Evaluating Differentiation Status of Mesenchymal Stem Cells by Label-Free Microscopy System and Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Culture Medium
2.2. Culture and Induction of MSCs
2.3. FLIM Imaging and Processing
2.4. SRS Imaging
2.5. ALP Staining of Osteogenic Differentiation and Imaging Methods
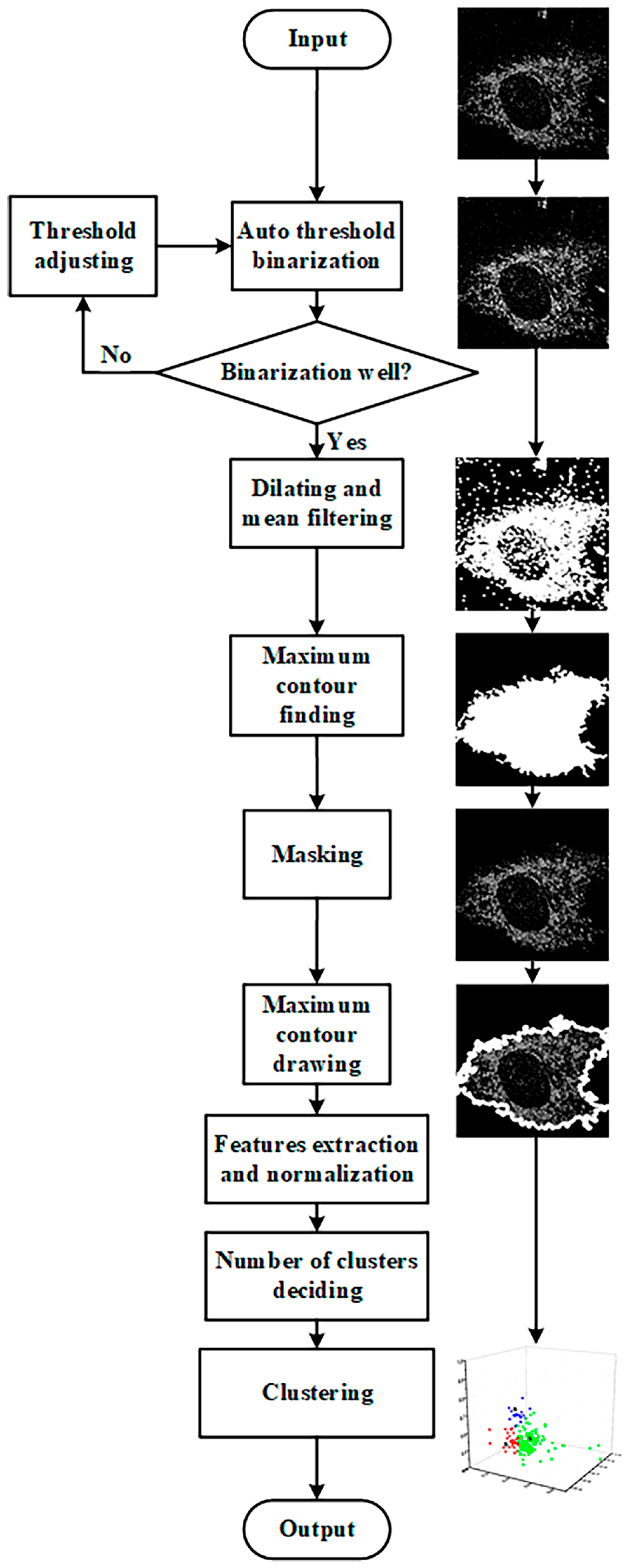
2.6. Image Segmentation
2.7. Kmeans++ Algorithm
2.8. Statistical Analysis
3. Results
3.1. Identification of Adipogenic Differentiation Status
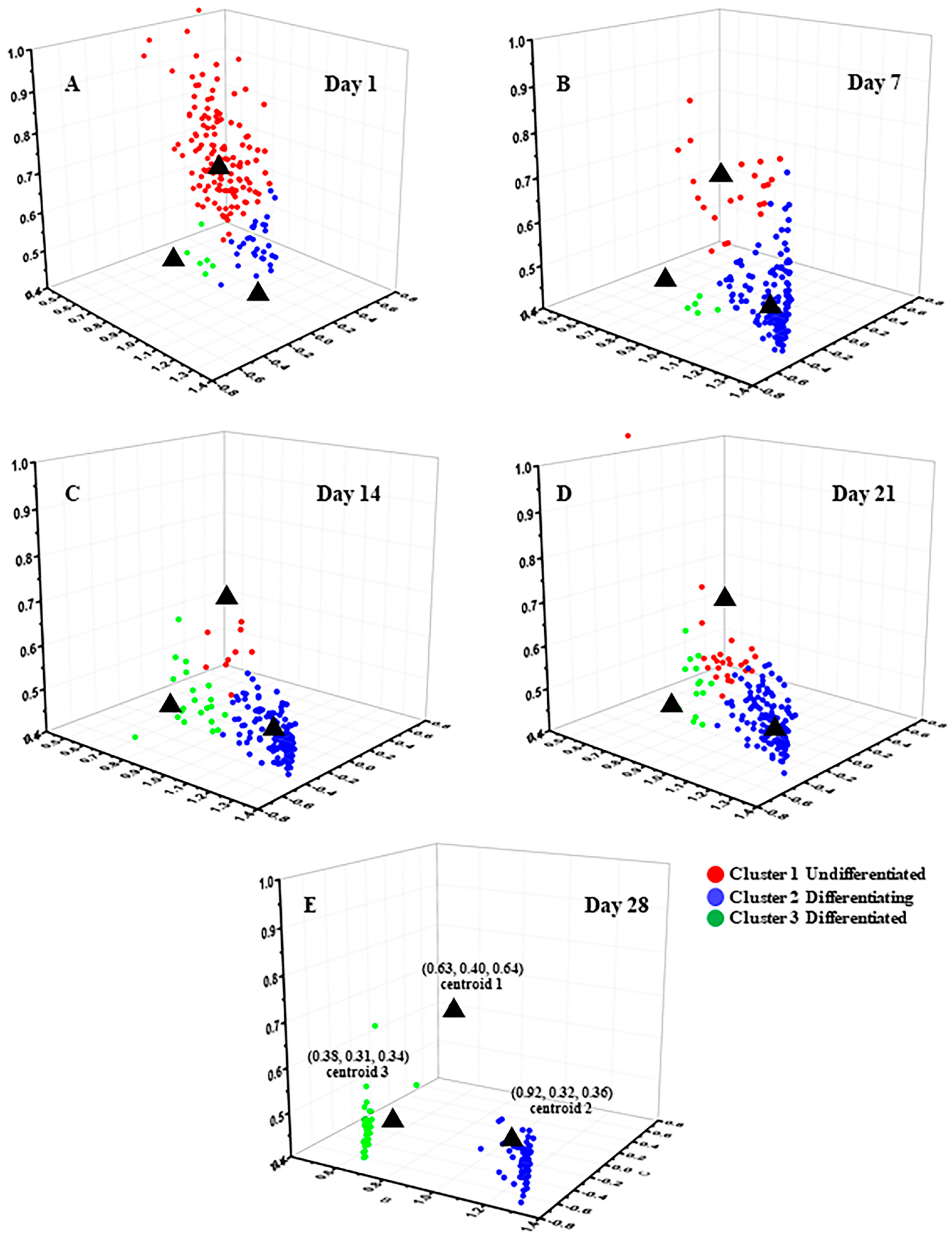
3.1.1. FLIM Images and K-Means++ Clustering
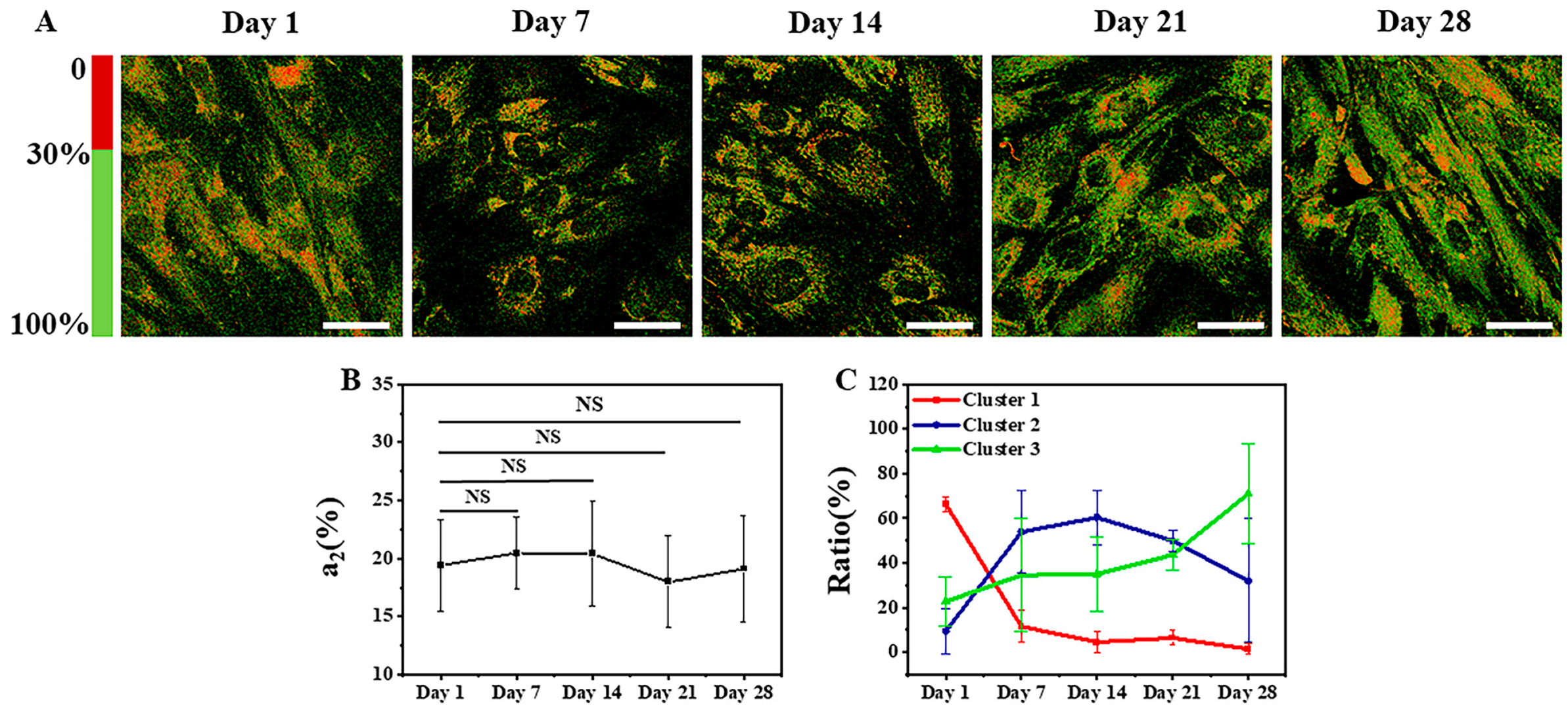
3.1.2. SRS Images of Lipids and K-Means++ Clustering
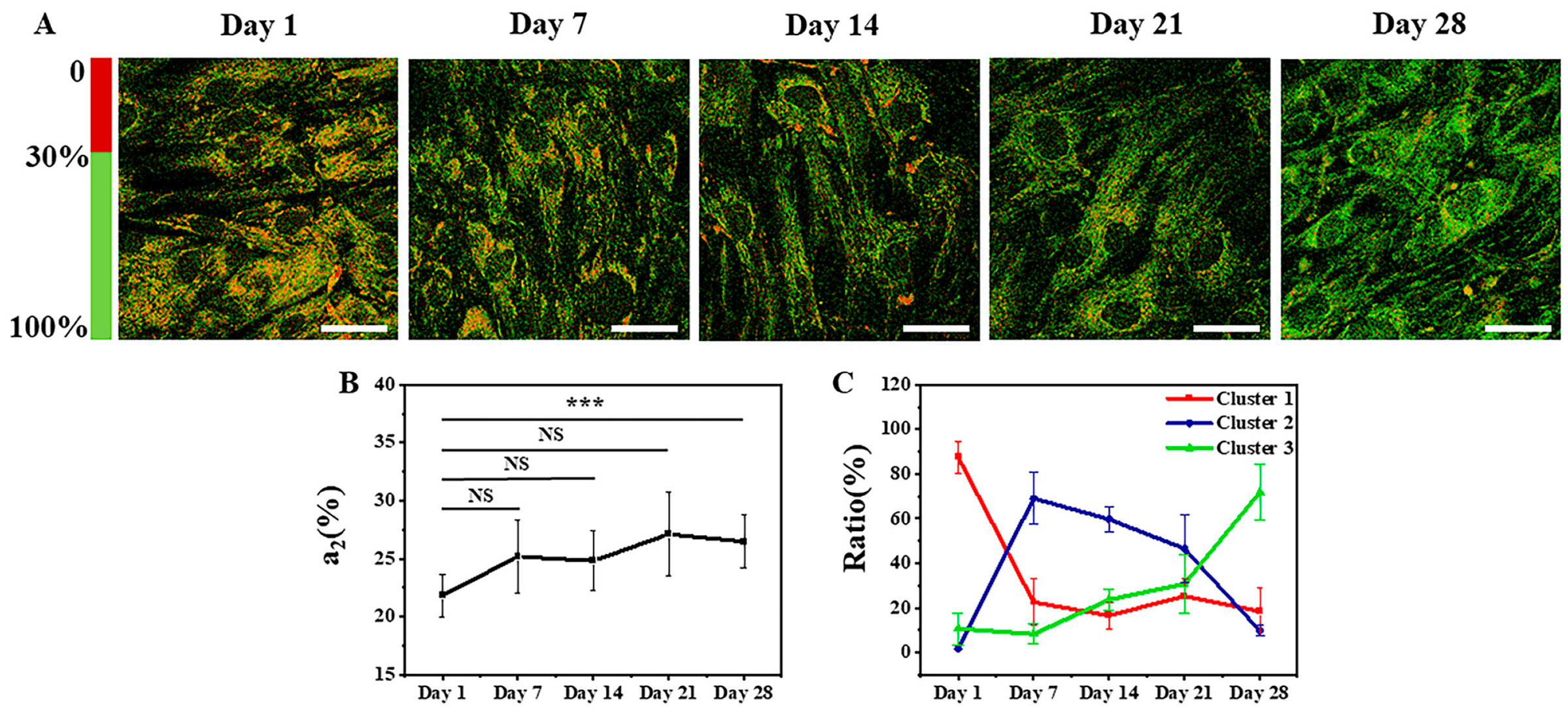
3.2. Identification of Osteogenic Differentiation Status
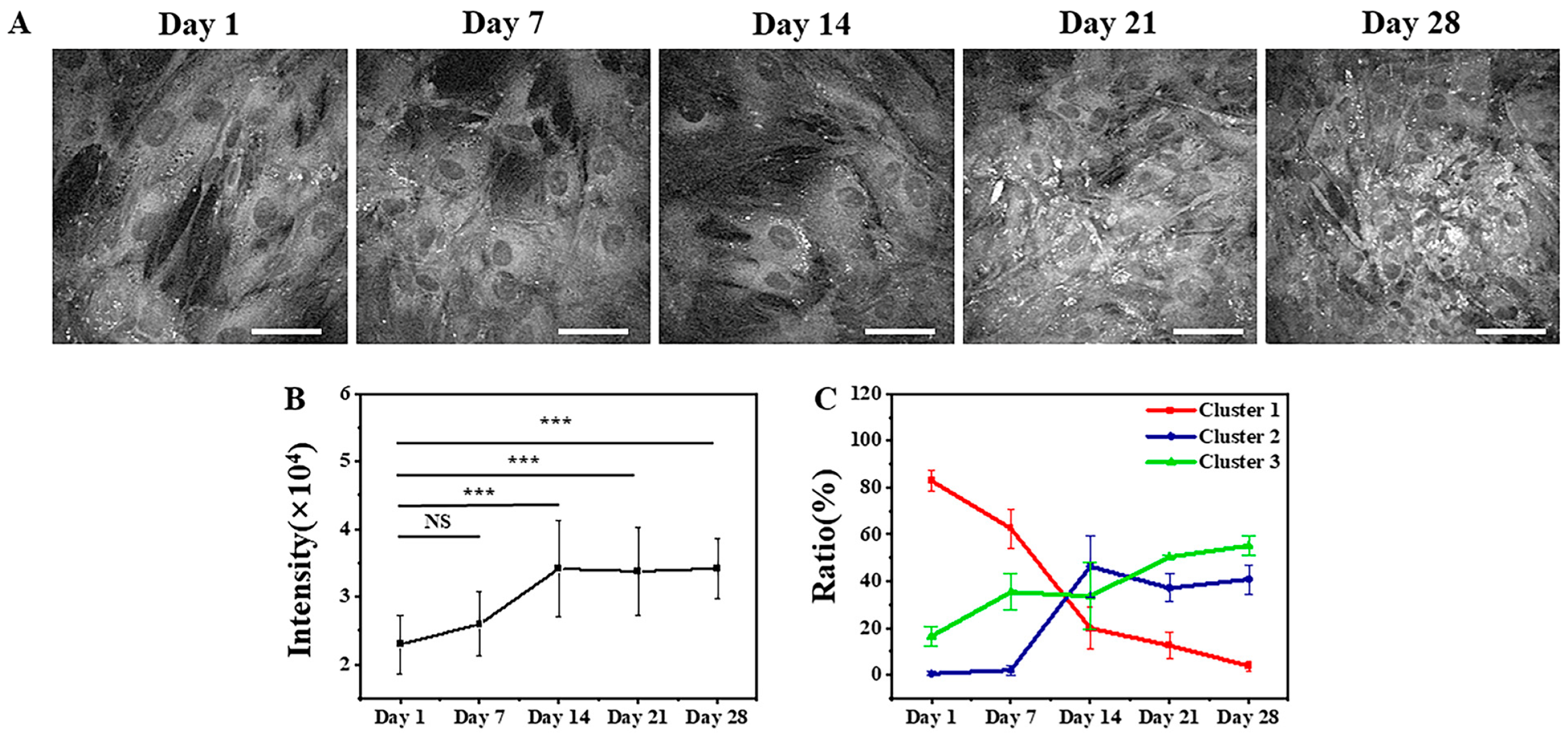
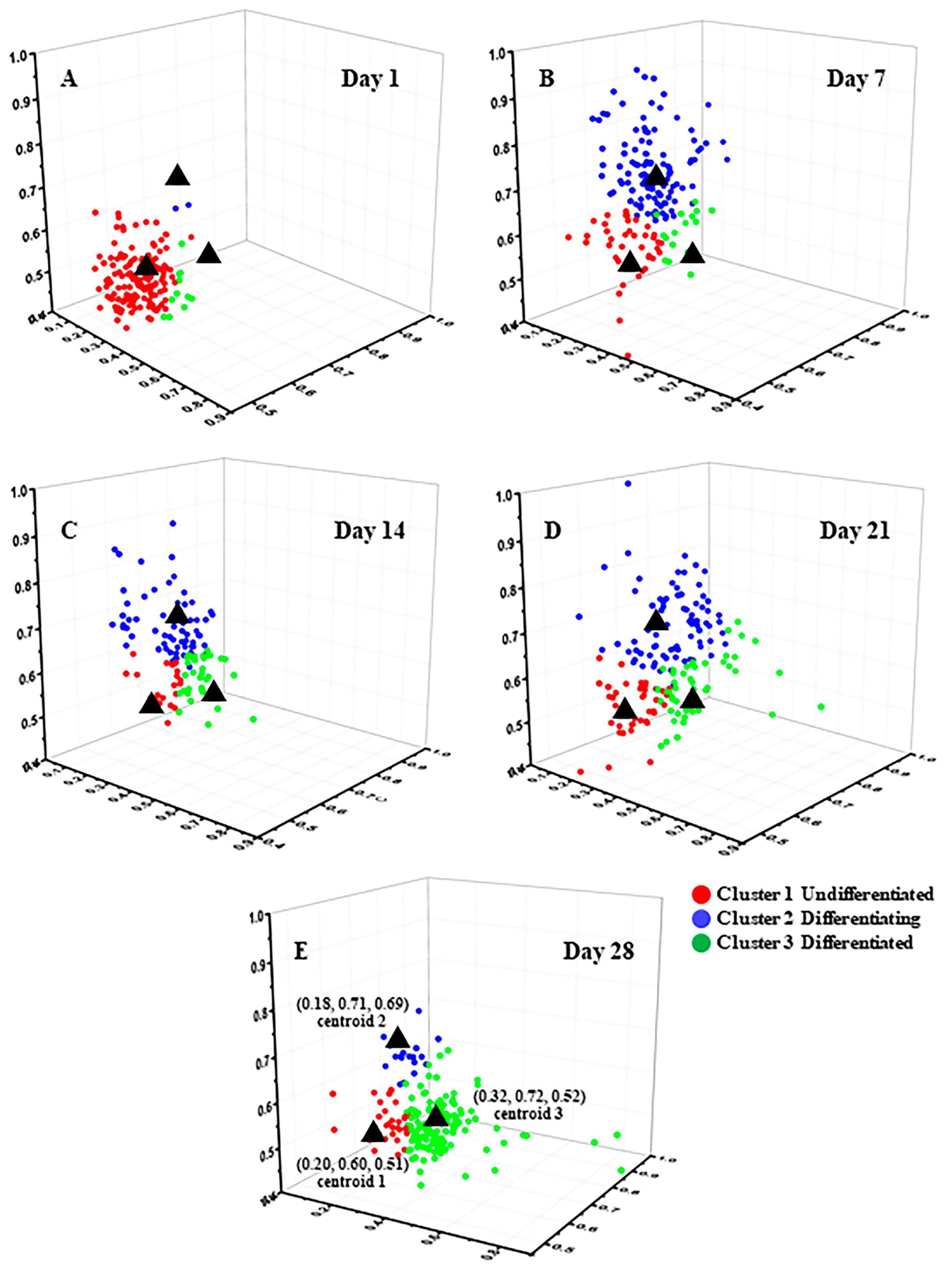
3.2.1. FLIM Images and K-Means++ Clustering
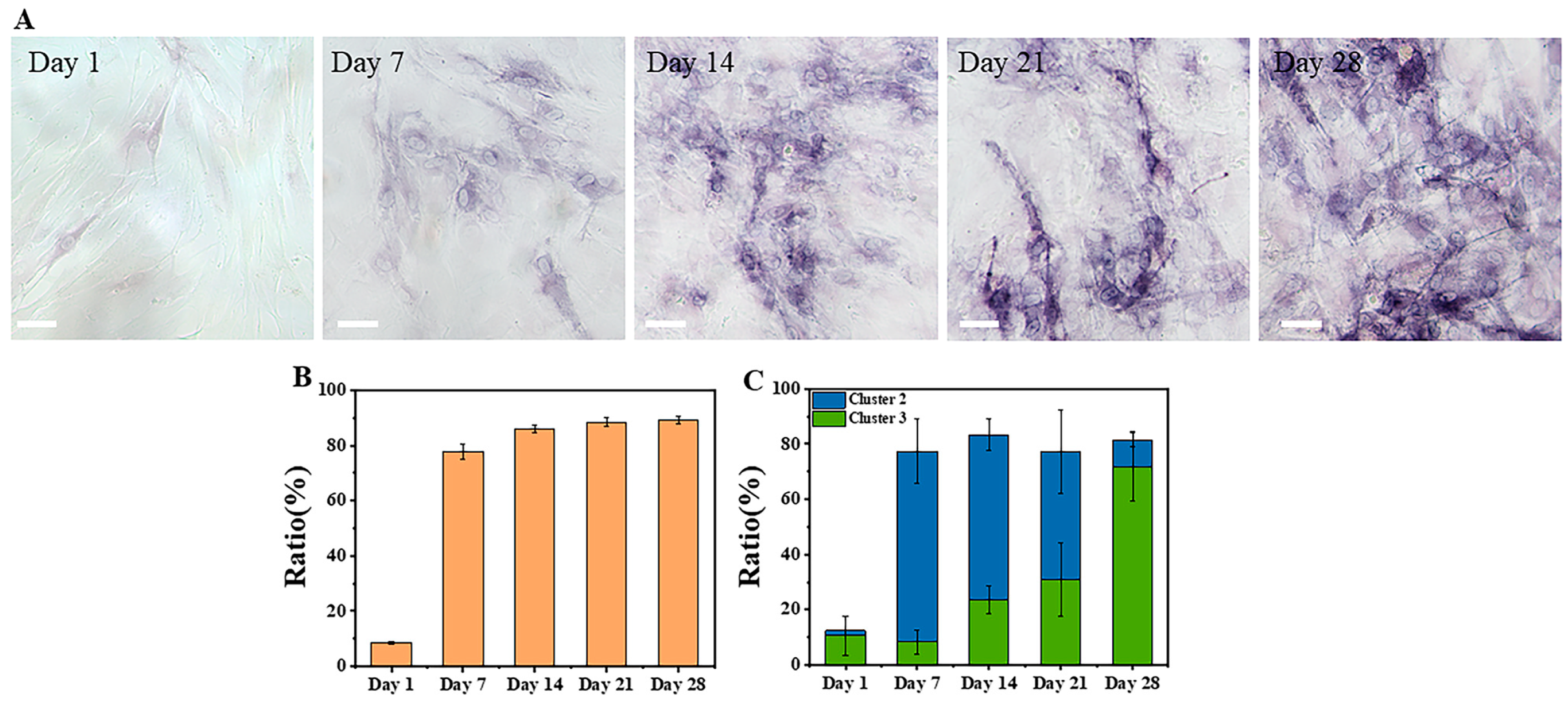
3.2.2. ALP Staining of Osteogenic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gimble, J.; Guilak, F. Adipose-Derived Adult Stem Cells: Isolation, Characterization, and Differentiation Potential. Cytotherapy 2003, 5, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of Proliferative and Multilineage Differentiation Potential of Human Mesenchymal Stem Cells Derived from Umbilical Cord and Bone Marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic Transplants of Bone Marrow—Analysis of Precursor Cells for Osteogenic and Hematopoietic Tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Pountos, I.; Corscadden, D.; Emery, P.; Giannoudis, P.V. Mesenchymal Stem Cell Tissue Engineering: Techniques for Isolation, Expansion and Application. Injury 2007, 38, S23–S33. [Google Scholar] [CrossRef]
