Pharmacodynamic Modeling to Evaluate the Impact of Cimetidine, an OCT2 Inhibitor, on the Anticancer Effects of Cisplatin
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs, Reagents, and Cell Line
2.2. CCK-8 Cell Viability Assay
2.3. Mathematical Modeling
2.3.1. Concentration-Response Relationships and Determination of IC50
2.3.2. Evaluation of Static Concentration-Response Drug-Drug Interactions
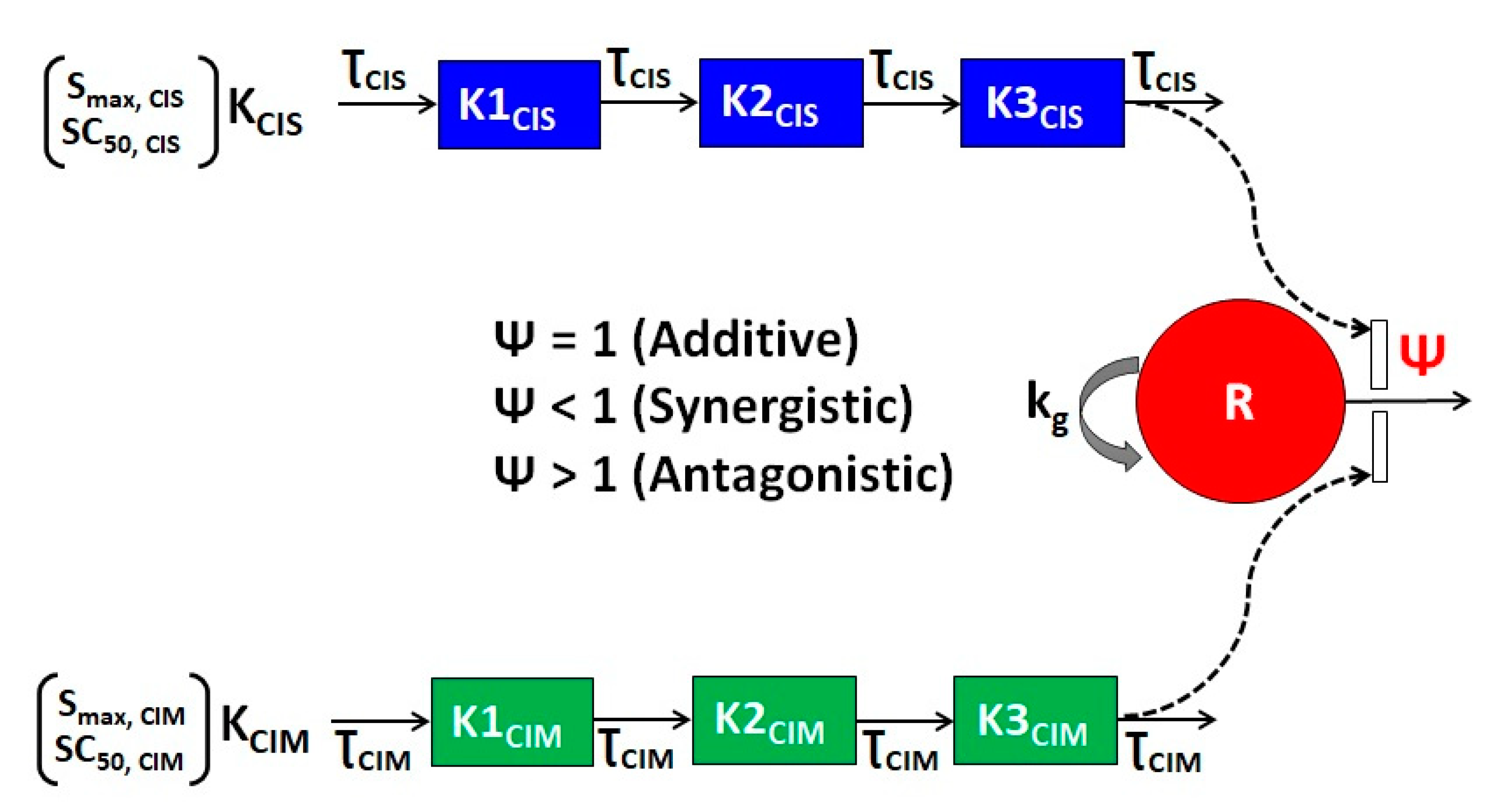
2.4. Development of the In Vitro Cellular Level Pharmacodynamic (PD) Response Model
2.4.1. Single-Agent PD Models
2.4.2. Drug Combination PD Models
3. Results
3.1. Determination of IC50 for CIS and CIM as Single Agents from Static Concentration-Response Curves
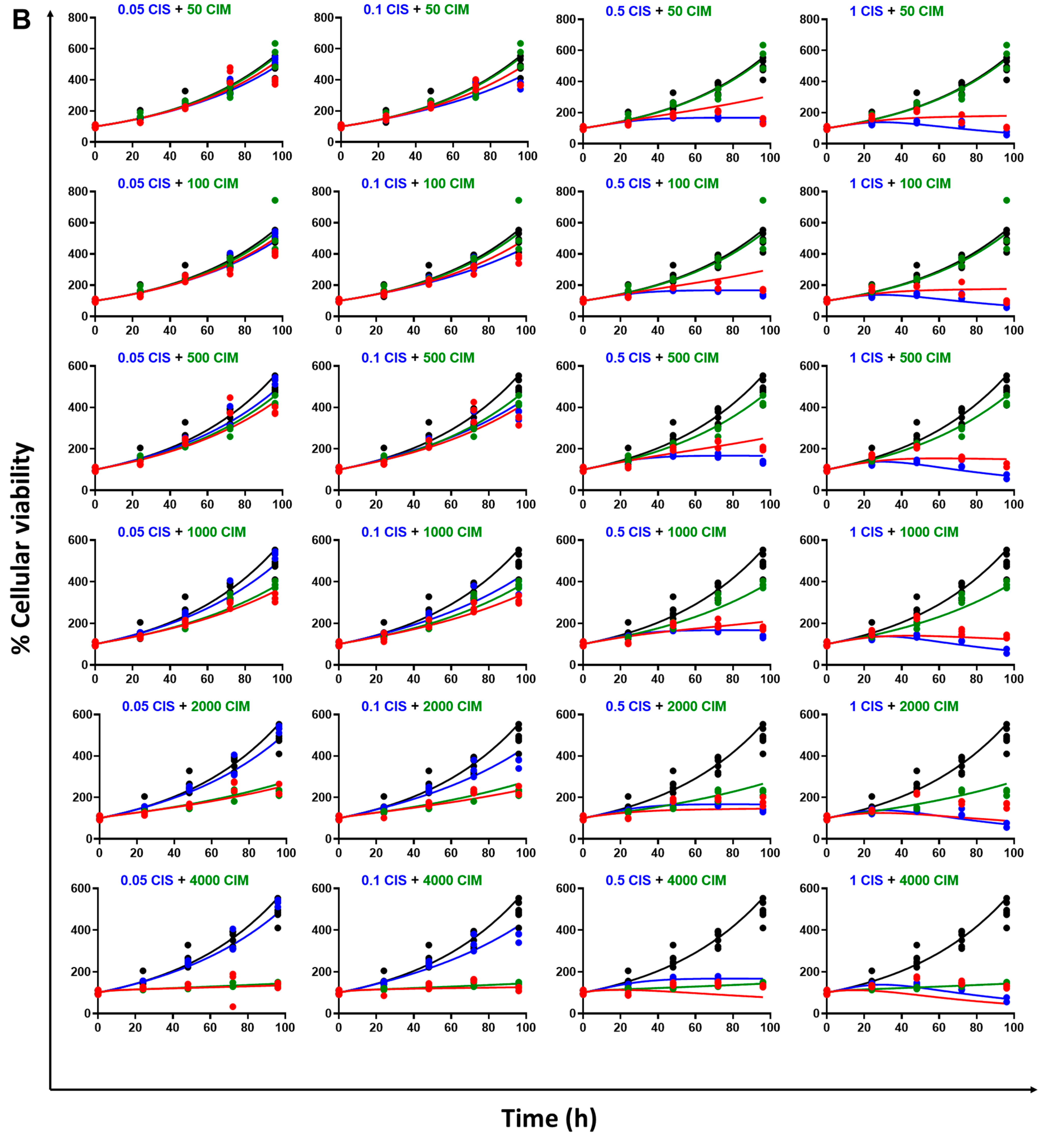
3.2. Static Concentration-Response Combinatorial Drug Effects of CIS and CIM
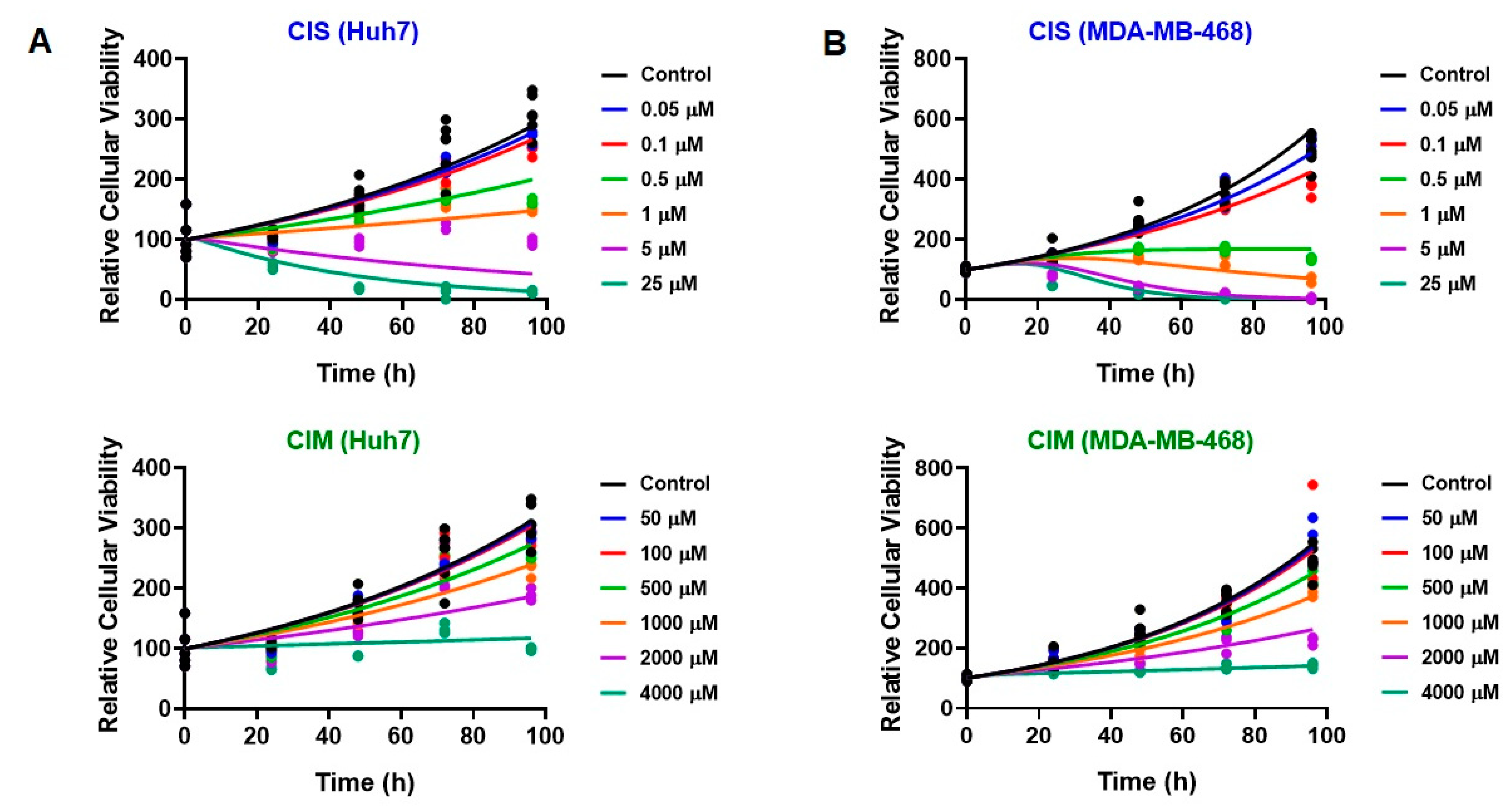
3.3. Time Course Effects of CIS and CIM Single Agents and Combination in Human Cancer Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciarimboli, G. Membrane transporters as mediators of cisplatin side-effects. Anticancer Res. 2014, 34, 547–550. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Ludwig, T.; Lang, D.; Pavenstäd, H.; Koepsell, H.; Piechota, H.-J.; Haier, J.; Zisowsky, J.; Schlatter, E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am. J. Pathol. 2005, 167, 1477–1484. [Google Scholar] [CrossRef]
