Transcriptomic Analysis Reveals Candidate Ligand-Receptor Pairs and Signaling Networks Mediating Intercellular Communication between Hair Matrix Cells and Dermal Papilla Cells from Cashmere Goats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Farm Animals and Chemical Reagents
2.2. Isolation, Mono-Culture and Co-Culture of Follicular Cells from Cashmere Goats
2.3. Transcriptome Sequencing
2.4. GO and KEGG Enrichment Analysis
2.5. Construction of Ligand-Receptor Pairs and Intercellular Communication Network
2.6. Cell Viability Assay
2.7. Flow Cytometry Analysis
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Hocheste 33,332 Staining
2.10. Statistical Analysis
3. Results
3.1. Coculture Altered the Cell Cycle Patterns of Goat DPCs and HMCs
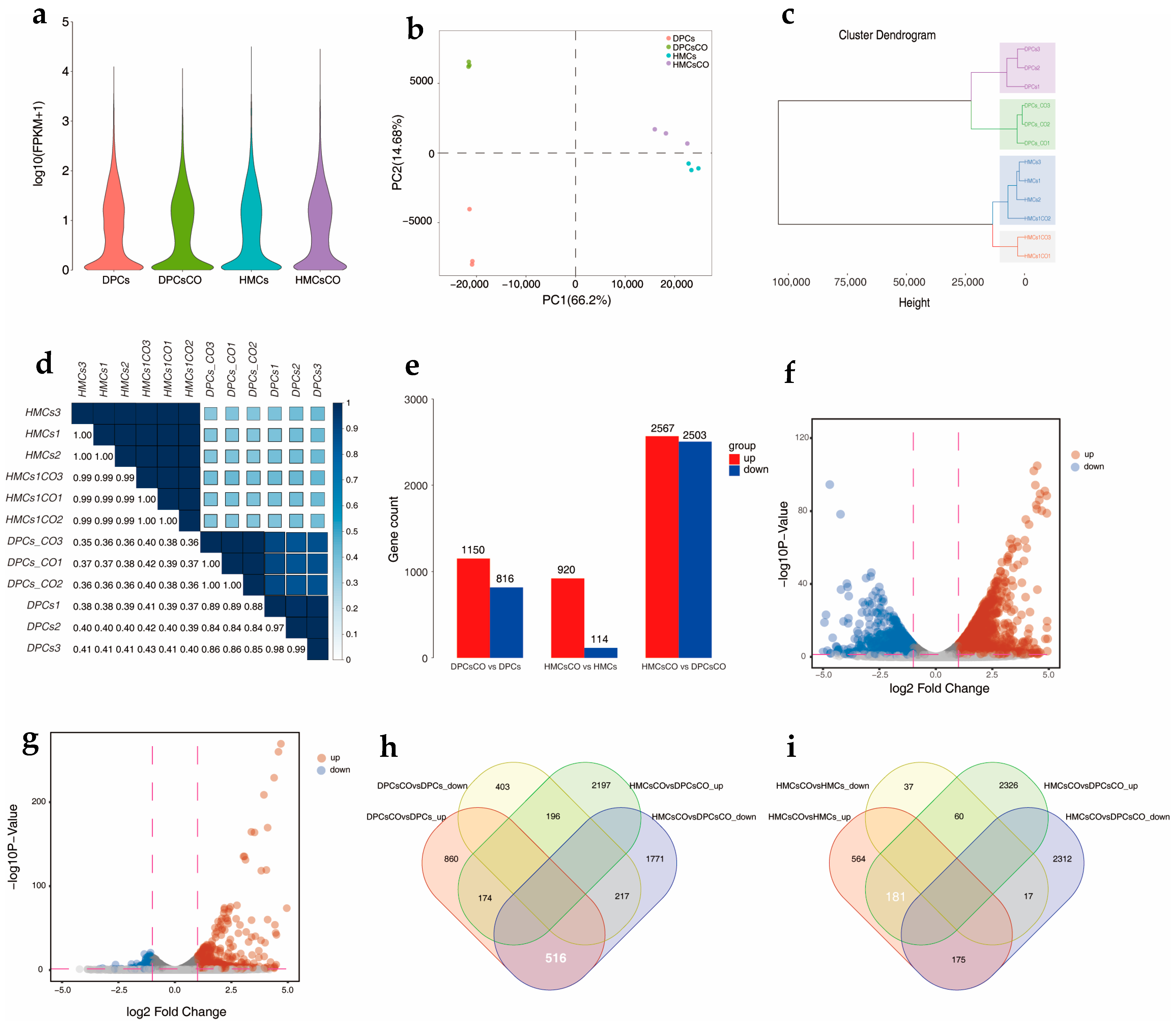
3.2. Coculture Changed the Transcriptomic Profiles of Goat DPCs and HMCs
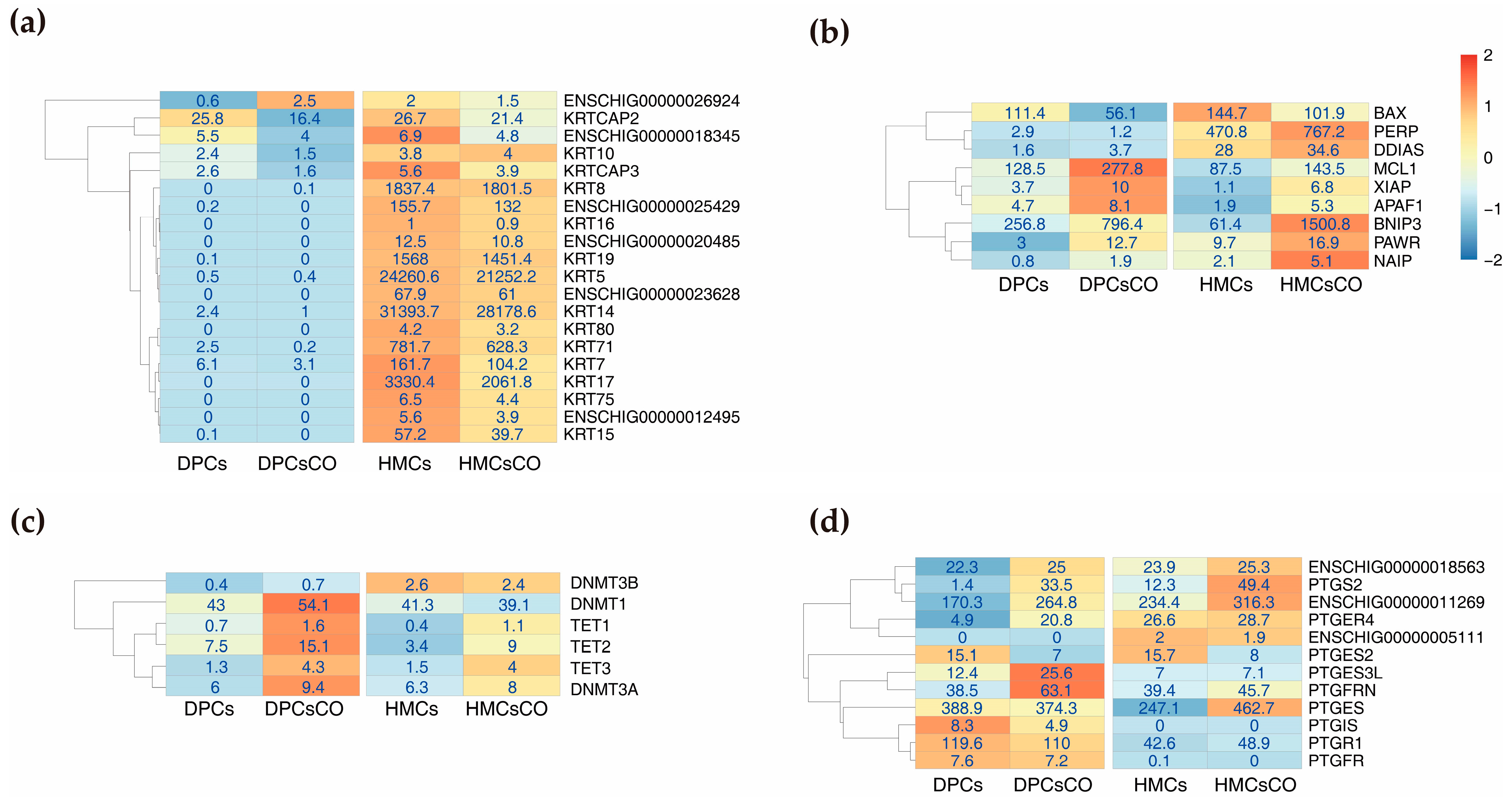
3.3. Unique Expression Patterns of Genes Related to Hair Composition, Cell Apoptosis, DNA Methylation, and Prostaglandin Metabolism
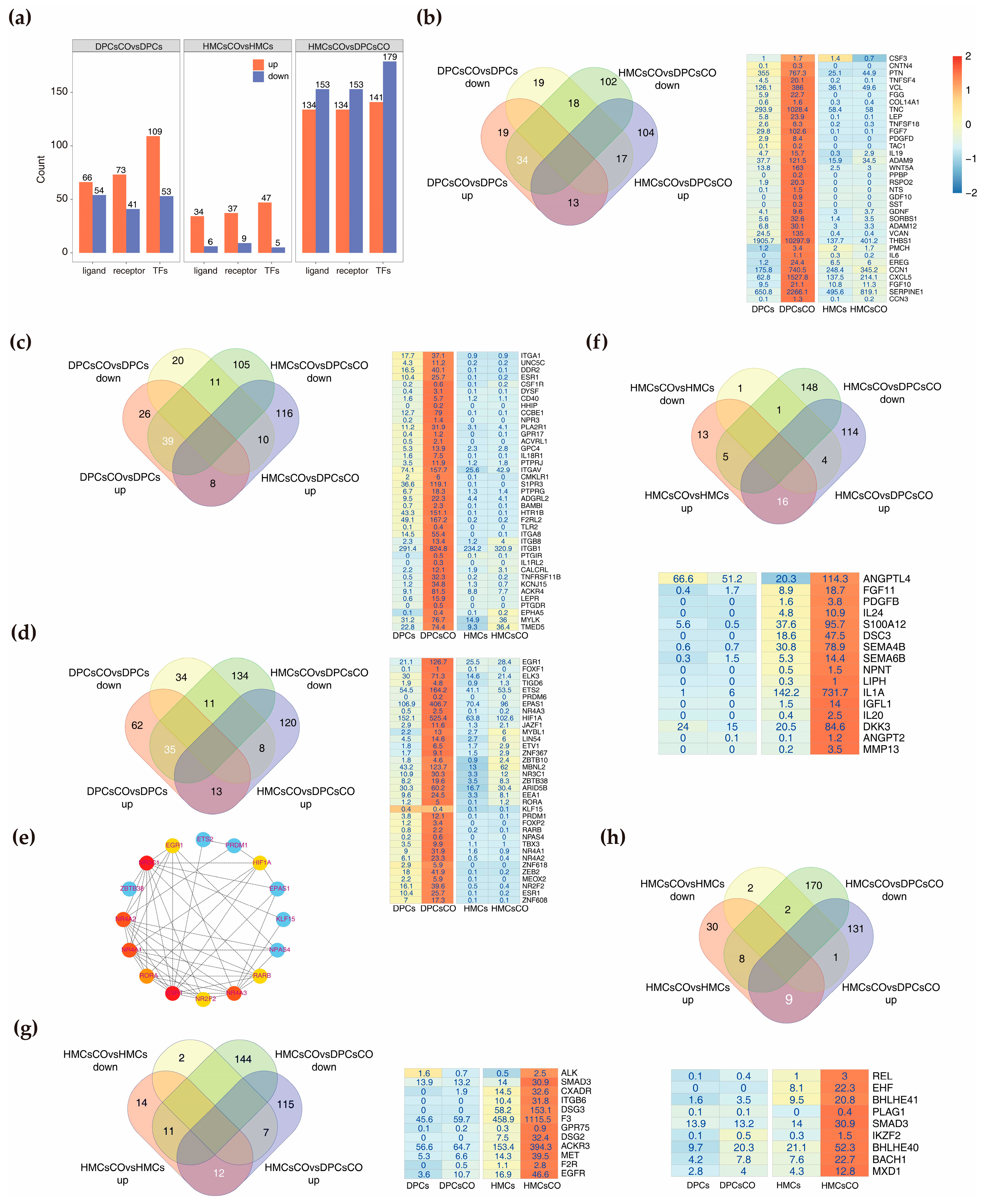
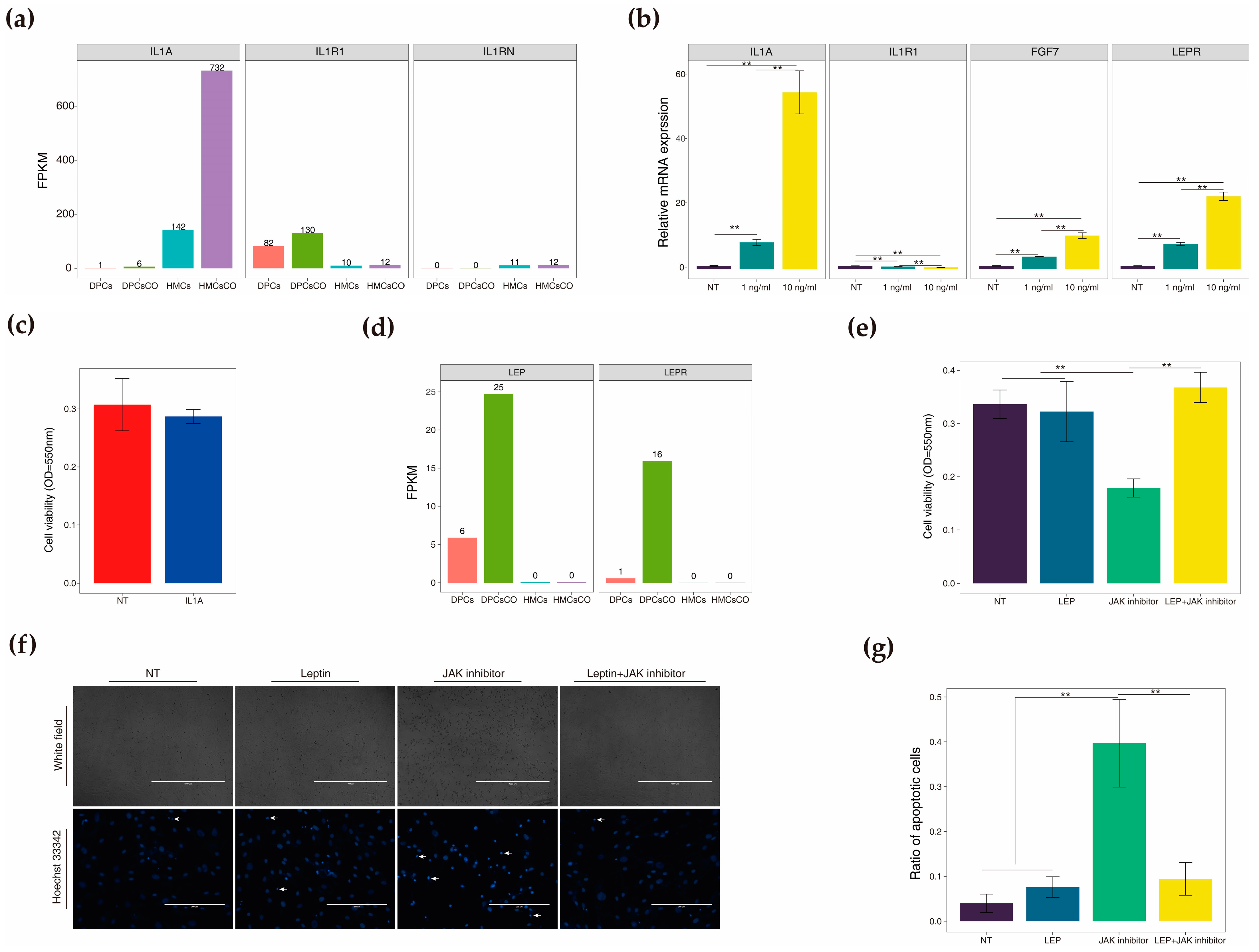
3.4. Identification of Core Ligands, Receptors, and Transcription Factors Affected by Coculture
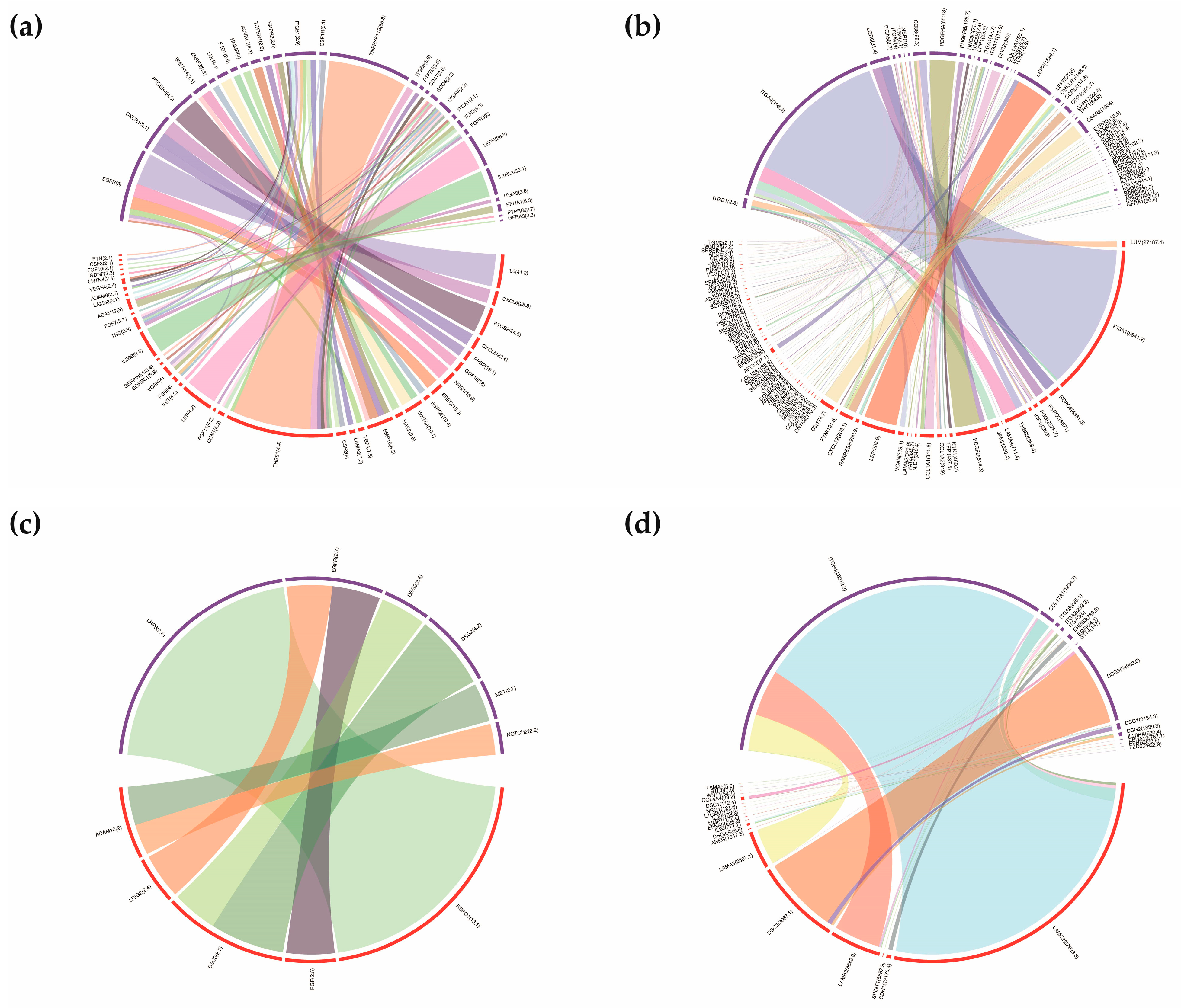
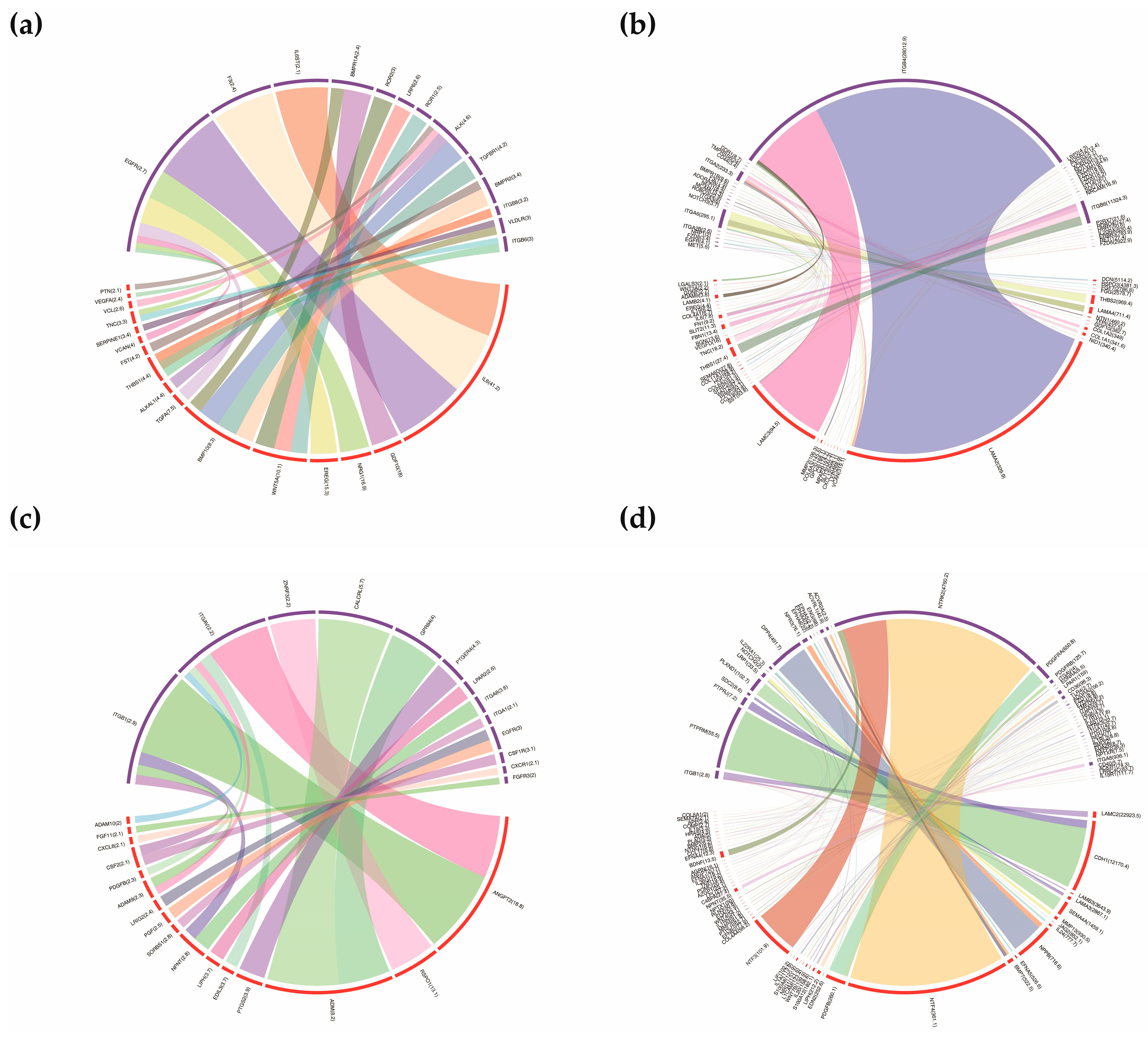
3.5. Construction of Ligand-Receptor Pairs Mediating the Autocrine and Paracrine Crosstalk between Homogenous and Heterogeneous Cell Types
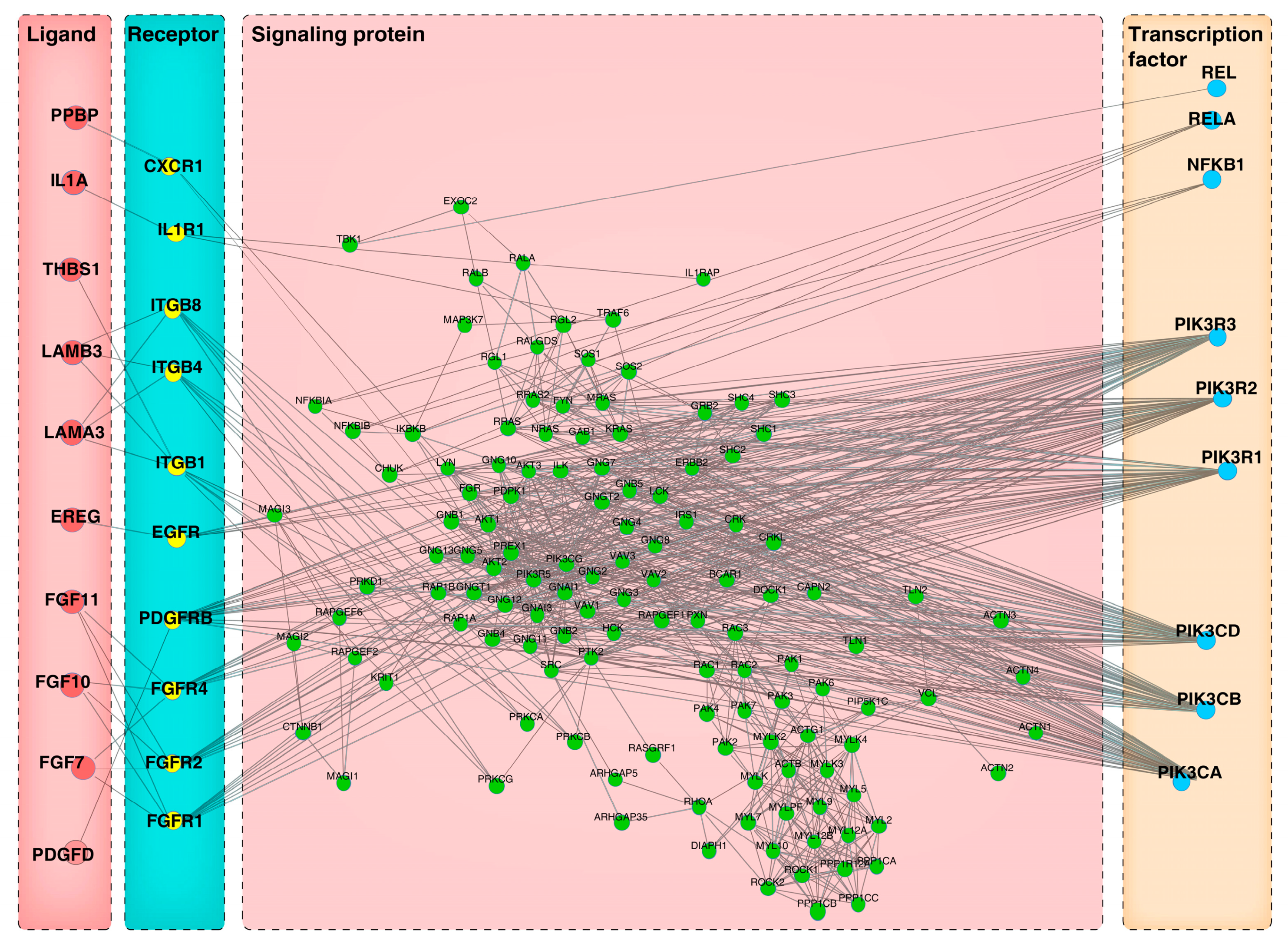
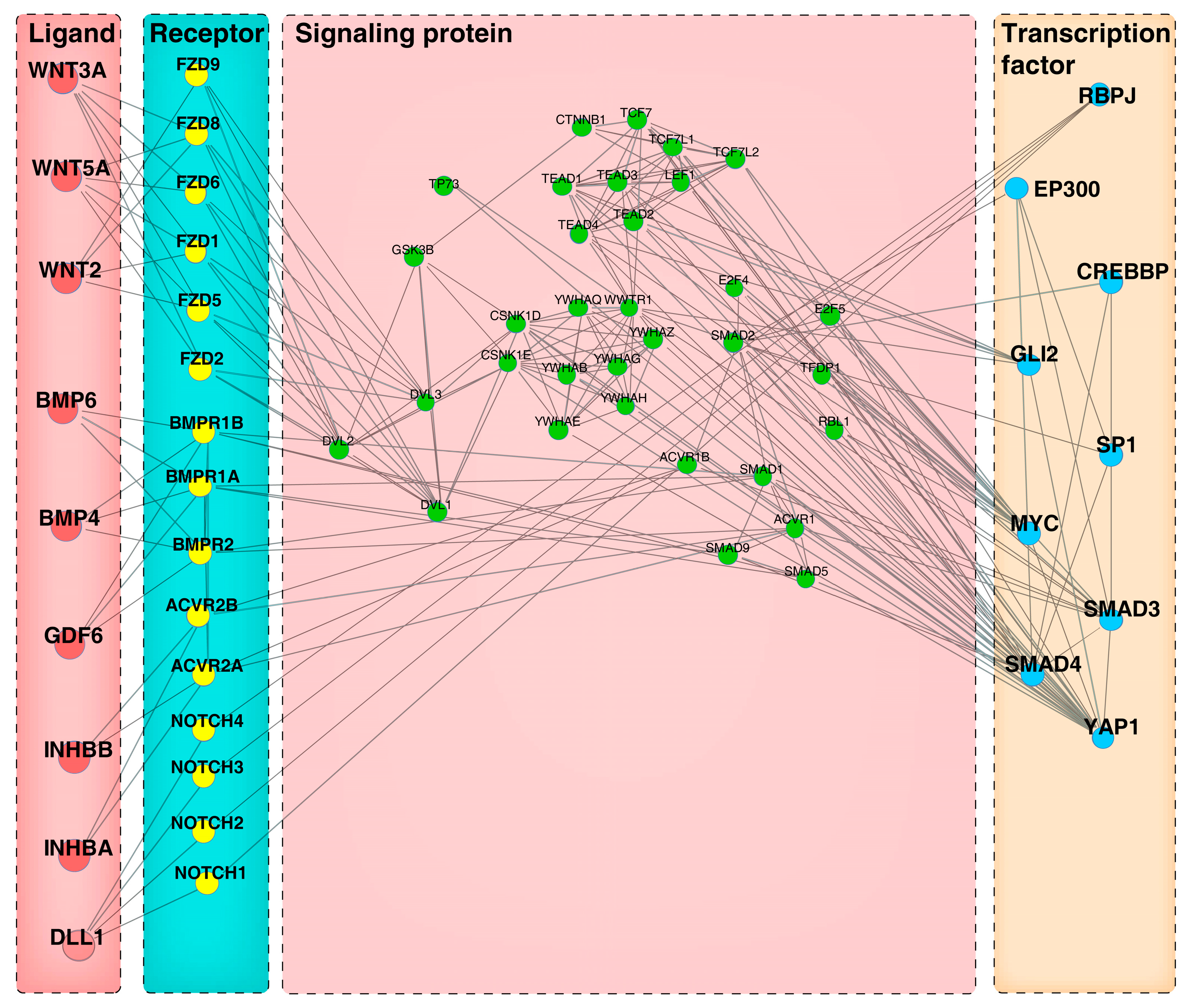
3.6. Construction of Ligand-Receptor-Signaling Protein-TFs Axis Mediating Autocrine and Paracrine Crosstalk in Cells
3.7. Construction of Ligand-Receptor-Signaling Protein-TFs Axis Mediating Autocrine and Paracrine Crosstalk in Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Galbraith, H. Fundamental Hair Follicle Biology and Fine Fibre Production in Animals. Animal 2010, 4, 1490–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allain, D.; Renieri, C. Genetics of Fibre Production and Fleece Characteristics in Small Ruminants, Angora Rabbit and South American Camelids. Animal 2010, 4, 1472–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenn, K.S.; Paus, R. Controls of Hair Follicle Cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Tumbar, T. Hairy Tale of Signaling in Hair Follicle Development and Cycling. Semin. Cell Dev. Biol. 2012, 23, 906–916. [Google Scholar] [CrossRef] [Green Version]
