Reciprocal Effect of Environmental Stimuli to Regulate the Adipogenesis and Osteogenesis Fate Decision in Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs)
Abstract
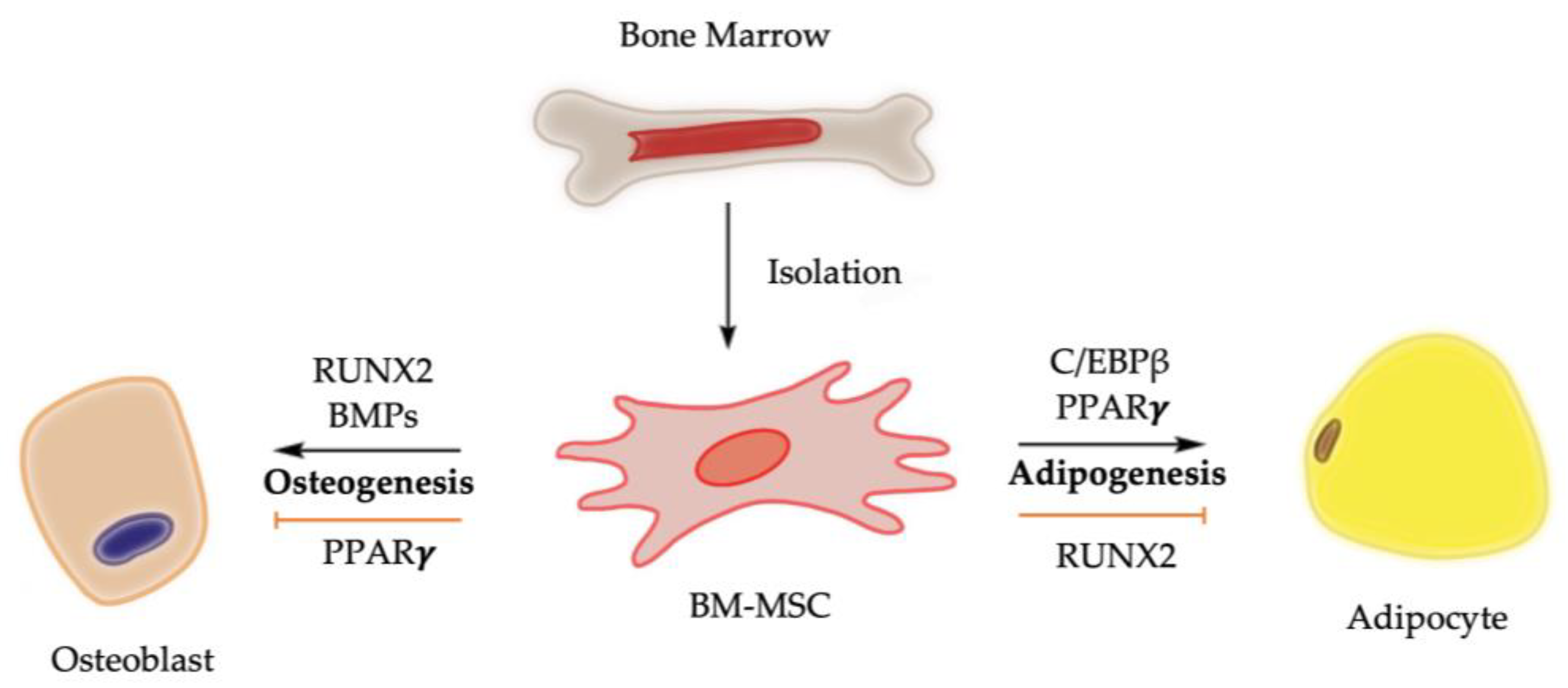
1. Introduction
2. Endocrine Disruptors
2.1. Phthalates and Organotins
2.2. Per- and Poly-fluoroalkyl Substances (PFASs)
2.3. Parabens
2.4. Organophosphate Ester and Halogenated Bisphenol A (BPA) Analogs
3. METALS
3.1. Lead (Pb)
3.2. Cerium (Ce) and Terbium (Tb)
3.3. Cadmium (Cd)
4. Dietary Factors
4.1. Resveratrol
4.2. Curcumin
4.3. Epimedium-Derived Phytoestrogen Flavonoids
4.4. Alcohol Consumption
5. Physical Factors
5.1. Microgravity
5.2. Mechanical Stretch
5.3. Mechanical Vibration
5.4. Electromagnetic Fields
5.5. Ionizing Radiation
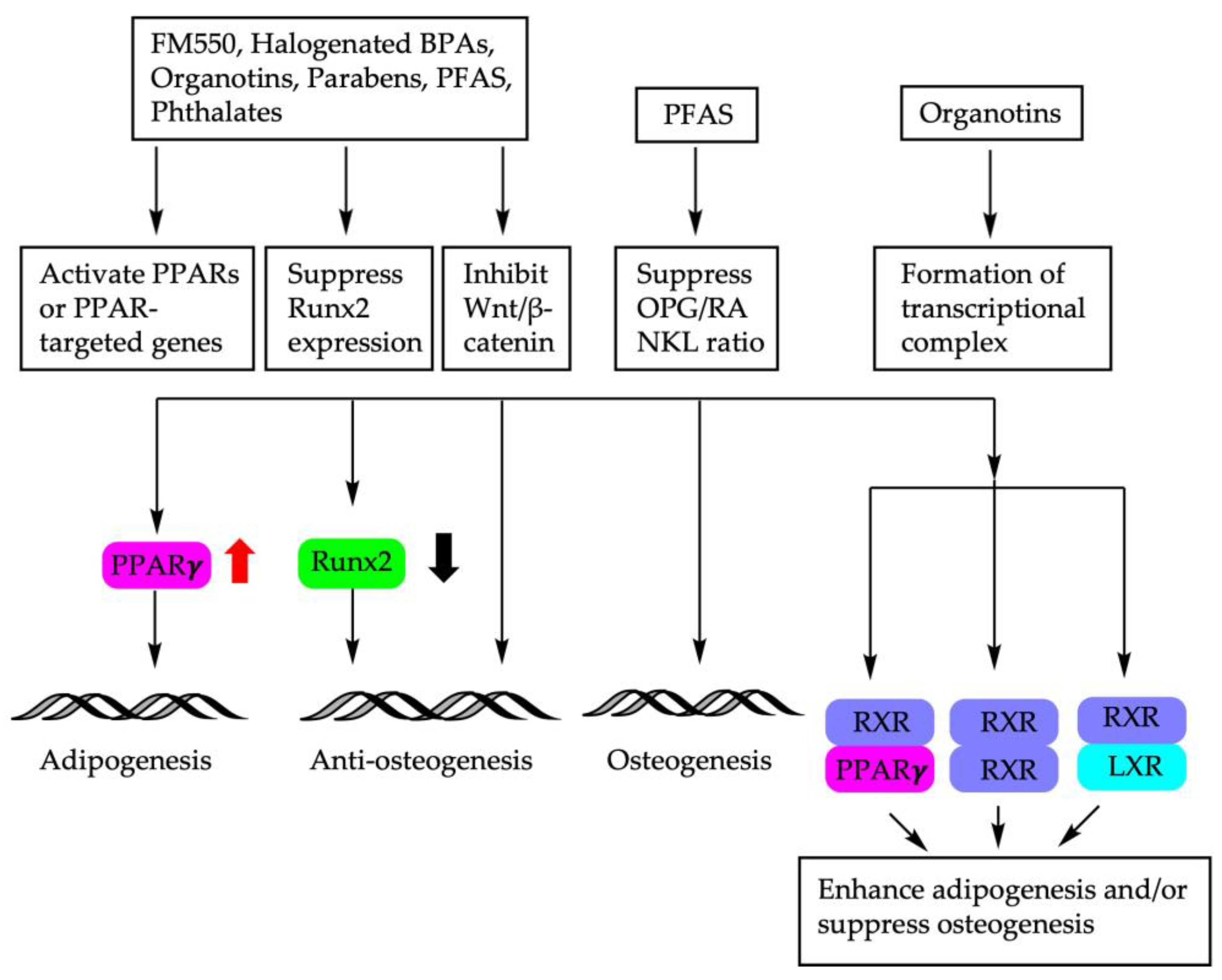
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| 3′UTR | Three prime untranslated region | |
| 6:2 Cl-PFESA | 6:2 chlorinated polyfluorinated ether sulfonate | |
| Abca1 | ATP binding cassette subfamily A member 1 | |
| AFVS | Acoustic frequency vertical sinusoid | |
| Akt | AKT serine/threonine kinase | |
| ALP | Alkaline phosphatase | |
| ASC | Adipose-derived multipotent stem cell | |
| ATF4 | Activating transcription factor 4 | |
| BGLAP | Bone gamma-carboxyglutamate protein | |
| BMD | Bone mineral density | |
| BM-MSCs | Bone marrow mesenchymal stem cells | |
| BMP | Bone morphogenetic protein | |
| BPA | Bisphenol A | |
| BPDP | Tert-butylphenyl diphenyl phosphate | |
| BSP | Bone sialoprotein | |
| cAMP | Cyclic adenosine monophosphate | |
| C/EBP | CCAAT enhancer binding protein | |
| CHOP | C/EBP homologous protein | |
| Col I | Type I collagen | |
| CREB | cAMP response element binding protein | |
| Crif1 | CR6-interacting factor 1 | |
| CTX-I | C-terminal telopeptide of type I collagen | |
| TCDD | 2,3,7,8-Tetrachlorodibenzo-p-dioxin | |
| DEHP | di-(2-ethylhexyl)-phthalate | |
| Dex | Dexamethasone | |
| Dmp1 | Dentin matrix acidic phosphoprotein 1 | |
| ECM | Extracellular matrix | |
| EMFs | Electromagnetic fields | |
| EMP | Human embryonic stem cell-derived mesenchymal progenitors | |
| EPFs | Epimedium brevicornum maxim–derived phytoestrogen flavonoids | |
| ER | Endoplasmic reticulum | |
| ER | Estrogen receptor | |
| ERK1/2 | Extracellular signal-regulated protein kinase 1/2 | |
| FABP4 | Fatty acid-binding protein | |
| FAK | Focal adhesion kinase | |
| FAS | Fatty acid synthase | |
| FRE | FOXO response element | |
| FOXO3A | Forkhead box 3A | |
| Fz receptor | Frizzled protein receptor | |
| GDF | Growth differentiation factor | |
| HFPO-DA | Hexafluoropropylene oxide dimer acid | |
| HFPO-TA | Hexafluoropropylene oxide trimer acid | |
| IκB | Inhibitor of κB | |
| IKK | IκB kinase | |
| ITP | Tri-isopropylated triaryl phosphates | |
| LEP | Leptin | |
| LMMS | Low magnitude mechanical signals | |
| LPL | Lipoprotein lipase | |
| LXR | Liver X receptor | |
| MEHP | Mono-(2-ethylhexyl) phthalate | |
| MEK/ERK1/2 | Mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 | |
| METBP | Mono-(2-ethylhexyl) tetrabromophthalate | |
| MMP13 | Matrix metalloproteinase 13 | |
| MSCs | Mesenchymal stem cells | |
| NF-κB | Nuclear factor κ B | |
| OCN | Osteocalcin | |
| OGN | Osteoglycin | |
| ONFH | Osteonecrosis of the femoral head | |
| OPG | Osteoprotegerin | |
| OPN | Osteopontin | |
| OSX | Osterix aka transcription factor | |
| P1NP | Procollagen type 1 N-terminal propeptide | |
| PBDEs | Polybrominated diphenyl ethers | |
| PEMFs | Pulsed electromagnetic fields | |
| PFHxS | Perfluorohexanesulfonic acid | |
| PFOA | Perfluorooctanoic acid | |
| PFOS | Perfluorooctanesulfonate | |
| PI3K | Phosphoinositide 3-kinases | |
| PKAα cat | Protein kinase A α catalytic subunit | |
| PLIN1 | Perilipin | |
| PPAR | Peroxisome proliferator-activated receptor | |
| RANK | Receptor activator of NF-κB | |
| RANKL | Receptor activator of NF-κB ligand | |
| RhoA | Ras homolog family member A | |
| Rosi | Rosiglitazone | |
| RS | Resveratrol | |
| RUNX2 | Runt-related transcription factor 2 | |
| RXR | Retinoid X receptor | |
| SIRT1 | Sirtuin 1 | |
| SMAD | Suppressor of mothers against decapentaplegic | |
| SMURF | SMAD specific E3 ubiquitin protein ligase | |
| TBB | Tetrabromobenzoate | |
| TBBPA | Tetrabromobisphenol A | |
| TBPH | bis-(2-ethylhexyl) tetrabromophthalate | |
| TBT | Tributyltin | |
| TCBPA | Tetrachlorobisphenol A | |
| TGFB | Transforming growth factor | |
| TGM2 | Transglutaminase 2 | |
| TNFα | Tumor necrosis factor | |
| TPhT | Triphenyltin | |
| TPP | Triphenyl phosphate | |
| TRACP5b | Tartrate-resistant acid phosphatase 5b | |
| WAT | White adipose tissue | |
| WNT | Wingless-related integration site | |
References
