Effect of Expansion Media on Functional Characteristics of Bone Marrow-Derived Mesenchymal Stromal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture of MSCs
2.1.1. Harvesting of Primary Material
2.1.2. Cell Expansion in Different Media
2.1.3. Determination of Glucose Consumption, Lactate Generation and Yield
2.2. Characterization of MSCs
2.2.1. Flow Cytometric Characterization of MSCs
2.2.2. Differentiation Assays
2.2.3. Scratch Wound Migration Assay
2.3. Proteome Analysis of MSCs and Media
2.4. Characterization of Media and Conditioned Media
2.4.1. Magnetic-Bead-Based Multiplex Analyses
2.4.2. TNF-Inducible Gene 6 (TSG-6) Enzyme-Linked Immune Sorbent Assay (ELISA)
2.5. Statistics
3. Results
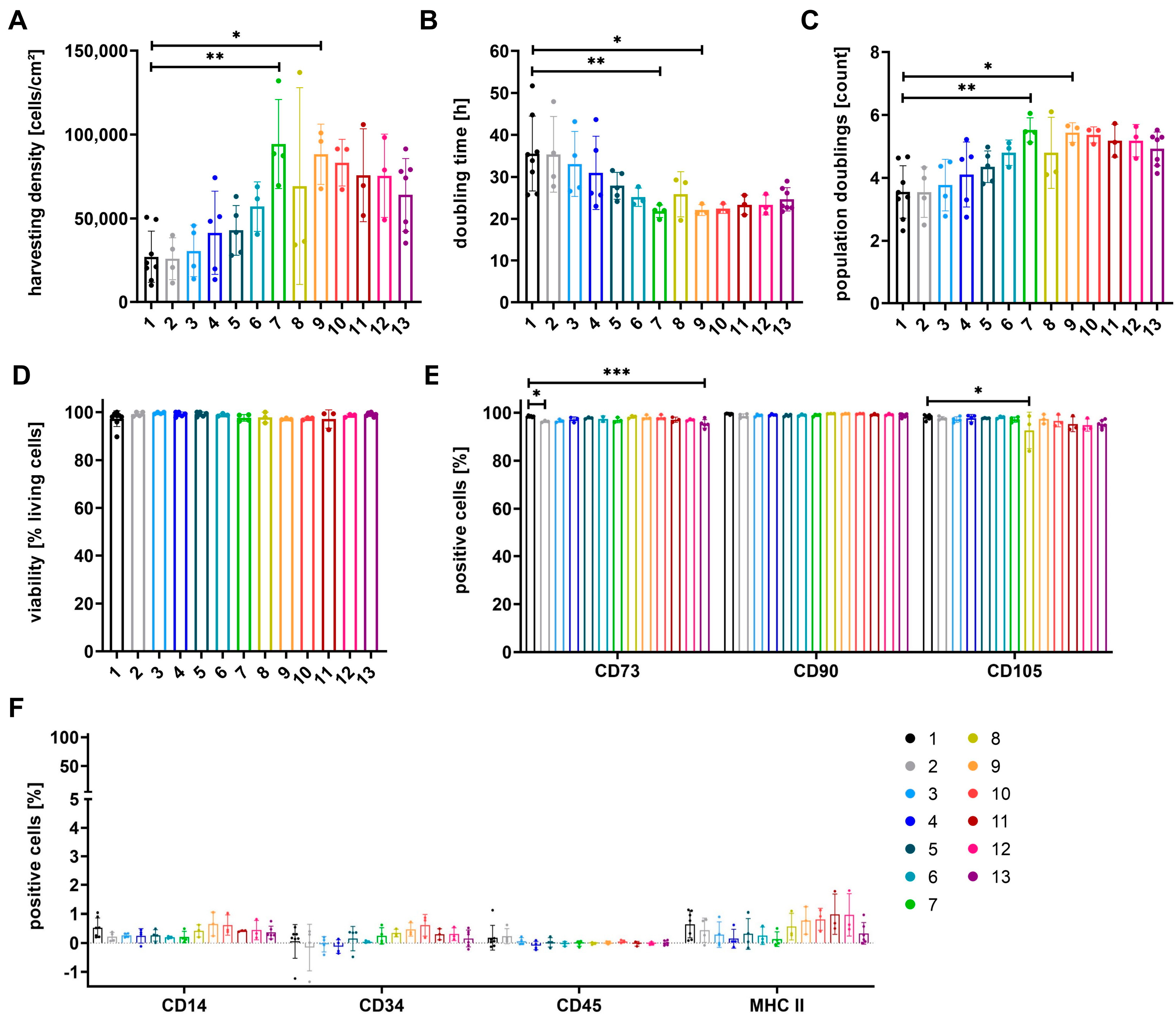
3.1. Proliferation of Cells Can Be Increased by Media Containing at Least 50% StemMACSTM
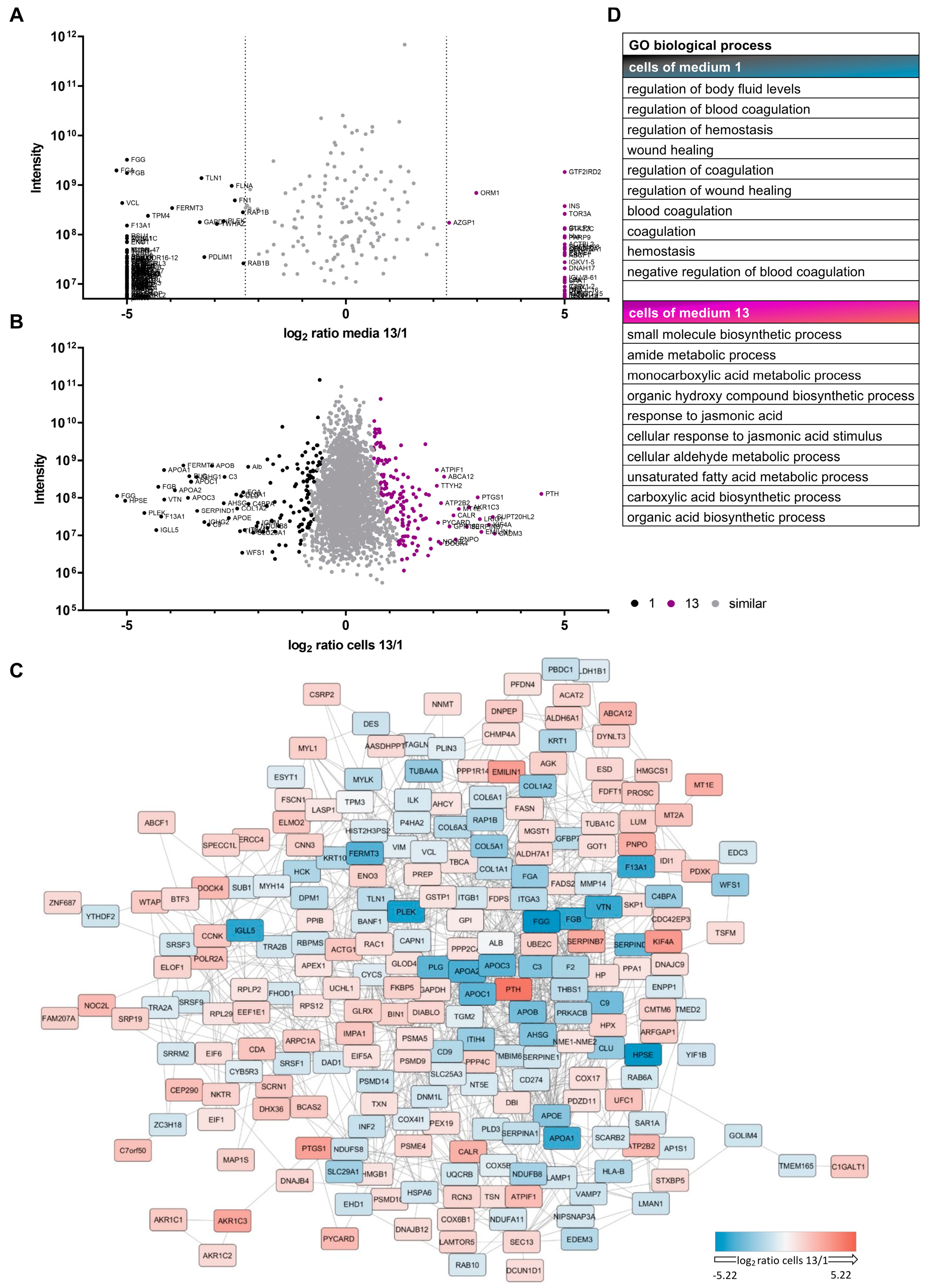
3.2. Proteomic Analyses Indicate Differences for Growth Media and Respective Cells
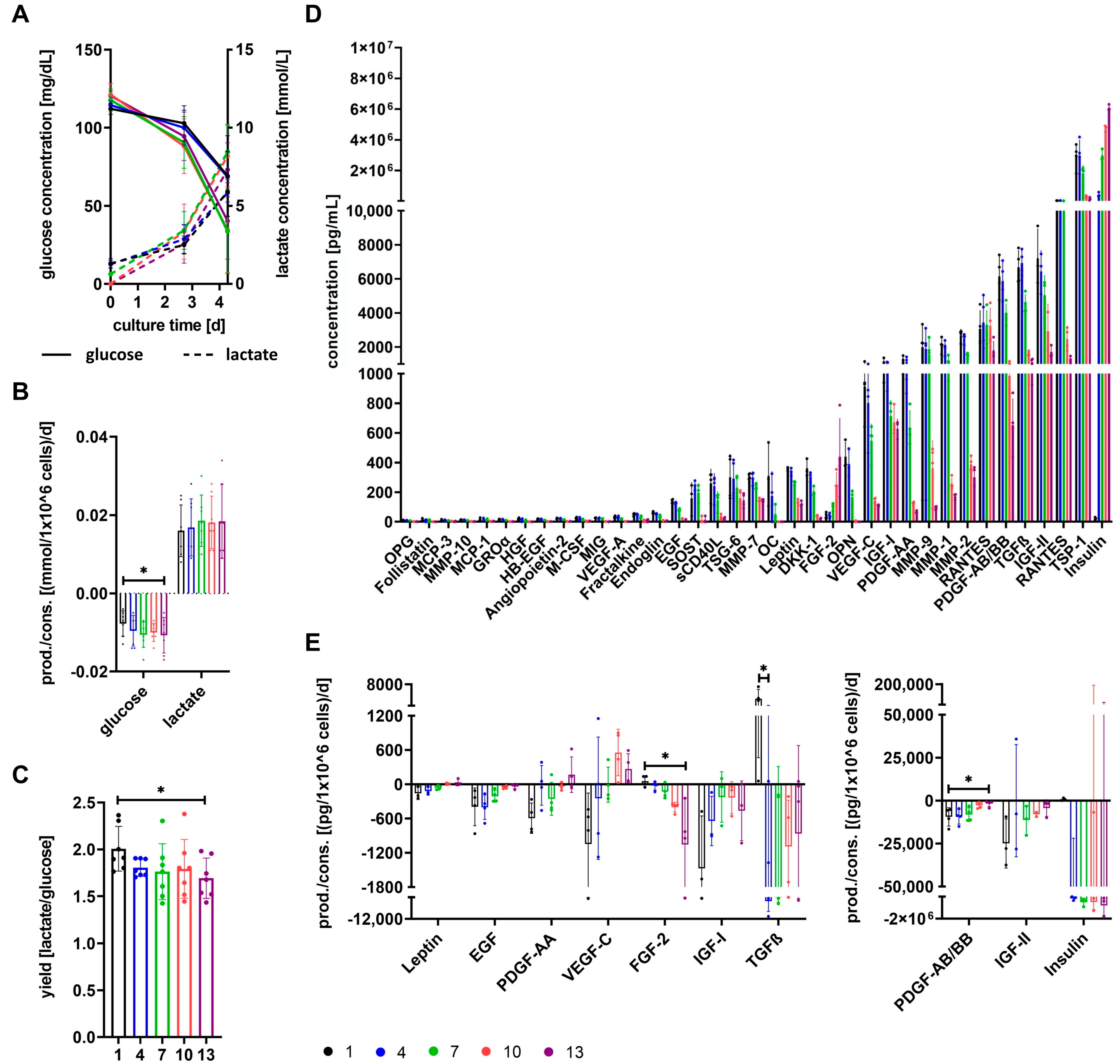
3.3. Cells Show Different Basic Metabolism Depending on Growth Media
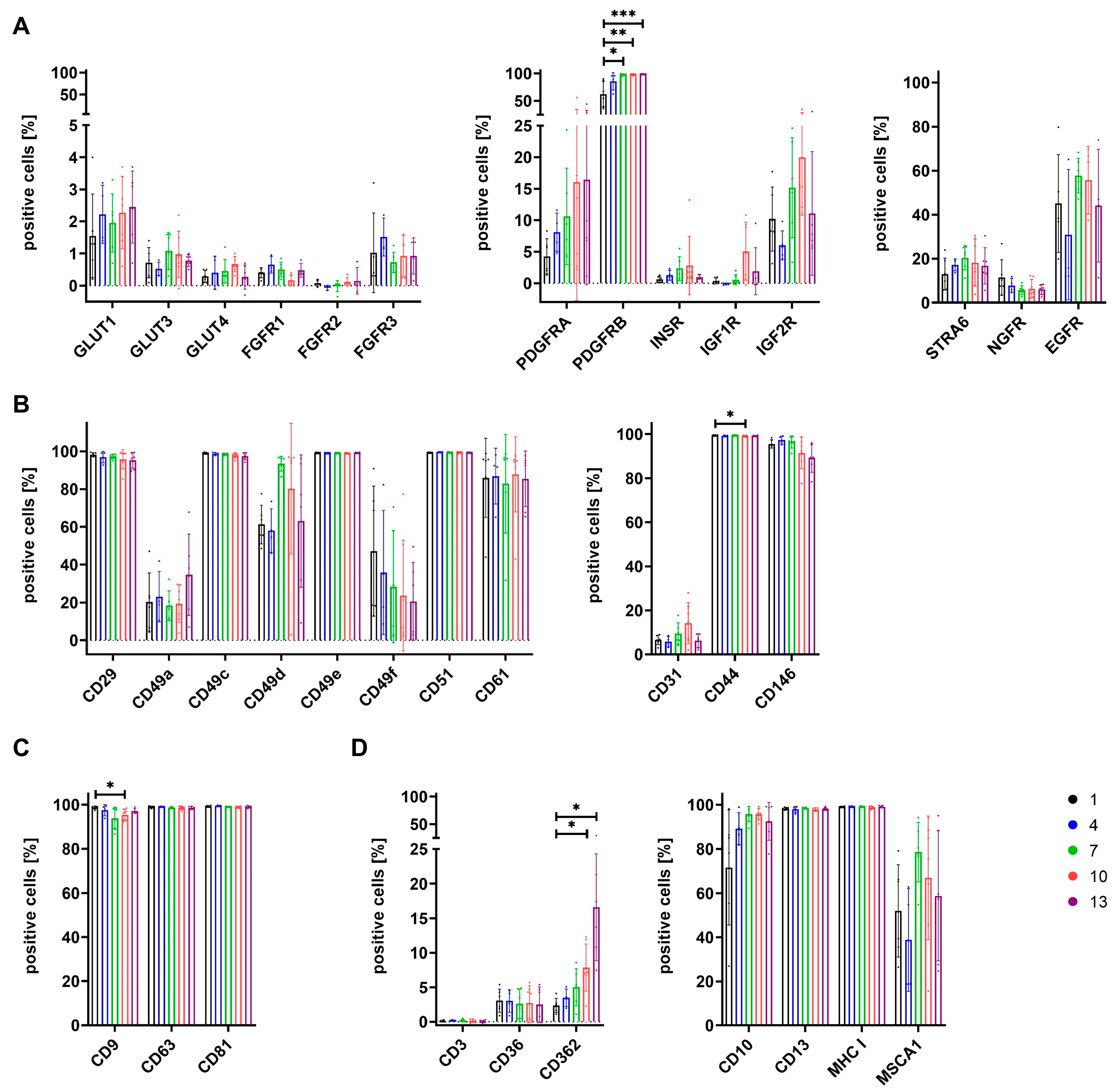
3.4. Expression of Surface Antigens Shows Alterations between Cells Grown in Different Media
3.5. The Secretome of Cells Varies after Expansion in Different Growth Media
3.6. Differentiation Potential of Cells Depends on Growth Media
3.7. SF/XF Culture Conditions Reduce Migratory Potential of MSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast Precursors in Normal and Irradiated Mouse Hematopoietic Organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Orozco, L.; Munar, A.; Huguet, M.; Lopez, R.; Vives, J.; Coll, R.; Codinach, M.; Garcia-Lopez, J. Final Results of a Phase I-Ii Trial Using Ex Vivo Expanded Autologous Mesenchymal Stromal Cells for the Treatment of Osteoarthritis of the Knee Confirming Safety and Suggesting Cartilage Regeneration. Knee 2016, 23, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell Therapy Induced Regeneration of Severely Atrophied Mandibular Bone in a Clinical Trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef]
- Gomez-Barrena, E.; Padilla-Eguiluz, N.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebe, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; et al. Early Efficacy Evaluation of Mesenchymal Stromal Cells (MSC) Combined to Biomaterials to Treat Long Bone Non-Unions. Injury 2020, 51 (Suppl. 1), S63–S73. [Google Scholar] [CrossRef]
- Gomez-Barrena, E.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebe, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; Padilla-Eguiluz, N.; et al. Feasibility and Safety of Treating Non-Unions in Tibia, Femur and Humerus with Autologous, Expanded, Bone Marrow-Derived Mesenchymal Stromal Cells Associated with Biphasic Calcium Phosphate Biomaterials in a Multicentric, Non-Comparative Trial. Biomaterials 2019, 196, 100–108. [Google Scholar] [CrossRef]
- Gomez-Barrena, E.; Padilla-Eguiluz, N.G.; Rosset, P.; Hernigou, P.; Baldini, N.; Ciapetti, G.; Gonzalo-Daganzo, R.M.; Avendano-Sola, C.; Rouard, H.; Giordano, R.; et al. Osteonecrosis of the Femoral Head Safely Healed with Autologous, Expanded, Bone Marrow-Derived Mesenchymal Stromal Cells in a Multicentric Trial with Minimum 5 Years Follow-Up. J. Clin. Med. 2021, 10, 508. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous Bone Marrow-Derived Cultured Mesenchymal Stem Cells Delivered in a Fibrin Spray Accelerate Healing in Murine and Human Cutaneous Wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Lataillade, J.J.; Doucet, C.; Bey, E.; Carsin, H.; Huet, C.; Clairand, I.; Bottollier-Depois, J.F.; Chapel, A.; Ernou, I.; Gourven, M.; et al. New Approach to Radiation Burn Treatment by Dosimetry-Guided Surgery Combined with Autologous Mesenchymal Stem Cell Therapy. Regen. Med. 2007, 2, 785–794. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Mitsuno, H.; Nonaka, I.; Sen, Y.; Kawanishi, K.; Inada, Y.; Takakura, Y.; Okuchi, K.; Nonomura, A. Wound Therapy by Marrow Mesenchymal Cell Transplantation. Plast. Reconstr. Surg. 2008, 121, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Dash, N.R.; Dash, S.N.; Routray, P.; Mohapatra, S.; Mohapatra, P.C. Targeting Nonhealing Ulcers of Lower Extremity In Human through Autologous Bone Marrow-Derived Mesenchymal Stem Cells. Rejuvenation Res. 2009, 12, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of Bone Marrow Mesenchymal Stem Cells with Bone Marrow-Derived Mononuclear Cells for Treatment of Diabetic Critical Limb Ischemia and Foot Ulcer: A Double-Blind, Randomized, Controlled Trial. Diabetes Res. Clin. Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous Mesenchymal Stem Cell Transplantation in Stroke Patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, N.K.; Kumar, S.K.; Balaraju, S.; Radhakrishnan, R.C.; Bansal, A.; Dixit, A.; Rao, D.K.; Das, M.; Jan, M.; Gupta, P.K.; et al. Open-Labeled Study of Unilateral Autologous Bone-Marrow-Derived Mesenchymal Stem Cell Transplantation in Parkinson’s Disease. Transl. Res. 2010, 155, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Barczewska, M.; Maksymowicz, S.; Zdolinska-Malinowska, I.; Siwek, T.; Grudniak, M. Umbilical Cord Mesenchymal Stem Cells in Amyotrophic Lateral Sclerosis: An Original Study. Stem Cell Rev. Rep. 2020, 16, 922–932. [Google Scholar] [CrossRef]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I.; Vaknin-Dembinsky, A.; Ben-Hur, T.; Offen, D.; Abramsky, O.; et al. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients with Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.; Petrou, P.; Ben-Hur, T.; Abramsky, O.; et al. Safety and Immunological Effects of Mesenchymal Stem Cell Transplantation in Patients with Multiple Sclerosis and Amyotrophic Lateral Sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Gotherstrom, C.; Hassan, M.; Uzunel, M.; Ringden, O. Treatment of Severe Acute Graft-Versus-Host Disease with Third Party Haploidentical Mesenchymal Stem Cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Boberg, E.; von Bahr, L.; Afram, G.; Lindstrom, C.; Ljungman, P.; Heldring, N.; Petzelbauer, P.; Garming Legert, K.; Kadri, N.; Le Blanc, K. Treatment of Chronic GvHD with Mesenchymal Stromal Cells Induces Durable Responses: A Phase Ii Study. Stem Cells Transl. Med. 2020, 9, 1190–1202. [Google Scholar] [CrossRef]
- Ringden, O.; Uzunel, M.; Rasmusson, I.; Remberger, M.; Sundberg, B.; Lonnies, H.; Marschall, H.U.; Dlugosz, A.; Szakos, A.; Hassan, Z.; et al. Mesenchymal Stem Cells for Treatment of Therapy-Resistant Graft-Versus-Host Disease. Transplantation 2006, 81, 1390–1397. [Google Scholar] [CrossRef]
- Dotoli, G.M.; De Santis, G.C.; Orellana, M.D.; de Lima Prata, K.; Caruso, S.R.; Fernandes, T.R.; Rensi Colturato, V.A.; Kondo, A.T.; Hamerschlak, N.; Simoes, B.P.; et al. Mesenchymal Stromal Cell Infusion to Treat Steroid-Refractory Acute Gvhd Iii/Iv after Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant. 2017, 52, 859–862. [Google Scholar] [CrossRef]
- Zhao, K.; Lou, R.; Huang, F.; Peng, Y.; Jiang, Z.; Huang, K.; Wu, X.; Zhang, Y.; Fan, Z.; Zhou, H.; et al. Immunomodulation Effects of Mesenchymal Stromal Cells on Acute Graft-Versus-Host Disease after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Guijo, F.; Caballero-Velazquez, T.; Lopez-Villar, O.; Redondo, A.; Parody, R.; Martinez, C.; Olavarria, E.; Andreu, E.; Prosper, F.; Diez-Campelo, M.; et al. Sequential Third-Party Mesenchymal Stromal Cell Therapy for Refractory Acute Graft-Versus-Host Disease. Biol. Blood Marrow Transplant. 2014, 20, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Muroi, K.; Miyamura, K.; Okada, M.; Yamashita, T.; Murata, M.; Ishikawa, T.; Uike, N.; Hidaka, M.; Kobayashi, R.; Imamura, M.; et al. Bone Marrow-Derived Mesenchymal Stem Cells (Jr-031) for Steroid-Refractory Grade Iii or Iv Acute Graft-Versus-Host Disease: A Phase Ii/Iii Study. Int. J. Hematol. 2016, 103, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in Mesenchymal Stromal Cells Induces In Vivo Recipient-Mediated Immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous Hmscs Improve Myocardial Infarction in Mice because Cells Embolized in Lung are Activated to Secrete the Anti-Inflammatory Protein Tsg-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Togel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered Mesenchymal Stem Cells Protect against Ischemic Acute Renal Failure through Differentiation-Independent Mechanisms. Am. J. Physiol. Ren. Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence Supporting Paracrine Hypothesis for Akt-Modified Mesenchymal Stem Cell-Mediated Cardiac Protection and Functional Improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Mildner, M.; Ankersmit, H.J. Cell Secretome based Drug Substances in Regenerative Medicine: When Regulatory Affairs Meet Basic Science. Ann. Transl. Med. 2017, 5, 170. [Google Scholar] [CrossRef]
