Reciprocal Regulation between lncRNA ANRIL and p15 in Steroid-Induced Glaucoma
Abstract
1. Introduction
2. Results
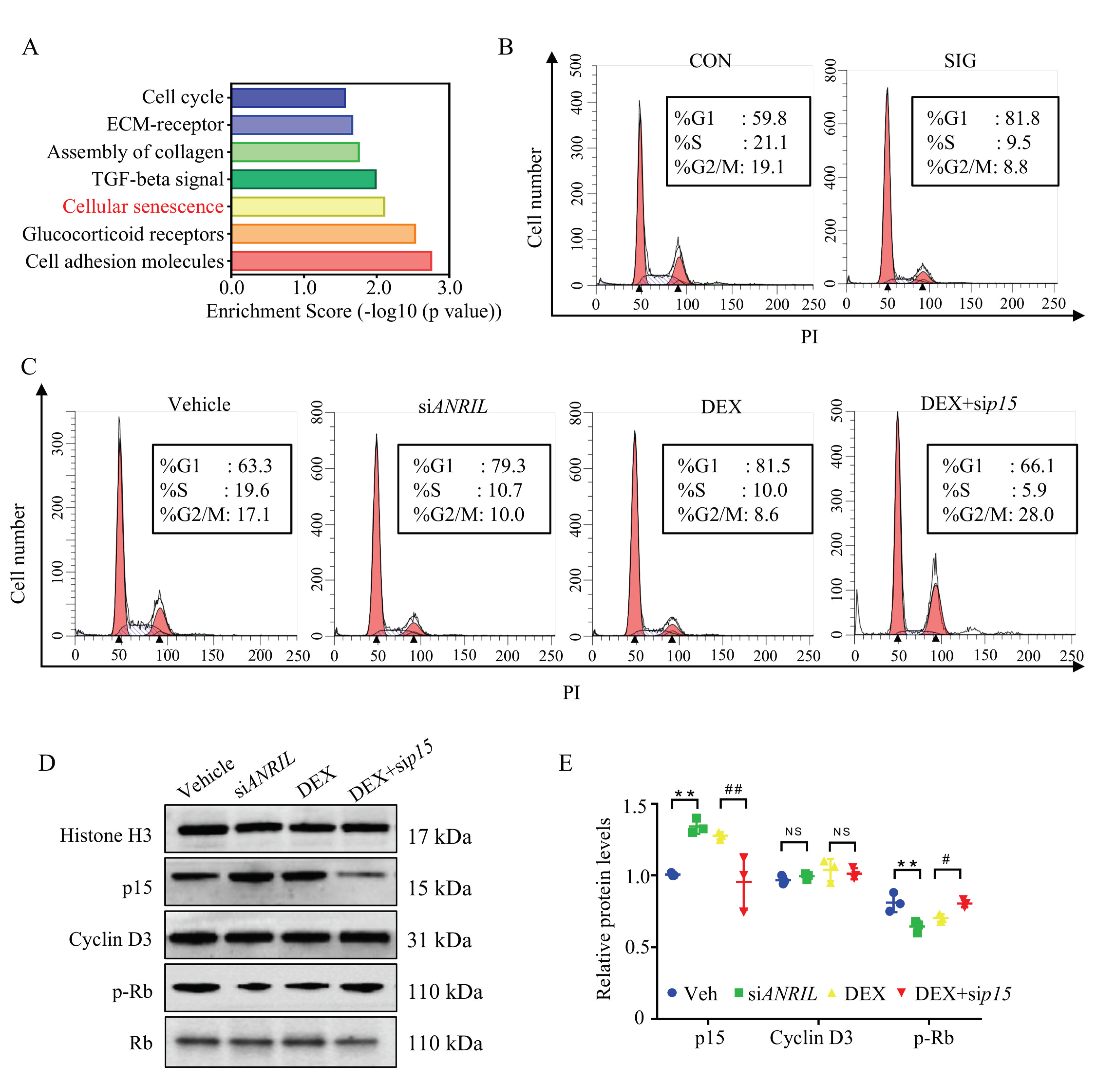
2.1. Transcriptome Landscape in TM from SIG Models
2.2. Correlation between ANRIL/p15 and SIG Clinical Manifestations
2.3. ANRIL Is Poorly Expressed in SIG Whilst p15 Is Highly Expressed
2.4. Role of ANRIL/p15 in Regulating Cellular Senescence in Response to Steroid
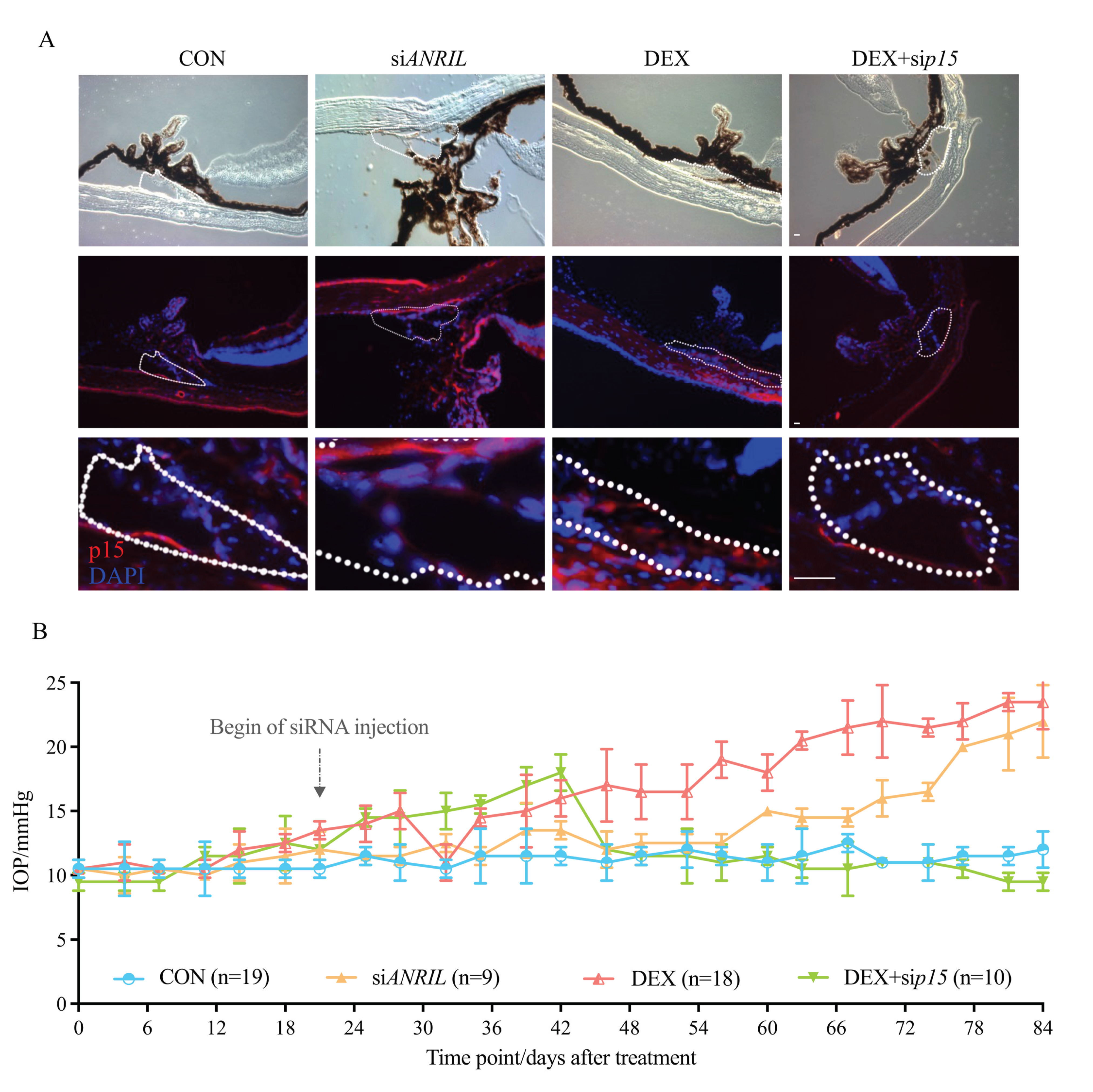
