Human Health during Space Travel: State-of-the-Art Review
Abstract
1. Introduction
1.1. Medical Screening and Certification Prior to Space Travel
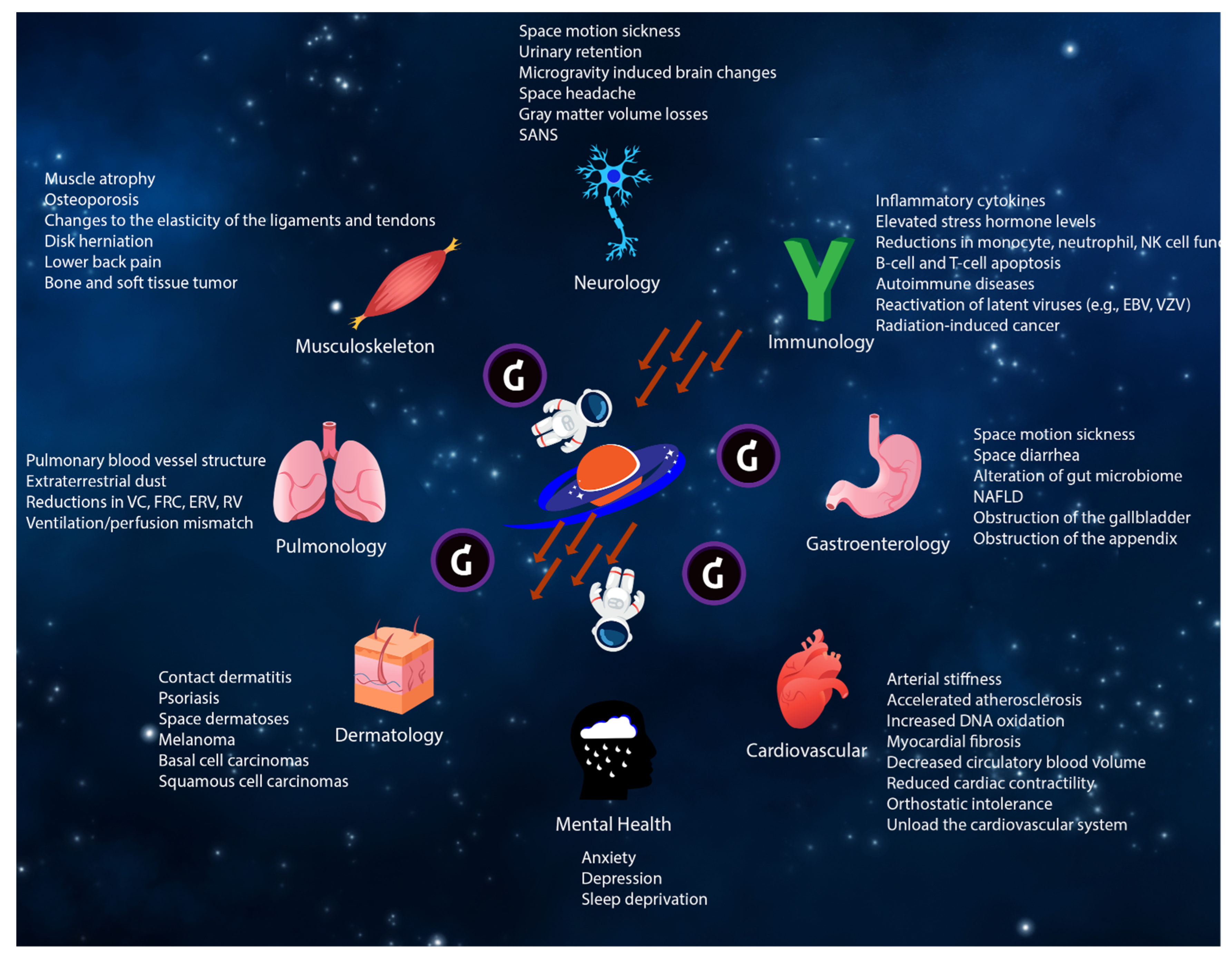
1.2. Effects on the Cardiovascular System
1.3. Effects on the Gastrointestinal System
1.4. Effects on the Immune System
1.5. Effects on the Hematologic System
1.6. Oncologic Effects
1.7. Effects on the Neurologic System
1.7.1. Effects on the Neuro-Ocular System
1.7.2. Effects on the Neuro-Behavioral System
1.8. Effects on the Musculoskeletal System
1.9. Effects on the Pulmonary System
1.10. Effects on the Dermatologic System
1.11. Diagnostic Imaging Modalities in Space
1.12. Medical and Surgical Procedures in Space
1.13. Lifestyle Management in Space
1.14. Future Directions for Precision Space Health with AI
2. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LEO | Low Earth Orbit |
| NASA | National Aeronautics and Space Administration |
| VO2max | Maximal oxygen consumption |
| AI | Artificial intelligence |
| ISS | International Space Station |
| AF | Atrial fibrillation |
| LET | linear energy transfer |
| MRI | Magnetic resonance imaging |
| Gy | Gray (unit) |
| BMI | Body mass index |
| NAFLD | Non-alcoholic fatty liver disease |
| EBV | Epstein-Bar Virus |
| VZV | Varicella-Zoster Virus |
| CMV | Cytomegalovirus |
| NCRP | National Council on Radiation Protection and Measurements |
| SMS | Space motion sickness |
| ICP | Intracranial pressure |
| PFMS | Post-flight motion sickness |
| CNS | Central nervous system |
| SANS | Spaceflight Associated Neuro-Ocular Syndrome |
| IOP | Intraocular pressure |
| FRC | Functional residual capacity |
| VC | Vital capacity |
| ERV | Expiratory reserve volume |
| RV | Residual volume |
| V/Q | Ventilation/perfusion |
| NEEMO | NASA Extreme Environment Missions Operations |
| FAST | Focused Assessment with Sonography for Trauma |
| PET | Positron emission tomography |
| CPR | Cardiopulmonary Resuscitation |
| CR | Caloric restriction |
| TPU | Tensor Processing Unit |
| PHQ-9 | Patient Health Questionnaire-9 |
| GAD-7 | General Anxiety Disorder-7 |
| GCR | Galactic Cosmic Ray |
| DVT | Deep vein thrombosis |
| ASIC | Application-specific integrated circuit |
| TB | Tuberculosis |
| PSA | Prostate-specific antigen |
| DXA | Dual-energy X-ray absorptiometry |
| T4 | Levothyroxine |
| TSH | Thyroid-stimulating hormone |
| GDR | Galactic cosmic radiation |
References
- The Evolving Landscape of 21st Century American Spaceflight. Available online: https://www.nasa.gov/sites/default/files/files/Emerging_Space_Report.pdf (accessed on 2 November 2022).
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, 6436. [Google Scholar] [CrossRef]
- Little, M.P.; Tawn, E.J.; Tzoulaki, I.; Wakeford, R.; Hildebrandt, G.; Paris, F.; Tapio, S.; Elliott, P. Review and meta-analysis of epidemiological associations between low/moderate doses of ionizing radiation and circulatory disease risks, and their possible mechanisms. Radiat. Environ. Biophys. 2010, 49, 139–153. [Google Scholar] [CrossRef]
- Yu, T.; Parks, B.W.; Yu, S.; Srivastava, R.; Gupta, K.; Wu, X.; Khaled, S.; Chang, P.Y.; Kabarowski, J.H.; Kucik, D.F. Iron-ion radiation accelerates atherosclerosis in apolipoprotein E-deficient mice. Radiat. Res. 2011, 175, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 2015, 1, 15013. [Google Scholar] [CrossRef] [PubMed]
- Donaubauer, A.-J.; Deloch, L.; Becker, I.; Fietkau, R.; Frey, B.; Gaipl, U.S. The Influence of Radiation on Bone and Bone Cells-Differential Effects on Osteoclasts and Osteoblasts. Int. J. Mol. Sci. 2020, 21, 6377. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huff, J.; Casey, R.; Kim, M.; Cucinotta, F. Risk of Acute Radiation Syndromes Due to Solar Particle Events. In Human Health and Performance Risks of Space Exploration Missions; NASA SP-2009-3405; NASA Human Research Program: Houston, TX, USA, 2009; pp. 171–190. [Google Scholar]
- Kanas, N. Psychiatric issues affecting long duration space missions. Aviat. Space Environ. Med. 1998, 69, 1211–1216. [Google Scholar] [PubMed]
- Arone, A.; Ivaldi, T.; Loganovsky, T.; Palermo, S.; Parra, E.; Flamini, W.; Marazziti, D. The Burden of Space Exploration on the Mental Health of Astronauts: A Narrative Review. Clin. Neuropsychiatry 2021, 18, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Barratt, M.R.; Baker, E.S.; Pool, S.L. (Eds.) Principal for Clinical Medicine for Space Flight, 2nd ed.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Available online: https://www.nasa.gov/sites/default/files/atoms/files/nasa-std-3001-vol-1a-chg1.pdf (accessed on 2 November 2022).
- Available online: https://lsda.jsc.nasa.gov/lsda_data/document/Project/MRID/MR009S.pdf (accessed on 2 November 2022).
- Astronaut Selection and Training. Available online: https://www.nasa.gov/centers/johnson/pdf/606877main_FS-2011-11-057-JSC-astro_trng.pdf (accessed on 2 November 2022).
- Siconolfi, S.F.; Lemoine, S.L. Graded exercise testing for spaceflight. Aviat. Space Environ. Med. 1995, 66, 435–439. [Google Scholar]
- Hamilton, D.R.; Murray, J.D.; Ball, C.G. Cardiac health for astronauts: Coronary calcification scores and CRP as criteria for selection and retention. Aviat. Space Environ. Med. 2006, 77, 377–387. [Google Scholar]
- Scheuring, R.A.; Mathers, C.H.; Jones, J.A.; Wear, M.L. Musculoskeletal injuries and minor trauma in space: Incidence and injury mechanisms in U.S. astronauts. Aviat. Space Environ. Med. 2009, 80, 117–124. [Google Scholar] [CrossRef]
- Gray, G.W.; Johnston, S.L.; Saary, J.; Cook, T. Medical Evaluations and Standards. In Principles of Clinical Medicine for Space Flight, 2nd ed.; Barratt, M.R., Baker, E.S., Pool, S.L., Eds.; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Lifetime Surveillance of Astronaut Health (LSAH). Available online: https://www.nasa.gov/feature/lifetime-surveillanceof-astronaut-health-lsah (accessed on 2 November 2022).
