Influence of Age on Hyperoxia-Induced Cardiac Pathophysiology in Type 1 Diabetes Mellitus (T1DM) Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Hyperoxia Exposure
2.3. Physical Parameters
2.4. Histological Analysis
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
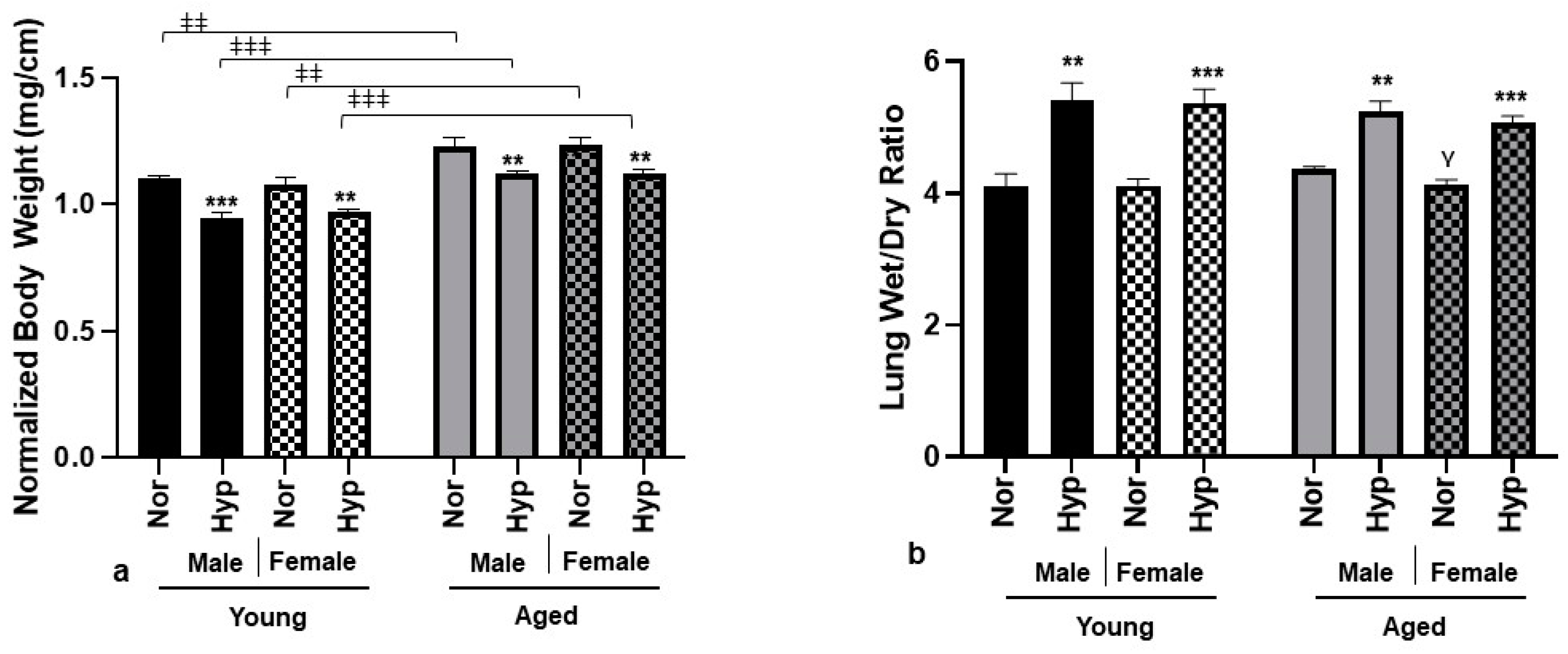
3.1. Physical Parameters
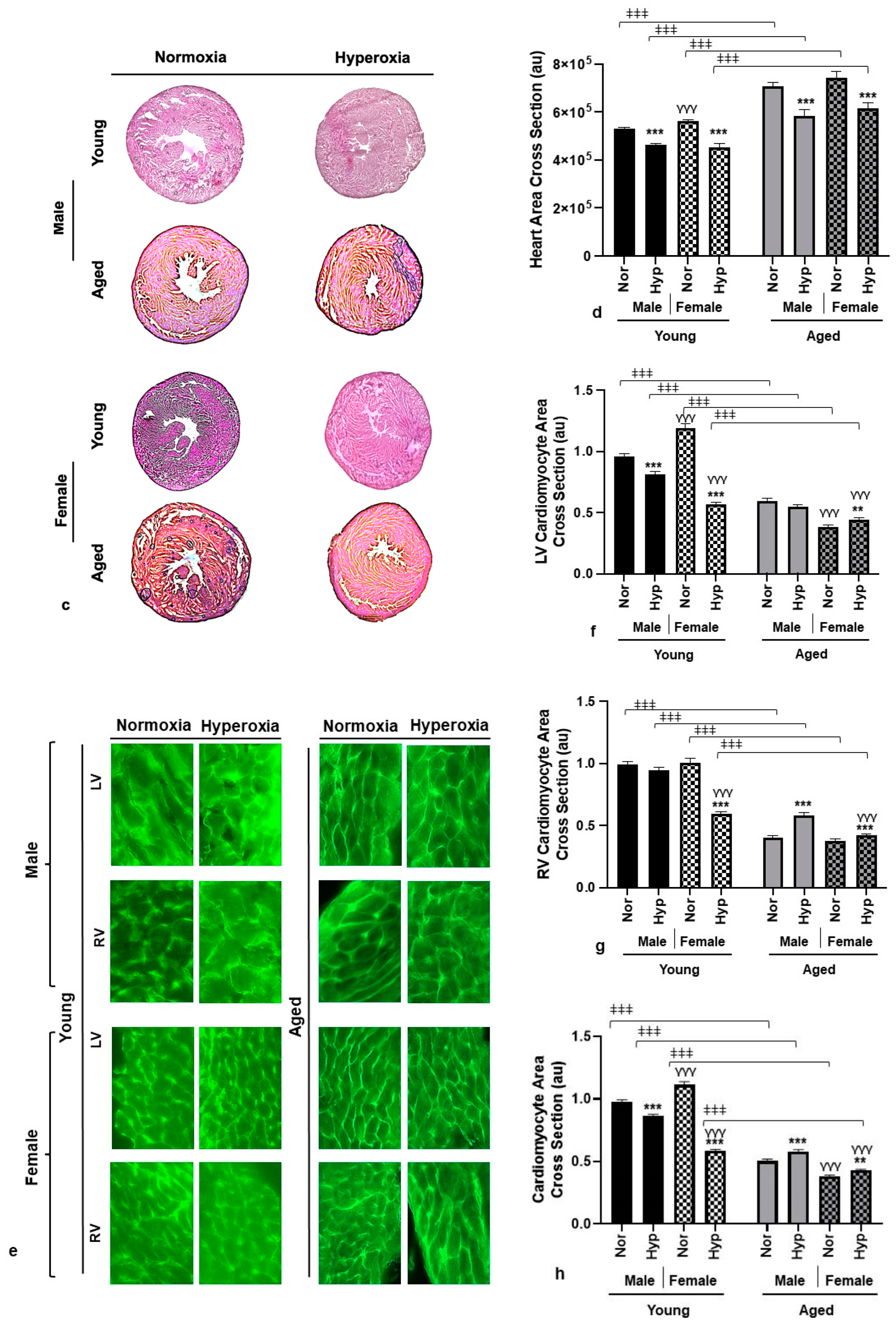
3.2. Histological Analysis
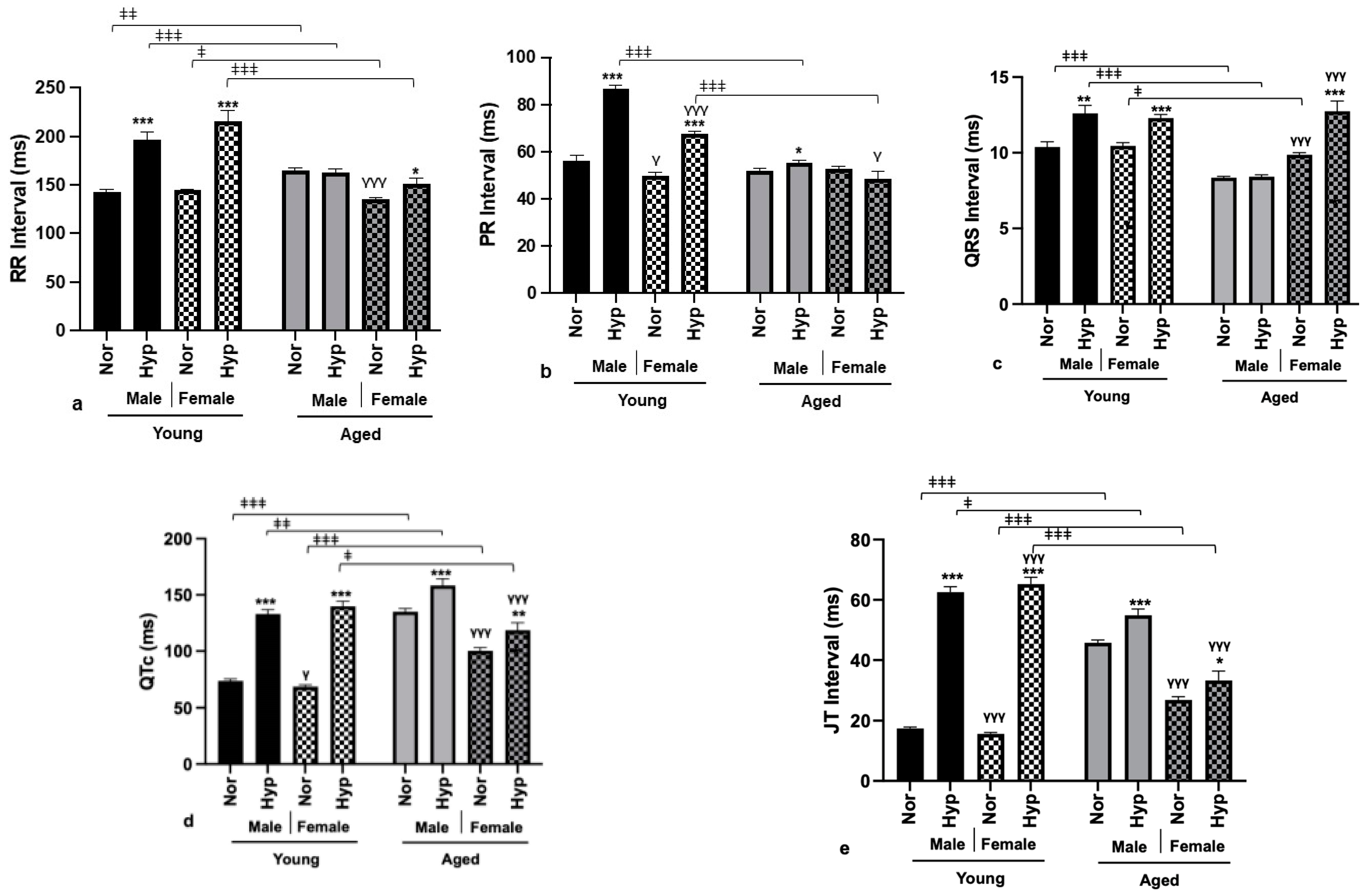
3.3. Electrophysiological Parameters
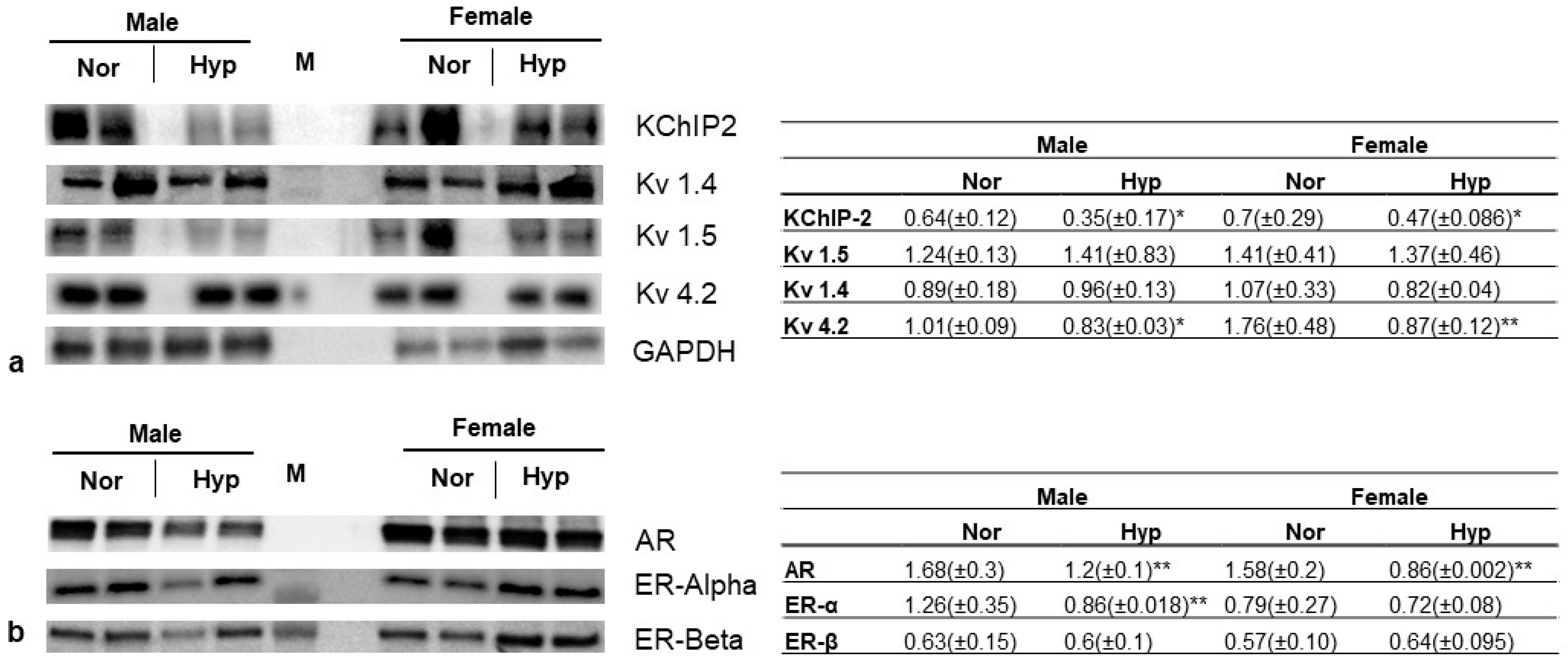
3.4. Gene Expression
4. Discussion
5. Conclusions
Limitations and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanidziar, D.; Robson, S.C. Hyperoxia and modulation of pulmonary vascular and immune responses in COVID-19. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 320, L12–L16. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Eastwood, G.M.; Peck, L.; Glassford, N.J.; Bellomo, R. Current oxygen management in mechanically ventilated patients: A prospective observational cohort study. J. Crit. Care 2013, 28, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Pala Cifci, S.; Urcan Tapan, Y.; Turemis Erkul, B.; Savran, Y.; Comert, B. The impact of hyperoxia on outcome of patients treated with noninvasive respiratory support. Can. Respir. J. 2020, 2020, 3953280. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.S.; Grocott, M.P.W. Oxygen therapy in critical illness: Precise control of arterial oxygenation and permissive hypoxemia. Crit. Care Med. 2013, 41, 423–432. [Google Scholar] [CrossRef]
- Ni, Y.-N.; Wang, Y.-M.; Liang, B.-M.; Liang, Z.-A. The effect of hyperoxia on mortality in critically ill patients: A systematic review and meta analysis. BMC Pulm. Med. 2019, 19, 53. [Google Scholar] [CrossRef]
- Li, L.-F.; Liao, S.-K.; Ko, Y.-S.; Lee, C.-H.; Quinn, D.A. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: A prospective, controlled animal experiment. Crit. Care 2007, 11, R25. [Google Scholar] [CrossRef]
