Scutellaria baicalensis Attenuated Neurological Impairment by Regulating Programmed Cell Death Pathway in Ischemic Stroke Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Scutellaria baicalensis Extraction
2.2. Animals
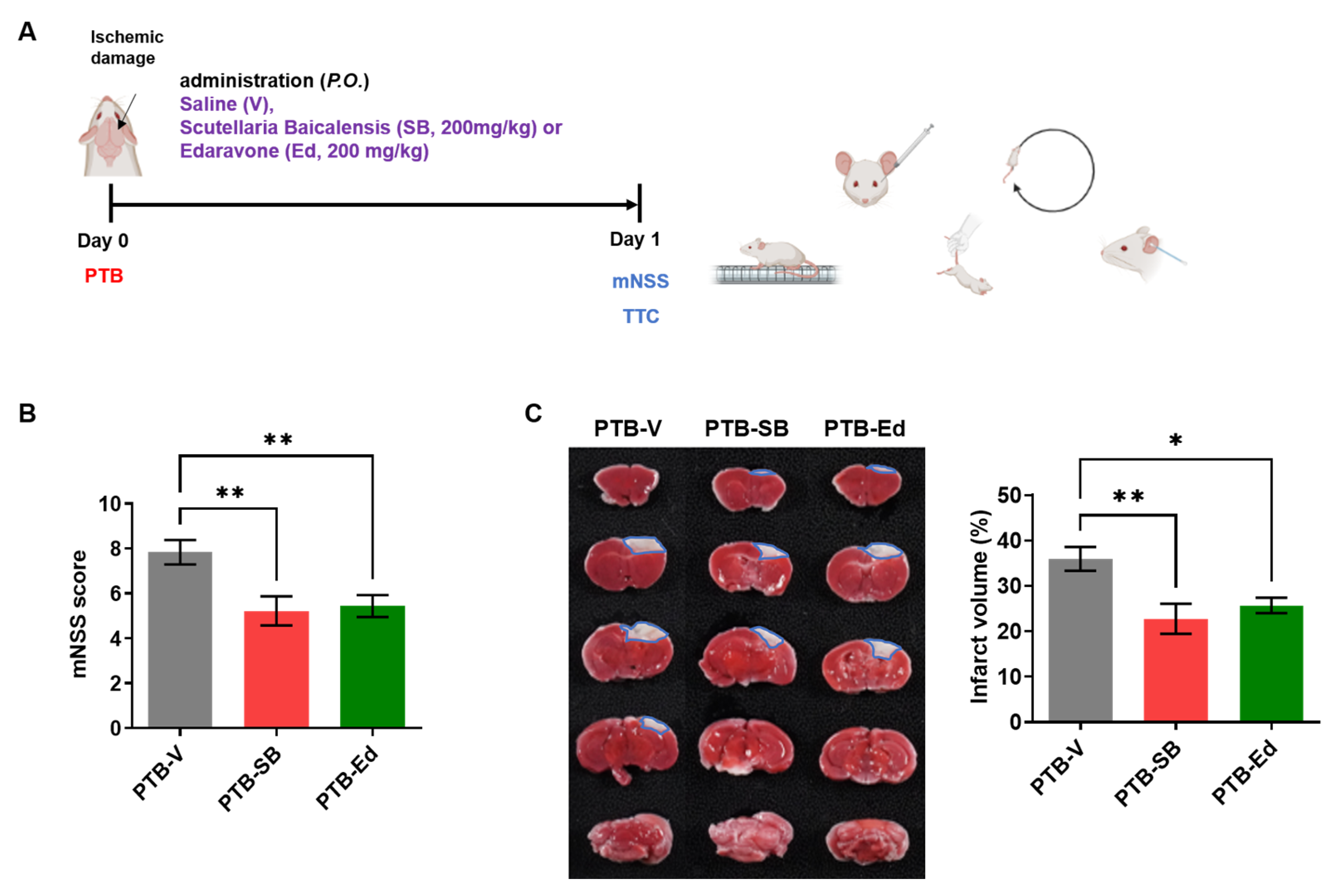
2.2.1. Photothrombotic Model
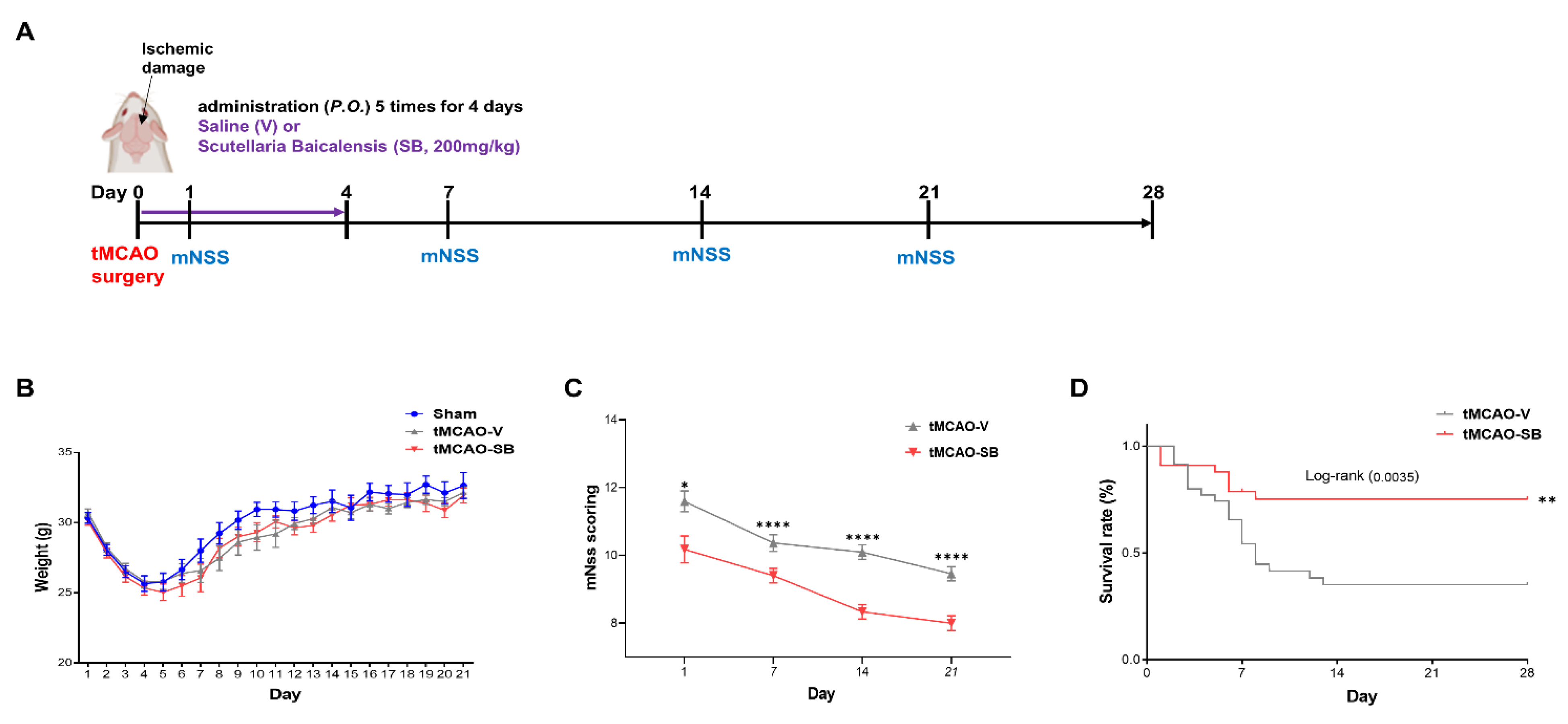
2.2.2. Transient Middle Cerebral Artery Occlusion Model
2.2.3. Scutellaria baicalensis Extraction Administration
2.3. Neurological Severity Score, Weight, and Survival Rate
2.4. 2,3,5-Triphenyl Tetrazolium Chloride Stain
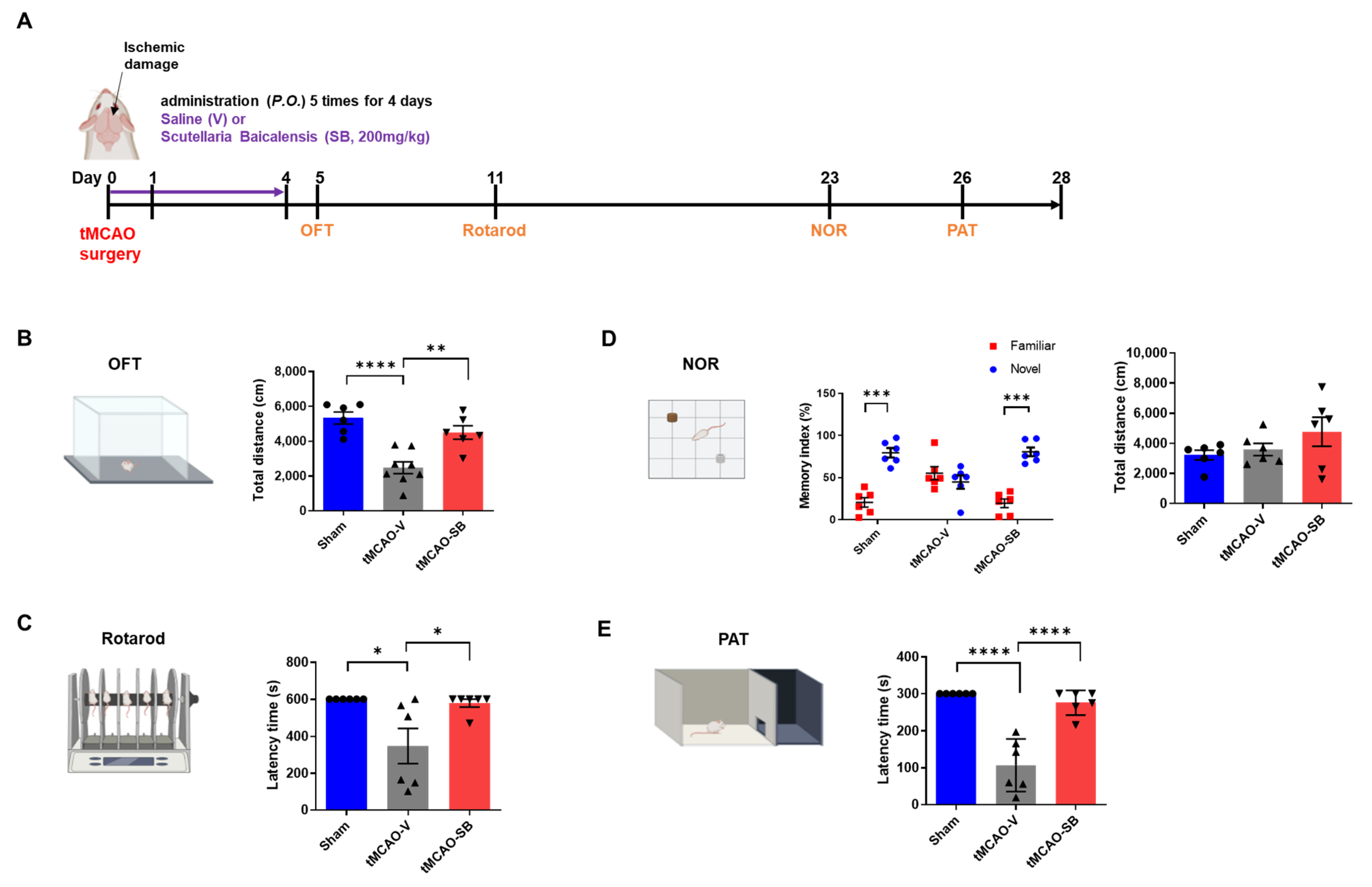
2.5. Behavior Tests
2.5.1. Open-Field Test
2.5.2. Rotarod Test
2.5.3. Novel Object Recognition Test
2.5.4. Passive Avoidance Test
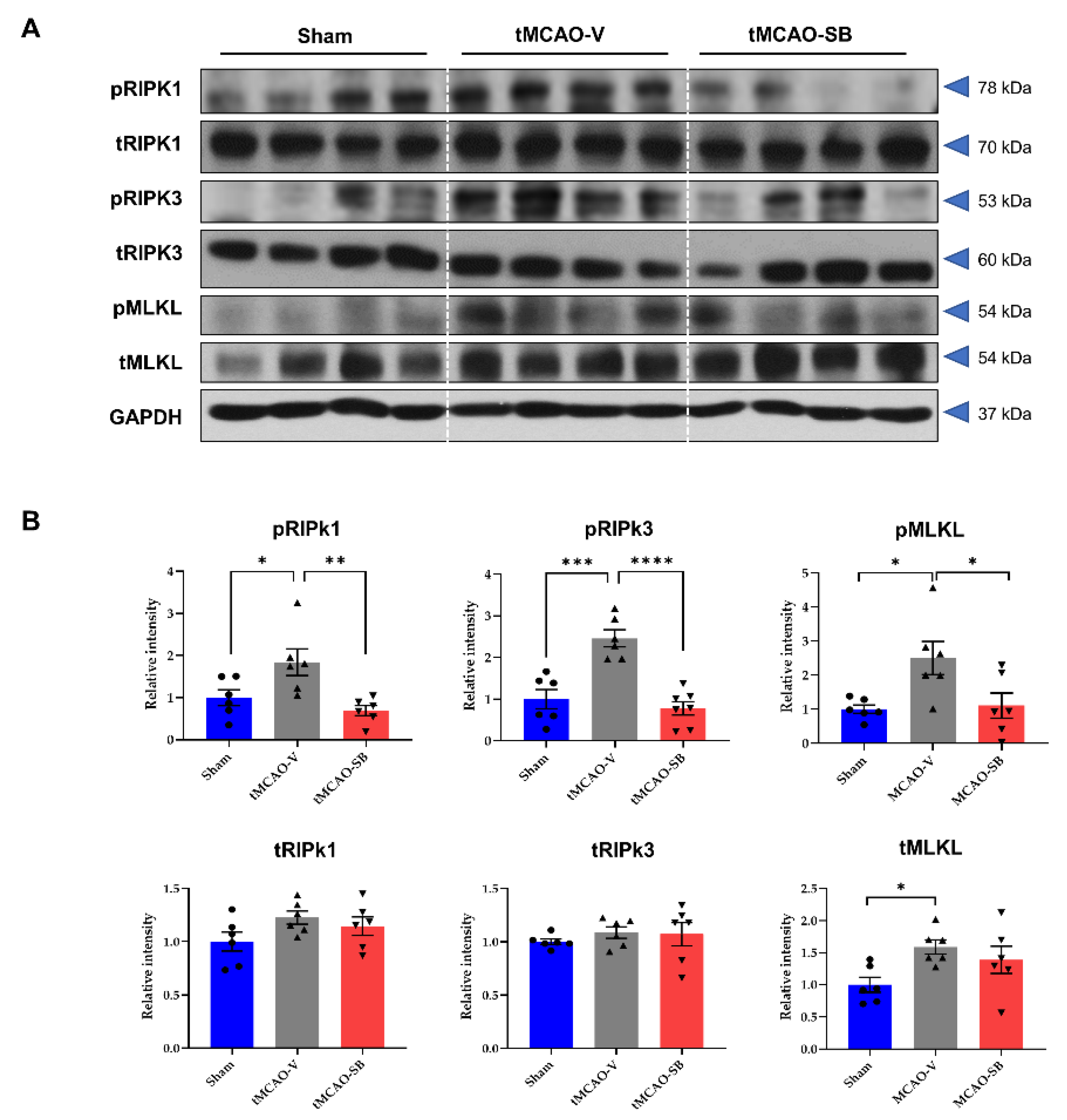
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. SB Improved Neurological Function in the Mouse Model of PTB Stroke
3.2. SB Downregulated the Key Regulators of Necroptosis in tMCAO Mice Brains
3.3. SB Downregulated NLRP3 Inflammasome and the Key Regulators of Pyroptosis in tMCAO Mice Brains
3.4. SB Administration Showed Long-Term Neuroprotective Effects against Brain Injuries following tMCAO
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paul; Candelario-Jalil, E. Emerging Neuroprotective Strategies for the Treatment of Ischemic Stroke: An Overview of Clinical and Preclinical Studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kroemer, G. Necroptosis: A specialized pathway of programmed necrosis. Cell 2008, 135, 1161–1163. [Google Scholar] [CrossRef]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Fann, D.Y.; Lim, Y.A.; Cheng, Y.L.; Lok, K.Z.; Chunduri, P.; Baik, S.H.; Drummond, G.R.; Dheen, S.T.; Sobey, C.G.; Jo, D.G.; et al. Evidence that NF-κB and MAPK Signaling Promotes NLRP Inflammasome Activation in Neurons Following Ischemic Stroke. Mol. Neurobiol. 