- Ludwig, T.; Riethmüller, C.; Gekle, M.; Schwerdt, G.; Oberleithner, H. Nephrotoxicity of platinum complexes is related to basolateral organic cation transport. Kidney Int. 2004, 66, 196–202. [Google Scholar] [CrossRef]
- Motohashi, H.; Sakurai, Y.; Saito, H.; Masuda, S.; Urakami, Y.; Goto, M.; Fukatsu, A.; Ogawa, O.; Inui, K.-I. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 2002, 13, 866–874. [Google Scholar] [CrossRef]
- Yonezawa, A.; Masuda, S.; Nishihara, K.; Ikuko, Y.; Katsura, T.; Inui, K.-I. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 2005, 70, 1823–1831. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Deuster, D.; Knief, A.; Sperling, M.; Holtkamp, M.; Edemir, B.; Pavenstädt, H.; Lanvers-Kaminsky, C.; am Zenhoff-Dinnesen, A.; Schinkel, A.H.; et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 2010, 176, 1169–1180. [Google Scholar] [CrossRef]
- Filipski, K.K.; Mathijssen, R.H.; Mikkelsen, T.S.; Schinkel, A.H.; Sparreboom, A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 2009, 86, 396–402. [Google Scholar] [CrossRef]
- Sprowl, J.A.; van Doorn, L.; van Gerven, L.; de Bruijn, P.; Gibson, A.A.; Mathijssen, R.H.; Sparreboom, A. Conjunctive therapy of cisplatin with the OCT2 inhibitor cimetidine: Influence on antitumor efficacy and systemic clearance. Clin. Pharmacol. Ther. 2013, 94, 585–592. [Google Scholar] [CrossRef]
