Calcium-Activated Big-Conductance (BK) Potassium Channels Traffic through Nuclear Envelopes into Kinocilia in Ray Electrosensory Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ampullae Dissection and Whole-Mount Immunostaining
2.2. Site-Directed Mutagenesis
2.3. HEK293 Transfections and Immunofluorescence Staining
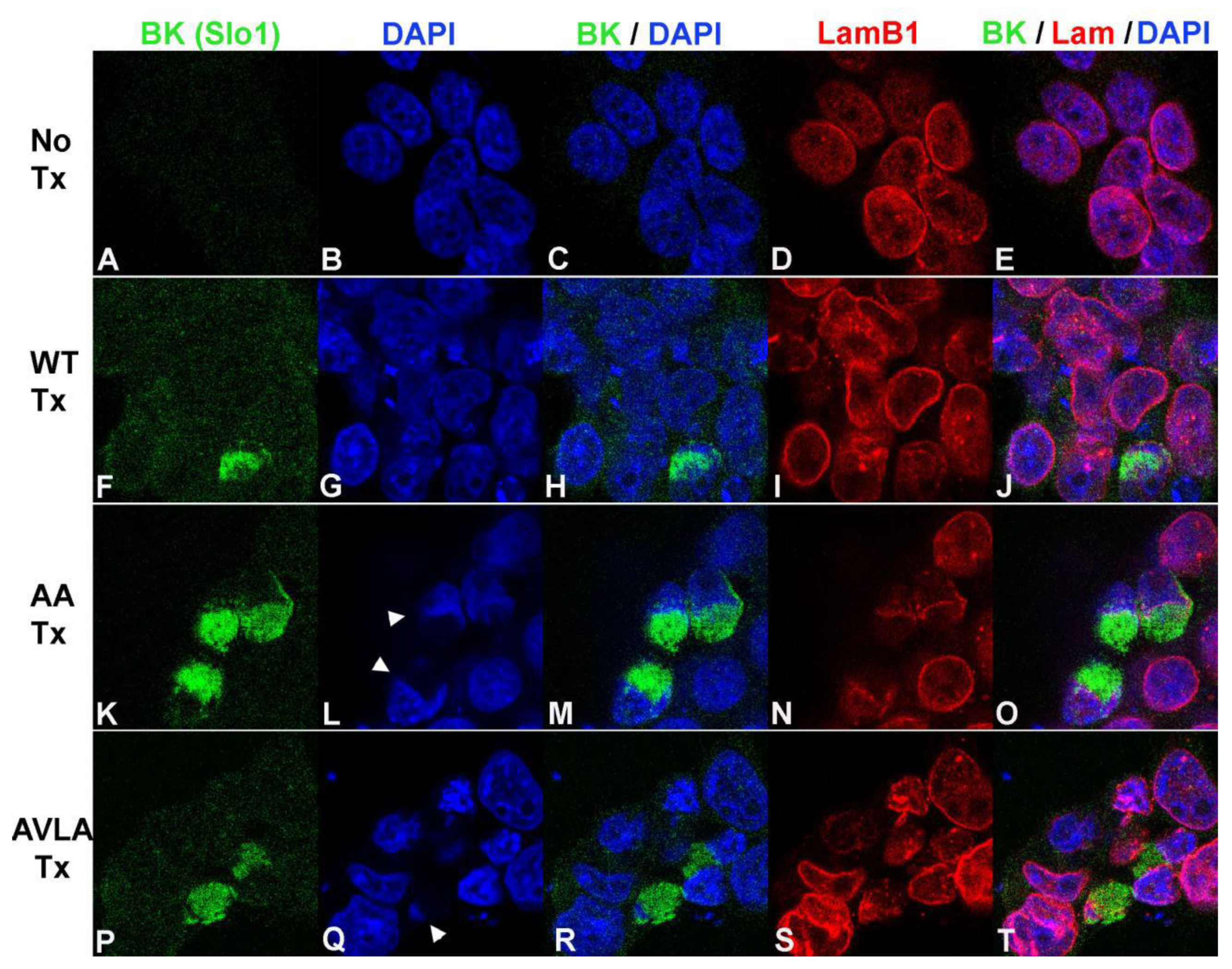
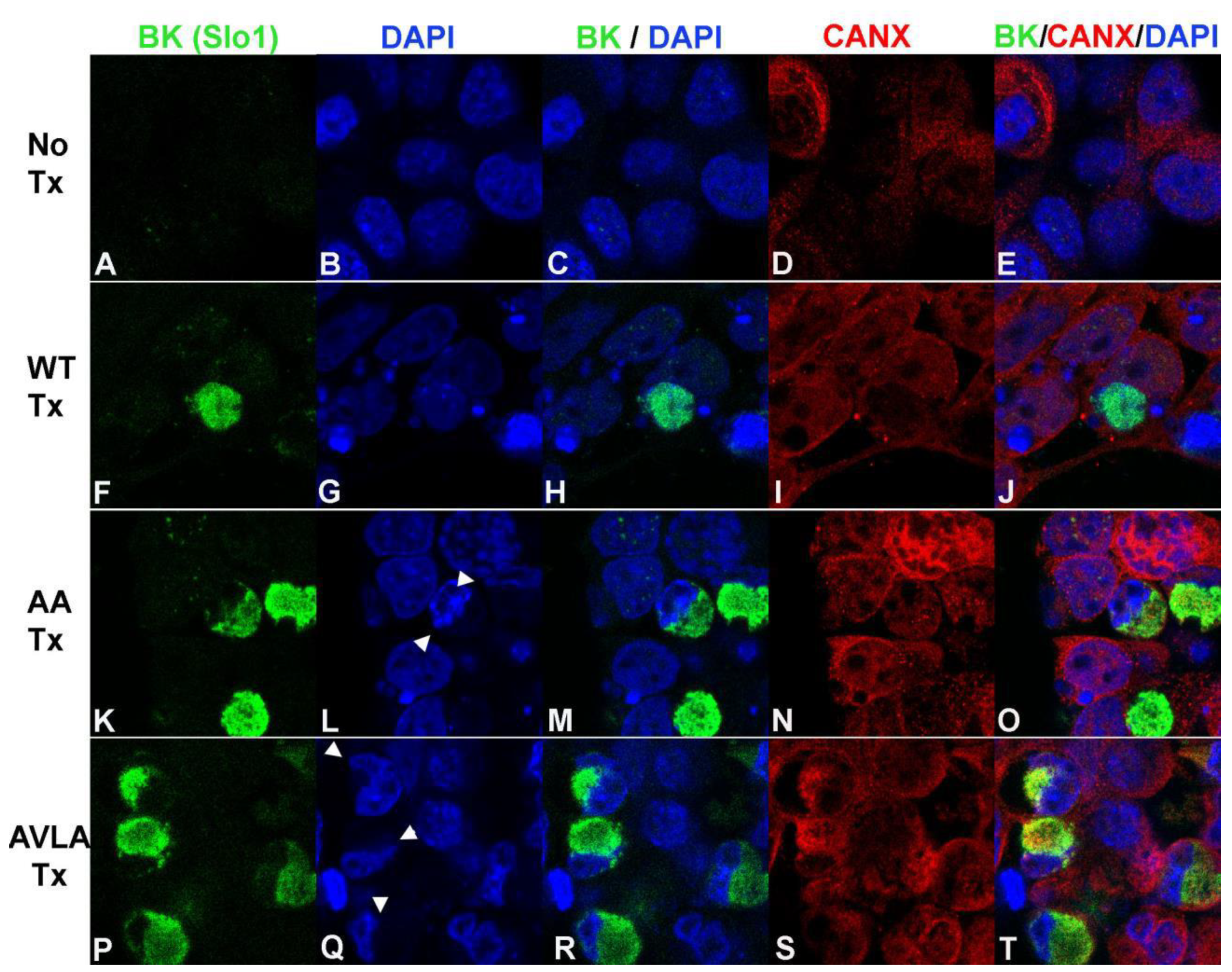
3. Results
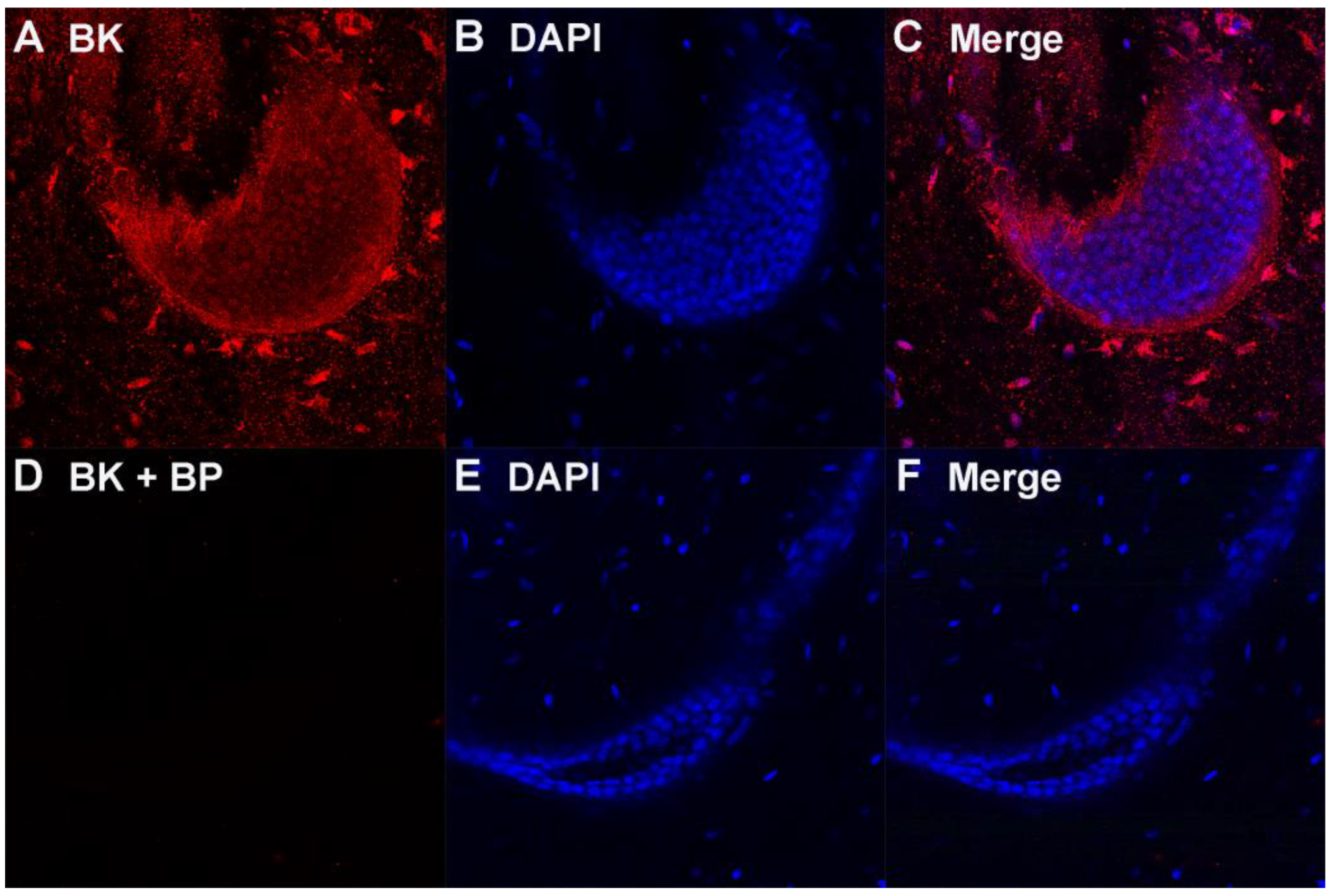
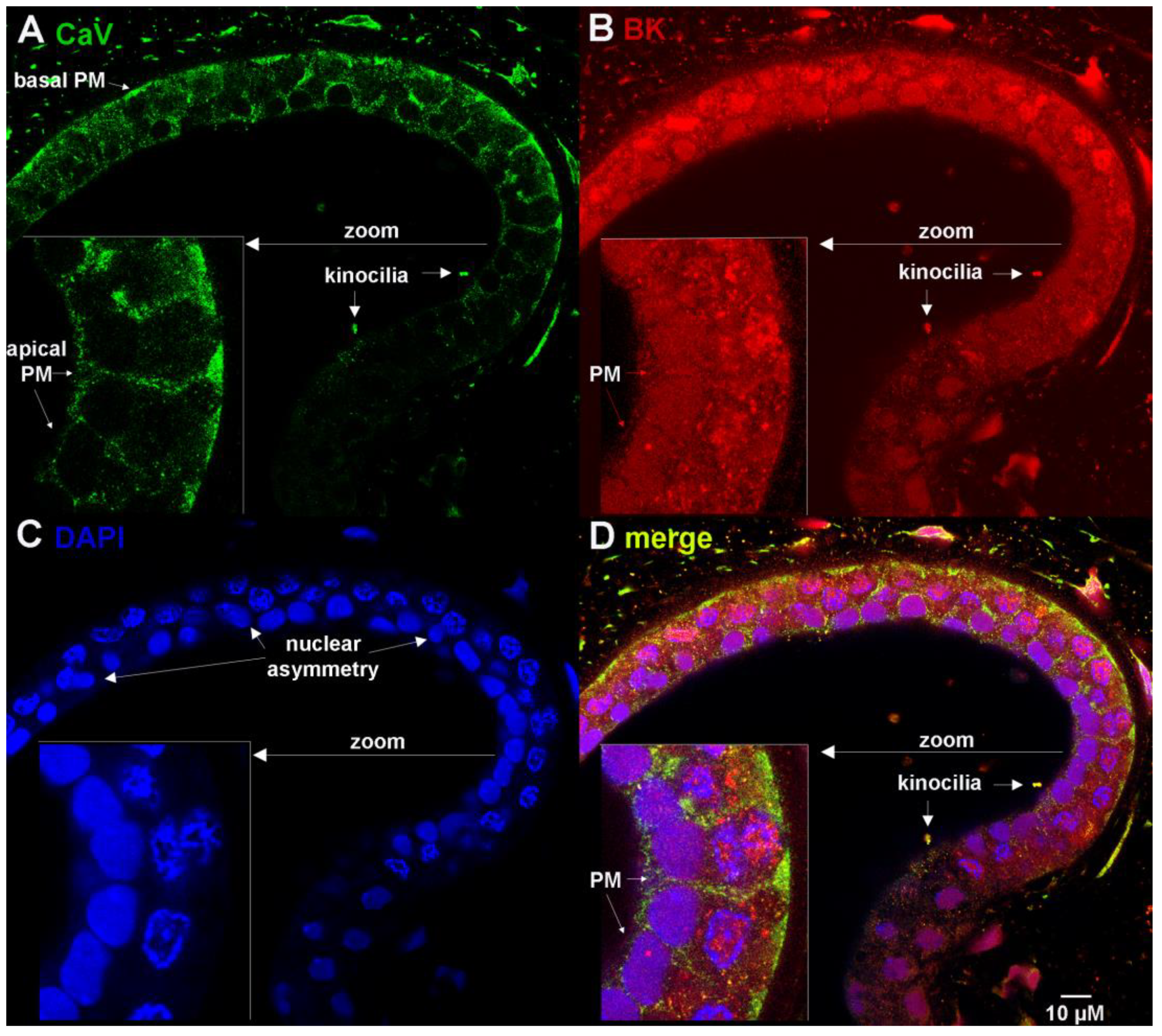
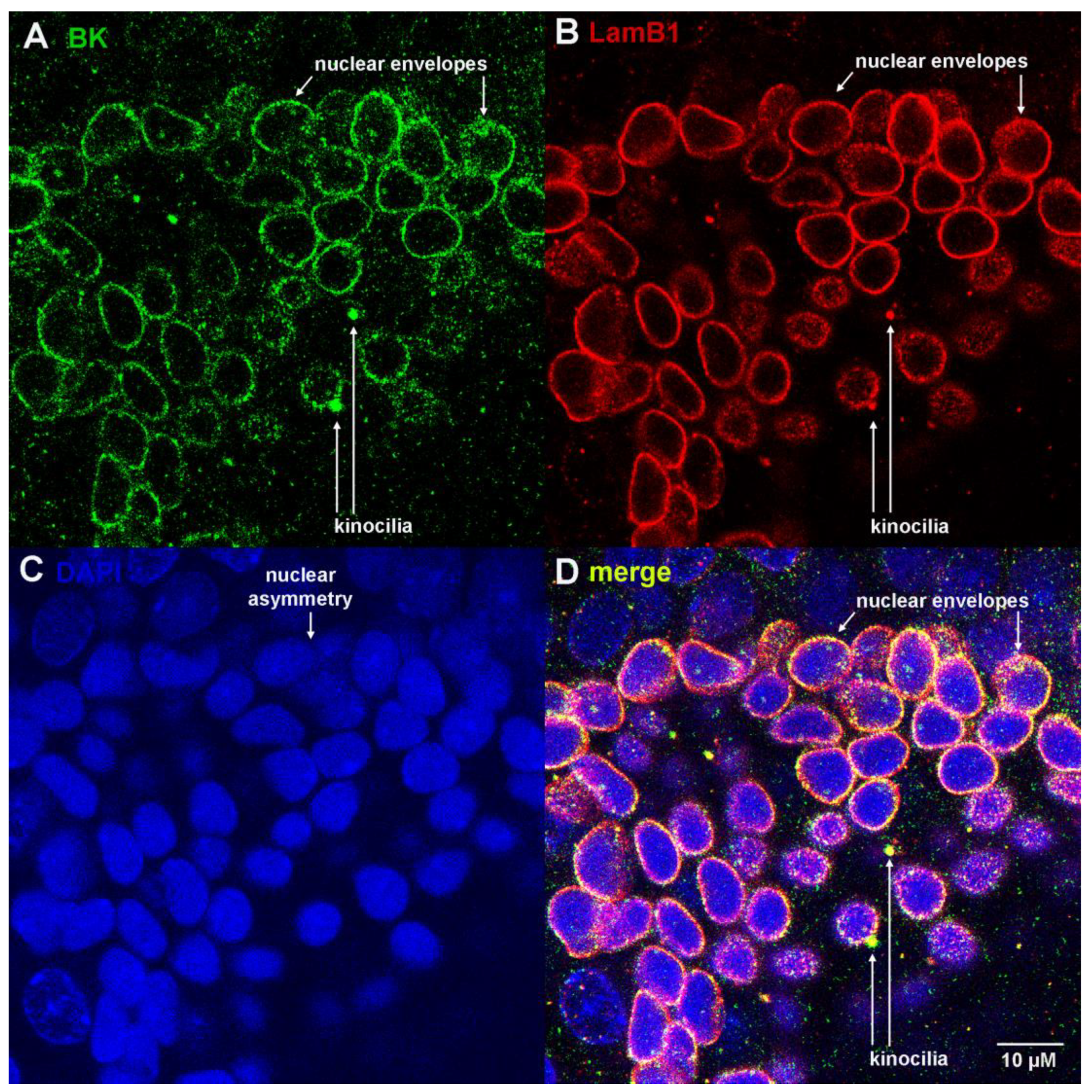
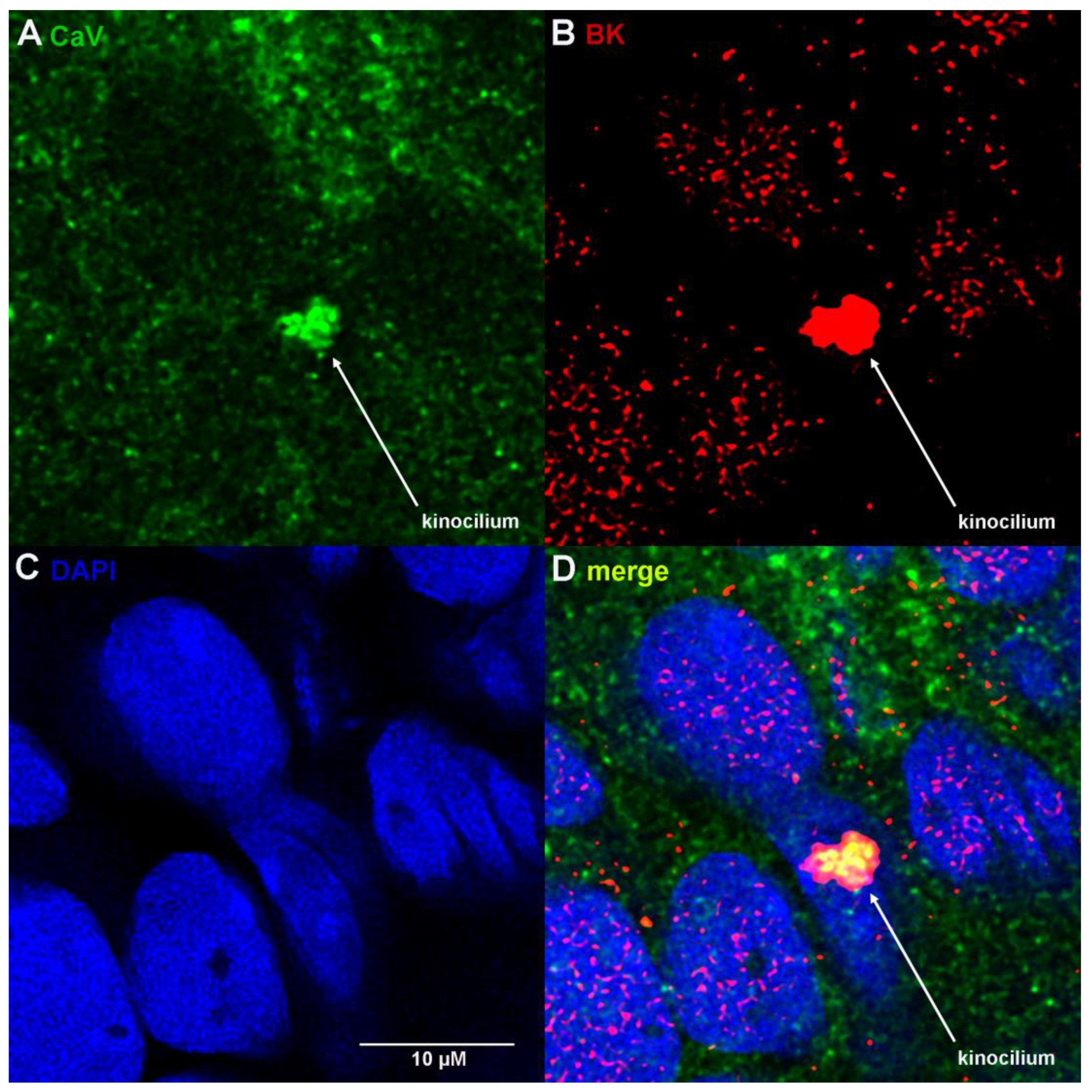
3.1. Ampulla Expression of BK, CaV1.3 and Lamin B1
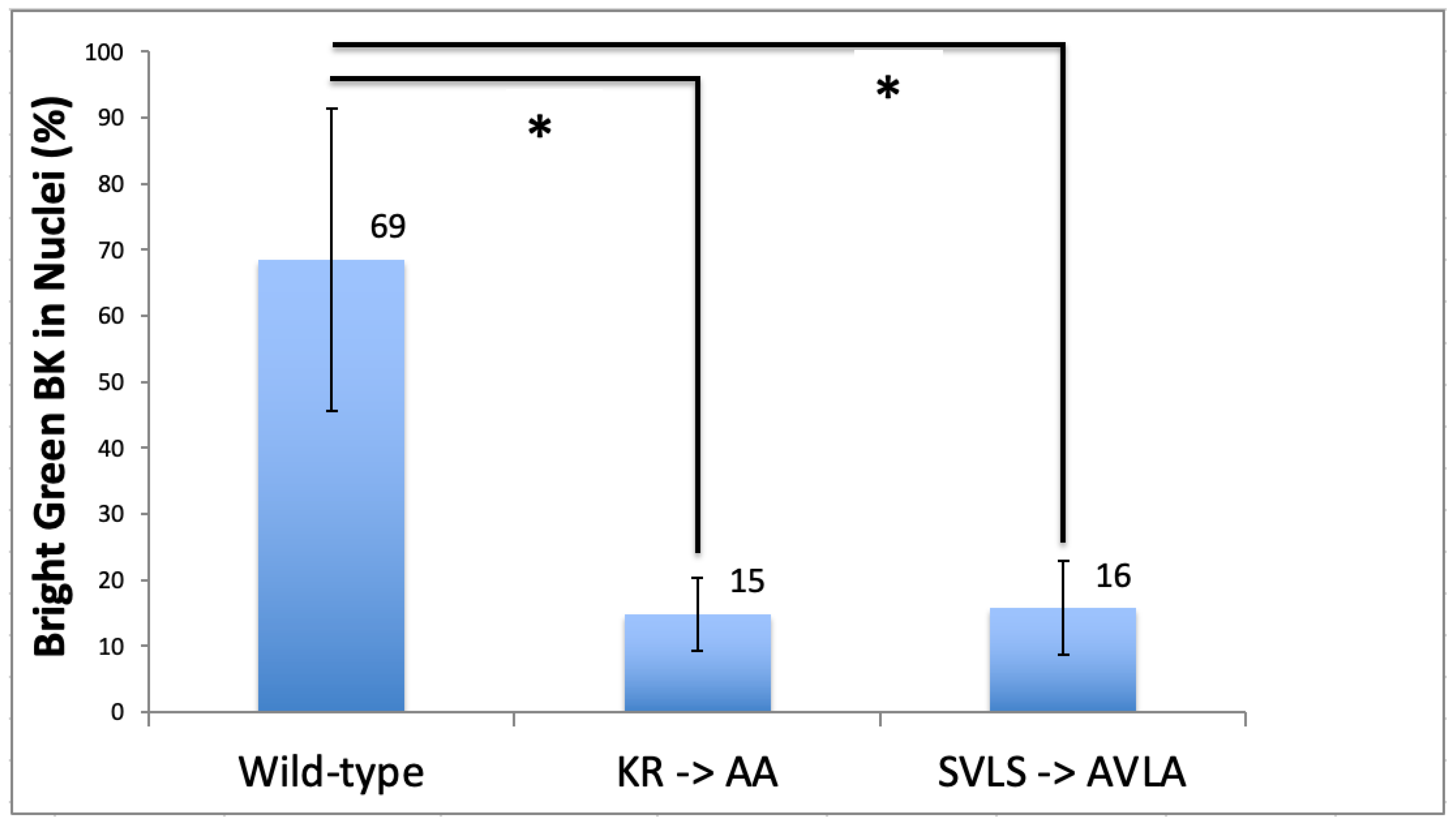
3.2. BK Nuclear Localization Sequence Characterization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalmijn, A.J. The electric sense of sharks and rays. J. Exp. Biol. 1971, 55, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Obara, S.; Bennett, M.V. Mode of operation of ampullae of Lorenzini of the skate, Raja. J. Gen. Physiol. 1972, 60, 534–557. [Google Scholar] [CrossRef] [PubMed]
- Sejnowski, T.J.; Yodlowski, M.L. A freeze-fracture study of the skate electroreceptor. J. Neurocytol. 1982, 11, 897–912. [Google Scholar] [CrossRef]
- Wueringer, B.E.; Tibbetts, I.R.; Whitehead, D.L. Ultrastructure of the ampullae of Lorenzini of Aptychotrema rostrata (Rhinobatidae). Zoomorphology 2009, 128, 45–52. [Google Scholar] [CrossRef]
