Improved Method for Dental Pulp Stem Cell Preservation and Its Underlying Cell Biological Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Dental Pulp Tissues
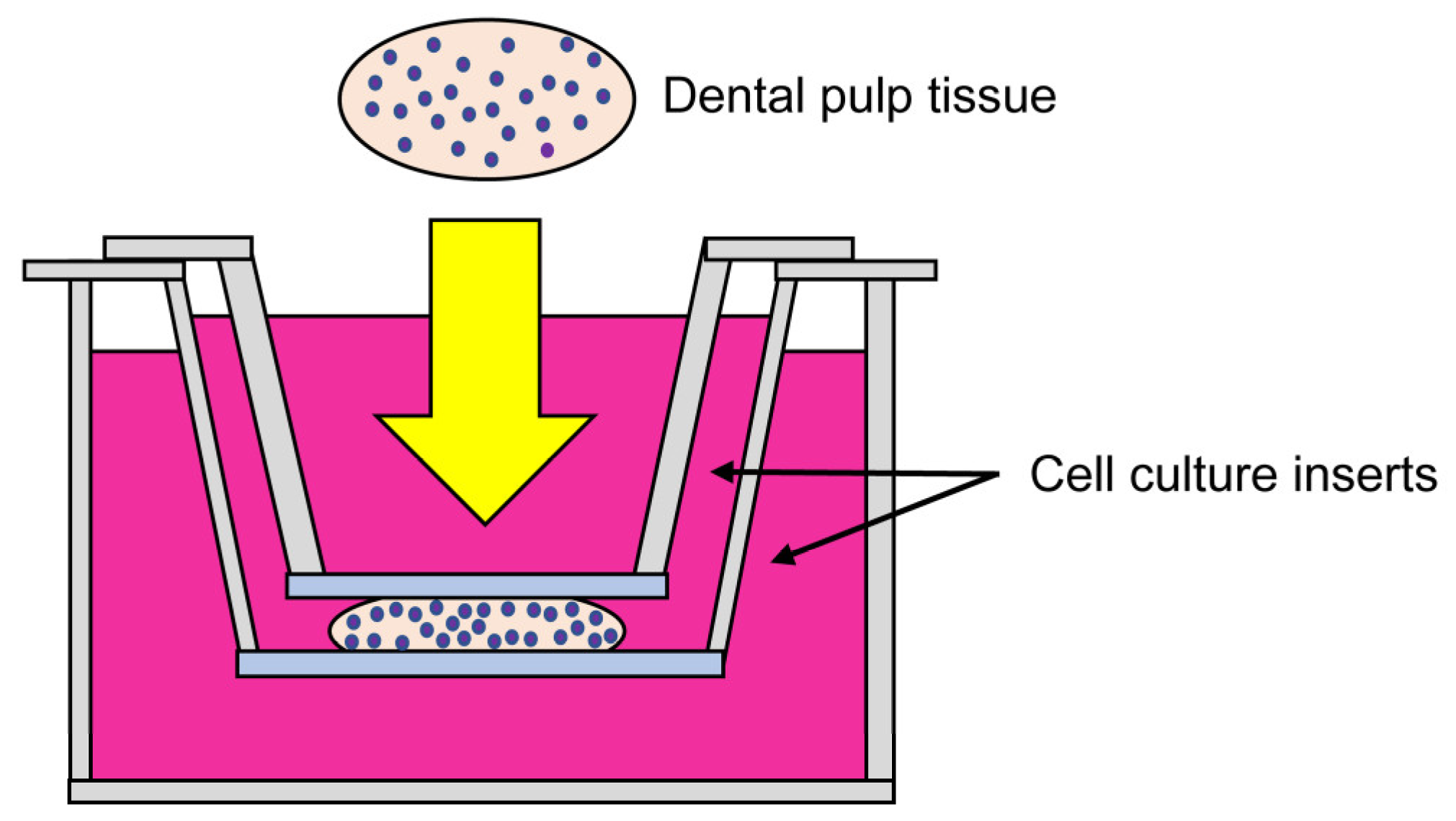
2.2. Improved Culture Method for Dental Pulp Tissues
2.3. Histological Examination of Dental Pulp Tissue Pieces Cultured Using the Improved CMDPT
2.4. Comparison with the NCM Method (Previous Study)
2.5. Immunohistochemical Examination
2.6. Cell Migration Assay
2.7. Effects of SDF1 Inhibition on the Migration of DPSCs Cultured Using the Improved CMDPT
2.8. Statistical Analysis
3. Results
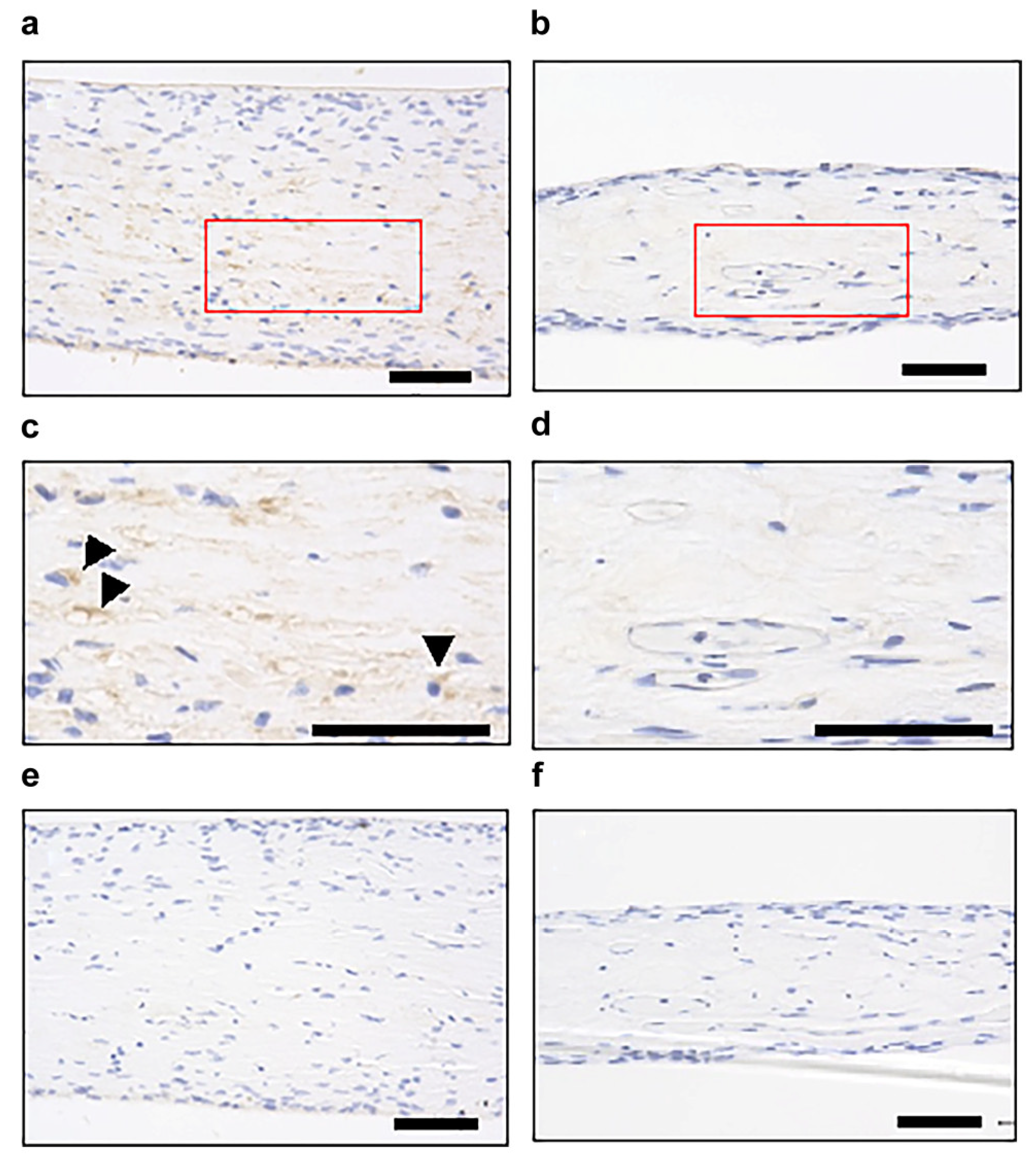
3.1. Histological Examination of Dental Pulp Tissue Cultured by the Improved CMDPT
3.2. Comparison of the Number of Cells Migrating to the Vicinity of the Membrane
3.3. Identification of Factors Involved in Cell Migration
3.4. Effect of SDF1 on the Migration of DPSCs In Vitro
3.5. Effect of SDF1neutralizing Antibody on the Migration of DPSCs under the Improved CMDPT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsutsui, T.W. Dental pulp stem cells: Advances to applications. Stem Cells Cloning 2020, 13, 33–42. [Google Scholar] [CrossRef]
- Ding, T.; Kang, W.; Li, J.; Yu, L.; Ge, S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J. Nanobiotechnol. 2021, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [PubMed]
- Kushibiki, T.; Saito, A.; Hayashi, S.; Ishii, K.; Sawa, Y.; Awazu, K. Quantitative estimates of vascularity in a collagen-based cell scaffold containing basic fibroblast growth factor by noninvasive near-infrared spectroscopy for regenerative medicine. Photomed. Laser Surg. 2008, 26, 247–250. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Zarrintaj, P.; Oftadeh, M.O.; Keramati, F.; Fouladiha, H.; Sohrabi-Jahromi, S.; Ziraksaz, Z. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol. Genet. Eng. Rev. 2017, 33, 144–172. [Google Scholar] [CrossRef]
- Collart-Dutilleul, P.Y.; Secret, E.; Panayotov, I.; Deville de Périère, D.; Martín-Palma, R.J.; Torres-Costa, V.; Martin, M.; Gergely, C.; Durand, J.O.; Cunin, F.; et al. Adhesion and proliferation of human mesenchymal stem cells from dental pulp on porous silicon scaffolds. ACS Appl. Mater. Interfaces 2014, 6, 1719–1728. [Google Scholar] [CrossRef]
- Klump, H.; Teichweyde, N.; Meyer, C.; Horn, P.A. Development of patient-specific hematopoietic stem and progenitor cell grafts from pluripotent stem cells, in vitro. Curr. Mol. Med. 2013, 13, 815–820. [Google Scholar] [CrossRef]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef]