- Han, L.; Wang, B.; Wang, R.; Gong, S.; Chen, G.; Xu, W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res. Ther. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Rodriguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.W.; Marcon, B.H.; Dallagiovanna, B.; Shigunov, P. Adipogenesis, Osteogenesis, and Chondrogenesis of Human Mesenchymal Stem/Stromal Cells: A Comparative Transcriptome Approach. Front. Cell Dev. Biol. 2020, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Zhang, X.; Zhu, C.; Tang, X.; Yu, F.; Shang, G.W.; Cai, X. Molecular Mechanisms of PPAR-gamma Governing MSC Osteogenic and Adipogenic Differentiation. Curr. Stem Cell Res. Ther. 2016, 11, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, K.; Tarnowski, M. Bone Marrow Adipocytes-Role in Physiology and Various Nutritional Conditions in Human and Animal Models. Nutrients 2021, 13, 1412. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T.; et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014, 20, 368–375. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Dong, J.C.; Bian, Q. Small molecules for mesenchymal stem cell fate determination. World J. Stem Cells 2019, 11, 1084–1103. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Maxson, S.; Burg, K.J. Conditioned media cause increases in select osteogenic and adipogenic differentiation markers in mesenchymal stem cell cultures. J. Tissue Eng. Regen. Med. 2008, 2, 147–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, D.; Delling, J.; Tobiasch, E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. Sci. World J. 2012, 2012, 793823. [Google Scholar] [CrossRef]
- Han, P.; Frith, J.E.; Gomez, G.A.; Yap, A.S.; O’Neill, G.M.; Cooper-White, J.J. Five Piconewtons: The Difference between Osteogenic and Adipogenic Fate Choice in Human Mesenchymal Stem Cells. ACS Nano 2019, 13, 11129–11143. [Google Scholar] [CrossRef] [PubMed]
- Adhami, M.; Ghori-Javed, F.Y.; Chen, H.; Gutierrez, S.E.; Javed, A. Runx2 regulates the gene network associated with insulin signaling and energy homeostasis. Cells Tissues Organs 2011, 194, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tang, W.; Li, Y. Matrix metalloproteinase 13 (MMP13) is a direct target of osteoblast-specific transcription factor osterix (Osx) in osteoblasts. PLoS ONE 2012, 7, e50525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Li, L.C. Genetic expression and functional characterization of the RUNX2 gene in human adult bone marrow mesenchymal stem cells. Genet. Mol. Res. 2015, 14, 18210–18217. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, I.E. Inflammation in bone physiology and pathology. Curr. Opin. Rheumatol. 2018, 30, 59–64. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef]
- Nardone, V.; D’Asta, F.; Brandi, M.L. Pharmacological management of osteogenesis. Clinics 2014, 69, 438–446. [Google Scholar] [CrossRef]
- Newell-Fugate, A.E. The role of sex steroids in white adipose tissue adipocyte function. Reproduction 2017, 153, R133–R149. [Google Scholar] [CrossRef]
- Szulc, P. Role of sex steroids hormones in the regulation of bone metabolism in men: Evidence from clinical studies. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101624. [Google Scholar] [CrossRef]
- Usategui-Martin, R.; Rigual, R.; Ruiz-Mambrilla, M.; Fernandez-Gomez, J.M.; Duenas, A.; Perez-Castrillon, J.L. Molecular Mechanisms Involved in Hypoxia-Induced Alterations in Bone Remodeling. Int. J. Mol. Sci. 2022, 23, 3233. [Google Scholar] [CrossRef]
- Chen, J.; Ahn, K.C.; Gee, N.A.; Ahmed, M.I.; Duleba, A.J.; Zhao, L.; Gee, S.J.; Hammock, B.D.; Lasley, B.L. Triclocarban enhances testosterone action: A new type of endocrine disruptor? Endocrinology 2008, 149, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Yaglova, N.V.; Yaglov, V.V. Endocrine Disruptors as a New Etiologic Factor of Bone Tissue Diseases (Review). Sovrem Tekhnologii Med. 2021, 13, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- Graceli, J.B.; Sena, G.C.; Lopes, P.F.; Zamprogno, G.C.; da Costa, M.B.; Godoi, A.F.; Dos Santos, D.M.; de Marchi, M.R.; Dos Santos, M.A.F. Organotins: A review of their reproductive toxicity, biochemistry, and environmental fate. Reprod. Toxicol. 2013, 36, 40–52. [Google Scholar] [CrossRef]
- Chamorro-Garcia, R.; Blumberg, B. Current Research Approaches and Challenges in the Obesogen Field. Front. Endocrinol. 2019, 10, 167. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, M.J. Phthalate exposure and childhood obesity. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 69–75. [Google Scholar] [CrossRef]
- Grun, F. Obesogens. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 453–459. [Google Scholar] [CrossRef]
- Feige, J.N.; Gelman, L.; Rossi, D.; Zoete, V.; Metivier, R.; Tudor, C.; Anghel, S.I.; Grosdidier, A.; Lathion, C.; Engelborghs, Y.; et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J. Biol. Chem. 2007, 282, 19152–19166. [Google Scholar] [CrossRef]
- Hiromori, Y.; Nishikawa, J.; Yoshida, I.; Nagase, H.; Nakanishi, T. Structure-dependent activation of peroxisome proliferator-activated receptor (PPAR) gamma by organotin compounds. Chem. Biol. Interact. 2009, 180, 238–244. [Google Scholar] [CrossRef]
- Yanik, S.C.; Baker, A.H.; Mann, K.K.; Schlezinger, J.J. Organotins are potent activators of PPARgamma and adipocyte differentiation in bone marrow multipotent mesenchymal stromal cells. Toxicol. Sci. 2011, 122, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Watt, J.; Schlezinger, J.J. Structurally-diverse, PPARgamma-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology 2015, 331, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Sun, S.C.; Chiang, C.K.; Wang, C.C.; Chan, D.C.; Chen, H.J.; Liu, S.H.; Yang, R.S. Plasticizer di(2-ethylhexyl)phthalate interferes with osteoblastogenesis and adipogenesis in a mouse model. J. Orthop. Res. 2018, 36, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Madan, B.; McDonald, M.J.; Foxa, G.E.; Diegel, C.R.; Williams, B.O.; Virshup, D.M. Bone loss from Wnt inhibition mitigated by concurrent alendronate therapy. Bone Res. 2018, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wei, X.; Guo, H.; Cheng, D.; Li, H.; Sun, L.; Wang, S.; Guo, D.; Yang, Y.; Si, J. Tributyltin reduces bone mineral density by reprograming bone marrow mesenchymal stem cells in rat. Environ. Toxicol. Pharmacol. 2020, 73, 103271. [Google Scholar] [CrossRef] [PubMed]