2018, 55, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Savage, C.D.; Lopez-Castejon, G.; Denes, A.; Brough, D. NLRP3-Inflammasome Activating DAMPs Stimulate an Inflammatory Response in Glia in the Absence of Priming Which Contributes to Brain Inflammation after Injury. Front. Immunol. 2012, 3, 288. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.N.; Nguyen, H.D.; Kim, Y.J.; Nguyen, T.T.; Lai, T.T.; Lee, Y.K.; Ma, H.I.; Kim, Y.E. Role of NLRP3 Inflammasome in Parkinson’s Disease and Therapeutic Considerations. J. Parkinsons Dis. 2022, 12, 2117–2133. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, J.; Zhang, P.; Li, H.; Shen, H.; Li, X.; Chen, G. Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J. Neurosci. Res. 2019, 97, 645–660. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef]
- Barrington, J.; Lemarchand, E.; Allan, S.M. A brain in flame; do inflammasomes and pyroptosis influence stroke pathology? Brain Pathol. 2017, 27, 205–212. [Google Scholar] [CrossRef]
- Dong, Z.; Pan, K.; Pan, J.; Peng, Q.; Wang, Y. The Possibility and Molecular Mechanisms of Cell Pyroptosis After Cerebral Ischemia. Neurosci. Bull. 2018, 34, 1131–1136. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Wang, L.P.; Wan, Q. Therapeutic targets of neuroprotection and neurorestoration in ischemic stroke: Applications for natural compounds from medicinal herbs. Biomed. Pharmacother. 2022, 148, 112719. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Zheng, W.X.; He, W.Q.; Zhang, Q.R.; Jia, J.X.; Zhao, S.; Wu, F.J.; Cao, X.L. Baicalin Inhibits NLRP3 Inflammasome Activity Via the AMPK Signaling Pathway to Alleviate Cerebral Ischemia-Reperfusion Injury. Inflammation 2021, 44, 2091–2105. [Google Scholar] [CrossRef]