- Sinclair, S.E.; Altemeier, W.A.; Matute-Bello, G.; Chi, E.Y. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit. Care Med. 2004, 32, 2496–2501. [Google Scholar] [CrossRef]
- Dias-Freitas, F.; Metelo-Coimbra, C.; Roncon-Albuquerque, R., Jr. Molecular mechanisms underlying hyperoxia acute lung injury. Respir. Med. 2016, 119, 23–28. [Google Scholar] [CrossRef]
- You, J.; Fan, X.; Bi, X.; Xian, Y.; Xie, D.; Fan, M.; Xu, W.; Zhang, K. Association between arterial hyperoxia and mortality in critically ill patients: A systematic review and meta-analysis. J. Crit. Care 2018, 47, 260–268. [Google Scholar] [CrossRef]
- Stub, D.; Smith, K.; Bernard, S.; Nehme, Z.; Stephenson, M.; Bray, J.E.; Cameron, P.; Barger, B.; Ellims, A.H.; Taylor, A.J. Air versus oxygen in ST-segment–elevation myocardial infarction. Circulation 2015, 131, 2143–2150. [Google Scholar] [CrossRef]
- Moradkhan, R.; Sinoway, L.I. Revisiting the role of oxygen therapy in cardiac patients. J. Am. Coll. Cardiol. 2010, 56, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Chapalamadugu, K.C.; Panguluri, S.K.; Bennett, E.S.; Kolliputi, N.; Tipparaju, S.M. High level of oxygen treatment causes cardiotoxicity with arrhythmias and redox modulation. Toxicol. Appl. Pharmacol. 2015, 282, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Panguluri, S.K.; Tur, J.; Fukumoto, J.; Deng, W.; Sneed, K.B.; Kolliputi, N.; Bennett, E.S.; Tipparaju, S.M. Hyperoxia-induced hypertrophy and ion channel remodeling in left ventricle. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1651–H1661. [Google Scholar] [CrossRef] [PubMed]
- Vichare, R.; Saleem, F.; Mansour, H.; Bojkovic, K.; Cheng, F.; Biswal, M.; Panguluri, S.K. Impact of age and sex on hyperoxia-induced cardiovascular pathophysiology. Mech. Ageing Dev. 2022, 208, 111727. [Google Scholar] [CrossRef]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular risks associated with gender and aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef]
