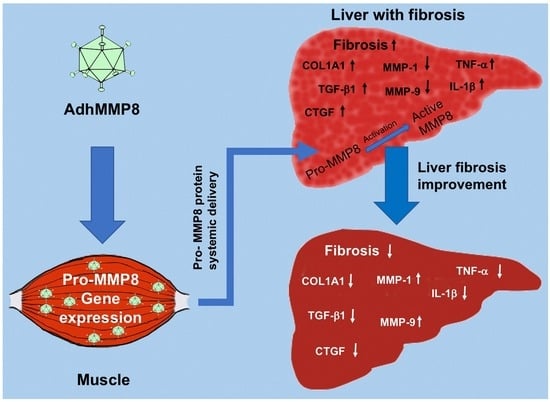
AdhMMP8 Vector Administration in Muscle: An Alternate Strategy to Regress Hepatic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Adenoviral Vectors Production
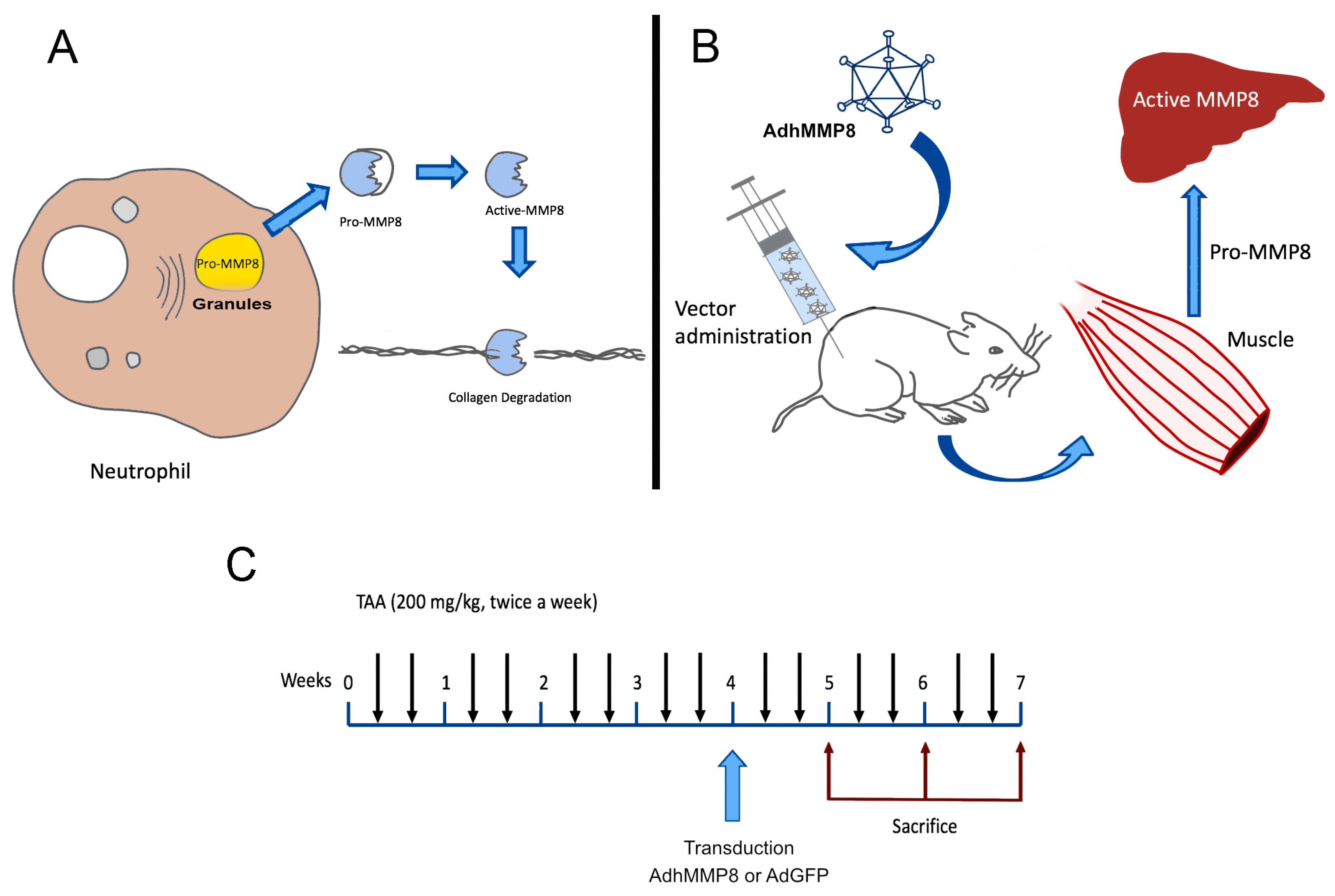
2.2. Animals and Experimental Design
2.3. TAA Liver Cirrhosis Model
2.4. Adenovirus Treatment in TAA-Intoxicated Rats
2.5. Quantitative Real-Time Reverse Transcriptase (RT-PCR)
2.6. Histological Examination of Liver Sections
2.7. Biochemical Assays
2.8. MMP-8 Protein Determination
2.9. Statistical Analysis
3. Results
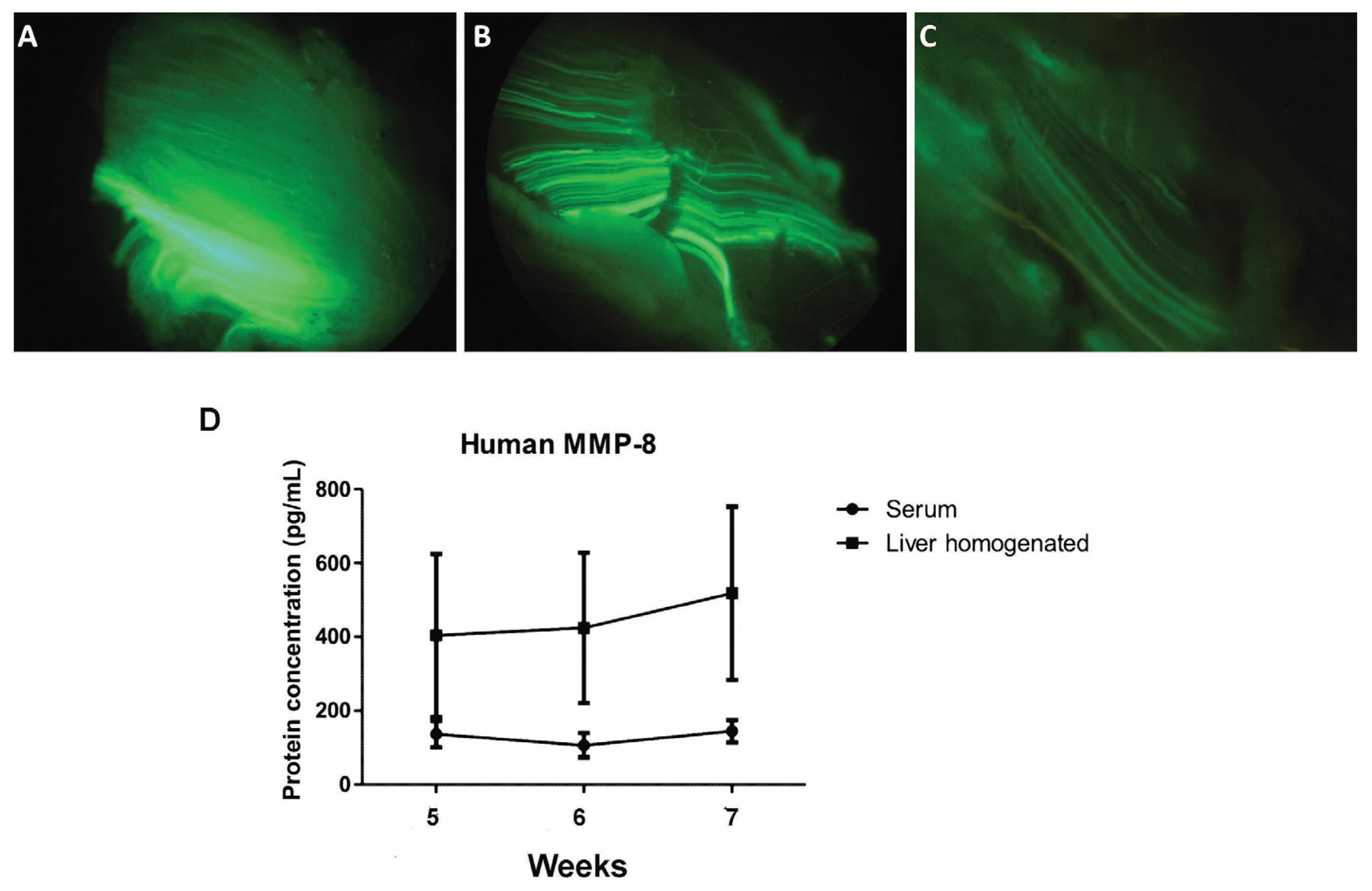
3.1. Adenoviral Vectors Amplification, Analysis of Muscle GFP Gene Expression, and Determination of Serum MMP-8 Protein
3.2. Liver Function after AdhMMP8 Intramuscular Administration
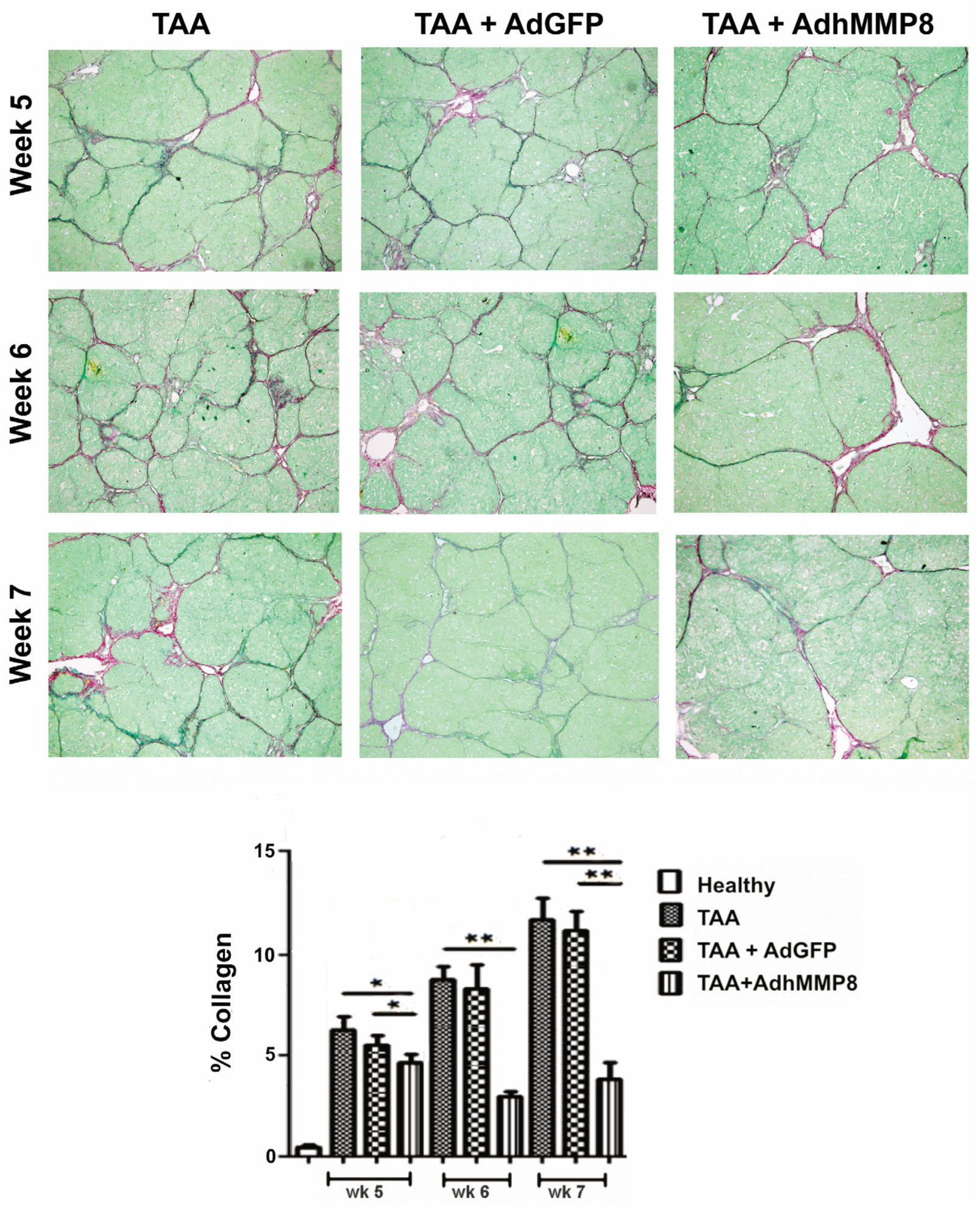
3.3. Liver Fibrosis Decreased after Intramuscular Injection of AdhMMP8
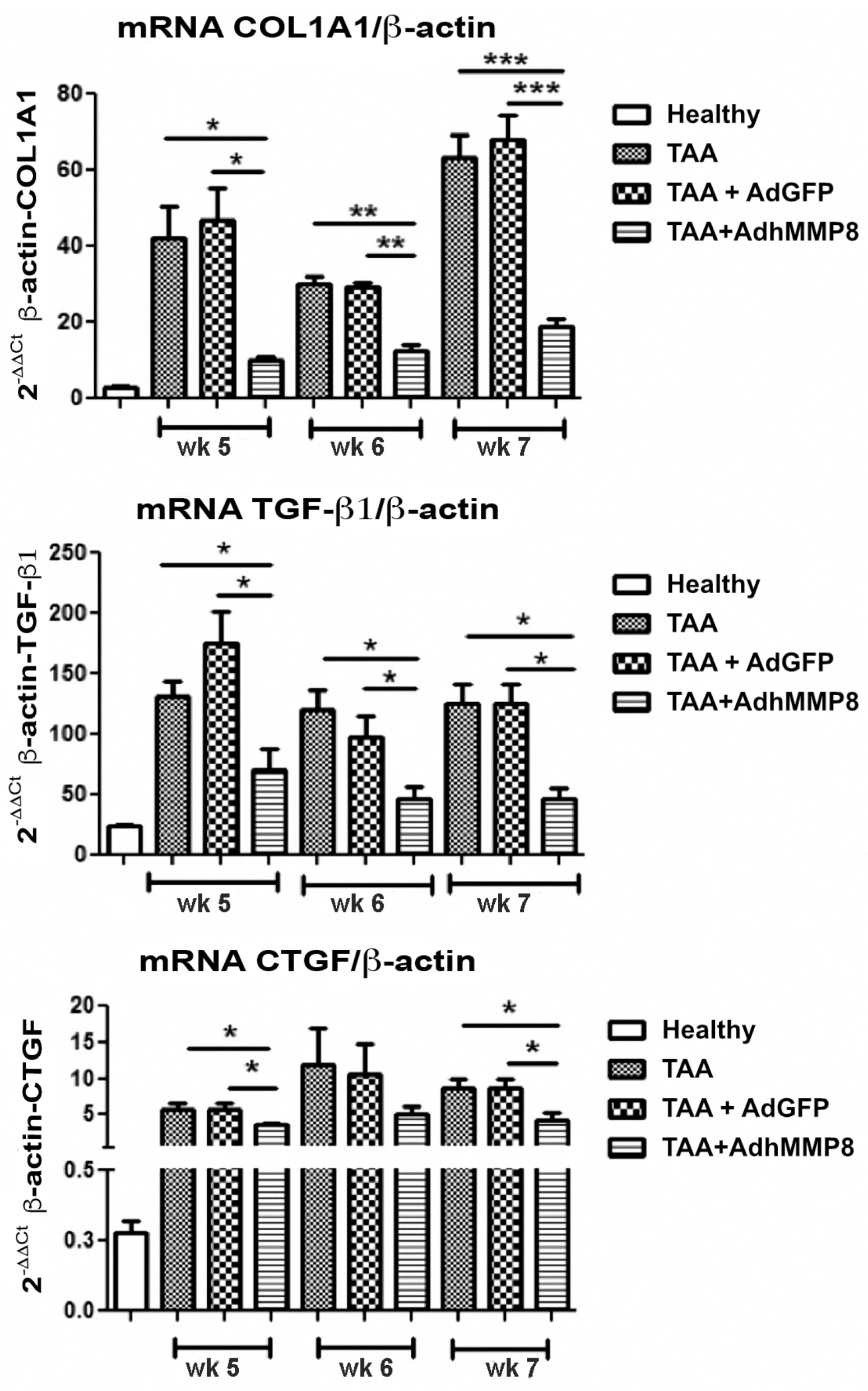
3.4. Fibrogenic Molecules Gene Expression after Intramuscular Injection of AdhMMP8
3.5. Antifibrogenic Molecules Gene Expression after Intramuscular Injection of AdhMMP8
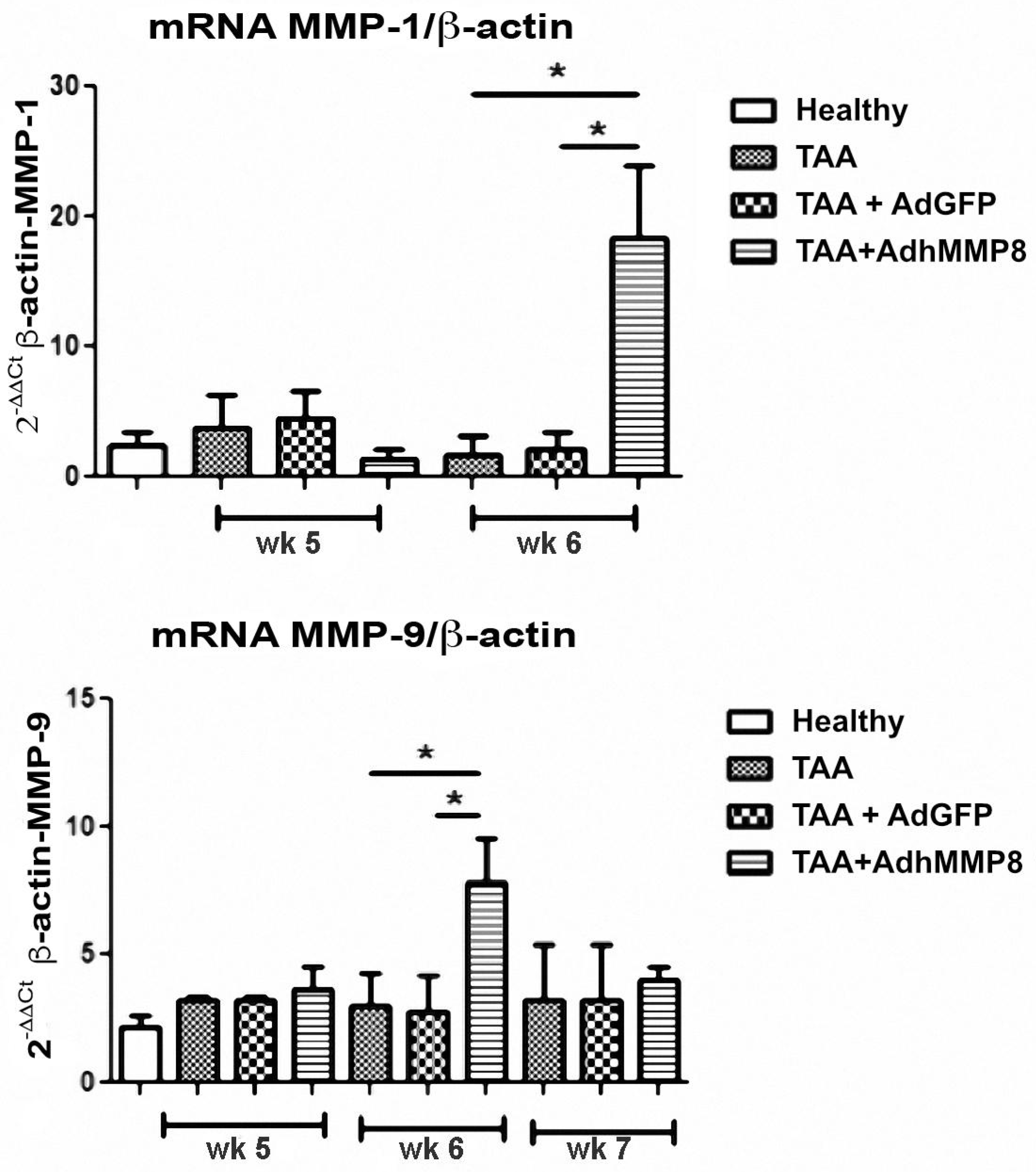
3.6. Inflammatory Gene Expression after Transduction with AdMMP8
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247 Pt 1, 1465–1468. [Google Scholar] [CrossRef]
- MacColl, G.S.; Goldspink, G.; Bouloux, P.M. Using skeletal muscle as an artificial endocrine tissue. J. Endocrinol. 1999, 162, 1–9. [Google Scholar] [CrossRef][Green Version]
- Haase, G.; Kennel, P.; Pettmann, B.; Vigne, E.; Akli, S.; Revah, F.; Schmalbruch, H.; Kahn, A. Gene therapy of murine motor neuron disease using adenoviral vectors for neurotrophic factors. Nat. Med. 1997, 3, 429–436. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Rockey, D.C. Fibrosis reversal after hepatitis C virus elimination. Curr. Opin. Gastroenterol. 2019, 35, 137–144. [Google Scholar] [CrossRef]