- le Maire, A.; Grimaldi, M.; Roecklin, D.; Dagnino, S.; Vivat-Hannah, V.; Balaguer, P.; Bourguet, W. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009, 10, 367–373. [Google Scholar] [CrossRef]
- Dawson, M.I.; Xia, Z. The retinoid X receptors and their ligands. Biochim. Biophys. Acta 2012, 1821, 21–56. [Google Scholar] [CrossRef]
- Cocci, P.; Mosconi, G.; Arukwe, A.; Mozzicafreddo, M.; Angeletti, M.; Aretusi, G.; Palermo, F.A. Effects of Diisodecyl Phthalate on PPAR:RXR-Dependent Gene Expression Pathways in Sea Bream Hepatocytes. Chem. Res. Toxicol. 2015, 28, 935–947. [Google Scholar] [CrossRef]
- Plutzky, J. The PPAR-RXR transcriptional complex in the vasculature: Energy in the balance. Circ. Res. 2011, 108, 1002–1016. [Google Scholar] [CrossRef]
- Baker, A.H.; Watt, J.; Huang, C.K.; Gerstenfeld, L.C.; Schlezinger, J.J. Tributyltin engages multiple nuclear receptor pathways and suppresses osteogenesis in bone marrow multipotent stromal cells. Chem. Res. Toxicol. 2015, 28, 1156–1166. [Google Scholar] [CrossRef]
- Kaartinen, M.T.; El-Maadawy, S.; Rasanen, N.H.; McKee, M.D. Tissue transglutaminase and its substrates in bone. J. Bone Miner. Res. 2002, 17, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Shoucri, B.M.; Martinez, E.S.; Abreo, T.J.; Hung, V.T.; Moosova, Z.; Shioda, T.; Blumberg, B. Retinoid X Receptor Activation Alters the Chromatin Landscape To Commit Mesenchymal Stem Cells to the Adipose Lineage. Endocrinology 2017, 158, 3109–3125. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Bai, Y.; Chang, K.C.; Wang, Y.; Burris, T.P.; Freedman, L.P.; Thompson, C.C.; Nagpal, S. Liver X receptor-retinoid X receptor (LXR-RXR) heterodimer cistrome reveals coordination of LXR and AP1 signaling in keratinocytes. J. Biol. Chem. 2011, 286, 14554–14563. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Djordjevic, A.B.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Niu, Y.; Luan, H.; Li, M.; Zheng, L.; Pan, Y.; Liu, W. Effects of legacy and emerging per- and polyfluoroalkyl substances on PPARalpha/beta/gamma regulation and osteogenic/adipogenic differentiation. Environ. Int. 2022, 170, 107584. [Google Scholar] [CrossRef]
- Peters, J.M.; Hollingshead, H.E.; Gonzalez, F.J. Role of peroxisome-proliferator-activated receptor beta/delta (PPARbeta/delta) in gastrointestinal tract function and disease. Clin. Sci. 2008, 115, 107–127. [Google Scholar] [CrossRef]
- Scholtysek, C.; Katzenbeisser, J.; Fu, H.; Uderhardt, S.; Ipseiz, N.; Stoll, C.; Zaiss, M.M.; Stock, M.; Donhauser, L.; Bohm, C.; et al. PPARbeta/delta governs Wnt signaling and bone turnover. Nat. Med. 2013, 19, 608–613. [Google Scholar] [CrossRef]
- Gong, K.; Qu, B.; Wang, C.; Zhou, J.; Liao, D.; Zheng, W.; Pan, X. Peroxisome Proliferator-Activated Receptor alpha Facilitates Osteogenic Differentiation in MC3T3-E1 Cells via the Sirtuin 1-Dependent Signaling Pathway. Mol. Cells 2017, 40, 393–400. [Google Scholar]
- Li, C.H.; Ren, X.M.; Guo, L.H. Adipogenic Activity of Oligomeric Hexafluoropropylene Oxide (Perfluorooctanoic Acid Alternative) through Peroxisome Proliferator-Activated Receptor gamma Pathway. Environ. Sci. Technol. 2019, 53, 3287–3295. [Google Scholar] [CrossRef]
- Liu, W.; Qin, H.; Pan, Y.; Luo, F.; Zhang, Z. Low concentrations of perfluorooctane sulfonate repress osteogenic and enhance adipogenic differentiation of human mesenchymal stem cells. Toxicol. Appl. Pharmacol. 2019, 367, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, H.; Terry, P.D.; Zhao, L.; Chen, J. Impact of Paraben Exposure on Adiposity-Related Measures: An Updated Literature Review of Population-Based Studies. Int. J. Environ. Res. Public Health 2022, 19, 16268. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Chen, X.; Whitener, R.J.; Boder, E.T.; Jones, J.O.; Porollo, A.; Chen, J.; Zhao, L. Effects of parabens on adipocyte differentiation. Toxicol. Sci. 2013, 131, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Kennedy, R.C.; Chen, X.; Zhang, J.; Shen, C.L.; Chen, J.; Zhao, L. Differential effects on adiposity and serum marker of bone formation by post-weaning exposure to methylparaben and butylparaben. Environ. Sci. Pollut. Res. Int. 2016, 23, 21957–21968. [Google Scholar] [CrossRef]
- Hu, P.; Overby, H.; Heal, E.; Wang, S.; Chen, J.; Shen, C.L.; Zhao, L. Methylparaben and butylparaben alter multipotent mesenchymal stem cell fates towards adipocyte lineage. Toxicol. Appl. Pharmacol. 2017, 329, 48–57. [Google Scholar] [CrossRef]
- Shea, C.M.; Edgar, C.M.; Einhorn, T.A.; Gerstenfeld, L.C. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J. Cell. Biochem. 2003, 90, 1112–1127. [Google Scholar] [CrossRef]
- Almalki, S.G.; Agrawal, D.K. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 2016, 92, 41–51. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, X.; Qian, S.W.; Guo, L.; Huang, H.Y.; He, Q.; Liu, Y.; Ma, C.G.; Tang, Q.Q. Down-regulation of type I Runx2 mediated by dexamethasone is required for 3T3-L1 adipogenesis. Mol. Endocrinol. 2012, 26, 798–808. [Google Scholar] [CrossRef]
- Krentzel, A.A.; Kimble, L.C.; Dorris, D.M.; Horman, B.M.; Meitzen, J.; Patisaul, H.B. FireMaster(R) 550 (FM 550) exposure during the perinatal period impacts partner preference behavior and nucleus accumbens core medium spiny neuron electrophysiology in adult male and female prairie voles, Microtus ochrogaster. Horm. Behav. 2021, 134, 105019. [Google Scholar] [CrossRef]