- Sinclair, A.; Saeedi, P.; Kaundal, A.; Karuranga, S.; Malanda, B.; Williams, R. Diabetes and global ageing among 65–99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2020, 162, 108078. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef]
- Rodgers, J.L.; Samal, E.; Mohapatra, S.; Panguluri, S.K. Hyperoxia-induced cardiotoxicity and ventricular remodeling in type-II diabetes mice. Heart Vessel. 2018, 33, 561–572. [Google Scholar] [CrossRef]
- Bajwa, S.J.S. Intensive care management of critically sick diabetic patients. Indian J. Endocrinol. Metab. 2011, 15, 349. [Google Scholar] [CrossRef]
- Halter, J.B.; Musi, N.; McFarland Horne, F.; Crandall, J.P.; Goldberg, A.; Harkless, L.; Hazzard, W.R.; Huang, E.S.; Kirkman, M.S.; Plutzky, J. Diabetes and cardiovascular disease in older adults: Current status and future directions. Diabetes 2014, 63, 2578–2589. [Google Scholar] [CrossRef]
- Bojkovic, K.; Rodgers, J.L.; Vichare, R.; Nandi, A.; Mansour, H.; Saleem, F.; Abidin, Z.U.; Vanthenapalli, S.; Cheng, F.; Panguluri, S.K. The implications of hyperoxia, type 1 diabetes and sex on cardiovascular physiology in mice. Sci. Rep. 2021, 11, 23086. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, V.; Stevens, I.; Mattock, M.; Ebeling, P.; Muggeo, M.; Stephenson, J.; Idzior-Walus, B.; Group, E.I.C.S. Cardiovascular disease and its risk factors in IDDM in Europe. Diabetes Care 1996, 19, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.L.; Iyer, D.; Rodgers, L.E.; Vanthenapalli, S.; Panguluri, S.K. Impact of hyperoxia on cardiac pathophysiology. J. Cell Physiol. 2019, 234, 12595–12603. [Google Scholar] [CrossRef] [PubMed]
- Barazzone-Argiroffo, C.; Muzzin, P.; Donati, Y.R.; Kan, C.-D.; Aubert, M.L.; Piguet, P.-F. Hyperoxia increases leptin production: A mechanism mediated through endogenous elevation of corticosterone. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L1150–L1156. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Kim, Y.E.; Lee, S.J.; Cho, Y.J.; Jeong, Y.Y.; Kim, H.C.; Lee, J.D.; Hwang, Y.S. The clinical significance of weight change in mechanical ventilated, critically ill patients of ICU. Acute Crit. Care 2011, 26, 139–144. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Tylavsky, F.; Kritchevsky, S.B.; Cauley, J.A.; Newman, A.B.; Blunt, B.A.; Harris, T.B. One-and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J. Appl. Physiol. 2003, 94, 2368–2374. [Google Scholar] [CrossRef]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef]
- Szadkowska, A.; Madej, A.; Ziółkowska, K.; Szymańska, M.; Jeziorny, K.; Mianowska, B.; Pietrzak, I. Gender and age-dependent effect of type 1 diabetes on obesity and altered body composition in young adults. Ann. Agric. Environ. Med. 2015, 22, 124–128. [Google Scholar] [CrossRef]
- Conway, B.; Miller, R.G.; Costacou, T.; Fried, L.; Kelsey, S.; Evans, R.; Orchard, T. Temporal patterns in overweight and obesity in type 1 diabetes. Diabet. Med. 2010, 27, 398–404. [Google Scholar] [CrossRef]
- Mottalib, A.; Kasetty, M.; Mar, J.Y.; Elseaidy, T.; Ashrafzadeh, S.; Hamdy, O. Weight management in patients with type 1 diabetes and obesity. Curr. Diabetes Rep. 2017, 17, 92. [Google Scholar] [CrossRef]
- Vilarrasa, N.; San Jose, P.; Rubio, M.Á.; Lecube, A. Obesity in patients with type 1 diabetes: Links, risks and management challenges. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 2807–2827. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.-S.; Masutani, K.; Tanaka, Y.; Noda, K.; Peng, X.; Takahashi, T.; Billiar, T.R. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L646–L656. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Sha, X.-L.; Li, Y.-L.; Li, C.-L.; Chen, S.-H.; Wang, J.-J.; Xia, Z. Lung injury induced by short-term mechanical ventilation with hyperoxia and its mitigation by deferoxamine in rats. BMC Anesthesiol. 2020, 20, 188. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.L.; Vanthenapalli, S.; Panguluri, S.K. Electrical remodeling and cardiotoxicity precedes structural and functional remodeling of mouse hearts under hyperoxia treatment. J. Cell Physiol. 