- Chang, D.G.; Healey, R.M.; Snyder, A.J.; Sayson, J.V.; Macias, B.R.; Coughlin, D.G.; Bailey, J.F.; Parazynski, S.E.; Lotz, J.C.; Hargens, A.R. Lumbar Spine Paraspinal Muscle and Intervertebral Disc Height Changes in Astronauts After Long-Duration Spaceflight on the International Space Station. Spine 2016, 41, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Sibonga, J.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Young, M.; Keyak, J.; Kohri, K.; et al. Resistive exercise in astronauts on prolonged spaceflights provides partial protection against spaceflight-induced bone loss. Bone 2019, 128, 112037. [Google Scholar] [CrossRef]
- Leblanc, A.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Evans, H.; Spector, E.; Ploutz-Snyder, R.; et al. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos. Int. 2013, 24, 2105–2114. [Google Scholar] [CrossRef]
- Roffino, S.; Carnino, A.; Chopard, A.; Mutin, M.; Marini, J.F. Structural remodeling of unweighted soleus myotendinous junction in monkey. Comptes Rendus Biol. 2006, 329, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Carnino, A.; Roffino, S.; Chopard, A.; Marini, J.F. Effects of a 14-day spaceflight on soleus myotendinous junction ultrastructure in the rhesus monkey. J. Gravit. Physiol. J. Int. Soc. Gravit. Physiol. 2000, 7, S65–S68. [Google Scholar]
- Delp, M.D. Unraveling the complex web of impaired wound healing with mechanical unloading and physical deconditioning. J. Appl. Physiol. 2008, 104, 1262–1263. [Google Scholar] [CrossRef] [PubMed]
- Clément, G.; Reschke, M.F. Neuroscience in Space; Springer: New York, NY, USA, 2010. [Google Scholar]
- Cheung, B.; Nakashima, A.M.; Hofer, K.D. Various anti-motion sickness drugs and core body temperature changes. Aviat. Space Environ. Med. 2011, 82, 409–415. [Google Scholar] [CrossRef]
- Vickers, Z.M.; Rice, B.L.; Rose, M.S.; Lane, H.W. Simulated microgravity [bed rest] has little influence on taste, odor or trigeminal sensitivity. J. Sens. Stud. 2001, 16, 23–32. [Google Scholar] [CrossRef]
- Auñón-Chancellor, S.M.; Pattarini, J.M.; Moll, S.; Sargsyan, A. Venous Thrombosis during Spaceflight. N. Engl. J. Med. 2020, 382, 89–90. [Google Scholar] [CrossRef]
- Marshall-Goebel, K.; Laurie, S.S.; Alferova, I.V.; Arbeille, P.; Auñón-Chancellor, S.M.; Ebert, D.J.; Lee, S.M.C.; Macias, B.R.; Martin, D.S.; Pattarini, J.M.; et al. Assessment of Jugular Venous Blood Flow Stasis and Thrombosis During Spaceflight. JAMA Netw. Open 2019, 2, e1915011. [Google Scholar] [CrossRef]
- Reschke, M.F.; Good, E.F.; Clément, G.R. Neurovestibular Symptoms in Astronauts Immediately after Space Shuttle and International Space Station Missions. OTO Open 2017, 1, 2473974x17738767. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Feiveson, A.H.; Sams, C.F.; Pierson, D.L. Latent virus reactivation in astronauts on the international space station. NPJ Microgravity 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Rooney, B.V.; Crucian, B.E.; Pierson, D.L.; Laudenslager, M.L.; Mehta, S.K. Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth. Front. Microbiol. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Makedonas, G.; Mehta, S.; Choukèr, A.; Simpson, R.J.; Marshall, G.; Orange, J.S.; Aunon-Chancellor, S.; Smith, S.M.; Zwart, S.R.; Stowe, R.P.; et al. Specific Immunologic Countermeasure Protocol for Deep-Space Exploration Missions. Front. Immunol. 2019, 10, 2407. [Google Scholar] [CrossRef] [PubMed]
- Cogoli, A. The effect of space flight on human cellular immunity. Environ. Med. Annu. Rep. Res. Inst. Environ. Med. Nagoya Univ. 1993, 37, 107–116. [Google Scholar]
- Turroni, S.; Magnani, M.; KC, P.; Lesnik, P.; Vidal, H.; Heer, M. Gut Microbiome and Space Travelers’ Health: State of the Art and Possible Pro/Prebiotic Strategies for Long-Term Space Missions. Front. Physiol. 2020, 11, 553929. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Hao, Z.; Li, Z.; Sahu, S.K.; Liu, H.; Xiao, L. Relationship between the Gut Microbiome and Energy/Nutrient Intake in a Confined Bioregenerative Life Support System. Appl. Environ. Microbiol. 2020, 86, e02465-19. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Shi, J.; Wang, J.; Hao, J.; Pang, X.; Huang, X.; Chen, X.; Li, Y.; Jin, R.; et al. Intestinal microbiota contributes to altered glucose metabolism in simulated microgravity mouse model. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 10140–10151. [Google Scholar] [CrossRef]
- Menezes, A.A.; Cumbers, J.; Hogan, J.A.; Arkin, A.P. Towards synthetic biological approaches to resource utilization on space missions. J. R. Soc. Interface 2015, 12, 20140715. [Google Scholar] [CrossRef]
- Campbell, M.R.; Johnston, S.L., 3rd; Marshburn, T.; Kane, J.; Lugg, D. Nonoperative treatment of suspected appendicitis in remote medical care environments: Implications for future spaceflight medical care. J. Am. Coll. Surg. 2004, 198, 822–830. [Google Scholar] [CrossRef]
- Ball, C.G.; Kirkpatrick, A.W.; Williams, D.R.; Jones, J.A.; Polk, J.D.; Vanderploeg, J.M.; Talamini, M.A.; Campbell, M.R.; Broderick, T.J. Prophylactic surgery prior to extended-duration space flight: Is the benefit worth the risk? Can. J. Surg. 2012, 55, 125–131. [Google Scholar] [CrossRef][Green Version]
- Buckey, J.C., Jr.; Lane, L.D.; Levine, B.D.; Watenpaugh, D.E.; Wright, S.J.; Moore, W.E.; Gaffney, F.A.; Blomqvist, C.G. Orthostatic intolerance after spaceflight. J. Appl. Physiol. 1996, 81, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Perhonen, M.A.; Franco, F.; Lane, L.D.; Buckey, J.C.; Blomqvist, C.G.; Zerwekh, J.E.; Peshock, R.M.; Weatherall, P.T.; Levine, B.D. Cardiac atrophy after bed rest and spaceflight. J. Appl. Physiol. 2001, 91, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.H.; Gibson, C.R.; Pass, A.F.; Kramer, L.A.; Lee, A.G.; Fogarty, J.; Tarver, W.J.; Dervay, J.P.; Hamilton, D.R.; Sargsyan, A.; et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 2011, 118, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Boyd, M.; Orengo, I. Dermatologic manifestations in spaceflight: A review. Dermatol. Online J. 2018, 24, 11. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Campbell, M.R.; Jones, J.A.; Broderick, T.J.; Ball, C.G.; McBeth, P.B.; McSwain, N.E.; Hamilton, D.R.; Holcomb, J.B. Extraterrestrial hemorrhage control: Terrestrial developments in technique, technology, and philosophy with applicability to traumatic hemorrhage control in long-duration spaceflight. J. Am. Coll. Surg. 2005, 200, 64–76. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Campbell, M.R.; Novinkov, O.L.; Goncharov, I.B.; Kovachevich, I.V. Blunt trauma and operative care in microgravity: A review of microgravity physiology and surgical investigations with implications for critical care and operative treatment in space. J. Am. Coll. Surg. 1997, 184, 441–453. [Google Scholar]
- Kirkpatrick, A.W.; Ball, C.G.; Campbell, M.; Williams, D.R.; Parazynski, S.E.; Mattox, K.L.; Broderick, T.J. Severe traumatic injury during long duration spaceflight: Light years beyond ATLS. J. Trauma Manag. Outcomes 2009, 3, 4. [Google Scholar] [CrossRef]
- Ferrone, K.L.; Guan, F.; Ma, J.; Peterson, L.E.; Willis, C.E.; Kry, S.F. Reducing space radiation cancer risk with magnetic shielding. Adv. Space Res. 2021, 68, 153–160. [Google Scholar] [CrossRef]
- Dobynde, M.I.; Shprits, Y.Y.; Drozdov, A.Y.; Hoffman, J.; Li, J. Beating 1 Sievert: Optimal Radiation Shielding of Astronauts on a Mission to Mars. Space Weather 2021, 19, e2021SW002749. [Google Scholar] [CrossRef]
- Trudel, G.; Shahin, N.; Ramsay, T.; Laneuville, O.; Louati, H. Hemolysis contributes to anemia during long-duration space flight. Nat. Med. 2022, 28, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Trudel, G.; Shafer, J.; Laneuville, O.; Ramsay, T. Characterizing the effect of exposure to microgravity on anemia: More space is worse. Am. J. Hematol. 2020, 95, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Liu, B.; Frost, J.L.; Lemere, C.A.; Williams, J.P.; Olschowka, J.A.; O’Banion, M.K. Galactic cosmic radiation leads to cognitive impairment and increased aβ plaque accumulation in a mouse model of Alzheimer’s disease. PLoS ONE 2012, 7, e53275. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, K.E.; McGregor, H.R.; Reuter-Lorenz, P.A.; Seidler, R.D. Microgravity effects on the human brain and behavior: Dysfunction and adaptive plasticity. Neurosci. Biobehav. Rev. 2021, 122, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Koppelmans, V.; Bloomberg, J.J.; Mulavara, A.P.; Seidler, R.D. Brain structural plasticity with spaceflight. NPJ Microgravity 2016, 2, 2. [Google Scholar] [CrossRef]
- Lee, J.K.; Koppelmans, V.; Riascos, R.F.; Hasan, K.M.; Pasternak, O.; Mulavara, A.P.; Bloomberg, J.J.; Seidler, R.D. Spaceflight-Associated Brain White Matter Microstructural Changes and Intracranial Fluid Redistribution. JAMA Neurol. 2019, 76, 412–419. [Google Scholar] [CrossRef]
- Kramer, L.A.; Hasan, K.M.; Stenger, M.B.; Sargsyan, A.; Laurie, S.S.; Otto, C.; Ploutz-Snyder, R.J.; Marshall-Goebel, K.; Riascos, R.F.; Macias, B.R. Intracranial Effects of Microgravity: A Prospective Longitudinal MRI Study. Radiology 2020, 295, 640–648. [Google Scholar] [CrossRef]
- Baselet, B.; Rombouts, C.; Benotmane, A.M.; Baatout, S.; Aerts, A. Cardiovascular diseases related to ionizing radiation: The risk of low-dose exposure (Review). Int. J. Mol. Med. 2016, 38, 1623–1641. [Google Scholar] [CrossRef]
- Hendry, J.H.; Akahoshi, M.; Wang, L.S.; Lipshultz, S.E.; Stewart, F.A.; Trott, K.R. Radiation-induced cardiovascular injury. Radiat. Environ. Biophys. 2008, 47, 189–193. [Google Scholar] [CrossRef]
- Soucy, K.G.; Lim, H.K.; Kim, J.H.; Oh, Y.; Attarzadeh, D.O.; Sevinc, B.; Kuo, M.M.; Shoukas, A.A.; Vazquez, M.E.; Berkowitz, D.E. HZE ⁵⁶Fe-ion irradiation induces endothelial dysfunction in rat aorta: Role of xanthine oxidase. Radiat. Res. 2011, 176, 474–485. [Google Scholar] [CrossRef]
- Yan, X.; Sasi, S.P.; Gee, H.; Lee, J.; Yang, Y.; Mehrzad, R.; Onufrak, J.; Song, J.; Enderling, H.; Agarwal, A.; et al. Cardiovascular risks associated with low dose ionizing particle radiation. PLoS ONE 2014, 9, e110269. [Google Scholar] [CrossRef] [PubMed]
- Lipman, R.M.; Tripathi, B.J.; Tripathi, R.C. Cataracts induced by microwave and ionizing radiation. Surv. Ophthalmol. 1988, 33, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Ainsbury, E.A.; Barnard, S.; Bright, S.; Dalke, C.; Jarrin, M.; Kunze, S.; Tanner, R.; Dynlacht, J.R.; Quinlan, R.A.; Graw, J.; et al. Ionizing radiation induced cataracts: Recent biological and mechanistic developments and perspectives for future research. Mutat. Research. Rev. Mutat. Res. 2016, 770, 238–261. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.M.; Powell, D.R.; Li, Z.; Bell, J.S.K.; Barwick, B.G.; Feng, H.; McCrary, M.R.; Dwivedi, B.; Kowalski, J.; Dynan, W.S.; et al. Galactic Cosmic Radiation Induces Persistent Epigenome Alterations Relevant to Human Lung Cancer. Sci. Rep. 2018, 8, 6709. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.nasa.gov/sites/default/files/human-adaptation-to-spaceflight-the-role-of-nutrition.pdf (accessed on 2 November 2022).
- Nicogossian, A.E.; Williams, R.S.; Huntoon, C.L.; Doarn, C.R.; Polk, J.D.; Schneider, V.S. Space Physiology and Medicine: From Evidence to Practice; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Kiecolt-Glaser, J.K.; Christian, L.; Preston, H.; Houts, C.R.; Malarkey, W.B.; Emery, C.F.; Glaser, R. Stress, inflammation, and yoga practice. Psychosom. Med. 2010, 72, 113–121. [Google Scholar] [CrossRef]
- Available online: https://www.psychologytoday.com/us/blog/home-in-the-cosmos/201902/astronauts-open-about-depression-and-isolation-in-space (accessed on 2 November 2022).
- Gavrilescu, M.; Vizireanu, N. Predicting Depression, Anxiety, and Stress Levels from Videos Using the Facial Action Coding System. Sensors 2019, 19, 3693. [Google Scholar] [CrossRef]
- Available online: https://www.forbes.com/sites/ganeskesari/2021/05/24/ai-can-now-detect-depression-from-just-your-voice/?sh=19ba3e364c8d (accessed on 2 November 2022).
- Available online: https://www.nasa.gov/feature/goddard/2021/lunanet-empowering-artemis-with-communications-and-navigation-interoperability (accessed on 2 November 2022).