- Sennett, R.; Rendl, M. Mesenchymal–Epithelial Interactions during Hair Follicle Morphogenesis and Cycling. Semin. Cell Dev. Biol. 2012, 23, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The Hair Follicle as a Dynamic Miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.-C.; Rose, C.; Yoon, G.S.; Lee, S.-J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Morgan, B.A. The Dermal Papilla: An Instructive Niche for Epithelial Stem and Progenitor Cells in Development and Regeneration of the Hair Follicle. Cold Spring Harb. Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef] [Green Version]
- Telerman, S.B.; Rognoni, E.; Sequeira, I.; Pisco, A.O.; Lichtenberger, B.M.; Culley, O.; Viswanathan, P.; Driskell, R.R.; Watt, F.M. Dermal Blimp1 Acts Downstream of Epidermal TGFβ and Wnt/β-Catenin to Regulate Hair Follicle Formation and Growth. J. Investig. Dermatol. 2017, 137, 2270–2281. [Google Scholar] [CrossRef] [Green Version]
- Woo, W.-M.; Zhen, H.H.; Oro, A.E. Shh Maintains Dermal Papilla Identity and Hair Morphogenesis via a Noggin–Shh Regulatory Loop. Genes Dev. 2012, 26, 1235–1246. [Google Scholar] [CrossRef] [Green Version]
- St-Jacques, B.; Dassule, H.R.; Karavanova, I.; Botchkarev, V.A.; Li, J.; Danielian, P.S.; McMahon, J.A.; Lewis, P.M.; Paus, R.; McMahon, A.P. Sonic Hedgehog Signaling Is Essential for Hair Development. Curr. Biol. 1998, 8, 1058–1069. [Google Scholar] [CrossRef] [Green Version]
- Rezza, A.; Wang, Z.; Sennett, R.; Qiao, W.; Wang, D.; Heitman, N.; Mok, K.W.; Clavel, C.; Yi, R.; Zandstra, P.; et al. Signaling Networks among Stem Cell Precursors, Transit-Amplifying Progenitors, and Their Niche in Developing Hair Follicles. Cell Rep. 2016, 14, 3001–3018. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, S.M.; Jankovic, S.V. The Control of Hair Growth. Dermatol. Online J. 1998, 4, 2. [Google Scholar] [CrossRef]
- Chen, C.-C.; Plikus, M.V.; Tang, P.-C.; Widelitz, R.B.; Chuong, C.M. The Modulatable Stem Cell Niche: Tissue Interactions during Hair and Feather Follicle Regeneration. J. Mol. Biol. 2016, 428, 1423–1440. [Google Scholar] [CrossRef] [Green Version]
- Rendl, M.; Lewis, L.; Fuchs, E. Molecular Dissection of Mesenchymal–Epithelial Interactions in the Hair Follicle. PLoS Biol. 2005, 3, e331. [Google Scholar] [CrossRef] [Green Version]