- Lee, A.S.; Tang, C.; Cao, F.; Xie, X.; Van Der Bogt, K.; Hwang, A.; Connolly, A.J.; Robbins, R.C.; Wu, J.C. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle 2009, 8, 2608–2612. [Google Scholar] [CrossRef]
- Olmedo-Moreno, L.; Aguilera, Y.; Baliña-Sánchez, C.; Martín-Montalvo, A.; Capilla-González, V. Heterogeneity of In Vitro Expanded Mesenchymal Stromal Cells and Strategies to Improve Their Therapeutic Actions. Pharmaceutics 2022, 14, 1112. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, S.; Wang, L.; Yang, H.; Tai, C.; Ling, L.; Chen, L.; Liu, S.; Bin Wang, B. Individual heterogeneity screened umbilical cord-derived mesenchymal stromal cells with high Treg promotion demonstrate improved recovery of mouse liver fibrosis. Stem Cell Res. Ther. 2021, 12, 359. [Google Scholar] [CrossRef]
- Donald, G.P.; Kopen, G.; Righter, W.; Webster, S.; Tremain, N.; Prockop, D.J. Donor Variation in the Growth Properties and Osteogenic Potential of Human Marrow Stromal Cells. J. Cell. Biochem. 1999, 75, 424–436. [Google Scholar]
- Hsu, Y.-C.; Wu, Y.-T.; Yu, T.-H.; Wei, Y.-H. Mitochondria in mesenchymal stem cell biology and cell therapy: From cellular differentiation to mitochondrial transfer. Semin. Cell Dev. Biol. 2016, 52, 119–131. [Google Scholar] [CrossRef]
- Guo, H.-W.; Yu, J.-S.; Hsu, S.-H.; Wei, Y.-H.; Lee, O.K.; Dong, C.-Y.; Wang, H.-W. Correlation of NADH fluorescence lifetime and oxidative phosphorylation metabolism in the osteogenic differentiation of human mesenchymal stem cell. J. Biomed. Opt. 2015, 20, 017004. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, E.; Ung, T.P.L.; del Carmen, A.A.; del Toro-Ríos, X.; Fajardo-Orduña, G.R.; Noriega, L.G.; Cortés-Morales, V.A.; Tovar, A.R.; Montesinos, J.J.; Orozco-Solís, R.; et al. Coordinated metabolic transitions and gene expression by NAD+ during adipogenesis. J. Cell Biol. 2022, 221, e202111137. [Google Scholar] [CrossRef]
