Unraveling White Adipose Tissue Heterogeneity and Obesity by Adipose Stem/Stromal Cell Biology and 3D Culture Models
Abstract
:1. Introduction
2. White Adipose Tissue
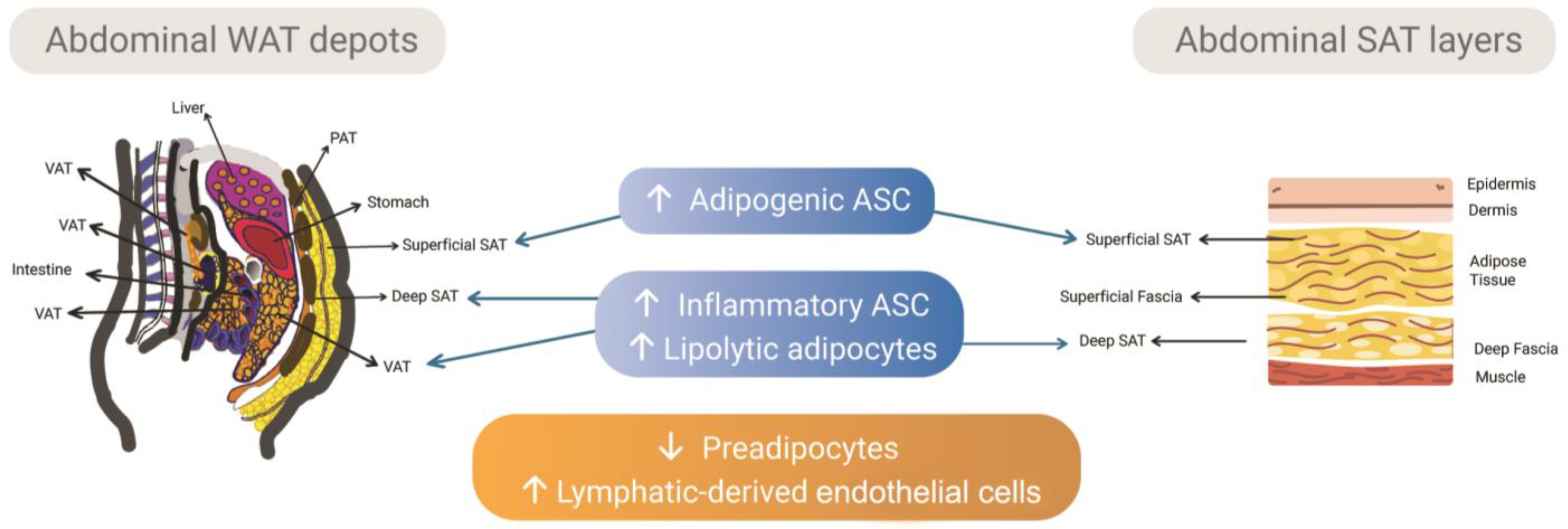
2.1. Subcutaneous and Visceral Depots and the Intrinsic Differences of Adipose Tissue-Derived Stem/Stromal Cells
2.2. Subcutaneous Adipose Tissue Layers as Adipose Tissue-Derived Stem/Stromal Cells Microenvironments
2.3. The Stem/Progenitor Cells Derived from the Fascial System of Subcutaneous Adipose Tissue
2.4. The Dermis Can Be Stratified According to Its Fibroblasts Subpopulations
3. Adipose Tissue 3D Models
3.1. Adipose Tissue Engineering
3.2. Adipose Tissue In Vitro 3D Models for Obesity
3.3. Organoids as Recent Advances in Obese Models
4. Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Habich, C.; Eckel, J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716. [Google Scholar] [CrossRef]
- Silva, K.R.; Baptista, L.S. Adipose-derived stromal/stem cells from different adipose depots in obesity development. World J. Stem. Cells 2019, 11, 147–166. [Google Scholar] [CrossRef]
- Baptista, L.S.; Côrtes, I.; Montenegro, B.; Claudio-da-Silva, C.; Bouschbacher, M.; Jobeili, L.; Auxenfans, C.; Sigaudo-Roussel, D. A novel conjunctive microenvironment derived from human subcutaneous adipose tissue contributes to physiology of its superficial layer. Stem. Cell Res. Ther. 2021, 12, 480. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Dong, Y.; Chen, T.; Xu, G. Generation of functional fat organoid from rat superficial fascia. Adipocyte 2022, 11, 287–300. [Google Scholar] [CrossRef]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose Tissue: Physiology to Metabolic Dysfunction. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Sahu, B.; Bal, N.C. Adipokines from white adipose tissue in regulation of whole body energy homeostasis. Biochimie 2022, 204, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fan, J.; Su, Q.; Yang, Z. Cytokines and Abnormal Glucose and Lipid Metabolism. Front. Endocrinol. (Lausanne) 2019, 10, 703. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. (Lausanne) 2016, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Hepler, C.; Gupta, R.K. The expanding problem of adipose depot remodeling and postnatal adipocyte progenitor recruitment. Mol. Cell. Endocrinol. 2017, 445, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.S.; Prakken, B.; Kalkhoven, E.; Boes, M. Adipose tissue-resident immune cells: Key players in immunometabolism. Trends Endocrinol. Metab. 2012, 23, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Colón-Mesa, I.; Fernández-Galilea, M.; Sáinz, N.; Lopez-Yus, M.; Artigas, J.M.; Arbonés-Mainar, J.M.; Félix-Soriano, E.; Escoté, X.; Moreno-Aliaga, M.J. Regulation of p27 and Cdk2 Expression in Different Adipose Tissue Depots in Aging and Obesity. Int. J. Mol. Sci. 2021, 22, 11745. [Google Scholar] [CrossRef]
- Chinnapaka, S.; Yang, K.S.; Flowers, Q.; Faisal, M.; Nerone, W.V.; Rubin, J.P.; Ejaz, A. Metformin Improves Stemness of Human Adipose-Derived Stem Cells by Downmodulation of Mechanistic Target of Rapamycin (mTOR) and Extracellular Signal-Regulated Kinase (ERK) Signaling. Biomedicines 2021, 9, 1782. [Google Scholar] [CrossRef]
- Silva, K.R.; Côrtes, I.; Liechocki, S.; Carneiro, J.R.I.; Souza, A.A.P.; Borojevic, R.; Maya-Monteiro, C.M.; Baptista, L.S. Characterization of stromal vascular fraction and adipose stem cells from subcutaneous, preperitoneal and visceral morbidly obese human adipose tissue depots. PLoS ONE 2017, 12, e0174115. [Google Scholar] [CrossRef] [Green Version]
- Raajendiran, A.; Ooi, G.; Bayliss, J.; O’brien, P.E.; Schittenhelm, R.B.; Clark, A.K.; Taylor, R.A.; Rodeheffer, M.S.; Burton, P.R.; Watt, M.J. Identification of Metabolically Distinct Adipocyte Progenitor Cells in Human Adipose Tissues. Cell. Rep. 2019, 27, 1528–1540.e7. [Google Scholar] [CrossRef] [Green Version]
