Blood and Sputum Eosinophils of COPD Patients Are Differently Polarized than in Asthma
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Study Design and Participants
2.2. Blood and Sputum Sampling and Processing
2.3. Assessment of Surface Receptor Expression by Flow Cytometry
2.4. Protein Analysis in Induced Sputum
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
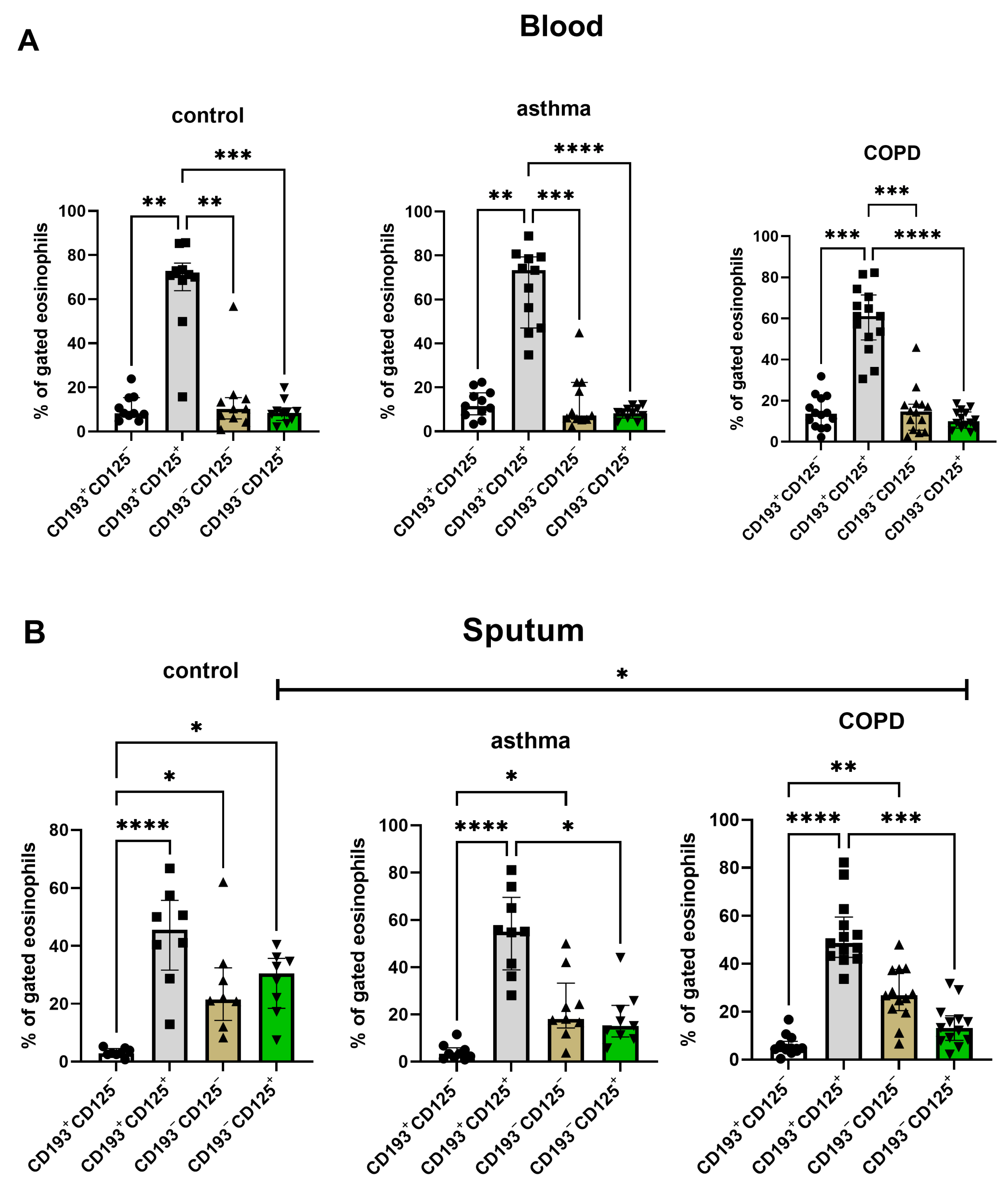
3.2. Expression of CD125, CD193, CD62L, CD66b, CD14, CD11b on Sputum and Blood Eosinophils
3.3. Cytokine and Eotaxin Concentrations in Sputum Samples
3.4. Correlations between Th2 Inflammation Biomarkers and Eosinophil Phenotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mycroft, K.; Krenke, R.; Górska, K. Eosinophils in COPD—Current Concepts and Clinical Implications. J. Allergy Clin. Immunol. Pract. 2020, 8, 2565–2574. [Google Scholar] [CrossRef] [PubMed]
- Salvo-Romero, E.; Rodiño-Janeiro, B.K.; Albert-Bayo, M.; Lobo, B.; Santos, J.; Farré, R.; Martinez, C.; Vicario, M. Eosinophils in the Gastrointestinal Tract: Key Contributors to Neuro-Immune Crosstalk and Potential Implications in Disorders of Brain-Gut Interaction. Cells 2022, 11, 1644. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.; Berti, A.; Cottini, M. The Emerging Roles of Eosinophils: Implications for the Targeted Treatment of Eosinophilic-Associated Inflammatory Conditions. Curr. Res. Immunol. 2022, 3, 42–53. [Google Scholar] [CrossRef]
- McBrien, C.N.; Menzies-Gow, A. The Biology of Eosinophils and Their Role in Asthma. Front. Med. 2017, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Akuthota, P.; Roufosse, F. Eosinophils and Eosinophilic Immune Dysfunction in Health and Disease. Eur. Respir. Rev. 2022, 31, 210150. [Google Scholar] [CrossRef]
- Schwartz, C.; Willebrand, R.; Huber, S.; Rupec, R.A.; Wu, D.; Locksley, R.; Voehringer, D. Eosinophil-Specific Deletion of IκBα in Mice Reveals a Critical Role of NF-ΚB-Induced Bcl-XL for Inhibition of Apoptosis. Blood 2015, 125, 3896–3904. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, N.; Rothenberg, M.E. Receptor Internalization Is Required for Eotaxin-Induced Responses in Human Eosinophils. J. Allergy Clin. Immunol. 2003, 111, 97–105. [Google Scholar] [CrossRef]
