Biodegradable Poly(D-L-lactide-co-glycolide) (PLGA)-Infiltrated Bioactive Glass (CAR12N) Scaffolds Maintain Mesenchymal Stem Cell Chondrogenesis for Cartilage Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
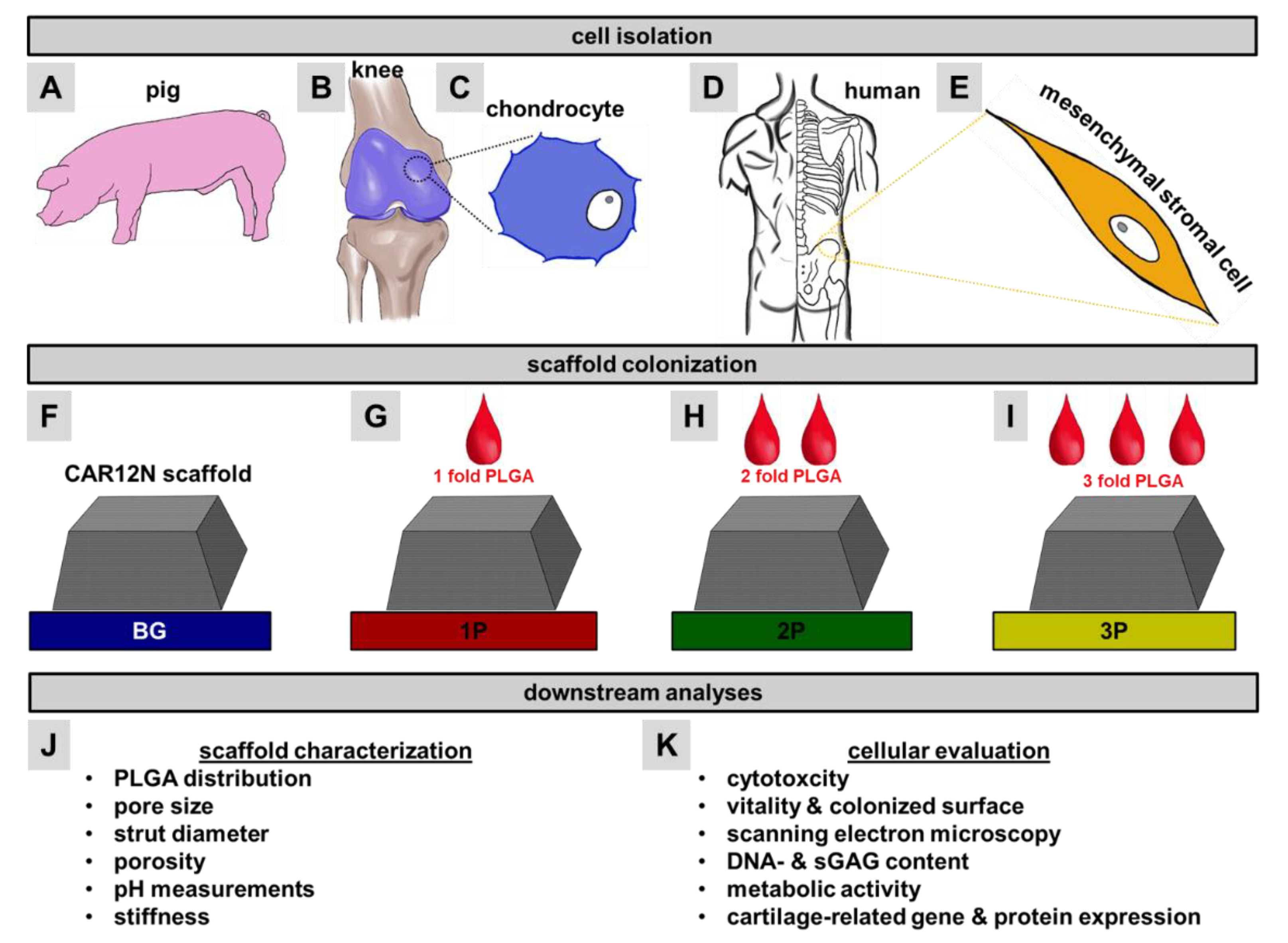
2.1. Bioactive Glass (BG) Scaffold Fabrication (CAR12N)
2.2. Preparation of PLGA-Infiltrated CAR12N Scaffolds
2.3. Scaffold Characterization by Light Microscopy
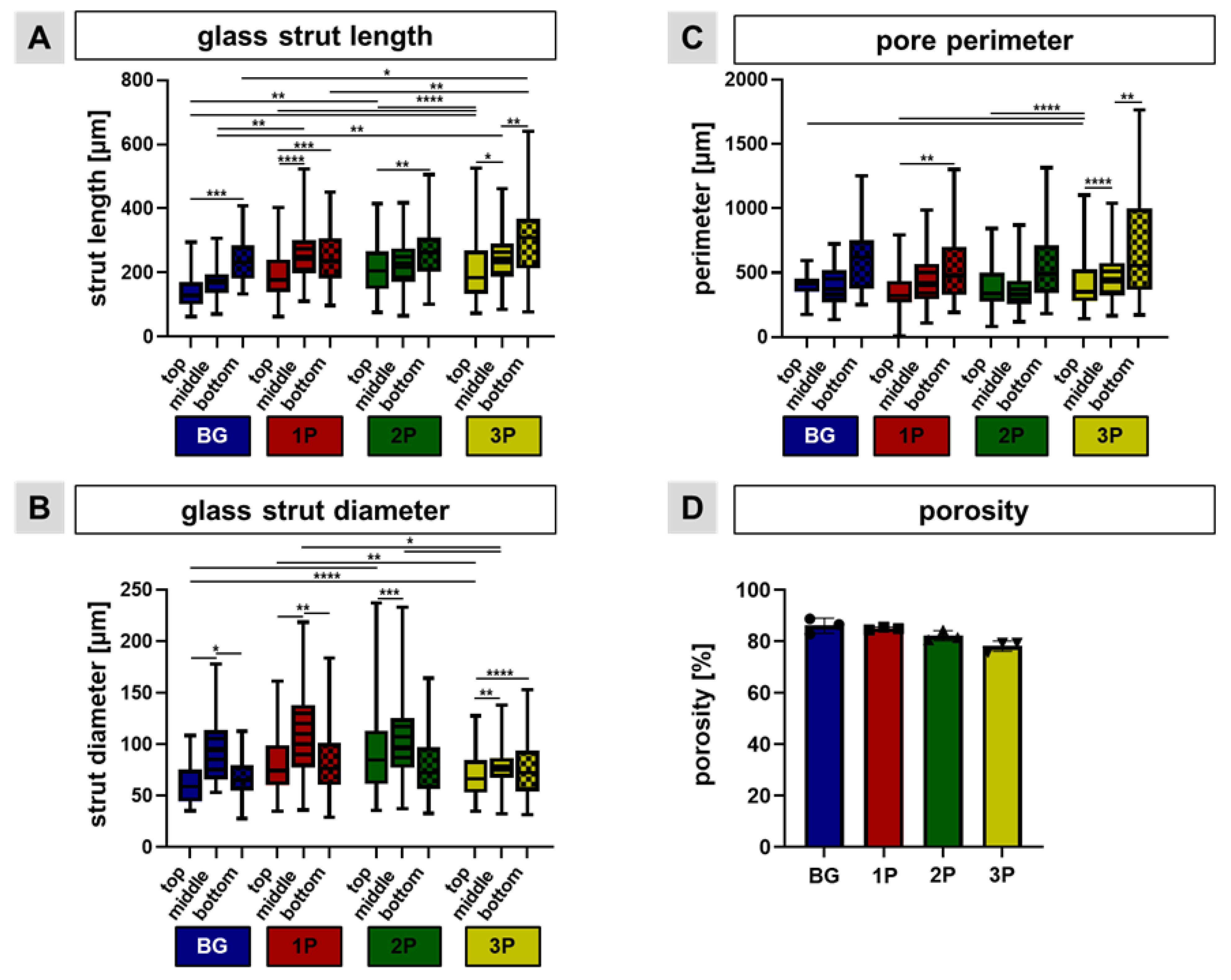
2.4. Pore Perimeter, Strut Length Measurement and Porosity
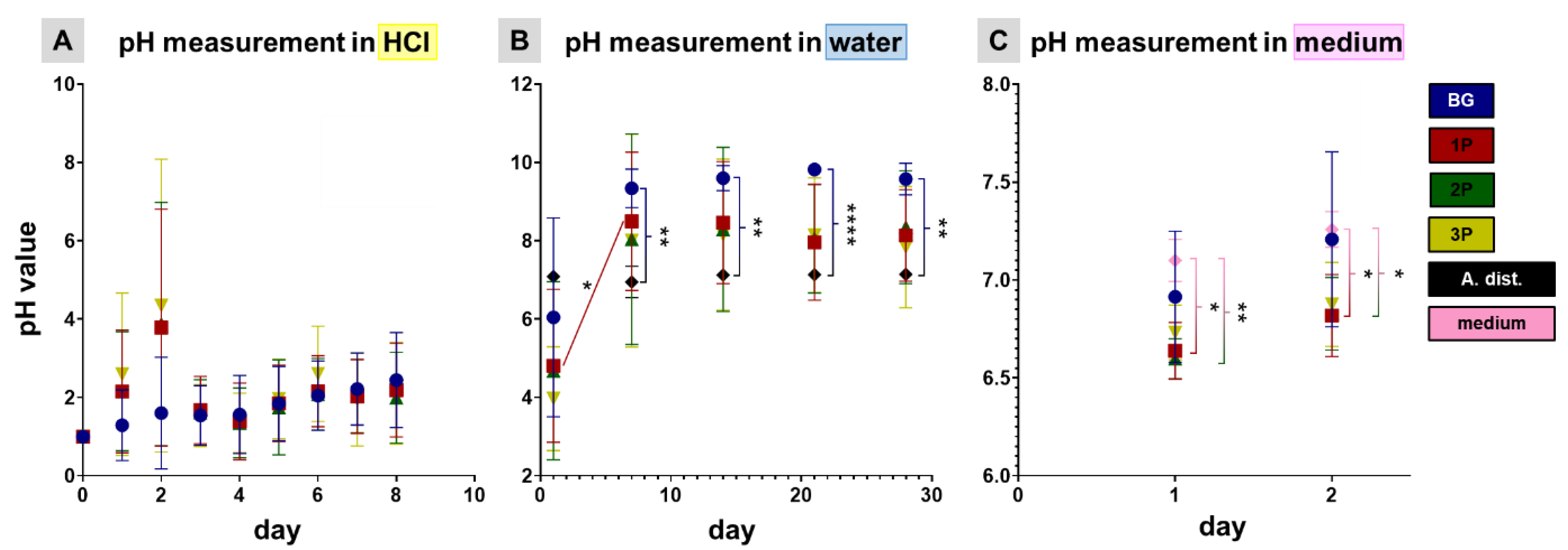
2.5. pH Measurements
2.6. Mechanical Strength Testing
2.7. Cell Isolation, Cultivation and Colonization
2.8. Cytotoxicity Testing
2.9. Viability Assay
2.10. Calculation of the Viability, Colonized Scaffold Surface and Cell Seeding Efficiency
2.11. Scanning Electron Microscopy
2.12. Measurement of Total DNA and sGAG Amount
2.13. Metabolic Activity Evaluation
2.14. Immunocytochemical Staining
2.15. RNA Isolation
2.16. Quantitative Real Time PCR
2.17. Statistical Analysis
3. Results
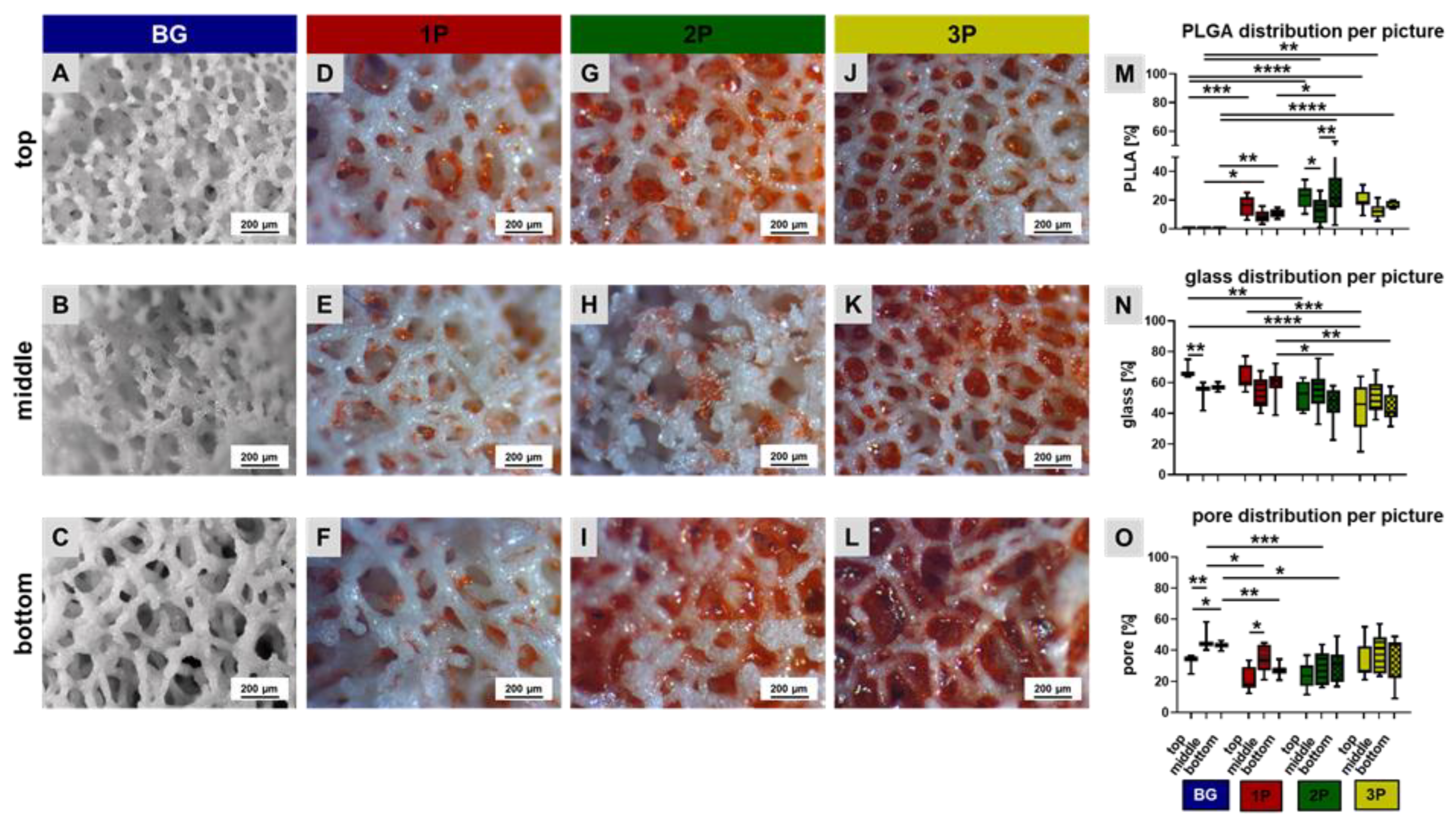
3.1. PLGA Distribution within the Scaffolds
3.2. Scaffold Topology and Polymer Infiltration
3.3. pH Profile during Leaching Process and Gel Layer Formation
3.4. Polymer Infiltration Strengthens Scaffold Structure
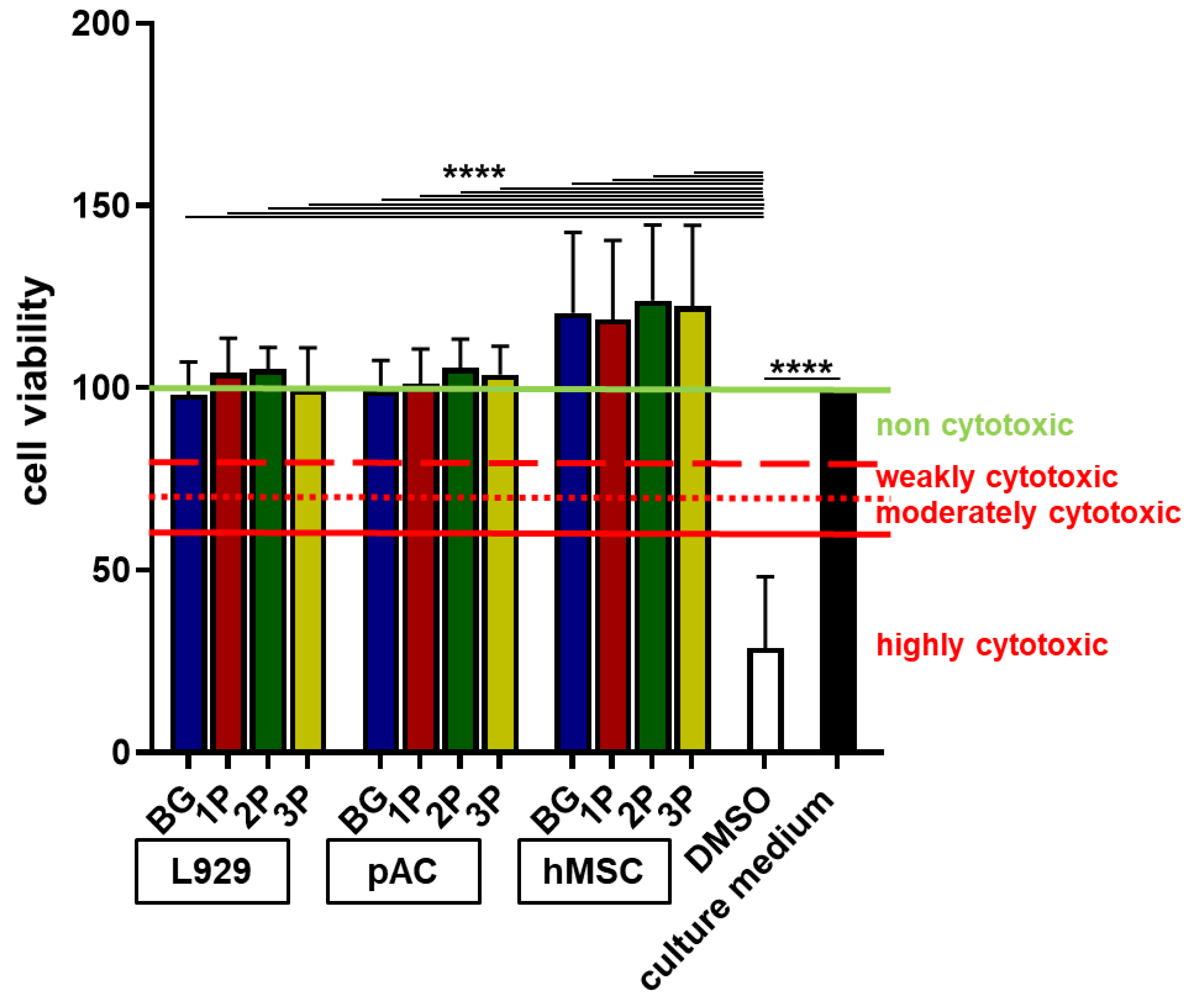
3.5. No Cytotoxic Effects by Polymer Infiltration
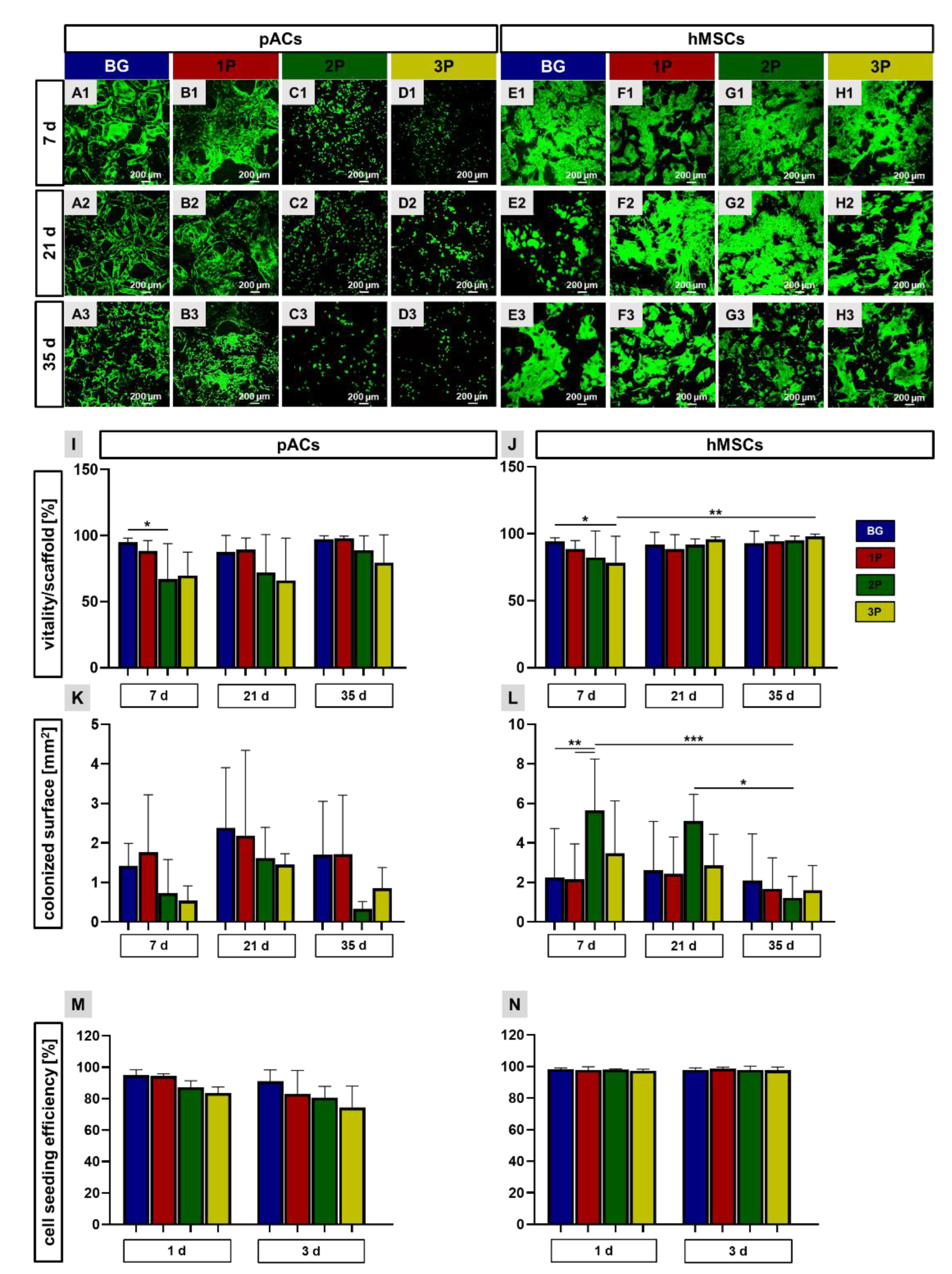
3.6. High Viability over 35 Days Cultivation Period
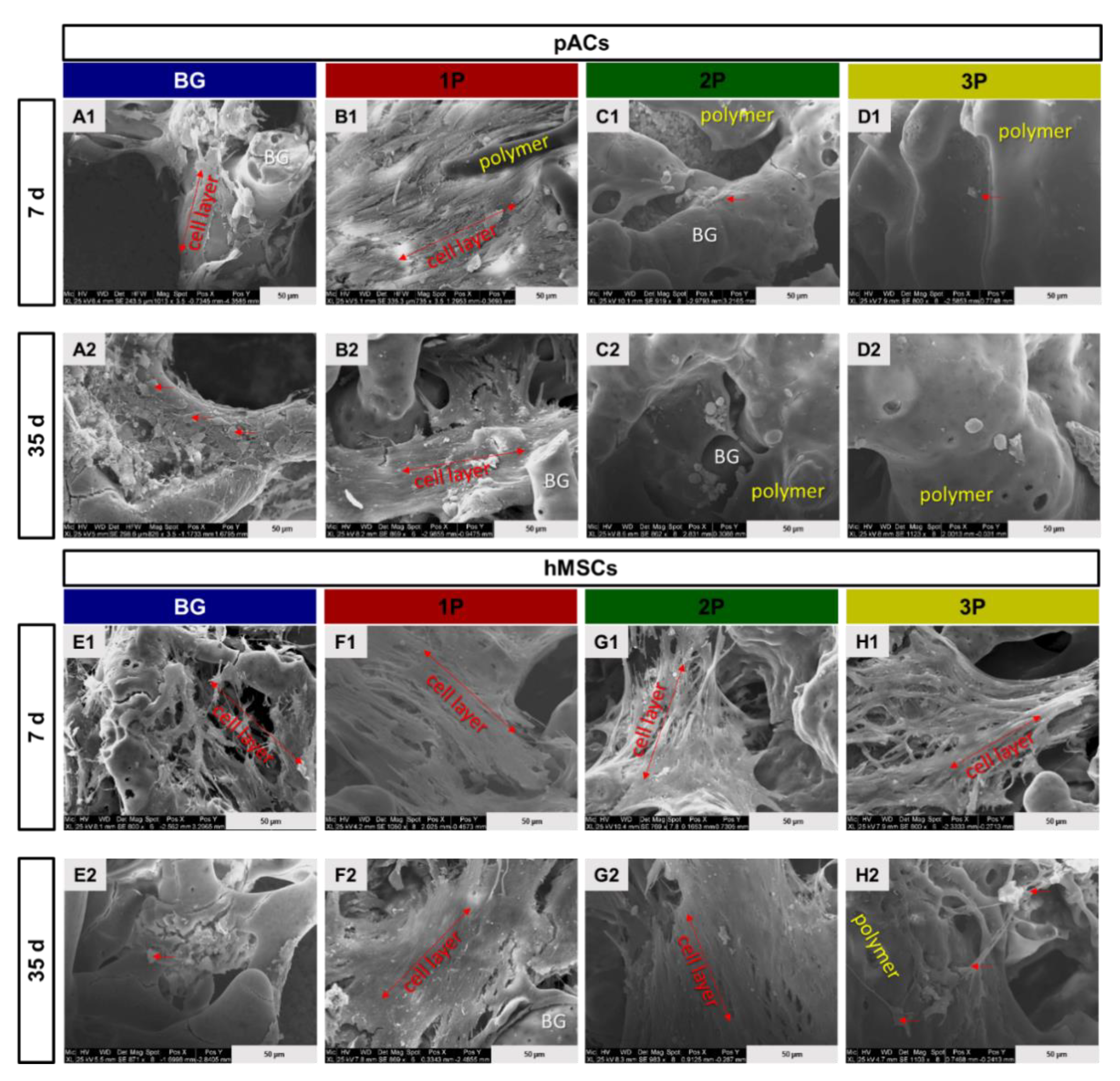
3.7. Scanning Electron Microscopy Analysis
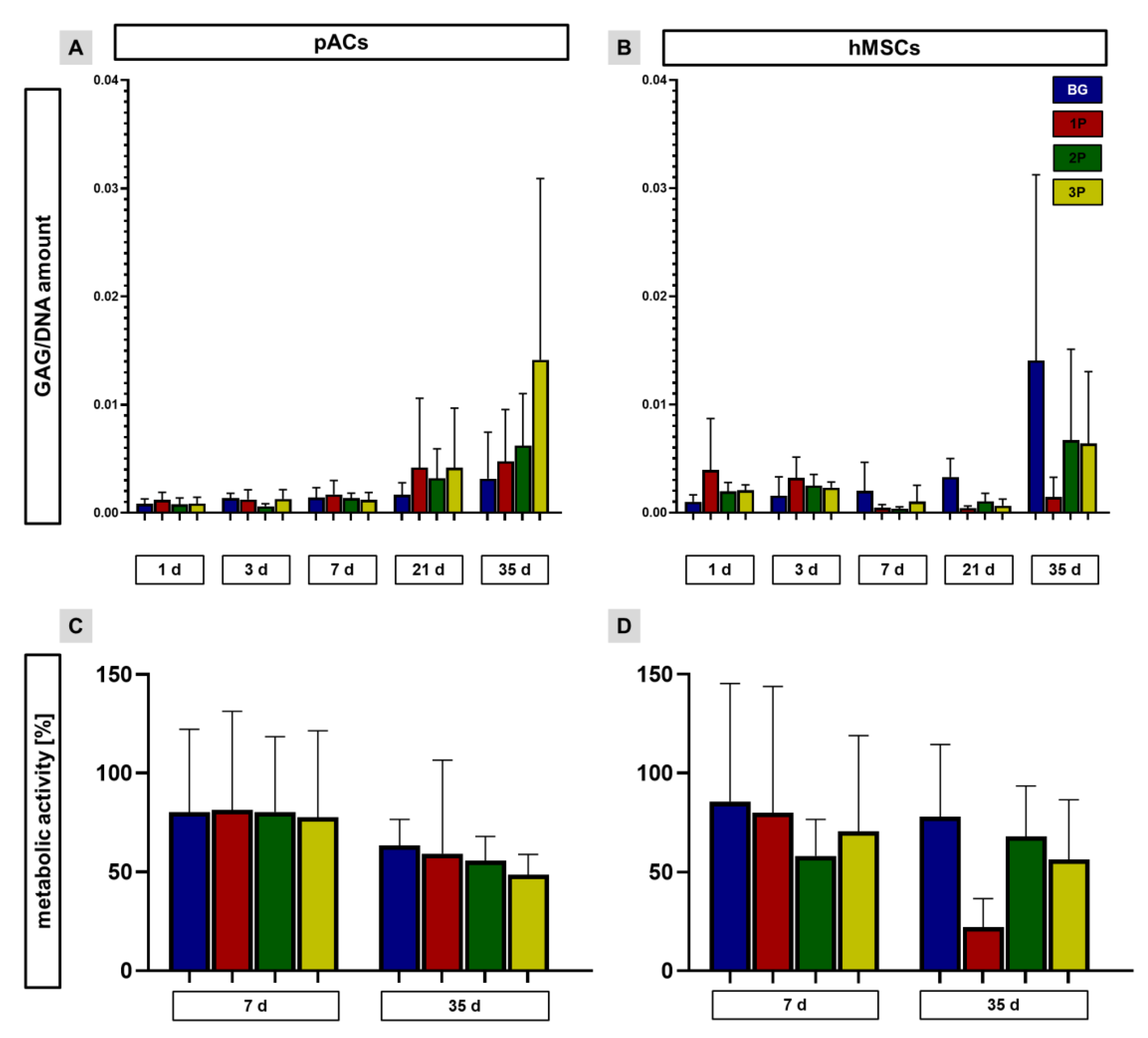
3.8. Sulfated Glycosaminoglycan per DNA Contents and Metabolic Activity Depend on Cell Type and Time Point of Culturing
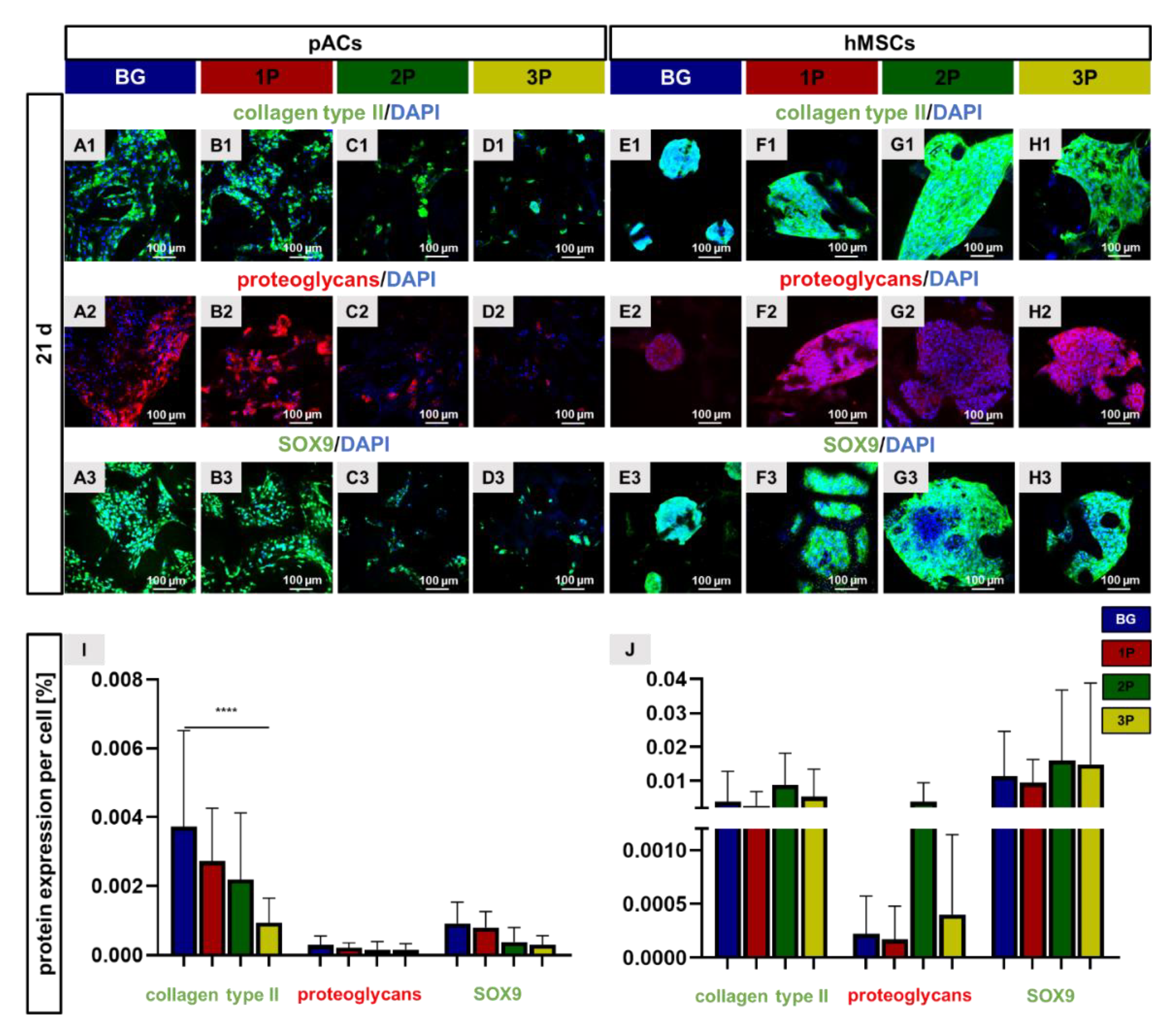
3.9. Cartilage Related Protein Expression
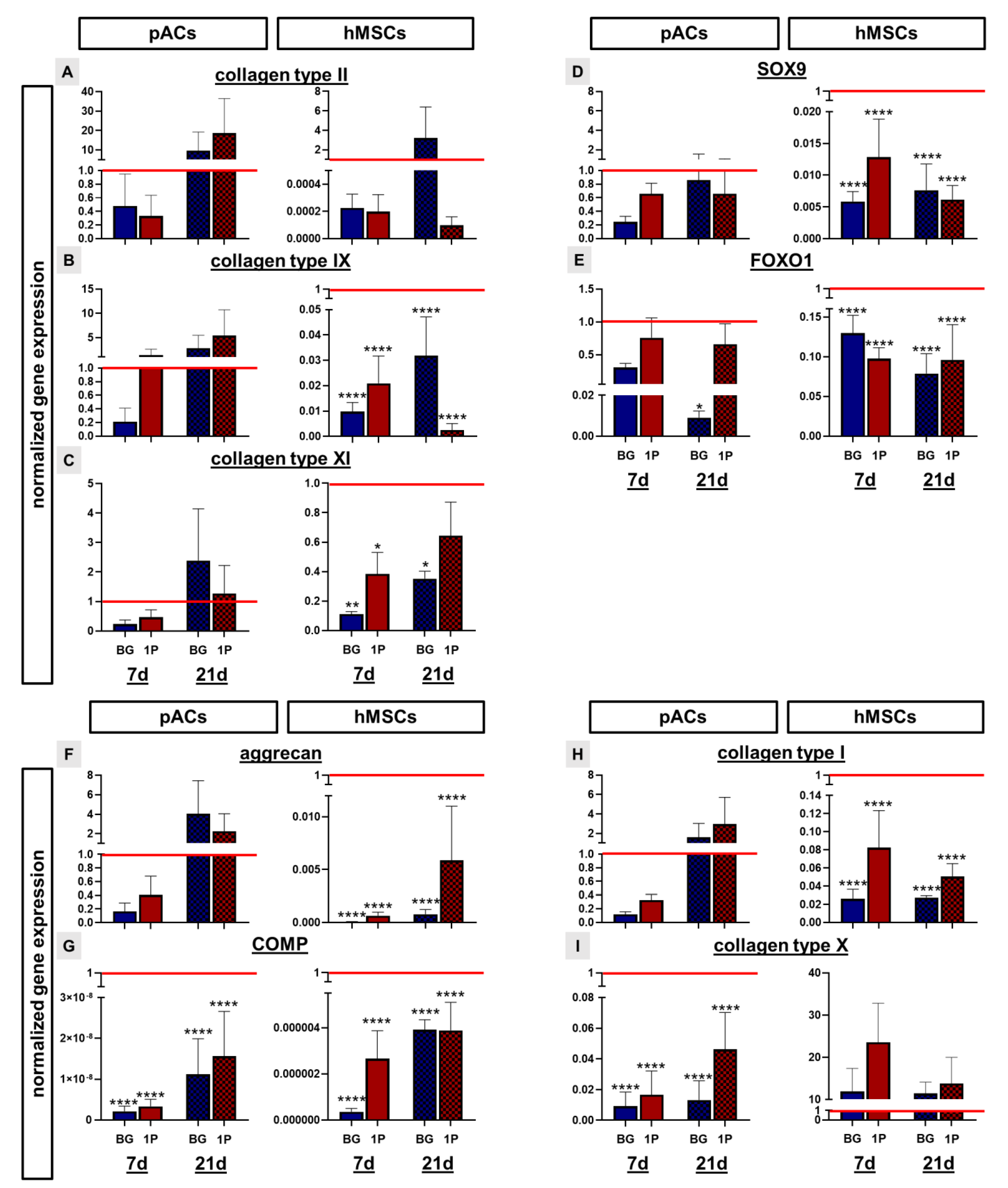
3.10. Non-Induced Human Mesenchymal Stem Cells Express Cartilage-Specific Genes
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Archer, C.W.; Francis-West, P. The chondrocyte. Int. J. Biochem. Cell Biol. 2003, 35, 401–404. [Google Scholar] [CrossRef]
- Stockwell, R.A. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J. Anat. 1971, 109, 411–421. [Google Scholar] [PubMed]
- Ulrich-Vinther, M.; Maloney, M.D.; Schwarz, E.M.; Rosier, R.; O’Keefe, R.J. Articular cartilage biology. J. Am. Acad. Orthop. Surg. 2003, 11, 421–430. [Google Scholar] [CrossRef]
- Chen, S.; Fu, P.; Wu, H.; Pei, M. Meniscus, articular cartilage and nucleus pulposus: A comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017, 370, 53–70. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, B.; Liu, W.; Wang, P.; Lv, X.; Chen, S.; Liu, H.; Shao, Z. Articular cartilage regeneration: The role of endogenous mesenchymal stem/progenitor cell recruitment and migration. Semin. Arthritis Rheum. 2020, 50, 198–208. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Cimmino, M.A.; Scarpa, R.; Caporali, R.; Parazzini, F.; Zaninelli, A.; Atzeni, F.; Canesi, B. Osteoarthritis: An overview of the disease and its treatment strategies. Semin. Arthritis Rheum. 2005, 35, 1–10. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G. Experimental Therapeutics for the Treatment of Osteoarthritis. J. Exp. Pharmacol. 2021, 13, 101–125. [Google Scholar] [CrossRef]
- Safari, R.; Jackson, J.; Sheffield, D. Digital Self-Management Interventions for People With Osteoarthritis: Systematic Review With Meta-Analysis. J. Med. Internet Res. 2020, 22, e15365. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Brittberg, M. Autologous chondrocyte transplantation. Clin. Orthop. Relat. Res. 1999, 367, S147–S155. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Peterson, L.; Sjogren-Jansson, E.; Tallheden, T.; Lindahl, A. Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J. Bone Joint Surg. Am. 2003, 85, 109–115. [Google Scholar] [CrossRef]