- Pham, P.V.; Vu, N.B.; Pham, V.M.; Truong, N.H.; Pham, T.L.; Dang, L.T.; Nguyen, T.T.; Bui, A.N.; Phan, N.K. Good Manufacturing Practice-Compliant Isolation and Culture of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. J. Transl. Med. 2014, 12, 56. [Google Scholar] [CrossRef]
- Sundin, M.; Ringden, O.; Sundberg, B.; Nava, S.; Gotherstrom, C.; Le Blanc, K. No Alloantibodies against Mesenchymal Stromal Cells, but Presence of Anti-Fetal Calf Serum Antibodies, after Transplantation in Allogeneic Hematopoietic Stem Cell Recipients. Haematologica 2007, 92, 1208–1215. [Google Scholar] [CrossRef]
- van der Valk, J. Fetal Bovine Serum-a Cell Culture Dilemma. Science 2022, 375, 143–144. [Google Scholar] [CrossRef]
- Fekete, N.; Rojewski, M.T.; Lotfi, R.; Schrezenmeier, H. Essential Components for Ex Vivo Proliferation of Mesenchymal Stromal Cells. Tissue Eng. Part C Methods 2014, 20, 129–139. [Google Scholar] [CrossRef]
- Fekete, N.; Gadelorge, M.; Furst, D.; Maurer, C.; Dausend, J.; Fleury-Cappellesso, S.; Mailander, V.; Lotfi, R.; Ignatius, A.; Sensebe, L.; et al. Platelet Lysate from Whole Blood-Derived Pooled Platelet Concentrates and Apheresis-Derived Platelet Concentrates for the Isolation and Expansion of Human Bone Marrow Mesenchymal Stromal Cells: Production Process, Content and Identification of Active Components. Cytotherapy 2012, 14, 540–554. [Google Scholar] [PubMed]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human Platelet Lysate: Replacing Fetal Bovine Serum as a Gold Standard for Human Cell Propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Schallmoser, K.; Bartmann, C.; Rohde, E.; Reinisch, A.; Kashofer, K.; Stadelmeyer, E.; Drexler, C.; Lanzer, G.; Linkesch, W.; Strunk, D. Human Platelet Lysate can Replace Fetal Bovine Serum for Clinical-Scale Expansion of Functional Mesenchymal Stromal Cells. Transfusion 2007, 47, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Rojewski, M.T.; Lotfi, R.; Gjerde, C.; Mustafa, K.; Veronesi, E.; Ahmed, A.B.; Wiesneth, M.; Korper, S.; Sensebe, L.; Layrolle, P.; et al. Translation of a Standardized Manufacturing Protocol for Mesenchymal Stromal Cells: A Systematic Comparison of Validation and Manufacturing Data. Cytotherapy 2019, 21, 468–482. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, M.H.; Jang, J.E.; Lee, J.E.; Yang, Y.; Choi, J.Y.; Kang, H.S.; Lee, U.; Choung, J.W.; Jung, H.; et al. Comparative Analysis of Mesenchymal Stem Cells Cultivated in Serum Free Media. Sci. Rep. 2022, 12, 8620. [Google Scholar] [CrossRef]
- Xu, J.; Lian, W.; Wu, H.; Wang, X.; Chen, J.; Yang, L.; Zhuang, X.; Li, L.; Huang, Z. Improved Therapeutic Consistency and Efficacy of Mesenchymal Stem Cells Expanded with Chemically Defined Medium for Systemic Lupus Erythematosus. Cell. Mol. Immunol. 2020, 17, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Chase, L.G.; Yang, S.; Zachar, V.; Yang, Z.; Lakshmipathy, U.; Bradford, J.; Boucher, S.E.; Vemuri, M.C. Development and Characterization of a Clinically Compliant Xeno-Free Culture Medium in Good Manufacturing Practice for Human Multipotent Mesenchymal Stem Cells. Stem Cells Transl. Med. 2012, 1, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Panchalingam, K.M.; Rosenberg, L.; Behie, L.A. Ex Vivo Expansion of Human Mesenchymal Stem Cells in Defined Serum-Free Media. Stem Cells Int. 2012, 2012, 123030. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, L.R.; Myers, M.; Screven, R.; Liu, Z.; Boxer, L. A Serum-Free Medium Formulation Efficiently Supports Isolation and Propagation of Canine Adipose-Derived Mesenchymal Stem/Stromal Cells. PLoS ONE 2019, 14, e0210250. [Google Scholar] [CrossRef] [PubMed]
- Chase, L.G.; Lakshmipathy, U.; Solchaga, L.A.; Rao, M.S.; Vemuri, M.C. A Novel Serum-Free Medium for the Expansion of Human Mesenchymal Stem Cells. Stem Cell Res. Ther. 2010, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Miltenyi Biotec, B.V.; Co, K.G. Miltenyi Biotec’s Cell Culture Media Definition. Available online: https://www.miltenyibiotec.com/DE-en/products/macs-cell-culture-and-stimulation/definition-of-cell-culture-media.html (accessed on 8 February 2023).