2.5. ANRIL/p15 Signaling Promotes IOP Elevation in Response to DEX
2.6. ANRIL/p15 Signaling Promotes SIG via TM Cell Senescence
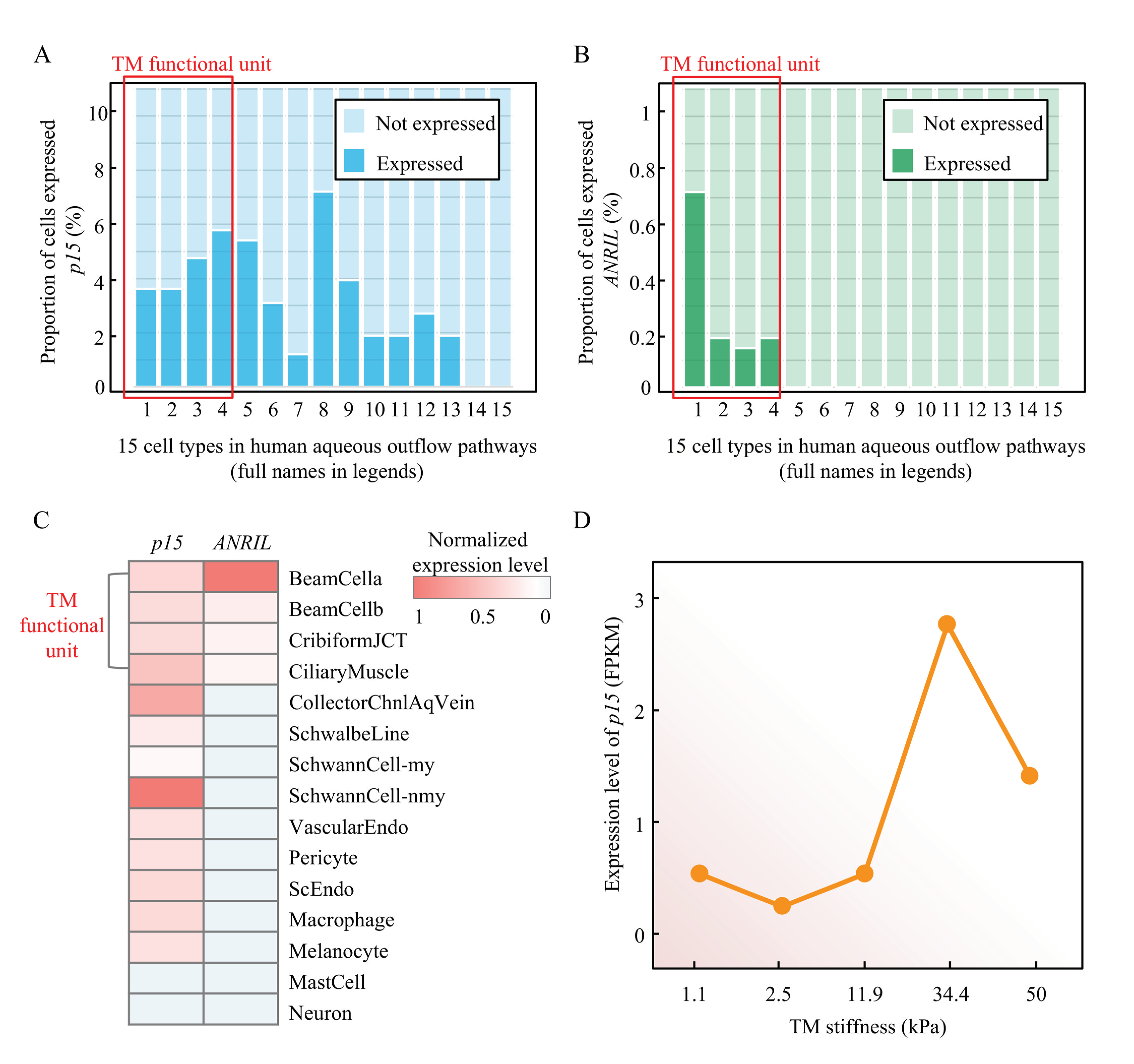
2.7. ANRIL/p15 Signaling Regulated TM Stiffness in HUMAN Samples
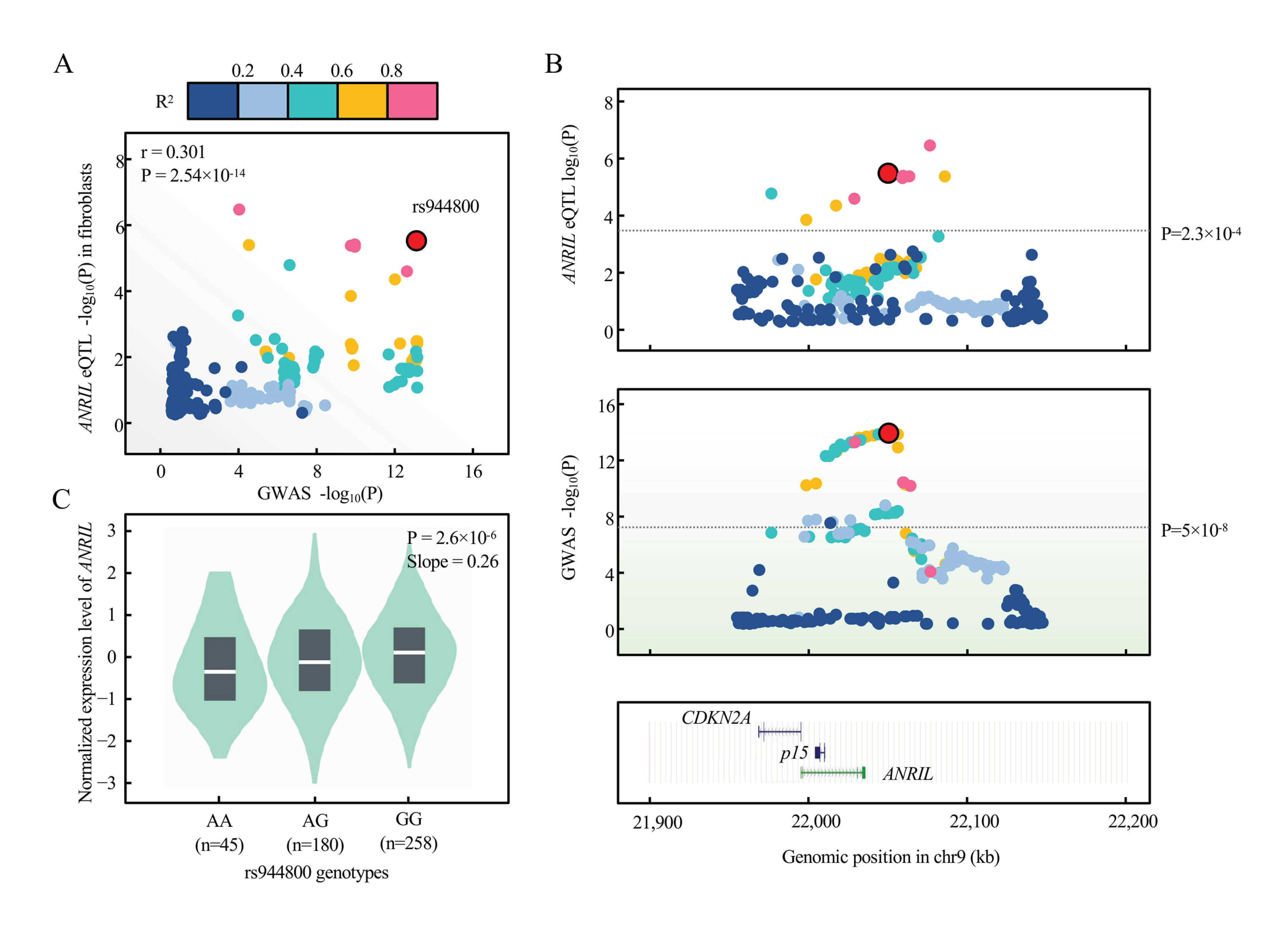
2.8. Evidence of ANRIL Underlying Glaucoma Genetic Susceptibility
3. Discussion
4. Materials and Methods
4.1. Establishment of the Steroid Induced Glaucoma (SIG) Model
4.2. Establishment of the Steroid Induced Glaucoma (SIG) Model
4.3. Intraocular Pressure (IOP) Measurement
4.4. Intracameral Delivery of siRNA
4.5. Mouse Anterior Segment Isolation
4.6. Mouse TM Tissues Isolation
4.7. Mouse TM Samples Microarray Profiling
4.8. Fundus and Optical Coherence Tomography (OCT) Examination
4.9. Mouse TM Cells Maintenance and Treatment
4.10. DEX Treatment in TM Cells
4.11. Gene Knockdown in TM Cells
4.12. Luciferase Report Assay
4.13. Cell Cycle Analysis
4.14. Immunofluorescence (IF) Staining
4.15. Immunohistochemistry Labeling
4.16. Nuclear Extracts
4.17. Real-Time PCR
4.18. Western Blot
4.19. Bioinformatic Analyses of Human TM Expression Profile
4.20. Colocalization between GWAS and eQTL Signals
4.21. Statistics and Reproducibility
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abuse, N.I.O.D. Steroids Trends & Statistics. 2019. Available online: https://www.drugabuse.gov/drug-topics/steroids-anabolic/steroids-trends-statistics (accessed on 19 September 2020).