- Rockey, D.C.; Friedman, S.L. Fibrosis Regression after Eradication of Hepatitis C Virus: From Bench to Bedside. Gastroenterology 2021, 160, 1502–1520.e1. [Google Scholar] [CrossRef]
- Elsharkawy, A.; Samir, R.; El-Kassas, M. Fibrosis regression following hepatitis C antiviral therapy. World J. Hepatol. 2022, 14, 1120–1130. [Google Scholar] [CrossRef]
- Yoo, H.W.; Park, J.Y.; Kim, S.G.; Jung, Y.K.; Lee, S.H.; Kim, M.Y.; Jun, D.W.; Jang, J.Y.; Lee, J.W.; Kwon, O.S. Regression of liver fibrosis and hepatocellular carcinoma development after HCV eradication with oral antiviral agents. Sci. Rep. 2022, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J. A review of liver fibrosis and cirrhosis regression. J. Pathol. Transl. Med. 2023, 57, 189–195. [Google Scholar] [CrossRef]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Sun, B. The Molecular Mechanisms of Liver Fibrosis and Its Potential Therapy in Application. Int. J. Mol. Sci. 2022, 23, 12572. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of Liver Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 425–456. [Google Scholar] [CrossRef]
- Iredale, J.P.; Murphy, G.; Hembry, R.M.; Friedman, S.L.; Arthur, M.J. Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1. Implications for regulation of matrix degradation in liver. J. Clin. Invest. 1992, 90, 282–287. [Google Scholar] [CrossRef]
- Murawaki, Y.; Yamamoto, H.; Kawasaki, H.; Shima, H. Serum tissue inhibitor of metalloproteinases in patients with chronic liver disease and with hepatocellular carcinoma. Clin. Chim. Acta 1993, 218, 47–58. [Google Scholar] [CrossRef]