- Song, X.; Gong, Z.; Liu, K.; Kou, J.; Liu, B.; Liu, K. Baicalin combats glutamate excitotoxicity via protecting glutamine synthetase from ROS-induced 20S proteasomal degradation. Redox Biol. 2020, 34, 101559. [Google Scholar] [CrossRef]
- Wang, P.; Cao, Y.; Yu, J.; Liu, R.; Bai, B.; Qi, H.; Zhang, Q.; Guo, W.; Zhu, H.; Qu, L. Baicalin alleviates ischemia-induced memory impairment by inhibiting the phosphorylation of CaMKII in hippocampus. Brain Res. 2016, 1642, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, H.; Yang, Y.; Wang, R.; Wang, Y.; Wu, C.; Du, G. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed. Pharmacother. 2019, 117, 109102. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Long, J.Y.; Xie, L.; Zhang, L.L.; Xie, Q.X.; Chen, H.J.; Deng, M.; Li, X.F. Applications, phytochemistry, pharmacological effects, pharmacokinetics, toxicity of Scutellaria baicalensis Georgi. and its probably potential therapeutic effects on COVID-19: A review. Chin. Med. 2020, 15, 102. [Google Scholar] [CrossRef]
- Schroeter, M.; Jander, S.; Stoll, G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: Characterization of inflammatory responses. J. Neurosci. Methods 2002, 117, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Ko, G.; Kim, J.; Jeon, Y.J.; Lee, D.; Baek, H.M.; Chang, K.A. Salvia miltiorrhiza Alleviates Memory Deficit Induced by Ischemic Brain Injury in a Transient MCAO Mouse Model by Inhibiting Ferroptosis. Antioxidants 2023, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mao, X.; Sun, C.; Zheng, P.; Gao, J.; Wang, X.; Min, D.; Sun, H.; Xie, N.; Cai, J. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res. Bull. 2011, 85, 396–402. [Google Scholar] [CrossRef]
