Crosstalk between the Rod Outer Segments and Retinal Pigmented Epithelium in the Generation of Oxidative Stress in an In Vitro Model
Abstract
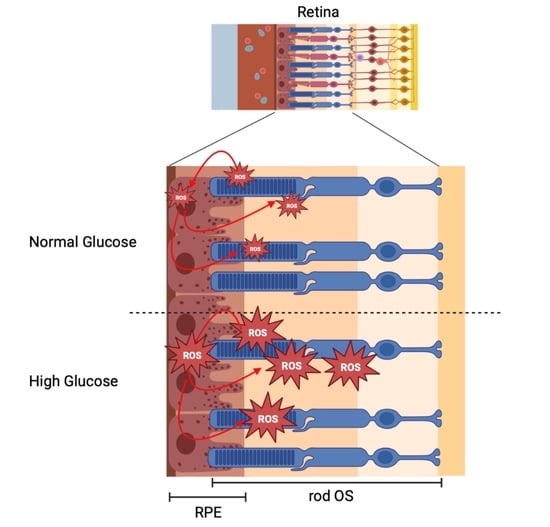
1. Introduction
2. Materials and Methods
2.1. Cell Line and Culture Conditions
2.2. Rod OS Isolation
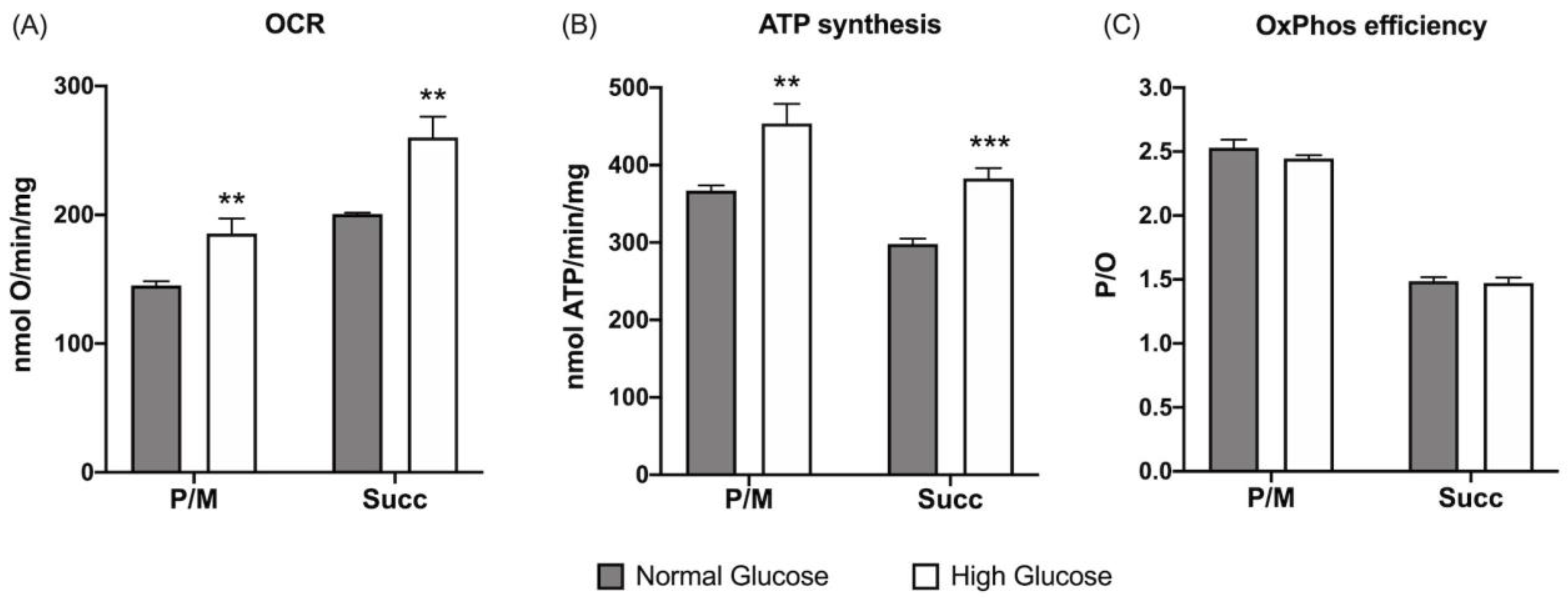
2.3. Oxygen Consumption Rate Evaluation
2.4. Aerobic ATP Synthesis Evaluation
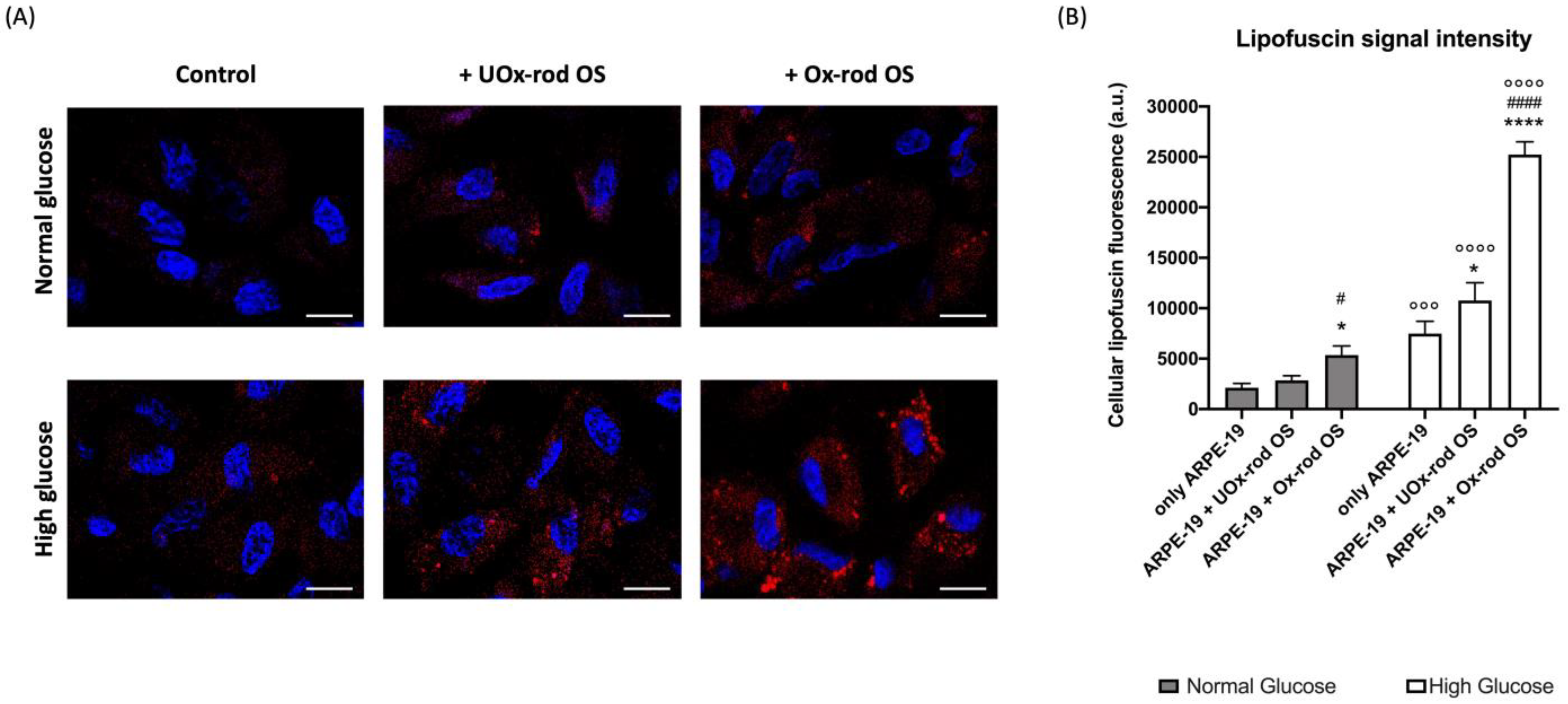
2.5. Evaluation of Lipofuscin Accumulation in ARPE-19 Cells by Confocal Microscopy
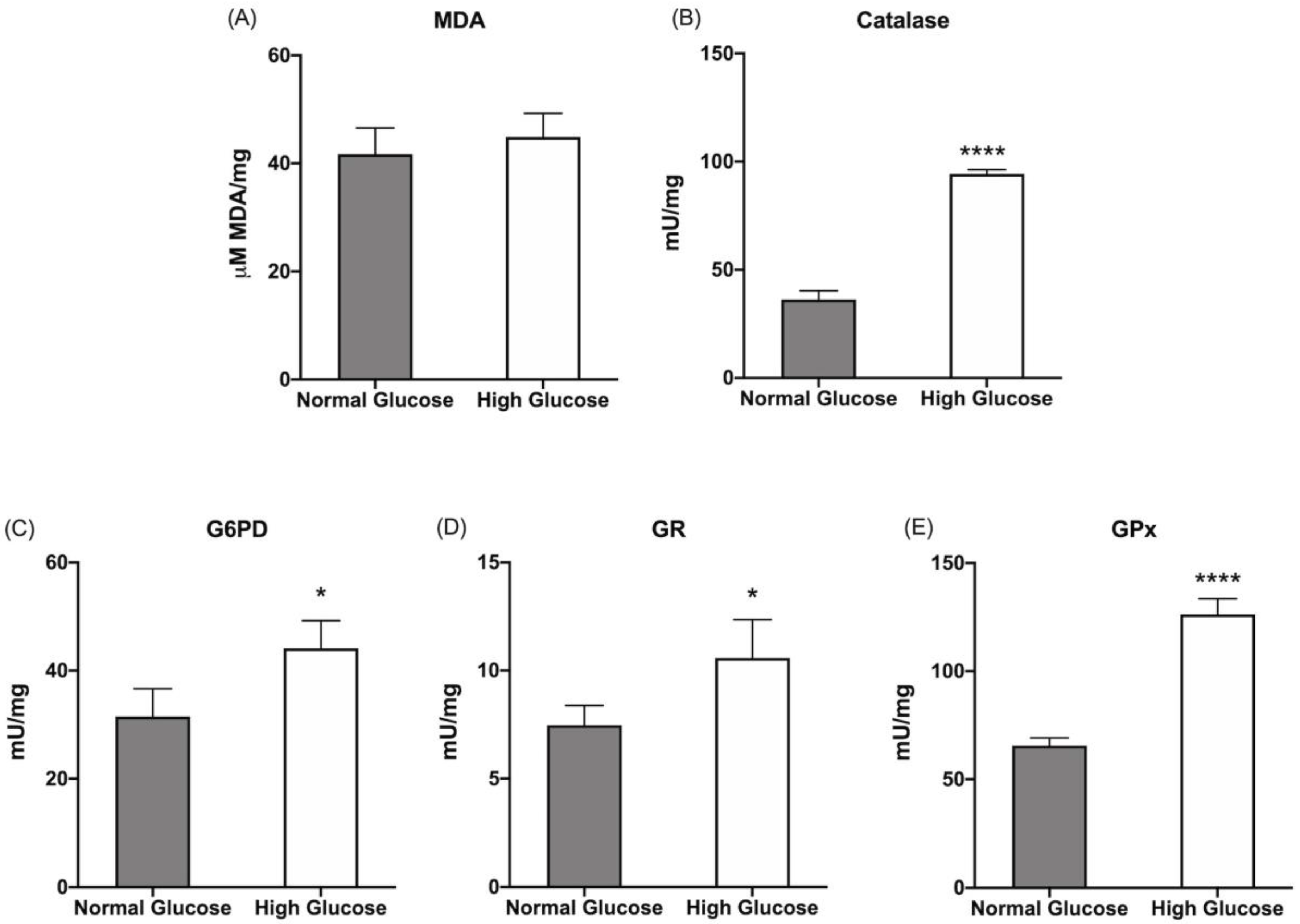
2.6. Lipoperoxidation Evaluation
2.7. Antioxidant Enzyme Activity Evaluation
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. ARPE-19 Cells Increase Aerobic Energy Metabolism Proportionally to Glucose Concentration without Causing an Increase in Oxidative Damage Due to the Activation of Endogenous Antioxidant Defenses
3.2. The Phagocytizing Capacity of ARPE-19 Cells Depends on the Glucose Concentration in the Medium and the Rod Outer-Segment Oxidative State
3.3. The Altered ARPE-19 Cell Phagocytosis Capacity Depends on the Accumulation of Oxidative Stress
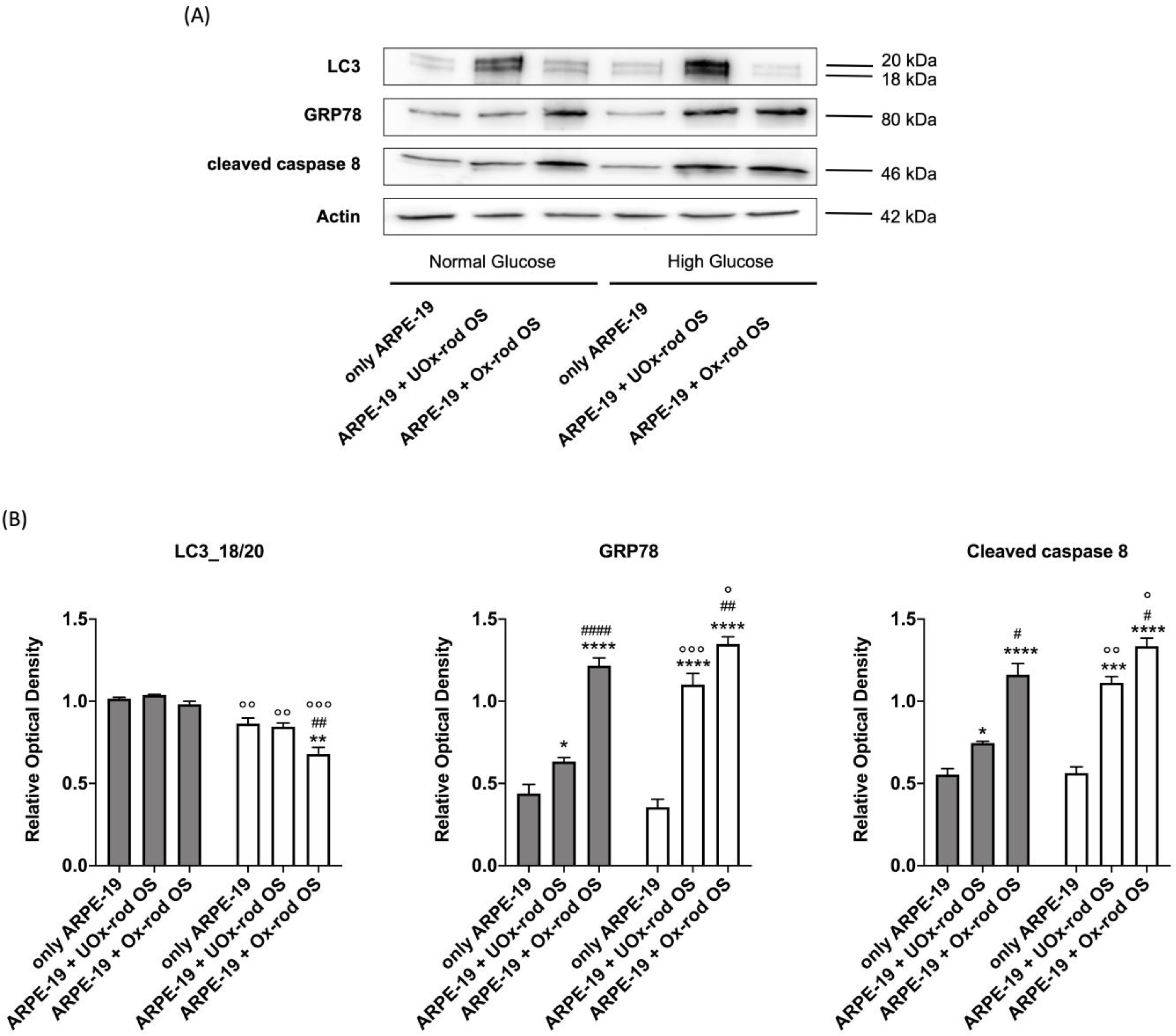
3.4. The Accumulation of Oxidative Stress due to the Phagocytosis of Ox-rod OSs Causes an Increase in the Expression of Markers of the Unfolding Protein Response and Pro-Apoptotic Signal and Reduces LC3 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caceres, P.S.; Rodriguez-Boulan, E. Retinal Pigment Epithelium Polarity in Health and Blinding Diseases. Curr. Opin. Cell Biol. 2020, 62, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative Stress, Innate Immunity, and Age-Related Macular Degeneration. AIMS Mol. Sci. 2016, 3, 196–221. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding Age-Related Macular Degeneration (AMD): Relationships between the Photoreceptor/Retinal Pigment Epithelium/Bruch’s Membrane/Choriocapillaris Complex. Mol. Aspects Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.P.; Wang, Z.; Chen, J.; Sapieha, P.; Smith, L.H.; Ma, J.X. Neurovascular Cross Talk in Diabetic Retinopathy: Pathophysiological Roles and Therapeutic Implications. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H738–H749. [Google Scholar] [CrossRef]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A. Photoreceptor Topography in Ageing and Age-Related Maculopathy. Eye 2001, 15, 376–383. [Google Scholar] [CrossRef]
- Lamb, T.D.; Curtin, J. Photoreceptor Physiology and Evolution: Cellular and Molecular Basis of Rod and Cone Phototransduction. J. Physiol. 2022, 600, 4585–4601. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chuang, J.Z.; Sung, C.H. Light Regulates the Ciliary Protein Transport and Outer Segment Disc Renewal of Mammalian Photoreceptors. Dev. Cell 2015, 32, 731–742. [Google Scholar] [CrossRef]
- Winkler, B.S. An Hypothesis to Account for the Renewal of Outer Segments in Rod and Cone Photoreceptor Cells: Renewal as a Surrogate Antioxidant. Invest. Ophthalmol. Vis. Sci. 2008, 49, 3259–3261. [Google Scholar] [CrossRef] [PubMed]
- Law, A.L.; Parinot, C.; Chatagnon, J.; Gravez, B.; Sahel, J.A.; Bhattacharya, S.S.; Nandrot, E.F. Cleavage of Mer Tyrosine Kinase (MerTK) from the Cell Surface Contributes to the Regulation of Retinal Phagocytosis. J. Biol. Chem. 2015, 290, 4941–4952. [Google Scholar] [CrossRef] [PubMed]
- Nandrot, E.F.; Silva, K.E.; Scelfo, C.; Finnemann, S.C. Retinal Pigment Epithelial Cells Use a MerTK-Dependent Mechanism to Limit the Phagocytic Particle Binding Activity of Avβ5 Integrin. Biol. Cell/Under Auspices Eur. Cell Biol. Organ. 2012, 104, 326. [Google Scholar] [CrossRef]