- Gupta, S.; Shah, P.; Grewal, S.; Chaurasia, A.K.; Gupta, V. Steroid-induced glaucoma and childhood blindness. Br. J. Ophthalmol. 2015, 99, 1454–1456. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.W.; Müskens, R.P.; Ramdas, W.D.; Wolfs, R.C.; De Jong, P.T.; Vingerling, J.R.; Hofman, A.; Stricker, B.H.; Jansonius, N.M. Corticosteroids and open-angle glaucoma in the elderly: A population-based cohort study. Drugs Aging 2012, 29, 963–970. [Google Scholar] [CrossRef]
- Bucolo, C.; Gozzo, L.; Longo, L.; Mansueto, S.; Vitale, D.C.; Drago, F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: A systematic review of real-world studies. J. Pharmacol. Sci. 2018, 138, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Armaly, M.F.; Becker, B. Intraocular pressure response to topical corticosteroids. Fed. Proc. 1965, 24, 1274–1278. [Google Scholar]
- Becker, B. Intraocular Pressure Response to Topical Corticosteroids. Investig. Ophthalmol. 1965, 4, 198–205. [Google Scholar]
- Bollinger, K.E.; Smith, S.D. Prevalence and management of elevated intraocular pressure after placement of an intravitreal sustained-release steroid implant. Curr. Opin. Ophthalmol. 2009, 20, 99–103. [Google Scholar] [CrossRef]
- Kersey, J.P.; Broadway, D.C. Corticosteroid-induced glaucoma: A review of the literature. Eye (Lond.) 2006, 20, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Phulke, S.; Kaushik, S.; Kaur, S.; Pandav, S.S. Steroid-induced Glaucoma: An Avoidable Irreversible Blindness. J. Curr. Glaucoma Pract. 2017, 11, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Nuyen, B.; Weinreb, R.N.; Robbins, S.L. Steroid-induced glaucoma in the pediatric population. J. AAPOS 2017, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Aga, M.; Bradley, J.M.; Kelley, M.J.; Acott, T.S. Extracellular matrix turnover and outflow resistance. Exp. Eye Res. 2009, 88, 676–682. [Google Scholar] [CrossRef]
- Johnson, D.H.; Bradley, J.M.; Acott, T.S. The effect of dexamethasone on glycosaminoglycans of human trabecular meshwork in perfusion organ culture. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2568–2571. [Google Scholar]
- Yun, A.J.; Murphy, C.G.; Polansky, J.R.; Newsome, D.A.; Alvarado, J.A. Proteins secreted by human trabecular cells. Glucocorticoid and other effects. Investig. Ophthalmol. Vis. Sci. 1989, 30, 2012–2022. [Google Scholar]
- Samples, J.R.; Alexander, J.P.; Acott, T.S. Regulation of the levels of human trabecular matrix metalloproteinases and inhibitor by interleukin-1 and dexamethasone. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3386–3395. [Google Scholar]
- Porter, K.; Hirt, J.; Stamer, W.D.; Liton, P.B. Autophagic dysregulation in glaucomatous trabecular meshwork cells. Biochim. Biophys. Acta 2015, 1852, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sacca, S.C.; Pascotto, A.; Camicione, P.; Capris, P.; Izzotti, A. Oxidative DNA damage in the human trabecular meshwork: Clinical correlation in patients with primary open-angle glaucoma. Arch. Ophthalmol. 2005, 123, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Zhavoronkov, A.; Izumchenko, E.; Kanherkar, R.R.; Teka, M.; Cantor, C.; Manaye, K.; Sidransky, D.; West, M.D.; Makarev, E.; Csoka, A.B. Pro-fibrotic pathway activation in trabecular meshwork and lamina cribrosa is the main driving force of glaucoma. Cell Cycle 2016, 15, 1643–1652. [Google Scholar] [CrossRef]
- Hong, M.; Tao, S.; Zhang, L.; Diao, L.T.; Huang, X.; Huang, S.; Xie, S.J.; Xiao, Z.D.; Zhang, H. RNA sequencing: New technologies and applications in cancer research. J. Hematol. Oncol. 2020, 13, 166. [Google Scholar] [CrossRef]
- Lu, D.; Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat. Rev. Cardiol. 2019, 16, 661–674. [Google Scholar] [CrossRef]
- Romano, G.L.; Platania, C.B.; Forte, S.; Salomone, S.; Drago, F.; Bucolo, C. MicroRNA target prediction in glaucoma. Prog. Brain Res. 2015, 220, 217–240. [Google Scholar] [CrossRef]
- Tan, P.; Guo, Y.H.; Zhan, J.K.; Long, L.M.; Xu, M.L.; Ye, L.; Ma, X.Y.; Cui, X.J.; Wang, H.Q. LncRNA-ANRIL inhibits cell senescence of vascular smooth muscle cells by regulating miR-181a/Sirt1. Biochem. Cell Biol. 2019, 97, 571–580. [Google Scholar] [CrossRef]
