid signaling in syndromic craniosynostosis. Am. J. Hum. Genet. 2023, 110, 846–862. [Google Scholar] [CrossRef]
- Helsmoortel, C.; Vulto-van Silfhout, A.T.; Coe, B.P.; Vandeweyer, G.; Rooms, L.; van den Ende, J.; Schuurs-Hoeijmakers, J.H.; Marcelis, C.L.; Willemsen, M.H.; Vissers, L.E.; et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat. Genet. 2014, 46, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Van Dijck, A.; Vulto-van Silfhout, A.T.; Cappuyns, E.; van der Werf, I.M.; Mancini, G.M.; Tzschach, A.; Bernier, R.; Gozes, I.; Eichler, E.E.; Romano, C.; et al. Clinical Presentation of a Complex Neurodevelopmental Disorder Caused by Mutations in ADNP. Biol. Psychiatry 2019, 85, 287–297. [Google Scholar] [CrossRef]
- Levine, J.; Hakim, F.; Kooy, R.F.; Gozes, I. Vineland Adaptive Behavior Scale in a Cohort of Four ADNP Syndrome Patients Implicates Age-Dependent Developmental Delays with Increased Impact of Activities of Daily Living. J. Mol. Neurosci. 2022, 72, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Arnett, A.B.; Beighley, J.S.; Kurtz-Nelson, E.C.; Hoekzema, K.; Wang, T.; Bernier, R.A.; Eichler, E.E. Developmental Predictors of Cognitive and Adaptive Outcomes in Genetic Subtypes of Autism Spectrum Disorder. Autism Res. 2020, 13, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; You, Z.M.; Chen, W.H.; Yang, S.; Feng, C.C.; Wang, H.Y.; Wang, T.; Zhu, Y.Y. Helsmoortel-van der Aa syndrome in a Chinese pediatric patient due to ADNP nonsense mutation: A case report. Front. Pediatr. 2023, 11, 1122513. [Google Scholar] [CrossRef]
- Hudac, C.M.; Friedman, N.R.; Ward, V.R.; Estreicher, R.E.; Dorsey, G.C.; Bernier, R.A.; Kurtz-Nelson, E.C.; Earl, R.K.; Eichler, E.E.; Neuhaus, E. Characterizing Sensory Phenotypes of Subgroups with a Known Genetic Etiology Pertaining to Diagnoses of Autism Spectrum Disorder and Intellectual Disability. J. Autism Dev. Disord. 2023, 1–16. [Google Scholar] [CrossRef]
- Fastman, J.; Kolevzon, A. ADNP Syndrome: A Qualitative Assessment of Symptoms, Therapies, and Challenges. Children 2023, 10, 593. [Google Scholar] [CrossRef]
- Georget, M.; Lejeune, E.; Buratti, J.; Servant, E.; le Guern, E.; Heron, D.; Keren, B.; de Sainte Agathe, J.M. Loss of function of ADNP by an intragenic inversion. Eur. J. Hum. Genet. 2023, 31, 967–970. [Google Scholar] [CrossRef]
- Bend, E.G.; Aref-Eshghi, E.; Everman, D.B.; Rogers, R.C.; Cathey, S.S.; Prijoles, E.J.; Lyons, M.J.; Davis, H.; Clarkson, K.; Gripp, K.W.; et al. Gene domain-specific DNA methylation episignatures highlight distinct molecular entities of ADNP syndrome. Clin. Epigenet. 2019, 11, 64. [Google Scholar] [CrossRef]
- Breen, M.S.; Garg, P.; Tang, L.; Mendonca, D.; Levy, T.; Barbosa, M.; Arnett, A.B.; Kurtz-Nelson, E.; Agolini, E.; Battaglia, A.; et al. Episignatures Stratifying Helsmoortel-Van Der Aa Syndrome Show Modest Correlation with Phenotype. Am. J. Hum. Genet. 2020, 107, 555–563. [Google Scholar] [CrossRef]
- Dingemans, A.J.M.; Hinne, M.; Truijen, K.M.G.; Goltstein, L.; van Reeuwijk, J.; de Leeuw, N.; Schuurs-Hoeijmakers, J.; Pfundt, R.; Diets, I.J.; den Hoed, J.; et al. PhenoScore quantifies phenotypic variation for rare genetic diseases by combining facial analysis with other clinical features using a machine-learning framework. Nat. Genet. 2023, 55, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Ivashko-Pachima, Y.; Hadar, A.; Grigg, I.; Korenkova, V.; Kapitansky, O.; Karmon, G.; Gershovits, M.; Sayas, C.L.; Kooy, R.F.; Attems, J.; et al. Discovery of autism/intellectual disability somatic mutations in Alzheimer’s brains: Mutated ADNP cytoskeletal impairments and repair as a case study. Mol. Psychiatry 2021, 26, 1619–1633. [Google Scholar] [CrossRef]