- Rice, W.L.; Kaplan, D.L.; Georgakoudi, I. Two-Photon Microscopy for Non-Invasive, Quantitative Monitoring of Stem Cell Differentiation. PLoS ONE 2010, 5, e10075. [Google Scholar]
- Chen, C.-T.; Shih, Y.-R.V.; Kuo, T.K.; Lee, O.K.; Wei, Y.-H. Coordinated Changes of Mitochondrial Biogenesis and Antioxidant Enzymes During Osteogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef]
- Meleshina, A.V.; Dudenkova, V.V.; Bystrova, A.S.; Kuznetsova, D.S.; Shirmanova, M.V.; Zagaynova, E.V. Two-photon FLIM of NAD(P)H and FAD in mesenchymal stem cells undergoing either osteogenic or chondrogenic differentiation. Stem Cell Res. Ther. 2017, 8, 15. [Google Scholar] [CrossRef]
- Guo, H.-W.; Chen, C.-T.; Wei, Y.-H.; Lee, O.K.; Gukassyan, V.; Kao, F.-J.; Wang, H.-W. Reduced nicotinamide adenine dinucleotide fluorescence lifetime separates human mesenchymal stem cells from differentiated progenies. J. Biomed. Opt. 2008, 13, 050505. [Google Scholar] [CrossRef] [PubMed]
- Meleshina, A.V.; Dudenkova, V.V.; Shirmanova, M.V.; Shcheslavskiy, V.I.; Becker, W.; Bystrova, A.S.; Cherkasova, E.I.; Zagaynova, E.V. Probing metabolic states of differentiating stem cells using two-photon FLIM. Sci. Rep. 2016, 6, 21853. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ou, M.H.; Kuo, J.C.; Chiou, A. A Comparative Study of Metabolic State of Stem Cells During Osteogenic and Adipogenic Differentiations Via Fluorescence Lifetime Imaging Microscopy. In Optics in Health Care and Biomedical Optics VII; SPIE: Bellingham, DC, USA, 2016. [Google Scholar]
