Cell Dome as an Evaluation Platform for Organized HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Enclosed in Cell Dome
2.3. Hydrogel Permeability
2.4. GSTP1 Enzymatic Activity
2.5. HIF-1α Gene Expression
2.6. Drug Treatment and Proliferation Inhibition
2.7. Statistical Analysis
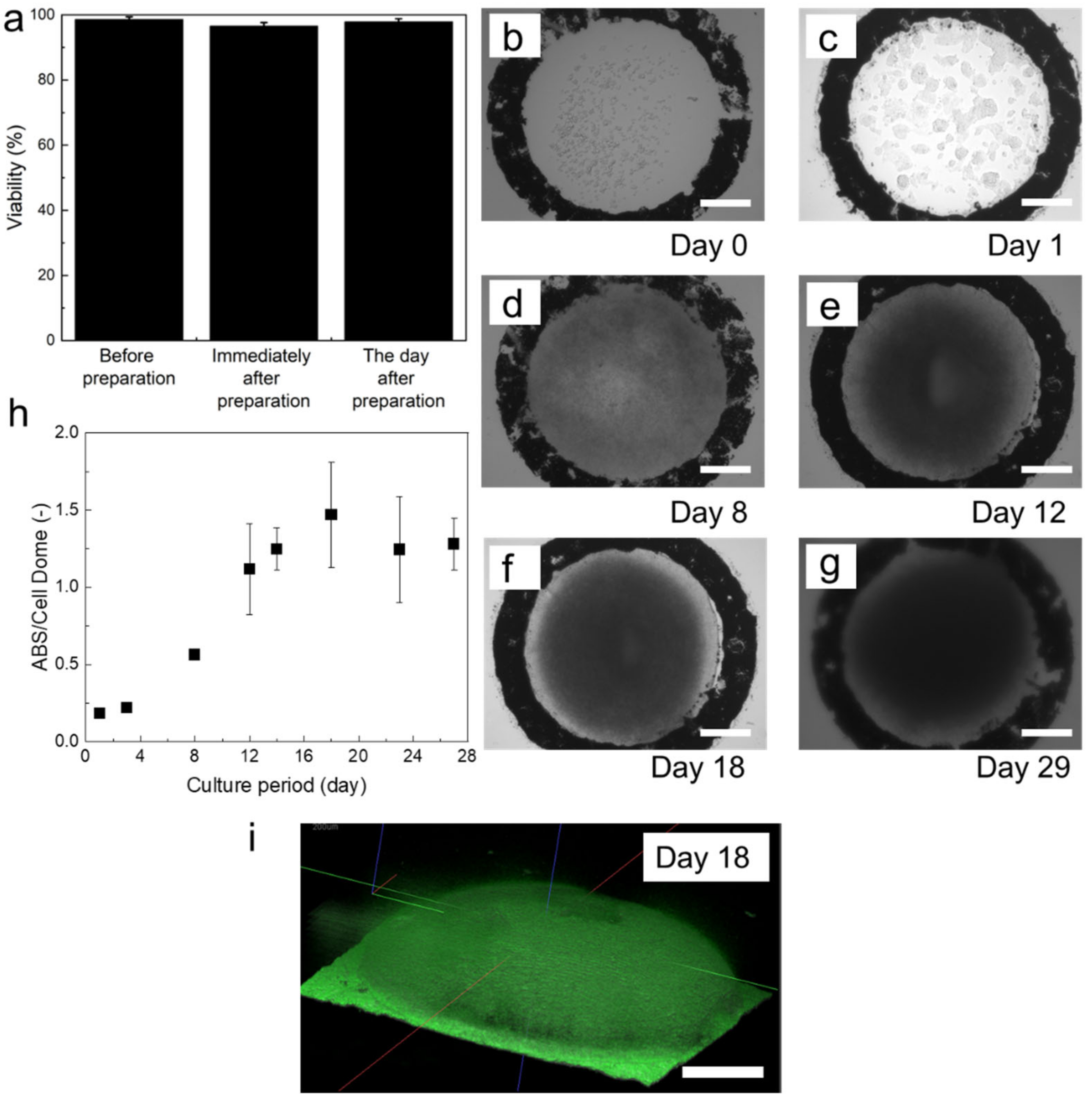
3. Results
3.1. Hydrogel Permeability
3.2. Cell Growth in Cell Dome
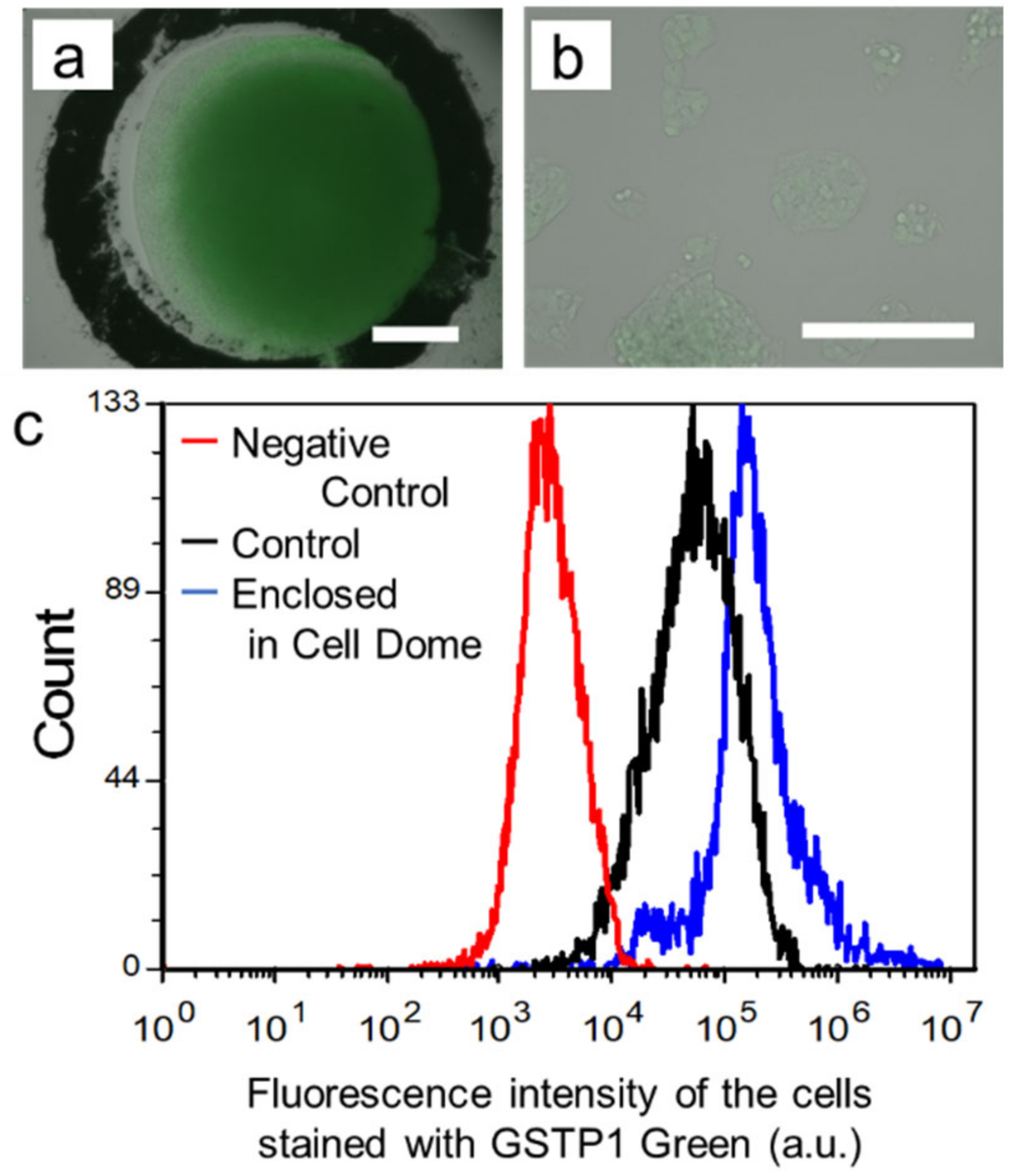
3.3. GSTP1 Enzymatic Activity
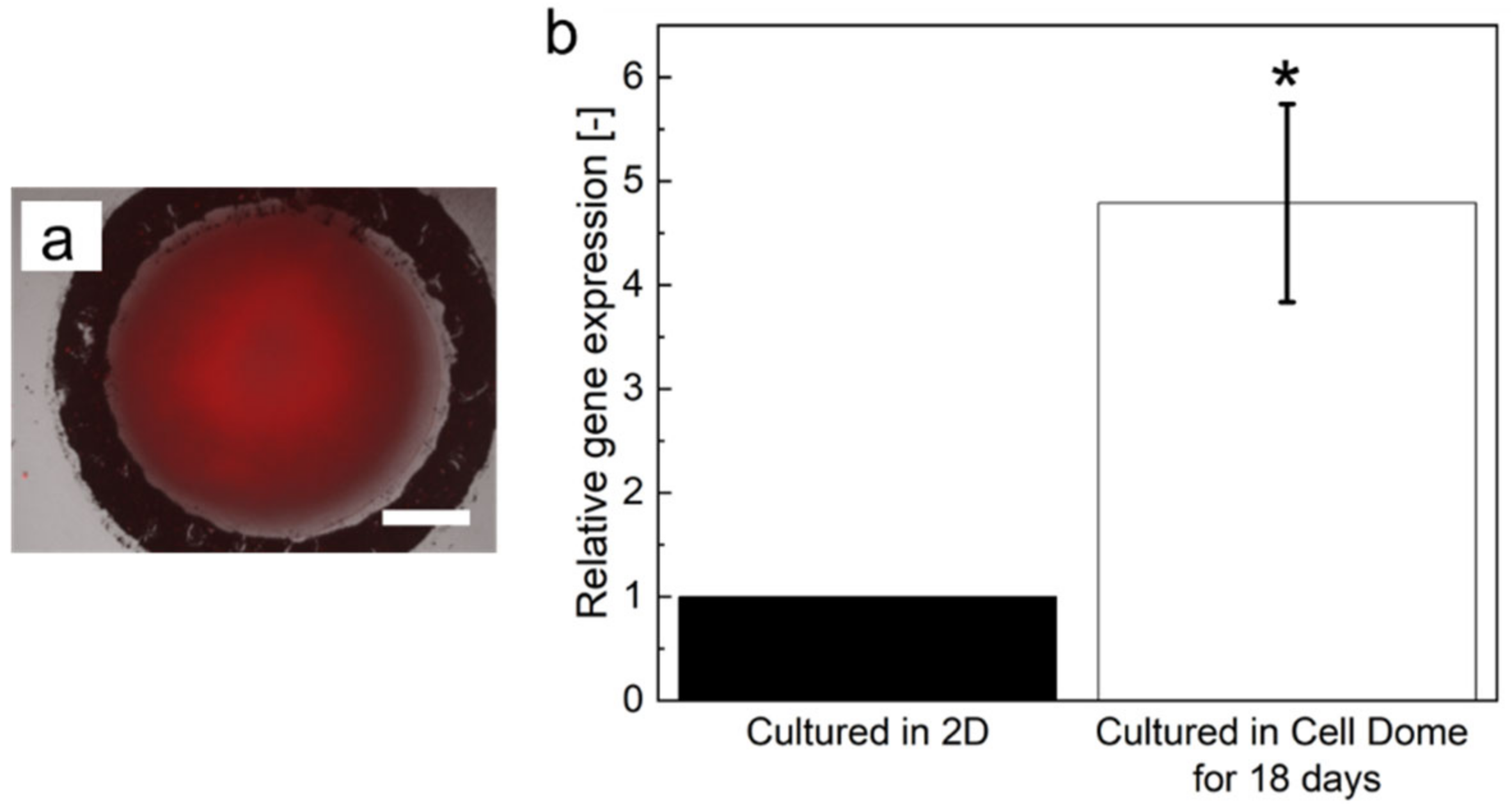
3.4. Expression of HIF-1α
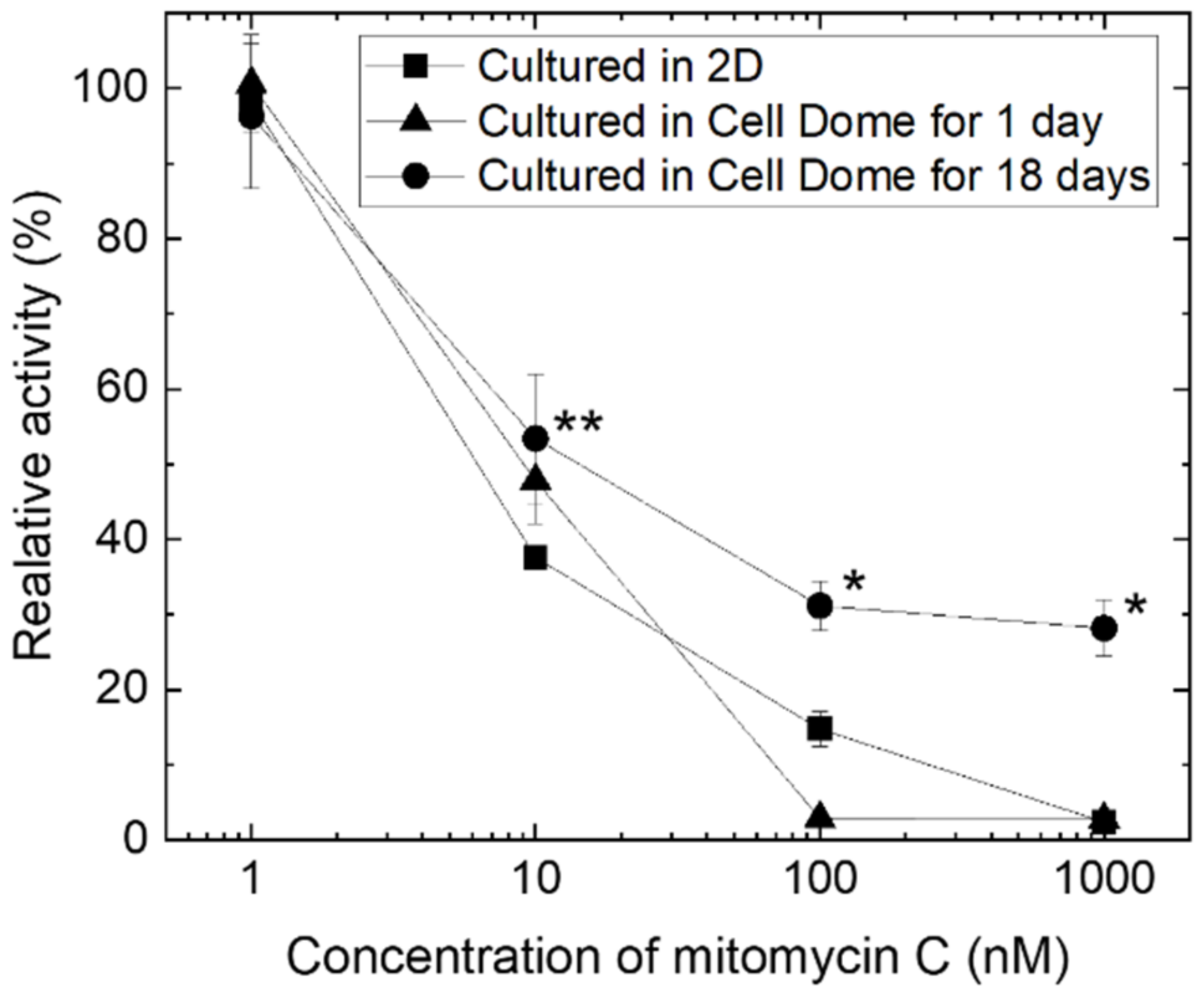
3.5. Drug Treatment of Enclosed Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramirez, T.; Strigun, A.; Verlohner, A.; Huener, H.A.; Peter, E.; Herold, M.; Bordag, N.; Mellert, W.; Walk, T.; Spitzer, M.; et al. Prediction of liver toxicity and mode of action using metabolomics in vitro in HepG2 cells. Arch. Toxicol. 2018, 92, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Beggs, K.M.; Maiuri, A.R.; Fullerton, A.M.; Poulsen, K.L.; Breier, A.B.; Ganey, P.E.; Roth, R.A. Trovafloxacin-induced replication stress sensitizes HepG2 cells to tumor necrosis factor-alpha-induced cytotoxicity mediated by extracellular signal-regulated kinase and ataxia telangiectasia and Rad3-related. Toxicology 2015, 331, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C.; Zheng, J.; Yourick, J.J.; Sprando, R.L.; Gao, X. Toxicogenomic responses of human liver HepG2 cells to silver nanoparticles. J. Appl. Toxicol. 2015, 35, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, K.; Arakawa, H.; Yano, K.; Koyama, S.; Kojima, H.; Ogihara, T. Utility of Three-Dimensional Cultures of Primary Human Hepatocytes (Spheroids) as Pharmacokinetic Models. Biomedicines 2020, 8, 374. [Google Scholar] [CrossRef]