- Macari, S.; Rock, K.D.; Santos, M.S.; Lima, V.T.M.; Szawka, R.E.; Moss, J.; Horman, B.; Patisaul, H.B. Developmental Exposure to the Flame Retardant Mixture Firemaster 550 Compromises Adult Bone Integrity in Male but not Female Rats. Int. J. Mol. Sci. 2020, 21, 2553. [Google Scholar] [CrossRef] [PubMed]
- Pillai, H.K.; Fang, M.; Beglov, D.; Kozakov, D.; Vajda, S.; Stapleton, H.M.; Webster, T.F.; Schlezinger, J.J. Ligand binding and activation of PPARgamma by Firemaster(R) 550: Effects on adipogenesis and osteogenesis in vitro. Environ. Health Perspect. 2014, 122, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Kawane, T.; Qin, X.; Jiang, Q.; Miyazaki, T.; Komori, H.; Yoshida, C.A.; Matsuura-Kawata, V.; Sakane, C.; Matsuo, Y.; Nagai, K.; et al. Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation by regulating Fgfr2 and Fgfr3. Sci. Rep. 2018, 8, 13551. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Hales, B.F. Effects of Organophosphate Ester Flame Retardants on Endochondral Ossification in Ex Vivo Murine Limb Bud Cultures. Toxicol. Sci. 2019, 168, 420–429. [Google Scholar] [CrossRef]
- Rashid, H.; Ma, C.; Chen, H.; Wang, H.; Hassan, M.Q.; Sinha, K.; de Crombrugghe, B.; Javed, A. Sp7 and Runx2 molecular complex synergistically regulate expression of target genes. Connect. Tissue Res. 2014, 55 (Suppl. S1), 83–87. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Song, M.; Liang, D.; Liang, Y.; Chen, M.; Wang, F.; Wang, H.; Jiang, G. Assessing developmental toxicity and estrogenic activity of halogenated bisphenol A on zebrafish (Danio rerio). Chemosphere 2014, 112, 275–281. [Google Scholar] [CrossRef]
- Riu, A.; Grimaldi, M.; le Maire, A.; Bey, G.; Phillips, K.; Boulahtouf, A.; Perdu, E.; Zalko, D.; Bourguet, W.; Balaguer, P. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 2011, 119, 1227–1232. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Sauk, J.; Somerman, M.; Todd, A.; McNeill, F.; Fowler, B.; Fontaine, A.; van Buren, J. Lead in bone: Storage site, exposure source, and target organ. Neurotoxicology 1993, 14, 225–236. [Google Scholar]
- Alissa, E.M.; Ferns, G.A. Heavy metal poisoning and cardiovascular disease. J. Toxicol. 2011, 2011, 870125. [Google Scholar] [CrossRef]
- Abdullah, M.; Rahman, F.A.; Gnanasegaran, N.; Govindasamy, V.; Abu Kasim, N.H.; Musa, S. Diverse effects of lead nitrate on the proliferation, differentiation, and gene expression of stem cells isolated from a dental origin. Sci. World J. 2014, 2014, 235941. [Google Scholar] [CrossRef] [PubMed]
- Escribano, A.; Revilla, M.; Hernandez, E.R.; Seco, C.; Gonzalez-Riola, J.; Villa, L.F.; Rico, H. Effect of lead on bone development and bone mass: A morphometric, densitometric, and histomorphometric study in growing rats. Calcif. Tissue Int. 1997, 60, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.N.; Gabrielli, M.; Bonifacino, G.; Codesido, M.M.; Vila, M.D.C. Lead enhancement of 3T3-L1 fibroblasts differentiation to adipocytes involves ERK, C/EBPbeta and PPARgamma activation. Mol. Cell. Biochem. 2018, 437, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Beier, E.E.; Maher, J.R.; Sheu, T.J.; Cory-Slechta, D.A.; Berger, A.J.; Zuscik, M.J.; Puzas, J.E. Heavy metal lead exposure, osteoporotic-like phenotype in an animal model, and depression of Wnt signaling. Environ. Health Perspect. 2013, 121, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Fricker, S.P. The therapeutic application of lanthanides. Chem. Soc. Rev. 2006, 35, 524–533. [Google Scholar] [CrossRef]
- Liu, D.D.; Zhang, J.C.; Zhang, Q.; Wang, S.X.; Yang, M.S. TGF-beta/BMP signaling pathway is involved in cerium-promoted osteogenic differentiation of mesenchymal stem cells. J. Cell. Biochem. 2013, 114, 1105–1114. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes. Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef]
- Koganti, P.; Levy-Cohen, G.; Blank, M. Smurfs in Protein Homeostasis, Signaling, and Cancer. Front. Oncol. 2018, 8, 295. [Google Scholar] [CrossRef]
- Liu, D.D.; Ge, K.; Jin, Y.; Sun, J.; Wang, S.X.; Yang, M.S.; Zhang, J.C. Terbium promotes adhesion and osteogenic differentiation of mesenchymal stem cells via activation of the Smad-dependent TGF-beta/BMP signaling pathway. J. Biol. Inorg. Chem. 2014, 19, 879–891. [Google Scholar] [CrossRef]
- Buha, A.; Jugdaohsingh, R.; Matovic, V.; Bulat, Z.; Antonijevic, B.; Kerns, J.G.; Goodship, A.; Hart, A.; Powell, J.J. Bone mineral health is sensitively related to environmental cadmium exposure- experimental and human data. Environ. Res. 2019, 176, 108539. [Google Scholar] [CrossRef]
- Wan, Y.; Mo, L.J.; Wu, L.; Li, D.L.; Song, J.; Hu, Y.K.; Huang, H.B.; Wei, Q.Z.; Wang, D.P.; Qiu, J.M.; et al. Bone morphogenetic protein 4 is involved in cadmium-associated bone damage. Toxicol. Sci. 2023, 191, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, L.; Chen, J. Physiologically achievable doses of resveratrol enhance 3T3-L1 adipocyte differentiation. Eur. J. Nutr. 2015, 54, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.C.; Hou, S.M.; Chen, R.J.; Peng, H.W.; Hsieh, C.F.; Kuo, M.L.; Yen, M.L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, J.C.; Jiang, Q.; Lee, W.Y. Role of sirtuins in bone biology: Potential implications for novel therapeutic strategies for osteoporosis. Aging Cell 2021, 20, e13301. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Gong, Y.; Xu, L.; Zhou, M.; Li, J.; Song, J. Bidirectional regulation of osteogenic differentiation by the FOXO subfamily of Forkhead transcription factors in mammalian MSCs. Cell Prolif. 2019, 52, e12540. [Google Scholar] [CrossRef] [PubMed]