- Lazarević, J.; Kukolj, T.; Bugarski, D.; Lazarević, N.; Popović, Z. Probing primary mesenchymal stem cells differentiation status by micro-Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Francesca, R.; Efeoglu, E.; Byrne, H.J. Monitoring Stem Cell Differentiation Using Raman Microspectroscopy: Chondrogenic Differentiation, Towards Cartilage Formation. Analyst 2012, 146, 322–337. [Google Scholar]
- Prince, R.C.; Frontiera, R.R.; Potma, E.O. Stimulated Raman Scattering: From Bulk to Nano. Chem. Rev. 2016, 117, 5070–5094. [Google Scholar] [CrossRef]
- Wang, M.C.; Min, W.; Freudiger, C.W.; Ruvkun, G.; Xie, X.S. RNAi screening for fat regulatory genes with SRS microscopy. Nat. Methods 2011, 8, 135–138. [Google Scholar] [CrossRef]
- Wang, P.; Liu, B.; Zhang, D.; Belew, M.Y.; Tissenbaum, H.A.; Cheng, J.-X. Imaging Lipid Metabolism in Live Caenorhabditis Elegans Using Fingerprint Vibrations. Angew. Chem. 2014, 126, 11981–11986. [Google Scholar] [CrossRef]
- Pouikli, A.; Tessarz, P. Metabolism and Chromatin: A Dynamic Duo That Regulates Development and Ageing: Elucidating the Metabolism-Chromatin Axis in Bone-Marrow Mesenchymal Stem Cell Fate Decisions. Bioessays 2021, 43, 2000273. [Google Scholar] [CrossRef]
- Mota, S.M.; Rogers, R.E.; Haskell, A.W.; McNeill, E.P.; Kaunas, R.R.; Gregory, C.A.; Giger, M.L.; Maitland, K.C. Automated mesenchymal stem cell segmentation and machine learning-based phenotype classification using morphometric and textural analysis. J. Med. Imaging 2021, 8, 014503. [Google Scholar] [CrossRef]
- Marklein, R.A.; Klinker, M.W.; Drake, K.A.; Polikowsky, H.; Lessey-Morillon, E.C.; Bauer, S.R. Morphological profiling using machine learning reveals emergent subpopulations of interferon-γ–stimulated mesenchymal stromal cells that predict immunosuppression. Cytotherapy 2018, 21, 17–31. [Google Scholar] [CrossRef]