- Oz, S.; Kapitansky, O.; Ivashco-Pachima, Y.; Malishkevich, A.; Giladi, E.; Skalka, N.; Rosin-Arbesfeld, R.; Mittelman, L.; Segev, O.; Hirsch, J.A.; et al. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol. Psychiatry 2014, 19, 1115–1124. [Google Scholar] [CrossRef]
- Vulih-Shultzman, I.; Pinhasov, A.; Mandel, S.; Grigoriadis, N.; Touloumi, O.; Pittel, Z.; Gozes, I. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J. Pharmacol. Exp. Ther. 2007, 323, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Hacohen-Kleiman, G.; Sragovich, S.; Karmon, G.; Gao, A.Y.L.; Grigg, I.; Pasmanik-Chor, M.; Le, A.; Korenkova, V.; McKinney, R.A.; Gozes, I. Activity-dependent neuroprotective protein deficiency models synaptic and developmental phenotypes of autism-like syndrome. J. Clin. Investig. 2018, 128, 4956–4969. [Google Scholar] [CrossRef] [PubMed]
- Ivashko-Pachima, Y.; Sayas, C.L.; Malishkevich, A.; Gozes, I. ADNP/NAP dramatically increase microtubule end-binding protein-Tau interaction: A novel avenue for protection against tauopathy. Mol. Psychiatry 2017, 22, 1335–1344. [Google Scholar] [CrossRef]
- Ivashko-Pachima, Y.; Gozes, I. NAP Protects against Tau Hyperphosphorylation Through GSK3. Curr. Pharm. Des. 2018, 24, 3868–3877. [Google Scholar] [CrossRef]
- Gozes, I.; Shazman, S. A novel davunetide (NAPVSIPQQ to NAPVSIPQE) point mutation in activity-dependent neuroprotective protein (ADNP) causes a mild developmental syndrome. Eur. J. Neurosci. 2023, 58, 2641–2652. [Google Scholar] [CrossRef]
- Amal, H. Sex and the Brain: Novel ADNP Syndrome Mice Are Protected by NAP. Biol. Psychiatry 2022, 92, 8–9. [Google Scholar] [CrossRef]
- Ganaiem, M.; Karmon, G.; Ivashko-Pachima, Y.; Gozes, I. Distinct Impairments Characterizing Different ADNP Mutants Reveal Aberrant Cytoplasmic-Nuclear Crosstalk. Cells 2022, 11, 2994. [Google Scholar] [CrossRef]
- Gozes, I.; Divinski, I. NAP, a neuroprotective drug candidate in clinical trials, stimulates microtubule assembly in the living cell. Curr. Alzheimer Res. 2007, 4, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.P.; Kang, S.C.; Gawelek, K.; Zacharias, R.A.; Anderson, S.R.; Turner, C.P.; Morris, J.K. In vivo and in vitro ketamine exposure exhibits a dose-dependent induction of activity-dependent neuroprotective protein in rat neurons. Neuroscience 2015, 290, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.P.; Gutierrez, S.; Liu, C.; Miller, L.; Chou, J.; Finucane, B.; Carnes, A.; Kim, J.; Shing, E.; Haddad, T.; et al. Strategies to defeat ketamine-induced neonatal brain injury. Neuroscience 2012, 210, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Kimhi, Y.; Palfrey, C.; Spector, I.; Barak, Y.; Littauer, U.Z. Maturation of neuroblastoma cells in the presence of dimethylsulfoxide. Proc. Natl. Acad. Sci. USA 1976, 73, 462–466. [Google Scholar] [CrossRef]
- Gozes, I.; Barnstable, C.J. Monoclonal antibodies that recognize discrete forms of tubulin. Proc. Natl. Acad. Sci. USA 1982, 79, 2579–2583. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Subcellular Fractionation Protocol. Available online: https://www.abcam.com/protocols/subcellular-fractionation-protocol (accessed on 1 March 2023).