- Katsuda, H.; Yamashita, M.; Katsura, H.; Yu, J.; Waki, Y.; Nagata, N.; Sai, Y.; Miyamoto, K.-I. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumor activity. Biol. Pharm. Bull. 2010, 33, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Dana, P.; Vaeteewoottacharn, K.; Kariya, R.; Matsuda, K.; Wongkham, S.; Okada, S. Repurposing cimetidine for cholangiocarcinoma: Antitumor effects in vitro and in vivo. Oncol. Lett. 2017, 13, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.G.; Liu, F.-R.; Li, J.-B.; Xu, H.-M. Cimetidine induces apoptosis in gastric cancer cells in vitro and inhibits tumor growth in vivo. Oncol. Rep 2010, 23, 693–700. [Google Scholar] [PubMed]
- Mackenzie, G.G.; Huang, L.; Alston, N.; Ouyang, N.; Vrankova, K.; Mattheolabakis, G.; Constantinides, P.P.; Rigas, B. Targeting mitochondrial STAT3 with the novel phospho-valproic acid (MDC-1112) inhibits pancreatic cancer growth in mice. PLoS One 2013, 8, e61532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Xu, J.; Sun, G.; Xu, Z. Cimetidine promotes STUB1-mediated degradation of tumoral FOXP3 by activating PI3K-Akt pathway in gastric cancer. Ann. Transl. Med. 2020, 8, 1304. [Google Scholar] [CrossRef] [PubMed]
- Ande, A.; Chaar, M.; Ait-Oudhia, S. Multiscale systems pharmacological analysis of everolimus action in hepatocellular carcinoma. J. Pharmacokinet. Pharmacodyn. 2018, 45, 607–620. [Google Scholar] [CrossRef]