- Whetstone, C.E.; Ranjbar, M.; Omer, H.; Cusack, R.P.; Gauvreau, G.M. The Role of Airway Epithelial Cell Alarmins in Asthma. Cells 2022, 11, 1105. [Google Scholar] [CrossRef]
- Proboszcz, M.; Mycroft, K.; Paplinska-Goryca, M.; Górska, K.; Nejman-Gryz, P.; Jankowski, P.; Zak, N.; Krenke, R. Relationship between Blood and Induced Sputum Eosinophils, Bronchial Hyperresponsiveness and Reversibility of Airway Obstruction in Mild-to-Moderate Chronic Obstructive Pulmonary Disease. COPD 2019, 16, 354–361. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Bafadhel, M.; Peterson, S.; De Blas, M.A.; Calverley, P.M.; Rennard, S.I.; Richter, K.; Fagerås, M. Predictors of Exacerbation Risk and Response to Budesonide in Patients with Chronic Obstructive Pulmonary Disease: A Post-Hoc Analysis of Three Randomised Trials. Lancet Respir. Med. 2018, 6, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.H.; Guasconi, A.; Vestbo, J.; Jones, P.; Agusti, A.; Paggiaro, P.; Wedzicha, J.A.; Singh, D. Blood Eosinophils: A Biomarker of Response to Extrafine Beclomethasone/Formoterol in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 523–525. [Google Scholar] [CrossRef] [Green Version]
- Adcock, I.M.; Bhatt, S.P.; Balkissoon, R.; Wise, R.A. The Use of Inhaled Corticosteroids for Patients with COPD Who Continue to Smoke Cigarettes: An Evaluation of Current Practice. Am. J. Med. 2022, 135, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Mkorombindo, T.; Dransfield, M.T. Mepolizumab in the Treatment of Eosinophilic Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1779–1787. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Cole, J.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N. Engl. J. Med 2023. [Google Scholar] [CrossRef] [PubMed]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-Resident Eosinophils Represent a Distinct Regulatory Eosinophil Subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, A.; Yun, Y.; Bui, D.V.; Nguyen, L.M.; Kobayashi, Y.; Suzuki, K.; Mitani, A.; Sawada, S.; Hamada, S.; Asako, M.; et al. The Multiple Functions and Subpopulations of Eosinophils in Tissues under Steady-State and Pathological Conditions. Allergol. Int. 2021, 70, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2020. Available online: http://goldcopd.org/ (accessed on 14 September 2020).