- Gauthier, A.R.G.; Whitehead, D.L.; Tibbetts, I.R.; Cribb, B.W.; Bennett, M.B. Morphological comparison of the ampullae of Lorenzini of three sympatric benthic rays. J. Fish. Biol. 2018, 92, 504–514. [Google Scholar] [CrossRef]
- Clusin, W.; Spray, D.C.; Bennett, M.V. Activation of a voltage-insensitive conductance by inward calcium current. Nature 1975, 256, 425–427. [Google Scholar] [CrossRef]
- Clusin, W.T.; Bennett, M.V. Calcium-activated conductance in skate electroreceptors: Current clamp experiments. J. Gen. Physiol. 1977, 69, 121–143. [Google Scholar] [CrossRef]
- Clusin, W.T.; Bennett, M.V. The oscillatory responses of skate electroreceptors to small voltage stimuli. J. Gen. Physiol. 1979, 73, 685–702. [Google Scholar] [CrossRef]
- Berkefeld, H.; Fakler, B.; Schulte, U. Ca2+-activated K+ channels: From protein complexes to function. Physiol. Rev. 2010, 90, 1437–1459. [Google Scholar] [CrossRef]
- Latorre, R.; Castillo, K.; Carrasquel-Ursulaez, W.; Sepulveda, R.V.; Gonzalez-Nilo, F.; Gonzalez, C.; Alvarez, O. Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol. Rev. 2017, 97, 39–87. [Google Scholar] [CrossRef]