- Fleisher, B.; Lezeau, J.; Werkman, C.; Jacobs, B.; Ait-Oudhia, S. In vitro to Clinical Translation of Combinatorial Effects of Doxorubicin and Abemaciclib in Rb-Positive Triple Negative Breast Cancer: A Systems-Based Pharmacokinetic/Pharmacodynamic Modeling Approach. Breast Cancer (Dove Med. Press) 2021, 13, 87–105. [Google Scholar] [CrossRef]
- Chakraborty, A.; Jusko, W.J. Pharmacodynamic interaction of recombinant human interleukin-10 and prednisolone using in vitro whole blood lymphocyte proliferation. J. Pharm. Sci. 2002, 91, 1334–1342. [Google Scholar] [CrossRef]
- Pawaskar, D.K.; Straubinger, R.M.; Fetterly, G.J.; Ma, W.W.; Jusko, W.J. Interactions of Everolimus and Sorafenib in Pancreatic Cancer Cells. AAPS J. 2013, 15, 78–84. [Google Scholar] [CrossRef]
- Miao, X.; Koch, G.; Straubinger, R.M.; Jusko, W.J. Pharmacodynamic modeling of combined chemotherapeutic effects predicts synergistic activity of gemcitabine and trabectedin in pancreatic cancer cells. Cancer Chemother. Pharmacol. 2016, 77, 181–193. [Google Scholar] [CrossRef]
- Zhu, X.; Straubinger, R.M.; Jusko, W.J. Mechanism-based mathematical modeling of combined gemcitabine and birinapant in pancreatic cancer cells. J. Pharmacokinet. Pharmacodyn. 2015, 42, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Ingar Lau, Y.-K.; Du, X.; Rayannavar, V.; Hopkins, B.; Shaw, J.; Bessler, E.; Thomas, T.; Pires, M.M.; Keniry, M.; Parsons, R.E.; et al. Metformin and erlotinib synergize to inhibit basal breast cancer. Oncotarget. 2014, 5, 10503–10517. [Google Scholar]