- Bian, M.; Yu, Y.; Li, Y.; Zhou, Z.; Wu, X.; Ye, X.; Yu, J. Upregulating the Expression of LncRNA ANRIL Promotes Osteogenesis via the miR-7-5p/IGF-1R Axis in the Inflamed Periodontal Ligament Stem Cells. Front. Cell Dev. Biol. 2021, 9, 604400. [Google Scholar] [CrossRef] [PubMed]
- Kotake, Y.; Nakagawa, T.; Kitagawa, K.; Suzuki, S.; Liu, N.; Kitagawa, M.; Xiong, Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 2011, 30, 1956–1962. [Google Scholar] [CrossRef]
- Qiu, J.J.; Wang, Y.; Liu, Y.L.; Zhang, Y.; Ding, J.X.; Hua, K.Q. The long non-coding RNA ANRIL promotes proliferation and cell cycle progression and inhibits apoptosis and senescence in epithelial ovarian cancer. Oncotarget 2016, 7, 32478–32492. [Google Scholar] [CrossRef]
- Naemura, M.; Murasaki, C.; Inoue, Y.; Okamoto, H.; Kotake, Y. Long Noncoding RNA ANRIL Regulates Proliferation of Non-small Cell Lung Cancer and Cervical Cancer Cells. Anticancer Res. 2015, 35, 5377–5382. [Google Scholar] [PubMed]
- Taneera, J.; Lang, S.; Sharma, A.; Fadista, J.; Zhou, Y.; Ahlqvist, E.; Jonsson, A.; Lyssenko, V.; Vikman, P.; Hansson, O.; et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 2012, 16, 122–134. [Google Scholar] [CrossRef]
- Aguilo, F.; Zhou, M.M.; Walsh, M.J. Long noncoding RNA, polycomb, and the ghosts haunting INK4b-ARF-INK4a expression. Cancer Res. 2011, 71, 5365–5369. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef] [PubMed]
- Soto Martinez, J.L.; Cabrera Morales, C.M.; Serrano Ortega, S.; Lopez-Nevot, M.A. Mutation and homozygous deletion analyses of genes that control the G1/S transition of the cell cycle in skin melanoma: p53, p21, p16 and p15. Clin. Transl. Oncol. 2005, 7, 156–164. [Google Scholar] [CrossRef]
- Segev, A.; Nili, N.; Qiang, B.; Osherov, A.B.; Giordano, F.J.; Jaffe, R.; Gauldie, J.; Sparkes, J.D.; Fraser, A.R.; Ladouceur-Wodzak, M.; et al. Inhibition of intimal hyperplasia after stenting by over-expression of p15: A member of the INK4 family of cyclin-dependent kinase inhibitors. J. Mol. Cell Cardiol. 2011, 50, 417–425. [Google Scholar] [CrossRef]
- Kahl, C.R.; Means, A.R. Regulation of cyclin D1/Cdk4 complexes by calcium/calmodulin-dependent protein kinase I. J. Biol. Chem. 2004, 279, 15411–15419. [Google Scholar] [CrossRef] [PubMed]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Sage, J.; Koivomagi, M.; Skotheim, J.M. Cyclin D-Cdk4,6 Drives Cell-Cycle Progression via the Retinoblastoma Protein’s C-Terminal Helix. Mol. Cell 2019, 74, 758–770.e4. [Google Scholar] [CrossRef]
- Aird, K.M.; Zhang, R. Detection of senescence-associated heterochromatin foci (SAHF). Methods Mol. Biol. 2013, 965, 185–196. [Google Scholar] [CrossRef]
- van Zyl, T.; Yan, W.; McAdams, A.; Peng, Y.R.; Shekhar, K.; Regev, A.; Juric, D.; Sanes, J.R. Cell atlas of aqueous humor outflow pathways in eyes of humans and four model species provides insight into glaucoma pathogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 10339–10349. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Morgan, J.T.; Park, S.A.; Weber, D.; Phinney, B.S.; Murphy, C.J.; Russell, P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4447–4459. [Google Scholar] [CrossRef]
- Tie, J.; Chen, D.; Guo, J.; Liao, S.; Luo, X.; Zhang, Y.; Guo, R.; Xu, C.; Huang, D.; Zhang, Y.; et al. Transcriptome-wide study of the response of human trabecular meshwork cells to the substrate stiffness increase. J. Cell Biochem. 2020, 121, 3112–3123. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Patel, G.; Fury, W.; Yang, H.; Gomez-Caraballo, M.; Bai, Y.; Yang, T.; Adler, C.; Wei, Y.; Ni, M.; Schmitt, H.; et al. Molecular taxonomy of human ocular outflow tissues defined by single-cell transcriptomics. Proc. Natl. Acad. Sci. USA 2020, 117, 12856–12867. [Google Scholar] [CrossRef]
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Eye Res. 2017, 158, 112–123. [Google Scholar] [CrossRef]