- Loureiro, Z.Y.; Solivan-Rivera, J.; Corvera, S. Adipocyte Heterogeneity Underlying Adipose Tissue Functions. Endocrinology 2022, 163, bqab138. [Google Scholar] [CrossRef]
- Joe, A.W.; Yi, L.; Even, Y.; Vogl, A.W.; Rossi, F.M. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 2009, 27, 2563–2570. [Google Scholar] [CrossRef]
- Macotela, Y.; Emanuelli, B.; Mori, M.A.; Gesta, S.; Schulz, T.J.; Tseng, Y.-H.; Kahn, C.R. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 2012, 61, 1691–1699. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, E.; Church, C.D.; Holtrup, B.; Colman, L.; Rodeheffer, M.S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell. Biol. 2015, 17, 376–385. [Google Scholar] [CrossRef]
- Shan, B.; Shao, M.; Zhang, Q.; Hepler, C.; Paschoal, V.A.; Barnes, S.D.; Vishvanath, L.; An, Y.A.; Jia, L.; Malladi, V.S.; et al. Perivascular mesenchymal cells control adipose-tissue macrophage accrual in obesity. Nat. Metab. 2020, 2, 1332–1349. [Google Scholar] [CrossRef] [PubMed]
- Vishvanath, L.; MacPherson, K.A.; Hepler, C.; Wang, Q.A.; Shao, M.; Spurgin, S.B.; Wang, M.Y.; Kusminski, C.M.; Morley, T.S.; Gupta, R.K. Pdgfrβ+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell. Metab. 2016, 23, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Vijay, J.; Gauthier, M.-F.; Biswell, R.L.; Louiselle, D.A.; Johnston, J.J.; Cheung, W.A.; Belden, B.; Pramatarova, A.; Biertho, L.; Gibson, M.; et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat. Metab. 2020, 2, 97–109. [Google Scholar] [CrossRef]
- Mathur, N.; Severinsen, M.C.K.; Jensen, M.E.; Naver, L.; Schrölkamp, M.; Laye, M.J.; Watt, M.J.; Nielsen, S.; Krogh-Madsen, R.; Pedersen, B.K.; et al. Human visceral and subcutaneous adipose stem and progenitor cells retain depot-specific adipogenic properties during obesity. Front. Cell. Dev. Biol. 2022, 10, 983899. [Google Scholar] [CrossRef] [PubMed]
- Divoux, A.; Erdos, E.; Whytock, K.; Osborne, T.F.; Smith, S.R. Transcriptional and DNA Methylation Signatures of Subcutaneous Adipose Tissue and Adipose-Derived Stem Cells in PCOS Women. Cells 2022, 11, 848. [Google Scholar] [CrossRef] [PubMed]
- Zuccarini, M.; Giuliani, P.; Di Liberto, V.; Frinchi, M.; Caciagli, F.; Caruso, V.; Ciccarelli, R.; Mudò, G.; Di Iorio, P. Adipose Stromal/Stem Cell-Derived Extracellular Vesicles: Potential Next-Generation Anti-Obesity Agents. Int. J. Mol. Sci. 2022, 23, 1543. [Google Scholar] [CrossRef] [PubMed]
- Abate, N.; Garg, A.; Peshock, R.M.; Stray-Gundersen, J.; Grundy, S.M. Relationships of generalized and regional adiposity to insulin sensitivity in men. J. Clin. Investig. 1995, 96, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, Y.; DeFronzo, R.A. Visceral fat dominant distribution in male type 2 diabetic patients is closely related to hepatic insulin resistance, irrespective of body type. Cardiovasc. Diabetol. 2009, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, T.; Lamendola, C.; Liu, A.; Abbasi, F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J. Clin. Endocrinol. Metab. 2011, 96, E1756–E1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotta, L.A.; Wittemans, L.B.L.; Zuber, V.; Stewart, I.D.; Sharp, S.J.; Luan, J.; Day, F.R.; Li, C.; Bowker, N.; Cai, L.; et al. Association of genetic variants related to gluteofemoral vs abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA 2018, 320, 2553–2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, B.; Barker, C.S.; Shao, M.; Zhang, Q.; Gupta, R.K.; Wu, Y. Multilayered omics reveal sex- and depot-dependent adipose progenitor cell heterogeneity. Cell. Metab. 2022, 34, 783–799.e7. [Google Scholar] [CrossRef]
- Ong, W.K.; Chakraborty, S.; Sugii, S. Adipose Tissue: Understanding the Heterogeneity of Stem Cells for Regenerative Medicine. Biomolecules 2021, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, R.; Rainer, P.; Deplancke, B. Toward a Consensus View of Mammalian Adipocyte Stem and Progenitor Cell Heterogeneity. Trends Cell. Biol. 2020, 30, 937–950. [Google Scholar] [CrossRef]