- Lauschke, V.M.; Hendriks, D.F.; Bell, C.C.; Andersson, T.B.; Ingelman-Sundberg, M. Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Chem. Res. Toxicol. 2016, 29, 1936–1955. [Google Scholar] [CrossRef]
- Donato, M.T.; Tolosa, L.; Gomez-Lechon, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Bokhari, M.; Carnachan, R.J.; Cameron, N.R.; Przyborski, S.A. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J. Anat. 2007, 211, 567–576. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- Ramaiahgari, S.C.; den Braver, M.W.; Herpers, B.; Terpstra, V.; Commandeur, J.N.; van de Water, B.; Price, L.S. A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Arch. Toxicol. 2014, 88, 1083–1095. [Google Scholar] [CrossRef]
- Aritomi, K.; Ishitsuka, Y.; Tomishima, Y.; Shimizu, D.; Abe, N.; Shuto, T.; Irikura, M.; Kai, H.; Irie, T. Evaluation of three-dimensional cultured HepG2 cells in a nano culture plate system: An in vitro human model of acetaminophen hepatotoxicity. J. Pharmacol. Sci. 2014, 124, 218–229. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, Y.; Lv, M.; Zang, G.; Ng, S.S.; Chen, X. Advances in 3D cell culture for liver preclinical studies. Acta Biochim. Biophys. Sin. 2021, 53, 643–651. [Google Scholar] [CrossRef]
- Wilkening, S.; Stahl, F.; Bader, A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003, 31, 1035–1042. [Google Scholar] [CrossRef]
- Mori, R.; Sakai, Y.; Nakazawa, K. Micropatterned organoid culture of rat hepatocytes and HepG2 cells. J. Biosci. Bioeng. 2008, 106, 237–242. [Google Scholar] [CrossRef]
- Liu, W.; Sun, M.; Lu, B.; Yan, M.; Han, K.; Wang, J. A microfluidic platform for multi-size 3D tumor culture, monitoring and drug resistance testing. Sens. Actuators B Chem. 2019, 292, 111–120. [Google Scholar] [CrossRef]
- Hurrell, T.; Ellero, A.A.; Masso, Z.F.; Cromarty, A.D. Characterization and reproducibility of HepG2 hanging drop spheroids toxicology in vitro. Toxicol. Vitr. 2018, 50, 86–94. [Google Scholar] [CrossRef]