- Bianchetti, G.; Ciccarone, F.; Ciriolo, M.R.; De Spirito, M.; Pani, G.; Maulucci, G. Label-free metabolic clustering through unsupervised pixel classification of multiparametric fluorescent images. Anal. Chim. Acta 2020, 1148, 238173. [Google Scholar] [CrossRef]
- Ji, M.; Zhong, J.; Xue, R.; Su, W.; Kong, Y.; Fei, Y.; Ma, J.; Wang, Y.; Mi, L. Early Detection of Cervical Cancer by Fluorescence Lifetime Imaging Microscopy Combined with Unsupervised Machine Learning. Int. J. Mol. Sci. 2022, 23, 11476. [Google Scholar] [CrossRef]
- Liu, Z.; Su, W.; Ao, J.; Wang, M.; Jiang, Q.; He, J.; Gao, H.; Lei, S.; Nie, J.; Yan, X.; et al. Instant diagnosis of gastroscopic biopsy via deep-learned single-shot femtosecond stimulated Raman histology. Nat. Commun. 2022, 13, 4050. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Zheng, B.; Su, L.; Chen, Y.; Ma, S.; Hu, Q.; Zou, X.; Yao, L.; Yang, Y.; et al. Rapid histology of laryngeal squamous cell carcinoma with deep-learning based stimulated Raman scattering microscopy. Theranostics 2019, 9, 2541–2554. [Google Scholar] [CrossRef]
- Ao, J.; Fang, X.; Miao, X.; Ling, J.; Kang, H.; Park, S.; Wu, C.; Ji, M. Switchable stimulated Raman scattering microscopy with photochromic vibrational probes. Nat. Commun. 2021, 12, 3089. [Google Scholar] [CrossRef]
- Crowley, J.L.; Draper, B.; Thonnat, M. Computer Vision Systems: 8th International Conference, ICVS 2011, Sophia Antipolis, France, September 20–22, 2011, Proceedings; Springer: Berlin/Heidelberg, Germany, 2011; Volume 6962. [Google Scholar]