- King, B.L.; Shi, L.F.; Kao, P.; Clusin, W.T. Calcium activated K(+) channels in the electroreceptor of the skate confirmed by cloning. Details of subunits and splicing. Gene 2016, 578, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Leitch, D.B.; Julius, D. Molecular basis of ancestral vertebrate electroreception. Nature 2017, 543, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, M.; DeFelice, L.J.; Cohn, J.; Malter, H. Ion channels in the nuclear envelope. Nature 1990, 343, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Tseng-Crank, J.; Foster, C.D.; Krause, J.D.; Mertz, R.; Godinot, N.; DiChiara, T.J.; Reinhart, P.H. Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron 1994, 13, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tian, L.; MacDonald, S.H.; McClafferty, H.; Hammond, M.S.; Huibant, J.M.; Ruth, P.; Knaus, H.G.; Shipston, M.J. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J. Biol. Chem. 2005, 280, 33599–33609. [Google Scholar] [CrossRef]
- Miranda-Rottmann, S.; Kozlov, A.S.; Hudspeth, A.J. Highly specific alternative splicing of transcripts encoding BK channels in the chicken’s cochlea is a minor determinant of the tonotopic gradient. Mol. Cell. Biol. 2010, 30, 3646–3660. [Google Scholar] [CrossRef]
- Fedorenko, O.; Yarotskyy, V.; Duzhyy, D.; Marchenko, S. The large-conductance ion channels in the nuclear envelope of central neurons. Pflugers Arch. 2010, 460, 1045–1050. [Google Scholar] [CrossRef]