- Sakai, S.; Ito, S.; Ogushi, Y.; Hashimoto, I.; Hosoda, N.; Sawae, Y.; Kawakami, K. Enzymatically fabricated and degradable microcapsules for production of multicellular spheroids with well-defined diameters of less than 150 µm. Biomaterials 2009, 30, 5937–5942. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Sakai, S.; Ashida, T.; Taya, M. Production of hyaluronic-acid-based cell-enclosing microparticles and microcapsules via enzymatic reaction using a microfluidic system. J. Appl. Polym. Sci. 2016, 133, 43107. [Google Scholar] [CrossRef]
- Wei, J.; Lei, D.; Chen, M.; Ran, P.; Li, X. Engineering HepG2 spheroids with injectable fiber fragments as predictable models for drug metabolism and tumor infiltration. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3331–3344. [Google Scholar] [CrossRef]
- Pandkar, M.R.; Dhamdhere, S.G.; Shukla, S. Oxygen gradient and tumor heterogeneity: The chronicle of a toxic relationship. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188553. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updates 2011, 14, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Wrzesinski, K.; Rogowska-Wrzesinska, A.; Kanlaya, R.; Borkowski, K.; Schwammle, V.; Dai, J.; Joensen, K.E.; Wojdyla, K.; Carvalho, V.B.; Fey, S.J. The cultural divide: Exponential growth in classical 2D and metabolic equilibrium in 3D environments. PLoS ONE 2014, 9, e106973. [Google Scholar] [CrossRef] [PubMed]
- Achilli, T.M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Kazama, R.; Sato, R.; Fujiwara, H.; Qu, Y.; Nakahata, M.; Kojima, M.; Fujita, S.; Sakai, S. Development of non-adherent cell-enclosing domes with enzymatically cross-linked hydrogel shell. Biofabrication 2023, 15, 015002. [Google Scholar] [CrossRef]