- Bhat, S.; Viswanathan, P.; Chandanala, S.; Prasanna, S.J.; Seetharam, R.N. Expansion and Characterization of Bone Marrow Derived Human Mesenchymal Stromal Cells in Serum-Free Conditions. Sci. Rep. 2021, 11, 3403. [Google Scholar] [CrossRef] [PubMed]
- Dolley-Sonneville, P.J.; Romeo, L.E.; Melkoumian, Z.K. Synthetic Surface for Expansion of Human Mesenchymal Stem Cells in Xeno-Free, Chemically Defined Culture Conditions. PLoS ONE 2013, 8, e70263. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Tran, N.T.; Than, U.T.T.; Nguyen, M.Q.; Tran, A.M.; Do, P.T.X.; Chu, T.T.; Nguyen, T.D.; Bui, A.V.; Ngo, T.A.; et al. Optimization of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell Isolation and Culture Methods in Serum- and Xeno-Free Conditions. Stem Cell Res. Ther. 2022, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Amann, E.M.; Gross, A.; Rojewski, M.T.; Kestler, H.A.; Kalbitz, M.; Brenner, R.E.; Huber-Lang, M.; Schrezenmeier, H. Inflammatory Response of Mesenchymal Stromal Cells after In Vivo Exposure with Selected Trauma-Related Factors and Polytrauma Serum. PLoS ONE 2019, 14, e0216862. [Google Scholar] [CrossRef]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 Primes Human Mesenchymal Stem Cells towards an Anti-Inflammatory and Pro-Trophic Phenotype In Vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Saether, E.E.; Chamberlain, C.S.; Aktas, E.; Leiferman, E.M.; Brickson, S.L.; Vanderby, R. Primed Mesenchymal Stem Cells Alter and Improve Rat Medial Collateral Ligament Healing. Stem Cell Rev. Rep. 2016, 12, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Gorin, C.; Rochefort, G.Y.; Bascetin, R.; Ying, H.; Lesieur, J.; Sadoine, J.; Beckouche, N.; Berndt, S.; Novais, A.; Lesage, M.; et al. Priming Dental Pulp Stem Cells with Fibroblast Growth Factor-2 Increases Angiogenesis of Implanted Tissue-Engineered Constructs through Hepatocyte Growth Factor and Vascular Endothelial Growth Factor Secretion. Stem Cells Transl. Med. 2016, 5, 392–404. [Google Scholar] [CrossRef]
- Schop, D.; Janssen, F.W.; van Rijn, L.D.; Fernandes, H.; Bloem, R.M.; de Bruijn, J.D.; van Dijkhuizen-Radersma, R. Growth, Metabolism, and Growth Inhibitors of Mesenchymal Stem Cells. Tissue Eng. Part A 2009, 15, 1877–1886. [Google Scholar] [CrossRef]
- Krutzke, L.; Rosler, R.; Allmendinger, E.; Engler, T.; Wiese, S.; Kochanek, S. Process- and Product-Related Impurities in the Chadox1 Ncov-19 Vaccine. eLife 2022, 11, eLife.78513. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.; Berghaus, N.; Schreiner, S.; Hegenbart, U.; Schonland, S.O.; Wiese, S.; Huhn, S.; Haupt, C. Identification of Al Proteins from 10 Lambda-Al Amyloidosis Patients by Mass Spectrometry Extracted from Abdominal Fat and Heart Tissue. Amyloid 2023, 30, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. Maxquant Enables High Peptide Identification Rates, Individualized P.P.B.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A Peptide Search Engine Integrated into the Maxquant Environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. String V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, S.; Moradi, B.; Frank, S.; Dreher, T.; Kammerer, P.W.; Richter, W.; Gotterbarm, T. Different Culture Media Affect Growth Characteristics, Surface Marker Distribution and Chondrogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stromal Cells. BMC Musculoskelet. Disord. 2013, 14, 223. [Google Scholar] [CrossRef]
- Lensch, M.; Muise, A.; White, L.; Badowski, M.; Harris, D. Comparison of Synthetic Media Designed for Expansion of Adipose-Derived Mesenchymal Stromal Cells. Biomedicines 2018, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Boucher, S.; Koh, S.; Sastry, K.S.; Chase, L.; Lakshmipathy, U.; Choong, C.; Yang, Z.; Vemuri, M.C.; Rao, M.S.; et al. Pdgf, Tgf-Beta, and Fgf Signaling is Important for Differentiation and Growth of Mesenchymal Stem Cells (Mscs): Transcriptional Profiling can Identify Markers and Signaling Pathways Important in Differentiation of Mscs into Adipogenic, Chondrogenic, and Osteogenic Lineages. Blood 2008, 112, 295–307. [Google Scholar]
- Munger, J.S.; Harpel, J.G.; Gleizes, P.E.; Mazzieri, R.; Nunes, I.; Rifkin, D.B. Latent Transforming Growth Factor-Beta: Structural Features and Mechanisms of Activation. Kidney Int. 1997, 51, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Stamenkovic, I. Cell Surface-Localized Matrix Metalloproteinase-9 Proteolytically Activates Tgf-Beta and Promotes Tumor Invasion and Angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [CrossRef]
- Hahn, O.; Ingwersen, L.C.; Soliman, A.; Hamed, M.; Fuellen, G.; Wolfien, M.; Scheel, J.; Wolkenhauer, O.; Koczan, D.; Kamp, G.; et al. TGF-ß1 Induces Changes in the Energy Metabolism of White Adipose Tissue-Derived Human Adult Mesenchymal Stem/Stromal Cells In Vitro. Metabolites 2020, 10, 59. [Google Scholar] [CrossRef]
- Ullrich, A.; Schlessinger, J. Signal Transduction by Receptors with Tyrosine Kinase Activity. Cell 1990, 61, 203–212. [Google Scholar] [CrossRef]
- Li, P.; Wei, J.; Gao, X.; Wei, B.; Lin, H.; Huang, R.; Niu, Y.; Lim, K.; Jing, K.; Chu, J. Insulin Promotes the Proliferation of Human Umbilical Cord Matrix-Derived Mesenchymal Stem Cells by Activating the Akt-Cyclin D1 Axis. Stem Cells Int. 2017, 2017, 7371615. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Kim, M.; Park, J.B. Insulin-Like Growth Factor 2-Enhanced Osteogenic Differentiation of Stem Cell Spheroids by Regulation of Runx2 and Col1 Expression. Exp. Ther. Med. 2021, 21, 383. [Google Scholar] [CrossRef]
- Feng, J.; Meng, Z. Insulin Growth Factor-1 Promotes the Proliferation and Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells through the Wnt/Beta-Catenin Pathway. Exp. Ther. Med. 2021, 22, 891. [Google Scholar] [CrossRef]
- Le Roith, D. The Insulin-Like Growth Factor System. Exp. Diabesity Res. 2003, 4, 205–212. [Google Scholar] [CrossRef]
- Foulstone, E.; Prince, S.; Zaccheo, O.; Burns, J.L.; Harper, J.; Jacobs, C.; Church, D.; Hassan, A.B. Insulin-Like Growth Factor Ligands, Receptors, and Binding Proteins in Cancer. J. Pathol. 2005, 205, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Aboalola, D.; Han, V.K. The Roles of Insulin-Like Growth Factors in Mesenchymal Stem Cell Niche. Stem Cells Int. 2017, 2017, 9453108. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Leav, B.A.; Yang, Z.; Rasmussen, A.; Pearce, A.; Zweibel, J.A.; Lippman, M.E.; Cullen, K.J. Affinity for the Insulin-like Growth Factor-Ii (Igf-Ii) Receptor Inhibits Autocrine Igf-Ii Activity in Mcf-7 Breast Cancer Cells. Mol. Endocrinol. 1996, 10, 286–297. [Google Scholar] [PubMed]
- Chen, Z.; Ge, Y.; Kang, J.X. Down-Regulation of the M6p/Igf-Ii Receptor Increases Cell Proliferation and Reduces Apoptosis in Neonatal Rat Cardiac Myocytes. BMC Cell Biol. 2004, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary Passage is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ponnazhagan, S. Bone Homing of Mesenchymal Stem Cells by Ectopic Alpha 4 Integrin Expression. FASEB J. 2007, 21, 3917–3927. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dong, P.; Fu, X.; Li, Q.; Ma, S.; Wu, D.; Kang, N.; Liu, X.; Yan, L.; Xiao, R. Cd49f Acts as an Inflammation Sensor to Regulate Differentiation, Adhesion, and Migration of Human Mesenchymal Stem Cells. Stem Cells 2015, 33, 2798–2810. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Li, X.; Tian, G.; Yang, Z.; Sun, Z.; Yang, Y.; Wei, F.; Huang, B.; Jiang, S.; Li, H.; et al. Evaluation of Cd49f as a Novel Surface Marker to Identify Functional Adipose-Derived Mesenchymal Stem Cell Subset. Cell Prolif. 2021, 54, e13017. [Google Scholar] [CrossRef]