- Misra, A.; Garg, A.; Abate, N.; Peshock, R.M.; Stray-Gundersen, J.; Grundy, S.M. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes. Res. 1997, 5, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Herlin, C.; Chica-Rosa, A.; Subsol, G.; Gilles, B.; Macri, F.; Beregi, J.P.; Captier, G. Three-dimensional study of the skin/subcutaneous complex using in vivo whole body 3T MRI: Review of the literature and confirmation of a generic pattern of organization. Surg. Radiol. Anat. 2015, 37, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Golan, R.; Shelef, I.; Rudich, A.; Gepner, Y.; Shemesh, E.; Chassidim, Y.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Ben Avraham, S.; et al. Abdominal superficial subcutaneous fat: A putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care 2012, 35, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Marinou, K.; Hodson, L.; Vasan, S.K.; Fielding, B.A.; Banerjee, R.; Brismar, K.; Koutsilieris, M.; Clark, A.; Neville, M.J.; Karpe, F. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care 2014, 37, 821–829. [Google Scholar] [CrossRef] [Green Version]
- Boulet, N.; Estève, D.; Bouloumié, A.; Galitzky, J. Cellular heterogeneity in superficial and deep subcutaneous adipose tissues in overweight patients. J. Physiol. Biochem. 2013, 19, 170–180. [Google Scholar] [CrossRef]
- Cancello, R.; Zulian, A.; Gentilini, D.; Maestrini, S.; Della Barba, A.; Invitti, C.; Corà, D.; Caselle, M.; Liuzzi, A.; Di Blasio, A.M. Molecular and morphologic characterization of superficial- and deep-subcutaneous adipose tissue subdivisions in human obesity. Obesity 2013, 21, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Kubota, Y.; Adachi, N.; Akita, S.; Sasahara, Y.; Kira, T.; Kuroda, M.; Mitsukawa, N.; Bujo, H.; Satoh, K. Human adipocytes from the subcutaneous superficial layer have greater adipogenic potential and lower PPAR-γ DNA methylation levels than deep layer adipocytes. Am. J. Physiol. Cell. Physiol. 2016, 311, C322–C329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.E.; Kim, S.J.; Yang, S.-J.; Joo, S.-Y.; Park, H.; Lee, K.W.; Yang, H.-M.; Park, J.B. Comparative characterization of mesenchymal stromal cells from multiple abdominal adipose tissues and enrichment of angiogenic ability via CD146 molecule. Cytotherapy 2017, 19, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Morandi, E.M.; Rainer, J.; Grubwieser, P.; Heinz, K.; Wolfram, D.; Bernhard, D.; Lobenwein, S.; Pierer, G.; Ploner, C. Human Macrophages Preferentially Infiltrate the Superficial Adipose Tissue. Int. J. Mol. Sci. 2018, 19, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Péault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal vascular progenitors in adult human adipose tissue. Cytom. A 2010, 77, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Chung, J.-H.; Song, S.-W.; Jung, W.S.; Lee, Y.-A.; Kim, H.-N. Relationship between deep subcutaneous abdominal adipose tissue and metabolic syndrome: A case control study. Diabetol. Metabol. Syndr. 2016, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Hausman, G.J. The origin and purpose of layers of subcutaneous adipose tissue in pigs and man. Horm. Mol. Biol. Clin. Investig. 2018, 33. [Google Scholar] [CrossRef]
- Monzon, J.R.; Basile, R.; Heneghan, S.; Udupi, V.; Green, A. Lipolysis in adipocytes isolated from deep and superficial subcutaneous adipose tissue. Obes. Res. 2002, 10, 266–269. [Google Scholar] [CrossRef]
- Bordoni, B.; Marelli, F.; Morabito, B.; Sacconi, B. The indeterminable resilience of the fascial system. J. Integr. Med. 2017, 15, 337–343. [Google Scholar] [CrossRef]