- Erdman, C.P.; Dosier, C.R.; Olivares-Navarrete, R.; Baile, C.; Guldberg, R.E.; Schwartz, Z.; Boyan, B.D. Effects of resveratrol on enrichment of adipose-derived stem cells and their differentiation to osteoblasts in two-and three-dimensional cultures. J. Tissue Eng. Regen. Med. 2012, 6 (Suppl. S3), s34–s46. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xin, Z.; Cai, M. The role of resveratrol in bone marrow-derived mesenchymal stem cells from patients with osteoporosis. J. Cell. Biochem. 2019, 120, 16634–16642. [Google Scholar] [CrossRef]
- Moon, D.K.; Kim, B.G.; Lee, A.R.; Choe, Y.I.; Khan, I.; Moon, K.M.; Jeon, R.H.; Byun, J.H.; Hwang, S.C.; Woo, D.K. Resveratrol can enhance osteogenic differentiation and mitochondrial biogenesis from human periosteum-derived mesenchymal stem cells. J. Orthop. Surg. Res. 2020, 15, 203. [Google Scholar] [CrossRef]
- Ali, D.; Chen, L.; Kowal, J.M.; Okla, M.; Manikandan, M.; AlShehri, M.; AlMana, Y.; AlObaidan, R.; AlOtaibi, N.; Hamam, R.; et al. Resveratrol inhibits adipocyte differentiation and cellular senescence of human bone marrow stromal stem cells. Bone 2020, 133, 115252. [Google Scholar] [CrossRef]
- Liu, S.H.; Wang, H.; Zhai, Y.M.; Zhu, X.Y.; Zhang, J.; Wan, Y.; Lu, W.; Shi, J. Comparison of Adipogenesis and Adipocyte Functions of 3T3-L1 Cells and Human Bone Marrow Mesenchymal Stem Cells In Vitro. J. Exp. Hematol. 2015, 23, 1729–1733. [Google Scholar]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, M.; Strauss, S.; Lazaridis, A.; Bucan, V.; Kuhbier, J.W.; Vogt, P.M.; Konneker, S. Influence of glucose and insulin in human adipogenic differentiation models with adipose-derived stem cells. Adipocyte 2019, 8, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 9607–9611. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Hatamipour, M.; Johnston, T.P.; Sahebkar, A. One Molecule, Many Targets and Numerous Effects: The Pleiotropy of Curcumin Lies in its Chemical Structure. Curr. Pharm. Des. 2018, 24, 2129–2136. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocinska, K.; Zielinska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Gu, Q.; Cai, Y.; Huang, C.; Shi, Q.; Yang, H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn. Mag. 2012, 8, 202–208. [Google Scholar]
- Yang, Q.; Leong, S.A.; Chan, K.P.; Yuan, X.L.; Ng, T.K. Complex effect of continuous curcumin exposure on human bone marrow-derived mesenchymal stem cell regenerative properties through matrix metalloproteinase regulation. Basic Clin. Pharmacol. Toxicol. 2021, 128, 141–153. [Google Scholar] [CrossRef]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31 (Suppl. S1), 177–183. [Google Scholar] [CrossRef] [PubMed]
- Alcorta-Sevillano, N.; Macias, I.; Infante, A.; Rodriguez, C.I. Deciphering the Relevance of Bone ECM Signaling. Cells 2020, 9, 2630. [Google Scholar] [CrossRef] [PubMed]
- Behonick, D.J.; Xing, Z.; Lieu, S.; Buckley, J.M.; Lotz, J.C.; Marcucio, R.S.; Werb, Z.; Miclau, T.; Colnot, C. Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration. PLoS ONE 2007, 2, e1150. [Google Scholar] [CrossRef]
- Hwang, J.H.; Byun, M.R.; Kim, A.R.; Kim, K.M.; Cho, H.J.; Lee, Y.H.; Kim, J.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. Extracellular Matrix Stiffness Regulates Osteogenic Differentiation through MAPK Activation. PLoS ONE 2015, 10, e0135519. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Ponte, F.; Kim, H.N.; Warren, A.; Iyer, S.; Han, L.; Mannen, E.; Gomez-Acevedo, H.; Nookaew, I.; Almeida, M.; Manolagas, S.C. Mmp13 deletion in mesenchymal cells increases bone mass and may attenuate the cortical bone loss caused by estrogen deficiency. Sci. Rep. 2022, 12, 10257. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Alimoradi, Z.; Haqi, P.; Mahdizad, F. Effects of phytoestrogens on bone mineral density during the menopause transition: A systematic review of randomized, controlled trials. Climacteric 2016, 19, 535–545. [Google Scholar] [CrossRef]
- Zhang, G.; Qin, L.; Shi, Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: A 24-month randomized, double-blind and placebo-controlled trial. J. Bone Miner. Res. 2007, 22, 1072–1079. [Google Scholar] [CrossRef]
- Heim, M.; Frank, O.; Kampmann, G.; Sochocky, N.; Pennimpede, T.; Fuchs, P.; Hunziker, W.; Weber, P.; Martin, I.; Bendik, I. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 2004, 145, 848–859. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, G.; He, Y.; Wang, X.; Leung, P.; Leung, K.; Qin, L. Epimedium-derived flavonoids promote osteoblastogenesis and suppress adipogenesis in bone marrow stromal cells while exerting an anabolic effect on osteoporotic bone. Bone 2009, 45, 534–544. [Google Scholar]
- Xu, Y.X.; Wu, C.L.; Wu, Y.; Tong, P.J.; Jin, H.T.; Yu, N.Z.; Xiao, L.W. Epimedium-derived flavonoids modulate the balance between osteogenic differentiation and adipogenic differentiation in bone marrow stromal cells of ovariectomized rats via Wnt/beta-catenin signal pathway activation. Chin. J. Integr. Med. 2012, 18, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, Y.; Jia, B.; Wang, Y.; Li, T. Icariin stimulates osteogenesis and suppresses adipogenesis of human bone mesenchymal stem cells via miR-23a-mediated activation of the Wnt/beta-catenin signaling pathway. Phytomedicine 2021, 85, 153485. [Google Scholar] [CrossRef] [PubMed]