- Bowles, A.C.; Kouroupis, D.; Willman, M.A.; Perucca Orfei, C.; Agarwal, A.; Correa, D. Signature Quality Attributes of Cd146(+) Mesenchymal Stem/Stromal Cells Correlate with High Therapeutic and Secretory Potency. Stem Cells 2020, 38, 1034–1049. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Z.; Wu, D.; Kou, X.; Mao, X.; Shi, S. Cd146 Controls the Quality of Clinical Grade Mesenchymal Stem Cells from Human Dental Pulp. Stem Cell Res. Ther. 2021, 12, 488. [Google Scholar] [CrossRef]
- Wu, C.C.; Liu, F.L.; Sytwu, H.K.; Tsai, C.Y.; Chang, D.M. Cd146+ Mesenchymal Stem Cells Display Greater Therapeutic Potential than Cd146- Cells for Treating Collagen-Induced Arthritis in Mice. Stem Cell Res. Ther. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Bikorimana, J.P.; Saad, W.; Abusarah, J.; Lahrichi, M.; Talbot, S.; Shammaa, R.; Rafei, M. CD146 Defines a Mesenchymal Stromal Cell Subpopulation with Enhanced Suppressive Properties. Cells 2022, 11, 2263. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yu, J.M.; Joo, H.J.; Kim, H.K.; Cho, H.H.; Bae, Y.C.; Jung, J.S. Role of CD9 in Proliferation and Proangiogenic Action of Human Adipose-Derived Mesenchymal Stem Cells. Pflugers Arch. 2007, 455, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Masterson, C.; Devaney, J.; Horie, S.; O’Flynn, L.; Deedigan, L.; Elliman, S.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Syndecan-2-positive, Bone Marrow-derived Human Mesenchymal Stromal Cells Attenuate Bacterial-Induced Acute Lung Injury and Enhance Resolution of Ventilator-induced Lung Injury in Rats. Anesthesiology 2018, 129, 502–516. [Google Scholar] [CrossRef]
- Horie, S.; Masterson, C.; Brady, J.; Loftus, P.; Horan, E.; O’Flynn, L.; Elliman, S.; Barry, F.; O’Brien, T.; Laffey, J.G.; et al. Umbilical Cord-Derived CD362(+) Mesenchymal Stromal Cells for E. coli Pneumonia: Impact of Dose Regimen, Passage, Cryopreservation, and Antibiotic Therapy. Stem Cell Res. Ther. 2020, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Shi, Y.; Willis, G.; Imani, J.; Kwon, M.Y.; Li, G.; Ayaub, E.; Ghanta, S.; Ng, J.; Hwang, N.; et al. Mesenchymal Stromal Cell-Derived Syndecan-2 Regulates the Immune Response during Sepsis to Foster Bacterial Clearance and Resolution of Inflammation. FEBS J. 2022, 289, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Graneli, C.; Thorfve, A.; Ruetschi, U.; Brisby, H.; Thomsen, P.; Lindahl, A.; Karlsson, C. Novel Markers of Osteogenic and Adipogenic Differentiation of Human Bone Marrow Stromal Cells Identified Using a Quantitative Proteomics Approach. Stem Cell Res. 2014, 12, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Sobiesiak, M.; Sivasubramaniyan, K.; Hermann, C.; Tan, C.; Orgel, M.; Treml, S.; Cerabona, F.; de Zwart, P.; Ochs, U.; Muller, C.A.; et al. The Mesenchymal Stem Cell Antigen Msca-1 is Identical to Tissue Non-Specific Alkaline Phosphatase. Stem Cells Dev. 2010, 19, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Umrath, F.; Thomalla, C.; Poschel, S.; Schenke-Layland, K.; Reinert, S.; Alexander, D. Comparative Study of Msca-1 and Cd146 Isolated Periosteal Cell Subpopulations. Cell. Physiol. Biochem. 2018, 51, 1193–1206. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.G.; Moon, M.Y.; Jeon, C.Y.; Won, H.Y.; Kim, H.J.; Jeon, Y.J.; Seo, J.Y.; Kim, J.I.; Kim, J.; et al. Transforming Growth Factor-Beta1 Regulates Macrophage Migration via RhoA. Blood 2006, 108, 1821–1829. [Google Scholar] [CrossRef]
- Crane, J.L.; Cao, X. Bone marrow Mesenchymal Stem Cells and TGF-beta Signaling in Bone Remodeling. J. Clin. Invest. 2014, 124, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Qiu, H.; Xue, M.; Zhang, S.; Zhang, X.; Xu, J.; Chen, J.; Yang, Y.; Xie, J. MSC-Secreted TGF-Beta Regulates Lipopolysaccharide-Stimulated Macrophage M2-like Polarization via the Akt/FoxO1 Pathway. Stem Cell Res. Ther. 2019, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Lee, S.; Yu, Y.; Yoo, S.M.; Baek, S.Y.; Jung, N.; Seo, K.W.; Kang, K.S. TGF-Beta Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Ameliorates Atopic Dermatitis by Inhibiting Secretion of TNF-Alpha and IgE. Stem Cells 2020, 38, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, X.Y.; Song, T.; Zhang, L.; Eirin, A.; Conley, S.; Tang, H.; Saadiq, I.; Jordan, K.; Lerman, A.; et al. Mesenchymal Stem Cells Protect Renal Tubular Cells via TSG-6 Regulating Macrophage Function and Phenotype Switching. Am. J. Physiol. Renal Physiol. 2021, 320, F454–F463. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Vander Beken, S.; Wlaschek, M.; et al. TSG-6 Released from Intradermally Injected Mesenchymal Stem Cells Accelerates Wound Healing and Reduces Tissue Fibrosis in Murine Full-Thickness Skin Wounds. J. Investig. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef]
- Liu, L.; Song, H.; Duan, H.; Chai, J.; Yang, J.; Li, X.; Yu, Y.; Zhang, X.; Hu, X.; Xiao, M.; et al. TSG-6 Secreted by Human Umbilical cord-MSCs Attenuates Severe Burn-Induced Excessive Inflammation via Inhibiting Activations of P38 and JNK Signaling. Sci. Rep. 2016, 6, 30121. [Google Scholar] [CrossRef]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-Inflammatory Protein TSG-6 Secreted by Activated MSCs Attenuates Zymosan-Induced Mouse Peritonitis by Decreasing TLR2/NF-kappaB Signaling in Resident Macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Giri, J.; Das, R.; Nylen, E.; Chinnadurai, R.; Galipeau, J. CCL2 and CXCL12 Derived from Mesenchymal Stromal Cells Cooperatively Polarize IL-10+ Tissue Macrophages to Mitigate Gut Injury. Cell Rep. 2020, 30, 1923–1934.e4. [Google Scholar] [CrossRef]
- Whelan, D.S.; Caplice, N.M.; Clover, A.J.P. Mesenchymal Stromal Cell Derived CCL2 is Required for Accelerated Wound Healing. Sci. Rep. 2020, 10, 2642. [Google Scholar] [CrossRef]
- Papa, S.; Vismara, I.; Mariani, A.; Barilani, M.; Rimondo, S.; De Paola, M.; Panini, N.; Erba, E.; Mauri, E.; Rossi, F.; et al. Mesenchymal Stem Cells Encapsulated into Biomimetic Hydrogel Scaffold Gradually Release CCL2 Chemokine In Situ Preserving Cytoarchitecture and Promoting Functional Recovery in Spinal Cord Injury. J. Control. Release 2018, 278, 49–56. [Google Scholar] [CrossRef]
- Meng, H.; Wei, F.; Zhou, Y.; Hu, L.; Ge, Z.; Jin, J.; Wang, H.; Wu, C.T. Overexpression of Hepatocyte Growth Factor in Dental Pulp Stem Cells Ameliorates the Severity of Psoriasis by Reducing Inflammatory Responses. Stem Cells Dev. 2021, 30, 876–889. [Google Scholar] [CrossRef]