- Puddu, A.; Ravera, S.; Panfoli, I.; Bertola, N.; Maggi, D. High Glucose Impairs Expression and Activation of MerTK in ARPE-19 Cells. Int. J. Mol. Sci. 2022, 23, 1144. [Google Scholar] [CrossRef]
- Pazour, G.J.; Baker, S.A.; Deane, J.A.; Cole, D.G.; Dickert, B.L.; Rosenbaum, J.L.; Witman, G.B.; Besharse, J.C. The Intraflagellar Transport Protein, IFT88, Is Essential for Vertebrate Photoreceptor Assembly and Maintenance. J. Cell Biol. 2002, 157, 103–113. [Google Scholar] [CrossRef]
- Insinna, C.; Besharse, J.C. Intraflagellar Transport and the Sensory Outer Segment of Vertebrate Photoreceptors. Dev. Dyn. 2008, 237, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.D.; Linsenmeier, R.A.; Goldstick, T.K. Oxygen Consumption in the Inner and Outer Retina of the Cat. Investig. Ophthalmol. Vis. Sci. 1995, 36, 542–554. [Google Scholar]
- Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Retinal Oxygen: Fundamental and Clinical Aspects. Arch. Ophthalmol. 2003, 121, 547–557. [Google Scholar] [CrossRef]
- Berdeaux, O.; Juaneda, P.; Martine, L.; Cabaret, S.; Bretillon, L.; Acar, N. Identification and Quantification of Phosphatidylcholines Containing Very-Long-Chain Polyunsaturated Fatty Acid in Bovine and Human Retina Using Liquid Chromatography/Tandem Mass Spectrometry. J. Chromatogr. A 2010, 1217, 7738–7748. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Domènech, E.B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: NRF2 as Therapeutic Target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Léveillard, T.; Mohand-Saïd, S.; Lorentz, O.; Hicks, D.; Fintz, A.C.; Clérin, E.; Simonutti, M.; Forster, V.; Cavusoglu, N.; Chalmel, F.; et al. Identification and Characterization of Rod-Derived Cone Viability Factor. Nat. Genet. 2004, 36, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.J.; et al. Retinal Neurodegeneration May Precede Microvascular Changes Characteristic of Diabetic Retinopathy in Diabetes Mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef]
