Abstract
The genus Aspergillus, one of the most abundant airborne fungi, is classified into hundreds of species that affect humans, animals, and plants. Among these, Aspergillus nidulans, as a key model organism, has been extensively studied to understand the mechanisms governing growth and development, physiology, and gene regulation in fungi. A. nidulans primarily reproduces by forming millions of asexual spores known as conidia. The asexual life cycle of A. nidulans can be simply divided into growth and asexual development (conidiation). After a certain period of vegetative growth, some vegetative cells (hyphae) develop into specialized asexual structures called conidiophores. Each A. nidulans conidiophore is composed of a foot cell, stalk, vesicle, metulae, phialides, and 12,000 conidia. This vegetative-to-developmental transition requires the activity of various regulators including FLB proteins, BrlA, and AbaA. Asymmetric repetitive mitotic cell division of phialides results in the formation of immature conidia. Subsequent conidial maturation requires multiple regulators such as WetA, VosA, and VelB. Matured conidia maintain cellular integrity and long-term viability against various stresses and desiccation. Under appropriate conditions, the resting conidia germinate and form new colonies, and this process is governed by a myriad of regulators, such as CreA and SocA. To date, a plethora of regulators for each asexual developmental stage have been identified and investigated. This review summarizes our current understanding of the regulators of conidial formation, maturation, dormancy, and germination in A. nidulans.
1. Introduction
Filamentous fungi are eukaryotic organisms that are ubiquitous in our surrounding environments, and they have large and small effects on human life [1,2,3]. Several fungi, such as Fusarium, Aspergillus, and Penicillium, produce mycotoxins that can cause plant diseases or contaminate stored foods, leading to economic losses [4]. Some fungi can directly exert adverse clinical effects on humans [1]. Conversely, other filamentous fungi have been used as industrial cell factories for producing various proteins because of their efficient secretion and sustainable production systems [5,6]. Therefore, to suppress side effects or maximize positive effects, it is important to understand the biological characteristics of filamentous fungi.
Aspergillus is one of the filamentous fungi that comprise the maximum proportion of airborne organisms. Among the numerous species, Aspergillus flavus is a saprophytic fungus that contaminates preharvest and postharvest crops and produces potent hepatocarcinogenic secondary metabolite aflatoxins; it is known to be the second leading cause of invasive aspergillosis [7,8]. Aspergillus fumigatus is an opportunistic pathogenic fungus that causes life-threatening disease (aspergillosis) in immunocompromised individuals [9,10]. In contrast, Aspergillus niger and Aspergillus oryzae are biochemical cell factories in the fermentation and enzyme industry, which efficiently produce several enzymes and useful secondary metabolites [11,12]. These Aspergillus species have some limitations in controlling and handling for research; therefore, many scientists have explored the specie Aspergillus nidulans for decades as a referential model organism to uncover fungal growth, asexual/sexual development, spore properties, germination, and secondary metabolites [13,14,15].
The asexual spore (conidium) is the primary reproductive structure with long-term viability and contains various secondary metabolites including mycotoxins [14,16,17]. Conidia, which float through the air, settle on crops, foods, or humans; consume organic/nonorganic nutrients; and grow vegetatively, by expanding their habitats. In the presence of some stimuli, growing hyphae form thick-walled foot cells and branch to the aerial stalks. Swollen stalks successively form multinucleate vesicles, metulae, and phialides and finally develop conidiophores with immature conidia [15,18,19]. Then, the conidia on the conidiophores are matured and remain in the dormant stage. They transform their external structures, tolerate environmental stresses, maintain long-term viability, and prepare for the next stage of development through the activity of transcription and translation [20,21]. Under favorable conditions, quiescent conidia establish isotropic growth and germinate by producing the germ tubes. This lifespan of asexual spores may be delicately regulated by complicated and efficient mechanisms of genetic regulators.
This review describes the genetic regulatory factors involved in each developmental stage of A. nidulans conidia, from conidiogenesis, conidial maturation and dormancy to conidial germination. A comprehensive understanding of the functions of various regulators in the model organism A. nidulans can help prevent the formation of conidia that act as infectious particles, i.e., spores, or induce the maximal production of desired enzymes and proteins in filamentous fungi.
2. Research on A. nidulans Asexual Spores
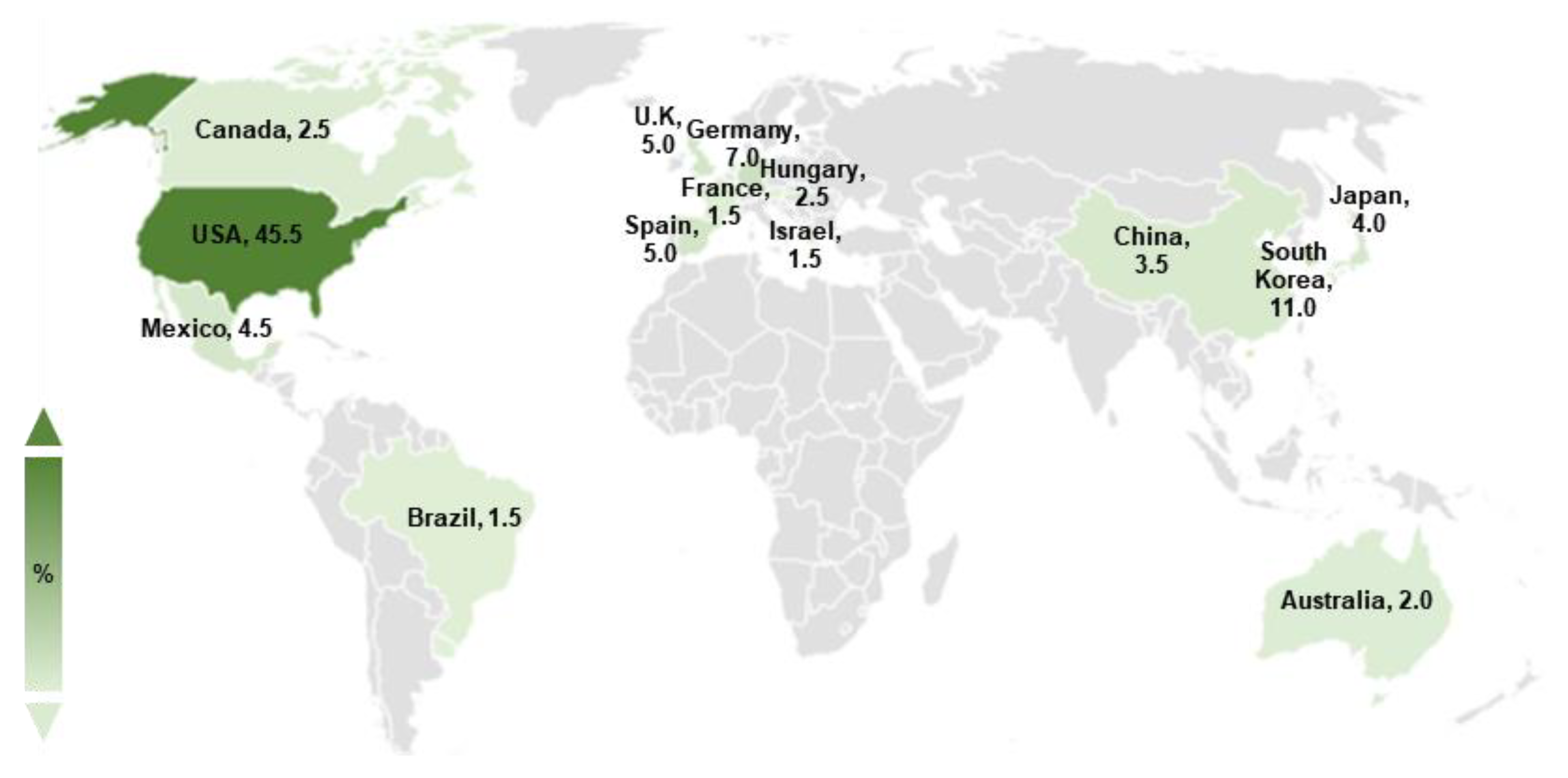
Basic scientific studies on A. nidulans spores have been conducted for decades. To gain a broad understanding of various genetic regulators in conidia, we searched the keyword “Aspergillus nidulans spore” in the PubMed database. We obtained 648 articles published from 1969 to 2022, of which 200 studies explored the genetic regulatory factors in A. nidulans asexual spores. Studies on the regulators of A. nidulans conidia have been actively conducted worldwide, including in America, Asia, and Europe, with scientists primarily located in the USA, South Korea, and Germany (Figure 1).
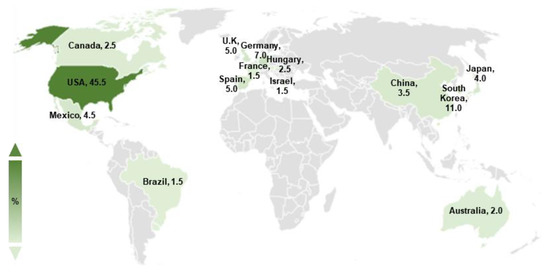
Figure 1.
Studies on Aspergillus nidulans asexual spores. The worldwide distribution of studies on A. nidulans spores. The darkness of color indicates a high ratio of published articles. Only countries where the ratio of studies on A. nidulans spores is >1% are indicated.
A. nidulans conidia undergo a series of processes to form a specialized developmental structure known as the conidiophore. At the tips of conidiophores, the matured conidia remain in the dormant state and then start germinating and producing germ tubes through the activation of transcription and translation [18,21]. The life cycle of A. nidulans conidia is closely related to many regulators. To understand the development of A. nidulans, all of the regulatory factors, searched in PubMed (200 papers), were summarized by each developmental stage. Among them, we focused on the transcription factors, and how they act and organize their regulatory network. We also described major signaling pathways in A. nidulans conidia.
3. Conidiogenesis
Differentiated vegetative hyphae develop thick-walled foot cells and form conidiophores. An overview of the roles of regulators coordinating the formation of asexual structures and spores in A. nidulans is provided in Figure 2 and Table 1 and Table 2.