- Barger, L.K.; Flynn-Evans, E.E.; Kubey, A.; Walsh, L.; Ronda, J.M.; Wang, W.; Wright, K.P., Jr.; Czeisler, C.A. Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: An observational study. Lancet Neurol. 2014, 13, 904–912. [Google Scholar] [CrossRef]
- Dijk, D.J.; Neri, D.F.; Wyatt, J.K.; Ronda, J.M.; Riel, E.; Ritz-De Cecco, A.; Hughes, R.J.; Elliott, A.R.; Prisk, G.K.; West, J.B.; et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2001, 281, R1647–R1664. [Google Scholar] [CrossRef]
- Prisk, G.K. Pulmonary challenges of prolonged journeys to space: Taking your lungs to the moon. Med. J. Aust. 2019, 211, 271–276. [Google Scholar] [CrossRef]
- Roller, C.A.; Clark, J.B. Short-duration space flight and hearing loss. Otolaryngol.–Head Neck Surg. 2003, 129, 98–106. [Google Scholar] [CrossRef]
- Taylor, P.W. Impact of space flight on bacterial virulence and antibiotic susceptibility. Infect. Drug Resist. 2015, 8, 249–262. [Google Scholar] [CrossRef]
- Bijlani, S.; Singh, N.K.; Eedara, V.V.R.; Podile, A.R.; Mason, C.E.; Wang, C.C.C.; Venkateswaran, K. Methylobacterium ajmalii sp. nov., Isolated From the International Space Station. Front. Microbiol. 2021, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Makedonas, G.; Mehta, S.K.; Scheuring, R.A.; Haddon, R.; Crucian, B.E. SARS-CoV-2 Pandemic Impacts on NASA Ground Operations to Protect ISS Astronauts. J. Allergy Clin. Immunol. Pract. 2020, 8, 3247–3250. [Google Scholar] [CrossRef] [PubMed]
- George, K.; Rhone, J.; Beitman, A.; Cucinotta, F.A. Cytogenetic damage in the blood lymphocytes of astronauts: Effects of repeat long-duration space missions. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2013, 756, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Rothfuss, A.; Steger-Hartmann, T.; Heinrich, N.; Wichard, J. Computational prediction of the chromosome-damaging potential of chemicals. Chem. Res. Toxicol. 2006, 19, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.; Chakrabarti, S. Artificial Intelligence (AI)-Based Systems Biology Approaches in Multi-Omics Data Analysis of Cancer. Front. Oncol. 2020, 10, 588221. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Hu, M.J.; Cui, Y.J.; Liang, L.; Zhou, M.M.; Yang, Y.W.; Huang, F. Carotid-Femoral Pulse Wave Velocity in the Prediction of Cardiovascular Events and Mortality: An Updated Systematic Review and Meta-Analysis. Angiology 2018, 69, 617–629. [Google Scholar] [CrossRef]
- Khera, A.; Budoff, M.J.; O’Donnell, C.J.; Ayers, C.A.; Locke, J.; de Lemos, J.A.; Massaro, J.M.; McClelland, R.L.; Taylor, A.; Levine, B.D. Astronaut Cardiovascular Health and Risk Modification (Astro-CHARM) Coronary Calcium Atherosclerotic Cardiovascular Disease Risk Calculator. Circulation 2018, 138, 1819–1827. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, D.; Lu, H.; Sun, J.; Lv, L.; Li, S.; Peng, G.; Ma, X.; Li, J.; Li, Z.; et al. Evaluation of Machine Learning Models for Predicting Antimicrobial Resistance of Actinobacillus pleuropneumoniae From Whole Genome Sequences. Front. Microbiol. 2020, 11, 48. [Google Scholar] [CrossRef]
- Budd, S.; Blaas, A.; Hoarfrost, A.; Khezeli, K.; Silva, K.D.; Soboczenski, F.; Mackintosh, G.; Chia, N.; Kalantari, J. Prototyping CRISP: A Causal Relation and Inference Search Platform applied to Colorectal Cancer Data. In Proceedings of the 2021 IEEE 3rd Global Conference on Life Sciences and Technologies (LifeTech), Nara, Japan, 9–11 March 2021; pp. 517–521. [Google Scholar]
- Aryal, S.; Alimadadi, A.; Manandhar, I.; Joe, B.; Cheng, X. Machine Learning Strategy for Gut Microbiome-Based Diagnostic Screening of Cardiovascular Disease. Hypertension 2020, 76, 1555–1562. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Ahern, A.; Carbone, C.; Temko, A.; Claesson, M.J.; Gasbarrini, A.; Tortora, G. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Estévez, O.; Anibarro, L.; Garet, E.; Pallares, Á.; Barcia, L.; Calviño, L.; Maueia, C.; Mussá, T.; Fdez-Riverola, F.; Glez-Peña, D.; et al. An RNA-seq Based Machine Learning Approach Identifies Latent Tuberculosis Patients With an Active Tuberculosis Profile. Front. Immunol. 2020, 11, 1470. [Google Scholar] [CrossRef]
- Andriasyan, V.; Yakimovich, A.; Petkidis, A.; Georgi, F.; Witte, R.; Puntener, D.; Greber, U.F. Microscopy deep learning predicts virus infections and reveals mechanics of lytic-infected cells. iScience 2021, 24, 102543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, Y.; Aouizerat, B.E.; Peng, G.; Marconi, V.C.; Corley, M.J.; Hulgan, T.; Bryant, K.J.; Zhao, H.; Krystal, J.H.; et al. Machine learning selected smoking-associated DNA methylation signatures that predict HIV prognosis and mortality. Clin. Epigenet. 2018, 10, 155. [Google Scholar] [CrossRef]
- Kist, A. Deep Learning on Edge TPUs. arXiv 2021, arXiv:2108.13732. [Google Scholar]
- Bizzi, A.; Pascuzzo, R.; Blevins, J.; Moscatelli, M.E.M.; Grisoli, M.; Lodi, R.; Doniselli, F.M.; Castelli, G.; Cohen, M.L.; Stamm, A.; et al. Subtype Diagnosis of Sporadic Creutzfeldt-Jakob Disease with Diffusion Magnetic Resonance Imaging. Ann. Neurol. 2021, 89, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Chuang, K.V.; DeCarli, C.; Jin, L.-W.; Beckett, L.; Keiser, M.J.; Dugger, B.N. Interpretable classification of Alzheimer’s disease pathologies with a convolutional neural network pipeline. Nat. Commun. 2019, 10, 2173. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Y.; Wang, Y.K.; Chen, H.P.; Gao, K.L.; Shu, C.; Wang, J.C.; Yan, L.F.; Yang, Y.G.; Xie, F.Y.; Liu, J. A Deep Learning Based Framework for Diagnosing Multiple Skin Diseases in a Clinical Environment. Front. Med. 2021, 8, 626369. [Google Scholar] [CrossRef]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. Jama 2016, 316, 2402–2410. [Google Scholar] [CrossRef]
- Toscano, W. Wearable Biosensor Monitor to Support Autonomous Crew Health and Readinessto Perform. 2017. Available online: https://ntrs.nasa.gov/citations/20190001996 (accessed on 2 November 2022).
- Castaldo, R.; Chappell, M.J.; Byrne, H.; Innominato, P.F.; Hughes, S.; Pescapè, A.; Pecchia, L. Detection of melatonin-onset in real settings via wearable sensors and artificial intelligence. A pilot study. Biomed. Signal Process. Control 2021, 65, 102386. [Google Scholar] [CrossRef]
- Cogswell, D.; Bisesi, P.; Markwald, R.R.; Cruickshank-Quinn, C.; Quinn, K.; McHill, A.; Melanson, E.L.; Reisdorph, N.; Wright, K.P., Jr.; Depner, C.M. Identification of a Preliminary Plasma Metabolome-based Biomarker for Circadian Phase in Humans. J. Biol. Rhythm. 2021, 36, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, E.; Ong, J.; Zaman, N.; Kamran, S.A.; Lee, A.G.; Tavakkoli, A. A non-invasive approach to monitor anemia during long-duration spaceflight with retinal fundus images and deep learning. Life Sci. Space Res. 2022, 33, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Kist, A.M.; Döllinger, M. Efficient Biomedical Image Segmentation on EdgeTPUs at Point of Care. IEEE Access 2020, 8, 139356–139366. [Google Scholar] [CrossRef]
- Hlushchuk, R.; Zubler, C.; Barré, S.; Correa Shokiche, C.; Schaad, L.; Röthlisberger, R.; Wnuk, M.; Daniel, C.; Khoma, O.; Tschanz, S.A.; et al. Cutting-edge microangio-CT: New dimensions in vascular imaging and kidney morphometry. Am. J. Physiol. Ren. Physiol. 2018, 314, F493–F499. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, J. NASA SpaceCube Edge TPU SmallSat Card for Autonomous Operations and Onboard Science-Data Analysis. In Proceedings of the Small Satellite Conference, Logan, UT, USA, 7–12 August 2021. [Google Scholar]
- Broderick, T.J.; Privitera, M.B.; Parazynski, S.E.; Cuttino, M. Simulated hand-assisted laparoscopic surgery (HALS) in microgravity. J. Laparoendosc. Adv. Surg. Tech. Part A 2005, 15, 145–148. [Google Scholar] [CrossRef]
- Tabaza, L.; Virk, H.u.H.; Janzer, S.; George, J.C. Robotic-assisted percutaneous coronary intervention in a COVID-19 patient. Catheter. Cardiovasc. Interv. 2021, 97, E343–E345. [Google Scholar] [CrossRef]
- Kane, W.J.; Charles, E.J.; Mehaffey, J.H.; Hawkins, R.B.; Meneses, K.B.; Tache-Leon, C.A.; Yang, Z. Robotic compared with laparoscopic cholecystectomy: A propensity matched analysis. Surgery 2020, 167, 432–435. [Google Scholar] [CrossRef]
- Garrow, C.R.; Kowalewski, K.F.; Li, L.; Wagner, M.; Schmidt, M.W.; Engelhardt, S.; Hashimoto, D.A.; Kenngott, H.G.; Bodenstedt, S.; Speidel, S.; et al. Machine Learning for Surgical Phase Recognition: A Systematic Review. Ann. Surg. 2021, 273, 684–693. [Google Scholar] [CrossRef]
- Fourati, F.; Alouini, M.-S. Artificial Intelligence for Satellite Communication: A Review. arXiv 2012, arXiv:2101.10899. [Google Scholar] [CrossRef]
- Chowell, D.; Yoo, S.-K.; Valero, C.; Pastore, A.; Krishna, C.; Lee, M.; Hoen, D.; Shi, H.; Kelly, D.W.; Patel, N.; et al. Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Westfall, S.; Carracci, F.; Estill, M.; Zhao, D.; Wu, Q.-l.; Shen, L.; Simon, J.; Pasinetti, G.M. Optimization of probiotic therapeutics using machine learning in an artificial human gastrointestinal tract. Sci. Rep. 2021, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://oig.nasa.gov/docs/IG-21-025.pdf (accessed on 2 November 2022).
- Matar, M.; Gokoglu, S.A.; Prelich, M.T.; Gallo, C.A.; Iqbal, A.K.; Britten, R.A.; Prabhu, R.K.; Myers, J.G., Jr. Machine Learning Models to Predict Cognitive Impairment of Rodents Subjected to Space Radiation. Front. Syst. Neurosci. 2021, 15, 713131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ng, D.W.H.; Park, H.S.; McAlpine, M.C. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat. Rev. Mater. 2021, 6, 27–47. [Google Scholar] [CrossRef]
- Available online: https://www.nasa.gov/sites/default/files/files/3D_Printing-v3.pdf (accessed on 2 November 2022).
- Galib, S.M.; Bhowmik, P.K.; Avachat, A.V.; Lee, H.K. A comparative study of machine learning methods for automated identification of radioisotopes using NaI gamma-ray spectra. Nucl. Eng. Technol. 2021, 53, 4072–4079. [Google Scholar] [CrossRef]
- Israel, D.J.; Mauldin, K.D.; Roberts, C.J.; Mitchell, J.W.; Pulkkinen, A.A.; Cooper, L.V.D.; Johnson, M.A.; Christe, S.D.; Gramling, C.J. LunaNet: A Flexible and Extensible Lunar Exploration Communications and Navigation Infrastructure. In Proceedings of the 2020 IEEE Aerospace Conference, Big Sky, MT, USA, 7–14 March 2020; pp. 1–14. [Google Scholar]
- Recenti, M.; Ricciardi, C.; Aubonnet, R.; Picone, I.; Jacob, D.; Svansson, H.Á.R.; Agnarsdóttir, S.; Karlsson, G.H.; Baeringsdóttir, V.; Petersen, H.; et al. Toward Predicting Motion Sickness Using Virtual Reality and a Moving Platform Assessing Brain, Muscles, and Heart Signals. Front. Bioeng. Biotechnol. 2021, 9, 635661. [Google Scholar] [CrossRef]
- O’Connell, M. Preventing and treating motion sickness using virtual reality. In Proceedings of the 43rd COSPAR Scientific Assembly, Sydney, Australia, 28 January–4 February 2021; p. 2071. [Google Scholar]
- Available online: https://ntrs.nasa.gov/citations/20130012776 (accessed on 2 November 2022).
- Lewis, J.E.; Kemp, M.L. Integration of machine learning and genome-scale metabolic modeling identifies multi-omics biomarkers for radiation resistance. Nat. Commun. 2021, 12, 2700. [Google Scholar] [CrossRef]
- Kim, E.; Chung, Y. Feasibility study of deep learning based radiosensitivity prediction model of National Cancer Institute-60 cell lines using gene expression. Nucl. Eng. Technol. 2021, 56, 103156. [Google Scholar] [CrossRef]
- Belli, M.; Tabocchini, M.A. Ionizing Radiation-Induced Epigenetic Modifications and Their Relevance to Radiation Protection. Int. J. Mol. Sci. 2020, 21, 5993. [Google Scholar] [CrossRef]
- Available online: https://www.4bridgeworks.com/interview-using-ai-to-revolutionise-data-acceleration/ (accessed on 2 November 2022).