- Petiot, A.; Conti, F.J.A.; Grose, R.; Revest, J.-M.; Hodivala-Dilke, K.M.; Dickson, C. A Crucial Role for Fgfr2-IIIb Signalling in Epidermal Development and Hair Follicle Patterning. Development 2003, 130, 5493–5501. [Google Scholar] [CrossRef] [Green Version]
- Michno, K.; Boras-Granic, K.; Mill, P.; Hui, C.C.; Hamel, P.A. Shh Expression Is Required for Embryonic Hair Follicle but Not Mammary Gland Development. Dev. Biol. 2003, 264, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. β-Catenin Activity in the Dermal Papilla Regulates Morphogenesis and Regeneration of Hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Soma, T.; Fujiwara, S.; Shirakata, Y.; Hashimoto, K.; Kishimoto, J. Hair-Inducing Ability of Human Dermal Papilla Cells Cultured under Wnt/β-Catenin Signalling Activation: Letter to the Editor. Exp. Dermatol. 2012, 21, 307–309. [Google Scholar] [CrossRef]
- Armingol, E.; Officer, A.; Harismendy, O.; Lewis, N.E. Deciphering Cell–Cell Interactions and Communication from Gene Expression. Nat. Rev. Genet. 2021, 22, 71–88. [Google Scholar] [CrossRef]
- Shao, X.; Liao, J.; Li, C.; Lu, X.; Cheng, J.; Fan, X. CellTalkDB: A Manually Curated Database of Ligand–Receptor Interactions in Humans and Mice. Brief. Bioinform. 2021, 22, bbaa269. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yu, D.; Yang, P.; Guo, R.; Kong, M.; Gao, Y.; Yu, X.; Lu, X.; Fan, X. Revealing the Transcriptional Heterogeneity of Organ-specific Metastasis in Human Gastric Cancer Using Single-cell RNA Sequencing. Clin. Transl. Med. 2022, 12, e730. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Sheng, J.; Gao, D.; Li, F.; Durrans, A.; Ryu, S.; Lee, S.B.; Narula, N.; Rafii, S.; Elemento, O.; et al. Transcriptome Analysis of Individual Stromal Cell Populations Identifies Stroma-Tumor Crosstalk in Mouse Lung Cancer Model. Cell Rep. 2015, 10, 1187–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Wang, Y.; Zhou, G.; Ding, Y.; Yang, Y.; Wang, X.; Zhang, E.; Chen, Y. Synchronous Profiling and Analysis of MRNAs and NcRNAs in the Dermal Papilla Cells from Cashmere Goats. BMC Genom. 2019, 20, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Wang, L.; Zong, B.; Wang, Y.; Wang, X.; Shi, Y.; Yang, Y.; Chen, Y. Cultivation of Hair Matrix Cells from Cashmere Goat Skins and Exemplified Applications. Animals 2020, 10, 1400. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential Gene and Transcript Expression Analysis of RNA-Seq Experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [Green Version]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize Implements and Enhances Circular Visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef] [Green Version]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- Colombe, L.; Vindrios, A.; Michelet, J.-F.; Bernard, B.A. Prostaglandin Metabolism in Human Hair Follicle. Exp. Dermatol. 2007, 16, 762–769. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. CytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [Green Version]
- Philpott, M.P.; Sanders, D.A.; Bowen, J.; Kealey, T. Effects of Interleukins, Colony-stimulating Factor and Tumour Necrosis Factor on Human Hair Follicle Growth in Vitro: A Possible Role for Interleukin-1 and Tumour Necrosis Factor-α in Alopecia Areata. Br. J. Dermatol. 1996, 135, 942–948. [Google Scholar] [CrossRef]
- Lee, P.; Gund, R.; Dutta, A.; Pincha, N.; Rana, I.; Ghosh, S.; Witherden, D.; Kandyba, E.; MacLeod, A.; Kobielak, K.; et al. Stimulation of Hair Follicle Stem Cell Proliferation through an IL-1 Dependent Activation of ΓδT-Cells. eLife 2017, 6, e28875. [Google Scholar] [CrossRef]
- Chedid, M.; Rubin, J.S.; Csaky, K.G.; Aaronson, S.A. Regulation of Keratinocyte Growth Factor Gene Expression by Interleukin 1. J. Biol. Chem. 1994, 269, 10753–10757. [Google Scholar] [CrossRef]
- Gao, J.; Tian, J.; Lv, Y.; Shi, F.; Kong, F.; Shi, H.; Zhao, L. Leptin Induces Functional Activation of Cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT Pathways in Human Endometrial Cancer Cells. Cancer Sci. 2009, 100, 389–395. [Google Scholar] [CrossRef]
- Lee, M.J.; Cha, H.J.; Lim, K.M.; Lee, O.-K.; Bae, S.; Kim, C.-H.; Lee, K.-H.; Lee, Y.N.; Ahn, K.J.; An, S. Analysis of the MicroRNA Expression Profile of Normal Human Dermal Papilla Cells Treated with 5α-Dihydrotestosterone. Mol. Med. Rep. 2015, 12, 1205–1212. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of Minoxidil on Proliferation and Apoptosis in Dermal Papilla Cells of Human Hair Follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef]