- Xu, J.; Xiang, L.; Liu, Q.; Gilmore, H.; Wu, J.; Tang, J.; Madabhushi, A. Stacked Sparse Autoencoder (SSAE) for Nuclei Detection on Breast Cancer Histopathology Images. IEEE Trans. Med. Imaging 2015, 35, 119–130. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, F.; Zhao, J.; Chu, J. Segmentation of White Blood Cells Image Using Adaptive Location and Iteration. IEEE J. Biomed. Health Inform. 2016, 21, 1644–1655. [Google Scholar] [CrossRef]
- Cao, F.; Liu, Y.; Huang, Z.; Chu, J.; Zhao, J. Effective Segmentations in White Blood Cell Images Using ϵ-Svr-Based Detection Method. Neural Comput. Appl. 2019, 31, 6767–6780. [Google Scholar] [CrossRef]
- Gurcan, M.N.; Pan, T.; Shimada, H.; Saltz, J. Image Analysis for Neuroblastoma Classification: Segmentation of Cell Nuclei. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006. [Google Scholar]
- Zhang, B.; Zimmer, C.; Olivo-Marin, J.-C. Tracking Fluorescent Cells with Coupled Geometric Active Contours. In Proceedings of the 2004 2nd IEEE International Symposium on Biomedical Imaging: Nano to Macro (IEEE Cat No. 04EX821), Arlington, VA, USA, 18 April 2004. [Google Scholar]
- Ran, X.; Zhou, X.; Lei, M.; Tepsan, W.; Deng, W. A Novel K-Means Clustering Algorithm with a Noise Algorithm for Capturing Urban Hotspots. Appl. Sci. 2021, 11, 11202. [Google Scholar] [CrossRef]
- Chen, B.; Lu, Y.; Pan, W.; Xiong, J.; Yang, Z.; Yan, W.; Liu, L.; Qu, J. Support Vector Machine Classification of Nonmelanoma Skin Lesions Based on Fluorescence Lifetime Imaging Microscopy. Anal. Chem. 2019, 91, 10640–10647. [Google Scholar] [CrossRef]