- Tu, Q.; Hao, J.; Zhou, X.; Yan, L.; Dai, H.; Sun, B.; Yang, D.; An, S.; Lv, L.; Jiao, B.; et al. CDKN2B deletion is essential for pancreatic cancer development instead of unmeaningful co-deletion due to juxtaposition to CDKN2A. Oncogene 2018, 37, 128–138. [Google Scholar] [CrossRef]
- Senturk, S.; Mumcuoglu, M.; Gursoy-Yuzugullu, O.; Cingoz, B.; Akcali, K.C.; Ozturk, M. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology 2010, 52, 966–974. [Google Scholar] [CrossRef]
- Congrains, A.; Kamide, K.; Oguro, R.; Yasuda, O.; Miyata, K.; Yamamoto, E.; Kawai, T.; Kusunoki, H.; Yamamoto, H.; Takeya, Y.; et al. Genetic variants at the 9p21 locus contribute to atherosclerosis through modulation of ANRIL and CDKN2A/B. Atherosclerosis 2012, 220, 449–455. [Google Scholar] [CrossRef]
- Skowronska-Krawczyk, D.; Zhao, L.; Zhu, J.; Weinreb, R.N.; Cao, G.; Luo, J.; Flagg, K.; Patel, S.; Wen, C.; Krupa, M.; et al. P16INK4a Upregulation Mediated by SIX6 Defines Retinal Ganglion Cell Pathogenesis in Glaucoma. Mol. Cell 2015, 59, 931–940. [Google Scholar] [CrossRef]
- Li, L.U.; Zhao, Y.; Zhang, H. P16INK4a upregulation mediated by TBK1 induces retinal ganglion cell senescence in ischemic injury. Cell Death Dis. 2017, 8, e2752. [Google Scholar] [CrossRef]
- Papageorgis, P. Complex Interplay Between Aging and Cancer: Role of TGF-beta Signaling. Crit. Rev. Oncog. 2017, 22, 313–321. [Google Scholar] [CrossRef]
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-beta Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect Biol. 2017, 9, a022145. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Borlon, C.; Pascal, T.; Royer, V.; Eliaers, F.; Ninane, N.; Carrard, G.; Friguet, B.; de Longueville, F.; Boffe, S.; et al. Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta1 signaling pathway. J. Cell Sci. 2005, 118 Pt 4, 743–758. [Google Scholar] [CrossRef]
- Minagawa, S.; Araya, J.; Numata, T.; Nojiri, S.; Hara, H.; Yumino, Y.; Kawaishi, M.; Odaka, M.; Morikawa, T.; Nishimura, S.L.; et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L391–L401. [Google Scholar] [CrossRef]
- Roberti, G.; Oddone, F.; Agnifili, L.; Katsanos, A.; Michelessi, M.; Mastropasqua, L.; Quaranta, L.; Riva, I.; Tanga, L.; Manni, G. Steroid-induced glaucoma: Epidemiology, pathophysiology, and clinical management. Surv. Ophthalmol. 2020, 65, 458–472. [Google Scholar] [CrossRef]
- Sihota, R.; Konkal, V.L.; Dada, T.; Agarwal, H.C.; Singh, R. Prospective, long-term evaluation of steroid-induced glaucoma. Eye (Lond) 2008, 22, 26–30. [Google Scholar] [CrossRef]
- Amadio, M.; Pascale, A.; Cupri, S.; Pignatello, R.; Osera, C.; D’agata, V.; D’Amico, A.G.; Leggio, G.M.; Ruozi, B.; Govoni, S.; et al. Nanosystems based on siRNA silencing HuR expression counteract diabetic retinopathy in rat. Pharmacol. Res. 2016, 111, 713–720. [Google Scholar] [CrossRef]
- Zode, G.S.; Sharma, A.B.; Lin, X.; Searby, C.C.; Bugge, K.; Kim, G.H.; Clark, A.F.; Sheffield, V.C. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J. Clin. Investig. 2014, 124, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Millar, J.C.; Pang, I.H.; Wax, M.B.; Clark, A.F. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4617–4621. [Google Scholar] [CrossRef] [PubMed]
- Gage, G.J.; Kipke, D.R.; Shain, W. Whole animal perfusion fixation for rodents. J. Vis. Exp. 2012, 30, 3564. [Google Scholar] [CrossRef]
- Mao, W.; Liu, Y.; Wordinger, R.J.; Clark, A.F. A magnetic bead-based method for mouse trabecular meshwork cell isolation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3600–3606. [Google Scholar] [CrossRef]
- Keller, K.E.; Bhattacharya, S.K.; Borras, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Narendran, S.; Pereira, F.; Yerramothu, P.; Apicella, I.; Wang, S.B.; Varshney, A.; Baker, K.L.; Marion, K.M.; Ambati, M.; Ambati, V.L.; et al. A Clinical Metabolite of Azidothymidine Inhibits Experimental Choroidal Neovascularization and Retinal Pigmented Epithelium Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4. [Google Scholar] [CrossRef]