- Suman, S.; Domingues, A.; Ratajczak, J.; Ratajczak, M.Z. Potential clinical applications of stem cells in regenerative medicine. Adv. Exp. Med. Biol. 2019, 1201, 1–22. [Google Scholar] [PubMed]
- Cable, J.; Fuchs, E.; Weissman, I.; Jasper, H.; Glass, D.; Rando, T.A.; Blau, H.; Debnath, S.; Oliva, A.; Park, S.; et al. Adult stem cells and regenerative medicine—A symposium report. Ann. N. Y. Acad. Sci. 2020, 1462, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Szade, K.; Szade, A.; Nowak, W.; Józkowicz, A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim. Pol. 2015, 62, 329–337. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug. Discov. 2017, 16, 115–130. [Google Scholar]
- Whiting, P.; Kerby, J.; Coffey, P.; da Cruz, L.; McKernan, R. Progressing a human embryonic stem-cell-based regenerative medicine therapy towards the clinic. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140375. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Zhang, X.; Liu, Y.; Chen, J.; Hu, B.; Song, J.; Zhang, Y. Strategies to optimize adult stem cell therapy for tissue regeneration. Int. J. Mol. Sci. 2016, 17, 982. [Google Scholar] [CrossRef]
- Yin, W.; Wang, J.; Jiang, L.; James Kang, Y.J. Cancer and stem cells. Exp. Biol. Med. 2021, 246, 1791–1801. [Google Scholar] [CrossRef]
- Kuhn, N.Z.; Tuan, R.S. Regulation of stemness and stem cell niche of mesenchymal stem cells: Implications in tumorigenesis and metastasis. J. Cell. Physiol. 2010, 222, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Prasongchean, W.; Ferretti, P. Autologous stem cells for personalised medicine. New Biotechnol. 2012, 29, 641–650. [Google Scholar]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal stem cells for regenerative medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Ahmed, G.M.; Abouauf, E.A.; AbuBakr, N.; Dörfer, C.E.; El-Sayed, K.F. Tissue engineering approaches for enamel, dentin, and pulp regeneration: An update. Stem Cells Int. 2020, 2020, 5734539. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Nakajima, K.; Awada, T.; Tsuka, Y.; Abe, T.; Ando, K.; Hiraki, T.; Kimura, A.; Tanimoto, K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 501, 193–198. [Google Scholar] [CrossRef]
- Sui, B.; Wu, D.; Xiang, L.; Fu, Y.; Kou, X.; Shi, S. Dental pulp stem cells: From discovery to clinical application. J. Endod. 2020, 46, S46–S55. [Google Scholar] [CrossRef]
- Pilbauerova, N.; Schmidt, J.; Soukup, T.; Koberova Ivancakova, R.K.; Suchanek, J. The effects of cryogenic storage on human dental pulp stem cells. Int. J. Mol. Sci. 2021, 22, 4432. [Google Scholar] [CrossRef]