- Lindner, G.; Botchkarev, V.A.; Botchkareva, N.V.; Ling, G.; van der Veen, C.; Paus, R. Analysis of Apoptosis during Hair Follicle Regression (Catagen). Am. J. Pathol. 1997, 151, 1601–1617. [Google Scholar]
- Tang, P.; Wang, X.; Zhang, M.; Huang, S.; Lin, C.; Yan, F.; Deng, Y.; Zhang, L.; Zhang, L. Activin B Stimulates Mouse Vibrissae Growth and Regulates Cell Proliferation and Cell Cycle Progression of Hair Matrix Cells through ERK Signaling. Int. J. Mol. Sci. 2019, 20, 853. [Google Scholar] [CrossRef] [Green Version]
- Kwack, M.H.; Ahn, J.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dihydrotestosterone-Inducible IL-6 Inhibits Elongation of Human Hair Shafts by Suppressing Matrix Cell Proliferation and Promotes Regression of Hair Follicles in Mice. J. Investig. Dermatol. 2012, 132, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inamatsu, M.; Matsuzaki, T.; Iwanari, H.; Yoshizato, K. Establishment of Rat Dermal Papilla Cell Lines That Sustain the Potency to Induce Hair Follicles from Afollicular Skin. J. Investig. Dermatol. 1998, 111, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohyama, M.; Kobayashi, T.; Sasaki, T.; Shimizu, A.; Amagai, M. Restoration of the Intrinsic Properties of Human Dermal Papilla in Vitro. J. Cell Sci. 2012, 125, 4114–4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Li, Y.; Zhou, G.; Gao, Y.; Ma, S.; Chen, Y.; Song, J.; Wang, X. Whole-Genome Bisulfite Sequencing of Goat Skins Identifies Signatures Associated with Hair Cycling. BMC Genom. 2018, 19, 638. [Google Scholar] [CrossRef]
- Cobb, J.E.; Wong, N.C.; Yip, L.W.; Martinick, J.; Bosnich, R.; Sinclair, R.D.; Craig, J.M.; Saffery, R.; Harrap, S.B.; Ellis, J.A. Evidence of Increased DNA Methylation of the Androgen Receptor Gene in Occipital Hair Follicles from Men with Androgenetic Alopecia. Br. J. Dermatol. 2011, 165, 210–213. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J.; Liu, M.; Yang, N.; Bao, Z.; Zhang, X.; Dai, Y.; Cai, J.; Chen, Y.; Wu, X. DNA Methylation Mediates LncRNA2919 Regulation of Hair Follicle Regeneration. Int. J. Mol. Sci. 2022, 23, 9481. [Google Scholar] [CrossRef]
- Sasaki, S.; Hozumi, Y.; Kondo, S. Influence of Prostaglandin F2α and Its Analogues on Hair Regrowth and Follicular Melanogenesis in a Murine Model. Exp. Dermatol. 2005, 14, 323–328. [Google Scholar] [CrossRef]