- Armstrong, C. The architecture and spatial organization of the living human body as revealed by intratissular endoscopy—An osteopathic perspective. Bodyw. Mov. Ther. 2020, 24, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Lyu, Y.; Wang, W.; Zhang, Y.; Li, D.; Wei, S.; Du, C.; Geng, B.; Sztalryd, C.; Xu, G. Fascia Origin of Adipose Cells. Stem Cells 2016, 34, 1407–1419. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, X.; Dong, Y.; Chen, T.; Zhang, Y.; Wu, B.; Li, H.; Sun, X.; Xia, L.; Zhang, D.; et al. Cytological and functional characteristics of fascia adipocytes in rats: A unique population of adipocytes. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2020, 1865, 158585. [Google Scholar] [CrossRef]
- Stecco, C.; Schleip, R. A Fascia and the Fascial System. Bodyw. Mov. Ther. 2016, 20, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, M.E.; Sorensen, A.M.; Banyard, D.A.; Sayadi, L.R.; Chnari, E.; Hatch, M.M.; Tassey, J.; Mirzakhanyan, Y.; Gershon, P.D.; Hughes, C.C.W.; et al. Deconstructing Allograft Adipose and Fascia Matrix: Fascia Matrix Improves Angiogenesis, Volume Retention, and Adipogenesis in a Rodent Model. Plast. Reconstr. Surg. 2023, 151, 108–117. [Google Scholar] [CrossRef]
- Baptista, L.S.; Silva, K.R.; Pedrosa, C.S.; Claudio-da-Silva, C.; Carneiro, J.R.; Aniceto, M.; de Mello-Coelho, V.; Takiya, C.M.; Rossi, M.I.; Borojevic, R. Adipose tissue of control and ex-obese patients exhibit differences in blood vessel content and resident mesenchymal stem cell population. Obes. Surg. 2009, 19, 1304–1312. [Google Scholar] [CrossRef]
- Silva, K.R.; Liechocki, S.; Carneiro, J.R.; Claudio-Da-Silva, C.; Maya-Monteiro, C.M.; Borojevic, R.; Baptista, L.S. Stromal-vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric surgery ex-obese women. Stem Cell. Res. Ther. 2015, 6, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauroy, P.; Barruche, V.; Marchand, L.; Nindorera-Badara, S.; Bordes, S.; Closs, B.; Ruggiero, F. Human Dermal Fibroblast Subpopulations Display Distinct Gene Signatures Related to Cell Behaviors and Matrisome. J. Investig. Dermatol. 2017, 137, 1787–1789. [Google Scholar] [CrossRef]
- Harper, R.A.; Grove, G. Human skin fibroblasts derived from papillary and reticular dermis: Differences in growth potential in vitro. Science 1979, 204, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Mine, S.; Fortunel, N.O.; Pageon, H.; Asselineau, D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: A new view of skin morphogenesis and aging. PLoS ONE 2008, 3, e4066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauroux, A.; Joncour, P.; Gillet, B.; Hughes, S.; Ardidie-Robouant, C.; Marchand, L.; Liabotis, A.; Mailly, P.; Monnot, C.; Germain, S.; et al. Papillary and Reticular Fibroblasts Generate Distinct Microenvironments that Differentially Impact Angiogenesis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, C.A.; Kretzschmar, K.; Watt, F.M. Reprogramming adult dermis to a neonatal state through epidermal activation of β-catenin. Development 2011, 138, 5189–5199. [Google Scholar] [CrossRef] [Green Version]
- Haydont, V.; Neiveyans, V.; Perez, P.; Busson, É.; Lataillade, J.-J.; Asselineau, D.; Fortunel, N.O. Fibroblasts from the Human Skin Dermo-Hypodermal Junction are Distinct from Dermal Papillary and Reticular Fibroblasts and from Mesenchymal Stem Cells and Exhibit a Specific Molecular Profile Related to Extracellular Matrix Organization and Modeling. Cells 2020, 9, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H.; Yang, J.-M.; Park, S.-B.; Kwak, S.-J.; Park, J.-S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jin, S.Y.; Song, J.S.; Seo, K.K.; Cho, K.H. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann. Dermatol. 2012, 24, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Broadbent, J.A.; Huan, J.; Yang, H. The Effects of Adipose Stem Cell-Conditioned Media on Fibrogenesis of Dermal Fibroblasts Stimulated by Transforming Growth Factor-β1. J. Burn Care Res. 2018, 39, 129–140. [Google Scholar] [CrossRef]