- Halleen, J.M.; Tiitinen, S.L.; Ylipahkala, H.; Fagerlund, K.M.; Vaananen, H.K. Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin. Lab. 2006, 52, 499–509. [Google Scholar]
- Nenonen, A.; Cheng, S.; Ivaska, K.K.; Alatalo, S.L.; Lehtimaki, T.; Schmidt-Gayk, H.; Uusi-Rasi, K.; Heinonen, A.; Kannus, P.; Sievanen, H.; et al. Serum TRACP 5b is a useful marker for monitoring alendronate treatment: Comparison with other markers of bone turnover. J. Bone Miner. Res. 2005, 20, 1804–1812. [Google Scholar] [CrossRef]
- Zhang, J.F.; Li, G.; Chan, C.Y.; Meng, C.L.; Lin, M.C.; Chen, Y.C.; He, M.L.; Leung, P.C.; Kung, H.F. Flavonoids of Herba Epimedii regulate osteogenesis of human mesenchymal stem cells through BMP and Wnt/beta-catenin signaling pathway. Mol. Cell. Endocrinol. 2010, 314, 70–74. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, T.L.; Chang, W.Z.; Liu, Y.; Centrella, M. Runx2 integrates estrogen activity in osteoblasts. J. Biol. Chem. 2003, 278, 43121–43129. [Google Scholar] [CrossRef]
- Mont, M.A.; Salem, H.S.; Piuzzi, N.S.; Goodman, S.B.; Jones, L.C. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today?: A 5-Year Update. J. Bone Jt. Surg. Am. 2020, 102, 1084–1099. [Google Scholar] [CrossRef]
- Gaddini, G.W.; Turner, R.T.; Grant, K.A.; Iwaniec, U.T. Alcohol: A Simple Nutrient with Complex Actions on Bone in the Adult Skeleton. Alcohol. Clin. Exp. Res. 2016, 40, 657–671. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, H.; Yin, Q.; Chen, L.; Dong, P.; Zhang, X.; Kang, J. ER stress activating ATF4/CHOP-TNF-alpha signaling pathway contributes to alcohol-induced disruption of osteogenic lineage of multipotential mesenchymal stem cell. Cell. Physiol. Biochem. 2013, 32, 743–754. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Mao, K.; Li, J.; Cui, Q.; Wang, G.J. Alcohol-induced adipogenesis in bone and marrow: A possible mechanism for osteonecrosis. Clin. Orthop. Relat. Res. 2003, 410, 213–224. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, Y.; Saleh, K.J.; Wang, G.J.; Balian, G. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. S3), 148–154. [Google Scholar]
- Yang, X.; Matsuda, K.; Bialek, P.; Jacquot, S.; Masuoka, H.C.; Schinke, T.; Li, L.; Brancorsini, S.; Sassone-Corsi, P.; Townes, T.M.; et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell 2004, 117, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Yoneshima, E.; Okamoto, K.; Sakai, E.; Nishishita, K.; Yoshida, N.; Tsukuba, T. The Transcription Factor EB (TFEB) Regulates Osteoblast Differentiation Through ATF4/CHOP-Dependent Pathway. J. Cell. Physiol. 2016, 231, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Jiang, D.; Ge, C.; Zhao, Z.; Lai, Y.; Boules, H.; Phimphilai, M.; Yang, X.; Karsenty, G.; Franceschi, R.T. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J. Biol. Chem. 2005, 280, 30689–30696. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Yin, Q.; Gao, H.; Dong, P.; Zhang, X.; Kang, J. Reciprocal interferences of TNF-alpha and Wnt1/beta-catenin signaling axes shift bone marrow-derived stem cells towards osteoblast lineage after ethanol exposure. Cell. Physiol. Biochem. 2013, 32, 755–765. [Google Scholar] [CrossRef]
- Xu, H.; Duan, J.; Ning, D.; Li, J.; Liu, R.; Yang, R.; Jiang, J.X.; Shang, P. Role of Wnt signaling in fracture healing. BMB Rep. 2014, 47, 666–672. [Google Scholar] [CrossRef]
- Joeng, K.S.; Lee, Y.C.; Lim, J.; Chen, Y.; Jiang, M.M.; Munivez, E.; Ambrose, C.; Lee, B.H. Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasis. J. Clin. Investig. 2017, 127, 2678–2688. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration—What Do We Know So Far. Front. Cell Dev. Biol. 2018, 6, 170. [Google Scholar] [CrossRef]
- Maul, T.M.; Chew, D.W.; Nieponice, A.; Vorp, D.A. Mechanical stimuli differentially control stem cell behavior: Morphology, proliferation, and differentiation. Biomech. Model Mechanobiol. 2011, 10, 939–953. [Google Scholar] [CrossRef]
- Prasad, B.; Grimm, D.; Strauch, S.M.; Erzinger, G.S.; Corydon, T.J.; Lebert, M.; Magnusson, N.E.; Infanger, M.; Richter, P.; Kruger, M. Influence of Microgravity on Apoptosis in Cells, Tissues, and Other Systems In Vivo and In Vitro. Int. J. Mol. Sci. 2020, 21, 9373. [Google Scholar] [CrossRef]
- Dai, Z.Q.; Wang, R.; Ling, S.K.; Wan, Y.M.; Li, Y.H. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif. 2007, 40, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, Z.Q.; Ling, S.K.; Zhang, H.Y.; Wan, Y.M.; Li, Y.H. Gravity, a regulation factor in the differentiation of rat bone marrow mesenchymal stem cells. J. Biomed. Sci. 2009, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Li, Y.; Chen, J. Duration of simulated microgravity affects the differentiation of mesenchymal stem cells. Mol. Med. Rep. 2017, 15, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M. The effect of exercise on bone mass and structural geometry during growth. Med. Sport Sci. 2007, 51, 33–49. [Google Scholar]
- Guadalupe-Grau, A.; Fuentes, T.; Guerra, B.; Calbet, J.A. Exercise and bone mass in adults. Sports Med. 2009, 39, 439–468. [Google Scholar] [CrossRef]
- David, V.; Martin, A.; Lafage-Proust, M.H.; Malaval, L.; Peyroche, S.; Jones, D.B.; Vico, L.; Guignandon, A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 2007, 148, 2553–2562. [Google Scholar] [CrossRef]