- Berkowitz, B.A. Preventing Diabetic Retinopathy by Mitigating Subretinal Space Oxidative Stress in Vivo. Vis. Neurosci. 2020, 37, E002. [Google Scholar] [CrossRef]
- Bek, T. Mitochondrial Dysfunction and Diabetic Retinopathy. Mitochondrion 2017, 36, 4–6. [Google Scholar] [CrossRef]
- Calzia, D.; Garbarino, G.; Caicci, F.; Pestarino, M.; Manni, L.; Traverso, C.E.; Panfoli, I.; Candiani, S. Evidence of Oxidative Phosphorylation in Zebrafish Photoreceptor Outer Segments at Different Larval Stages. J. Histochem. Cytochem. 2018, 66, 497–509. [Google Scholar] [CrossRef]
- Panfoli, I.; Calzia, D.; Bianchini, P.; Ravera, S.; Diaspro, A.; Candiano, G.; Bachi, A.; Monticone, M.; Aluigi, M.G.; Barabino, S.; et al. Evidence for Aerobic Metabolism in Retinal Rod Outer Segment Disks. Int. J. Biochem. Cell Biol. 2009, 41, 2555–2565. [Google Scholar] [CrossRef]
- Calzia, D.; Degan, P.; Caicci, F.; Bruschi, M.; Manni, L.; Ramenghi, L.A.; Candiano, G.; Traverso, C.E.; Panfoli, I. Modulation of the Rod Outer Segment Aerobic Metabolism Diminishes the Production of Radicals Due to Light Absorption. Free Radic. Biol. Med. 2018, 117, 110–118. [Google Scholar] [CrossRef]
- Calzia, D.; Oneto, M.; Caicci, F.; Bianchini, P.; Ravera, S.; Bartolucci, M.; Diaspro, A.; Degan, P.; Manni, L.; Traverso, C.E.; et al. Effect of Polyphenolic Phytochemicals on Ectopic Oxidative Phosphorylation in Rod Outer Segments of Bovine Retina. Br. J. Pharmacol. 2015, 172, 3890–3903. [Google Scholar] [CrossRef]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Keeling, E.; Culling, A.J.; Johnston, D.A.; Chatelet, D.S.; Page, A.; Tumbarello, D.A.; Lotery, A.J.; Ratnayaka, J.A. An In-Vitro Cell Model of Intracellular Protein Aggregation Provides Insights into RPE Stress Associated with Retinopathy. Int. J. Mol. Sci. 2020, 21, 6647. [Google Scholar] [CrossRef]