- Ivashko-Pachima, Y.; Maor-Nof, M.; Gozes, I. NAP (davunetide) preferential interaction with dynamic 3-repeat Tau explains differential protection in selected tauopathies. PLoS ONE 2019, 14, e0213666. [Google Scholar] [CrossRef]
- Sragovich, S.; Malishkevich, A.; Piontkewitz, Y.; Giladi, E.; Touloumi, O.; Lagoudaki, R.; Grigoriadis, N.; Gozes, I. The autism/neuroprotection-linked ADNP/NAP regulate the excitatory glutamatergic synapse. Transl. Psychiatry 2019, 9, 2. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Kapitansky, O.; Sragovich, S.; Jaljuli, I.; Hadar, A.; Giladi, E.; Gozes, I. Age and Sex-Dependent ADNP Regulation of Muscle Gene Expression Is Correlated with Motor Behavior: Possible Feedback Mechanism with PACAP. Int. J. Mol. Sci. 2020, 21, 6715. [Google Scholar] [CrossRef]
- Ivashko-Pachima, Y.; Gozes, I. A Novel Microtubule-Tau Association Enhancer and Neuroprotective Drug Candidate: Ac-SKIP. Front. Cell. Neurosci. 2019, 13, 435. [Google Scholar] [CrossRef] [PubMed]
- TASSER Server for Protein Structure and Function Prediction. Available online: https://zhanggroup.org/I-TASSER (accessed on 20 December 2022).
- HDOCK SERVER. Available online: http://hdock.phys.hust.edu.cn/ (accessed on 20 December 2022).
- SwissDock SERVER. Available online: http://www.swissdock.ch/ (accessed on 22 January 2023).
- Ivashko-Pachima, Y.; Gozes, I. Activity-dependent neuroprotective protein (ADNP)-end-binding protein (EB) interactions regulate microtubule dynamics toward protection against tauopathy. Prog. Mol. Biol. Transl. Sci. 2021, 177, 65–90. [Google Scholar] [CrossRef] [PubMed]
- Biedzinski, S.; Agsu, G.; Vianay, B.; Delord, M.; Blanchoin, L.; Larghero, J.; Faivre, L.; Thery, M.; Brunet, S. Microtubules control nuclear shape and gene expression during early stages of hematopoietic differentiation. EMBO J. 2020, 39, e103957. [Google Scholar] [CrossRef] [PubMed]
- Gerlitz, G.; Reiner, O.; Bustin, M. Microtubule dynamics alter the interphase nucleus. Cell. Mol. Life Sci. CMLS 2013, 70, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Aboonq, M.S.; Vasiliou, S.A.; Haddley, K.; Quinn, J.P.; Bubb, V.J. Activity-dependent neuroprotective protein modulates its own gene expression. J. Mol. Neurosci. 2012, 46, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Michael, S.; Alvarado-Valverde, J.; Meszaros, B.; Samano-Sanchez, H.; Zeke, A.; Dobson, L.; Lazar, T.; Ord, M.; Nagpal, A.; et al. The Eukaryotic Linear Motif resource: 2022 release. Nucleic Acids Res. 2022, 50, D497–D508. [Google Scholar] [CrossRef] [PubMed]
- Oz, S.; Ivashko-Pachima, Y.; Gozes, I. The ADNP derived peptide, NAP modulates the tubulin pool: Implication for neurotrophic and neuroprotective activities. PLoS ONE 2012, 7, e51458. [Google Scholar] [CrossRef]
- Jouroukhin, Y.; Ostritsky, R.; Assaf, Y.; Pelled, G.; Giladi, E.; Gozes, I. NAP (davunetide) modifies disease progression in a mouse model of severe neurodegeneration: Protection against impairments in axonal transport. Neurobiol. Dis. 2013, 56, 79–94. [Google Scholar] [CrossRef]