- Singh, H.; Stefani, E.; Toro, L. Intracellular BK(Ca) (iBK(Ca)) channels. J. Physiol. 2012, 590, 5937–5947. [Google Scholar] [CrossRef]
- Kyle, B.D.; Braun, A.P. The regulation of BK channel activity by pre- and post-translational modifications. Front. Physiol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Li, B.; Jie, W.; Huang, L.; Wei, P.; Li, S.; Luo, Z.; Friedman, A.K.; Meredith, A.L.; Han, M.H.; Zhu, X.H.; et al. Nuclear BK channels regulate gene expression via the control of nuclear calcium signaling. Nat. Neurosci. 2014, 17, 1055–1063. [Google Scholar] [CrossRef]
- Li, B.; Gao, T.M. Functional Role of Mitochondrial and Nuclear BK Channels. Int. Rev. Neurobiol. 2016, 128, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanabria, N.; Echeverria, F.; Segura, I.; Alvarado-Sanchez, R.; Latorre, R. BK in Double-Membrane Organelles: A Biophysical, Pharmacological, and Functional Survey. Front. Physiol. 2021, 12, 761474. [Google Scholar] [CrossRef] [PubMed]
- Berkefeld, H.; Sailer, C.A.; Bildl, W.; Rohde, V.; Thumfart, J.O.; Eble, S.; Klugbauer, N.; Reisinger, E.; Bischofberger, J.; Oliver, D.; et al. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 2006, 314, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Vivas, O.; Moreno, C.M.; Santana, L.F.; Hille, B. Proximal clustering between BK and CaV1.3 channels promotes functional coupling and BK channel activation at low voltage. Elife 2017, 6, e28029. [Google Scholar] [CrossRef]