- Lynn, S.A.; Ward, G.; Keeling, E.; Scott, J.A.; Cree, A.J.; Johnston, D.A.; Page, A.; Cuan-Urquizo, E.; Bhaskar, A.; Grossel, M.C.; et al. Ex-Vivo Models of the Retinal Pigment Epithelium (RPE) in Long-Term Culture Faithfully Recapitulate Key Structural and Physiological Features of Native RPE. Tissue Cell 2017, 49, 447–460. [Google Scholar] [CrossRef]
- Chowers, I.; Kim, Y.; Farkas, R.H.; Gunatilaka, T.L.; Hackam, A.S.; Campochiaro, P.A.; Finnemann, S.C.; Zack, D.J. Changes in Retinal Pigment Epithelial Gene Expression Induced by Rod Outer Segment Uptake. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2098–2106. [Google Scholar] [CrossRef][Green Version]
- Panfoli, I.; Calzia, D.; Ravera, S.; Bianchini, P.; Diaspro, A. Maximizing the Rod Outer Segment Yield in Retinas Extracted from Cattle Eyes. Bio Protoc. 2022, 12, e4474. [Google Scholar] [CrossRef] [PubMed]
- Schnetkamp, P.P.M.; Daemen, F.J.M. Isolation and Characterization of Osmotically Sealed Bovine Rod Outer Segments. Methods Enzymol. 1982, 81, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, E.; Cuccarolo, P.; Stroppiana, G.; Miano, M.; Bottega, R.; Cossu, V.; Degan, P.; Ravera, S. Defects in Mitochondrial Energetic Function Compels Fanconi Anaemia Cells to Glycolytic Metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, E.; Degan, P.; Bruno, S.; Pierri, F.; Miano, M.; Raggi, F.; Farruggia, P.; Mecucci, C.; Crescenzi, B.; Naim, V.; et al. The Passage from Bone Marrow Niche to Bloodstream Triggers the Metabolic Impairment in Fanconi Anemia Mononuclear Cells. Redox Biol. 2020, 36, 101618. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, P.C. P/O Ratios of Mitochondrial Oxidative Phosphorylation. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.N.; Maksimov, E.G.; Moysenovich, A.M.; Yakovleva, M.A.; Tsoraev, G.V.; Ramonova, A.A.; Shirshin, E.A.; Sluchanko, N.N.; Feldman, T.B.; Rubin, A.B.; et al. Protein-Mediated Carotenoid Delivery Suppresses the Photoinducible Oxidation of Lipofuscin in Retinal Pigment Epithelial Cells. Antioxidants 2023, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Höhn, A.; Grune, T. Lipofuscin: Detection and Quantification by Microscopic Techniques. Methods Mol. Biol. 2010, 594, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Ravera, S.; Traverso, C.; Amaro, A.; Piaggio, F.; Emionite, L.; Bachetti, T.; Pfeffer, U.; Raffaghello, L. Curcumin Induces a Fatal Energetic Impairment in Tumor Cells in Vitro and in Vivo by Inhibiting ATP-Synthase Activity. Carcinogenesis 2018, 39, 1141–1150. [Google Scholar] [CrossRef]
- Miceli, A.; Cossu, V.; Marini, C.; Castellani, P.; Raffa, S.; Donegani, M.I.; Bruno, S.; Ravera, S.; Emionite, L.; Orengo, A.M.; et al. 18F-Fluorodeoxyglucose Positron Emission Tomography Tracks the Heterogeneous Brain Susceptibility to the Hyperglycemia-Related Redox Stress. Int. J. Mol. Sci. 2020, 21, 8154. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Bertola, N.; Pasquale, C.; Bruno, S.; Benedicenti, S.; Ferrando, S.; Zekiy, A.; Arany, P.; Amaroli, A. 808-Nm Photobiomodulation Affects the Viability of a Head and Neck Squamous Carcinoma Cellular Model, Acting on Energy Metabolism and Oxidative Stress Production. Biomedicines 2021, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bernacchia, A.; Biondi, A.; Genova, M.L.; Lenaz, G.; Falasca, A. The Various Sources of Mitochondrial Oxygen Radicals: A Minireview. Toxicol. Mech. Methods 2004, 14, 25–30. [Google Scholar] [CrossRef]