- Moshiri, A.; Humpal, D.; Leonard, B.C.; Imai, D.M.; Tham, A.; Bower, L.; Clary, D.; Glaser, T.M.; Lloyd, K.C.; Murphy, C.J. Arap1 Deficiency Causes Photoreceptor Degeneration in Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1709–1718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stone, R.A.; Kuwayama, Y.; Laties, A.M.; Marangos, P.J. Neuron-specific enolase-containing cells in the rhesus monkey trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1332–1334. [Google Scholar]
- Wang, C.; Li, L.; Liu, Z. Experimental research on the relationship between the stiffness and the expressions of fibronectin proteins and adaptor proteins of rat trabecular meshwork cells. BMC Ophthalmol. 2017, 17, 268. [Google Scholar] [CrossRef]
- Du, Y.; Yun, H.; Yang, E.; Schuman, J.S. Stem cells from trabecular meshwork home to TM tissue in vivo. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1450–1459. [Google Scholar] [CrossRef]
- Shiga, Y.; Akiyama, M.; Nishiguchi, K.M.; Sato, K.; Shimozawa, N.; Takahashi, A.; Momozawa, Y.; Hirata, M.; Matsuda, K.; Yamaji, T.; et al. Genome-wide association study identifies seven novel susceptibility loci for primary open-angle glaucoma. Hum. Mol. Genet. 2018, 27, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet. 2020, 16, e1008720. [Google Scholar] [CrossRef] [PubMed]
- Valette, K.; Li, Z.; Bon-Baret, V.; Chignon, A.; Berube, J.C.; Eslami, A.; Lamothe, J.; Gaudreault, N.; Joubert, P.; Obeidat, M.; et al. Prioritization of candidate causal genes for asthma in susceptibility loci derived from UK Biobank. Commun. Biol. 2021, 4, 700. [Google Scholar] [CrossRef] [PubMed]




| PMID | Rsnum | Chr | Position | Nearest Gene | Significant eQTL in ANRIL |
|---|---|---|---|---|---|
| 22419738 | rs1063192 | 9 | 22003368 | ANRIL | N/A |
| 22792221 | rs523096 | 9 | 22019130 | ANRIL | p = 1.6 × 10−5 in prostate |
| 22428042 | rs7865618 | 9 | 22031006 | ANRIL | N/A |
| 22570617 32514122 25861811 | rs2157719 | 9 | 22033367 | ANRIL | N/A |
| 26752265 33627673 29891935 | rs1333037 | 9 | 22040766 | ANRIL | N/A |
| 33627673 | rs1412829 | 9 | 22043927 | ANRIL | N/A |
| 29891935 | rs10811645 | 9 | 22049657 | ANRIL | N/A |
| 29452408 | rs944800 | 9 | 22050899 | ANRIL | p = 2.6 × 10−6 in fibroblast cells |
| 30054594 29891935 31959993 33627673 | rs944801 | 9 | 22051671 | ANRIL | N/A |
| 30104761 33627673 | rs6475604 | 9 | 22052735 | ANRIL | N/A |
| 33627673 | rs7853090 | 9 | 22056296 | ANRIL | N/A |
| 27623284 | rs7866783 | 9 | 22056360 | ANRIL | N/A |
| 21532571 25173105 | rs4977756 | 9 | 22068653 | ANRIL | N/A |
| Manufacturer | Protein Size | Dilution | |
|---|---|---|---|
| p15 antibody | Abcam | 15 kDa | WB (1:1000), IF (1:200) |
| H3K9Me2 antibody | Abcam | 17 kDa | IF (1:200) |
| HP-1α antibody | Boster | 20 kDa | IF (1:100) |
| Phospho-Rb (Ser807/811) antibody | Abcam | 110 kDa | WB (1:500) |
| Histone H3 antibody | CST | 17 kDa | WB (1:2000) |
| Cyclin D3 antibody | Beyotime | 31 kDa | WB (1:1000) |
| Primer Name | Sequence (5′ to 3′) |
|---|---|
| β-actin-forward primer | TTCTGCTCTTCGGTTCTGCC |
| β-actin-forward primer | GCCGTGTAGGTCGAAACAGA |
| p15-forward primer | GGGACTAGTGGAGAAGGTGC |
| p15-reverse primer | CATCATCATGACCTGGATCGC |
| ANRIL-forward primer | GCGCCGGACTAGGACTATTT |
| ANRIL-reverse primer | GCCAGGACGGAGATCAGATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, P.; Huang, S.; Luo, Y.; Deng, C.; Zhou, J.; Long, E.; Zhuo, Y. Reciprocal Regulation between lncRNA ANRIL and p15 in Steroid-Induced Glaucoma. Cells 2022, 11, 1468. https://doi.org/10.3390/cells11091468
Wan P, Huang S, Luo Y, Deng C, Zhou J, Long E, Zhuo Y. Reciprocal Regulation between lncRNA ANRIL and p15 in Steroid-Induced Glaucoma. Cells. 2022; 11(9):1468. https://doi.org/10.3390/cells11091468
Chicago/Turabian StyleWan, Peixing, Siyu Huang, Yanting Luo, Caibin Deng, Jiajian Zhou, Erping Long, and Yehong Zhuo. 2022. "Reciprocal Regulation between lncRNA ANRIL and p15 in Steroid-Induced Glaucoma" Cells 11, no. 9: 1468. https://doi.org/10.3390/cells11091468
APA StyleWan, P., Huang, S., Luo, Y., Deng, C., Zhou, J., Long, E., & Zhuo, Y. (2022). Reciprocal Regulation between lncRNA ANRIL and p15 in Steroid-Induced Glaucoma. Cells, 11(9), 1468. https://doi.org/10.3390/cells11091468