- Nachury, M.V. How do cilia organize signalling cascades? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130465. [Google Scholar] [CrossRef]
- Falk, N.; Losl, M.; Schroder, N.; Giessl, A. Specialized Cilia in Mammalian Sensory Systems. Cells 2015, 4, 500–519. [Google Scholar] [CrossRef]
- Williams, D.B. Beyond lectins: The calnexin/calreticulin chaperone system of the endoplasmic reticulum. J. Cell Sci. 2006, 119, 615–623. [Google Scholar] [CrossRef]
- Malhas, A.; Goulbourne, C.; Vaux, D.J. The nucleoplasmic reticulum: Form and function. Trends Cell Biol. 2011, 21, 362–373. [Google Scholar] [CrossRef]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef]
- Shipston, M.J.; Duncan, R.R.; Clark, A.G.; Antoni, F.A.; Tian, L. Molecular components of large conductance calcium-activated potassium (BK) channels in mouse pituitary corticotropes. Mol. Endocrinol. 1999, 13, 1728–1737. [Google Scholar] [CrossRef]
- Yan, J.; Olsen, J.V.; Park, K.S.; Li, W.; Bildl, W.; Schulte, U.; Aldrich, R.W.; Fakler, B.; Trimmer, J.S. Profiling the phospho-status of the BKCa channel alpha subunit in rat brain reveals unexpected patterns and complexity. Mol. Cell Proteom. 