2021, 236, 4482–4495. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.D.; Lehmann, M.H.; Ritchie, R.H.; Gwathmey, J.K.; Green, G.E.; Schiebinger, R.J. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation 1998, 98, 256–261. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Pavez, M.; Wilson, C.; Lagos, D.; Duran, J.; Ramos, S.; Barrientos, G.; Silva, P.; Llanos, P.; Basualto-Alarcón, C. Testosterone activates glucose metabolism through AMPK and androgen signaling in cardiomyocyte hypertrophy. Biol. Res. 2021, 54, 3. [Google Scholar] [CrossRef]
- Zia, M.I.; Ghugre, N.R.; Roifman, I.; Strauss, B.H.; Walcarius, R.; Mohammed, M.; Sparkes, J.D.; Dick, A.J.; Wright, G.A.; Connelly, K.A. Comparison of the Frequencies of Myocardial Edema Determined by Cardiac Magnetic Resonance in Diabetic Versus Nondiabetic Patients Having Percutaneous Coronary Intervention for ST Elevation Myocardial Infarction. Am. J. Cardiol. 2014, 113, 607–612. [Google Scholar] [CrossRef]
- Li, C.-J.; Lv, L.; Li, H.; Yu, D.-M. Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc. Diabetol. 2012, 11, 73. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Wang, H.-L.; Peng, J.; Zhu, Y.; Zhang, H.-G.; Tang, F.-Q.; Jian, Z.; Xiao, Y.-B. Multinucleated polyploid cardiomyocytes undergo an enhanced adaptability to hypoxia via mitophagy. J. Mol. Cell Cardiol. 2020, 138, 115–135. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Ali, S.R.; Menendez-Montes, I.; Warshaw, J.; Xiao, F.; Sadek, H.A. Homotypic Fusion Generates Multinucleated Cardiomyocytes in the Murine Heart. Circulation 2020, 141, 1940–1942. [Google Scholar] [CrossRef] [PubMed]
- Giovanardi, P.; Vernia, C.; Tincani, E.; Giberti, C.; Silipo, F.; Fabbo, A. Combined effects of age and comorbidities on electrocardiographic parameters in a large non-selected population. J. Clin. Med. 2022, 11, 3737. [Google Scholar] [CrossRef]