- Kim, W.-S.; Park, B.-S.; Park, S.-H.; Kim, H.-K.; Sung, J.-H. Antiwrinkle effect of adipose-derived stem cell: Activation of dermal fibroblast by secretory factors. J. Dermatol. Sci. 2009, 53, 96–102. [Google Scholar] [CrossRef]
- Auxenfans, C.; Mojallal, A.; Rachidi, W.; Metral, E.; Thepot, A.; Damour, O.; dos Santos, M. Adipose-derived Stem Cells Promote Skin Homeostasis and Prevent its Senescence in an In vitro Skin Model. J. Stem Cell. Res. Ther. 2014, 4. [Google Scholar] [CrossRef]
- Søndergaard, R.H.; Højgaard, L.D.; Reese-Petersen, A.L.; Hoeeg, C.; Mathiasen, A.B.; Haack-Sørensen, M.; Follin, B.; Genovese, F.; Kastrup, J.; Juhl, M.; et al. Adipose-derived stromal cells increase the formation of collagens through paracrine and juxtacrine mechanisms in a fibroblast co-culture model utilizing macromolecular crowding. Stem Cell. Res. Ther. 2022, 13, 250. [Google Scholar] [CrossRef]
- Spiekman, M.; Przybyt, E.; Plantinga, J.A.; Gibbs, S.; van der Lei, B.; Harmsen, M.C. Adipose tissue-derived stromal cells inhibit TGF-β1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast. Reconstr. Surg. 2014, 134, 699–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrelli, M.R.; Patel, R.A.; Adem, S.; Diaz Deleon, N.M.; Shen, A.H.; Sokol, J.; Yen, S.; Chang, E.Y.; Nazerali, R.; Nguyen, D.; et al. The antifibrotic adipose-derived stromal cell: Grafted fat enriched with CD74+ adipose-derived stromal cells reduces chronic radiation-induced skin fibrosis. Stem Cells Transl. Med. 2020, 9, 1401–1413. [Google Scholar] [CrossRef]
- Su, W.; Yu, S.; Yin, Y.; Li, B.; Xue, J.; Wang, J.; Gu, Y.; Zhang, H.; Lyu, Z.; Mu, Y.; et al. Diabetic microenvironment preconditioning of adipose tissue-derived mesenchymal stem cells enhances their anti-diabetic, anti-long-term complications, and anti-inflammatory effects in type 2 diabetic rats. Stem Cell. Res. Ther. 2022, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Kenny, E.M.; Egro, F.M.; Ejaz, A.; Coleman, S.R.; Greenberger, J.S.; Rubin, J.P. Fat Grafting in Radiation-Induced Soft-Tissue Injury: A Narrative Review of the Clinical Evidence and Implications for Future Studies. Plast. Reconstr. Surg. 2021, 147, 819–838. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Shen, A.H.; Lee, G.K.; Momeni, A.; Longaker, M.T.; Wan, D.C. Radiation-Induced Skin Fibrosis: Pathogenesis, Current Treatment Options, and Emerging Therapeutics. Ann. Plast. Surg. 2019, 83, S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Deleon, N.M.D.; Adem, S.; Lavin, C.V.; Abbas, D.B.; Griffin, M.; King, M.E.; Borrelli, M.R.; Patel, R.A.; Fahy, E.J.; Lee, D.; et al. Angiogenic CD34+CD146+ adipose-derived stromal cells augment recovery of soft tissue after radiotherapy. J. Tissue Eng. Regen. Med. 2021, 15, 1105–1117. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, C. Tissue engineering strategies for adipose tissue repair. Anat. Rec. 2001, 263, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Halbleib, M.; Skurk, T.; de Luca, C.; von Heimburg, D.; Hauner, H. Tissue engineering of white adipose tissue using hyaluronic acid-based scaffolds. I: In vitro differentiation of human adipocyte precursor cells on scaffolds. Biomaterials 2003, 24, 3125–3132. [Google Scholar] [CrossRef]
- Mauney, J.R.; Nguyen, T.; Gillen, K.; Kirker-Head, C.; Gimble, J.M.; Kaplan, D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 2007, 28, 5280–5290. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Jiang, X.; Yang, Z.; Lai, X.; He, X.; Wu, P.; Liu, H. Xenograft-decellularized adipose tissue supports adipose remodeling in rabbit. Biochem. Biophys. Res. Commun. 2022, 635, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yuan, Z.; Guo, L.; Nurzat, Y.; Xu, H.; Zhang, Y. Construction of adipose tissue using a silica expander capsule and cell sheet-assembled of decellularized adipose tissue. Acta Biomater. 2022, 141, 89–101. [Google Scholar] [CrossRef]