- Brittberg, M. Autologous chondrocyte implantation--technique and long-term follow-up. Injury 2008, 39, S40–S49. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.L.; Schenck, R.C., Jr.; Wascher, D.C.; Treme, G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health 2016, 8, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brittberg, M.; Recker, D.; Ilgenfritz, J.; Saris, D.B.F.; Group, S.E.S. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am. J. Sports Med. 2018, 46, 1343–1351. [Google Scholar] [CrossRef]
- Brittberg, M. Cell carriers as the next generation of cell therapy for cartilage repair: A review of the matrix-induced autologous chondrocyte implantation procedure. Am. J. Sports Med. 2010, 38, 1259–1271. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Gillogly, S.D.; Cole, B.J.; Farr, J.; Arnold, R.; Hussey, K.; Minas, T. Autologous chondrocyte implantation in the patella: A multicenter experience. Am. J. Sports Med. 2014, 42, 1074–1081. [Google Scholar] [CrossRef]
- Kreuz, P.C.; Kalkreuth, R.H.; Niemeyer, P.; Uhl, M.; Erggelet, C. Long-Term Clinical and MRI Results of Matrix-Assisted Autologous Chondrocyte Implantation for Articular Cartilage Defects of the Knee. Cartilage 2019, 10, 305–313. [Google Scholar] [CrossRef]
- Nawaz, S.Z.; Bentley, G.; Briggs, T.W.; Carrington, R.W.; Skinner, J.A.; Gallagher, K.R.; Dhinsa, B.S. Autologous chondrocyte implantation in the knee: Mid-term to long-term results. J. Bone Joint Surg. Am. 2014, 96, 824–830. [Google Scholar] [CrossRef]
- Ogura, T.; Mosier, B.A.; Bryant, T.; Minas, T. A 20-Year Follow-up After First-Generation Autologous Chondrocyte Implantation. Am. J. Sports Med. 2017, 45, 2751–2761. [Google Scholar] [CrossRef]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.C.; Metcalfe, A.; Waugh, N. Autologous chondrocyte implantation in the knee: Systematic review and economic evaluation. Health Technol Assess 2017, 21, 1–294. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Schaumburger, J.; Schubert, T.; Grifka, J.; Behrens, P. [Matrix-associated autologous chondrocyte transplantation (MACT). Minimally invasive technique in the knee]. Oper. Orthop. Traumatol. 2008, 20, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, M.; Marlovits, S.; Eckhoff, G.; Fichtel, I.; Gotzen, L.; Vecsei, V.; Schlegel, J. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthr. Cartil. 2002, 10, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze-Tanzil, G. Activation and dedifferentiation of chondrocytes: Implications in cartilage injury and repair. Ann. Anat. 2009, 191, 325–338. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, K.; Litherland, G.J.; Rai, T.S. Cellular senescence in osteoarthritis pathology. Aging Cell 2017, 16, 210–218. [Google Scholar] [CrossRef]
- Li, J.; Pei, M. Cell senescence: A challenge in cartilage engineering and regeneration. Tissue Eng. Part B Rev. 2012, 18, 270–287. [Google Scholar] [CrossRef]
- Portron, S.; Hivernaud, V.; Merceron, C.; Lesoeur, J.; Masson, M.; Gauthier, O.; Vinatier, C.; Beck, L.; Guicheux, J. Inverse regulation of early and late chondrogenic differentiation by oxygen tension provides cues for stem cell-based cartilage tissue engineering. Cell Physiol. Biochem. 2015, 35, 841–857. [Google Scholar] [CrossRef]
- Hubka, K.M.; Dahlin, R.L.; Meretoja, V.V.; Kasper, F.K.; Mikos, A.G. Enhancing chondrogenic phenotype for cartilage tissue engineering: Monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng. Part B Rev. 2014, 20, 641–654. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.; Zhai, D.Y.; Mauck, R.L.; Burdick, J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng. Part A 2011, 17, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Wasyleczko, M.; Sikorska, W.; Chwojnowski, A. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Stampoultzis, T.; Karami, P.; Pioletti, D.P. Thoughts on cartilage tissue engineering: A 21st century perspective. Curr. Res. Transl. Med. 2021, 69, 103299. [Google Scholar] [CrossRef]
- Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalkan, R.; Nwekwo, C.W.; Adali, T. The Use of Scaffolds in Cartilage Regeneration. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Wang, X.; Li, J.; Deng, C.; Liu, Y.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. 3D printing of Mo-containing scaffolds with activated anabolic responses and bi-lineage bioactivities. Theranostics 2018, 8, 4372–4392. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Marquardt, L.M.; Day, D.; Sakiyama-Elbert, S.E.; Harkins, A.B. Effects of borate-based bioactive glass on neuron viability and neurite extension. J. Biomed. Mater. Res. A 2014, 102, 2767–2775. [Google Scholar] [CrossRef]
- Marza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Pestean, C.; Nagy, A.; Tabaran, F.; Licarete, E.; Suarasan, S.; Dreanca, A.; et al. Skin wound regeneration with bioactive glass-gold nanoparticles ointment. Biomed. Mater. 2019, 14, 025011. [Google Scholar] [CrossRef]
- Lin, R.; Deng, C.; Li, X.; Liu, Y.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Wu, C. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics 2019, 9, 6300–6313. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1245–1256. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109895. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Fonseca, G.F.; Avelino, S.O.M.; Mello, D.C.R.; do Prado, R.F.; Campos, T.M.B.; de Vasconcellos, L.M.R.; de Sousa Triches, E.; Borges, A.L.S. Scaffolds of PCL combined to bioglass: Synthesis, characterization and biological performance. J. Mater. Sci. Mater. Med. 2020, 31, 41. [Google Scholar] [CrossRef]
- Bellucci, D.; Salvatori, R.; Giannatiempo, J.; Anesi, A.; Bortolini, S.; Cannillo, V. A New Bioactive Glass/Collagen Hybrid Composite for Applications in Dentistry. Materials 2019, 12, 2079. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Requena, C.; Weaver, J.D.; Clark, A.Y.; Clift, D.A.; Perez-Amodio, S.; Castano, O.; Zhou, D.W.; Garcia, A.J.; Engel, E. PEG hydrogel containing calcium-releasing particles and mesenchymal stromal cells promote vessel maturation. Acta Biomater. 2018, 67, 53–65. [Google Scholar] [CrossRef]
- Alksne, M.; Kalvaityte, M.; Simoliunas, E.; Rinkunaite, I.; Gendviliene, I.; Locs, J.; Rutkunas, V.; Bukelskiene, V. In vitro comparison of 3D printed polylactic acid/hydroxyapatite and polylactic acid/bioglass composite scaffolds: Insights into materials for bone regeneration. J. Mech. Behav. Biomed. Mater. 2020, 104, 103641. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, E.; Mehdikhani-Nahrkhalaji, M.; Hashemi-Beni, B.; Zargar-Kharazi, A.; Kharaziha, M.J.P.M.S. Preparation, characterization and mechanical assessment of poly (lactide-co-glycolide)/hyaluronic acid/fibrin/bioactive glass nano-composite scaffolds for cartilage tissue engineering applications. Procedia Mater. Sci. 2015, 11, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Conoscenti, G.; Carfi Pavia, F.; Ongaro, A.; Brucato, V.; Goegele, C.; Schwarz, S.; Boccaccini, A.R.; Stoelzel, K.; La Carrubba, V.; Schulze-Tanzil, G. Human nasoseptal chondrocytes maintain their differentiated phenotype on PLLA scaffolds produced by thermally induced phase separation and supplemented with bioactive glass 1393. Connect. Tissue Res. 2019, 60, 344–357. [Google Scholar] [CrossRef]
- Goegele, C.; Wiltzsch, S.; Lenhart, A.; Civilleri, A.; Weiger, T.M.; Schaefer-Eckart, K.; Minnich, B.; Forchheimer, L.; Hornfeck, M.; Schulze-Tanzil, G. Highly porous novel chondro-instructive bioactive glass scaffolds tailored for cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112421. [Google Scholar] [CrossRef]