- Monitillo, F.; Leone, M.; Rizzo, C.; Passantino, A.; Iacoviello, M. Ventricular repolarization measures for arrhythmic risk stratification. World J. Cardiol. 2016, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Ahnve, S. QT interval prolongation in acute myocardial infarction. Eur. Heart J. 1985, 6, 85–95. [Google Scholar] [CrossRef]
- Taubel, J.; Pimenta, D. Considering the risk of QTc prolongation in patients with diabetes mellitus. J. Cardiol. Pract. 2022, 22, 8. [Google Scholar]
- Stettler, C.; Bearth, A.; Allemann, S.; Zwahlen, M.; Zanchin, L.; Deplazes, M.; Christ, E.; Teuscher, A.; Diem, P. QT c interval and resting heart rate as long-term predictors of mortality in type 1 and type 2 diabetes mellitus: A 23-year follow-up. Diabetologia 2007, 50, 186–194. [Google Scholar] [CrossRef]
- Shulman, E.; Aagaard, P.; Kargoli, F.; Hoch, E.; Zheng, L.; Di Biase, L.; Fisher, J.; Gross, J.; Kim, S.; Ferrick, K. Validation of PR interval length as a criterion for development of atrial fibrillation in non-Hispanic whites, African Americans and Hispanics. J. Electrocardiol. 2015, 48, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.W.; Wang, N.; Nelson, K.P.; Connelly, S.; Deo, R.; Rodondi, N.; Schelbert, E.B.; Garcia, M.E.; Phillips, C.L.; Shlipak, M.G. Electrocardiographic PR interval and adverse outcomes in older adults: The Health, Aging, and Body Composition study. Circ. Arrhythmia Electrophysiol. 2013, 6, 84–90. [Google Scholar] [CrossRef]
- Aiba, T.; Tomaselli, G.F. Electrical remodeling in the failing heart. Curr. Opin. Cardiol. 2010, 25, 29. [Google Scholar] [CrossRef]
- Barry, D.M.; Xu, H.; Schuessler, R.B.; Nerbonne, J.M. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 α subunit. Circ. Res. 1998, 83, 560–567. [Google Scholar] [CrossRef]
- Grubb, S.; Calloe, K.; Thomsen, M.B. Impact of KChIP2 on cardiac electrophysiology and the progression of heart failure. Front. Physiol. 2012, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Soltysinska, E.; Olesen, S.-P.; Christ, T.; Wettwer, E.; Varró, A.; Grunnet, M.; Jespersen, T. Transmural expression of ion channels and transporters in human nondiseased and end-stage failing hearts. Pflügers Arch. Eur. J. Physiol. 2009, 459, 11–23. [Google Scholar] [CrossRef]