- Gaire, B.P.; Sapkota, A.; Choi, J.W. BMS-986020, a Specific LPA(1) Antagonist, Provides Neuroprotection against Ischemic Stroke in Mice. Antioxidants 2020, 9, 1097. [Google Scholar] [CrossRef]
- Popp, A.; Jaenisch, N.; Witte, O.W.; Frahm, C. Identification of ischemic regions in a rat model of stroke. PLoS ONE 2009, 4, e4764. [Google Scholar] [CrossRef]
- Kim, J.; Suh, Y.H.; Chang, K.A. Interleukin-17 induced by cumulative mild stress promoted depression-like behaviors in young adult mice. Mol. Brain 2021, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A. A critical assessment of edaravone acute ischemic stroke efficacy trials: Is edaravone an effective neuroprotective therapy? Expert. Opin. Pharmacother. 2010, 11, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wu, H.; Yang, J.; Ye, M.; Liu, D.; Li, Y.; Zhang, D.; Zhang, J.; Yang, Q.; Liu, Y. Liraglutide Attenuates Myocardial Ischemia/Reperfusion Injury Through the Inhibition of Necroptosis by Activating GLP-1R/PI3K/Akt Pathway. Cardiovasc. Toxicol. 2023, 23, 161–175. [Google Scholar] [CrossRef]
- Chen, A.Q.; Fang, Z.; Chen, X.L.; Yang, S.; Zhou, Y.F.; Mao, L.; Xia, Y.P.; Jin, H.J.; Li, Y.N.; You, M.F.; et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019, 10, 487. [Google Scholar] [CrossRef]
- Miller, D.R.; Cramer, S.D.; Thorburn, A. The interplay of autophagy and non-apoptotic cell death pathways. Int. Rev. Cell Mol. Biol. 2020, 352, 159–187. [Google Scholar] [CrossRef]