- Patel, S. The effects of microgravity and space radiation on cardiovascular health: From low-Earth orbit and beyond. IJC Heart Vasc. 2020, 30, 100595. [Google Scholar] [CrossRef]
- Levine, B.D.; Lane, L.D.; Watenpaugh, D.E.; Gaffney, F.A.; Buckey, J.C.; Blomqvist, C.G. Maximal exercise performance after adaptation to microgravity. J. Appl. Physiol. 1996, 81, 686–694. [Google Scholar] [CrossRef]
- Watenpaugh, D.E.; Hargens, A.R. The Cardiovascular System in Microgravity. In Comprehensive Physiology; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 631–674. [Google Scholar]
- Norsk, P. Blood pressure regulation IV: Adaptive responses to weightlessness. Eur. J. Appl. Physiol. 2014, 114, 481–497. [Google Scholar] [CrossRef]
- Ertl, A.C.; Diedrich, A.; Biaggioni, I.; Levine, B.D.; Robertson, R.M.; Cox, J.F.; Zuckerman, J.H.; Pawelczyk, J.A.; Ray, C.A.; Buckey, J.C., Jr.; et al. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. J. Physiol. 2002, 538, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Shibata, S.; Hastings, J.L.; Platts, S.H.; Hamilton, D.M.; Bungo, M.W.; Stenger, M.B.; Ribeiro, C.; Adams-Huet, B.; Levine, B.D. Impact of Prolonged Spaceflight on Orthostatic Tolerance During Ambulation and Blood Pressure Profiles in Astronauts. Circulation 2019, 140, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Khine, H.W.; Steding-Ehrenborg, K.; Hastings, J.L.; Kowal, J.; Daniels, J.D.; Page, R.L.; Goldberger, J.J.; Ng, J.; Adams-Huet, B.; Bungo, M.W.; et al. Effects of Prolonged Spaceflight on Atrial Size, Atrial Electrophysiology, and Risk of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2018, 11, e005959. [Google Scholar] [CrossRef] [PubMed]
- Wuu, Y.R.; Hu, B.; Okunola, H.; Paul, A.M.; Blaber, E.A.; Cheng-Campbell, M.; Beheshti, A.; Grabham, P. LET-Dependent Low Dose and Synergistic Inhibition of Human Angiogenesis by Charged Particles: Validation of miRNAs that Drive Inhibition. iScience 2020, 23, 101771. [Google Scholar] [CrossRef]
- Vernice, N.A.; Meydan, C.; Afshinnekoo, E.; Mason, C.E. Long-term spaceflight and the cardiovascular system. Precis. Clin. Med. 2020, 3, 284–291. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Ethier, J.L.; Lee, D.S.; Thavendiranathan, P.; Amir, E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 53, 120–127. [Google Scholar] [CrossRef]
- Shrestha, S.; Bates, J.E.; Liu, Q.; Smith, S.A.; Oeffinger, K.C.; Chow, E.J.; Gupta, A.C.; Owens, C.A.; Constine, L.S.; Hoppe, B.S.; et al. Radiation therapy related cardiac disease risk in childhood cancer survivors: Updated dosimetry analysis from the Childhood Cancer Survivor Study. Radiother. Oncol. 2021, 163, 199–208. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kodama, K.; Nishi, N.; Kasagi, F.; Suyama, A.; Soda, M.; Grant, E.J.; Sugiyama, H.; Sakata, R.; Moriwaki, H.; et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ 2010, 340, b5349. [Google Scholar] [CrossRef]
- Killander, F.; Wieslander, E.; Karlsson, P.; Holmberg, E.; Lundstedt, D.; Holmberg, L.; Werner, L.; Koul, S.; Haghanegi, M.; Kjellen, E.; et al. No Increased Cardiac Mortality or Morbidity of Radiation Therapy in Breast Cancer Patients After Breast-Conserving Surgery: 20-Year Follow-up of the Randomized SweBCGRT Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.; Babiak-Vazquez, A.; Johnston, S.; Pierson, D.L.; Ott, C.M.; Sams, C. Incidence of clinical symptoms during long-duration orbital spaceflight. Int. J. Gen. Med. 2016, 9, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Thornton, W.E.; Linder, B.J.; Moore, T.P.; Pool, S.L. Gastrointestinal motility in space motion sickness. Aviat. Space Environ. Med. 1987, 58, A16–A21. [Google Scholar] [PubMed]
- Bae, J.H.; Kim, J.G.; Heo, K.; Yang, K.; Kim, T.O.; Yi, J.M. Identification of radiation-induced aberrant hypomethylation in colon cancer. BMC Genom. 2015, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.C.; Slaba, T.C.; Guida, P.; Rusek, A. NASA’s first ground-based Galactic Cosmic Ray Simulator: Enabling a new era in space radiobiology research. PLoS Biol. 2020, 18, e3000669. [Google Scholar] [CrossRef] [PubMed]
- Voorhies, A.A.; Mark Ott, C.; Mehta, S.; Pierson, D.L.; Crucian, B.E.; Feiveson, A.; Oubre, C.M.; Torralba, M.; Moncera, K.; Zhang, Y.; et al. Study of the impact of long-duration space missions at the International Space Station on the astronaut microbiome. Sci. Rep. 2019, 9, 9911. [Google Scholar] [CrossRef]
- Shao, D.; Yao, L.; Riaz, M.S.; Zhu, J.; Shi, J.; Jin, M.; Huang, Q.; Yang, H. Simulated microgravity affects some biological characteristics of Lactobacillus acidophilus. Appl. Microbiol. Biotechnol. 2017, 101, 3439–3449. [Google Scholar] [CrossRef]
- Benoit, M.R.; Li, W.; Stodieck, L.S.; Lam, K.S.; Winther, C.L.; Roane, T.M.; Klaus, D.M. Microbial antibiotic production aboard the International Space Station. Appl. Microbiol. Biotechnol. 2006, 70, 403–411. [Google Scholar] [CrossRef]
- Jiang, P.; Green, S.J.; Chlipala, G.E.; Turek, F.W.; Vitaterna, M.H. Reproducible changes in the gut microbiome suggest a shift in microbial and host metabolism during spaceflight. Microbiome 2019, 7, 113. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Hamilton, D.R.; McKee, J.L.; MacDonald, B.; Pelosi, P.; Ball, C.G.; Roberts, D.; McBeth, P.B.; Cocolini, F.; Ansaloni, L.; et al. Do we have the guts to go? The abdominal compartment, intra-abdominal hypertension, the human microbiome and exploration class space missions. Can. J. Surg. 2020, 63, E581–E593. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Meydan, C.; Schmidt, C.M.; Afshinnekoo, E.; Mason, C.E. The NASA Twins Study: The Effect of One Year in Space on Long-Chain Fatty Acid Desaturases and Elongases. Lifestyle Genom. 2020, 13, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, A.; Chakravarty, K.; Fogle, H.; Fazelinia, H.; Silveira, W.A.D.; Boyko, V.; Polo, S.L.; Saravia-Butler, A.M.; Hardiman, G.; Taylor, D.; et al. Multi-omics analysis of multiple missions to space reveal a theme of lipid dysregulation in mouse liver. Sci. Rep. 2019, 9, 19195. [Google Scholar] [CrossRef] [PubMed]
- Jonscher, K.R.; Alfonso-Garcia, A.; Suhalim, J.L.; Orlicky, D.J.; Potma, E.O.; Ferguson, V.L.; Bouxsein, M.L.; Bateman, T.A.; Stodieck, L.S.; Levi, M.; et al. Spaceflight Activates Lipotoxic Pathways in Mouse Liver. PLoS ONE 2016, 11, e0152877. [Google Scholar] [CrossRef]
- Krieger, S.S.; Zwart, S.R.; Mehta, S.; Wu, H.; Simpson, R.J.; Smith, S.M.; Crucian, B. Alterations in Saliva and Plasma Cytokine Concentrations During Long-Duration Spaceflight. Front. Immunol. 2021, 12, 725748. [Google Scholar] [CrossRef]
- Crucian, B.E.; Zwart, S.R.; Mehta, S.; Uchakin, P.; Quiriarte, H.D.; Pierson, D.; Sams, C.F.; Smith, S.M. Plasma cytokine concentrations indicate that in vivo hormonal regulation of immunity is altered during long-duration spaceflight. J. Interferon Cytokine Res. 2014, 34, 778–786. [Google Scholar] [CrossRef]
- Stowe, R.P.; Sams, C.F.; Pierson, D.L. Effects of mission duration on neuroimmune responses in astronauts. Aviat. Space Environ. Med. 2003, 74, 1281–1284. [Google Scholar]
- Buravkova, L.B.; Rykova, M.P.; Grigorieva, V.; Antropova, E.N. Cell interactions in microgravity: Cytotoxic effects of natural killer cells in vitro. J. Gravit. Physiol. A J. Int. Soc. Gravit. Physiol. 2004, 11, P177–P180. [Google Scholar]
- Bigley, A.B.; Agha, N.H.; Baker, F.L.; Spielmann, G.; Kunz, H.E.; Mylabathula, P.L.; Rooney, B.V.; Laughlin, M.S.; Mehta, S.K.; Pierson, D.L.; et al. NK cell function is impaired during long-duration spaceflight. J. Appl. Physiol. 2019, 126, 842–853. [Google Scholar] [CrossRef]
- Spielmann, G.; Agha, N.; Kunz, H.; Simpson, R.J.; Crucian, B.; Mehta, S.; Laughlin, M.; Campbell, J. B cell homeostasis is maintained during long-duration spaceflight. J. Appl. Physiol. 2019, 126, 469–476. [Google Scholar] [CrossRef]
- Kaur, I.; Simons, E.R.; Castro, V.A.; Ott, C.M.; Pierson, D.L. Changes in monocyte functions of astronauts. Brain Behav. Immun. 2005, 19, 547–554. [Google Scholar] [CrossRef]
- Schwarzenberg, M.; Pippia, P.; Meloni, M.A.; Cossu, G.; Cogoli-Greuter, M.; Cogoli, A. Signal transduction in T lymphocytes—A comparison of the data from space, the free fall machine and the random positioning machine. Adv. Space Res. 1999, 24, 793–800. [Google Scholar] [CrossRef]
- Stowe, R.P.; Sams, C.F.; Mehta, S.K.; Kaur, I.; Jones, M.L.; Feeback, D.L.; Pierson, D.L. Leukocyte subsets and neutrophil function after short-term spaceflight. J. Leukoc. Biol. 1999, 65, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Grove, D.S.; Pishak, S.A.; Mastro, A.M. The effect of a 10-day space flight on the function, phenotype, and adhesion molecule expression of splenocytes and lymph node lymphocytes. Exp. Cell Res. 1995, 219, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.V.; Konstantinova, I.V.; Fuchs, B.B.; Rakhmilevich, A.L.; Lesnyak, A.T.; Mastro, A.M. Effect of spaceflight on lymphocyte proliferation and interleukin-2 production. J. Appl. Physiol. 1992, 73, 186S–190S. [Google Scholar] [CrossRef] [PubMed]
- Stahn, A.C.; Werner, A.; Opatz, O.; Maggioni, M.A.; Steinach, M.; von Ahlefeld, V.W.; Moore, A.; Crucian, B.E.; Smith, S.M.; Zwart, S.R.; et al. Increased core body temperature in astronauts during long-duration space missions. Sci. Rep. 2017, 7, 16180. [Google Scholar] [CrossRef]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Crucian, B.E.; Makedonas, G.; Sams, C.F.; Pierson, D.L.; Simpson, R.; Stowe, R.P.; Smith, S.M.; Zwart, S.R.; Krieger, S.S.; Rooney, B.; et al. Countermeasures-based Improvements in Stress, Immune System Dysregulation and Latent Herpesvirus Reactivation onboard the International Space Station—Relevance for Deep Space Missions and Terrestrial Medicine. Neurosci. Biobehav. Rev. 2020, 115, 68–76. [Google Scholar] [CrossRef]
- Rodman, C.; Almeida-Porada, G.; George, S.K.; Moon, J.; Soker, S.; Pardee, T.; Beaty, M.; Guida, P.; Sajuthi, S.P.; Langefeld, C.D.; et al. In vitro and in vivo assessment of direct effects of simulated solar and galactic cosmic radiation on human hematopoietic stem/progenitor cells. Leukemia 2017, 31, 1398–1407. [Google Scholar] [CrossRef]
- Crucian, B.; Johnston, S.; Mehta, S.; Stowe, R.; Uchakin, P.; Quiriarte, H.; Pierson, D.; Laudenslager, M.L.; Sams, C. A case of persistent skin rash and rhinitis with immune system dysregulation onboard the International Space Station. J. Allergy Clin. Immunol. Pract. 2016, 4, 759–762.e758. [Google Scholar] [CrossRef]
- Nickerson, C.A.; Ott, C.M.; Wilson, J.W.; Ramamurthy, R.; Pierson, D.L. Microbial responses to microgravity and other low-shear environments. Microbiol. Mol. Biol. Rev. 2004, 68, 345–361. [Google Scholar] [CrossRef]
- Nickerson, C.A.; Ott, C.M.; Mister, S.J.; Morrow, B.J.; Burns-Keliher, L.; Pierson, D.L. Microgravity as a novel environmental signal affecting Salmonella enterica serovar Typhimurium virulence. Infect. Immun. 2000, 68, 3147–3152. [Google Scholar] [CrossRef]
- Alfrey, C.P.; Udden, M.M.; Huntoon, C.L.; Driscoll, T. Destruction of newly released red blood cells in space flight. Med. Sci. Sport. Exerc. 1996, 28, S42–S44. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Red blood cell and iron metabolism during space flight. Nutrition 2002, 18, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.; Quiriarte, H.; Simpson, R.J.; Ploutz-Snyder, R.; McMonigal, K.; Sams, C.; Crucian, B. Alterations in hematologic indices during long-duration spaceflight. BMC Hematol. 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.L.; Ron, E.; Tokuoka, S.; Funamoto, S.; Nishi, N.; Soda, M.; Mabuchi, K.; Kodama, K. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat. Res. 2007, 168, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Cardis, E.; Krewski, D.; Boniol, M.; Drozdovitch, V.; Darby, S.C.; Gilbert, E.S.; Akiba, S.; Benichou, J.; Ferlay, J.; Gandini, S.; et al. Estimates of the cancer burden in Europe from radioactive fallout from the Chernobyl accident. Int. J. Cancer 2006, 119, 1224–1235. [Google Scholar] [CrossRef]
- National Council on Radiation Protection and Measurements. Uncertainties in Fatal Cancer Risk Estimates Used in Radiation Protection; NCRP Report 126; National Council on Radiation Protection and Measurements: Bethesda, MD, USA, 1997. [Google Scholar]