- Dozza, B.; Lesci, I.G.; Duchi, S.; Della Bella, E.; Martini, L.; Salamanna, F.; Falconi, M.; Cinotti, S.; Fini, M.; Lucarelli, E.; et al. When size matters: Differences in demineralized bone matrix particles affect collagen structure, mesenchymal stem cell behavior, and osteogenic potential. J. Biomed. Mater. Res. A 2017, 105, 1019–1033. [Google Scholar] [CrossRef]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s CT difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Okido, M. Hydroxyapatite coating of titanium implants using hydroprocessing and evaluation of their osteoconductivity. Bioinorg. Chem. Appl. 2012, 2012, 730693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrere, F.; van der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.; de Groot, K.; Layrolle, P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 67, 655–665. [Google Scholar] [CrossRef]
- Shu, R.; McMullen, R.; Baumann, M.J.; McCabe, L.R. Hydroxyapatite accelerates differentiation and suppresses growth of MC3T3-E1 osteoblasts. J. Biomed. Mater. Res. A 2003, 67, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Hukkanen, M.V.; Batten, J.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: Implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.; Costa, M.; Lopes, M.; Silva, R.; Santos, J.; Fernandes, M.J.B. Si3N4-bioglass composites stimulate the proliferation of MG63 osteoblast-like cells and support the osteogenic differentiation of human bone marrow cells. Biomaterials 2002, 23, 4897–4906. [Google Scholar] [CrossRef]
- Johari, B.; Kadivar, M.; Lak, S.; Gholipourmalekabadi, M.; Urbanska, A.M.; Mozafari, M.; Ahmadzadehzarajabad, M.; Azarnezhad, A.; Afshari, S.; Zargan, J.; et al. Osteoblast-seeded bioglass/gelatin nanocomposite: A promising bone substitute in critical-size calvarial defect repair in rat. Int. J. Artif. Organs 2016, 39, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Huang, J. Design and Development of Ceramics and Glasses. In Biology and Engineering of Stem Cell Niches; Academic Press: Cambridge, MA, USA, 2017; pp. 315–329. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate-based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef] [Green Version]
- Goudarzi, Z.; Parvin, N.; Sharifianjazi, F.J.C.I. Formation of hydroxyapatite on surface of SiO2–P2O5–CaO–SrO–ZnO bioactive glass synthesized through sol-gel route. Ceram. Int. 2019, 45, 19323–19330. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, D. Cartilage tissue engineering using PHBV and PHBV/Bioglass scaffolds. Mol. Med. Rep. 2014, 10, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Xue, K.; Li, H.; Sun, J.; Liu, K. Improvement of PHBV scaffolds with bioglass for cartilage tissue engineering. PLoS ONE 2013, 8, e71563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, Y.G.; Lee, J.A.; Kim, Y.S.; Lee, H.Y.; Kim, H.J.; Kang, K.T. Optimal mechanical properties of a scaffold for cartilage regeneration using finite element analysis. J. Tissue Eng. 2019, 10, 2041731419832133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.S.; Jin, R.L.; Oh, H.J.; Truong, M.D.; Choi, B.H.; Park, S.H.; Park, D.Y.; Min, B.H. Sizable Scaffold-Free Tissue-Engineered Articular Cartilage Construct for Cartilage Defect Repair. Artif. Organs 2019, 43, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.; Bass, L.M.; Goldberg, D.J.; Graivier, M.H.; Lorenc, Z.P. Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA). Aesthet. Surg. J. 2018, 38, S13–S17. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Conoscenti, G.; Schneider, T.; Stoelzel, K.; Carfi Pavia, F.; Brucato, V.; Goegele, C.; La Carrubba, V.; Schulze-Tanzil, G. PLLA scaffolds produced by thermally induced phase separation (TIPS) allow human chondrocyte growth and extracellular matrix formation dependent on pore size. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Lara-Padilla, H.; Mendoza-Buenrostro, C.; Cardenas, D.; Rodriguez-Garcia, A.; Rodriguez, C.A. Influence of Controlled Cooling in Bimodal Scaffold Fabrication Using Polymers with Different Melting Temperatures. Materials 2017, 10, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Roether, J.; Boccaccini, A.J.T.i.t.e. Tissue engineering scaffolds from bioactive glass and composite materials. Top. Tissue Eng. 2008, 4, 1–27. [Google Scholar]