- Nishitani, Y.; Maruyama, Y.; Itoh, T.; Mikami, B.; Hashimoto, W.; Murata, K. Recognition of heteropolysaccharide alginate by periplasmic solute-binding proteins of a bacterial ABC transporter. Biochemistry 2012, 51, 3622–3633. [Google Scholar] [CrossRef]
- Nagano, H.; Kato, E.; Yamamura, S.; Ueda, M. Fluorescence studies on nyctinasty which suggest the existence of genus-specific receptors for leaf-movement factor. Rep. Prog. Phys. 2013, 76, 046602. [Google Scholar] [CrossRef]
- Sakai, S.; Kawakami, K. Synthesis and characterization of both ionically and enzymatically cross-linkable alginate. Acta Biomater. 2007, 3, 495–501. [Google Scholar] [CrossRef]
- Liu, Y.; Sakai, S.; Taya, M. Impact of the composition of alginate and gelatin derivatives in bioconjugated hydrogels on the fabrication of cell sheets and spherical tissues with living cell sheaths. Acta Biomater. 2013, 9, 6616–6623. [Google Scholar] [CrossRef]
- Hofling, F.; Franosch, T. Anomalous transport in the crowded world of biological cells. Rep. Prog. Phys. 2013, 76, 046602. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yamakoshi, K.; Yajima, Y.; Utoh, R.; Yamada, M.; Seki, M. Preparation of stripe-patterned heterogeneous hydrogel sheets using microfluidic devices for high-density coculture of hepatocytes and fibroblasts. J. Biosci. Bioeng. 2013, 116, 761–767. [Google Scholar] [CrossRef]
- Kim, J.M.; Hwang, I.H.; Jang, I.S.; Kim, M.; Bang, I.S.; Park, S.J.; Chung, Y.J.; Joo, J.C.; Lee, M.G. Houttuynia cordata Thunb Promotes Activation of HIF-1A-FOXO3 and MEF2A Pathways to Induce Apoptosis in Human HepG2 Hepatocellular Carcinoma Cells. Integr. Cancer Ther. 2017, 16, 360–372. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Gaedtke, L.; Thoenes, L.; Culmsee, C.; Mayer, B.; Wagner, E. Proteomic analysis reveals differences in protein expression in spheroid versus monolayer cultures of low-passage colon carcinoma cells. J. Proteome Res. 2007, 6, 4111–4118. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Meng, Q. Three-dimensional culture of hepatocytes for prediction of drug-induced hepatotoxicity. Expert Opin. Drug Metab. Toxicol. 2010, 6, 733–746. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, T.; Chen, D.; Wang, Q.; Zhang, L.W. Three-dimensional liver models: State of the art and their application for hepatotoxicity evaluation. Crit. Rev. Toxicol. 2020, 50, 279–309. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug. Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef]
- Basharat, A.; Rollison, H.E.; Williams, D.P.; Ivanov, D.P. HepG2 (C3A) spheroids show higher sensitivity compared to HepaRG spheroids for drug-induced liver injury (DILI). Toxicol. Appl. Pharmacol. 2020, 408, 115279. [Google Scholar] [CrossRef] [PubMed]
- Eilenberger, C.; Rothbauer, M.; Ehmoser, E.K.; Ertl, P.; Kupcu, S. Effect of Spheroidal Age on Sorafenib Diffusivity and Toxicity in a 3D HepG2 Spheroid Model. Sci. Rep. 2019, 9, 4863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wu, Q.; He, Y.; Gao, M.; Li, Y.; Peng, W.; Li, S.; Liu, Y.; Zhang, R.; Bao, J. Fabrication of Size-Controllable and Arrangement-Orderly HepG2 Spheroids for Drug Screening via Decellularized Liver Matrix-Derived Micropattern Array Chips. ACS Omega 2022, 7, 2364–2376. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, J.; Sakai, Y.; Nakazawa, K. Novel hepatocyte culture system developed using microfabrication and collagen/polyethylene glycol microcontact printing. Biomaterials 2006, 27, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Tsukamoto, Y.; Yoshida, H.; Sanae, H.; Mir, T.A.; Sakai, S.; Yoshida, T.; Okabe, M.; Nikaido, T.; Taya, M.; et al. The development of cell-adhesive hydrogel for 3D printing. Int. J. Bioprinting 2016, 2, 153–162. [Google Scholar] [CrossRef]