- Rockey, D.C. Gene therapy for hepatic fibrosis-bringing treatment into the new millennium. Hepatology 1999, 30, 816–818. [Google Scholar] [CrossRef]
- Van Lint, P.; Libert, C. Matrix metalloproteinase-8: Cleavage can be decisive. Cytokine Growth Factor Rev. 2006, 17, 217–223. [Google Scholar] [CrossRef]
- Khokha, R.; Murthy, A.; Weiss, A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 649–665. [Google Scholar] [CrossRef]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Ueki, T.; Kaneda, Y.; Tsutsui, H.; Nakanishi, K.; Sawa, Y.; Morishita, R.; Matsumoto, K.; Nakamura, T.; Takahashi, H.; Okamoto, E.; et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat. Med. 1999, 5, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Siller-López, F.; García-Bañuelos, J.; Hasty, K.A.; Segura, J.; Ramos-Márquez, M.; Qoronfleh, M.W.; Aguilar-Cordova, E.; Armendáriz-Borunda, J. Truncated active matrix metalloproteinase-8 gene expression in HepG2 cells is active against native type I collagen. J. Hepatol. 2000, 33, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Salgado, S.; Garcia, J.; Vera, J.; Siller, F.; Bueno, M.; Miranda, A.; Segura, A.; Grijalva, G.; Segura, J.; Orozco, H.; et al. Liver cirrhosis is reverted by urokinase-type plasminogen activator gene therapy. Mol. Ther. 2000, 2, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bañuelos, J.; Siller-Lopez, F.; Miranda, A.; Aguilar, L.K.; Aguilar-Cordova, E.; Armendariz-Borunda, J. Cirrhotic rat livers with extensive fibrosis can be safely transduced with clinical-grade adenoviral vectors. Evidence of cirrhosis reversion. Gene Ther. 2002, 9, 127–134. [Google Scholar] [CrossRef]