- Dehghani, F.; Annabi, N. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666. [Google Scholar] [CrossRef]
- Niu, Y.; Guo, L.; Liu, J.; Shen, H.; Su, J.; An, X.; Yu, B.; Wei, J.; Shin, J.W.; Guo, H.; et al. Bioactive and degradable scaffolds of the mesoporous bioglass and poly(l-lactide) composite for bone tissue regeneration. J. Mater. Chem. B 2015, 3, 2962–2970. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Hillmyer, M.A.; Francis, L.F. Processing and properties of porous poly(L-lactide)/bioactive glass composites. Biomaterials 2004, 25, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jerome, R. Porous poly(alpha-hydroxyacid)/Bioglass composite scaffolds for bone tissue engineering. I: Preparation and in vitro characterisation. Biomaterials 2004, 25, 4185–4194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larranaga, A.; Alonso-Varona, A.; Palomares, T.; Rubio-Azpeitia, E.; Aldazabal, P.; Martin, F.J.; Sarasua, J.R. Effect of bioactive glass particles on osteogenic differentiation of adipose-derived mesenchymal stem cells seeded on lactide and caprolactone based scaffolds. J. Biomed. Mater. Res. A 2015, 103, 3815–3824. [Google Scholar] [CrossRef]
- Tian, T.; Xie, W.; Gao, W.; Wang, G.; Zeng, L.; Miao, G.; Lei, B.; Lin, Z.; Chen, X. Micro-Nano Bioactive Glass Particles Incorporated Porous Scaffold for Promoting Osteogenesis and Angiogenesis in vitro. Front. Chem. 2019, 7, 186. [Google Scholar] [CrossRef]
- Xie, Y.; Lan, X.R.; Bao, R.Y.; Lei, Y.; Cao, Z.Q.; Yang, M.B.; Yang, W.; Wang, Y.B. High-performance porous polylactide stereocomplex crystallite scaffolds prepared by solution blending and salt leaching. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 602–609. [Google Scholar] [CrossRef]
- Rakovsky, A.; Gotman, I.; Rabkin, E.; Gutmanas, E.Y. beta-TCP-polylactide composite scaffolds with high strength and enhanced permeability prepared by a modified salt leaching method. J. Mech. Behav. Biomed. Mater. 2014, 32, 89–98. [Google Scholar] [CrossRef]
- Cannillo, V.; Chiellini, F.; Fabbri, P.; Sola, A.J.C.S. Production of Bioglass® 45S5–Polycaprolactone composite scaffolds via salt-leaching. Compos. Struct. 2010, 92, 1823–1832. [Google Scholar] [CrossRef]
- Pamula, E.; Menaszek, E. In vitro and in vivo degradation of poly(L: -lactide-co-glycolide) films and scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 2063–2070. [Google Scholar] [CrossRef]
- Li, H.; Chang, J. pH-compensation effect of bioactive inorganic fillers on the degradation of PLGA. Compos. Sci. Technol. 2005, 65, 2226–2232. [Google Scholar] [CrossRef]
- Gentleman, E.; Stevens, M.M.; Hill, R.G.; Brauer, D.S. Surface properties and ion release from fluoride-containing bioactive glasses promote osteoblast differentiation and mineralization in vitro. Acta Biomater. 2013, 9, 5771–5779. [Google Scholar] [CrossRef] [Green Version]
- Blochberger, M.; Hupa, L.; Brauer, D.S. Influence of zinc and magnesium substitution on ion release from Bioglass 45S5 at physiological and acidic pH. Biomed. Glas. 2015, 1. [Google Scholar] [CrossRef]