- Barthels, D.; Das, H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, T.; Asai, T.; Yanagida, Y.; Namba, M.; Koide, H.; Shimizu, K.; Oku, N. Combination therapy with liposomal neuroprotectants and tissue plasminogen activator for treatment of ischemic stroke. FASEB J. 2017, 31, 1879–1890. [Google Scholar] [CrossRef]
- Rangaraju, S.; Edwards, A.; Dehkharghani, S.; Nahab, F. Perfusion imaging in the 3-hour time window predicts a tPA-associated hemorrhage in acute ischemic stroke. Neurologist 2015, 19, 68–69. [Google Scholar] [CrossRef]
- Datta, A.; Sarmah, D.; Mounica, L.; Kaur, H.; Kesharwani, R.; Verma, G.; Veeresh, P.; Kotian, V.; Kalia, K.; Borah, A.; et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl. Stroke Res. 2020, 11, 1185–1202. [Google Scholar] [CrossRef] [PubMed]
- Sekerdag, E.; Solaroglu, I.; Gursoy-Ozdemir, Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018, 16, 1396–1415. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Naito, M.G.; Xu, D.; Amin, P.; Lee, J.; Wang, H.; Li, W.; Kelliher, M.; Pasparakis, M.; Yuan, J. Sequential activation of necroptosis and apoptosis cooperates to mediate vascular and neural pathology in stroke. Proc. Natl. Acad. Sci. USA 2020, 117, 4959–4970. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Li, X.; Zhang, H.; Wang, L.; Wu, X.; Zhang, H.; Luo, Y. Catalytically inactive RIP1 and RIP3 deficiency protect against acute ischemic stroke by inhibiting necroptosis and neuroinflammation. Cell Death Dis. 2020, 11, 565. [Google Scholar] [CrossRef]
- Jun-Long, H.; Yi, L.; Bao-Lian, Z.; Jia-Si, L.; Ning, Z.; Zhou-Heng, Y.; Xue-Jun, S.; Wen-Wu, L. Necroptosis Signaling Pathways in Stroke: From Mechanisms to Therapies. Curr. Neuropharmacol. 2018, 16, 1327–1339. [Google Scholar] [CrossRef]