2008, 7, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Lu, R.; Bopassa, J.C.; Meredith, A.L.; Stefani, E.; Toro, L. MitoBK(Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc. Natl. Acad. Sci. USA 2013, 110, 10836–10841. [Google Scholar] [CrossRef] [PubMed]
- Shelley, C.; Whitt, J.P.; Montgomery, J.R.; Meredith, A.L. Phosphorylation of a constitutive serine inhibits BK channel variants containing the alternate exon “SRKR”. J. Gen. Physiol. 2013, 142, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Katta, S.S.; Smoyer, C.J.; Jaspersen, S.L. Destination: Inner nuclear membrane. Trends Cell Biol. 2014, 24, 221–229. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yoshino, M.; Sui, J.L.; Wakui, M.; Kao, P.N.; Kao, C.Y. Potassium currents in freshly dissociated uterine myocytes from nonpregnant and late-pregnant rats. J. Gen. Physiol. 1998, 112, 737–756. [Google Scholar] [CrossRef]
- Eghbali, M.; Toro, L.; Stefani, E. Diminished surface clustering and increased perinuclear accumulation of large conductance Ca2+-activated K+ channel in mouse myometrium with pregnancy. J. Biol. Chem. 2003, 278, 45311–45317. [Google Scholar] [CrossRef]
- Goldman, R.D.; Shumaker, D.K.; Erdos, M.R.; Eriksson, M.; Goldman, A.E.; Gordon, L.B.; Gruenbaum, Y.; Khuon, S.; Mendez, M.; Varga, R.; et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 8963–8968. [Google Scholar] [CrossRef]