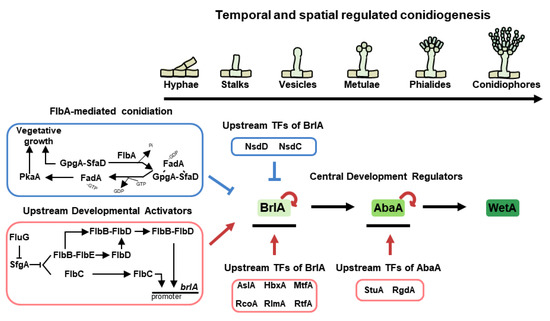
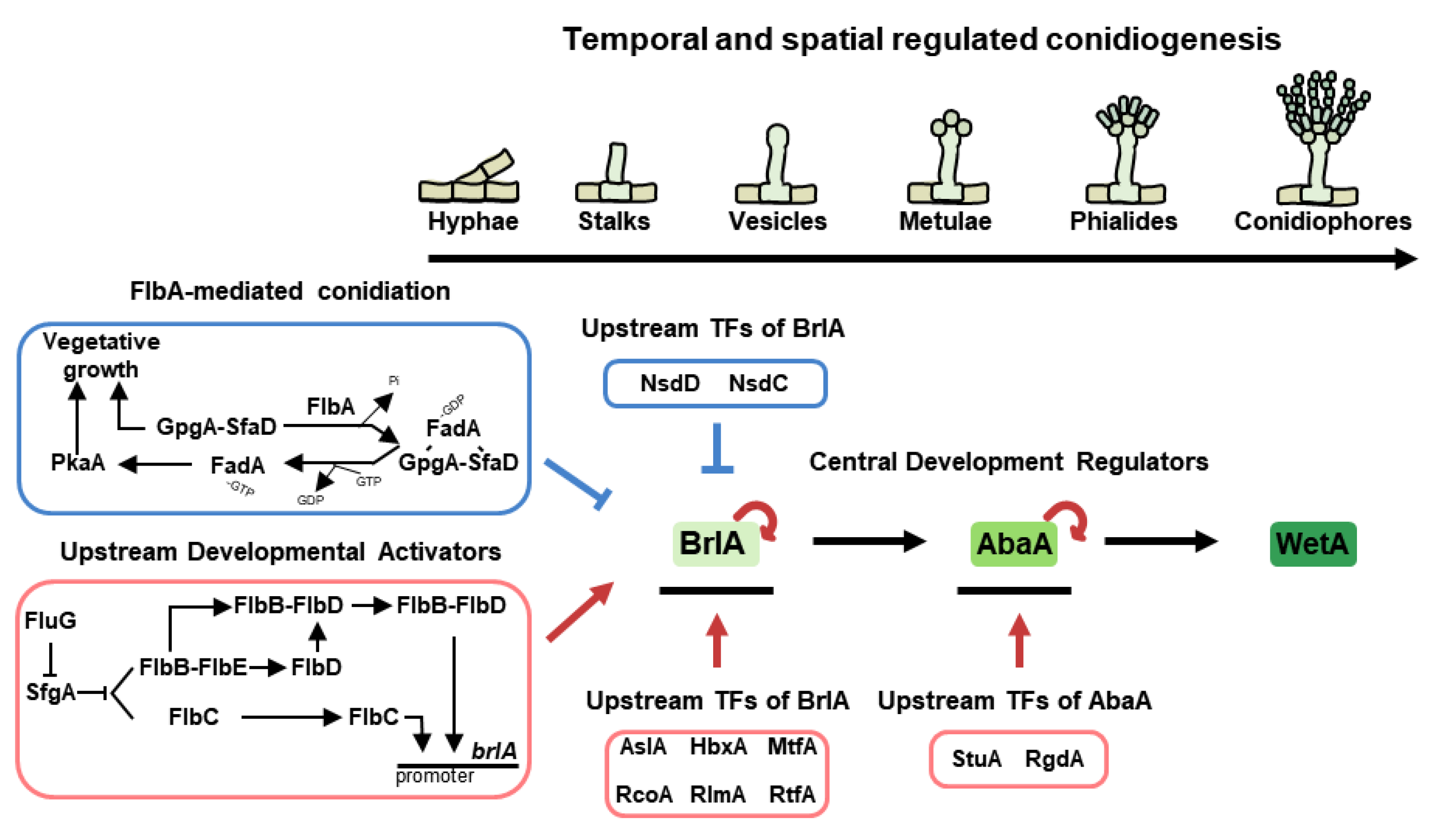
Figure 2.
Asexual development in A. nidulans. The temporal and spatial central regulators of conidiation in A. nidulans lifespan. Each regulator affects the expression of BrlA as upstream developmental activators (UDA) and upstream regulators of brlA.

Table 1.
List of Aspergillus nidulans genes involved in different asexual developmental stages.
3.1. Temporal and Spatial Central Regulators of Conidiation
Approximately 50 years ago, the genes brlA, abaA, and wetA were morphologically investigated, which demonstrated that their appropriate temporal and spatial expression results in normal conidiophores formation in A. nidulans [36,37]. Scientists have named them central regulators of conidiation (Figure 2). BrlA is composed of 432 amino acids having two C2H2 zinc finger domains [38]. It has demonstrated that the brlA null mutant blocksin a conidial formation, exhibiting defective vesicles and loss of pigment [36]. The gene is named brlA (bristle-like) because the shape of the mutant resembles bristles. The brlA is primarily expressed in the early asexual development stage, and BrlA protein controls its own expression and that of various conidiation-related genes by binding to their promoters. The binding site is termed the BrlA-response element (BRE) and the sequence is 5′-(C/A)(G/A)AGGG (G/A)-3′ in A. nidulans [39].
One of the target genes directly regulated by BrlA is abaA (abascus). The abaA deletion mutant shows rod-like, aberrant conidiophores and fails to form proper metulae and phialides at intervals in place of chains of conidia [36,37,40]. As with brlA, it is named abaA because of the morphological characteristics of the mutant [41]. During the middle stage of conidiophore development, BrlA activates abaA mRNA expression. Then, AbaA, which has ATTS/TEA DNA binding domain factor and a potential leucine zipper, regulates its own expression and controls brlAα or other developmental genes (such as wetA and vosA) by recognizing the AbaA-response element (ARE) in their promoters [42]. The ARE is 5′-CATTCY-3′, where Y is a pyrimidine.

Table 2.
List of Aspergillus nidulans genes involved in the proper formation of asexual spores.
Table 2.
List of Aspergillus nidulans genes involved in the proper formation of asexual spores.
| Name | Conidiogenesis | Description | Reference(s) |
|---|---|---|---|
| AclA/B | Activation | ATP-citrate lyase | [43] |
| AcoA~B | Activation | Aconidial genes, encoding putative aconitate hydratase | [44,45] |
| AflR | Activation | Sterigmatocystin/Aflatoxin Zn(II)2Cys6 transcriptional factor | [46] |
| AspE | Activation | Aspergillus septin E (septin protein) | [47] |
| BasA | Activation | Sphingolipid C4-hydroxylase, homolog of S.cerevisiae Sur2 | [48] |
| Bud4 | Activation | Bud site selection protein | [31] |
| CalB~H | Activation | Calcoflour hypersensitivity | [49] |
| CandA-C | Activation | Cullin-associated-nedd8-dissociated protein | [50] |
| ChsA | Activation | Chitin synthase encoding the fungal cell-wall integrity signaling (CWIS) pathway | [51] |
| FhbA/B | Activation | Flavohaemoglobins | [52,53] |
| GfsA | Activation | Galactofuranosyltransferase | [54] |
| GmcA | Activation | Glucose-methanol-choline oxidoreductase | [55] |
| KfsA | Activation | Kinase for septation | [56] |
| OdeA | Activation | Oleate Δ12 desaturases | [57] |
| PchA | Activation | Homolog of S. cerevisiae cyclin T | [58] |
| PclB | Activation | Homolog of S. cerevisiae pcl cyclins | [58] |
| PhnA | Repression | Phosducin-like protein (PhLP) | [59] |
| PhoA | Activation | PSTAIRE-containing kinase | [60] |
| PmtA/B | Activation | Protein O-mannosyltransferase involved in protein glycosylation | [61] |
| PmtC | Repression | Protein O-mannosyltransferase involved in protein glycosylation | [61,62] |
| PpoA/B | Repression | Psi-producing oxygenase involved in oxylipin biosynthesis | [63] |
| PpoC | Activation | Psi-producing oxygenase involved in oxylipin biosynthesis | [64] |
| PufA | Activation | Pumilio/fem-3 binding factor | [65] |
| SnaA~E | Activation | Suppressor of nudA1 | [66] |
| StcE/J/U | Repression | Sterigmatocystin biosynthetic gene cluster | [46] |
| SdeA/B | Activation | Δ9-stearic acid desaturases | [67] |
| SumO | Activation | Small ubiquitin-like modifier involved in SUMOylation | [68,69] |
| UgeA | Activation | UDP-glucose-4-epimerase | [70] |
| VapA | Activation | FYVE-like zinc finger protein, one of the VipC-associated protein | [71] |
| VapB | Activation | H3-K9 specific histone methyltransferase, one of the VipC-associated proteins | [71] |
| VipC | Repression | H3-K9 specific histone methyltransferase, one of the Velvet interacting proteins | [71] |
| WscA/B | Activation | Homolog of S. cerevisiae Wsc1 involved in the fungal cell-wall integrity signaling pathway | [72] |
In the late stage of conidiation, wetA (wet-white) is activated by AbaA. The null strain of wetA produces normal conidiophores, but it produces colorless, immature conidia in A. nidulans [36]. The wetA mutant conidia might undergo autolysis and show reduced viability. Moreover, the deletion strains of wetA have permeable conidia, whose wall layers are less condensed than those of the wild-type strain [73]. WetA has a conserved ESC1/WetA-related DNA binding domain that binds to the WetA-response element (WRE), 5′-CCGYTTGCGGC-3′ (Y = pyrimidine). The WetA, accumulated in conidia, recognizes WRE in the promoters of spore-specific genes and regulates their expression (wA, yA, vosA, and atfB) [74,75].
3.2. Upstream Developmental Activators (UDAs)
Previous studies have demonstrated the importance of UDAs in the development of A. nidulans. In 1994, the Adams group revealed that some developmental mutants formed cotton-like colonies with a “fluffy” morphology [76]. These included a mutant of fluG (fluffy locus A), and flbA~E (fluffy low brlA expression) genes. The fluG is required to produce an extracellular signal (a diorcinol-dehydroaustinol adduct) that initiates programmed asexual sporulation, and the fluG-derived signal can generate sparse conidiation and brlA expression [76]. Even the overexpression of fluG overcomes the developmental block and results in the formation of conidiophores in submerged culture [77]. In addition to brlA, the expressions of other early developmental regulatory genes (flb genes) are affected by FluG. The FluG-mediated developmental regulation is divided into two independent pathways; the activation of the Flb protein-mediated asexual development and the inhibition of FlbA-mediated vegetative growth (Figure 2).
3.2.1. Flb Protein-Mediated Asexual Development
The transmitted FluG signal activates conidiation by derepressing SfgA-related pathways. SfgA, one of the suppressors of fluG, is a Gal4-type Zn(II)2Cys6 transcription factor. The sfgA null mutant exhibits hyperactive sporulation in liquid-submerged cultures, whereas overexpressed mutant exhibits the inhibition of conidiation. In other words, SfgA plays a role in the repression of asexual development [78]. Moreover, based on genetic analyses, SfgA has been demonstrated as a downstream factor of FluG but an upstream regulator of Flb proteins (flbB, flbC, flbD, and flbE, excluding flbA). As shown in Figure 2, FluG inhibits the expression of SfgA, counteracting the inhibitory effect of SfgA on Flb proteins. Coordinated by FluG and SfgA, four Flb proteins block hyphal growth and timely mediate the asexual development by positively affecting brlA expression [76]. Among these proteins, FlbB is a basic zipper-type transcription factor localized in the nucleus and apical extension (Spitzenkörper). The flbB null mutant exhibits blockage of the synthesis of an extracellular signaling compound, which is expressed in the early phases of vegetative growth for proper conidiation [79]. Further studies report that FlbB cooperates with other UDAs for regulating conidiation. First, FlbB physically interacts with FlbE and colocalizes at hyphal tips. FlbB-FlbE heterocomplex directly binds to the promoter of brlA to express brlA mRNA levels for proper asexual development. Furthermore, this complex can activate transcriptional levels of flbD for proper fungal development. FlbE is expressed throughout the life cycle of A. nidulans and has two conserved but hitherto uncharacterized domains [80]. The deletion and overexpression of flbE cause defective development, cell autolysis, and delayed brlA expression, indicating that the appropriate amount of flbE is crucial for proper fungal growth and asexual development [81]. Second, the FlbB complexes with FlbD, expressed by the FlbB-FlbE heterocomplex, which jointly bind to the brlA promoter for the activation of brlA expression [82]. FlbD is a c-Myb transcription factor, primarily found in the nucleus. Like other flb null strains, flbD absence mutant shows fluffy colonies and aconidial phenotypes, whereas the flbD-overexpressed strain causes inappropriate activation of brlA expression and the production of complex conidiophores [82,83]. Meanwhile, FlbC is not affected by other Flb proteins and acts independently for asexual development. FlbC has two C2H2 zinc fingers and one activation domain. FlbC is localized in the nucleus and binds to the promoters of brlA, abaA, and vosA, regulating their expression levels. Deletion of flbC causes delayed vegetative growth and reduced conidiation. Moreover, the overexpression of flbC results in restricted hyphal growth and reduced cellular activity. These indicate that modulated flbC is essential for balancing growth and development in A. nidulans [76,84].