- Wang, H.; Sun, R.T.; Li, Y.; Yang, Y.F.; Xiao, F.J.; Zhang, Y.K.; Wang, S.X.; Sun, H.Y.; Zhang, Q.W.; Wu, C.T.; et al. HGF Gene Modification in Mesenchymal Stem Cells Reduces Radiation-Induced Intestinal Injury by Modulating Immunity. PLoS ONE 2015, 10, e0124420. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.P.; Han, D.M.; Zhao, L.; Guo, Z.K.; Xiao, F.J.; Zhang, Y.K.; Zhang, X.Y.; Wang, L.S.; Wang, H.X.; Wang, H. Hepatocyte Growth Factor Enhances the Inflammation-Alleviating Effect of Umbilical Cord-Derived Mesenchymal Stromal Cells in a Bronchiolitis Obliterans Model. Cytotherapy 2016, 18, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.F.; Zhao, L.; Xiao, F.J.; Zhang, Q.W.; Wen, M.L.; Wu, C.T.; Peng, R.Y.; Wang, L.S. Hepatocyte Growth Factor Gene-Modified Mesenchymal Stem Cells Reduce Radiation-Induced Lung Injury. Hum. Gene Ther. 2013, 24, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Yew, T.L.; Hung, Y.T.; Li, H.Y.; Chen, H.W.; Chen, L.L.; Tsai, K.S.; Chiou, S.H.; Chao, K.C.; Huang, T.F.; Chen, H.L.; et al. Enhancement of Wound Healing by Human Multipotent Stromal Cell Conditioned Medium: The Paracrine Factors and p38 MAPK Activation. Cell Transplant. 2011, 20, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Y.; Lu, Y.; Du, P.; Li, X.; Wang, C.; Guo, P.; Diao, L.; Lu, G. Mesenchymal Stromal Cells Pretreated with Proinflammatory Cytokines Enhance Skin Wound Healing via IL-6-Dependent M2 Polarization. Stem Cell Res. Ther. 2022, 13, 414. [Google Scholar] [CrossRef]
- Dorronsoro, A.; Lang, V.; Ferrin, I.; Fernandez-Rueda, J.; Zabaleta, L.; Perez-Ruiz, E.; Sepulveda, P.; Trigueros, C. Intracellular Role of IL-6 in Mesenchymal Stromal Cell Immunosuppression and Proliferation. Sci. Rep. 2020, 10, 21853. [Google Scholar] [CrossRef]
- Raffaghello, L.; Bianchi, G.; Bertolotto, M.; Montecucco, F.; Busca, A.; Dallegri, F.; Ottonello, L.; Pistoia, V. Human Mesenchymal Stem Cells Inhibit Neutrophil Apoptosis: A Model for Neutrophil Preservation in the Bone Marrow Niche. Stem Cells 2008, 26, 151–162. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent Mesenchymal Stromal Cells and the Innate Immune System. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Yang, S.; Xiao, Y.; Jia, Y.; Zhong, J.; Gao, Q.; Zhang, X. Umbilical Cord-Derived Mesenchymal Stem Cells Promote Myeloid-Derived Suppressor Cell Enrichment by Secreting CXCL1 to Prevent Graft-Versus-Host Disease after Hematopoietic Stem Cell Transplantation. Cytotherapy 2021, 23, 996–1006. [Google Scholar] [CrossRef]
- Huang, J.; Jochems, C.; Talaie, T.; Anderson, A.; Jales, A.; Tsang, K.Y.; Madan, R.A.; Gulley, J.L.; Schlom, J. Elevated Serum Soluble CD40 Ligand in Cancer Patients may Play an Immunosuppressive Role. Blood 2012, 120, 3030–3038. [Google Scholar] [CrossRef]
- Suga, H.; Eto, H.; Shigeura, T.; Inoue, K.; Aoi, N.; Kato, H.; Nishimura, S.; Manabe, I.; Gonda, K.; Yoshimura, K. IFATS Collection: Fibroblast Growth Factor-2-Induced Hepatocyte Growth Factor Secretion by Adipose-Derived Stromal Cells Inhibits Postinjury Fibrogenesis through a c-Jun N-Terminal Kinase-Dependent Mechanism. Stem Cells 2009, 27, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; Yasuda, H.; Yano, K.; Morinaga, T.; Higashio, K. RANK is the Essential Signaling Receptor for Osteoclast Differentiation Factor in Osteoclastogenesis. Biochem. Biophys. Res. Commun. 1998, 253, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Gori, F.; Hofbauer, L.C.; Dunstan, C.R.; Spelsberg, T.C.; Khosla, S.; Riggs, B.L. The Expression of Osteoprotegerin and RANK Ligand and the Support of Osteoclast Formation by Stromal-Osteoblast Lineage Cells is Developmentally Regulated. Endocrinology 2000, 141, 4768–4776. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Kim, K.S.; Bae, S.; Son, H.K.; Myung, P.K.; Hong, H.J.; Kim, H. Cytokine Secretion Profiling of Human Mesenchymal Stem Cells by Antibody Array. Int. J. Stem Cells 2009, 2, 59–68. [Google Scholar] [CrossRef]
- Qiao, B.; Shui, W.; Cai, L.; Guo, S.; Jiang, D. Human Mesenchymal Stem Cells as Delivery of Osteoprotegerin Gene: Homing and Therapeutic Effect for Osteosarcoma. Drug Des. Dev. Ther. 2015, 9, 969–976. [Google Scholar] [CrossRef]
- Oshita, K.; Yamaoka, K.; Udagawa, N.; Fukuyo, S.; Sonomoto, K.; Maeshima, K.; Kurihara, R.; Nakano, K.; Saito, K.; Okada, Y.; et al. Human Mesenchymal Stem Cells Inhibit Osteoclastogenesis through Osteoprotegerin Production. Arthritis Rheum. 2011, 63, 1658–1667. [Google Scholar] [CrossRef]
- Cho, K.A.; Park, M.; Kim, Y.H.; Ryu, K.H.; Woo, S.Y. Mesenchymal Stem Cells Inhibit RANK-RANKL Interactions between Osteoclasts and Th17 Cells via Osteoprotegerin Activity. Oncotarget 2017, 8, 83419–83431. [Google Scholar] [CrossRef]
- Prystaz, K.; Kaiser, K.; Kovtun, A.; Haffner-Luntzer, M.; Fischer, V.; Rapp, A.E.; Liedert, A.; Strauss, G.; Waetzig, G.H.; Rose-John, S.; et al. Distinct Effects of IL-6 Classic and Trans-Signaling in Bone Fracture Healing. Am. J. Pathol. 2018, 188, 474–490. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xia, X.; Yeh, J.; Kua, H.; Liu, H.; Mishina, Y.; Hao, A.; Li, B. PDGF-AA Promotes Osteogenic Differentiation and Migration of Mesenchymal Stem Cell by Down-Regulating PDGFRalpha and Derepressing BMP-Smad1/5/8 Signaling. PLoS ONE 2014, 9, e113785. [Google Scholar]
- Zheng, B.; Jiang, J.; Luo, K.; Liu, L.; Lin, M.; Chen, Y.; Yan, F. Increased Osteogenesis in Osteoporotic Bone Marrow Stromal Cells by Overexpression of Leptin. Cell Tissue Res. 2015, 361, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Jing, Y.; Zhang, Y.; Yue, Z.; Hu, X.; Wang, L.; Liang, J.; Liu, J. Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Adenovirus-Mediated Expression of Leptin. Regul. Pept. 2010, 163, 107–112. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Huang, Y.J.; Wu, H.H.; Liu, Y.A.; Liu, Y.S.; Lee, O.K. Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef]
- Liu, Y.C.; Kao, Y.T.; Huang, W.K.; Lin, K.Y.; Wu, S.C.; Hsu, S.C.; Schuyler, S.C.; Li, L.Y.; Leigh Lu, F.; Lu, J. CCL5/RANTES is Important for Inducing Osteogenesis of Human Mesenchymal Stem Cells and is Regulated by Dexamethasone. Biosci. Trends 2014, 8, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zhang, L.; Xu, C.; Zheng, C.; Han, Y.; Li, W.; Huang, Y.; Zhang, X.; Shao, C.; et al. An Osteopontin-Integrin Interaction Plays a Critical Role in Directing Adipogenesis and Osteogenesis by Mesenchymal Stem Cells. Stem Cells 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, Y.; Zhang, X.; Chu, Z.; Liu, Y.; Tang, C. MMP-1 Promotes Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells via the JNK and ERK Pathway. Int. J. Biochem. Cell Biol. 2020, 129, 105880. [Google Scholar] [CrossRef]
- Nakamura, A.; Dohi, Y.; Akahane, M.; Ohgushi, H.; Nakajima, H.; Funaoka, H.; Takakura, Y. Osteocalcin Secretion as an Early Marker of In Vitro Osteogenic Differentiation of Rat Mesenchymal Stem Cells. Tissue Eng. Part C Methods 2009, 15, 169–180. [Google Scholar] [CrossRef]
- Pinzone, J.J.; Hall, B.M.; Thudi, N.K.; Vonau, M.; Qiang, Y.W.; Rosol, T.J.; Shaughnessy, J.D., Jr. The Role of Dickkopf-1 in Bone Development, Homeostasis, and Disease. Blood 2009, 113, 517–525. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-beta1-Induced Migration of Bone Mesenchymal Stem Cells Couples Bone Resorption with Formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef]
- Wan, M.; Li, C.; Zhen, G.; Jiao, K.; He, W.; Jia, X.; Wang, W.; Shi, C.; Xing, Q.; Chen, Y.F.; et al. Injury-Activated Transforming Growth Factor Beta Controls Mobilization of Mesenchymal Stem Cells for Tissue Remodeling. Stem Cells 2012, 30, 2498–2511. [Google Scholar] [CrossRef] [PubMed]