- Meng, H.; Wei, F.; Ge, Z.; Jin, J.; Wang, H.; Wang, L.S.; Wu, C.T. Long-term hypoxia inhibits the passage-dependent stemness decrease and senescence increase of human dental pulp stem cells. Tissue Cell 2022, 76, 101819. [Google Scholar] [CrossRef]
- Ferrúa, C.P.; Centeno, E.G.Z.; Rosa, L.C.D.; Amaral, C.C.D.; Severo, R.F.; Sarkis-Onofre, R.; Nascimento, G.G.; Cordenonzi, G.; Bast, R.K.; Demarco, F.F.; et al. How has dental pulp stem cells isolation been conducted? A scoping review. Braz. Oral Res. 2017, 31, e87. [Google Scholar] [CrossRef] [PubMed]
- Mundra, V.; Gerling, I.C.; Mahato, R.I. Mesenchymal stem cell-based therapy. Mol. Pharm. 2013, 10, 77–89. [Google Scholar] [CrossRef]
- Takebe, Y.; Tatehara, S.; Fukushima, T.; Tokuyama-Toda, R.; Yasuhara, R.; Mishima, K.; Satomura, K. Cryopreservation method for the effective collection of dental pulp stem cells. Tissue Eng. Part C Methods 2017, 23, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Huang, G.W.; Shiung, J.N.; Huang, Y.H.; Jeng, J.H.; Kuo, T.F.; Yang, J.C.; Yang, W.C.V. Magnetic cryopreservation for dental pulp stem cells. Cells Tissues Organs 2012, 196, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Woods, E.J.; Perry, B.C.; Hockema, J.J.; Larson, L.; Zhou, D.; Goebel, W.S. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology 2009, 59, 150–157. [Google Scholar] [CrossRef]
- Das, M.; Das, A.; Barui, A.; Paul, R.R. Comparative evaluation of proliferative potential and replicative senescence associated changes in mesenchymal stem cells derived from dental pulp and umbilical cord. Cell Tissue Bank. 2022, 23, 157–170. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Apatzidou, D.; Aggelidou, E.; Gousopoulou, E.; Leyhausen, G.; Volk, J.; Kritis, A.; Koidis, P.; Geurtsen, W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res. Ther. 2017, 8, 247. [Google Scholar] [CrossRef]
- Yi, Q.; Liu, O.; Yan, F.; Lin, X.; Diao, S.; Wang, L.; Jin, L.; Wang, S.; Lu, Y.; Fan, Z. Analysis of senescence-related differentiation potentials and gene expression profiles in human dental pulp stem cells. Cells Tissues Organs 2017, 203, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.C.N.; Le, S.H.; Doan, V.N.; Ngo, L.T.Q.; Tran, H.L.B. Simplified conditions for storing and cryopreservation of dental pulp stem cells. Arch. Oral Biol. 2017, 84, 74–81. [Google Scholar] [CrossRef]