3.2.2. Inhibition of FlbA-Mediated Stimulation of Vegetative Growth
FlbA encodes 120 amino acids, having an RGS domain for the regulator of G protein signaling, which negatively regulates vegetative growth signaling [85]. Deletion of flbA results in unusual growth and abnormal conidiophores, whereas its overexpression causes hyphal tips to differentiate into spore-producing structures [76,86]. In other words, this protein plays an important role in mycelial proliferation and activation of asexual development. Commonly, RGS domain-containing proteins sense and respond to appropriate physiological and biochemical cues and function as GTPase-activating proteins in conjunction with α subunit of heterotrimeric G-proteins. As regulators of G proteins, they promote GTP hydrolysis of Gα to GDT, inactivating the G proteins (Figure 2). In A. nidulans, FadA is one of the Gα proteins, and the fadA deletion strain exhibits reduced growth without the impairment of sporulation [87]. In the heterotrimer status affected by FlbA, inactivated FadA (GDP-bound) exists with SfaD (suppressors of the flbA-loss of function mutations, Gβ) and GpgA (G protein gamma A, Gɤ) and represses vegetative growth [88]. When GTP is bound to FadA, this protein dissociates from the complex, and the separated FadA activates PkaA, which suppresses brlA levels, contributing to normal vegetative fungal growth and the inhibition of asexual development [89]. The remainders (GpgA and SfaD) separated from the complex also positively regulate hyphal proliferation and repress asexual developmental progression [90,91].
3.3. Upstream Regulators of BrlA
For hyphal cells to enter the stage of asexual development, appropriate temporal and spatial control of brlA expression must be applied in A. nidulans. Here, we describe the other upstream transcription factors of BrlA involved in the asexual development of A. nidulans, in addition to the UDAs that regulate brlA expression (Figure 2). StuA (stunted), studied in 1969, is known as a developmental modifier and is necessary for proper conidiophore formation [36]. StuA is one of the APSES transcription factors required for the transient and spatial regulation of brlA and abaA expression [92]. RgdA, named after retarded growth and development, is a putative APSES transcription factor that is important for the orderly organization of phialide formation [93]. RgdA affects brlA and abaA expression, but it is not influenced by brlA or stuA. RlmA, a S. cerevisiae ortholog of rlm1, is a MADS-box family transcription factor that is involved in proper brlA expression and phialide development, as well as in cell wall remodeling [94]. AslA, implicated in asexual differentiation with low-level conidiation, encodes a C2H2 zinc finger transcription factor that is related to normal asexual development and functions as an upstream activator of brlA [95]. MtfA is denominated from master transcription factor A, which encodes a C2H2 zinc finger domain. This protein has been demonstrated as a key factor for normal brlA expression, asexual development, and the production of several secondary metabolites in A. nidulans [96]. RcoA is a member of the WD repeat proteins that plays important roles in proper vegetative growth, asexual development, and carbon catabolite repression. This transcription factor also affects the expression of brlA, but not the signal transduction of flbA and fluG [97]. RtfA, encoding RNA-pol II transcription factor-like protein, regulates proper branched hyphal growth and conidiophore morphology by the accumulation of brlA transcript. Moreover, this protein affects the biosynthesis of several secondary metabolites, including sterigmatocystin and penicillin [98]. HbxA, a member of homeobox proteins, acts as an activator of asexual development and affects brlA mRNA expression. Deletion of hbxA results in aberrant asexual structures, whereas its overexpression results in enhanced production of conidiophores in liquid-submerged cultures [99].
Although Nsd (never in sexual development) proteins were previously described as transcription factors affecting the activation of asexual development, they are key negative regulators of conidiation. NsdC, with two C2H2 zinc fingers and a C2HC motif, is not only required for vegetative growth and sexual development but also negatively regulates asexual sporulation by repressing the brlA expression [100]. Like NsdC, one of the GATA transcription factors, NsdD, also affects conidiation by binding to the brlA promoter [101].
3.4. Other Key Regulators of Asexual Development
MedA (medusa) is also investigated in 1969 with StuA as mentioned above. This protein, which does not have any conserved domain, is essential for proper conidiophore formation [36]. MedA, known as an Neurospora crassa ortholog of acon-3, primarily modulates the expression of core conidiation genes (brlA and abaA) for the timely formation of metulae and phialides [102,103]. One of the WOPR fungi-specific DNA-binding proteins, OsaA (orchestrator of sexual and asexual development), indirectly controls conidiation by repressing downstream of the velvet regulator veA, which acts as a balancer between asexual and sexual development [104].
4. Conidial Maturation and Dormancy
Immature spores, formed at the ends of conidiophores, mature and stay in a dormant state. Dormant conidia modify the conidial wall, tolerate external stimuli, and maintain their viability for a long duration. The functions of controllers related to the maturation and quiescent phases in A. nidulans are shown in Figure 3 and Table 3.
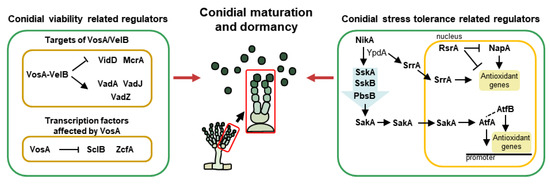
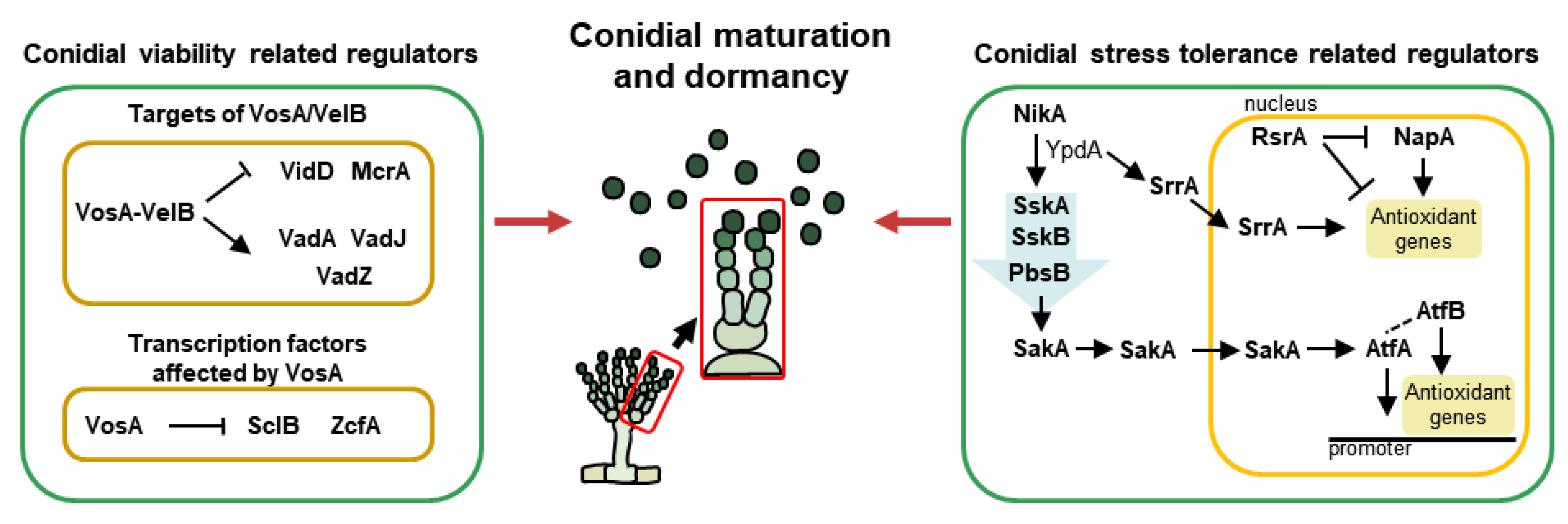
Figure 3.
The genetic regulators of maturation and dormancy in A. nidulans conidia. A simplified model for conidial maturation and dormancy, including velvet proteins, transcription factors, and MAPK-mediated regulators.

Table 3.
List of Aspergillus nidulans genes involved in conidial maturation and dormancy.
4.1. The Velvet Family
Members of the velvet family are known as essential regulators of fungal growth, development, and secondary metabolism in ascomycetes and basidiomycetes [18]. They commonly share a “velvet” DNA-binding domain that is composed of 150 amino acids [119]. There are four velvet proteins, VeA, VelB, VelC, and VosA, in A. nidulans, which interact with each other or non-velvet regulators. Through the interaction of various combinations, A. nidulans can control development and conidiation in a temporal and spatial manner. Among the velvet proteins, VelB and VosA play pivotal roles in the maturation and dormancy of asexual spores (Figure 3). VelB (velvet-like protein B) acts as a positive regulator of asexual development and mediates spore viability, trehalose biosynthesis, and conidial pigmentation [120]. VosA (viability of spores A) also regulates tolerance to several stresses and the long-term viability of conidia and trehalose biogenesis [14]. Moreover, the VosA and VelB form a heterocomplex in conidia and play important roles in conidial maturation, cell wall composition, and spore germination. One example is that the VosA-VelB complex directly binds to the promoter of fksA and controls β-glucan biosynthesis in asexual spores [121]. This heterocomplex also modulates the expression of spore-specific structural and regulatory genes during conidiogenesis. Representatively, VadA (VosA/VelB-activated developmental gene A) is known as a gene affected by the VosA-VelB complex in conidia. VadA functions in conidial trehalose and β-glucan biogenesis, stress tolerance, spore viability, and germination in A. nidulans [122,123]. VadJ, regulated by the VosA-VelB complex, is one of the highly conserved sensor histidine kinases in A. nidulans. VadJ is required for the proper formation of asexual spores and maintenance of conidial viability [124]. Another Vad protein, VadZ, is a GAL4-like Zn(II)2Cys6 transcription factor that is essential for conidiogenesis and spore longevity [125].
In contrast, VidA (VosA/VelB-inhibited developmental gene A), repressed by VosA and VelB, has two C2H2 zinc finger domains at the C-terminus. This protein is involved in conidial trehalose and β-glucan biogenesis in A. nidulans [126]. There exists another Vid gene, vidD, which does not contain any known domains, but VidD is essential for normal fungal development, trehalose biosynthesis, and conidial long-term viability in A. nidulans [127].
4.2. Transcription Factors Involved in Conidial Maturation and Dormancy
McrA (Multicluster regulator A) is one of the putative GAL4-like Zn(II)2Cys6 transcription factors and is highly expressed in late asexual development. This protein affects proper conidiation by modulating brlA expression through the life cycle. Furthermore, McrA is known as a direct target of the VosA-VelB heterodimer and modulates long-term spore viability, trehalose and β-glucan biogenesis, and proper conidial pigmentation [128]. One of the VosA-controlled regulatory genes, sclB (sclerotia-like), contains a Zn(II)2Cys6 zinc cluster fungal-type DNA binding domain. Unlike VosA, which represses the premature induction of brlA, SclB induces the early activators of asexual development (FlbC, FlbD, and BrlA) and influences conidiogenesis. In addition, SclB plays important roles in conidial viability and tolerance to oxidative stress [129]. Another Zn cluster family member, zcfA encodes a Zn(II)2Cys6 zinc finger protein and is known as a putative VosA target gene in conidia. The deletion of zcfA results in increased asexual spore formation and induced mRNA levels of brlA. Phenotypic analysis of ΔzcfA conidia shows that ZcfA plays a key role in conidial viability, trehalose biogenesis, and thermal stress resistance [130]. HbxB, one of the homeobox family members, is highly expressed in asexual spores and modulates the production of asexual spores and conidial stress resistance to thermal, oxidative, and UV stresses [99]. As a transcription factor, HbxB affects the transcriptomic levels of various genes, regulating trehalose biosynthesis, and β-glucan degradation in conidia [131]. CsgA is a GAL4-like Zn(II)2Cys6 transcription factor specifically expressed in A. nidulans conidia. The deletion of csgA results in an increased number of conidia, and csgA-deleted conidia exhibit augmented trehalose contents and increased tolerance to thermal, oxidative, and UV stresses compared to WT conidia. In addition, CsgA is required for normal conidial viability and germination [132].