- Lenaz, G. The Mitochondrial Production of Reactive Oxygen Species: Mechanisms and Implications in Human Pathology. IUBMB Life 2001, 52, 159–164. [Google Scholar] [CrossRef]
- Lenaz, G. Role of Mitochondria in Oxidative Stress and Ageing. Biochim. Biophys. Acta -Bioenerg. 1998, 1366, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Carlini, L.; Tancreda, G.; Iobbi, V.; Caicci, F.; Bruno, S.; Esposito, A.; Calzia, D.; Benini, S.; Bisio, A.; Manni, L.; et al. The Flavone Cirsiliol from Salvia x Jamensis Binds the F1 Moiety of ATP Synthase, Modulating Free Radical Production. Cells 2022, 11, 3169. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Qian, H. OCT Imaging of Rod Mitochondrial Respiration in Vivo. Exp. Biol. Med. 2021, 46, 2151–2158. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative Stress and Epigenetic Modifications in the Pathogenesis of Diabetic Retinopathy. Prog. Retin. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.; Petretto, A.; Caicci, F.; Bartolucci, M.; Calzia, D.; Santucci, L.; Manni, L.; Ramenghi, L.A.; Ghiggeri, G.; Traverso, C.E.; et al. Proteome of Bovine Mitochondria and Rod Outer Segment Disks: Commonalities and Differences. J. Proteome Res. 2018, 17, 918–925. [Google Scholar] [CrossRef]
- Bruschi, M.; Bartolucci, M.; Petretto, A.; Calzia, D.; Caicci, F.; Manni, L.; Traverso, C.E.; Candiano, G.; Panfoli, I. Differential Expression of the Five Redox Complexes in the Retinal Mitochondria or Rod Outer Segment Disks Is Consistent with Their Different Functionality. FASEB Bioadv. 2020, 2, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Calzia, D.; Barabino, S.; Bianchini, P.; Garbarino, G.; Oneto, M.; Caicci, F.; Diaspro, A.; Tacchetti, C.; Manni, L.; Candiani, S.; et al. New Findings in ATP Supply in Rod Outer Segments: Insights for Retinopathies. Biol. Cell 2013, 105, 345–358. [Google Scholar] [CrossRef]