- Ogata, K.; Moriyama, M.; Matsumura-Kawashima, M.; Kawado, T.; Yano, A.; Nakamura, S. The therapeutic potential of secreted factors from dental pulp stem cells for various diseases. Biomedicines 2022, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Eubanks, E.J.; Tarle, S.A.; Kaigler, D. Tooth storage, dental pulp stem cell isolation, and clinical scale expansion without animal serum. J. Endod. 2014, 40, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L.; Xiao, L.; Zhang, D. Recycle the dental fairy’s package: Overview of dental pulp stem cell. Stem Cell Res. Ther. 2018, 9, 347. [Google Scholar] [CrossRef]
- Suchanek, J.; Nasry, S.A.; Soukup, T. The differentiation potential of human natal dental pulp stem cells into insulin-producing cells. Folia Biol. 2017, 63, 132–138. [Google Scholar]
- Piva, E.; Tarlé, S.A.; Nör, J.E.; Zou, D.; Hatfield, E.; Guinn, T.; Eubanks, E.J.; Kaigler, D. Dental pulp tissue regeneration using dental pulp stem cells isolated and expanded in human serum. J. Endod. 2017, 43, 568–574. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Wang, S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional dental pulp regeneration: Basic research and clinical translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef]
- Ferro, F.; Spelat, R.; Baheney, C.S. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. Methods Mol. Biol. 2014, 1210, 91–115. [Google Scholar] [PubMed]
- Xie, J.; Zhao, Y.M.; Rao, N.Q.; Wang, X.T.; Fang, T.J.; Li, X.X.; Zhai, Y.; Li, J.Z.; Ge, L.H.; Wang, Y.Y. Comparative study of differentiation potential of mesenchymal stem cells derived from orofacial system into vascular endothelial cells. Beijing Da Xue Xue Bao Yi Xue Ban 2019, 51, 900–906. [Google Scholar] [PubMed]
- Nuti, N.; Corallo, C.; Chan, B.M.F.; Ferrari, M.; Gerami-Naini, B. Multipotent differentiation of human dental pulp stem cells: A literature review. Stem Cell Rev. Rep. 2016, 12, 511–523. [Google Scholar]
- Hata, M.; Omi, M.; Kobayashi, Y.; Nakamura, N.; Miyabe, M.; Ito, M.; Ohno, T.; Imanishi, Y.; Himeno, T.; Kamiya, H.; et al. Sustainable effects of human dental pulp stem cell transplantation on diabetic polyneuropathy in streptozotocine-induced type 1 diabetes model mice. Cells 2021, 10, 2473. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Hata, M.; Nakamura, N.; Miyabe, M.; Ozawa, S.; Nukada, H.; Tsukamoto, M.; Sango, K.; Himeno, T.; Kamiya, H.; et al. Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res. Ther. 2017, 8, 279. [Google Scholar] [CrossRef]