- Cullings, H.M.; Pierce, D.A.; Kellerer, A.M. Accounting for neutron exposure in the Japanese atomic bomb survivors. Radiat. Res. 2014, 182, 587–598. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Golge, S.; Slaba, T.C. NASA Galactic Cosmic Radiation Environment Model: Badhwar-O’Neill (2014). In Proceedings of the International Cosmic Ray Conference (ICRC), Moscow, Russia, 25–29 August 2016. [Google Scholar]
- Cucinotta, F.A.; Kim, M.-H.Y.; Chappell, L.J.; Huff, J.L. How Safe Is Safe Enough? Radiation Risk for a Human Mission to Mars. PLoS ONE 2013, 8, e74988. [Google Scholar] [CrossRef]
- Tubiana, M. Prevention of cancer and the dose-effect relationship: The carcinogenic effects of ionizing radiations. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2009, 13, 238–258. [Google Scholar] [CrossRef]
- Council, N.R. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; The National Academies Press: Washington, DC, USA, 2006; p. 422. [Google Scholar]
- Heuskin, A.C.; Osseiran, A.I.; Tang, J.; Costes, S.V. Simulating Space Radiation-Induced Breast Tumor Incidence Using Automata. Radiat. Res. 2016, 186, 27–38. [Google Scholar] [CrossRef]
- Suman, S.; Kumar, S.; Fornace, A.J.; Datta, K. Space radiation exposure persistently increased leptin and IGF1 in serum and activated leptin-IGF1 signaling axis in mouse intestine. Sci. Rep. 2016, 6, 31853. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Brown, N.; Finnon, R.; Warner, C.L.; Liu, X.; Genik, P.C.; Callan, M.A.; Ray, F.A.; Borak, T.B.; Badie, C.; et al. Radiation leukemogenesis in mice: Loss of PU.1 on chromosome 2 in CBA and C57BL/6 mice after irradiation with 1 GeV/nucleon 56Fe ions, X rays or gamma rays. Part I. Experimental observations. Radiat. Res. 2009, 171, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Ogiu, T.; Nishizaki, M.; Fujimoto, N.; Kido, S.; Ishimura, Y.; Shiraki, K.; Kuramoto, K.; Hirata, S.; Shoji, S.; et al. Induction of ovarian tumors by heavy ion irradiation in B6C3F1 mice. Oncol. Rep. 1998, 5, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt-Ohmann, H.; Genik, P.C.; Fallgren, C.M.; Ullrich, R.L.; Weil, M.M. Animal studies of charged particle-induced carcinogenesis. Health Phys. 2012, 103, 568–576. [Google Scholar] [CrossRef]
- Lahtz, C.; Bates, S.E.; Jiang, Y.; Li, A.X.; Wu, X.; Hahn, M.A.; Pfeifer, G.P. Gamma irradiation does not induce detectable changes in DNA methylation directly following exposure of human cells. PLoS ONE 2012, 7, e44858. [Google Scholar] [CrossRef]
- Borak, T.B.; Heilbronn, L.H.; Townsend, L.W.; McBeth, R.A.; de Wet, W. Quality factors for space radiation: A new approach. Life Sci. Space Res. 2014, 1, 96–102. [Google Scholar] [CrossRef]
- Available online: https://www.nationalacademies.org/news/2021/06/nasa-should-update-astronaut-radiation-exposure-limits-improve-communication-of-cancer-risks#:~:text=WASHINGTON%20%E2%80%94%20To%20protect%20astronauts%20from,Sciences%2C%20Engineering%2C%20and%20Medicine (accessed on 2 November 2022).
- Hsu, J.; Gore-Panter, S.; Tchou, G.; Castel, L.; Lovano, B.; Moravec, C.S.; Pettersson, G.B.; Roselli, E.E.; Gillinov, A.M.; McCurry, K.R.; et al. Genetic Control of Left Atrial Gene Expression Yields Insights into the Genetic Susceptibility for Atrial Fibrillation. Circ. Genom. Precis. Med. 2018, 11, e002107. [Google Scholar] [CrossRef]
- Harris, L.R.; Jenkin, M.; Jenkin, H.; Zacher, J.E.; Dyde, R.T. The effect of long-term exposure to microgravity on the perception of upright. NPJ Microgravity 2017, 3, 3. [Google Scholar] [CrossRef]
- Clément, G.; Skinner, A.; Lathan, C. Distance and Size Perception in Astronauts during Long-Duration Spaceflight. Life 2013, 3, 524–537. [Google Scholar] [CrossRef]
- Young, L.R.; Oman, C.M.; Watt, D.G.; Money, K.E.; Lichtenberg, B.K. Spatial orientation in weightlessness and readaptation to earth’s gravity. Science 1984, 225, 205–208. [Google Scholar] [CrossRef]
- Benson, A.J.; Kass, J.R.; Vogel, H. European vestibular experiments on the Spacelab-1 mission: 4. Thresholds of perception of whole-body linear oscillation. Exp. Brain Res. 1986, 64, 264–271. [Google Scholar] [CrossRef] [PubMed]
- DiZio, P.; Lackner, J.R. The effects of gravitoinertial force level and head movements on post-rotational nystagmus and illusory after-rotation. Exp. Brain Res. 1988, 70, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, A.; Hummel, R.; Lavrentyev, V.; Derry, W.; Williams, D.; Merrell, R.C. Microgravity effects on fine motor skills: Tying surgical knots during parabolic flight. Aviat. Space Environ. Med. 2006, 77, 852–856. [Google Scholar] [PubMed]
- Mulavara, A.P.; Peters, B.T.; Miller, C.A.; Kofman, I.S.; Reschke, M.F.; Taylor, L.C.; Lawrence, E.L.; Wood, S.J.; Laurie, S.S.; Lee, S.M.C.; et al. Physiological and Functional Alterations after Spaceflight and Bed Rest. Med. Sci. Sport. Exerc. 2018, 50, 1961–1980. [Google Scholar] [CrossRef]
- Tays, G.D.; Hupfeld, K.E.; McGregor, H.R.; Salazar, A.P.; De Dios, Y.E.; Beltran, N.E.; Reuter-Lorenz, P.A.; Kofman, I.S.; Wood, S.J.; Bloomberg, J.J.; et al. The Effects of Long Duration Spaceflight on Sensorimotor Control and Cognition. Front. Neural Circuits 2021, 15, 723504. [Google Scholar] [CrossRef]
- Taylor, A.J.; Beauchamp, J.D.; Briand, L.; Heer, M.; Hummel, T.; Margot, C.; McGrane, S.; Pieters, S.; Pittia, P.; Spence, C. Factors affecting flavor perception in space: Does the spacecraft environment influence food intake by astronauts? Compr. Rev. Food Sci. Food Saf. 2020, 19, 3439–3475. [Google Scholar] [CrossRef]
- Olabi, A.A.; Lawless, H.T.; Hunter, J.B.; Levitsky, D.A.; Halpern, B.P. The effect of microgravity and space flight on the chemical senses. J. Food Sci. 2002, 67, 468–478. [Google Scholar] [CrossRef]
- Bershad, E.M.; Urfy, M.Z.; Calvillo, E.; Tang, R.; Cajavilca, C.; Lee, A.G.; Venkatasubba Rao, C.P.; Suarez, J.I.; Chen, D. Marked olfactory impairment in idiopathic intracranial hypertension. J. Neurol. Neurosurg. Psychiatry 2014, 85, 959–964. [Google Scholar] [CrossRef]
- Wood, S.J.; Paloski, W.H.; Clark, J.B. Assessing Sensorimotor Function Following ISS with Computerized Dynamic Posturography. Aerosp. Med. Hum. Perform. 2015, 86, A45–A53. [Google Scholar] [CrossRef]
- Miller, C.A.; Kofman, I.S.; Brady, R.R.; May-Phillips, T.R.; Batson, C.D.; Lawrence, E.L.; Taylor, L.C.; Peters, B.T.; Mulavara, A.P.; Feiveson, A.H.; et al. Functional Task and Balance Performance in Bed Rest Subjects and Astronauts. Aerosp. Med. Hum. Perform. 2018, 89, 805–815. [Google Scholar] [CrossRef]
- Moore, S.T.; Dilda, V.; Morris, T.R.; Yungher, D.A.; MacDougall, H.G.; Wood, S.J. Long-duration spaceflight adversely affects post-landing operator proficiency. Sci. Rep. 2019, 9, 2677. [Google Scholar] [CrossRef] [PubMed]
- Gazenko, O.G.; Genin, A.M.; Egorov, A.D. Major Medical Results Of The Salyut-6—Soyuz 185-Day Space Flight. In Space Mankind’s Fourth Environment; Napolitano, L.G., Ed.; Elsevier: Pergamon, Turkey, 1982; pp. 275–293. [Google Scholar]
- Pechenkova, E.; Nosikova, I.; Rumshiskaya, A.; Litvinova, L.; Rukavishnikov, I.; Mershina, E.; Sinitsyn, V.; Van Ombergen, A.; Jeurissen, B.; Jillings, S.; et al. Alterations of Functional Brain Connectivity After Long-Duration Spaceflight as Revealed by fMRI. Front. Physiol. 2019, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, K.E.; McGregor, H.R.; Koppelmans, V.; Beltran, N.E.; Kofman, I.S.; De Dios, Y.E.; Riascos, R.F.; Reuter-Lorenz, P.A.; Wood, S.J.; Bloomberg, J.J.; et al. Brain and Behavioral Evidence for Reweighting of Vestibular Inputs with Long-Duration Spaceflight. Cereb. Cortex 2021, 32, bhab239. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.H.; Gibson, C.R.; Miller, N.R.; Subramanian, P.S.; Patel, N.B.; Lee, A.G. An overview of spaceflight-associated neuro-ocular syndrome (SANS). Neurol. India 2019, 67, S206–S211. [Google Scholar] [CrossRef]
- Lee, A.G.; Mader, T.H.; Gibson, C.R.; Tarver, W.; Rabiei, P.; Riascos, R.F.; Galdamez, L.A.; Brunstetter, T. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: A review and an update. NPJ Microgravity 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Wåhlin, A.; Holmlund, P.; Fellows, A.M.; Malm, J.; Buckey, J.C.; Eklund, A. Optic Nerve Length before and after Spaceflight. Ophthalmology 2021, 128, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Gibson, C.R.; Mader, T.H.; Ericson, K.; Ploutz-Snyder, R.; Heer, M.; Smith, S.M. Vision changes after spaceflight are related to alterations in folate- and vitamin B-12-dependent one-carbon metabolism. J. Nutr. 2012, 142, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Fleischman, D.; Lee, A.G.; Killer, H.E.; Chen, J.J.; Bhatti, M.T. Current concepts of cerebrospinal fluid dynamics and the translaminar cribrosa pressure gradient: A paradigm of optic disk disease. Surv. Ophthalmol. 2020, 65, 48–66. [Google Scholar] [CrossRef]
- Wostyn, P.; De Winne, F.; Stern, C.; Mader, T.H.; Gibson, C.R.; De Deyn, P.P. Potential Involvement of the Ocular Glymphatic System in Optic Disc Edema in Astronauts. Aerosp. Med. Hum. Perform. 2020, 91, 975–977. [Google Scholar] [CrossRef]
- Wostyn, P.; Gibson, C.R.; Mader, T.H. The odyssey of the ocular and cerebrospinal fluids during a mission to Mars: The “ocular glymphatic system” under pressure. Eye 2021, 36, 686–691. [Google Scholar] [CrossRef]
- Mader, T.H.; Gibson, C.R.; Barratt, M.R.; Miller, N.R.; Subramanian, P.S.; Killer, H.E.; Tarver, W.J.; Sargsyan, A.E.; Garcia, K.; Hart, S.F.; et al. Persistent Globe Flattening in Astronauts following Long-Duration Spaceflight. Neuro-Ophthalmol. (Aeolus Press) 2021, 45, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Billica, R. Inflight medical events for U.S. astronauts during space shuttle programs STS-1 through STS-89, April 1981–January 1998. In Presentation to the Institute of Medicine Committee on Creating a Vision for Space Medicine During Travel Beyond Earth Orbit; NASA Johnson Space Center, Houston, 22 February. As cited in: IOM, 2001. Safe passage: Astronaut care for exploration missions; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Kanas, N.; Manzey, D. Space Psychology and Psychiatry; Springer: Dordrecht, The Netherlands, 2003; Volume 16. [Google Scholar]
- Hanes, D.A.; McCollum, G. Cognitive-vestibular interactions: A review of patient difficulties and possible mechanisms. J. Vestib. Res. Equilib. Orientat. 2006, 16, 75–91. [Google Scholar] [CrossRef]
- Belavy, D.L.; Adams, M.; Brisby, H.; Cagnie, B.; Danneels, L.; Fairbank, J.; Hargens, A.R.; Judex, S.; Scheuring, R.A.; Sovelius, R.; et al. Disc herniations in astronauts: What causes them, and what does it tell us about herniation on earth? Eur. Spine J. 2016, 25, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.F.; Miller, S.L.; Khieu, K.; O’Neill, C.W.; Healey, R.M.; Coughlin, D.G.; Sayson, J.V.; Chang, D.G.; Hargens, A.R.; Lotz, J.C. From the international space station to the clinic: How prolonged unloading may disrupt lumbar spine stability. Spine J. 2018, 18, 7–14. [Google Scholar] [CrossRef]
- Fincke, E.M.; Padalka, G.; Lee, D.; van Holsbeeck, M.; Sargsyan, A.E.; Hamilton, D.R.; Martin, D.; Melton, S.L.; McFarlin, K.; Dulchavsky, S.A. Evaluation of shoulder integrity in space: First report of musculoskeletal US on the International Space Station. Radiology 2005, 234, 319–322. [Google Scholar] [CrossRef]
- Anderson, A.P.; Newman, D.J.; Welsch, R.E. Statistical Evaluation of Causal Factors Associated with Astronaut Shoulder Injury in Space Suits. Aerosp. Med. Hum. Perform. 2015, 86, 606–613. [Google Scholar] [CrossRef]
- Fitzgerald, J. Cartilage breakdown in microgravity-a problem for long-term spaceflight? NPJ Regen. Med. 2017, 2, 1–2. [Google Scholar] [CrossRef]
- Winnard, A.; Debuse, D.; Caplan, N. Countermeasure Development for Lumbopelvic Deconditioning in Space; IntechOpen: London, UK, 2018; pp. 121–138. [Google Scholar]
- Snijders, C.J.D.L.; Hides, J.A.; Pool-Goudzwaard, A.L.; Richardson, C.A. Study of Low Back Pain in Crew Members During Space Flight (Muscle); University of Rotterdam: Rotterdam, The Netherlands, 2016. Available online: https://www.nasa.gov/mission_pages/station/research/experiments/176.html (accessed on 2 November 2022).