- Da Silva, J.G.; Babb, R.; Salzlechner, C.; Sharpe, P.T.; Brauer, D.S.; Gentleman, E. Optimisation of lithium-substituted bioactive glasses to tailor cell response for hard tissue repair. J. Mater. Sci. 2017, 52, 8832–8844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Fiqi, A.; Mandakhbayar, N.; Jo, S.B.; Knowles, J.C.; Lee, J.H.; Kim, H.W. Nanotherapeutics for regeneration of degenerated tissue infected by bacteria through the multiple delivery of bioactive ions and growth factor with antibacterial/angiogenic and osteogenic/odontogenic capacity. Bioact. Mater. 2021, 6, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Cholewa-Kowalska, K.; Kokoszka, J.; Laczka, M.; Niedzwiedzki, L.; Madej, W.; Osyczka, A.M. Gel-derived bioglass as a compound of hydroxyapatite composites. Biomed. Mater. 2009, 4, 055007. [Google Scholar] [CrossRef]
- Xie, D.; Zhao, J.; Weng, Y.; Park, J.G.; Jiang, H.; Platt, J.A. Bioactive glass-ionomer cement with potential therapeutic function to dentin capping mineralization. Eur. J. Oral. Sci. 2008, 116, 479–487. [Google Scholar] [CrossRef]
- Yli-Urpo, H.; Vallittu, P.K.; Narhi, T.O.; Forsback, A.P.; Vakiparta, M. Release of silica, calcium, phosphorus, and fluoride from glass ionomer cement containing bioactive glass. J. Biomater. Appl. 2004, 19, 5–20. [Google Scholar] [CrossRef]
- Jebahi, S.; Oudadesse, H.; Jardak, N.; Khayat, I.; Keskes, H.; Khabir, A.; Rebai, T.; El Feki, H. Biological therapy of strontium-substituted bioglass for soft tissue wound-healing: Responses to oxidative stress in ovariectomised rats. Ann. Pharm. Fr. 2013, 71, 234–242. [Google Scholar] [CrossRef] [Green Version]
- de Laia, A.G.S.; Barrioni, B.R.; Valverde, T.M.; de Goes, A.M.; de Sá, M.A.; Pereira, M.d.M.J.J.o.M.S. Therapeutic cobalt ion incorporated in poly (vinyl alcohol)/bioactive glass scaffolds for tissue engineering. J. Mater. Sci. 2020, 55, 8710–8727. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, L.; Liu, Z.; Geng, B.; Teng, Y.; Liu, X.; Yi, Q.; Yu, D.; Chen, X.; Zhao, D.; et al. Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: Potential therapeutic targets for osteoarthritis. Channels 2021, 15, 339–359. [Google Scholar] [CrossRef]
- Melchels, F.P.; Barradas, A.M.; van Blitterswijk, C.A.; de Boer, J.; Feijen, J.; Grijpma, D.W. Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomater. 2010, 6, 4208–4217. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Song, K.; Jiang, S.; Chen, J.; Tang, L.; Li, S.; Fan, J.; Wang, Y.; Zhao, J.; Liu, T. Numerical Simulation of Mass Transfer and Three-Dimensional Fabrication of Tissue-Engineered Cartilages Based on Chitosan/Gelatin Hybrid Hydrogel Scaffold in a Rotating Bioreactor. Appl. Biochem. Biotechnol. 2017, 181, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Eswaramoorthy, R.; Mulhall, K.J.; Kelly, D.J. Decellularization of porcine articular cartilage explants and their subsequent repopulation with human chondroprogenitor cells. J. Mech. Behav. Biomed. Mater. 2015, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, H.; Rais, Y.; Miosge, N.; Ornan, E.M. The primary cilium as a dual sensor of mechanochemical signals in chondrocytes. Cell Mol. Life Sci. 2012, 69, 2101–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGlashan, S.R.; Jensen, C.G.; Poole, C.A. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J. Histochem. Cytochem. 2006, 54, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Kim, Y.H.; Park, K.D.; Jee, K.S.; Shin, J.W.; Hahn, S.B. Importance of integrin beta1-mediated cell adhesion on biodegradable polymers under serum depletion in mesenchymal stem cells and chondrocytes. Biomaterials 2004, 25, 1901–1909. [Google Scholar] [CrossRef]
- Chan, T.M.; Harn, H.J.; Lin, H.P.; Chou, P.W.; Chen, J.Y.; Ho, T.J.; Chiou, T.W.; Chuang, H.M.; Chiu, S.C.; Chen, Y.C.; et al. Improved human mesenchymal stem cell isolation. Cell Transplant. 2014, 23, 399–406. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Zhu, X.; Assoian, R.K. Integrin-dependent activation of MAP kinase: A link to shape-dependent cell proliferation. Mol. Biol. Cell 1995, 6, 273–282. [Google Scholar] [CrossRef] [Green Version]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, V.; Angelozzi, M.; Haseeb, A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019, 61, 39–47. [Google Scholar] [CrossRef]