- Siller-López, F.; Sandoval, A.; Salgado, S.; Salazar, A.; Bueno, M.; Garcia, J.; Vera, J.; Gálvez, J.; Hernández, I.; Ramos, M. Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology 2004, 126, 1122–1133; discussion 949. [Google Scholar] [CrossRef]
- Gálvez-Gastélum, F.J.; Garcia-Bañuelos, J.J.; Beas-Zárate, C.; Segura-Flores, A.; González, H.; Chaparro-Huerta, V.; Salazar-Montes, A.; Sandoval-Rodriguez, A.S.; Bueno-Topete, M.; Lucano-Landeros, S.; et al. Combinatorial gene therapy induces regression of hepatic encephalopathy. Gene Ther. 2011, 18, 88–94. [Google Scholar] [CrossRef][Green Version]
- Meza-Ríos, A.; García-Benavides, L.; García-Bañuelos, J.; Salazar-Montes, A.; Armendáriz-Borunda, J.; Sandoval-Rodríguez, A. Simultaneous Administration of ADSCs-Based Therapy and Gene Therapy Using Ad-huPA Reduces Experimental Liver Fibrosis. PLoS ONE 2016, 11, e0166849. [Google Scholar] [CrossRef][Green Version]
- Sobrevilla-Navarro, A.A.; Sandoval-Rodríguez, A.; García-Bañuelos, J.J.; Armendariz-Borunda, J.; Salazar-Montes, A.M. Interferon-α Silencing by Small Interference RNA Increases Adenovirus Transduction and Transgene Expression in Huh7 Cells. Mol. Biotechnol. 2018, 60, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Wang, Y.; Rong, H.; Wang, K.; Huang, X. Human Adenovirus Associated Hepatic Injury. Front. Public Health 2022, 10, 878161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X. Adenovirus vector-attributed hepatotoxicity blocks clinical application in gene therapy. Cytotherapy 2021, 23, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Seki, E. In Vivo and In Vitro Models to Study Liver Fibrosis: Mechanisms and Limitations. Cell Mol. Gastroenterol. Hepatol. 2023, 16, 355–367. [Google Scholar] [CrossRef]