- Sakai, S.; Hashimoto, I.; Ogushi, Y.; Kawakami, K. Peroxidase-catalyzed cell encapsulation in subsieve-size capsules of alginate with phenol moieties in water-immiscible fluid dissolving H2O2. Biomacromolecules 2007, 8, 2622–2626. [Google Scholar] [CrossRef]
- Sakai, S.; Mochizuki, K.; Qu, Y.; Mail, M.; Nakahata, M.; Taya, M. Peroxidase-catalyzed microextrusion bioprinting of cell-laden hydrogel constructs in vaporized ppm-level hydrogen peroxide. Biofabrication 2018, 10, 045007. [Google Scholar] [CrossRef]
- Sakai, S.; Yoshii, A.; Sakurai, S.; Horii, K.; Nagasuna, O. Silk fibroin nanofibers: A promising ink additive for extrusion three-dimensional bioprinting. Mater. Today Bio. 2020, 8, 100078. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Sakai, S.; Yamada, Y.; Zenke, T.; Kawakami, K. Novel chitosan derivative soluble at neutral pH and in-situ gellable via peroxidase-catalyzed enzymatic reaction. J. Mater. Chem. 2009, 19, 230–235. [Google Scholar] [CrossRef]
- Kyffin, J.A.; Sharma, P.; Leedale, J.; Colley, H.E.; Murdoch, C.; Mistry, P.; Webb, S.D. Impact of cell types and culture methods on the functionality of in vitro liver systems—A review of cell systems for hepatotoxicity assessment. Toxicol. In Vitro 2018, 48, 262–275. [Google Scholar] [CrossRef]
- Chen, H.; Wei, X.; Chen, H.; Wei, H.; Wang, Y.; Nan, W.; Zhang, Q.; Wen, X. The study of establishment of an in vivo tumor model by three-dimensional cells culture systems methods and evaluation of antitumor effect of biotin-conjugated pullulan acetate nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 123–131. [Google Scholar] [CrossRef]
- Xinwei, Z.; Chang, L.; Xinru, Z.; Wang, X. A 3D bioprinting liver umor model for drug screening. World J. Pharm. Pharm. Sci. 2016, 5, 196–213. [Google Scholar] [CrossRef]