4.3. Genetic Regulators Related to Stress Tolerance in Conidia
4.3.1. Mitogen-Activated Protein Kinase (MAPK) Cascades
During maturation, the cellular composition of immature spores is altered by several regulators, and consequently, they possess the ability to withstand various external stresses. Representatively, the histidine-to-aspartate (His-Asp) phosphorelay systems actively function so that spores can survive for a long time even under extreme environmental conditions. In A. nidulans, NikA is a histidine-specific protein kinase (HK) that first recognizes stimuli. NikA plays important roles in proper conidial reproduction and conidial resistance to certain fungicides and osmotic stress. In the presence of stimuli, NikA responds and activates downstream stress-related regulators [133]. Upon activation by NikA, YpdA transmits the signals to the response regulator, SskA. The deletion of sskA results in defective asexual development, conidial viability, and sensitivity against cold and oxidative stresses (H2O2). SskA phosphorylates SskB (MAPKKK), which subsequently phosphorylates PbsB (MAPKK) and the ortholog of Hog1 (MAPK). There are two homologs of S. cerevisiae Hog1 in A. nidulans and other Aspergilli: SakA (stress-activated MAP kinase) and MpkC. Both of these homologs physically interact with the upstream regulator PbsB, but they have the same or different functions in A. nidulans [134]. As a member of the Hog1/Sty1/p38 family, SakA is a well-known key regulator of spore stress tolerance in filamentous fungi. Similarly, in A. nidulans, the deletion mutant of sakA exhibits defective conidial production and ΔsakA is sensitive to thermal, oxidative, and cell wall stresses. Conversely, the deletion of mpkC results in increased conidial production and resistance to oxidative stress. However, both Hog1 homologs, SakA and MpkC, are essential for the long-term survival of conidia [135]. Another MAPK, MpkB, is phosphorylated by other MAPK components (pheromone MAPK pathway), and activated MpkB is also involved in fungal development. MpkB, regulated by VosA, is pivotal for spore viability and inhibits conidial germination. MpkB also influences fungal autolysis [136]. The other MAPK, MpkC, is activated by the fungal cell wall integrity signal (CWIS). Phosphorylated MpkC modulates proper conidial germination as well as conidial cell wall integrity by upregulating genes related to α-, β-1,3-glucans and chitin biosynthesis [137].
4.3.2. Other Transcription Factors Related to Stress Tolerance in Conidia
SrrA is one of the response regulators and components of a stress-sensing phosphorelay system in A. nidulans. Like SskA, SrrA is activated by YpdA. However, unlike SskA, SrrA directly translocates into the nucleus. As a specific transcription factor, SrrA affects conidial resistance against oxidative stress by regulating antioxidant genes such as catB. Furthermore, SrrA plays important roles in conidial formation and spore viability [138]. AtfA, an ortholog of Schizosaccharomyces pombe atf1, is a member of the activating transcription factor/cAMP-responsive element-binding protein (ATF/CREB) family. AtfA, containing a bZIP domain, permanently exists in the nucleus and responds to oxidative stress during spore development. When SakA accumulates in the conidial nucleus by oxidative stress, it physically interacts with AtfA, regulating the expression of antioxidant-related genes in conidia. Consequently, AtfA plays pivotal roles in the conidial antioxidant response (tBOOH and H2O2) and long-term viability [139,140]. Recent research shows that AtfA interacts with another bZIP transcription factor, AtfB, for coordinating asexual development and stress tolerance [140]. Although AtfA appears to be more important than AtfB in the oxidative stress defense system of A. nidulans spores, AtfB is also essential for tolerating thermal and oxidative stresses. Another transcription factor related to oxidative response is NapA, which is an S. cerevisiae Yap1 functional homolog and contains a bZIP domain. Similar to the functions of AtfA, NapA is involved in oxidant detoxification (menadione and H2O2). When stimulated by oxidants, NapA protects by positively regulating both nonenzymatic (e.g., glutathione and thioredoxin) and enzymatic (e.g., catalases and superoxide dismutases) pathways in conidia. Moreover, NapA regulates asexual development and carbon utilization [141]. RsrA (regulator of stress response) is known as a C2H2 zinc finger transcription factor that is required for fungal growth and sporulation. RsrA directly represses antioxidant genes such as glrA, trxA, and catB as well as NapA in the presence of reactive oxygen species such as tBOOH and H2O2 [142].
5. Conidial Germination
Under appropriate conditions, the resting conidia break the quiescent state and modify their morphology. Through the alteration of cell wall composition and molecular organization, they swell and produce the germ tube (polarized growth). The germinated spores can expand their habitats such as humans, animals, and plants. Therefore, it is important to understand the regulatory mechanisms related to conidial germination. Although there are several coordinators mediating conidial germination (Figure 4 and Table 4), we focus on the genetic regulators and transcription factors.
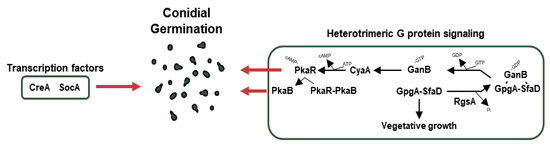
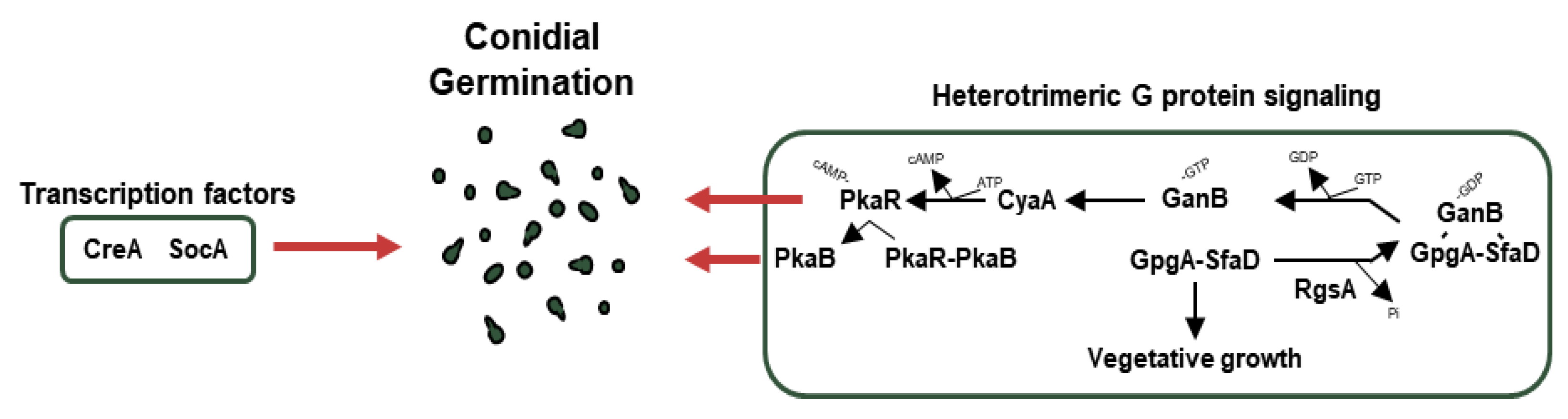
Figure 4.
Genetic regulators of germination in A. nidulans conidia. A schematic presentation of the genetic regulators of conidial germination.

Table 4.
List of Aspergillus nidulans genes involved in asexual spore germination.
5.1. Transcription Factors Involved in Germination
CreA is a Cys2His2 transcription factor for carbon catabolite repression (CCR). In the presence of glucose as a carbon source, CreA directly or indirectly represses genes encoding enzymes (cellulases, xylanases) for degrading alternative carbon sources. However, in the absence of glucose, CreA is ubiquitinated and targeted by the proteasomes. As a result of the degradation of CreA, enzymes for alternative carbon sources are biosynthesized in A. nidulans [179]. In addition to CCR, the ΔcreA strain shows defective spore germination [180]. SocA, a Zn(II)2Cys6 transcription factor, was first discovered by mutagenesis and isolation of FLIP (Fluffy in Phosphate) mutant. The null mutant of socA shows deficient colony growth and abnormal morphogenesis as well as an altered germination pattern [62].
5.2. Other Regulators Related to Germination
The cAMP-PKA pathway (PKA pathway) is closely related to A. nidulans conidial germination. When GanB, which is Gα forming a heterotrimer with SfaD (Gβ) and GpgA (Gγ) mentioned earlier, separates from the complex and then activates CyaA, the activated CyaA as the adenylate cyclase produces cAMP from ATP, which attaches to PkaR and promotes conidial germination [169]. In addition, PkaB, a secondary protein kinase A catalytic subunit that is separated from PkaR by cAMP binding, promotes conidial germination and spore resistance to oxidative stress and inhibits conidiation while remaining alone [181]. Meanwhile, the dissociated GanB and SfaD-GpgA dimers are reunited by RgsA (regulator of G-protein signaling family), which indirectly affects asexual development and inhibits vegetative growth. RgsA plays a vital role in proper conidial germination and tolerance to thermal and oxidative stresses [182].
6. Conclusions
The genus of Aspergillus is one of the most abundant filamentous fungi in the air. They proliferate by producing a number of asexual spores, which constitute the major reproductive mode. Floating at short and long distances, conidia take root in the appropriate environment or host and grow by changing their morphology. They undergo asexual development and produce conidiophores. The matured conidia on the conidiophores stay in the resting phase and prepare for the next generation. These processes are coordinated by several genetic regulators or signaling pathways, which have been investigated by researchers. In this review, we summarized the key genetic regulators and their roles in each stage of asexual reproduction in the model organism A. nidulans. This information will help us gain a better understanding of the organizational and systematic developmental process and may help prevent the development of pathogenic spores or maximize the production of desired ones. Nevertheless, scientific studies must be continuously and deeply scrutinized as there are still several unexplored regulators.