- Armendáriz-Borunda, J.; Bastidas-Ramírez, B.E.; Sandoval-Rodríguez, A.; González-Cuevas, J.; Gómez-Meda, B.; García-Bañuelos, J. Production of first generation adenoviral vectors for preclinical protocols: Amplification, purification and functional titration. J. Biosci. Bioeng. 2011, 112, 415–421. [Google Scholar] [CrossRef]
- Chilakapati, J.; Shankar, K.; Korrapati, M.C.; Hill, R.A.; Mehendale, H.M. Saturation toxicokinetics of thioacetamide: Role in initiation of liver injury. Drug Metab. Dispos. 2005, 33, 1877–1885. [Google Scholar] [CrossRef]
- Dashti, H.; Jeppsson, B.; Hägerstrand, I.; Hultberg, B.; Srinivas, U.; Abdulla, M.; Bengmark, S. Thioacetamide- and carbon tetrachloride-induced liver cirrhosis. Eur. Surg Res. 1989, 21, 83–91. [Google Scholar] [CrossRef]
- Li, X.; Benjamin, I.S.; Alexander, B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J. Hepatol. 2002, 36, 488–493. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta] CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N., Jr. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Soboleski, M.R.; Oaks, J.; Halford, W.P. Green fluorescent protein is a quantitative reporter of gene expression in individual eukaryotic cells. FASEB J. 2005, 19, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Sakamoto, T.; Nakamura, T.; Qi, Z.; Astuchi, N.; Takeshita, A.; Shimizu, K.; Ohashi, H. A soluble transforming growth factor beta receptor expressed in muscle prevents liver fibrogenesis and dysfunction in rats. Hum. Gene Ther. 2000, 11, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.A.; Nagase, H.; Harris, E.D., Jr. Proactivator-dependent activation of procollagenase induced by treatment with EGTA. Biochem J. 1986, 237, 853–858. [Google Scholar] [CrossRef]