- Choi, Y.M.; Diehl, J.; Levins, P.C. Promising Alternative Clinical Uses of Prostaglandin F2α Analogs: Beyond the Eyelashes. J. Am. Acad. Dermatol. 2015, 72, 712–716. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; You, X.; Leimert, K.; Popowycz, K.; Fang, X.; Wood, S.L.; Slater, D.M.; Sun, Q.; Gu, H.; et al. PGF 2α Modulates the Output of Chemokines and pro-Inflammatory Cytokines in Myometrial Cells from Term Pregnant Women through Divergent Signaling Pathways. Mol. Hum. Reprod. 2015, 21, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Lefort, K.; Qiu, W.; Nguyen, B.-C.; Rajaram, R.D.; Castillo, E.; He, F.; Chen, Y.; Angel, P.; Brisken, C.; et al. Control of Hair Follicle Cell Fate by Underlying Mesenchyme through a CSL–Wnt5a–FoxN1 Regulatory Axis. Genes Dev. 2010, 24, 1519–1532. [Google Scholar] [CrossRef] [Green Version]
- Hansen, L.A.; Alexander, N.; Hogan, M.E.; Sundberg, J.P.; Dlugosz, A.; Threadgill, D.W.; Magnuson, T.; Yuspa, S.H. Genetically Null Mice Reveal a Central Role for Epidermal Growth Factor Receptor in the Differentiation of the Hair Follicle and Normal Hair Development. Am. J. Pathol. 1997, 150, 1959–1975. [Google Scholar]
- Gugasyan, R.; Voss, A.; Varigos, G.; Thomas, T.; Grumont, R.J.; Kaur, P.; Grigoriadis, G.; Gerondakis, S. The Transcription Factors C-Rel and RelA Control Epidermal Development and Homeostasis in Embryonic and Adult Skin via Distinct Mechanisms. Mol. Cell Biol. 2004, 24, 5733–5745. [Google Scholar] [CrossRef] [Green Version]
- Pablos, J.; Everett, E.T.; Leroy, E.C.; Norris, J.S. Thrombospondin 1 Is Expressed by Mesenchymal Cells in Mouse Post-Natal Skin and Hair Follicle Development. Histochem. J. 1998, 30, 451–465. [Google Scholar] [CrossRef]
- Zannettino, A.C.W.; Holding, C.A.; Diamond, P.; Atkins, G.J.; Kostakis, P.; Farrugia, A.; Gamble, J.; To, L.B.; Findlay, D.M.; Haynes, D.R. Osteoprotegerin (OPG) is localized to the Weibel-Palade bodies of human vascular endothelial cells and is physically associated with von Willebrand factor. J. Cell. Physiol. 2005, 204, 714–723. [Google Scholar] [CrossRef]
- Kitajima, Y. New Insights into Desmosome Regulation and Pemphigus Blistering as a Desmosome-Remodeling Disease. Kaohsiung J. Med. Sci. 2013, 29, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kurzen, H.; Schäfer, S.; Franke, W.W.; Moll, I.; Moll, R.; Simics, E.; Amagai, M.; Wheelock, M.J. Compositionally Different Desmosomes in the Various Compartments of the Human Hair Follicle. Differentiation 1998, 63, 295–304. [Google Scholar] [CrossRef]