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-Ethnic Reference Values for Spirometry for the 3–95-Yr Age Range: The Global Lung Function 2012 Equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- GINA. Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma—Global Initiative for Asthma—GINA. 2020. Available online: ginasthma.org (accessed on 14 September 2020).
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Djukanović, R.; Sterk, P.J.; Fahy, J.V.; Hargreave, F.E. Standardised Methodology of Sputum Induction and Processing. Eur. Respir. J. 2002, 20 (Suppl. 37), 1s–2s. [Google Scholar] [CrossRef] [Green Version]
- Górska, K.; Paplińska-Goryca, M.; Nejman-Gryz, P.; Goryca, K.; Krenke, R. Eosinophilic and Neutrophilic Airway Inflammation in the Phenotyping of Mild-to-Moderate Asthma and Chronic Obstructive Pulmonary Disease. J. Chronic Obstr. Pulm. Dis. 2017, 14, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Tak, T.; Hilvering, B.; Tesselaar, K.; Koenderman, L. Similar Activation State of Neutrophils in Sputum of Asthma Patients Irrespective of Sputum Eosinophilia. Clin. Exp. Immunol. 2015, 182, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, M.; Lacy, P.; Ueki, S. Eosinophil Extracellular Traps and Inflammatory Pathologies—Untangling the Web! Front. Immunol. 2018, 9, 2763. [Google Scholar] [CrossRef]
- Smyth, L.J.C.; Starkey, C.; Gordon, F.S.; Vestbo, J.; Singh, D. CD8 Chemokine Receptors in Chronic Obstructive Pulmonary Disease. Clin. Exp. Immunol. 2008, 154, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bocchino, V.; Bertorelli, G.; Bertrand, C.P.; Ponath, P.D.; Newman, W.; Franco, C.; Marruchella, A.; Merlini, S.; Del Donno, M.; Zhuo, X.; et al. Eotaxin and CCR3 Are up-Regulated in Exacerbations of Chronic Bronchitis. Allergy 2002, 57, 17–22. [Google Scholar]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef] [Green Version]
- Van Hove, C.L.; Moerloose, K.; Maes, T.; Joos, G.F.; Tournoy, K.G. Cigarette Smoke Enhances Th-2 Driven Airway Inflammation and Delays Inhalational Tolerance. Respir. Res. 2008, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, D.K.; Hirata, F.; Rishi, A.K.; Gairola, C.G. Cigarette Smoke, Inflammation, and Lung Injury: A Mechanistic Perspective. J. Toxicol. Environ. Health Part B Crit. Rev. 2009, 12, 45–64. [Google Scholar] [CrossRef]
- Kelly, M.M.; Leigh, R.; Carruthers, S.; Horsewood, P.; Gleich, G.J.; Hargreave, F.E.; Cox, G. Increased Detection of Interleukin-;5 in Sputum by Addition of Protease Inhibitors. Eur. Respir. J. 2001, 18, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marc-Malovrh, M.; Camlek, L.; Škrgat, S.; Kern, I.; Fležar, M.; Dežman, M.; Korošec, P. Elevated Eosinophils, IL5 and IL8 in Induced Sputum in Asthma Patients with Accelerated FEV1 Decline. Respir. Med. 2020, 162, 105875. [Google Scholar] [CrossRef] [PubMed]