- Nathan, C. Role of iNOS in human host defense. Science 2006, 312, 1874–1875. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Goldwasser, E.; Lu, M.M.; Barr, E.; Leiden, J.M. Stable delivery of physiologic levels of recombinant erythropoietin to the systemic circulation by intramuscular injection of replication-defective adenovirus. Proc. Natl. Acad. Sci. USA 1994, 91, 11557–11561. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 2, 827–839. [Google Scholar] [CrossRef]
- Iimuro, Y.; Brenner, D.A. Matrix Metalloproteinase Gene Delivery for Liver Fibrosis. Pharm. Res. 2008, 25, 249–258. [Google Scholar] [CrossRef]
- Porter, W.R.; Gudzinowicz, M.J.; Neal, R.A. Thioacetamide-induced hepatic necrosis. II. Pharmacokinetics of thioacetamide and thioacetamide-S-oxide in the rat. J. Pharmacol. Exp. Ther. 1979, 208, 386–391. [Google Scholar]
- Mullen, K.D.; McCullough, A.J. Problems with animal models of chronic liver disease: Suggestions for improvement in standardization. Hepatology 1989, 9, 500–503. [Google Scholar] [CrossRef]
- Moreira, E.; Fontana, L.; Torres, M.I.; Fernández, I.; Ríos, A.; Sánchez de Medina, F.; Gil, A. Dietary long-chain polyunsaturated fatty acids influence the recovery of thioacetamide-induced liver cirrhosis in rats. JPEN J. Parenter. Enteral. Nutr. 1995, 19, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Masumi, S.; Moriyama, M.; Kannan, Y.; Ohta, M.; Sugano, T.; Yamate, J. Population of hepatic macrophages and response of perfused liver to platelet-activating factor during production of thioacetamide-induced cirrhosis in rats. Hepatology 1996, 24, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Cawston, T.E.; Murphy, G. Mammalian collagenases. Methods Enzymol. 1981, 80 Pt C, 711–722. [Google Scholar] [CrossRef]