- Zebisch, M.; Xu, Y.; Krastev, C.; MacDonald, B.T.; Chen, M.; Gilbert, R.J.C.; He, X.; Jones, E.Y. Structural and Molecular Basis of ZNRF3/RNF43 Transmembrane Ubiquitin Ligase Inhibition by the Wnt Agonist R-Spondin. Nat. Commun. 2013, 4, 2787. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.-X.; Xie, Y.; Zhang, Y.; Charlat, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 Promotes Wnt Receptor Turnover in an R-Spondin-Sensitive Manner. Nature 2012, 485, 195–200. [Google Scholar] [CrossRef]
- Rajbhandari, P.; Thomas, B.J.; Feng, A.-C.; Hong, C.; Wang, J.; Vergnes, L.; Sallam, T.; Wang, B.; Sandhu, J.; Seldin, M.M.; et al. IL-10 Signaling Remodels Adipose Chromatin Architecture to Limit Thermogenesis and Energy Expenditure. Cell 2018, 172, 218–233.e17. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.W.; Houston-Hawkins, D.E.; Woodruff, T.K.; Matzuk, M.M. Insertion of Inhbb into the Inhba Locus Rescues the Inhba-Null Phenotype and Reveals New Activin Functions. Nat. Genet. 2000, 25, 453–457. [Google Scholar] [CrossRef]
- Lodberg, A. Principles of the Activin Receptor Signaling Pathway and Its Inhibition. Cytokine Growth Factor Rev. 2021, 60, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Shen, X.; Liu, I.; Semenov, M.; He, X.; Wang, X.-F. Smad3-Dependent Nuclear Translocation of β-Catenin Is Required for TGF-Β1-Induced Proliferation of Bone Marrow-Derived Adult Human Mesenchymal Stem Cells. Genes Dev. 2006, 20, 666–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.; Hollenbeck, S.T.; Ryer, E.J.; Edlin, R.; Yamanouchi, D.; Kundi, R.; Wang, C.; Liu, B.; Kent, K.C. TGF-β through Smad3 Signaling Stimulates Vascular Smooth Muscle Cell Proliferation and Neointimal Formation. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H540–H549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Chen, H.; Chen, Q.; Qiu, J.; Qahar, M.; Fan, Z.; Chu, W.; Tredget, E.E.; Wu, Y. Injury-Induced Interleukin-1 Alpha Promotes Lgr5 Hair Follicle Stem Cells de Novo Regeneration and Proliferation via Regulating Regenerative Microenvironment in Mice. Inflamm. Regen. 2023, 43, 14. [Google Scholar] [CrossRef] [PubMed]
- Sumikawa, Y.; Inui, S.; Nakajima, T.; Itami, S. Hair Cycle Control by Leptin as a New Anagen Inducer. Exp. Dermatol. 2014, 23, 27–32. [Google Scholar] [CrossRef]