Author Contributions
Conceptualization, writing—original draft preparation, view, editing; and funding acquisition, Y.-E.S., J.-H.Y. and H.-S.P.; supervision and project administration J.-H.Y. and H.-S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant to HSP funded by the Korean government (NRF-2020R1C1C1004473) and a project to train professional personnel in biological materials by the Ministry of Environment. The work by YES was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2021R1A6A3A13044577). The work at UW-Madison was supported by Food Research Institute.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Egbuta, M.A.; Mwanza, M.; Babalola, O.O. Health Risks Associated with Exposure to Filamentous Fungi. Int. J. Environ. Res. Public Health 2017, 14, 719. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Okmen, B.; Zhu, W.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5, FUNK-0023-2016. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Sakekar, A.A.; Gaikwad, S.R.; Punekar, N.S. Protein expression and secretion by filamentous fungi. J. Biosci. 2021, 46, 5. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, C.; Xiao, H. Genetic Engineering of Filamentous Fungi for Efficient Protein Expression and Secretion. Front. Bioeng. Biotechnol. 2020, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Latge, J.P. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef]
- Latge, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef]
- Cairns, T.C.; Nai, C.; Meyer, V. How a fungus shapes biotechnology: 100 years of Aspergillus niger research. Fungal Biol. Biotechnol. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.J.; Hu, S.; Wang, B.T.; Jin, L. Advances in Genetic Engineering Technology and Its Application in the Industrial Fungus Aspergillus oryzae. Front. Microbiol. 2021, 12, 644404. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W. Aspergillus: A primer for the novice. Med. Mycol. 2009, 47 (Suppl. 1), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Yu, J.H. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS ONE 2007, 2, e970. [Google Scholar] [CrossRef]
- Etxebeste, O.; Garzia, A.; Espeso, E.A.; Ugalde, U. Aspergillus nidulans asexual development: Making the most of cellular modules. Trends Microbiol. 2010, 18, 569–576. [Google Scholar] [CrossRef]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef]
- Adams, T.H.; Wieser, J.K.; Yu, J.H. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 1998, 62, 35–54. [Google Scholar] [CrossRef]
- Park, H.S.; Yu, J.H. Genetic control of asexual sporulation in filamentous fungi. Curr. Opin. Microbiol. 2012, 15, 669–677. [Google Scholar] [CrossRef]
- Krijgsheld, P.; Bleichrodt, R.; van Veluw, G.J.; Wang, F.; Muller, W.H.; Dijksterhuis, J.; Wosten, H.A. Development in Aspergillus. Stud. Mycol. 2013, 74, 1–29. [Google Scholar] [CrossRef]
- Wang, F.; Sethiya, P.; Hu, X.H.; Guo, S.H.; Chen, Y.Y.; Li, A.; Tan, K.L.; Wong, K.H. Transcription in fungal conidia before dormancy produces phenotypically variable conidia that maximize survival in different environments. Nat. Microbiol. 2021, 6, 1066–1081. [Google Scholar] [CrossRef]
- Baltussen, T.J.H.; Zoll, J.; Verweij, P.E.; Melchers, W.J.G. Molecular Mechanisms of Conidial Germination in Aspergillus spp. Microbiol. Mol. Biol. Rev. 2020, 84, e00049-19. [Google Scholar] [CrossRef] [PubMed]
- Schultzhaus, Z.; Cunningham, G.A.; Mourino-Perez, R.R.; Shaw, B.D. The phospholipid flippase DnfD localizes to late Golgi and is involved in asexual differentiation in Aspergillus nidulans. Mycologia 2019, 111, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.W.; Jiang, P.; Qiao, W.R.; Zhang, Y.W.; Wei, W.F.; Lu, L. Protein phosphatase 2A (PP2A) regulatory subunits ParA and PabA orchestrate septation and conidiation and are essential for PP2A activity in Aspergillus nidulans. Eukaryot. Cell 2014, 13, 1494–1506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ye, X.S.; Lee, S.L.; Wolkow, T.D.; McGuire, S.L.; Hamer, J.E.; Wood, G.C.; Osmani, S.A. Interaction between developmental and cell cycle regulators is required for morphogenesis in Aspergillus nidulans. EMBO J. 1999, 18, 6994–7001. [Google Scholar] [CrossRef] [PubMed]
- Kadry, A.A.; El-Ganiny, A.M.; Mosbah, R.A.; Kaminskyj, S.G.W. Deletion of Aspergillus nidulans GDP-mannose transporters affects hyphal morphometry, cell wall architecture, spore surface character, cell adhesion, and biofilm formation. Med. Mycol. 2018, 56, 621–630. [Google Scholar] [CrossRef]
- Karos, M.; Fischer, R. Molecular characterization of HymA, an evolutionarily highly conserved and highly expressed protein of Aspergillus nidulans. Mol. Gen. Genet. 1999, 260, 510–521. [Google Scholar] [CrossRef]
- Leeder, A.C.; Turner, G. Characterisation of Aspergillus nidulans polarisome component BemA. Fungal Genet. Biol. 2008, 45, 897–911. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, H.; Long, N.; Carbo, N.; Chen, P.; Aguilar, P.S.; Lu, L. FigA, a putative homolog of low-affinity calcium system member Fig1 in Saccharomyces cerevisiae, is involved in growth and asexual and sexual development in Aspergillus nidulans. Eukaryot. Cell 2014, 13, 295–303. [Google Scholar] [CrossRef]
- Canovas, D.; Marcos, A.T.; Gacek, A.; Ramos, M.S.; Gutierrez, G.; Reyes-Dominguez, Y.; Strauss, J. The histone acetyltransferase GcnE (GCN5) plays a central role in the regulation of Aspergillus asexual development. Genetics 2014, 197, 1175–1189. [Google Scholar] [CrossRef]
- El-Ganiny, A.M.; Sanders, D.A.; Kaminskyj, S.G. Aspergillus nidulans UDP-galactopyranose mutase, encoded by ugmA plays key roles in colony growth, hyphal morphogensis, and conidiation. Fungal Genet. Biol. 2008, 45, 1533–1542. [Google Scholar] [CrossRef]
- Si, H.; Rittenour, W.R.; Xu, K.; Nicksarlian, M.; Calvo, A.M.; Harris, S.D. Morphogenetic and developmental functions of the Aspergillus nidulans homologues of the yeast bud site selection proteins Bud4 and Axl2. Mol. Microbiol. 2012, 85, 252–270. [Google Scholar] [CrossRef] [PubMed]
- Schier, N.; Liese, R.; Fischer, R. A Pcl-like cyclin of Aspergillus nidulans is transcriptionally activated by developmental regulators and is involved in sporulation. Mol. Cell. Biol. 2001, 21, 4075–4088. [Google Scholar] [CrossRef]
- Liu, B.; Morris, N.R. A spindle pole body-associated protein, SNAD, affects septation and conidiation in Aspergillus nidulans. Mol. Gen. Genet. 2000, 263, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Gems, D.H.; Clutterbuck, A.J. Enhancers of conidiation mutants in Aspergillus nidulans. Genetics 1994, 137, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, M.V.C.L.; McPheat, W.L.; Stark, M.J.R. A novel ‘two-component’ protein containing histidine kinase and response regulator domains required for sporulation in Aspergillus nidulans. Curr. Genet. 2000, 37, 364–372. [Google Scholar] [CrossRef]
- Clutterbuck, A.J. A mutational analysis of conidial development in Aspergillus nidulans. Genetics 1969, 63, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Sewall, T.C. Cellular effects of misscheduled brlA, abaA, and wetA expression in Aspergillus nidulans. Can. J. Microbiol. 1994, 40, 1035–1042. [Google Scholar] [CrossRef]
- Adams, T.H.; Boylan, M.T.; Timberlake, W.E. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 1988, 54, 353–362. [Google Scholar] [CrossRef]
- Chang, Y.C.; Timberlake, W.E. Identification of Aspergillus brlA response elements (BREs) by genetic selection in yeast. Genetics 1993, 133, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Boylan, M.T.; Mirabito, P.M.; Willett, C.E.; Zimmerman, C.R.; Timberlake, W.E. Isolation and physical characterization of three essential conidiation genes from Aspergillus nidulans. Mol. Cell. Biol. 1987, 7, 3113–3118. [Google Scholar] [CrossRef]
- Sewall, T.C.; Mims, C.W.; Timberlake, W.E. abaA controls phialide differentiation in Aspergillus nidulans. Plant Cell 1990, 2, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Andrianopoulos, A.; Timberlake, W.E. The Aspergillus nidulans abaA gene encodes a transcriptional activator that acts as a genetic switch to control development. Mol. Cell. Biol. 1994, 14, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Hynes, M.J.; Murray, S.L. ATP-citrate lyase is required for production of cytosolic acetyl coenzyme A and development in Aspergillus nidulans. Eukaryot. Cell 2010, 9, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Butnick, N.Z.; Yager, L.N.; Hermann, T.E.; Kurtz, M.B.; Champe, S.P. Mutants of Aspergillus nidulans blocked at an early stage of sporulation secrete an unusual metabolite. J. Bacteriol. 1984, 160, 533–540. [Google Scholar] [CrossRef]
- Butnick, N.Z.; Yager, L.N.; Kurtz, M.B.; Champe, S.P. Genetic analysis of mutants of Aspergillus nidulans blocked at an early stage of sporulation. J. Bacteriol. 1984, 160, 541–545. [Google Scholar] [CrossRef]
- Wilkinson, H.H.; Ramaswamy, A.; Sim, S.C.; Keller, N.P. Increased conidiation associated with progression along the sterigmatocystin biosynthetic pathway. Mycologia 2004, 96, 1190–1198. [Google Scholar] [CrossRef]
- Hernandez-Rodriguez, Y.; Masuo, S.; Johnson, D.; Orlando, R.; Smith, A.; Couto-Rodriguez, M.; Momany, M. Distinct septin heteropolymers co-exist during multicellular development in the filamentous fungus Aspergillus nidulans. PLoS ONE 2014, 9, e92819. [Google Scholar] [CrossRef]
- Li, S.; Bao, D.; Yuen, G.; Harris, S.D.; Calvo, A.M. basA regulates cell wall organization and asexual/sexual sporulation ratio in Aspergillus nidulans. Genetics 2007, 176, 243–253. [Google Scholar] [CrossRef][Green Version]
- Hill, T.W.; Loprete, D.M.; Momany, M.; Ha, Y.; Harsch, L.M.; Livesay, J.A.; Mirchandani, A.; Murdock, J.J.; Vaughan, M.J.; Watt, M.B. Isolation of cell wall mutants in Aspergillus nidulans by screening for hypersensitivity to Calcofluor White. Mycologia 2006, 98, 399–409. [Google Scholar] [CrossRef]
- Kohler, A.M.; Harting, R.; Langeneckert, A.E.; Valerius, O.; Gerke, J.; Meister, C.; Strohdiek, A.; Braus, G.H. Integration of Fungus-Specific CandA-C1 into a Trimeric CandA Complex Allowed Splitting of the Gene for the Conserved Receptor Exchange Factor of CullinA E3 Ubiquitin Ligases in Aspergilli. mBio 2019, 10, e01094-19. [Google Scholar] [CrossRef]