- Hu, R.; Liang, J.; Ding, L.; Zhang, W.; Wang, Y.; Zhang, Y.; Zhang, D.; Pei, L.; Liu, X.; Xia, Z.; et al. Gasdermin D inhibition ameliorates neutrophil mediated brain damage in acute ischemic stroke. Cell Death Discov. 2023, 9, 50. [Google Scholar] [CrossRef]
- Hsu, S.K.; Li, C.Y.; Lin, I.L.; Syue, W.J.; Chen, Y.F.; Cheng, K.C.; Teng, Y.N.; Lin, Y.H.; Yen, C.H.; Chiu, C.C. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics 2021, 11, 8813–8835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.S.; Chen, J.; Tan, H.Y.; Wang, N.; Chen, Z.; Feng, Y. Scutellaria baicalensis and Cancer Treatment: Recent Progress and Perspectives in Biomedical and Clinical Studies. Am. J. Chin. Med. 2018, 46, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Yingrui, W.; Zheng, L.; Guoyan, L.; Hongjie, W. Research progress of active ingredients of Scutellaria baicalensis in the treatment of type 2 diabetes and its complications. Biomed. Pharmacother. 2022, 148, 112690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Wang, Z.; Zhang, X.Y.; Ying, K.; Liu, J.X.; Wang, Y.Y. Gene expression profile induced by oral administration of baicalin and gardenin after focal brain ischemia in rats. Acta Pharmacol. Sin. 2005, 26, 307–314. [Google Scholar] [CrossRef]
- Liang, W.; Huang, X.; Chen, W. The Effects of Baicalin and Baicalein on Cerebral Ischemia: A Review. Aging Dis. 2017, 8, 850–867. [Google Scholar] [CrossRef]
- Li, M.; Meng, Z.; Yu, S.; Li, J.; Wang, Y.; Yang, W.; Wu, H. Baicalein ameliorates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3 axis. Chem. Biol. Interact. 2022, 366, 110137. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Gu, Y.; Peng, T.; Yang, D.; Chang, R.C.; So, K.F.; Liu, K.; Shen, J. Baicalin can scavenge peroxynitrite and ameliorate endogenous peroxynitrite-mediated neurotoxicity in cerebral ischemia-reperfusion injury. J. Ethnopharmacol. 2013, 150, 116–124. [Google Scholar] [CrossRef]