- Larose, M.-C.; Chakir, J.; Archambault, A.-S.; Joubert, P.; Provost, V.; Laviolette, M.; Flamand, N. Correlation between CCL26 Production by Human Bronchial Epithelial Cells and Airway Eosinophils: Involvement in Patients with Severe Eosinophilic Asthma. J. Allergy Clin. Immunol. 2015, 136, 904–913. [Google Scholar] [CrossRef] [Green Version]
- Lokwani, R.; Wark, P.A.; Baines, K.J.; Fricker, M.; Barker, D.; Simpson, J.L. Blood Neutrophils in COPD but Not Asthma Exhibit a Primed Phenotype with Downregulated CD62L Expression. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2517–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veen, J.C.I.; Grootendorst, D.C.; Bel, E.H.; Smits, H.H.; Van Der Keur, M.; Sterk, P.J.; Hiemstra, P.S. CD11b and L-Selectin Expression on Eosinophils and Neutrophils in Blood and Induced Sputum of Patients with Asthma Compared with Normal Subjects. Clin. Exp. Allergy 1998, 28, 606–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockfelt, M.; Christenson, K.; Andersson, A.; Björkman, L.; Padra, M.; Brundin, B.; Ganguly, K.; Asgeirsdottir, H.; Lindén, S.; Qvarfordt, I.; et al. Increased CD11b and Decreased CD62L in Blood and Airway Neutrophils from Long-Term Smokers with and without COPD. J. Innate Immun. 2020, 12, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; van Staveren, S.; van Grinsven, E.; Bartels, M.; Tesselaar, K.; Leijte, G.; Kox, M.; Pickkers, P.; Vrisekoop, N.; Koenderman, L. Characterization of the Phenotype of Human Eosinophils and Their Progenitors in the Bone Marrow of Healthy Individuals. Haematologica 2020, 105, e52–e56. [Google Scholar] [CrossRef]
- Yoon, J.; Terada, A.; Kita, H. CD66b Regulates Adhesion and Activation of Human Eosinophils. J. Immunol. 2007, 179, 8454–8462. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.; Zündorf, J.; Grüger, T.; Brandenburg, K.; Reiners, A.-L.; Zinserling, J.; Schnitzler, N. CD66b Overexpression and Homotypic Aggregation of Human Peripheral Blood Neutrophils after Activation by a Gram-Positive Stimulus. J. Leukoc. Biol. 2012, 91, 791–802. [Google Scholar] [CrossRef]
- Curto, E.; Mateus-Medina, É.F.; Crespo-Lessmann, A.; Osuna-Gómez, R.; Ujaldón-Miró, C.; García-Moral, A.; Galván-Blasco, P.; Soto-Retes, L.; Ramos-Barbón, D.; Plaza, V. Identification of Two Eosinophil Subsets in Induced Sputum from Patients with Allergic Asthma According to CD15 and CD66b Expression. Int. J. Environ. Res. Public Health 2022, 19, 13400. [Google Scholar] [CrossRef]
- Barnig, C.; Alsaleh, G.; Jung, N.; Dembélé, D.; Paul, N.; Poirot, A.; Uring-Lambert, B.; Georgel, P.; de Blay, F.; Bahram, S. Circulating Human Eosinophils Share a Similar Transcriptional Profile in Asthma and Other Hypereosinophilic Disorders. PLoS ONE 2015, 10, e0141740. [Google Scholar] [CrossRef]