- Alliston, T.; Choy, L.; Ducy, P.; Karsenty, G.; Derynck, R. TGF-Beta-Induced Repression of CBFA1 by Smad3 Decreases cbfa1 and Osteocalcin Expression and Inhibits Osteoblast Differentiation. EMBO J. 2001, 20, 2254–2272. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.S.; Ferrara, N.; Orci, L.; Montesano, R. Potent Synergism between Vascular Endothelial Growth Factor and Basic Fibroblast Growth Factor in the Induction of Angiogenesis In Vitro. Biochem. Biophys. Res. Commun. 1992, 189, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Aizman, I.; Vinodkumar, D.; McGrogan, M.; Bates, D. Cell Injury-Induced Release of Fibroblast Growth Factor 2: Relevance to Intracerebral Mesenchymal Stromal Cell Transplantations. Stem Cells Dev. 2015, 24, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Bhang, S.H.; Yang, H.S.; La, W.G.; Yoon, H.H.; Shin, J.Y.; Seong, J.Y.; Shin, H.; Kim, B.S. Enhancement of Long-Term Angiogenic Efficacy of Adipose Stem Cells by Delivery of FGF2. Microvasc. Res. 2012, 84, 1–8. [Google Scholar] [CrossRef]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast Growth Factor-2 (FGF-2) Induces Vascular Endothelial Growth Factor (VEGF) Expression in the Endothelial Cells of Forming Capillaries: An Autocrine Mechanism Contributing to Angiogenesis. J. Cell Biol. 1998, 141, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Zhang, H.; Hou, J.; Wan, L.; Cheng, W.; Wang, X.; Dong, D.; Chen, C.; Xia, J.; Guo, J.; et al. VEGF Secreted by Mesenchymal Stem Cells Mediates the Differentiation of Endothelial Progenitor Cells into Endothelial Cells via Paracrine Mechanisms. Mol. Med. Rep. 2018, 17, 1667–1675. [Google Scholar] [CrossRef]
- Lee, E.J.; Choi, E.K.; Kang, S.K.; Kim, G.H.; Park, J.Y.; Kang, H.J.; Lee, S.W.; Kim, K.H.; Kwon, J.S.; Lee, K.H.; et al. N-Cadherin Determines Individual Variations in the Therapeutic Efficacy of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells in a Rat Model of Myocardial Infarction. Mol. Ther. 2012, 20, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Hur, S.M.; Park, K.Y.; Kim, C.K.; Kim, Y.M.; Kim, H.S.; Shin, H.C.; Won, M.H.; Ha, K.S.; Kwon, Y.G.; et al. Multiple Paracrine Factors Secreted by Mesenchymal Stem Cells Contribute to Angiogenesis. Vascul Pharmacol. 2014, 63, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, M.A.; Shevchenko, E.K.; Molokotina, Y.D.; Makarevich, P.I.; Beloglazova, I.B.; Zubkova, E.S.; Dergilev, K.V.; Tsokolaeva, Z.I.; Penkov, D.; Hsu, M.N.; et al. Transplantation of Adipose Stromal Cell Sheet Producing Hepatocyte Growth Factor Induces Pleiotropic Effect in Ischemic Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 3088. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, J.; Wu, J.; Yang, L.; Dai, S.; Tan, X.; Liang, L. Locally Overexpressing Hepatocyte Growth Factor Prevents Post-Ischemic Heart Failure by Inhibition of Apoptosis via Calcineurin-Mediated Pathway and Angiogenesis. Arch. Med. Res. 2008, 39, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.F.; Wu, C.T.; Wu, D.L.; Lu, Y.; Liu, H.J.; Ha, X.Q.; Zhang, Q.W.; Wang, H.; Jia, X.X.; Wang, L.S. Treatment of Myocardial Ischemia with Bone Marrow-Derived Mesenchymal Stem Cells Overexpressing Hepatocyte Growth Factor. Mol. Ther. 2003, 8, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Su, G.H.; Sun, Y.F.; Lu, Y.X.; Shuai, X.X.; Liao, Y.H.; Liu, Q.Y.; Han, J.; Luo, P. Hepatocyte Growth Factor Gene-Modified Bone Marrow-Derived Mesenchymal Stem Cells Transplantation Promotes Angiogenesis in a Rat Model of Hindlimb Ischemia. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Kim, P.H.; Cho, H.M.; Yum, S.Y.; Choi, Y.J.; Son, Y.; Lee, D.; Kang, I.; Kang, K.S.; Jang, G.; et al. Inducible HGF-secreting Human Umbilical Cord Blood-derived MSCs Produced via TALEN-mediated Genome Editing Promoted Angiogenesis. Mol. Ther. 2016, 24, 1644–1654. [Google Scholar] [CrossRef]
- Fahmy-Garcia, S.; Farrell, E.; Witte-Bouma, J.; Robbesom-van den Berge, I.; Suarez, M.; Mumcuoglu, D.; Walles, H.; Kluijtmans, S.; van der Eerden, B.C.J.; van Osch, G.; et al. Follistatin Effects in Migration, Vascularization, and Osteogenesis in vitro and Bone Repair in vivo. Front. Bioeng. Biotechnol. 2019, 7, 38. [Google Scholar] [CrossRef]
- Park, H.Y.; Kwon, H.M.; Lim, H.J.; Hong, B.K.; Lee, J.Y.; Park, B.E.; Jang, Y.; Cho, S.Y.; Kim, H.S. Potential role of leptin in angiogenesis: Leptin Induces Endothelial Cell Proliferation and Expression of Matrix Metalloproteinases In Vivo and In Vitro. Exp. Mol. Med. 2001, 33, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.B.; Besner, G.E. HB-EGF Promotes Angiogenesis in Endothelial Cells via PI3-Kinase and MAPK Signaling Pathways. Growth Factors 2007, 25, 253–263. [Google Scholar] [CrossRef]
- Ren, B.; Yee, K.O.; Lawler, J.; Khosravi-Far, R. Regulation of Tumor Angiogenesis by Thrombospondin-1. Biochim. Biophys. Acta 2006, 1765, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lennon, D.P.; Caplan, A.I.; DeChant, A.; Hecker, J.; Kranso, J.; Zaremba, A.; Miller, R.H. Hepatocyte Growth Factor Mediates Mesenchymal Stem Cell-Induced Recovery in Multiple Sclerosis Models. Nat. Neurosci. 2012, 15, 862–870. [Google Scholar] [CrossRef]
- Jia, Y.; Cao, N.; Zhai, J.; Zeng, Q.; Zheng, P.; Su, R.; Liao, T.; Liu, J.; Pei, H.; Fan, Z.; et al. HGF Mediates Clinical-Grade Human Umbilical Cord-Derived Mesenchymal Stem Cells Improved Functional Recovery in a Senescence-Accelerated Mouse Model of Alzheimer’s Disease. Adv. Sci. 2020, 7, 1903809. [Google Scholar] [CrossRef]
- Liu, A.M.; Lu, G.; Tsang, K.S.; Li, G.; Wu, Y.; Huang, Z.S.; Ng, H.K.; Kung, H.F.; Poon, W.S. Umbilical Cord-Derived Mesenchymal Stem Cells with Forced Expression of Hepatocyte Growth Factor Enhance Remyelination and Functional Recovery in a Rat Intracerebral Hemorrhage Model. Neurosurgery 2010, 67, 357–365; discussion 365–356. [Google Scholar] [CrossRef]
- Kim, D.H.; Lim, H.; Lee, D.; Choi, S.J.; Oh, W.; Yang, Y.S.; Oh, J.S.; Hwang, H.H.; Jeon, H.B. Thrombospondin-1 Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Rescues Neurons from Synaptic Dysfunction in Alzheimer’s Disease Model. Sci. Rep. 2018, 8, 354. [Google Scholar] [CrossRef]
- Yu, K.; Ge, J.; Summers, J.B.; Li, F.; Liu, X.; Ma, P.; Kaminski, J.; Zhuang, J. TSP-1 Secreted by Bone Marrow Stromal Cells Contributes to Retinal Ganglion Cell Neurite Outgrowth and Survival. PLoS ONE 2008, 3, e2470. [Google Scholar] [CrossRef]
- Giunti, D.; Parodi, B.; Usai, C.; Vergani, L.; Casazza, S.; Bruzzone, S.; Mancardi, G.; Uccelli, A. Mesenchymal Stem Cells Shape Microglia Effector Functions through the Release of CX3CL1. Stem Cells 2012, 30, 2044–2053. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Shirakata, Y.; Kimura, R.; Nanba, D.; Iwamoto, R.; Tokumaru, S.; Morimoto, C.; Yokota, K.; Nakamura, M.; Sayama, K.; Mekada, E.; et al. Heparin-Binding EGF-Like Growth Factor Accelerates Keratinocyte Migration and Skin Wound Healing. J. Cell Sci. 2005, 118, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Cahill, E.F.; Kennelly, H.; Carty, F.; Mahon, B.P.; English, K. Hepatocyte Growth Factor Is Required for Mesenchymal Stromal Cell Protection Against Bleomycin-Induced Pulmonary Fibrosis. Stem Cells Transl. Med. 2016, 5, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, S.; Zhou, Y.; Feng, F.; Wang, Q.; Zhu, X.; Ai, H.; Huang, X.; Zhang, X. Hepatocyte Growth Factor Gene-Modified Adipose-Derived Mesenchymal Stem Cells Ameliorate Radiation Induced Liver Damage in a Rat Model. PLoS ONE 2014, 9, e114670. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, L.; Qian, X.; Chen, N.; Yao, A.; Pu, L.; Zhang, F.; Li, X.; Kong, L.; Sun, B.; et al. Antifibrotic Effect of Hepatocyte Growth Factor-Expressing Mesenchymal Stem Cells in Small-for-Size Liver Transplant Rats. Stem Cells Dev. 2010, 19, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wan, J.; Zhang, F.; Zhang, R.; Zhou, Z.; You, D. Influence of Hepatocyte Growth Factor-Transfected Bone Marrow-Derived Mesenchymal Stem Cells towards Renal Fibrosis in Rats. Indian J. Med. Res. 2019, 149, 508–516. [Google Scholar] [PubMed]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New Insights into TGF-beta/Smad Signaling in Tissue Fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, L.; Hou, X.G.; Hou, W.K.; Dong, J.J.; Sun, L.; Tang, K.X.; Wang, B.; Song, J.; Li, H.; et al. Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells from Diabetic Patients into Insulin-Producing Cells In Vitro. Chin. Med. J. 2007, 120, 771–776. [Google Scholar] [CrossRef]