- Young, D.A.; Ibrahim, D.O.; Hu, D.; Christman, K.L. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 2011, 7, 1040–1049. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Johnson, J.A.; Zhang, Q.; Beahm, E.K. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomater. 2013, 9, 8921–8931. [Google Scholar] [CrossRef] [Green Version]
- Cheung, H.K.; Han, T.T.Y.; Marecak, D.M.; Watkins, J.F.; Amsden, B.G.; Flynn, L.E. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials 2014, 35, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, O.A.; Motherwell, J.M.; Rogers, E.; Bratton, M.R.; Zhang, Q.; Wang, G.; Bunnell, B.; Hayes, D.J.; Gimble, J.M. Characterization and Proteomic Analysis of Decellularized Adipose Tissue Hydrogels Derived from Lean and Overweight/Obese Human Donors. Adv. Biosyst. 2020, 4, e2000124. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Yang, H.-J.; Kim, B.S.; Kim, J.D.; Lee, S.H.; Lee, E.K.; Park, K.; Cho, Y.W.; Lee, H.Y. Fabrication of porous extracellular matrix scaffolds from human adipose tissue. Tissue Eng. Part C Methods 2010, 16, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Mohiuddin, O.A.; O’donnell, B.T.; Poche, J.N.; Iftikhar, R.; Wise, R.M.; Motherwell, J.M.; Campbell, B.; Savkovic, S.D.; Bunnell, B.A.; Hayes, D.J.; et al. Human. adipose-derived hydrogel characterization based on in vitro ASC biocompatibility and differentiation. Stem Cells Int. 2019, 2019, 9276398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, L.E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 2010, 31, 4715–4724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, J.; Bai, S. Biocompatibility of injectable hydrogel from decellularized human adipose tissue in vitro and in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1684–1694. [Google Scholar] [CrossRef]
- Han, T.T.Y.; Toutounji, S.; Amsden, B.G.; Flynn, L.E. Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials 2015, 72, 125–137. [Google Scholar] [CrossRef]
- Sawadkar, P.; Mandakhbayar, N.; Patel, K.D.; Buitrago, J.O.; Kim, T.H.; Rajasekar, P.; Lali, F.; Kyriakidis, C.; Rahmani, B.; Mohanakrishnan, J.; et al. Three dimensional porous scaffolds derived from collagen, elastin and fibrin proteins orchestrate adipose tissue regeneration. J. Tissue Eng. 2021, 12, 20417314211019238. [Google Scholar] [CrossRef] [PubMed]
- Baptista, L.S.; Kronemberger, G.S.; Côrtes, I.; Charelli, L.E.; Matsui, R.A.M.; Palhares, T.N.; Sohier, J.; Rossi, A.M.; Granjeiro, J.M. Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1285. [Google Scholar] [CrossRef] [Green Version]
- Achilli, T.M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert. Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [Green Version]
- Ida, Y.; Sato, T.; Umetsu, A.; Watanabe, M.; Furuhashi, M.; Hikage, F.; Ohguro, H. Addition of ROCK Inhibitors Alleviates Prostaglandin-Induced Inhibition of Adipogenesis in 3T3L-1 Spheroids. Bioengineering 2022, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, A.; Ida, Y.; Sato, T.; Watanabe, M.; Tsugeno, Y.; Furuhashi, M.; Hikage, F.; Ohguro, H. Brimonidine Modulates the ROCK1 Signaling Effects on Adipogenic Differentiation in 2D and 3D 3T3-L1 Cells. Bioengineering 2022, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Avelino, T.M.; García-Arévalo, M.; Torres, F.R.; Goncalves Dias, M.M.; Domingues, R.R.; de Carvalho, M.; Fonseca, M.C.; Rodrigues, V.K.T.; Leme, A.F.P.; Figueira, A.C.M. Mass spectrometry-based proteomics of 3D cell culture: A useful tool to validate culture of spheroids and organoids. SLAS Discov. 2022, 27, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Ohguro, H.; Ida, Y.; Hikage, F.; Umetsu, A.; Ichioka, H.; Watanabe, M.; Furuhashi, M. STAT3 Is the Master Regulator for the Forming of 3D Spheroids of 3T3-L1 Preadipocytes. Cells 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Song, L.; Wang, J.; Yan, S.; Li, G.; Cui, L.; Yin, J. Strategy for constructing vascularized adipose units in poly(l-glutamic acid) hydrogel porous scaffold through inducing in-situ formation of ASCs spheroids. Acta Biomater. 2017, 51, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Ader, I.; Creff, J.; Leménager, H.; Achard, P.; Casteilla, L.; Sensebé, L.; Carrière, A.; Deschaseaux, F. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci. Rep. 2019, 9, 7250. [Google Scholar] [CrossRef]