| Variable | Control (n = 11) | Asthma (n = 14) | COPD (n = 15) | p-Value |
|---|---|---|---|---|
| Age (years) | 58 (35–63) | 46 (39–61) | 64 (55–72) | 0.046 |
| BMI (kg/m2) | 28.3 (24.1–32.3) | 26 (23–32.5) | 27.5 (23–32) | 0.832 |
| Females, n (%) | 6 (55%) | 10 (71%) | 6 (40%) | 0.47 |
| Atopy, n (%) | 5 (45%) | 10 (71%) | 4 (27%) | 0.049 |
| FEV1 (% pred.) | 89 (86–102) | 89 (84–95) | 66 (59–84) | 0.002 |
| FEV1/FVC% | 71 (70–78) | 74 (64–76) | 53 (41–62) | <0.001 |
| COPD severity according to GOLD 2020 | ||||
| GOLD A, n (%) | n/a | n/a | 4 (27%) | |
| GOLD B, n (%) | n/a | n/a | 10 (68%) | |
| GOLD C, n (%) | n/a | n/a | 1 (7%) | |
| Asthma severity according to GINA 2019 | ||||
| GINA 1, n (%) | n/a | 9 (64%) | n/a | |
| GINA 2, n (%) | n/a | 2 (14%) | n/a | |
| GINA 3, n (%) | n/a | 3 (21%) | n/a | |
| Total IgE (IU/mL) | 73.6 (11.3–270.1) | 49.2 (33.6–150.1) | 30.7 (8.8–79.5) | 0.848 |
| CRP (mg/L) | 2.9 (2.5–3.2) | 2.5 (0.4–4.7) | 1.9 (0.85–3.8) | 0.834 |
| Blood eosinophil count (cells/µL) | 171 (55–185) | 219 (137–390) | 175 (127–282) | 0.554 |
| Blood eosinophil (%) | 2 (1–2) | 3 (4–7) | 2 (1–5) | 0.019 |
| Sputum eosinophil (%) | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0.88 |
| Control (n = 9) | Asthma (n = 12) | COPD (n = 14) | p-Value | |
|---|---|---|---|---|
| IL-5 (pg/mL) | 1.95 (1.61–2.27) | 1.84 (1–1.99) | 2.16 (0.91–2.91) | 0.48 |
| Eotaxin-3 (pg/mL) | 0.22 (0–0.6) | 0 (0–0.13) | 0.26 (0–0.7) | 0.17 |
| IL-13 (pg/m) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.95 |
| CCL5 (pg/mL) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mycroft, K.; Paplińska-Goryca, M.; Proboszcz, M.; Nejman-Gryz, P.; Krenke, R.; Górska, K. Blood and Sputum Eosinophils of COPD Patients Are Differently Polarized than in Asthma. Cells 2023, 12, 1631. https://doi.org/10.3390/cells12121631
Mycroft K, Paplińska-Goryca M, Proboszcz M, Nejman-Gryz P, Krenke R, Górska K. Blood and Sputum Eosinophils of COPD Patients Are Differently Polarized than in Asthma. Cells. 2023; 12(12):1631. https://doi.org/10.3390/cells12121631
Chicago/Turabian StyleMycroft, Katarzyna, Magdalena Paplińska-Goryca, Małgorzata Proboszcz, Patrycja Nejman-Gryz, Rafał Krenke, and Katarzyna Górska. 2023. "Blood and Sputum Eosinophils of COPD Patients Are Differently Polarized than in Asthma" Cells 12, no. 12: 1631. https://doi.org/10.3390/cells12121631
APA StyleMycroft, K., Paplińska-Goryca, M., Proboszcz, M., Nejman-Gryz, P., Krenke, R., & Górska, K. (2023). Blood and Sputum Eosinophils of COPD Patients Are Differently Polarized than in Asthma. Cells, 12(12), 1631. https://doi.org/10.3390/cells12121631