- Yuan, P.; Ito, K.; Perez-Lorenzo, R.; Del Guzzo, C.; Hyun Lee, J.; Shen, C.-H.; Bosenberg, M.W.; McMahon, M.; Cantley, L.C.; Zheng, B. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc. Natl. Acad. Sci. USA 2013, 110, 18226–18231. [Google Scholar] [CrossRef] [PubMed]
- Heise, M.; Lautem, A.; Knapstein, J.; Schattenberg, J.M.; Hoppe-Lotichius, M.; Foltys, D.; Weiler, N.; Zimmermann, A.; Schad, A.; Gründemann, D.; et al. Downregulation of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and their prognostic significance. BMC Cancer 2012, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, C.; Ahlin, G.; Seithel, A.; Artursson, P.; Ungell, A.-L.; Karlsson, J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab. Dispos. 2007, 35, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Yasuda, M.; Suzuki, R.; Fukushima, S. Intracellular uptake of an antitumor-active azole-bridged dinuclear platinum(II) complex in cisplatin-resistant tumor cells. Biometals 2016, 29, 1075–1083. [Google Scholar] [CrossRef]
- Miyata, M.; Morishita, A.; Sakamoto, T.; Katsura, A.; Kato, K.; Nishioka, T.; Toyota, Y.; Fujita, K.; Maeda, E.; Nomura, T.; et al. MicroRNA profiles in cisplatin-induced apoptosis of hepatocellular carcinoma cells. Int. J. Oncol. 2015, 47, 535–542. [Google Scholar] [CrossRef]