- Culp, D.W.; Dodge, C.L.; Miao, Y.; Li, L.; Sag-Ozkal, D.; Borgia, P.T. The chsA gene from Aspergillus nidulans is necessary for maximal conidiation. FEMS Microbiol. Lett. 2000, 182, 349–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marcos, A.T.; Ramos, M.S.; Marcos, J.F.; Carmona, L.; Strauss, J.; Canovas, D. Nitric oxide synthesis by nitrate reductase is regulated during development in Aspergillus. Mol. Microbiol. 2016, 99, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Marcos, A.T.; Ramos, M.S.; Schinko, T.; Strauss, J.; Canovas, D. Nitric oxide homeostasis is required for light-dependent regulation of conidiation in Aspergillus. Fungal Genet. Biol. 2020, 137, 103337. [Google Scholar] [CrossRef] [PubMed]
- Komachi, Y.; Hatakeyama, S.; Motomatsu, H.; Futagami, T.; Kizjakina, K.; Sobrado, P.; Ekino, K.; Takegawa, K.; Goto, M.; Nomura, Y.; et al. GfsA encodes a novel galactofuranosyltransferase involved in biosynthesis of galactofuranose antigen of O-glycan in Aspergillus nidulans and Aspergillus fumigatus. Mol. Microbiol. 2013, 90, 1054–1073. [Google Scholar] [CrossRef]
- Etxebeste, O.; Herrero-García, E.; Cortese, M.S.; Garzia, A.; Oiartzabal-Arano, E.; Ríos, V.D.L.; Ugalde, U.; Espeso, E.A. GmcA is a putative glucose-methanol-choline oxidoreductase required for the induction of asexual development in Aspergillus nidulans. PLoS ONE 2012, 7, e40292. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, N.; Vienken, K.; Rolbetzki, A.; Fischer, R. The Aspergillus nidulans putative kinase, KfsA (kinase for septation), plays a role in septation and is required for efficient asexual spore formation. Fungal Genet. Biol. 2007, 44, 1205–1214. [Google Scholar] [CrossRef]
- Shimizu, K.; Keller, N.P. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 2001, 157, 591–600. [Google Scholar] [CrossRef]
- Kempf, C.; Bathe, F.; Fischer, R. Evidence that two Pcl-like cyclins control Cdk9 activity during cell differentiation in Aspergillus nidulans asexual development. Eukaryot. Cell 2013, 12, 23–36. [Google Scholar] [CrossRef]
- Seo, J.A.; Yu, J.H. The phosducin-like protein PhnA is required for Gβγ-mediated signaling for vegetative growth, developmental control, and toxin biosynthesis in Aspergillus nidulans. Eukaryot. Cell 2006, 5, 400–410. [Google Scholar] [CrossRef]
- Bussink, H.J.; Osmani, S.A. A cyclin-dependent kinase family member (PHOA) is required to link developmental fate to environmental conditions in Aspergillus nidulans. EMBO J. 1998, 17, 3990–4003. [Google Scholar] [CrossRef]
- Le, T.H.T.; Oki, A.; Goto, M.; Shimizu, K. Protein O-mannosyltransferases are required for sterigmatocystin production and developmental processes in Aspergillus nidulans. Curr. Genet. 2018, 64, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Otamendi, A.; Espeso, E.A.; Etxebeste, O. Identification and Characterization of Aspergillus nidulans Mutants Impaired in Asexual Development under Phosphate Stress. Cells 2019, 8, 1520. [Google Scholar] [CrossRef] [PubMed]
- Tsitsigiannis, D.I.; Zarnowski, R.; Keller, N.P. The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulans. J. Biol. Chem. 2004, 279, 11344–11353. [Google Scholar] [CrossRef]
- Tsitsigiannis, D.I.; Kowieski, T.M.; Zarnowski, R.; Keller, N.P. Endogenous lipogenic regulators of spore balance in Aspergillus nidulans. Eukaryot. Cell 2004, 3, 1398–1411. [Google Scholar] [CrossRef]
- Son, S.H.; Jang, S.Y.; Park, H.S. Functions of PUF Family RNA-Binding Proteins in Aspergillus nidulans. J. Microbiol. Biotechnol. 2021, 31, 676–685. [Google Scholar] [CrossRef]
- Goldman, G.H.; Morris, N.R. Extragenic suppressors of a dynein mutation that blocks nuclear migration in Aspergillus nidulans. Genetics 1995, 139, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Chang, P.K.; Dobrzyn, A.; Ntambi, J.M.; Zarnowski, R.; Keller, N.P. Two Δ9-stearic acid desaturases are required for Aspergillus nidulans growth and development. Fungal Genet. Biol. 2004, 41, 501–509. [Google Scholar] [CrossRef]
- Wong, K.H.; Todd, R.B.; Oakley, B.R.; Oakley, C.E.; Hynes, M.J.; Davis, M.A. Sumoylation in Aspergillus nidulans: sumO inactivation, overexpression and live-cell imaging. Fungal Genet. Biol. 2008, 45, 728–737. [Google Scholar] [CrossRef]
- Harting, R.; Bayram, O.; Laubinger, K.; Valerius, O.; Braus, G.H. Interplay of the fungal sumoylation network for control of multicellular development. Mol. Microbiol. 2013, 90, 1125–1145. [Google Scholar] [CrossRef]
- El-Ganiny, A.M.; Sheoran, I.; Sanders, D.A.; Kaminskyj, S.G. Aspergillus nidulans UDP-glucose-4-epimerase UgeA has multiple roles in wall architecture, hyphal morphogenesis, and asexual development. Fungal Genet. Biol. 2010, 47, 629–635. [Google Scholar] [CrossRef]
- Sarikaya-Bayram, O.; Bayram, O.; Feussner, K.; Kim, J.H.; Kim, H.S.; Kaever, A.; Feussner, I.; Chae, K.S.; Han, D.M.; Han, K.H.; et al. Membrane-bound methyltransferase complex VapA-VipC-VapB guides epigenetic control of fungal development. Dev. Cell 2014, 29, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Futagami, T.; Nakao, S.; Kido, Y.; Oka, T.; Kajiwara, Y.; Takashita, H.; Omori, T.; Furukawa, K.; Goto, M. Putative stress sensors WscA and WscB are involved in hypo-osmotic and acidic pH stress tolerance in Aspergillus nidulans. Eukaryot. Cell 2011, 10, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Sewall, T.C.; Mims, C.W.; Timberlake, W.E. Conidium differentiation in Aspergillus nidulans wild-type and wet-white (wetA) mutant strains. Dev. Biol. 1990, 138, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Mead, M.E.; Lee, M.K.; Loss, E.M.O.; Kim, S.C.; Rokas, A.; Yu, J.H. Systematic Dissection of the Evolutionarily Conserved WetA Developmental Regulator across a Genus of Filamentous Fungi. mBio 2018, 9, e01130-18. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Mead, M.E.; Lee, M.K.; Neuhaus, G.F.; Adpressa, D.A.; Martien, J.I.; Son, Y.E.; Moon, H.; Amador-Noguez, D.; Han, K.H.; et al. Transcriptomic, Protein-DNA Interaction, and Metabolomic Studies of VosA, VelB, and WetA in Aspergillus nidulans Asexual Spores. mBio 2021, 12, e03128-20. [Google Scholar] [CrossRef] [PubMed]
- Wieser, J.; Lee, B.N.; Fondon, J., 3rd; Adams, T.H. Genetic requirements for initiating asexual development in Aspergillus nidulans. Curr. Genet. 1994, 27, 62–69. [Google Scholar] [CrossRef]
- Lee, B.N.; Adams, T.H. FluG and flbA function interdependently to initiate conidiophore development in Aspergillus nidulans through brlA β activation. EMBO J. 1996, 15, 299–309. [Google Scholar] [CrossRef]
- Seo, J.A.; Guan, Y.; Yu, J.H. FluG-dependent asexual development in Aspergillus nidulans occurs via derepression. Genetics 2006, 172, 1535–1544. [Google Scholar] [CrossRef]
- Etxebeste, O.; Ni, M.; Garzia, A.; Kwon, N.J.; Fischer, R.; Yu, J.H.; Espeso, E.A.; Ugalde, U. Basic-zipper-type transcription factor FlbB controls asexual development in Aspergillus nidulans. Eukaryot. Cell 2008, 7, 38–48. [Google Scholar] [CrossRef]
- Garzia, A.; Etxebeste, O.; Herrero-Garcia, E.; Fischer, R.; Espeso, E.A.; Ugalde, U. Aspergillus nidulans FlbE is an upstream developmental activator of conidiation functionally associated with the putative transcription factor FlbB. Mol. Microbiol. 2009, 71, 172–184. [Google Scholar] [CrossRef]
- Kwon, N.J.; Shin, K.S.; Yu, J.H. Characterization of the developmental regulator FlbE in Aspergillus fumigatus and Aspergillus nidulans. Fungal Genet. Biol. 2010, 47, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Garzia, A.; Etxebeste, O.; Herrero-Garcia, E.; Ugalde, U.; Espeso, E.A. The concerted action of bZip and cMyb transcription factors FlbB and FlbD induces brlA expression and asexual development in Aspergillus nidulans. Mol. Microbiol. 2010, 75, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Wieser, J.; Adams, T.H. flbD encodes a Myb-like DNA-binding protein that coordinates initiation of Aspergillus nidulans conidiophore development. Genes Dev. 1995, 9, 491–502. [Google Scholar] [CrossRef]
- Kwon, N.J.; Garzia, A.; Espeso, E.A.; Ugalde, U.; Yu, J.H. FlbC is a putative nuclear C2H2 transcription factor regulating development in Aspergillus nidulans. Mol. Microbiol. 2010, 77, 1203–1219. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Yu, J.H.; Keller, N.P.; Adams, T.H. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA G alpha protein-dependent signaling pathway. EMBO J. 1997, 16, 4916–4923. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.N.; Adams, T.H. Overexpression of flbA, an early regulator of Aspergillus asexual sporulation, leads to activation of brlA and premature initiation of development. Mol. Microbiol. 1994, 14, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Wieser, J.; Adams, T.H. The Aspergillus FlbA RGS domain protein antagonizes G protein signaling to block proliferation and allow development. EMBO J. 1996, 15, 5184–5190. [Google Scholar] [CrossRef]
- Wieser, J.; Yu, J.H.; Adams, T.H. Dominant mutations affecting both sporulation and sterigmatocystin biosynthesis in Aspergillus nidulans. Curr. Genet. 1997, 32, 218–224. [Google Scholar] [CrossRef]
- Calvo, A.M.; Gardner, H.W.; Keller, N.P. Genetic connection between fatty acid metabolism and sporulation in Aspergillus nidulans. J. Biol. Chem. 2001, 276, 25766–25774. [Google Scholar] [CrossRef]
- Seo, J.A.; Han, K.H.; Yu, J.H. Multiple roles of a heterotrimeric G-protein γ-subunit in governing growth and development of Aspergillus nidulans. Genetics 2005, 171, 81–89. [Google Scholar] [CrossRef]
- Rosen, S.; Yu, J.H.; Adams, T.H. The Aspergillus nidulans sfaD gene encodes a G protein β subunit that is required for normal growth and repression of sporulation. EMBO J. 1999, 18, 5592–5600. [Google Scholar] [CrossRef]
- Miller, K.Y.; Wu, J.; Miller, B.L. StuA is required for cell pattern formation in Aspergillus. Genes Dev. 1992, 6, 1770–1782. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, L.H.; Kim, H.E.; Park, J.S.; Han, K.H.; Han, D.M. A putative APSES transcription factor is necessary for normal growth and development of Aspergillus nidulans. J. Microbiol. 2013, 51, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.; Szarka, M.; Kovacs, S.; Boczonadi, I.; Emri, T.; Abe, K.; Pocsi, I.; Pusztahelyi, T. Effect of cell wall integrity stress and RlmA transcription factor on asexual development and autolysis in Aspergillus nidulans. Fungal Genet. Biol. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yu, Y.M.; Maeng, P.J. Differential Control of Asexual Development and Sterigmatocystin Biosynthesis by a Novel Regulator in Aspergillus nidulans. Sci. Rep. 2017, 7, 46340. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Dhingra, S.; Kincaid, A.; Shantappa, S.; Feng, X.; Calvo, A.M. The putative C2H2 transcription factor MtfA is a novel regulator of secondary metabolism and morphogenesis in Aspergillus nidulans. PLoS ONE 2013, 8, e74122. [Google Scholar] [CrossRef]