- Russell, A.L.; Lefavor, R.; Durand, N.; Glover, L.; Zubair, A.C. Modifiers of mesenchymal stem cell quantity and quality. Transfusion 2018, 58, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Russell, A.L.; Lefavor, R.; Durand, N.C.; James, E.; Harvey, L.; Zhang, C.; Countryman, S.; Stodieck, L.; Zubair, A.C. Feasibility, potency, and safety of growing human mesenchymal stem cells in space for clinical application. NPJ Microgravity 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.A.; Filograna, A.; Ranjan, R.; Corda, D.; Valente, C.; Sirleto, L. Three-dimensional label-free imaging throughout adipocyte differentiation by stimulated Raman microscopy. PLoS ONE 2019, 14, e0216811. [Google Scholar] [CrossRef] [PubMed]
- D’arco, A.; Brancati, N.; Ferrara, M.A.; Indolfi, M.; Frucci, M.; Sirleto, L. Subcellular chemical and morphological analysis by stimulated Raman scattering microscopy and image analysis techniques. Biomed. Opt. Express 2016, 7, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Ferrara, M.; Filograna, A.; Valente, C.; Sirleto, L. Femtosecond Stimulated Raman microscopy: Home-built realization and a case study of biological imaging. J. Instrum. 2019, 14, P09008. [Google Scholar] [CrossRef]
- O’Melia, M.J.; Wallrabe, H.; Svindrych, Z.; Rehman, S.; Periasamy, A. Flim Data Analysis of Nadh and Tryptophan Autofluorescence in Prostate Cancer Cells. Multiphoton Microsc. Biomed. Sci. XVI 2016, 9712, 261–266. [Google Scholar]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekström, K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE 2018, 13, e0193059. [Google Scholar] [CrossRef]
- Mehta, N.; Shaik, S.; Prasad, A.; Chaichi, A.; Sahu, S.P.; Liu, Q.; Hasan, S.M.A.; Sheikh, E.; Donnarumma, F.; Murray, K.K.; et al. Multimodal Label-Free Monitoring of Adipogenic Stem Cell Differentiation Using Endogenous Optical Biomarkers. Adv. Funct. Mater. 2021, 31, 2103955. [Google Scholar] [CrossRef]