- Turner, P.A.; Tang, Y.; Weiss, S.J.; Janorkar, A.V. Three-dimensional spheroid cell model of in vitro adipocyte inflammation. Tissue Eng. Part A 2015, 21, 1837–1847. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.A.; Gurumurthy, B.; Bailey, J.L.; Elks, C.M.; Janorkar, A.V. Adipogenic Differentiation of Human Adipose-Derived Stem Cells Grown as Spheroids. Process. Biochem. 2017, 59, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Pieters, V.M.; Rjaibi, S.T.; Singh, K.; Li, N.T.; Khan, S.T.; Nunes, S.S.; Cin, A.D.; Gilbert, P.M.; McGuigan, A.P. A three-dimensional human adipocyte model of fatty acid-induced obesity. Biofabrication 2022, 14, 045009. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.B.; Kim, Y.E.; Yoo, H.M.; Hong, J.; Choi, K.-J.; Kim, K.Y.; Kang, D. iTRAQ-Based Quantitative Proteomic Comparison of 2D and 3D Adipocyte Cell Models Co-cultured with Macrophages Using Online 2D-nanoLC-ESI-MS/MS. Sci. Rep. 2019, 9, 16746. [Google Scholar] [CrossRef] [Green Version]
- Park, S.B.; Lee, S.Y.; Jung, W.H.; Lee, J.; Jeong, H.G.; Hong, J.; Kang, D.; Kim, K.Y. Development of in vitro three-dimensional co-culture system for metabolic syndrome therapeutic agents. Diabetes Obes. Metab. 2019, 21, 1146–1157. [Google Scholar] [CrossRef]
- Choi, K.J.; Lee, J.H.; Park, S.B.; Na, Y.-J.; Jung, W.H.; Lee, H.; Kim, K.Y. Development of in vitro three-dimensional drug screening system for obesity-related metabolic syndrome. J. Pharmacol. Sci. 2022, 148, 377–386. [Google Scholar] [CrossRef]
- Park, S.B.; Koh, B.; Jung, W.H.; Choi, K.J.; Na, Y.J.; Yoo, H.M.; Lee, S.; Kang, D.; Lee, D.; Kim, K.Y. Development of a three-dimensional in vitro co-culture model to increase drug selectivity for humans. Diabetes Obes. Metab. 2020, 22, 1302–1315. [Google Scholar] [CrossRef]
- Guria, S.; Hoory, A.; Das, S.; Chattopadhyay, D.; Mukherjee, S. Adipose tissue macrophages and their role in obesity-associated insulin resistance: An overview of the complex dynamics at play. Biosci. Rep. 2023, 43, BSR20220200. [Google Scholar] [CrossRef]
- Rajangam, T.; Park, M.H.; Kim, S.-H. 3D Human Adipose-Derived Stem Cell Clusters as a Model for In Vitro Fibrosis. Tissue Eng. Part C Methods 2016, 22, 679–690. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Daquinag, A.C.; Souza, G.R.; Kolonin, M.G. Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng. Part C Methods 2013, 19, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Ioannidou, A.; Alatar, S.; Schipper, R.; Baganha, F.; Åhlander, M.; Hornell, A.; Fisher, R.M.; Hagberg, C.E. Hypertrophied human adipocyte spheroids as in vitro model of weight gain and adipose tissue dysfunction. J. Physiol. 2022, 600, 869–883. [Google Scholar] [CrossRef]
- Panoutsopoulos, A.A. Organoids, assembloids, and novel biotechnology: Steps forward in developmental and disease-related neuroscience. Neuroscientist 2021, 27, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Baptista, L.S.; Porrini, C.; Kronemberger, G.S.; Kelly, D.J.; Perrault, C.M. 3D organ-on-a-chip: The convergence of microphysiological systems and organoids. Front. Cell. Dev. Biol. 2022, 10, 1043117. [Google Scholar] [CrossRef]