- Song, J.; Kim, Y.S.; Lee, D.; Kim, H. Safety evaluation of root extract of Pueraria lobata and Scutellaria baicalensis in rats. BMC Complement. Med. Ther. 2020, 20, 226. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Millerot-Serrurot, E.; Bertrand, N.; Mossiat, C.; Faure, P.; Prigent-Tessier, A.; Garnier, P.; Bejot, Y.; Giroud, M.; Beley, A.; Marie, C. Temporal changes in free iron levels after brain ischemia Relevance to the timing of iron chelation therapy in stroke. Neurochem. Int. 2008, 52, 1442–1448. [Google Scholar] [CrossRef]
- Dietrich, W.D.; Watson, B.D.; Busto, R.; Ginsberg, M.D.; Bethea, J.R. Photochemically induced cerebral infarction. I. Early microvascular alterations. Acta Neuropathol. 1987, 72, 315–325. [Google Scholar] [CrossRef]
- Wood, N.I.; Sopesen, B.V.; Roberts, J.C.; Pambakian, P.; Rothaul, A.L.; Hunter, A.J.; Hamilton, T.C. Motor dysfunction in a photothrombotic focal ischaemia model. Behav. Brain Res. 1996, 78, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Labat-gest, V.; Tomasi, S. Photothrombotic ischemia: A minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Cheng, O.; Li, Z.; Han, Y.; Jiang, Q.; Yan, Y.; Cheng, K. Baicalin improved the spatial learning ability of global ischemia/reperfusion rats by reducing hippocampal apoptosis. Brain Res. 2012, 1470, 111–118. [Google Scholar] [CrossRef]