- Pablo, R.J.; González, M.; Ríos, S.; Cambiazo, V. Cytoskeletal Organization of Human Mesenchymal Stem Cells (Msc) Changes During Their Osteogenic Differentiation. J. Cell. Biochem. 2004, 93, 721–731. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Xie, Z.; Song, P.; Zhao, R.C.H.; Guo, L.; Liu, Z.; Wu, Y. Epigenetic Dysregulation in Mesenchymal Stem Cell Aging and Spontaneous Differentiation. PLoS ONE 2011, 6, e20526. [Google Scholar] [CrossRef]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone Marrow Stromal Stem Cells: Nature, Biology, and Potential Applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.; Ao, J.; Chen, Q.; Su, W.; Zhao, Y.; Fei, Y.; Ma, J.; Ji, M.; Mi, L. Evaluating Differentiation Status of Mesenchymal Stem Cells by Label-Free Microscopy System and Machine Learning. Cells 2023, 12, 1524. https://doi.org/10.3390/cells12111524
Kong Y, Ao J, Chen Q, Su W, Zhao Y, Fei Y, Ma J, Ji M, Mi L. Evaluating Differentiation Status of Mesenchymal Stem Cells by Label-Free Microscopy System and Machine Learning. Cells. 2023; 12(11):1524. https://doi.org/10.3390/cells12111524
Chicago/Turabian StyleKong, Yawei, Jianpeng Ao, Qiushu Chen, Wenhua Su, Yinping Zhao, Yiyan Fei, Jiong Ma, Minbiao Ji, and Lan Mi. 2023. "Evaluating Differentiation Status of Mesenchymal Stem Cells by Label-Free Microscopy System and Machine Learning" Cells 12, no. 11: 1524. https://doi.org/10.3390/cells12111524
APA StyleKong, Y., Ao, J., Chen, Q., Su, W., Zhao, Y., Fei, Y., Ma, J., Ji, M., & Mi, L. (2023). Evaluating Differentiation Status of Mesenchymal Stem Cells by Label-Free Microscopy System and Machine Learning. Cells, 12(11), 1524. https://doi.org/10.3390/cells12111524