- Mori, M.; Fujikawa, Y.; Kikkawa, M.; Shino, M.; Sawane, M.; Sato, S.; Inoue, H. A highly selective fluorogenic substrate for imaging glutathione S-transferase P1: Development and cellular applicability in epigenetic studies. Chem. Commun. 2019, 55, 8122–8125. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Ohkura, T.; Ohta, K.; Nagao, T.; Kusumoto, K.; Koeda, A.; Ueda, T.; Jomura, T.; Ikeya, T.; Ozeki, E.; Wada, K.; et al. Evaluation of human hepatocytes cultured by three-dimensional spheroid systems for drug metabolism. Drug Metab. Pharmacokinet. 2014, 29, 373–378. [Google Scholar] [CrossRef]
- Fu, J.; Li, X.B.; Wang, L.X.; Lv, X.H.; Lu, Z.; Wang, F.; Xia, Q.; Yu, L.; Li, C.M. One-Step Dip-Coating-Fabricated Core-Shell Silk Fibroin Rice Paper Fibrous Scaffolds for 3D Tumor Spheroid Formation. ACS Appl. Bio. Mater. 2020, 3, 7462–7471. [Google Scholar] [CrossRef]
- Li, S.; Yuan, S.; Zhao, Q.; Wang, B.; Wang, X.; Li, K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed. Pharmacother. 2018, 100, 441–447. [Google Scholar] [CrossRef]
- Mo, W.; Zhang, J.T. Human ABCG2: Structure, function, and its role in multidrug resistance. Int. J. Biochem. Mol. Biol. 2012, 3, 1–27. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazama, R.; Fujita, S.; Sakai, S. Cell Dome as an Evaluation Platform for Organized HepG2 Cells. Cells 2023, 12, 69. https://doi.org/10.3390/cells12010069
Kazama R, Fujita S, Sakai S. Cell Dome as an Evaluation Platform for Organized HepG2 Cells. Cells. 2023; 12(1):69. https://doi.org/10.3390/cells12010069
Chicago/Turabian StyleKazama, Ryotaro, Satoshi Fujita, and Shinji Sakai. 2023. "Cell Dome as an Evaluation Platform for Organized HepG2 Cells" Cells 12, no. 1: 69. https://doi.org/10.3390/cells12010069
APA StyleKazama, R., Fujita, S., & Sakai, S. (2023). Cell Dome as an Evaluation Platform for Organized HepG2 Cells. Cells, 12(1), 69. https://doi.org/10.3390/cells12010069



.png)