- Hicks, J.; Lockington, R.A.; Strauss, J.; Dieringer, D.; Kubicek, C.P.; Kelly, J.; Keller, N. RcoA has pleiotropic effects on Aspergillus cellular development. Mol. Microbiol. 2001, 39, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Shantappa, S.; Dhingra, S.; Calvo, A.M. veA-dependent RNA-pol II transcription elongation factor-like protein, RtfA, is associated with secondary metabolism and morphological development in Aspergillus nidulans. Mol. Microbiol. 2012, 85, 795–814. [Google Scholar] [CrossRef]
- Son, S.H.; Son, Y.E.; Cho, H.J.; Chen, W.; Lee, M.K.; Kim, L.H.; Han, D.M.; Park, H.S. Homeobox proteins are essential for fungal differentiation and secondary metabolism in Aspergillus nidulans. Sci. Rep. 2020, 10, 6094. [Google Scholar] [CrossRef]
- Kim, H.R.; Chae, K.S.; Han, K.H.; Han, D.M. The nsdC gene encoding a putative C2H2-type transcription factor is a key activator of sexual development in Aspergillus nidulans. Genetics 2009, 182, 771–783. [Google Scholar] [CrossRef]
- Lee, M.K.; Kwon, N.J.; Lee, I.S.; Jung, S.; Kim, S.C.; Yu, J.H. Negative regulation and developmental competence in Aspergillus. Sci. Rep. 2016, 6, 28874. [Google Scholar] [CrossRef] [PubMed]
- Busby, T.M.; Miller, K.Y.; Miller, B.L. Suppression and enhancement of the Aspergillus nidulans medusa mutation by altered dosage of the bristle and stunted genes. Genetics 1996, 143, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.W.; Greenwald, C.; Upadhyay, S.; Ding, S.; Wilkinson, H.H.; Ebbole, D.J.; Shaw, B.D. acon-3, the Neurospora crassa ortholog of the developmental modifier, medA, complements the conidiation defect of the Aspergillus nidulans mutant. Fungal Genet. Biol. 2011, 48, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Alkahyyat, F.; Ni, M.; Kim, S.C.; Yu, J.H. The WOPR Domain Protein OsaA Orchestrates Development in Aspergillus nidulans. PLoS ONE 2015, 10, e0137554. [Google Scholar] [CrossRef]
- Navarro, R.E.; Hansberg, W.; Timberlake, W.E.; Stringer, M.A. catA, a new Aspergillus nidulans gene encoding a developmentally regulated catalase. Curr. Genet. 1996, 29, 352–359. [Google Scholar] [CrossRef]
- Kawasaki, L.; Wysong, D.; Diamond, R.; Aguirre, J. Two divergent catalase genes are differentially regulated during Aspergillus nidulans development and oxidative stress. J. Bacteriol. 1997, 179, 3284–3292. [Google Scholar] [CrossRef]
- Wang, S.; Cao, J.; Liu, X.; Hu, H.; Shi, J.; Zhang, S.; Keller, N.P.; Lu, L. Putative calcium channels CchA and MidA play the important roles in conidiation, hyphal polarity and cell wall components in Aspergillus nidulans. PLoS ONE 2012, 7, e46564. [Google Scholar] [CrossRef]
- Feng, X.; Ramamoorthy, V.; Pandit, S.S.; Prieto, A.; Espeso, E.A.; Calvo, A.M. cpsA regulates mycotoxin production, morphogenesis and cell wall biosynthesis in the fungus Aspergillus nidulans. Mol. Microbiol. 2017, 105, 1–24. [Google Scholar] [CrossRef]
- Stringer, M.A.; Timberlake, W.E. dewA encodes a fungal hydrophobin component of the Aspergillus spore wall. Mol. Microbiol. 1995, 16, 33–44. [Google Scholar] [CrossRef]
- Grunbacher, A.; Throm, T.; Seidel, C.; Gutt, B.; Rohrig, J.; Strunk, T.; Vincze, P.; Walheim, S.; Schimmel, T.; Wenzel, W.; et al. Six hydrophobins are involved in hydrophobin rodlet formation in Aspergillus nidulans and contribute to hydrophobicity of the spore surface. PLoS ONE 2014, 9, e94546. [Google Scholar] [CrossRef]
- Wartenberg, D.; Vodisch, M.; Kniemeyer, O.; Albrecht-Eckardt, D.; Scherlach, K.; Winkler, R.; Weide, M.; Brakhage, A.A. Proteome analysis of the farnesol-induced stress response in Aspergillus nidulans--The role of a putative dehydrin. J. Proteom. 2012, 75, 4038–4049. [Google Scholar] [CrossRef]
- Son, Y.E.; Cho, H.J.; Chen, W.; Son, S.H.; Lee, M.K.; Yu, J.H.; Park, H.S. The role of the VosA-repressed dnjA gene in development and metabolism in Aspergillus species. Curr. Genet. 2020, 66, 621–633. [Google Scholar] [CrossRef]
- Karacsony, Z.; Gacser, A.; Vagvolgyi, C.; Scazzocchio, C.; Hamari, Z. A dually located multi-HMG-box protein of Aspergillus nidulans has a crucial role in conidial and ascospore germination. Mol. Microbiol. 2014, 94, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E.; Barnett, Y.A.; McCullough, W. Germinating conidiospores of Aspergillus amino acid auxotrophs are hypersensitive to heat shock, oxidative stress and DNA damage. FEBS Lett. 1994, 355, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.E.; Park, H.S. Conserved Roles of MonA in Fungal Growth and Development in Aspergillus Species. Mycobiology 2019, 47, 457–465. [Google Scholar] [CrossRef]
- Lim, J.Y.; Jang, S.H.; Park, H.M. Mannitol-1-phosphate dehydrogenase, MpdA, is required for mannitol production in vegetative cells and involved in hyphal branching, heat resistance of conidia and sexual development in Aspergillus nidulans. Curr. Genet. 2021, 67, 613–630. [Google Scholar] [CrossRef]
- Futagami, T.; Seto, K.; Kajiwara, Y.; Takashita, H.; Omori, T.; Takegawa, K.; Goto, M. The putative stress sensor protein MtlA is required for conidia formation, cell wall stress tolerance, and cell wall integrity in Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2014, 78, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, S.; Chaveroche, M.K.; van Dijck, P.; de Vries, R.; Ruijter, G.; Thevelein, J.; d’Enfert, C. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology 2001, 147, 1851–1862. [Google Scholar] [CrossRef]
- Ahmed, Y.L.; Gerke, J.; Park, H.S.; Bayram, O.; Neumann, P.; Ni, M.; Dickmanns, A.; Kim, S.C.; Yu, J.H.; Braus, G.H.; et al. The velvet family of fungal regulators contains a DNA-binding domain structurally similar to NF-κB. PLoS Biol. 2013, 11, e1001750. [Google Scholar] [CrossRef]
- Park, H.S.; Ni, M.; Jeong, K.C.; Kim, Y.H.; Yu, J.H. The role, interaction and regulation of the velvet regulator VelB in Aspergillus nidulans. PLoS ONE 2012, 7, e45935. [Google Scholar] [CrossRef]
- Park, H.-S.; Yu, Y.M.; Lee, M.-K.; Maeng, P.J.; Kim, S.C.; Yu, J.-H. Velvet-mediated repression of β-glucan synthesis in Aspergillus nidulans spores. Sci. Rep. 2015, 5, 10199. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, M.K.; Kim, S.C.; Yu, J.H. The role of VosA/VelB-activated developmental gene vadA in Aspergillus nidulans. PLoS ONE 2017, 12, e0177099. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.E.; Park, H.S. Genome Wide Analysis Reveals the Role of VadA in Stress Response, Germination, and Sterigmatocystin Production in Aspergillus nidulans Conidia. Microorganisms 2020, 8, 1319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lee, M.K.; Lim, J.; Moon, H.; Park, H.S.; Zheng, W.; Yu, J.H. The putative sensor histidine kinase VadJ coordinates development and sterigmatocystin production in Aspergillus nidulans. J. Microbiol. 2021, 59, 746–752. [Google Scholar] [CrossRef]
- Zhao, Y.; Lee, M.K.; Lim, J.; Moon, H.; Park, H.S.; Zheng, W.; Yu, J.H. The velvet-activated putative C6 transcription factor VadZ regulates development and sterigmatocystin production in Aspergillus nidulans. Fungal Biol. 2022, 126, 421–428. [Google Scholar] [CrossRef]
- Kim, M.J.; Jung, W.H.; Son, Y.E.; Yu, J.H.; Lee, M.K.; Park, H.S. The velvet repressed vidA gene plays a key role in governing development in Aspergillus nidulans. J. Microbiol. 2019, 57, 893–899. [Google Scholar] [CrossRef]
- Son, Y.E.; Park, H.S. Unveiling the Functions of the VosA-VelB Target Gene vidD in Aspergillus nidulans. Mycobiology 2021, 49, 258–266. [Google Scholar] [CrossRef]
- Lee, M.K.; Son, Y.E.; Park, H.S.; Alshannaq, A.; Han, K.H.; Yu, J.H. Velvet activated McrA plays a key role in cellular and metabolic development in Aspergillus nidulans. Sci. Rep. 2020, 10, 15075. [Google Scholar] [CrossRef]
- Thieme, K.G.; Gerke, J.; Sasse, C.; Valerius, O.; Thieme, S.; Karimi, R.; Heinrich, A.K.; Finkernagel, F.; Smith, K.; Bode, H.B.; et al. Velvet domain protein VosA represses the zinc cluster transcription factor SclB regulatory network for Aspergillus nidulans asexual development, oxidative stress response and secondary metabolism. PLoS Genet. 2018, 14, e1007511. [Google Scholar] [CrossRef]
- Son, Y.E.; Cho, H.J.; Lee, M.K.; Park, H.S. Characterizing the role of Zn cluster family transcription factor ZcfA in governing development in two Aspergillus species. PLoS ONE 2020, 15, e0228643. [Google Scholar] [CrossRef]
- Son, S.H.; Lee, M.K.; Son, Y.E.; Park, H.S. HbxB Is a Key Regulator for Stress Response and β-Glucan Biogenesis in Aspergillus nidulans. Microorganisms 2021, 9, 144. [Google Scholar] [CrossRef]
- Cho, H.J.; Park, H.S. The function of a conidia specific transcription factor CsgA in Aspergillus nidulans. Sci. Rep. 2022, 12, 15588. [Google Scholar] [CrossRef]
- Hagiwara, D.; Matsubayashi, Y.; Marui, J.; Furukawa, K.; Yamashino, T.; Kanamaru, K.; Kato, M.; Abe, K.; Kobayashi, T.; Mizuno, T. Characterization of the NikA histidine kinase implicated in the phosphorelay signal transduction of Aspergillus nidulans, with special reference to fungicide responses. Biosci. Biotechnol. Biochem. 2007, 71, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Jaimes-Arroyo, R.; Lara-Rojas, F.; Bayram, O.; Valerius, O.; Braus, G.H.; Aguirre, J. The SrkA Kinase Is Part of the SakA Mitogen-Activated Protein Kinase Interactome and Regulates Stress Responses and Development in Aspergillus nidulans. Eukaryot. Cell 2015, 14, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Bazan, V.; Jaimes-Arroyo, R.; Sanchez, O.; Lara-Rojas, F.; Aguirre, J. SakA and MpkC Stress MAPKs Show Opposite and Common Functions During Stress Responses and Development in Aspergillus nidulans. Front. Microbiol. 2018, 9, 2518. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Chun, J.; Jun, S.C.; Han, D.M.; Chae, K.S.; Jahng, K.Y. The MpkB MAP kinase plays a role in autolysis and conidiation of Aspergillus nidulans. Fungal Genet. Biol. 2013, 61, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Mizutani, O.; Furukawa, K.; Sato, N.; Yoshimi, A.; Yamagata, Y.; Nakajima, T.; Abe, K. MpkA-Dependent and -independent cell wall integrity signaling in Aspergillus nidulans. Eukaryot. Cell 2007, 6, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Perez, I.; Sanchez, O.; Kawasaki, L.; Georgellis, D.; Aguirre, J. Response regulators SrrA and SskA are central components of a phosphorelay system involved in stress signal transduction and asexual sporulation in Aspergillus nidulans. Eukaryot. Cell 2007, 6, 1570–1583. [Google Scholar] [CrossRef]
- Lara-Rojas, F.; Sanchez, O.; Kawasaki, L.; Aguirre, J. Aspergillus nidulans transcription factor AtfA interacts with the MAPK SakA to regulate general stress responses, development and spore functions. Mol. Microbiol. 2011, 80, 436–454. [Google Scholar] [CrossRef]