- Yang, X.; Cai, X.; Wang, J.; Tang, H.; Yuan, Q.; Gong, P.; Lin, Y. Mechanical stretch inhibits adipogenesis and stimulates osteogenesis of adipose stem cells. Cell Prolif. 2012, 45, 158–166. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, Y.R.; Park, M.S.; Shin, J.W.; Park, K.D.; Kim, S.J.; Lee, J.W. ERK 1/2 activation in enhanced osteogenesis of human mesenchymal stem cells in poly(lactic-glycolic acid) by cyclic hydrostatic pressure. J. Biomed. Mater. Res. A 2007, 80, 826–836. [Google Scholar] [CrossRef]
- Kiel, D.P.; Hannan, M.T.; Barton, B.A.; Bouxsein, M.L.; Sisson, E.; Lang, T.; Allaire, B.; Dewkett, D.; Carroll, D.; Magaziner, J.; et al. Low-Magnitude Mechanical Stimulation to Improve Bone Density in Persons of Advanced Age: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2015, 30, 1319–1328. [Google Scholar] [CrossRef]
- Pham, M.H.; Buser, Z.; Wang, J.C.; Acosta, F.L. Low-magnitude mechanical signals and the spine: A review of current and future applications. J. Clin. Neurosci. 2017, 40, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.K.; Capilla, E.; Rosen, C.J.; Gilsanz, V.; Pessin, J.E.; Judex, S.; Rubin, C.T. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J. Bone Miner. Res. 2009, 24, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, F.; Zhong, D.Y.; Luo, Z.P. Acoustic-frequency vibratory stimulation regulates the balance between osteogenesis and adipogenesis of human bone marrow-derived mesenchymal stem cells. Biomed. Res. Int. 2015, 2015, 540731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guan, X.; Zhu, Z.; Gao, S.; Zhang, C.; Li, C.; Zhou, K.; Hou, W.; Yu, H. Osteogenic differentiation of bone marrow-derived mesenchymal stromal cells on bone-derived scaffolds: Effect of microvibration and role of ERK1/2 activation. Eur. Cell Mater. 2011, 22, 12–25. [Google Scholar] [CrossRef]
- Judex, S.; Lei, X.; Han, D.; Rubin, C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J. Biomech. 2007, 40, 1333–1339. [Google Scholar] [CrossRef]
- Oh, E.S.; Seo, Y.K.; Yoon, H.H.; Cho, H.; Yoon, M.Y.; Park, J.K. Effects of sub-sonic vibration on the proliferation and maturation of 3T3-L1 cells. Life Sci. 2011, 88, 169–177. [Google Scholar] [CrossRef]
- Yang, X.; He, H.; Ye, W.; Perry, T.A.; He, C. Effects of Pulsed Electromagnetic Field Therapy on Pain, Stiffness, Physical Function, and Quality of Life in Patients With Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Phys. Ther. 2020, 100, 1118–1131. [Google Scholar] [CrossRef]
- Leo, M.; Milena, F.; Ruggero, C.; Stefania, S.; Giancarlo, T. Biophysical stimulation in osteonecrosis of the femoral head. Indian J. Orthop. 2009, 43, 17–21. [Google Scholar]
- Di Bartolomeo, M.; Cavani, F.; Pellacani, A.; Grande, A.; Salvatori, R.; Chiarini, L.; Nocini, R.; Anesi, A. Pulsed Electro-Magnetic Field (PEMF) Effect on Bone Healing in Animal Models: A Review of Its Efficacy Related to Different Type of Damage. Biology 2022, 11, 402. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Xie, S.; Huang, J.; Song, K.; He, C. Effects of Pulsed Electromagnetic Field Therapy at Different Frequencies on Bone Mass and Microarchitecture in Osteoporotic Mice. Bioelectromagnetics 2021, 42, 441–454. [Google Scholar] [CrossRef]
- Li, J.P.; Chen, S.; Peng, H.; Zhou, J.L.; Fang, H.S. Pulsed electromagnetic fields protect the balance between adipogenesis and osteogenesis on steroid-induced osteonecrosis of femoral head at the pre-collapse stage in rats. Bioelectromagnetics 2014, 35, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhao, D.; Wei, S.; Liu, C.; Liu, Y.; Wang, B.; Zhao, W.; Yang, K.; Yang, Y.; Wu, H. The effect of electromagnetic fields on the proliferation and the osteogenic or adipogenic differentiation of mesenchymal stem cells modulated by dexamethasone. Bioelectromagnetics 2014, 35, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Marampon, F.; Gravina, G.L.; Di Rocco, A.; Bonfili, P.; Di Staso, M.; Fardella, C.; Polidoro, L.; Ciccarelli, C.; Festuccia, C.; Popov, V.M.; et al. MEK/ERK inhibitor U0126 increases the radiosensitivity of rhabdomyosarcoma cells in vitro and in vivo by downregulating growth and DNA repair signals. Mol. Cancer Ther. 2011, 10, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xu, L.; Zhou, C.; Lee, W.Y.; Wu, T.; Cui, L.; Li, G. Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. Int. J. Biochem. Cell Biol. 2014, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.F.; Chaudhary, L.; Fausto, A.; Halstead, L.R.; Ory, D.S.; Avioli, L.V.; Cheng, S.L. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J. Biol. Chem. 2001, 276, 14443–14450. [Google Scholar] [CrossRef]
- Jaiswal, R.K.; Jaiswal, N.; Bruder, S.P.; Mbalaviele, G.; Marshak, D.R.; Pittenger, M.F. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J. Biol. Chem. 2000, 275, 9645–9652. [Google Scholar] [CrossRef]
- Majeed, H.; Gupta, V. Adverse Effects of Radiation Therapy; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wang, Y.; Zhu, G.; Wang, J.; Chen, J. Irradiation alters the differentiation potential of bone marrow mesenchymal stem cells. Mol. Med. Rep. 2016, 13, 213–223. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, L.; Ran, Q.; Liu, Y.; Xiang, Y.; Xiao, Y.; Chen, L.; Li, F.; Zhong, J.F.; Li, Z. Crif1 Promotes Adipogenic Differentiation of Bone Marrow Mesenchymal Stem Cells After Irradiation by Modulating the PKA/CREB Signaling Pathway. Stem Cells 2015, 33, 1915–1926. [Google Scholar] [CrossRef]