- Ota, K.; Stetler-Stevenson, W.G.; Yang, Q.; Kumar, A.; Wada, J.; Kashihara, N.; Wallner, E.I.; Kanwar, Y.S. Cloning of murine membrane-type-1-matrix metalloproteinase (MT-1-MMP) and its metanephric developmental regulation with respect to MMP-2 and its inhibitor. Kidney Int. 1998, 54, 131–142. [Google Scholar] [CrossRef]
- Issa, R.; Zhou, X.; Trim, N.; Millward-Sadler, H.; Krane, S.; Benyon, C.; Iredale, J. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 2003, 17, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cheng, X.; Guo, Z.; Wang, Z.; Li, D.; Kang, F.; Li, H.; Li, B.; Cao, Z.; Nassal, M.; et al. Truncated Active Human Matrix Metalloproteinase-8 Delivered by a Chimeric Adenovirus-Hepatitis B Virus Vector Ameliorates Rat Liver Cirrhosis. PLoS ONE 2013, 8, e53392. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.X.; Zou, X.H.; Jiang, S.Y.; Lu, N.N.; Han, M.; Zhao, J.H.; Guo, X.J.; Zhao, S.C.; Lu, Z.Z. Prevalence of serum neutralizing antibodies to adenovirus type 5 (Ad5) and 41 (Ad41) in children is associated with age and sanitary conditions. Vaccine 2016, 34, 5579–5586. [Google Scholar] [CrossRef]
- Coughlan, L. Factors Which Contribute to the Immunogenicity of Non-replicating Adenoviral Vectored Vaccines. Front. Immunol. 2020, 11, 909–928. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]


| Study Group (n = 15) | Time after TAA Intoxication | |||||
|---|---|---|---|---|---|---|
| Week 5 (n = 5) | Week 6 (n = 5) | Week 7 (n = 5) | ||||
| AST | ALT | AST | ALT | AST | ALT | |
| Healthy | 226.0 ± 61.2 | 64.7 ± 3.5 | 226.0 ± 61.2 | 64.7 ± 3.6 | 226.0 ± 61.2 | 64.7 ± 3.6 |
| TAA | 311.0 ± 112.1 | 182.4 ± 12.8 | 551.5 ± 76.4 | 171.6 ± 42.4 | 270.0 ± 58.9 | 200.0 ± 183.8 |