- Kocsis, B.; Lee, M.K.; Yu, J.H.; Nagy, T.; Daroczi, L.; Batta, G.; Pocsi, I.; Leiter, E. Functional analysis of the bZIP-type transcription factors AtfA and AtfB in Aspergillus nidulans. Front. Microbiol. 2022, 13, 1003709. [Google Scholar] [CrossRef]
- Mendoza-Martinez, A.E.; Lara-Rojas, F.; Sanchez, O.; Aguirre, J. NapA Mediates a Redox Regulation of the Antioxidant Response, Carbon Utilization and Development in Aspergillus nidulans. Front. Microbiol. 2017, 8, 516. [Google Scholar] [CrossRef]
- Bok, J.W.; Wiemann, P.; Garvey, G.S.; Lim, F.Y.; Haas, B.; Wortman, J.; Keller, N.P. Illumina identification of RsrA, a conserved C2H2 transcription factor coordinating the NapA mediated oxidative stress signaling pathway in Aspergillus. BMC Genom. 2014, 15, 1011. [Google Scholar] [CrossRef]
- Fischer, R.; Timberlake, W.E. Aspergillus nidulans apsA (anucleate primary sterigmata) encodes a coiled-coil protein required for nuclear positioning and completion of asexual development. J. Cell Biol. 1995, 128, 485–498. [Google Scholar] [CrossRef]
- Clutterbuck, A.J. Mutants of Aspergillus nidulans deficient in nuclear migration during hyphal growth and conidiation. Microbiology 1994, 140 Pt 5, 1169–1174. [Google Scholar] [CrossRef][Green Version]
- Lindsey, R.; Cowden, S.; Hernandez-Rodriguez, Y.; Momany, M. Septins AspA and AspC are important for normal development and limit the emergence of new growth foci in the multicellular fungus Aspergillus nidulans. Eukaryot. Cell 2010, 9, 155–163. [Google Scholar] [CrossRef]
- Hernandez-Rodriguez, Y.; Hastings, S.; Momany, M. The septin AspB in Aspergillus nidulans forms bars and filaments and plays roles in growth emergence and conidiation. Eukaryot. Cell 2012, 11, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Belaish, R.; Sharon, H.; Levdansky, E.; Greenstein, S.; Shadkchan, Y.; Osherov, N. The Aspergillus nidulans cetA and calA genes are involved in conidial germination and cell wall morphogenesis. Fungal Genet. Biol. 2008, 45, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.D.; Lu, K.P.; Means, R.L.; Means, A.R. Calmodulin and cell cycle control. J. Physiol. 1992, 86, 83–88. [Google Scholar] [CrossRef]
- Yamazaki, H.; Tanaka, A.; Kaneko, J.; Ohta, A.; Horiuchi, H. Aspergillus nidulans ChiA is a glycosylphosphatidylinositol (GPI)-anchored chitinase specifically localized at polarized growth sites. Fungal Genet. Biol. 2008, 45, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.D.; Means, A.R. Identification and characterization of two Ca2+/CaM-dependent protein kinases required for normal nuclear division in Aspergillus nidulans. J. Biol. Chem. 2000, 275, 38230–38238. [Google Scholar] [CrossRef]
- Suzuki, S.; Bayram, O.S.; Bayram, O.; Braus, G.H. conF and conJ contribute to conidia germination and stress response in the filamentous fungus Aspergillus nidulans. Fungal Genet. Biol. 2013, 56, 42–53. [Google Scholar] [CrossRef]
- Johns, S.A.; Leeder, A.C.; Safaie, M.; Turner, G. Depletion of Aspergillus nidulans cotA causes a severe polarity defect which is not suppressed by the nuclear migration mutation nudA2. Mol. Genet. Genom. 2006, 275, 593–604. [Google Scholar] [CrossRef]
- Shi, J.; Chen, W.; Liu, Q.; Chen, S.; Hu, H.; Turner, G.; Lu, L. Depletion of the MobB and CotA complex in Aspergillus nidulans causes defects in polarity maintenance that can be suppressed by the environment stress. Fungal Genet. Biol. 2008, 45, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wang, L.; Liu, Z.; Kwon, N.J.; Kim, S.C.; Yu, J.H. Gβ-like CpcB plays a crucial role for growth and development of Aspergillus nidulans and Aspergillus fumigatus. PLoS ONE 2013, 8, e70355. [Google Scholar] [CrossRef] [PubMed]
- Schultzhaus, Z.; Yan, H.; Shaw, B.D. Aspergillus nidulans flippase DnfA is cargo of the endocytic collar and plays complementary roles in growth and phosphatidylserine asymmetry with another flippase, DnfB. Mol. Microbiol. 2015, 97, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Rohrig, J.; Kastner, C.; Fischer, R. Light inhibits spore germination through phytochrome in Aspergillus nidulans. Curr. Genet. 2013, 59, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Harispe, L.; Portela, C.; Scazzocchio, C.; Penalva, M.A.; Gorfinkiel, L. Ras GTPase-activating protein regulation of actin cytoskeleton and hyphal polarity in Aspergillus nidulans. Eukaryot. Cell 2008, 7, 141–153. [Google Scholar] [CrossRef]
- Bakti, F.; Kiraly, A.; Orosz, E.; Miskei, M.; Emri, T.; Leiter, E.; Pocsi, I. Study on the glutathione metabolism of the filamentous fungus Aspergillus nidulans. Acta Microbiol. Immunol. Hung. 2017, 64, 255–272. [Google Scholar] [CrossRef][Green Version]
- Dos Reis, T.F.; Mellado, L.; Lohmar, J.M.; Silva, L.P.; Zhou, J.J.; Calvo, A.M.; Goldman, G.H.; Brown, N.A. GPCR-mediated glucose sensing system regulates light-dependent fungal development and mycotoxin production. PLoS Genet. 2019, 15, e1008419. [Google Scholar] [CrossRef]
- Amon, J.; Varga, G.; Pfeiffer, I.; Farkas, Z.; Karacsony, Z.; Hegedus, Z.; Vagvolgyi, C.; Hamari, Z. The role of the Aspergillus nidulans high mobility group B protein HmbA, the orthologue of Saccharomyces cerevisiae Nhp6p. Sci. Rep. 2022, 12, 17334. [Google Scholar] [CrossRef]
- Kang, E.H.; Kim, J.A.; Oh, H.W.; Park, H.M. LAMMER Kinase LkhA plays multiple roles in the vegetative growth and asexual and sexual development of Aspergillus nidulans. PLoS ONE 2013, 8, e58762. [Google Scholar] [CrossRef]
- Harris, S.D. Morphogenesis is coordinated with nuclear division in germinating Aspergillus nidulans conidiospores. Microbiology 1999, 145 Pt 10, 2747–2756. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.M.; Song, H.Y.; Choi, H.J.; So, K.K.; Kim, D.H.; Chae, K.S.; Han, D.M.; Jahng, K.Y. Characterization of NpgA, a 4′-phosphopantetheinyl transferase of Aspergillus nidulans, and evidence of its involvement in fungal growth and formation of conidia and cleistothecia for development. J. Microbiol. 2015, 53, 21–31. [Google Scholar] [CrossRef]
- Hynes, M.J.; Murray, S.L.; Khew, G.S.; Davis, M.A. Genetic analysis of the role of peroxisomes in the utilization of acetate and fatty acids in Aspergillus nidulans. Genetics 2008, 178, 1355–1369. [Google Scholar] [CrossRef]
- Ahn, C.S.; Oh, Y.; Kim, J.G.; Han, K.H.; Lee, C.W.; Kim, J.W. The observation of plcA mutation and localization in Aspergillus nidulans. J. Microbiol. 2014, 52, 590–596. [Google Scholar] [CrossRef]
- Jiang, P.; Wei, W.F.; Zhong, G.W.; Zhou, X.G.; Qiao, W.R.; Fisher, R.; Lu, L. The function of the three phosphoribosyl pyrophosphate synthetase (Prs) genes in hyphal growth and conidiation in Aspergillus nidulans. Microbiology 2017, 163, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Osherov, N.; May, G. Conidial germination in Aspergillus nidulans requires RAS signaling and protein synthesis. Genetics 2000, 155, 647–656. [Google Scholar] [CrossRef]
- Kwon, N.J.; Park, H.S.; Jung, S.; Kim, S.C.; Yu, J.H. The putative guanine nucleotide exchange factor RicA mediates upstream signaling for growth and development in Aspergillus. Eukaryot. Cell 2012, 11, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, S.; Chaveroche, M.K.; Shimizu, K.; Keller, N.; d’Enfert, C. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 2002, 44, 1001–1016. [Google Scholar] [CrossRef]
- Harris, S.D.; Hofmann, A.F.; Tedford, H.W.; Lee, M.P. Identification and characterization of genes required for hyphal morphogenesis in the filamentous fungus Aspergillus nidulans. Genetics 1999, 151, 1015–1025. [Google Scholar] [CrossRef]
- Whittaker, S.L.; Lunness, P.; Milward, K.J.; Doonan, J.H.; Assinder, S.J. sodVIC is an α-COP-related gene which is essential for establishing and maintaining polarized growth in Aspergillus nidulans. Fungal Genet. Biol. 1999, 26, 236–252. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kang, E.H.; Park, Y.H.; Kook, J.H.; Park, H.M. Survival factor SvfA plays multiple roles in differentiation and is essential for completion of sexual development in Aspergillus nidulans. Sci. Rep. 2020, 10, 5586. [Google Scholar] [CrossRef]
- Upadhyay, S.; Shaw, B.D. A phosphoglucose isomerase mutant in Aspergillus nidulans is defective in hyphal polarity and conidiation. Fungal Genet. Biol. 2006, 43, 739–751. [Google Scholar] [CrossRef]
- Momany, M.; Westfall, P.J.; Abramowsky, G. Aspergillus nidulans swo mutants show defects in polarity establishment, polarity maintenance and hyphal morphogenesis. Genetics 1999, 151, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Higashitsuji, Y.; Herrero, S.; Takeshita, N.; Fischer, R. The cell end marker protein TeaC is involved in growth directionality and septation in Aspergillus nidulans. Eukaryot. Cell 2009, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Afroz, S.; El-Ganiny, A.M.; Sanders, D.A.; Kaminskyj, S.G. Roles of the Aspergillus nidulans UDP-galactofuranose transporter, UgtA in hyphal morphogenesis, cell wall architecture, conidiation, and drug sensitivity. Fungal Genet. Biol. 2011, 48, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, H.O.; Zheng, L.; Ohta, A.; Horiuchi, H. A Wiskott-Aldrich syndrome protein is involved in endocytosis in Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2016, 80, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Colabardini, A.C.; Brown, N.A.; Savoldi, M.; Goldman, M.H.; Goldman, G.H. Functional characterization of Aspergillus nidulans ypkA, a homologue of the mammalian kinase SGK. PLoS ONE 2013, 8, e57630. [Google Scholar] [CrossRef]
- Ries, L.N.; Beattie, S.R.; Espeso, E.A.; Cramer, R.A.; Goldman, G.H. Diverse Regulation of the CreA Carbon Catabolite Repressor in Aspergillus nidulans. Genetics 2016, 203, 335–352. [Google Scholar] [CrossRef]
- Dowzer, C.E.; Kelly, J.M. Analysis of the creA gene, a regulator of carbon catabolite repression in Aspergillus nidulans. Mol. Cell. Biol. 1991, 11, 5701–5709. [Google Scholar] [CrossRef]
- Ni, M.; Rierson, S.; Seo, J.A.; Yu, J.H. The pkaB gene encoding the secondary protein kinase A catalytic subunit has a synthetic lethal interaction with pkaA and plays overlapping and opposite roles in Aspergillus nidulans. Eukaryot. Cell 2005, 4, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Seo, J.A.; Yu, J.H. Regulators of G-protein signalling in Aspergillus nidulans: RgsA downregulates stress response and stimulates asexual sporulation through attenuation of GanB (Gα) signalling. Mol. Microbiol. 2004, 53, 529–540. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).