- Tu, X.K.; Yang, W.Z.; Shi, S.S.; Wang, C.H.; Chen, C.M. Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia. Neurochem. Res. 2009, 34, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, P.; Fernández-López, A. PANoptosis: New insights in regulated cell death in ischemia/reperfusion models. Neural Regen. Res. 2023, 18, 342–343. [Google Scholar] [CrossRef]
- Wang, Y.; Kanneganti, T.D. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput. Struct. Biotechnol. J. 2021, 19, 4641–4657. [Google Scholar] [CrossRef]
- Samir, P.; Malireddi, R.K.S.; Kanneganti, T.D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell Infect. Microbiol. 2020, 10, 238. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.-w.; Ha, T.-Y.; Ko, G.; Jang, A.; Choi, J.-W.; Lee, D.-h.; Chang, K.-A. Scutellaria baicalensis Attenuated Neurological Impairment by Regulating Programmed Cell Death Pathway in Ischemic Stroke Mice. Cells 2023, 12, 2133. https://doi.org/10.3390/cells12172133
Seo H-w, Ha T-Y, Ko G, Jang A, Choi J-W, Lee D-h, Chang K-A. Scutellaria baicalensis Attenuated Neurological Impairment by Regulating Programmed Cell Death Pathway in Ischemic Stroke Mice. Cells. 2023; 12(17):2133. https://doi.org/10.3390/cells12172133
Chicago/Turabian StyleSeo, Ho-won, Tae-Young Ha, Geon Ko, Aram Jang, Ji-Woong Choi, Dong-hun Lee, and Keun-A Chang. 2023. "Scutellaria baicalensis Attenuated Neurological Impairment by Regulating Programmed Cell Death Pathway in Ischemic Stroke Mice" Cells 12, no. 17: 2133. https://doi.org/10.3390/cells12172133
APA StyleSeo, H.-w., Ha, T.-Y., Ko, G., Jang, A., Choi, J.-W., Lee, D.-h., & Chang, K.-A. (2023). Scutellaria baicalensis Attenuated Neurological Impairment by Regulating Programmed Cell Death Pathway in Ischemic Stroke Mice. Cells, 12(17), 2133. https://doi.org/10.3390/cells12172133