| TAA + AdGFP | 346.8 ± 185.8 | 195.6 ± 20.7 | 484.0 ± 164.8 | 172.8 ± 43.0 | 276.8 ± 57.2 | 130.0 ± 47.4 |
| TAA + AdhMMP8 | 214.0 ± 11.2 | 117.5 ± 46.8 | 320.5 ± 137.4 * | 133.5 ± 25.7 | 159.0 ± 26.6 | 89.0 ± 13.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Bañuelos, J.; Oceguera-Contreras, E.; Sandoval-Rodríguez, A.; Bastidas-Ramírez, B.E.; Lucano-Landeros, S.; Gordillo-Bastidas, D.; Gómez-Meda, B.C.; Santos, A.; Cerda-Reyes, E.; Armendariz-Borunda, J. AdhMMP8 Vector Administration in Muscle: An Alternate Strategy to Regress Hepatic Fibrosis. Cells 2023, 12, 2127. https://doi.org/10.3390/cells12172127
García-Bañuelos J, Oceguera-Contreras E, Sandoval-Rodríguez A, Bastidas-Ramírez BE, Lucano-Landeros S, Gordillo-Bastidas D, Gómez-Meda BC, Santos A, Cerda-Reyes E, Armendariz-Borunda J. AdhMMP8 Vector Administration in Muscle: An Alternate Strategy to Regress Hepatic Fibrosis. Cells. 2023; 12(17):2127. https://doi.org/10.3390/cells12172127
Chicago/Turabian StyleGarcía-Bañuelos, Jesús, Edén Oceguera-Contreras, Ana Sandoval-Rodríguez, Blanca Estela Bastidas-Ramírez, Silvia Lucano-Landeros, Daniela Gordillo-Bastidas, Belinda C. Gómez-Meda, Arturo Santos, Eira Cerda-Reyes, and Juan Armendariz-Borunda. 2023. "AdhMMP8 Vector Administration in Muscle: An Alternate Strategy to Regress Hepatic Fibrosis" Cells 12, no. 17: 2127. https://doi.org/10.3390/cells12172127
APA StyleGarcía-Bañuelos, J., Oceguera-Contreras, E., Sandoval-Rodríguez, A., Bastidas-Ramírez, B. E., Lucano-Landeros, S., Gordillo-Bastidas, D., Gómez-Meda, B. C., Santos, A., Cerda-Reyes, E., & Armendariz-Borunda, J. (2023). AdhMMP8 Vector Administration in Muscle: An Alternate Strategy to Regress Hepatic Fibrosis. Cells, 12(17), 2127. https://doi.org/10.3390/cells12172127