- Gabr, M.M.; Zakaria, M.M.; Refaie, A.F.; Ismail, A.M.; Abou-El-Mahasen, M.A.; Ashamallah, S.A.; Khater, S.M.; El-Halawani, S.M.; Ibrahim, R.Y.; Uin, G.S.; et al. Insulin-Producing Cells from Adult Human Bone Marrow Mesenchymal Stem Cells Control Streptozotocin-Induced Diabetes in Nude Mice. Cell Transplant. 2013, 22, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Levi, E.; Fridman, R.; Miao, H.Q.; Ma, Y.S.; Yayon, A.; Vlodavsky, I. Matrix Metalloproteinase 2 Releases Active Soluble Ectodomain of Fibroblast Growth Factor Receptor 1. Proc. Natl. Acad. Sci. USA 1996, 93, 7069–7074. [Google Scholar] [CrossRef]
- McQuibban, G.A.; Gong, J.H.; Tam, E.M.; McCulloch, C.A.; Clark-Lewis, I.; Overall, C.M. Inflammation Dampened by Gelatinase A Cleavage of Monocyte Chemoattractant Protein-3. Science 2000, 289, 1202–1206. [Google Scholar] [CrossRef]
- Li, Q.; Park, P.W.; Wilson, C.L.; Parks, W.C. Matrilysin Shedding of Syndecan-1 Regulates Chemokine Mobilization and Transepithelial Efflux of Neutrophils in Acute Lung Injury. Cell 2002, 111, 635–646. [Google Scholar] [CrossRef]
- Gearing, A.J.; Beckett, P.; Christodoulou, M.; Churchill, M.; Clements, J.; Davidson, A.H.; Drummond, A.H.; Galloway, W.A.; Gilbert, R.; Gordon, J.L.; et al. Processing of Tumour Necrosis Factor-Alpha Precursor by Metalloproteinases. Nature 1994, 370, 555–557. [Google Scholar] [CrossRef]
- Ito, A.; Mukaiyama, A.; Itoh, Y.; Nagase, H.; Thogersen, I.B.; Enghild, J.J.; Sasaguri, Y.; Mori, Y. Degradation of Interleukin 1beta by Matrix Metalloproteinases. J. Biol. Chem. 1996, 271, 14657–14660. [Google Scholar] [CrossRef]
- Ho, I.A.; Chan, K.Y.; Ng, W.H.; Guo, C.M.; Hui, K.M.; Cheang, P.; Lam, P.Y. Matrix Metalloproteinase 1 is Necessary for the Migration of Human Bone Marrow-Derived Mesenchymal Stem Cells toward Human Glioma. Stem Cells 2009, 27, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.P.; Lam, A.T.L.; Newman, J.P.; Chua, K.L.M.; Kok, C.Y.L.; Chong, S.T.; Chua, M.L.K.; Lam, P.Y.P. Matrix Metalloproteinase-1 Facilitates MSC Migration via Cleavage of IGF-2/IGFBP2 Complex. FEBS Open Bio. 2018, 8, 15–26. [Google Scholar] [CrossRef] [PubMed]
- De Becker, A.; Van Hummelen, P.; Bakkus, M.; Vande Broek, I.; De Wever, J.; De Waele, M.; Van Riet, I. Migration of Culture-Expanded Human Mesenchymal Stem Cells through Bone Marrow Endothelium is Regulated by Matrix Metalloproteinase-2 and Tissue Inhibitor of Metalloproteinase-3. Haematologica 2007, 92, 440–449. [Google Scholar] [CrossRef]
- Ries, C.; Egea, V.; Karow, M.; Kolb, H.; Jochum, M.; Neth, P. MMP-2, MT1-MMP, and TIMP-2 are Essential for the Invasive Capacity of Human Mesenchymal Stem Cells: Differential Regulation by Inflammatory Cytokines. Blood 2007, 109, 4055–4063. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Mal, N.; Finan, A.; Zhang, M.; Kiedrowski, M.; Popovic, Z.; McCarthy, P.M.; Penn, M.S. Monocyte Chemotactic Protein-3 is a Myocardial Mesenchymal Stem Cell Homing Factor. Stem Cells 2007, 25, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.J.; Zhou, Y.; Chen, C.L.; Darbyshire, A.; Besner, G.E. Heparin-Binding Epidermal Growth Factor-Like Growth Factor Protects Mesenchymal Stem Cells. J. Surg. Res. 2012, 177, 359–364. [Google Scholar] [CrossRef][Green Version]
- Boomsma, R.A.; Geenen, D.L. Mesenchymal Stem Cells Secrete Multiple Cytokines that Promote Angiogenesis and Have Contrasting Effects on Chemotaxis and Apoptosis. PLoS ONE 2012, 7, e35685. [Google Scholar] [CrossRef]
- Cheng, J.; Diaz Encarnacion, M.M.; Warner, G.M.; Gray, C.E.; Nath, K.A.; Grande, J.P. TGF-beta1 Stimulates Monocyte Chemoattractant Protein-1 Expression in Mesangial Cells through a Phosphodiesterase Isoenzyme 4-Dependent Process. Am. J. Physiol. Cell Physiol. 2005, 289, C959–C970. [Google Scholar] [CrossRef]
- Takeshita, A.; Chen, Y.; Watanabe, A.; Kitano, S.; Hanazawa, S. TGF-Beta Induces Expression of Monocyte Chemoattractant JE/Monocyte Chemoattractant Protein 1 via Transcriptional Factor AP-1 Induced by Protein Kinase in Osteoblastic cells. J. Immunol. 1995, 155, 419–426. [Google Scholar] [CrossRef]
- Zhang, F.; Tsai, S.; Kato, K.; Yamanouchi, D.; Wang, C.; Rafii, S.; Liu, B.; Kent, K.C. Transforming Growth Factor-Beta Promotes Recruitment of Bone Marrow Cells and Bone Marrow-Derived Mesenchymal Stem Cells through Stimulation of MCP-1 Production in Vascular Smooth Muscle Cells. J. Biol. Chem. 2009, 284, 17564–17574. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal Stem Cell-Derived Microvesicles Protect against Acute Tubular Injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, Y.; Lan, B.; Wang, J.; Zhang, Z.; Zhang, L.; Xiao, P.; Meng, Q.; Geng, Y.J.; Yu, X.Y.; et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. Biomed. Res. Int. 2017, 2017, 4150705. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef] [PubMed]
- Jakl, V.; Ehmele, M.; Winkelmann, M.; Ehrenberg, S.; Eiseler, T.; Friemert, B.; Rojewski, M.T.; Schrezenmeier, H. A Novel Approach for Large-Scale Manufacturing of Small Extracellular Vesicles from Bone Marrow-Derived Mesenchymal Stromal Cells Using a Hollow Fiber Bioreactor. Front. Bioeng. Biotechnol. 2023, 11, 1107055. [Google Scholar] [CrossRef] [PubMed]



| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aMEM+8%PL [%] | 100 | 99 | 97.5 | 95 | 90 | 75 | 50 | 25 | 10 | 5 | 2.5 | 1 | 0 |
| StemMACSTM [%] | 0 | 1 | 2.5 | 5 | 10 | 25 | 50 | 75 | 90 | 95 | 97.5 | 99 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakl, V.; Popp, T.; Haupt, J.; Port, M.; Roesler, R.; Wiese, S.; Friemert, B.; Rojewski, M.T.; Schrezenmeier, H. Effect of Expansion Media on Functional Characteristics of Bone Marrow-Derived Mesenchymal Stromal Cells. Cells 2023, 12, 2105. https://doi.org/10.3390/cells12162105
Jakl V, Popp T, Haupt J, Port M, Roesler R, Wiese S, Friemert B, Rojewski MT, Schrezenmeier H. Effect of Expansion Media on Functional Characteristics of Bone Marrow-Derived Mesenchymal Stromal Cells. Cells. 2023; 12(16):2105. https://doi.org/10.3390/cells12162105
Chicago/Turabian StyleJakl, Viktoria, Tanja Popp, Julian Haupt, Matthias Port, Reinhild Roesler, Sebastian Wiese, Benedikt Friemert, Markus T. Rojewski, and Hubert Schrezenmeier. 2023. "Effect of Expansion Media on Functional Characteristics of Bone Marrow-Derived Mesenchymal Stromal Cells" Cells 12, no. 16: 2105. https://doi.org/10.3390/cells12162105
APA StyleJakl, V., Popp, T., Haupt, J., Port, M., Roesler, R., Wiese, S., Friemert, B., Rojewski, M. T., & Schrezenmeier, H. (2023). Effect of Expansion Media on Functional Characteristics of Bone Marrow-Derived Mesenchymal Stromal Cells. Cells, 12(16), 2105. https://doi.org/10.3390/cells12162105