- Kwok, A.T.; Mohamed, N.S.; Plate, J.F.; Yammani, R.R.; Rosas, S.; Bateman, T.A.; Livingston, E.; Moore, J.E.; Kerr, B.A.; Lee, J.; et al. Spaceflight and hind limb unloading induces an arthritic phenotype in knee articular cartilage and menisci of rodents. Sci. Rep. 2021, 11, 10469. [Google Scholar] [CrossRef]
- Frost, H.M. Mechanical determinants of bone modeling. Metab. Bone Dis. Relat. Res. 1982, 4, 217–229. [Google Scholar] [CrossRef]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef]
- Vico, L.; van Rietbergen, B.; Vilayphiou, N.; Linossier, M.-T.; Locrelle, H.; Normand, M.; Zouch, M.; Gerbaix, M.; Bonnet, N.; Novikov, V.; et al. Cortical and Trabecular Bone Microstructure Did Not Recover at Weight-Bearing Skeletal Sites and Progressively Deteriorated at Non-Weight-Bearing Sites During the Year Following International Space Station Missions. J. Bone Miner. Res. 2017, 32, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Vico, L.; Collet, P.; Guignandon, A.; Lafage-Proust, M.H.; Thomas, T.; Rehaillia, M.; Alexandre, C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 2000, 355, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Van Loon, J.J.W.A.; Bloomfield, S.; Vico, L.; Chopard, A.; Rittweger, J.; Kyparos, A.; Blottner, D.; Vuori, I.; Gerzer, R.; et al. Towards human exploration of space: The THESEUS review series on muscle and bone research priorities. NPJ Microgravity 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.R.; Prisk, G.K.; Guy, H.J.; West, J.B. Lung volumes during sustained microgravity on Spacelab SLS-1. J. Appl. Physiol. 1994, 77, 2005–2014. [Google Scholar] [CrossRef]
- Agostoni, E.; Hyatt, R.E. Static Behavior of the Respiratory System. In Comprehensive Physiology; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 113–130. [Google Scholar]
- Paiva, M.; Verbanck, S.; Estenne, M. Chest wall mechanics in microgravity: Results from parabolic flights. Life Sci. Res. Space 1994, 366, 307. [Google Scholar]
- Prisk, G.K.; Guy, H.J.; Elliott, A.R.; West, J.B. Inhomogeneity of pulmonary perfusion during sustained microgravity on SLS-1. J. Appl. Physiol. 1994, 76, 1730–1738. [Google Scholar] [CrossRef]
- Guy, H.J.; Prisk, G.K.; Elliott, A.R.; Deutschman, R.A., 3rd; West, J.B. Inhomogeneity of pulmonary ventilation during sustained microgravity as determined by single-breath washouts. J. Appl. Physiol. 1994, 76, 1719–1729. [Google Scholar] [CrossRef]
- Russomano, T.; Evetts, S.N.; Castro, J.; Dos Santos, M.A.; Gavillon, J.; Azevedo, D.F.; Whittle, J.; Coats, E.; Ernsting, J. A device for sampling arterialized earlobe blood in austere environments. Aviat. Space Environ. Med. 2006, 77, 453–455. [Google Scholar]
- Prisk, G.K.; Fine, J.M.; Cooper, T.K.; West, J.B. Vital capacity, respiratory muscle strength, and pulmonary gas exchange during long-duration exposure to microgravity. J. Appl. Physiol. 2006, 101, 439–447. [Google Scholar] [CrossRef][Green Version]
- Prisk, G.K.; Fine, J.M.; Cooper, T.K.; West, J.B. Lung function is unchanged in the 1 G environment following 6-months exposure to microgravity. Eur. J. Appl. Physiol. 2008, 103, 617–623. [Google Scholar] [CrossRef]
- Tronnier, H.; Wiebusch, M.; Heinrich, U. Change in skin physiological parameters in space--report on and results of the first study on man. Ski. Pharmacol. Physiol. 2008, 21, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, D.E.; Manning, F.J.; Worth, M.H., Jr. (Eds.) Review of NASA’s Longitudinal Study of Astronaut Health; National Academy of Sciences: Washington, DC, USA, 2004. [Google Scholar]
- Garcia, K.M.; Harrison, M.F.; Sargsyan, A.E.; Ebert, D.; Dulchavsky, S.A. Real-time Ultrasound Assessment of Astronaut Spinal Anatomy and Disorders on the International Space Station. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2018, 37, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Arbeille, P.; Chaput, D.; Zuj, K.; Depriester, A.; Maillet, A.; Belbis, O.; Benarroche, P.; Barde, S. Remote Echography between a Ground Control Center and the International Space Station Using a Tele-operated Echograph with Motorized Probe. Ultrasound Med. Biol. 2018, 44, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Downs, M.; Martin, D.S.; Hougland, E.; Sarmiento, L.; Arzeno, N.; Pettit, D.R.; Ploutz-Snyder, R.; Cunningham, D.; Jones, L.W.; et al. Teleguided self-ultrasound scanning for longitudinal monitoring of muscle mass during spaceflight. iScience 2021, 24, 102344. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.S.; South, D.A.; Garcia, K.M.; Arbeille, P. Ultrasound in space. Ultrasound Med. Biol. 2003, 29, 1–12. [Google Scholar] [CrossRef]
- Martin, D.S.; Caine, T.L.; Matz, T.; Lee, S.M.; Stenger, M.B.; Sargsyan, A.E.; Platts, S.H. Virtual guidance as a tool to obtain diagnostic ultrasound for spaceflight and remote environments. Aviat. Space Environ. Med. 2012, 83, 995–1000. [Google Scholar] [CrossRef]
- Jones, J.A.; Sargsyan, A.E.; Barr, Y.R.; Melton, S.; Hamilton, D.R.; Dulchavsky, S.A.; Whitson, P.A. Diagnostic ultrasound at MACH 20: Retroperitoneal and pelvic imaging in space. Ultrasound Med. Biol. 2009, 35, 1059–1067. [Google Scholar] [CrossRef]
- Law, J.; Macbeth, P.B. Ultrasound: From Earth to space. McGill J. Med. MJM Int. Forum Adv. Med. Sci. By Stud. 2011, 13, 59. [Google Scholar] [CrossRef]
- Dietrich, D.; Dekova, R.; Davy, S.; Fahrni, G.; Geissbühler, A. Applications of Space Technologies to Global Health: Scoping Review. J. Med. Internet Res. 2018, 20, e230. [Google Scholar] [CrossRef]
- Available online: https://jscfeatures.jsc.nasa.gov/pages.ashx/1065/New%20Innovative%20Technology%20to%20Keep%20Astronauts%20Healthy (accessed on 2 November 2022).
- Cramer, A.; Hecla, J.; Wu, D.; Lai, X.; Boers, T.; Yang, K.; Moulton, T.; Kenyon, S.; Arzoumanian, Z.; Krull, W.; et al. Stationary Computed Tomography for Space and other Resource-constrained Environments. Sci. Rep. 2018, 8, 14195. [Google Scholar] [CrossRef]
- Hamilton, D.R.; Sargsyan, A.E.; Kirkpatrick, A.W.; Nicolaou, S.; Campbell, M.; Dawson, D.L.; Melton, S.L.; Beck, G.; Guess, T.; Rasbury, J.; et al. Sonographic detection of pneumothorax and hemothorax in microgravity. Aviat. Space Environ. Med. 2004, 75, 272–277. [Google Scholar]
- Kirkpatrick, A.W.; Hamilton, D.R.; Nicolaou, S.; Sargsyan, A.E.; Campbell, M.R.; Feiveson, A.; Dulchavsky, S.A.; Melton, S.; Beck, G.; Dawson, D.L. Focused Assessment with Sonography for Trauma in weightlessness: A feasibility study. J. Am. Coll. Surg. 2003, 196, 833–844. [Google Scholar] [CrossRef]
- Johnston, S.L.; Campbell, M.R.; Billica, R.D.; Gilmore, S.M. Cardiopulmonary resuscitation in microgravity: Efficacy in the swine during parabolic flight. Aviat. Space Environ. Med. 2004, 75, 546–550. [Google Scholar]
- Kansagra, A.P.; Shute, T.S. Space: The Final Frontier for IR. J. Vasc. Interv. Radiol. JVIR 2015, 26, 825–828. [Google Scholar] [CrossRef]
- Hinkelbein, J.; Kerkhoff, S.; Adler, C.; Ahlbäck, A.; Braunecker, S.; Burgard, D.; Cirillo, F.; De Robertis, E.; Glaser, E.; Haidl, T.K.; et al. Cardiopulmonary resuscitation (CPR) during spaceflight—A guideline for CPR in microgravity from the German Society of Aerospace Medicine (DGLRM) and the European Society of Aerospace Medicine Space Medicine Group (ESAM-SMG). Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 108. [Google Scholar] [CrossRef]
- Komorowski, M.; Fleming, S.; Kirkpatrick, A.W. Fundamentals of Anesthesiology for Spaceflight. J. Cardiothorac. Vasc. Anesth. 2016, 30, 781–790. [Google Scholar] [CrossRef]
- Kaczka, D.W.; Beck, G. Mechanical ventilation in orbit: Emphasis on closed-loop ventilation. Respir. Care Clin. North Am. 2004, 10, 369–400, vii. [Google Scholar] [CrossRef]
- Starck, C.; Thierry, S.; Bernard, C.I.; Morineau, T.; Jaulin, F.; Chapelain, P.; Komorowski, M. Tracheal intubation in microgravity: A simulation study comparing direct laryngoscopy and videolaryngoscopy(†). Br. J. Anaesth. 2020, 125, e47–e53. [Google Scholar] [CrossRef]
- Campbell, M.R.; Billica, R.D.; Johnston, S.L., 3rd; Muller, M.S. Performance of advanced trauma life support procedures in microgravity. Aviat. Space Environ. Med. 2002, 73, 907–912. [Google Scholar]
- Campbell, M.R. A review of surgical care in space. J. Am. Coll. Surg. 2002, 194, 802–812. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Nicolaou, S.; Campbell, M.R.; Sargsyan, A.E.; Dulchavsky, S.A.; Melton, S.; Beck, G.; Dawson, D.L.; Billica, R.D.; Johnston, S.L.; et al. Percutaneous aspiration of fluid for management of peritonitis in space. Aviat. Space Environ. Med. 2002, 73, 925–930. [Google Scholar] [PubMed]
- Kirkpatrick, A.W.; Keaney, M.; Kmet, L.; Ball, C.G.; Campbell, M.R.; Kindratsky, C.; Groleau, M.; Tyssen, M.; Keyte, J.; Broderick, T.J. Intraperitoneal gas insufflation will be required for laparoscopic visualization in space: A comparison of laparoscopic techniques in weightlessness. J. Am. Coll. Surg. 2009, 209, 233–241. [Google Scholar] [CrossRef]
- Campbell, M.R.; Kirkpatrick, A.W.; Billica, R.D.; Johnston, S.L.; Jennings, R.; Short, D.; Hamilton, D.; Dulchavsky, S.A. Endoscopic surgery in weightlessness: The investigation of basic principles for surgery in space. Surg. Endosc. 2001, 15, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.R.; Williams, D.R.; Buckey, J.C., Jr.; Kirkpatrick, A.W. Animal surgery during spaceflight on the Neurolab Shuttle mission. Aviat. Space Environ. Med. 2005, 76, 589–593. [Google Scholar] [PubMed]
- Panesar, S.S.; Ashkan, K. Surgery in space. Br. J. Surg. 2018, 105, 1234–1243. [Google Scholar] [CrossRef]
- Kirkpatrick, A.W.; Mckee, J.L.; Tien, H.C.-N.; Laporta, A.J.; Lavell, K.; Leslie, T.; King, D.; McBeth, P.B.; Brien, S.; Roberts, D.J.; et al. Damage control surgery in weightlessness: A comparative study of simulated torso hemorrhage control comparing terrestrial and weightless conditions. J. Trauma Acute Care Surg. 2017, 82, 392–399. [Google Scholar] [CrossRef]
- Raison, N.; Khan, M.S.; Challacombe, B. Telemedicine in Surgery: What are the Opportunities and Hurdles to Realising the Potential? Curr. Urol. Rep. 2015, 16, 43. [Google Scholar] [CrossRef]
- Korte, C.; Nair, S.S.; Nistor, V.; Low, T.P.; Doarn, C.R.; Schaffner, G. Determining the threshold of time-delay for teleoperation accuracy and efficiency in relation to telesurgery. Telemed. J. e-Health 2014, 20, 1078–1086. [Google Scholar] [CrossRef]
- Hughson, R.L.; Robertson, A.D.; Arbeille, P.; Shoemaker, J.K.; Rush, J.W.; Fraser, K.S.; Greaves, D.K. Increased postflight carotid artery stiffness and inflight insulin resistance resulting from 6-mo spaceflight in male and female astronauts. Am. J. Physiology. Heart Circ. Physiol. 2016, 310, H628–H638. [Google Scholar] [CrossRef]
- Arbeille, P.; Provost, R.; Zuj, K. Carotid and Femoral Artery Intima-Media Thickness During 6 Months of Spaceflight. Aerosp. Med. Hum. Perform. 2016, 87, 449–453. [Google Scholar] [CrossRef]
- Després, J.P. Physical Activity, Sedentary Behaviours, and Cardiovascular Health: When Will Cardiorespiratory Fitness Become a Vital Sign? Can. J. Cardiol. 2016, 32, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Boerma, M.; Zhou, D. Ionizing Radiation-Induced Endothelial Cell Senescence and Cardiovascular Diseases. Radiat. Res. 2016, 186, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P. Weight, muscle and bone loss during space flight: Another perspective. Eur. J. Appl. Physiol. 2013, 113, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Clément, G.R.; Bukley, A.P.; Paloski, W.H. Artificial gravity as a countermeasure for mitigating physiological deconditioning during long-duration space missions. Front. Syst. Neurosci. 2015, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.E. Orbit Dynamics and Habitability Considerations for a Space Hotel with Artificial Gravity. In AIAA SPACE 2014 Conference and Exposition; AIAA SPACE Forum; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2014. [Google Scholar]
- Mehta, S.; Crucian, B.; Pierson, D.; Sams, C.; Stowe, R. Artificial Gravity And The Immune System Function. In Artificial Gravity; Clément, G., Bukley, A., Eds.; Springer: New York, NY, USA, 2007; pp. 271–286. [Google Scholar]
- Available online: https://www.nasa.gov/feature/goddard/2021/-ai-could-speed-fault-diagnosis-in-spacecraft (accessed on 2 November 2022).