- Zaltzman, R.; Beni, S.M.; Giladi, E.; Pinhasov, A.; Steingart, R.A.; Romano, J.; Shohami, E.; Gozes, I. Injections of the neuroprotective peptide NAP to newborn mice attenuate head-injury-related dysfunction in adults. Neuroreport 2003, 14, 481–484. [Google Scholar] [CrossRef]
- Romano, J.; Beni-Adani, L.; Nissenbaum, O.L.; Brenneman, D.E.; Shohami, E.; Gozes, I. A single administration of the peptide NAP induces long-term protective changes against the consequences of head injury: Gene Atlas array analysis. J. Mol. Neurosci. 2002, 18, 37–45. [Google Scholar] [CrossRef]
- Zaltzman, R.; Alexandrovich, A.; Trembovler, V.; Shohami, E.; Gozes, I. The influence of the peptide NAP on Mac-1-deficient mice following closed head injury. Peptides 2005, 26, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Bennison, S.A.; Blazejewski, S.M.; Liu, X.; Hacohen-Kleiman, G.; Sragovich, S.; Zoidou, S.; Touloumi, S.; Grigoriadis, N.; Gozes, I.; Toyo-Oka, K. The cytoplasmic localization of ADNP through 14-3-3 promotes sex-dependent neuronal morphogenesis, cortical connectivity, and calcium signaling. Mol. Psychiatry 2023. [CrossRef] [PubMed]
- Merenlender-Wagner, A.; Shemer, Z.; Touloumi, O.; Lagoudaki, R.; Giladi, E.; Andrieux, A.; Grigoriadis, N.C.; Gozes, I. New horizons in schizophrenia treatment: Autophagy protection is coupled with behavioral improvements in a mouse model of schizophrenia. Autophagy 2014, 10, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.; Gui, T.; Bourgeois, B.; Koyani, C.N.; Ulz, P.; Heitzer, E.; von Lewinski, D.; Burgering, B.M.T.; Malle, E.; Madl, T. beta-catenin regulates FOXP2 transcriptional activity via multiple binding sites. FEBS J. 2021, 288, 3261–3284. [Google Scholar] [CrossRef]
- Vaisburd, S.; Shemer, Z.; Yeheskel, A.; Giladi, E.; Gozes, I. Risperidone and NAP protect cognition and normalize gene expression in a schizophrenia mouse model. Sci. Rep. 2015, 5, 16300. [Google Scholar] [CrossRef]
- Hacohen-Kleiman, G.; Moaraf, S.; Kapitansky, O.; Gozes, I. Sex-and Region-Dependent Expression of the Autism-Linked ADNP Correlates with Social- and Speech-Related Genes in the Canary Brain. J. Mol. Neurosci. 2020, 70, 1671–1683. [Google Scholar] [CrossRef]
- Kolevzon, A.; Levy, T.; Barkley, S.; Bedrosian-Sermone, S.; Davis, M.; Foss-Feig, J.; Halpern, D.; Keller, K.; Kostic, A.; Layton, C.; et al. An open-label study evaluating the safety, behavioral, and electrophysiological outcomes of low-dose ketamine in children with ADNP syndrome. HGG Adv. 2022, 3, 100138. [Google Scholar] [CrossRef]
- Gozes, I.; Shazman, S. STOP Codon Mutations at Sites of Natural Caspase Cleavage Are Implicated in Autism and Alzheimer’s Disease: The Case of ADNP. Front. Endocrinol. 2022, 13, 867442. [Google Scholar] [CrossRef]
- Li, X.; Saiyin, H.; Chen, X.; Yu, Q.; Ma, L.; Liang, W. Ketamine impairs growth cone and synaptogenesis in human GABAergic projection neurons via GSK-3beta and HDAC6 signaling. Mol. Psychiatry 2022. [CrossRef]