- Ng, L.J.; Wheatley, S.; Muscat, G.E.; Conway-Campbell, J.; Bowles, J.; Wright, E.; Bell, D.M.; Tam, P.P.; Cheah, K.S.; Koopman, P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 1997, 183, 108–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, H.; Oh, C.D.; Chen, D.; de Crombrugghe, B.; Kim, J.H. A Novel Regulatory Mechanism of Type II Collagen Expression via a SOX9-dependent Enhancer in Intron 6. J. Biol. Chem. 2017, 292, 528–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szychlinska, M.A.; Calabrese, G.; Ravalli, S.; Parrinello, N.L.; Forte, S.; Castrogiovanni, P.; Pricoco, E.; Imbesi, R.; Castorina, S.; Leonardi, R.; et al. Cycloastragenol as an Exogenous Enhancer of Chondrogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. A Morphological Study. Cells 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, D.A.; Larsson, H.C.E. The tissues and regulatory pattern of limb chondrogenesis. Dev. Biol. 2020, 463, 124–134. [Google Scholar] [CrossRef]
- Wang, C.; Shen, J.; Ying, J.; Xiao, D.; O’Keefe, R.J. FoxO1 is a crucial mediator of TGF-beta/TAK1 signaling and protects against osteoarthritis by maintaining articular cartilage homeostasis. Proc. Natl. Acad. Sci. USA 2020, 117, 30488–30497. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Alvarez-Garcia, O.; Mokuda, S.; Nagira, K.; Olmer, M.; Gamini, R.; Miyata, K.; Akasaki, Y.; Su, A.I.; Asahara, H.; et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Meng, Q.C.; Yang, X.F.; Mu, W.D. TGF-beta1/WISP1/Integrin-alpha interaction mediates human chondrocytes dedifferentiation. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8675–8684. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Onnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef] [Green Version]
- Dudhia, J. Aggrecan, aging and assembly in articular cartilage. Cell Mol. Life Sci. 2005, 62, 2241–2256. [Google Scholar] [CrossRef]
- Wang, P.Y.; Chow, H.H.; Tsai, W.B.; Fang, H.W. Modulation of gene expression of rabbit chondrocytes by dynamic compression in polyurethane scaffolds with collagen gel encapsulation. J. Biomater. Appl. 2009, 23, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Chery, D.R.; Han, B.; Zhou, Y.; Wang, C.; Adams, S.M.; Chandrasekaran, P.; Kwok, B.; Heo, S.J.; Enomoto-Iwamoto, M.; Lu, X.L.; et al. Decorin regulates cartilage pericellular matrix micromechanobiology. Matrix Biol. 2021, 96, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, P.; Siegrist, M.; Hunziker, E.B.; Wong, M. The mechanosensitivity of cartilage oligomeric matrix protein (COMP). Biorheology 2003, 40, 101–109. [Google Scholar] [PubMed]
- Antunes, B.P.; Vainieri, M.L.; Alini, M.; Monsonego-Ornan, E.; Grad, S.; Yayon, A. Enhanced chondrogenic phenotype of primary bovine articular chondrocytes in Fibrin-Hyaluronan hydrogel by multi-axial mechanical loading and FGF18. Acta Biomater. 2020, 105, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Zanardi, L.; Hench, L.; Cannas, M. Type I collagen production by osteoblast-like cells cultured in contact with different bioactive glasses. J. Biomed. Mater. Res. 2002, 64A, 189–195. [Google Scholar] [CrossRef]
- Meretoja, V.V.; Dahlin, R.L.; Wright, S.; Kasper, F.K.; Mikos, A.G. Articular chondrocyte redifferentiation in 3D co-cultures with mesenchymal stem cells. Tissue Eng. Part C Methods 2014, 20, 514–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Chemical | Patented Mass [%] |
|---|---|
| Silicon dioxide (SiO2) | 62.7 |
| Sodium oxide (Na2O) | 25.6 |
| Diphospho pentoxide (P2O5) | 7.0 |
| Boron oxide (B2O3) | 2.0 |
| Potassium oxide (K2O) | 1.0 |
| Potassium nitrate (KNO3) | 0.5 |
| Sodium sulfate (Na2SO4) | 0.4 |