- Doarn, C.R.; Nicogossian, A.E.; Merrell, R.C. Applications of telemedicine in the United States space program. Telemed. J. 1998, 4, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Chelmins, D.T.; Briones, J.; Downey, J.; Clark, G.; Gannon, A. Cognitive communications for NASA space systems. In Proceedings of the International Communications Satellite Systems Conference, Washington, DA, USA, 28–29 September 2021. [Google Scholar]
- Laperre, B.; Amaya, J.; Lapenta, G. Dynamic Time Warping as a New Evaluation for Dst Forecast With Machine Learning. Front. Astron. Space Sci. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Rayman, R.; Croome, K.; Galbraith, N.; McClure, R.; Morady, R.; Peterson, S.; Smith, S.; Subotic, V.; Van Wynsberghe, A.; Primak, S. Long-distance robotic telesurgery: A feasibility study for care in remote environments. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS 2006, 2, 216–224. [Google Scholar] [CrossRef]
- Chen, A.I.; Balter, M.L.; Maguire, T.J.; Yarmush, M.L. Deep learning robotic guidance for autonomous vascular access. Nat. Mach. Intell. 2020, 2, 104–115. [Google Scholar] [CrossRef]
- Pantalone, D.; Faini, G.S.; Cialdai, F.; Sereni, E.; Bacci, S.; Bani, D.; Bernini, M.; Pratesi, C.; Stefàno, P.; Orzalesi, L.; et al. Robot-assisted surgery in space: Pros and cons. A review from the surgeon’s point of view. NPJ Microgravity 2021, 7, 56. [Google Scholar] [CrossRef]
- Available online: https://www.nasa.gov/mission_pages/NEEMO/index.html (accessed on 2 November 2022).
- Available online: https://www.nasa.gov/risky-space-business-challenge (accessed on 2 November 2022).
- Isaksson, L.J.; Pepa, M.; Zaffaroni, M.; Marvaso, G.; Alterio, D.; Volpe, S.; Corrao, G.; Augugliaro, M.; Starzyńska, A.; Leonardi, M.C.; et al. Machine Learning-Based Models for Prediction of Toxicity Outcomes in Radiotherapy. Front. Oncol. 2020, 10, 790. [Google Scholar] [CrossRef]
- Krittanawong, C. Big Data Analytics, the Microbiome, Host-omic and Bug-omic Data and Risk for Cardiovascular Disease. Heart Lung Circ. 2018, 27, e26–e27. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, W.A.; Fazelinia, H.; Rosenthal, S.B.; Laiakis, E.C.; Kim, M.S.; Meydan, C.; Kidane, Y.; Rathi, K.S.; Smith, S.M.; Stear, B.; et al. Comprehensive Multi-omics Analysis Reveals Mitochondrial Stress as a Central Biological Hub for Spaceflight Impact. Cell 2020, 183, 1185–1201.e1120. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Rogers, A.J.; Johnson, K.W.; Wang, Z.; Turakhia, M.P.; Halperin, J.L.; Narayan, S.M. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat. Rev. Cardiol. 2021, 18, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Chriskos, P.; Frantzidis, C.A.; Gkivogkli, P.T.; Bamidis, P.D.; Kourtidou-Papadeli, C. Achieving Accurate Automatic Sleep Staging on Manually Pre-processed EEG Data Through Synchronization Feature Extraction and Graph Metrics. Front. Hum. Neurosci. 2018, 12, 110. [Google Scholar] [CrossRef]
- Available online: https://www.businesswire.com/news/home/20190416005378/en/Recovery-Force-Receives-Prestigious-1.8M-Grant-National (accessed on 2 November 2022).
- Nie, G.Y.; Duh, H.B.L.; Liu, Y.; Wang, Y. Analysis on Mitigation of Visually Induced Motion Sickness by Applying Dynamical Blurring on a User’s Retina. IEEE Trans. Vis. Comput. Graph. 2020, 26, 2535–2545. [Google Scholar] [CrossRef]
- Varker, T.; Brand, R.M.; Ward, J.; Terhaag, S.; Phelps, A. Efficacy of synchronous telepsychology interventions for people with anxiety, depression, posttraumatic stress disorder, and adjustment disorder: A rapid evidence assessment. Psychol. Serv. 2019, 16, 621–635. [Google Scholar] [CrossRef]

| Scheme | Pre-Flight | In-Flight | Post-Flight |
|---|---|---|---|
| Physical Fitness Assessment | Cardiovascular fitness: VO2max measured cycle ergometry Flexibility: Sit & reach; shoulder flexibility Muscular strength and endurance: Maximum push-ups in 2 min; maximum sit-ups in 2 min; maximum pull-ups; handgrip strength with dynamometer | Cardiovascular fitness: Flight day (FD) +30, +120, return day (R) −30 VO2max FD+3 through day prior to return. Resistance: 3x/wk (60 min) Aerobic: 3x/wk (30 min) | Functional fitness assessment: R+7, R+30 Cardiovascular fitness: R+7, R+30 VO2max |
| Cardiovascular | Coronary artery calcium (CAC) score every 5 years Echocardiogram annually VO2max cycle test annually Fasting lipid panel High Sensitivity C-Reactive Peptide (HS-CRP) Carotid Intima-Media Thickness (CIMT) annually Individuals with cardiovascular disease history (e.g., compensated heart failure, asymptomatic CAD, arrhythmias) are not currently eligible for either long- or short-term space travel | Quarterly VO2max cycle testing Quarterly vascular US Monthly health surveillance exam with blood pressure measurement | VO2max cycle test at R+7, R+30 Resting 12-lead ECG annually Blood pressure measurement Echocardiogram Fasting lipid panel HS-CRP Hypertension screening using a sphygmomanometer annually and as clinically indicated Cardiovascular health screening annually |
| Pulmonology | Pulmonary function testing annually 6-Minute Walk Test for patients with chronic pulmonary disease | None | Pulmonary function testing Purified Protein Derivative (PPD) (tuberculin) skin test for tuberculosis screening, unless clinically contraindicated |
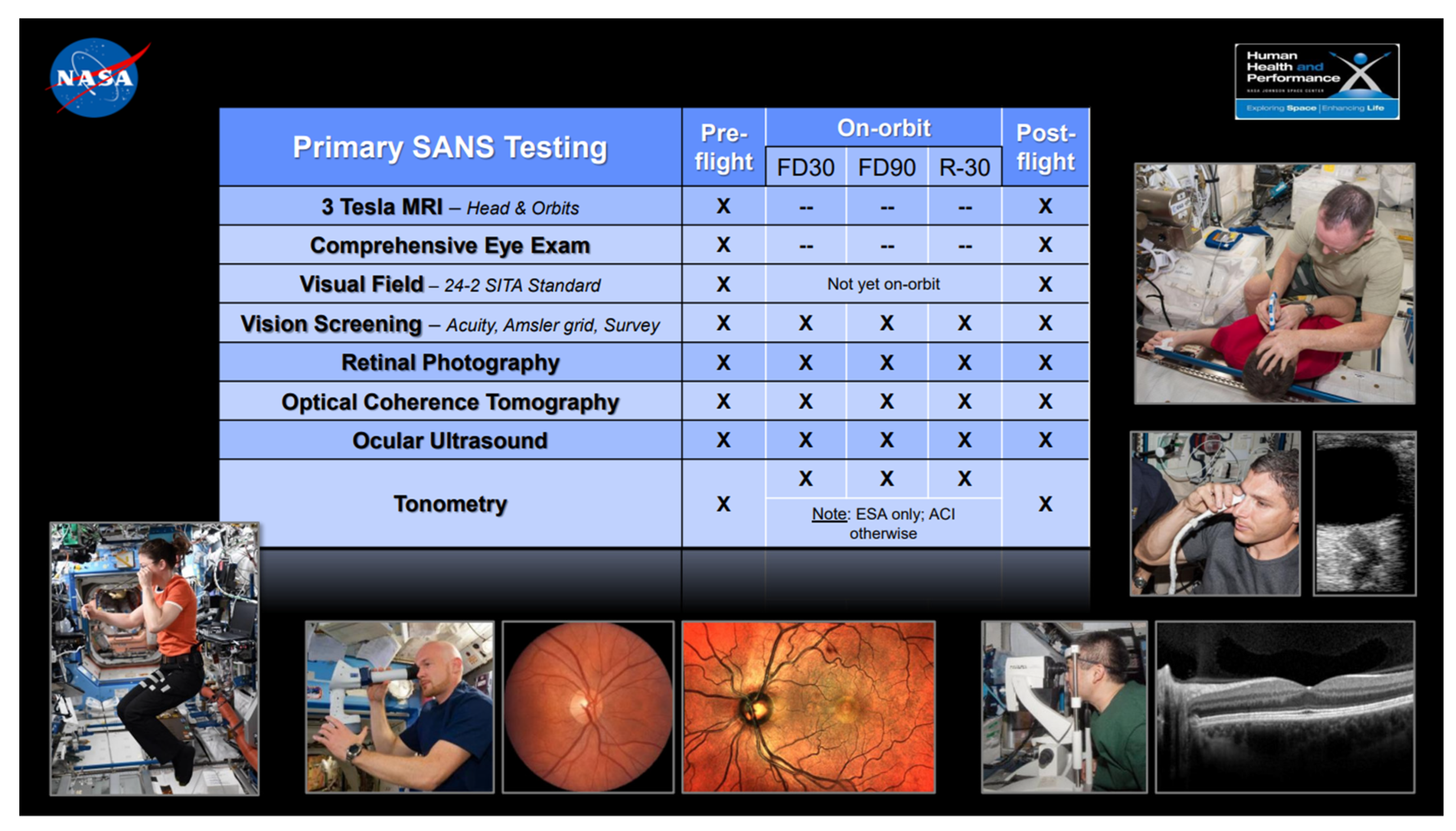
| Ophthalmology | SANS-surveillance, to include baseline testing of: Visual acuity OCT US MRI orbits Tonometry Ophthalmologic examination Color vision Phoria testing Ophthalmologic screening for ocular health and visual status (e.g., testing for refractive errors or cataracts) is recommended | See attached chart for inflight scheduling details | Visual acuity, color vision, and extraocular muscle testing (annually) Tonometry (annually) Dilated fundus exam, retinal photographs, and optical coherence tomography (OCT) |
| Otorhinolaryngology | Screening audiometry Tympanography | Quarterly On Orbit Hearing Assessment (OOHA) | Hearing questionnaire and pure-tone audiometry testing (annually) |
| Hematology | Complete Blood Count (CBC) with differential Iron studies | CBC every 60 days | CBC, CMP (annually) |
| Immunology | Administration of vaccines (e.g., Shingrix) for prevention of inflight VZV reactivation Immunoglobulins (annually) TB screening with PPD, Quantiferon Gold | None | NASA has recommended post-flight quarantine and immune status monitoring (i.e., immune-boosting protocol) to mitigate the risk of infection |
| Gastroenterology | Age appropriate colon cancer screening | None | Lifetime Surveillance of Astronaut Health (LSAH) |
| Musculoskeletal | Annual DXA for osteoporosis screening for postmenopausal females and males >50 years old Cervical and thoracic spine MRI | None | Post-flight DXA for BMD Spine MRI |
| Dermatology | Annual skin examination | None | Visual exam of the skin with photo documentation of any abnormalities, particularly for melanoma and non-melanoma skin cancers (R+0/1 day and annually) |
| Genitourinary | PSA in males Annual urinalysis, 24-h urine collection | Quarterly 24-h urine collection | Urinalysis 24-h urine collection |
| Neurology | MRI brain Neurovestibular platform test | None | Neurovestibular platform test |
| Endocrine | TSH, free T4 Thyroid US | None | Annual TSH, free T4 |
| Dental | Annual dental examinations Orthopantomogram X-rays | None | Post-flight dental examination |
| Oncology | Age-appropriate pap smear and mammogram in females Age-appropriate colon cancer screening |
| Tread/Hazards | Health Risks | Current Countermeasure |
|---|---|---|
| Microgravity | ||
| Musculoskeletal | Muscle and bone atrophy [19] | A combination of adequate exercise and antiresorptive medications [20,21] |
| Impaired wound healing [22,23,24] | No specific countermeasures exist | |
| Neurology | Space motion sickness (SMS) [25] | Scopolamine administered orally or via transdermal patch with or without an amphetamine (to counteract drowsiness), or intramuscular Promethazine [26] |
| Alterations of smell and taste [27] | No specific countermeasures exist | |
| Self-reported congestion [27] | Lower body negative pressure may help, but the effects are transient | |
| Internal jugular vein thrombosis [28,29] | No specific countermeasures exist | |
| Post-flight motion sickness [30] | No specific countermeasures exist | |
| Immunology | Reactivation of latent viruses [31,32] | Preflight vaccination [32] Treatment with polyclonal immunoglobulin (IG) and Interleukin-2 (SC) [33] Adequate exercise and nutrition [33] |
| Hypersensitivity [34] | No specific countermeasures exist | |
| Gastroenterology | Gut microbiota dysbiosis [2] | Probiotics, synthetic peptides, synthetic biology [35,36,37,38] |
| Acute appendicitis and cholecystitis | Inflight medical management or minimally invasive procedures (e.g., ultrasound guided percutaneous drainage) of traditionally “surgical” conditions. [39] Alternatively, can consider prophylactic minimally invasive surgery prior to prolonged space flight in at-risk individuals [40] | |
| Cardiovascular | Orthostatic intolerance [41] | Regular exercise training and adequate hydration prior to landing |
| Cardiac myocyte atrophy [42] | Restores back to baseline after returning from spaceflight | |