- Blaj, C.; Bringmann, A.; Schmidt, E.M.; Urbischek, M.; Lamprecht, S.; Frohlich, T.; Arnold, G.J.; Krebs, S.; Blum, H.; Hermeking, H.; et al. ADNP Is a Therapeutically Inducible Repressor of WNT Signaling in Colorectal Cancer. Clin. Cancer Res. 2017, 23, 2769–2780. [Google Scholar] [CrossRef]
- Bove, M.; Schiavone, S.; Tucci, P.; Sikora, V.; Dimonte, S.; Colia, A.L.; Morgese, M.G.; Trabace, L. Ketamine administration in early postnatal life as a tool for mimicking Autism Spectrum Disorders core symptoms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 117, 110560. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xiao, L.; Fan, W.; Yang, H.; Fu, Y.; Ye, Y.; Wang, X.; Wen, D.; Cheng, A.; Liao, L. Comprehensive metabolomic characterization of the hippocampus in a ketamine mouse model of schizophrenia. Biochem. Biophys. Res. Commun. 2022, 632, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.K.; Pinals, D.A.; Adler, C.M.; Elman, I.; Clifton, A.; Pickar, D.; Breier, A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 1997, 17, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Wink, L.K.; Reisinger, D.L.; Horn, P.; Shaffer, R.C.; O’Brien, K.; Schmitt, L.; Dominick, K.R.; Pedapati, E.V.; Erickson, C.A. Brief Report: Intranasal Ketamine in Adolescents and Young Adults with Autism Spectrum Disorder-Initial Results of a Randomized, Controlled, Crossover, Pilot Study. J. Autism Dev. Disord. 2021, 51, 1392–1399. [Google Scholar] [CrossRef]
- Gozes, I. The ADNP Syndrome and CP201 (NAP) Potential and Hope. Front. Neurol. 2020, 11, 608444. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, B.H.; Fox, A.W.; Stewart, A.J.; Gold, M. Davunetide: A review of safety and efficacy data with a focus on neurodegenerative diseases. Expert Rev. Clin. Pharmacol. 2013, 6, 483–502. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganaiem, M.; Gildor, N.D.; Shazman, S.; Karmon, G.; Ivashko-Pachima, Y.; Gozes, I. NAP (Davunetide): The Neuroprotective ADNP Drug Candidate Penetrates Cell Nuclei Explaining Pleiotropic Mechanisms. Cells 2023, 12, 2251. https://doi.org/10.3390/cells12182251
Ganaiem M, Gildor ND, Shazman S, Karmon G, Ivashko-Pachima Y, Gozes I. NAP (Davunetide): The Neuroprotective ADNP Drug Candidate Penetrates Cell Nuclei Explaining Pleiotropic Mechanisms. Cells. 2023; 12(18):2251. https://doi.org/10.3390/cells12182251
Chicago/Turabian StyleGanaiem, Maram, Nina D. Gildor, Shula Shazman, Gidon Karmon, Yanina Ivashko-Pachima, and Illana Gozes. 2023. "NAP (Davunetide): The Neuroprotective ADNP Drug Candidate Penetrates Cell Nuclei Explaining Pleiotropic Mechanisms" Cells 12, no. 18: 2251. https://doi.org/10.3390/cells12182251
APA StyleGanaiem, M., Gildor, N. D., Shazman, S., Karmon, G., Ivashko-Pachima, Y., & Gozes, I. (2023). NAP (Davunetide): The Neuroprotective ADNP Drug Candidate Penetrates Cell Nuclei Explaining Pleiotropic Mechanisms. Cells, 12(18), 2251. https://doi.org/10.3390/cells12182251