- Xiang, L.X.; Ran, Q.; Chen, L.; Xiang, Y.; Li, F.J.; Zhang, X.M.; Xiao, Y.N.; Zou, L.Y.; Zhong, J.F.; Li, S.C.; et al. CR6-interacting factor-1 contributes to osteoclastogenesis by inducing receptor activator of nuclear factor kappaB ligand after radiation. World J. Stem Cells 2020, 12, 222–240. [Google Scholar] [CrossRef]
- Ryu, M.J.; Kim, S.J.; Kim, Y.K.; Choi, M.J.; Tadi, S.; Lee, M.H.; Lee, S.E.; Chung, H.K.; Jung, S.B.; Kim, H.J.; et al. Crif1 deficiency reduces adipose OXPHOS capacity and triggers inflammation and insulin resistance in mice. PLoS Genet. 2013, 9, e1003356. [Google Scholar] [CrossRef]
- Kim, J.; Adachi, T. Cell-fate decision of mesenchymal stem cells toward osteocyte differentiation is committed by spheroid culture. Sci. Rep. 2021, 11, 13204. [Google Scholar] [CrossRef] [PubMed]
- Mollentze, J.; Durandt, C.; Pepper, M.S. An In Vitro and In Vivo Comparison of Osteogenic Differentiation of Human Mesenchymal Stromal/Stem Cells. Stem Cells Int. 2021, 2021, 9919361. [Google Scholar] [CrossRef]
- Jones, D.L.; Rando, T.A. Emerging models and paradigms for stem cell ageing. Nat. Cell Biol. 2011, 13, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Nikodemova, M.; Watters, J.J. Outbred ICR/CD1 mice display more severe neuroinflammation mediated by microglial TLR4/CD14 activation than inbred C57Bl/6 mice. Neuroscience 2011, 190, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.R., Jr.; Khazai, N.B.; Bouloux, G.F.; Camalier, C.E.; Lin, Y.; Garneys, L.M.; Siqueira, J.; Peng, L.; Pasquel, F.; Umpierrez, D.; et al. The effects of thiazolidinediones on human bone marrow stromal cell differentiation in vitro and in thiazolidinedione-treated patients with type 2 diabetes. Transl. Res. 2013, 161, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zimmerlin, L.; Marra, K.G.; Donnenberg, V.S.; Donnenberg, A.D.; Rubin, J.P. Adipogenic potential of adipose stem cell subpopulations. Plast. Reconstr. Surg. 2011, 128, 663–672. [Google Scholar] [CrossRef]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef]
- Kirchner, S.; Kieu, T.; Chow, C.; Casey, S.; Blumberg, B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol. Endocrinol. 2010, 24, 526–539. [Google Scholar] [CrossRef]
- Engin, A. Fat Cell and Fatty Acid Turnover in Obesity. Adv. Exp. Med. Biol. 2017, 960, 135–160. [Google Scholar]
- Wang, J.; Liu, S.; Li, J.; Zhao, S.; Yi, Z. Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2019, 10, 197. [Google Scholar] [CrossRef]
- Teta, D.; Tedjani, A.; Burnier, M.; Bevington, A.; Brown, J.; Harris, K. Glucose-containing peritoneal dialysis fluids regulate leptin secretion from 3T3-L1 adipocytes. Nephrol. Dial. Transplant. 2005, 20, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Fernandes, A.; Demaegdt, H.; Vandermeiren, K.; Hectors, T.L.; Jorens, P.G.; Blust, R.; Vanparys, C. Evaluation of a screening system for obesogenic compounds: Screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS ONE 2013, 8, e77481. [Google Scholar] [CrossRef] [PubMed]
- Caporale, N.; Leemans, M.; Birgersson, L.; Germain, P.L.; Cheroni, C.; Borbely, G.; Engdahl, E.; Lindh, C.; Bressan, R.B.; Cavallo, F.; et al. From cohorts to molecules: Adverse impacts of endocrine disrupting mixtures. Science 2022, 375, eabe8244. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ji, K. Identification of combinations of endocrine disrupting chemicals in household chemical products that require mixture toxicity testing. Ecotoxicol. Environ. Saf. 2022, 240, 113677. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Axelstad, M.; Scholze, M.; Johansson, H.K.L.; Hass, U.; Mandrup, K.; Frandsen, H.L.; Frederiksen, H.; Isling, L.K.; Boberg, J. Grouping of endocrine disrupting chemicals for mixture risk assessment—Evidence from a rat study. Environ. Int. 2020, 142, 105870. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, H.; Yuzuriha, T.; Akiyama, E.; Nakao, T.; Ohta, S. Complex toxicity as disruption of adipocyte or osteoblast differentiation in human mesenchymal stem cells under the mixed condition of TBBPA and TCDD. Toxicol. Rep. 2018, 5, 737–743. [Google Scholar] [CrossRef]
- Tung, E.W.; Boudreau, A.; Wade, M.G.; Atlas, E. Induction of adipocyte differentiation by polybrominated diphenyl ethers (PBDEs) in 3T3-L1 cells. PLoS ONE 2014, 9, e94583. [Google Scholar] [CrossRef]
- Tencerova, M.; Kassem, M. The Bone Marrow-Derived Stromal Cells: Commitment and Regulation of Adipogenesis. Front. Endocrinol. 2016, 7, 127. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zhao, L.; Terry, P.D.; Chen, J. Reciprocal Effect of Environmental Stimuli to Regulate the Adipogenesis and Osteogenesis Fate Decision in Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs). Cells 2023, 12, 1400. https://doi.org/10.3390/cells12101400
Xu X, Zhao L, Terry PD, Chen J. Reciprocal Effect of Environmental Stimuli to Regulate the Adipogenesis and Osteogenesis Fate Decision in Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs). Cells. 2023; 12(10):1400. https://doi.org/10.3390/cells12101400
Chicago/Turabian StyleXu, Xinyun, Ling Zhao, Paul D. Terry, and Jiangang Chen. 2023. "Reciprocal Effect of Environmental Stimuli to Regulate the Adipogenesis and Osteogenesis Fate Decision in Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs)" Cells 12, no. 10: 1400. https://doi.org/10.3390/cells12101400
APA StyleXu, X., Zhao, L., Terry, P. D., & Chen, J. (2023). Reciprocal Effect of Environmental Stimuli to Regulate the Adipogenesis and Osteogenesis Fate Decision in Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs). Cells, 12(10), 1400. https://doi.org/10.3390/cells12101400