| Ophthalmology | Spaceflight-Associated Neuro-Ocular Syndrome (SANS) [43] | The lower body negative pressure device has been proposed as a countermeasure but is currently unproven |
| Dermatology | Skin tinning, dry skin, delayed wound healing, or skin infections [44] | No specific countermeasures exist |
| Traumatology | Increased risk of traumatic injury with construction, inadvertent acceleration of large masses when in zero gravity, impaired physiologic responses to hemorrhage and shock in zero gravity, bone demineralization increases risk of traumatic fractures, micrometeorites in EVA activities [45,46,47] | No specific countermeasures exist |
| Radiation | ||
| Most systems | Cancer induction | Shielding, including advanced shielding options. [48,49] Optimize mission according to solar cycle [48,49] Sex- and age-based selection criteria for astronauts [48] |
| Musculoskeletal | Bone cell remodeling [6] | Shielding [7] |
| Hematology | Acute hematopoietic effects [7] | Shielding [7] |
| Space-related hemolytic anemia [50,51] | Screening and monitoring for hemolysis | |
| Neurology | Neuro-degenerative disorders and central nervous system changes [52,53,54,55,56] | Adaptive visuo-motor training, active sensory feedback to guide task performance, galvanic vestibular stimulation (GVS) |
| Gastroenterology | Gut microbiota dysbiosis [2] | Probiotics, synthetic peptides, synthetic biology [35,36,37,38] |
| Cardiovascular | Accelerated atherosclerosis [3,4,57,58,59,60] | Shielding [7] |
| Ophthalmology | Radiation-induced cataract [61,62] | No specific countermeasures exist |
| Pulmonology | Lung adenocarcinomas and squamous cell carcinomas [63] | Shielding [7] |
| Dermatology | Melanoma and non-melanoma skin cancers [44] | Inflight telediagnostic system |
| Others | ||
| Nutrition | Nutritional deficiencies [64] | Telehealth and routine lab work for identification of nutritional deficiencies, and adequate supplementation as needed |
| Mental health | Psychologic distress [10,65] | Stress-relieving breathing exercises and/or mindfulness/positive visualization exercises [66] |
| Depression, confinement, isolation, immobilization lack of social interaction [10,65,67] | Telepsychiatry, telepsychotherapy, space social media using LunaNet [68,69,70]. | |
| Altered circadian rhythms | Circadian de-synchronization and sleep loss [71] | Melatonin supplementation may be useful [72] |
| Dust exposure | Extraterrestrial dust exposure [73] | No specific countermeasures exist |
| Auditory exposures | Noise generated from man-made sources such as mechanical equipment (Noise/sound does not propagate in the vacuum of space and it requires an atmosphere to propagate, e.g., the pressurized spacecraft interior) [74] | Hearing protection in the form of foam ear plugs [74] |
| Unknown infections | Mission-threatening risks from unknown infections or novel bacterial/viral phenotypes [75,76] | PPE and post-exposure quarantine [77] |
| Potential AI Applications |
|---|
| Risk Prediction Models |
| Risk prediction models for development of cancer due to ionizing radiation exposure based on animal models [78,79] |
| Inflight AI assisted multi-omic research using the Edge TPU to generate novel risk prediction scores [80] |
| Risk prediction models for the development of cardiovascular disease based on carotid-femoral pulse wave velocity obtained via Doppler ultrasound or AI-guided analysis of 3D printed tissue models [81] |
| Risk prediction models such as the modified Astro-CHARM model which combines a patient’s CAC score with non-traditional risk factors associated with space travel (e.g., radiation exposure, microgravity exposure, dysbiosis of gut microbiota, etc.) to identify those at risk for accelerated atherosclerosis [82] |
| Predicting antimicrobial resistance of novel bacterial species (e.g., Methylobacterium ajmalii sp. nov., Methylobacterium rhodesianum, etc.) based on whole genome sequencing data [83] |
| AI-based models for the identification of causal relationships between various exposures and subsequent cancer development (e.g., CRISP) [84] |
| Medical Screening Preflight and Inflight Monitoring |
| AI monitoring for gut microbiota dysbiosis using smart toilets during pre-, intra-, and post-flight periods [2,85,86] |
| Screening for latent virus infection or reactivation based on immunological variables, which can then be used as a biomarker for assessment of adaptive immune function [87,88] |
| The Edge TPU and other similar low-power AI ASICs could be used to monitor genomic imprinting (e.g., DNA methylation signatures or epigenetic/transcriptomic-based tests) for inflight lung cancer screening (may be equivalent to low-dose CT chest screening) [63,89,90] |
| AI screening for mental illness through facial recognition and vocal analysis [68,69] |
| A combination of AI and imaging could potentially identity abnormal or misfolded proteins [91,92] |
| Deep learning algorithms for the inflight telediagnosis of various conditions (e.g., skin lesions or SANS) [93,94,95] |
| AI-integrated wearable technology for space suits (e.g., exoskeletons) to monitor biodata (e.g., electrocardiogram, blood pressure, sleep cycle, body temperature, etc.) [96,97] |
| AI monitoring of metabolomics-based biomarkers for assessment of sleep quality and circadian rhythm [98] |
| AI can be used to analyze retinal fundus photography to identify and monitor space anemia [99] |
| Medical Diagnostic Tools |
| The Edge TPU and other similar low-power AI ASICs (i.e., Google Coral Edge TPU, NVIDIA Jetson Nano, Intel Neural Compute Stick 2) could advance image segmentation AI models, allowing for these models to be feasibly deployed in ultrasound point-of-care settings (e.g., detection of DVT, structural heart disease, hemodynamic changes), even procedural guidance [100]. |
| AI-based system designed to guide non-physicians on the proper acquisition of medical diagnostic testing using The Edge TPU and deep reinforcement learning |
| AI-enhanced 3D-imaging technology (e.g., micro-CT scanners) [101] |
| Utilization of the Edge TPU and other similar low-power AI ASICs to provide the necessary processing power for high-performance parallel-processing space research [102] |
| Intervention |
| AI-guided minor surgical procedures [such as incision and drainage (I&D)] using next-generation High Performance Spaceflight Computing (HPSC) [103] |
| AI-assisted, remotely controlled robotic PCI and robotic laparoscopic surgery (e.g., telecholecystectomy and teleappendectomy); made possible by a reduction in communication latency beyond a lag of 200 ms [103,104,105,106,107] |
| Using AI predictions of drug metabolism and effectiveness based on an individual’s multiomic data prior to medication or supplement distribution (e.g., melatonin, immune supplements, probiotics) [108,109] |
| Disease Prevention |
| AI-integrated space suits (e.g., exoskeletons) to maximize EVA time and operating pressure, and minimize space radiation exposure [110,111] |
| 3D printing of personalized devices (e.g., ear plugs to prevent noise source generated from man-made sources), space shields, space suits for use in emergency scenarios [112,113,114] |
| AI-based chatbots or social media could potentially be used to prevent anxiety and depression during long-duration space travel. The Edge TPU could potentially be used in advancing an internet or social media for the moon, known as LunaNet [115]. |
| AI-improved augmented reality/virtual reality to reduce/prevent Spaceflight-Associated Neuro-Ocular Syndrome (SANS), space motion sickness (SMS), and post-flight motion sickness (PFMS) [116,117,118] |
| AI identification of an individual’s radiosensitivity (genotype-phenotype) using multiomics to prevent the negative effects of ionizing radiation [119,120,121] |
| Emerging technology could allow for the transfer of large volumes of data at faster rates in order to facilitate medical research in space [122] |
| AI can be used to create novel metrics (e.g., travelers’ satisfactions, post spaceflight measurements for travelers) and ethics (e.g., health insurance) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krittanawong, C.; Singh, N.K.; Scheuring, R.A.; Urquieta, E.; Bershad, E.M.; Macaulay, T.R.; Kaplin, S.; Dunn, C.; Kry, S.F.; Russomano, T.; et al. Human Health during Space Travel: State-of-the-Art Review. Cells 2023, 12, 40. https://doi.org/10.3390/cells12010040
Krittanawong C, Singh NK, Scheuring RA, Urquieta E, Bershad EM, Macaulay TR, Kaplin S, Dunn C, Kry SF, Russomano T, et al. Human Health during Space Travel: State-of-the-Art Review. Cells. 2023; 12(1):40. https://doi.org/10.3390/cells12010040
Chicago/Turabian StyleKrittanawong, Chayakrit, Nitin Kumar Singh, Richard A. Scheuring, Emmanuel Urquieta, Eric M. Bershad, Timothy R. Macaulay, Scott Kaplin, Carly Dunn, Stephen F. Kry, Thais Russomano, and et al. 2023. "Human Health during Space Travel: State-of-the-Art Review" Cells 12, no. 1: 40. https://doi.org/10.3390/cells12010040
APA StyleKrittanawong, C., Singh, N. K., Scheuring, R. A., Urquieta, E., Bershad, E. M., Macaulay, T. R., Kaplin, S., Dunn, C., Kry, S. F., Russomano, T., Shepanek, M., Stowe, R. P., Kirkpatrick, A. W., Broderick, T. J., Sibonga, J. D., Lee, A. G., & Crucian, B. E. (2023). Human Health during Space Travel: State-of-the-Art Review. Cells, 12(1), 40. https://doi.org/10.3390/cells12010040