- Zironi, I.; Gavoci, E.; Lattanzi, G.; Virelli, A.; Amorini, F.; Remondini, D.; Castellani, G. BK channel overexpression on plasma membrane of fibroblasts from Hutchinson-Gilford progeria syndrome. Aging 2018, 10, 3148–3160. [Google Scholar] [CrossRef]
- Holder, M.; Schwitzgebel, V. Early Onset Diabetes in Two Children due to Progeria, a Monogenic Disease of DNA Repair. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 315–318. [Google Scholar] [CrossRef]
- Joukov, V.; De Nicolo, A. The Centrosome and the Primary Cilium: The Yin and Yang of a Hybrid Organelle. Cells 2019, 8, 701. [Google Scholar] [CrossRef]
- Johnson, C.A.; Malicki, J.J. The Nuclear Arsenal of Cilia. Dev. Cell 2019, 49, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Brugerolle, G.; Mignot, J.P. The rhizoplast of chrysomonads, a basal body-nucleus connector that polarises the dividing spindle. Protoplasma 2003, 222, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, J.L.; Lechtreck, K.F. Chlamydomonas Basal Bodies as Flagella Organizing Centers. Cells 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.R.; You, L.R.; Wang, W.J.; Huang, W.S.; Chu, C.T.; Chi, Y.H.; Chen, H.C. Lamin A-mediated nuclear lamina integrity is required for proper ciliogenesis. EMBO Rep. 2020, 21, e49680. [Google Scholar] [CrossRef] [PubMed]