- Tryfonopoulos, D.; Walsh, S.; Collins, D.M.; Flanagan, L.; Quinn, C.; Corkery, B.; McDermott, E.W.; Evoy, D.; Pierce, A.; O’Donovan, N.; et al. Src: A potential target for the treatment of triple-negative breast cancer. Ann. Oncol. 2011, 22, 2234–2240. [Google Scholar] [CrossRef]
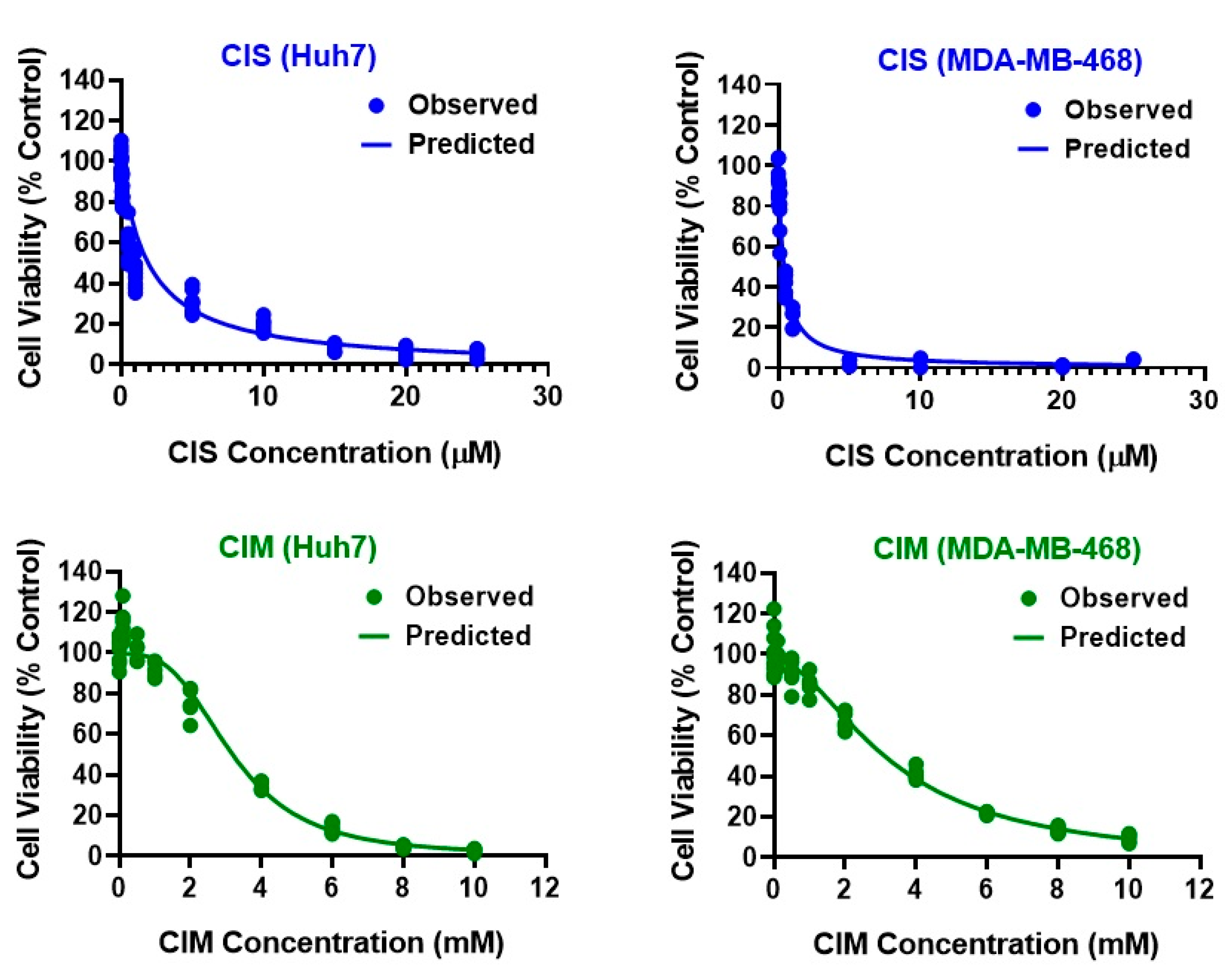


| Parameter (Units) | Definition | Estimate (% RSE) | |||
|---|---|---|---|---|---|
| CIS Huh7 | CIS MDA-MB-468 | CIM Huh7 | CIM MDA-MB-468 | ||
| R0 (%) | Baseline % Cell Viability | 100 (Fixed) | 100 (Fixed) | 99.5 (2.87) | 97.4 (2.17) |
| IC50 (µM or mM) | Drug concentration inducing 50% of maximal effect | 1.96 µM (9.82) | 0.39 µM (11.8) | 3.19 mM (4.66) | 3.28 mM (3.85) |
| Imax | Maximal effect | 1 (0.99) | 1 (Fixed) | 0.99 (0.74) | 1 (2.78) |
| γ | Hill coefficient | 1 (Fixed) | 1 (Fixed) | 3.12 (9.87) | 1.83 (10.5) |
| Parameter (Units) | Definition | Estimate (% RSE) | |
|---|---|---|---|
| Huh7 | MDA-MB-468 | ||
| R0 (%) | Baseline % cell viability | 100 (Fixed) | 100 (Fixed) |
| kg (h−1) | First-order growth rate constant | 0.011 (3.32) | 0.018 (1.4) |
| Smax,CIS (h−1) | Maximal killing rate constant of CIS | 0.038 (9.63) | 0.108 (5.75) |
| SC50,CIS (µM) | CIS concentration inducing 50% of maximal killing rate | 4.27 (6.32) | 2.48 (8.13) |
| 1/ƮCIS (h−1) | Transit constant for the stimulation of death by CIS | 1.17 (0.565) | 0.101 (7.94) |