- Olchawa, M.M.; Szewczyk, G.M.; Zadlo, A.C.; Sarna, M.W.; Wnuk, D.; Sarna, T.J. The Effect of Antioxidants on Photoreactivity and Phototoxic Potential of RPE Melanolipofuscin Granules from Human Donors of Different Age. Antioxidants 2020, 9, 1044. [Google Scholar] [CrossRef]
- Liang, F.Q.; Godley, B.F. Oxidative Stress-Induced Mitochondrial DNA Damage in Human Retinal Pigment Epithelial Cells: A Possible Mechanism for RPE Aging and Age-Related Macular Degeneration. Exp. Eye Res. 2003, 76, 397–403. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravera, S.; Bertola, N.; Puddu, A.; Bruno, S.; Maggi, D.; Panfoli, I. Crosstalk between the Rod Outer Segments and Retinal Pigmented Epithelium in the Generation of Oxidative Stress in an In Vitro Model. Cells 2023, 12, 2173. https://doi.org/10.3390/cells12172173
Ravera S, Bertola N, Puddu A, Bruno S, Maggi D, Panfoli I. Crosstalk between the Rod Outer Segments and Retinal Pigmented Epithelium in the Generation of Oxidative Stress in an In Vitro Model. Cells. 2023; 12(17):2173. https://doi.org/10.3390/cells12172173
Chicago/Turabian StyleRavera, Silvia, Nadia Bertola, Alessandra Puddu, Silvia Bruno, Davide Maggi, and Isabella Panfoli. 2023. "Crosstalk between the Rod Outer Segments and Retinal Pigmented Epithelium in the Generation of Oxidative Stress in an In Vitro Model" Cells 12, no. 17: 2173. https://doi.org/10.3390/cells12172173
APA StyleRavera, S., Bertola, N., Puddu, A., Bruno, S., Maggi, D., & Panfoli, I. (2023). Crosstalk between the Rod Outer Segments and Retinal Pigmented Epithelium in the Generation of Oxidative Stress in an In Vitro Model. Cells, 12(17), 2173. https://doi.org/10.3390/cells12172173