- Douin, D.J.; Anderson, E.L.; Dylla, L.; Rice, J.D.; Jackson, C.L.; Wright, F.L.; Bebarta, V.S.; Schauer, S.G.; Ginde, A.A. Association between hyperoxia, supplemental oxygen, and mortality in critically injured patients. Crit. Care Explor. 2021, 3, e0418. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.E.; Park, J.; Kompaniyets, L.; Baggs, J.; Cheng, Y.J.; Zhang, P.; Imperatore, G.; Pavkov, M.E. Intensive care unit admission, mechanical ventilation, and mortality among patients with type 1 diabetes hospitalized for COVID-19 in the US. Diabetes Care 2021, 44, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, F.; Mansour, N.; Vichare, R.; Ayalasomayajula, Y.; Yassine, J.; Hesaraghatta, A.; Panguluri, S.K. Influence of Age on Hyperoxia-Induced Cardiac Pathophysiology in Type 1 Diabetes Mellitus (T1DM) Mouse Model. Cells 2023, 12, 1457. https://doi.org/10.3390/cells12111457
Saleem F, Mansour N, Vichare R, Ayalasomayajula Y, Yassine J, Hesaraghatta A, Panguluri SK. Influence of Age on Hyperoxia-Induced Cardiac Pathophysiology in Type 1 Diabetes Mellitus (T1DM) Mouse Model. Cells. 2023; 12(11):1457. https://doi.org/10.3390/cells12111457
Chicago/Turabian StyleSaleem, Faizan, Noah Mansour, Riddhi Vichare, Yashwant Ayalasomayajula, Jenna Yassine, Anagha Hesaraghatta, and Siva Kumar Panguluri. 2023. "Influence of Age on Hyperoxia-Induced Cardiac Pathophysiology in Type 1 Diabetes Mellitus (T1DM) Mouse Model" Cells 12, no. 11: 1457. https://doi.org/10.3390/cells12111457
APA StyleSaleem, F., Mansour, N., Vichare, R., Ayalasomayajula, Y., Yassine, J., Hesaraghatta, A., & Panguluri, S. K. (2023). Influence of Age on Hyperoxia-Induced Cardiac Pathophysiology in Type 1 Diabetes Mellitus (T1DM) Mouse Model. Cells, 12(11), 1457. https://doi.org/10.3390/cells12111457