| Sodium chloride (NaCl) | 0.3 |
| Sodium fluoride (NaF) | 0.2 |
| Titanium oxide (TiO2) | 0.12 |
| Yttria stable Zirconia (ZrO2 + Y2O3) | 0.1 |
| Zinc oxide (ZnO) | 0.06 |
| Copper oxide (CuO) | 0.02 |
| total | 100.00 |
| Target | Primary Antibody | Dilution | Secondary Antibody | Dilution |
|---|---|---|---|---|
| type II collagen | Rabbit anti human, Acris Laboratories, Hiddenhausen, Germany | 1:50 | donkey-anti-rabbit, Alexa Fluor 488, Invitrogen | 1:200 |
| cartilage proteoglycans | Mouse anti human, Chemicon International, CA, USA | 1:70 | Donkey-anti-mouse; Cy3, Invitrogen | 1:200 |
| SOX9 | Rabbit anti human, Merck, Darmstadt, Germany | 1:100 | donkey-anti-rabbit, Alexa Fluor 488, Invitrogen | 1:200 |
| Gene Symbol | Species | Gene Name | Efficacy | Amplicon Length (Base Pairs) | Assay ID # |
|---|---|---|---|---|---|
| ACAN | Homo sapiens | Aggrecan | 1.95 | 93 | Hs00202971_m1 |
| ACAN | Sus scrofa | Aggrecan | 1.69 | 60 | Ss03374823_m1 |
| ACTB | Homo sapiens | β-actin | 1.89 | 171 | Hs99999903_m1 |
| ACTB | Sus scrofa | β-actin | 1.71 | 77 | Ss03376081_u1 |
| Col1A1 | Homo sapiens | Type I collagen | 2.06 | 66 | Hs00164004_m1 |
| Col1A1 | Sus scrofa | Type I collagen | 1.53 | 74 | Ss03373340_m1 |
| Col2A1 | Homo sapiens | Type II collagen | 2.06 (1.9 *) | 124 | Hs00264051_m1 |
| Col9A3 | Homo sapiens | Type IX collagen | 1.99 # | 52 | Hs00951243_m1 |
| Col9A3 | Sus scrofa | Type IX collagen | 1.92 | 70 | Ss06885389_m1 |
| Col10A1 | Homo sapiens | Type X collagen | 1.75 | 76 | Hs00166657_m1 |
| Col10A1 | Sus scrofa | Type X collagen | 1.87 | 85 | Ss03391766_m1 |
| Col11A1 | Homo sapiens | Type XI collagen | 1.80 # | 98 | Hs00266273_m1 |
| Col11A1 | Sus scrofa | Type XI collagen | 1.74 | 86 | SS03373534_m1 |
| COMP | Homo sapiens | Cartilage oligomeric matrix proteine | 2.21# | 101 | Hs00164359_m1 |
| COMP | Sus scrofa | Cartilage oligomeric matrix proteine | 1.76 | 117 | Ss03375728_u1 |
| FOXO1 | Homo sapiens | Forkhead box O 1 | 1.84 # | 103 | Hs 00231106_m1 |
| FOXO1 | Sus scrofa | Forkhead box O 1 | 1.56 | 107 | Ss03388140_s1 |
| SOX9 | Homo sapiens | SOX9 | 1.92 | 102 | Hs00165814_m1 |
| SOX9 | Sus scrofa | SOX9 | 1.57 | 145 | Ss03392406_m1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gögele, C.; Müller, S.; Belov, S.; Pradel, A.; Wiltzsch, S.; Lenhart, A.; Hornfeck, M.; Kerling, V.; Rübling, A.; Kühl, H.; et al. Biodegradable Poly(D-L-lactide-co-glycolide) (PLGA)-Infiltrated Bioactive Glass (CAR12N) Scaffolds Maintain Mesenchymal Stem Cell Chondrogenesis for Cartilage Tissue Engineering. Cells 2022, 11, 1577. https://doi.org/10.3390/cells11091577
Gögele C, Müller S, Belov S, Pradel A, Wiltzsch S, Lenhart A, Hornfeck M, Kerling V, Rübling A, Kühl H, et al. Biodegradable Poly(D-L-lactide-co-glycolide) (PLGA)-Infiltrated Bioactive Glass (CAR12N) Scaffolds Maintain Mesenchymal Stem Cell Chondrogenesis for Cartilage Tissue Engineering. Cells. 2022; 11(9):1577. https://doi.org/10.3390/cells11091577
Chicago/Turabian StyleGögele, Clemens, Silvana Müller, Svetlana Belov, Andreas Pradel, Sven Wiltzsch, Armin Lenhart, Markus Hornfeck, Vera Kerling, Achim Rübling, Hannes Kühl, and et al. 2022. "Biodegradable Poly(D-L-lactide-co-glycolide) (PLGA)-Infiltrated Bioactive Glass (CAR12N) Scaffolds Maintain Mesenchymal Stem Cell Chondrogenesis for Cartilage Tissue Engineering" Cells 11, no. 9: 1577. https://doi.org/10.3390/cells11091577
APA StyleGögele, C., Müller, S., Belov, S., Pradel, A., Wiltzsch, S., Lenhart, A., Hornfeck, M., Kerling, V., Rübling, A., Kühl, H., Schäfer-Eckart, K., Minnich, B., Weiger, T. M., & Schulze-Tanzil, G. (2022). Biodegradable Poly(D-L-lactide-co-glycolide) (PLGA)-Infiltrated Bioactive Glass (CAR12N) Scaffolds Maintain Mesenchymal Stem Cell Chondrogenesis for Cartilage Tissue Engineering. Cells, 11(9), 1577. https://doi.org/10.3390/cells11091577