| Smax,CIM (h−1) | Maximal killing rate constant of CIM | 0.106 (2.25) | 0.096 (17.1) |
| SC50,CIM (µM) | CIM concentration inducing 50% of maximal killing rate | 36,886.97 (22.7) | 21,453.95 (21.3) |
| 1/ƮCIM (h−1) | Transit constant for the stimulation of death by CIM | 1.92 (0.698) | 0.523 (4.69) |
| ψ | Interaction parameter | 0.96 (0.017) | 2.2 (4.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mody, H.; Vaidya, T.R.; Lesko, L.J.; Ait-Oudhia, S. Pharmacodynamic Modeling to Evaluate the Impact of Cimetidine, an OCT2 Inhibitor, on the Anticancer Effects of Cisplatin. Cells 2023, 12, 57. https://doi.org/10.3390/cells12010057
Mody H, Vaidya TR, Lesko LJ, Ait-Oudhia S. Pharmacodynamic Modeling to Evaluate the Impact of Cimetidine, an OCT2 Inhibitor, on the Anticancer Effects of Cisplatin. Cells. 2023; 12(1):57. https://doi.org/10.3390/cells12010057
Chicago/Turabian StyleMody, Hardik, Tanaya R. Vaidya, Lawrence J. Lesko, and Sihem Ait-Oudhia. 2023. "Pharmacodynamic Modeling to Evaluate the Impact of Cimetidine, an OCT2 Inhibitor, on the Anticancer Effects of Cisplatin" Cells 12, no. 1: 57. https://doi.org/10.3390/cells12010057
APA StyleMody, H., Vaidya, T. R., Lesko, L. J., & Ait-Oudhia, S. (2023). Pharmacodynamic Modeling to Evaluate the Impact of Cimetidine, an OCT2 Inhibitor, on the Anticancer Effects of Cisplatin. Cells, 12(1), 57. https://doi.org/10.3390/cells12010057