- Mandl, M.; Viertler, H.P.; Hatzmann, F.M.; Brucker, C.; Großmann, S.; Waldegger, P.; Rauchenwald, T.; Mattesich, M.; Zwierzina, M.; Pierer, G.; et al. An organoid model derived from human adipose stem/progenitor cells to study adipose tissue physiology. Adipocyte 2022, 11, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; McCarthy, M.; Brown, T.; Bukowska, J.; Smith, S.; Abbott, R.D.; Kaplan, D.L.; Williams, C.; Wade, J.W.; Alarcon, A.; et al. Human adipose derived cells in two- and three-dimensional cultures: Functional validation of an in vitro fat construct. Stem Cells Int. 2020, 2020, 4242130. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; Kamm, R.D.; Lopes, S.M.C.d.S.; Lancaster, M.A.; Weiss, R.; Trepat, X.; Hyun, I.; Montserrat, N. Rethinking organoid technology through bioengineering. Nat. Mater. 2021, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef]
- Paek, J.; Park, S.E.; Lu, Q.; Park, K.-T.; Cho, M.; Oh, J.M.; Kwon, K.W.; Yi, Y.-S.; Song, J.W.; Edelstein, H.I.; et al. Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues. ACS Nano 2019, 13, 7627–7643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Deng, P.; Tao, T.; Liu, H.; Wu, S.; Chen, W.; Qin, J. Modeling human nonalcoholic fatty liver disease (NAFLD) with an organoids-on-a-chip system. ACS Biomater. Sci. Eng. 2020, 6, 5734–5743. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Brown, T.; Alarcon, M.A.; Williams, C.; Wu, X.; Abbott, R.D.; Gimble, J.M.; Frazier, T. Fat-On-A-Chip Models for Research and Discovery in Obesity and Its Metabolic Comorbidities. Tissue Eng. Part B Rev. 2020, 26, 586–595. [Google Scholar] [CrossRef]
- Pope, B.D.; Warren, C.R.; Dahl, M.O.; Pizza, C.V.; Henze, D.E.; Sinatra, N.R.; Gonzalez, G.M.; Chang, H.; Liu, Q.; Glieberman, A.L.; et al. Fattening chips: Hypertrophy, feeding, and fasting of human white adipocytes in vitro. Lab. Chip 2020, 20, 4152–4165. [Google Scholar] [CrossRef] [PubMed]
- Rogal, J.; Roosz, J.; Teufel, C.; Cipriano, M.; Xu, R.; Eisler, W.; Weiss, M.; Schenke-Layland, K.; Loskill, P. Autologous Human Immunocompetent White Adipose Tissue-on-Chip. Adv. Sci. 2022, 9, e2104451. [Google Scholar] [CrossRef] [PubMed]
- Compera, N.; Atwell, S.; Wirth, J.; von Törne, C.; Hauck, S.M.; Meier, M. Adipose microtissue-on-chip: A 3D cell culture platform for differentiation, stimulation, and proteomic analysis of human adipocytes. Lab. Chip 2022, 22, 3172–3186. [Google Scholar] [CrossRef] [PubMed]


| Spheroids | Organoids | |
|---|---|---|
| Type of cells | Mature, progenitor or stem cells | Stem and progenitor cells |
| Self-assembly | Yes | In epithelial tissues is guided by the presence of a hydrogel |
| Morphology | Spheroidal | Diverse |
| Extracellular matrix | Strictly synthesized by cells | May contain exogenous components |
| 3D architecture | Non-mimetic | Mimetic |
| Physiological function | Not necessarily | Yes |
| Morphogenesis | Only present when spheroid is derived from progenitor or stem cells | Present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, L.S.; Silva, K.R.; Jobeili, L.; Guillot, L.; Sigaudo-Roussel, D. Unraveling White Adipose Tissue Heterogeneity and Obesity by Adipose Stem/Stromal Cell Biology and 3D Culture Models. Cells 2023, 12, 1583. https://doi.org/10.3390/cells12121583
Baptista LS, Silva KR, Jobeili L, Guillot L, Sigaudo-Roussel D. Unraveling White Adipose Tissue Heterogeneity and Obesity by Adipose Stem/Stromal Cell Biology and 3D Culture Models. Cells. 2023; 12(12):1583. https://doi.org/10.3390/cells12121583
Chicago/Turabian StyleBaptista, Leandra S., Karina R. Silva, Lara Jobeili, Lucile Guillot, and Dominique Sigaudo-Roussel. 2023. "Unraveling White Adipose Tissue Heterogeneity and Obesity by Adipose Stem/Stromal Cell Biology and 3D Culture Models" Cells 12, no. 12: 1583. https://doi.org/10.3390/cells12121583
APA StyleBaptista, L. S., Silva, K. R., Jobeili, L., Guillot, L., & Sigaudo-Roussel, D. (2023). Unraveling White Adipose Tissue Heterogeneity and Obesity by Adipose Stem/Stromal Cell Biology and 3D Culture Models. Cells, 12(12), 1583. https://doi.org/10.3390/cells12121583