- Mu, X.; Liu, H.; Yang, S.; Li, Y.; Xiang, L.; Hu, M.; Wang, X. Chitosan tubes inoculated with dental pulp stem cells and stem cell factor enhance facial nerve-vascularized regeneration in rabbits. ACS Omega 2022, 7, 18509–18520. [Google Scholar] [CrossRef] [PubMed]
- Victor, A.K.; Reiter, L.T. Dental pulp stem cells for the study of neurogenetic disorders. Hum. Mol. Genet. 2017, 26, R166–R171. [Google Scholar] [CrossRef] [PubMed]
- Raik, S.; Kumar, A.; Rattan, V.; Seth, S.; Kaur, A.; Charyya, S.B. Assessment of post-thaw quality of dental mesenchymal stromal cells after long-term cryopreservation by uncontrolled freezing. Appl. Biochem. Biotechnol. 2020, 191, 728–743. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Zheng, Y.; Dai, Y.; Lingling, E.; Zhang, R.; Zhang, S. Genome-wide screening of differentially expressed genes and their potential associations with aging dental pulp stem cells. Comb. Chem. High Throughput Screen. 2023, 26, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.W.; Ling, J.Q.; Gong, Q.M. The expression of stromal cell-derived factor 1 (SDF-1) in inflamed human dental pulp. J. Endod. 2008, 34, 1351–1354. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, Y.Q.; Du, R.; Gu, Y.X.; Xia, L.; Qin, F.; Ritchie, H.H. The expression and role of stromal cell-derived factor-1alpha-CXCR4 axis in human dental pulp. J. Endod. 2008, 34, 939–944. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeshita-Umehara, M.; Tokuyama-Toda, R.; Takebe, Y.; Terada-Ito, C.; Tadokoro, S.; Inoue, A.; Ijichi, K.; Yudo, T.; Satomura, K. Improved Method for Dental Pulp Stem Cell Preservation and Its Underlying Cell Biological Mechanism. Cells 2023, 12, 2138. https://doi.org/10.3390/cells12172138
Takeshita-Umehara M, Tokuyama-Toda R, Takebe Y, Terada-Ito C, Tadokoro S, Inoue A, Ijichi K, Yudo T, Satomura K. Improved Method for Dental Pulp Stem Cell Preservation and Its Underlying Cell Biological Mechanism. Cells. 2023; 12(17):2138. https://doi.org/10.3390/cells12172138
Chicago/Turabian StyleTakeshita-Umehara, Mai, Reiko Tokuyama-Toda, Yusuke Takebe, Chika Terada-Ito, Susumu Tadokoro, Akemi Inoue, Kohei Ijichi, Toshio Yudo, and Kazuhito Satomura. 2023. "Improved Method for Dental Pulp Stem Cell Preservation and Its Underlying Cell Biological Mechanism" Cells 12, no. 17: 2138. https://doi.org/10.3390/cells12172138
APA StyleTakeshita-Umehara, M., Tokuyama-Toda, R., Takebe, Y., Terada-Ito, C., Tadokoro, S., Inoue, A., Ijichi, K., Yudo, T., & Satomura, K. (2023). Improved Method for Dental Pulp Stem Cell Preservation and Its Underlying Cell Biological Mechanism. Cells, 12(17), 2138. https://doi.org/10.3390/cells12172138