| King, KJ756351 | KRIKKCGCKRL * * * * * * * * * * *QDENPSVLSPKKKQRNG |
| Bellono, KY355737 | KRIKKCGCKRPRYGYNGYLSTIQDENPSVLSPKKKQRNG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.L.; Wu, T.-H.; Shi, L.; Clusin, W.T.; Kao, P.N. Calcium-Activated Big-Conductance (BK) Potassium Channels Traffic through Nuclear Envelopes into Kinocilia in Ray Electrosensory Cells. Cells 2023, 12, 2125. https://doi.org/10.3390/cells12172125
Chen AL, Wu T-H, Shi L, Clusin WT, Kao PN. Calcium-Activated Big-Conductance (BK) Potassium Channels Traffic through Nuclear Envelopes into Kinocilia in Ray Electrosensory Cells. Cells. 2023; 12(17):2125. https://doi.org/10.3390/cells12172125
Chicago/Turabian StyleChen, Abby L., Ting-Hsuan Wu, Lingfang Shi, William T. Clusin, and Peter N. Kao. 2023. "Calcium-Activated Big-Conductance (BK) Potassium Channels Traffic through Nuclear Envelopes into Kinocilia in Ray Electrosensory Cells" Cells 12, no. 17: 2125. https://doi.org/10.3390/cells12172125
APA StyleChen, A. L., Wu, T.-H., Shi, L., Clusin, W. T., & Kao, P. N. (2023). Calcium-Activated Big-Conductance (BK) Potassium Channels Traffic through Nuclear Envelopes into Kinocilia in Ray Electrosensory Cells. Cells, 12(17), 2125. https://doi.org/10.3390/cells12172125