- Tasaki, N.; Minematsu, T.; Mugita, Y.; Ikeda, S.; Nakagami, G.; Sanada, H. Telogen Elongation in the Hair Cycle of Ob/Ob Mice. Biosci. Biotechnol. Biochem. 2016, 80, 74–79. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Choi, Y.S.; Choi, J.-H. Leptin Promotes Human Endometriotic Cell Migration and Invasion by Up-Regulating MMP-2 through the JAK2/STAT3 Signaling Pathway. Mol. Hum. Reprod. 2015, 21, 792–802. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Nie, W.; Li, W.; Zhou, X.; Sun, H.; Zhu, J.; Chen, J.; Peng, J. Interferon-β combined with all-trans retinoic acid supresses proliferation and promote apoptosis by inhibiting JAK2/STAT3 pathway in HepG2 human hepatocellular carcinoma cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016, 32, 901–905. [Google Scholar]
- Ma, S.; Zhou, G.; Chen, Y. Effects of All-Trans Retinoic Acid on Goat Dermal Papilla Cells Cultured in Vitro. Electron. J. Biotechnol. 2018, 34, 43–50. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Ji, D.; Wang, X.; Yang, Y.; Shi, Y.; Chen, Y. Transcriptomic Analysis Reveals Candidate Ligand-Receptor Pairs and Signaling Networks Mediating Intercellular Communication between Hair Matrix Cells and Dermal Papilla Cells from Cashmere Goats. Cells 2023, 12, 1645. https://doi.org/10.3390/cells12121645
Ma S, Ji D, Wang X, Yang Y, Shi Y, Chen Y. Transcriptomic Analysis Reveals Candidate Ligand-Receptor Pairs and Signaling Networks Mediating Intercellular Communication between Hair Matrix Cells and Dermal Papilla Cells from Cashmere Goats. Cells. 2023; 12(12):1645. https://doi.org/10.3390/cells12121645
Chicago/Turabian StyleMa, Sen, Dejun Ji, Xiaolong Wang, Yuxin Yang, Yinghua Shi, and Yulin Chen. 2023. "Transcriptomic Analysis Reveals Candidate Ligand-Receptor Pairs and Signaling Networks Mediating Intercellular Communication between Hair Matrix Cells and Dermal Papilla Cells from Cashmere Goats" Cells 12, no. 12: 1645. https://doi.org/10.3390/cells12121645
APA StyleMa, S., Ji, D., Wang, X., Yang, Y., Shi, Y., & Chen, Y. (2023). Transcriptomic Analysis Reveals Candidate Ligand-Receptor Pairs and Signaling Networks Mediating Intercellular Communication between Hair Matrix Cells and Dermal Papilla Cells from Cashmere Goats. Cells, 12(12), 1645. https://doi.org/10.3390/cells12121645





