The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Placental Tissues
2.2. Fluidigm BiomarkTM Array
2.3. Independent Validation by Using Real-Time PCR
2.4. 3D Cultures of Trophoblast Organoids In Vitro
2.5. Trophoblast-Derived Cell-Line, BeWo In Vitro
2.6. Placental NLRP3 Expression in an Inflammation-Induced Murine Model of FGR, In Vivo
2.7. Immunohistochemistry
2.8. Immunofluorescence
2.9. Western Immunoblotting
2.10. Immunoassays
2.11. Caspase 3 and Caspase 8 Activity Assays
2.12. Data Analysis
3. Results
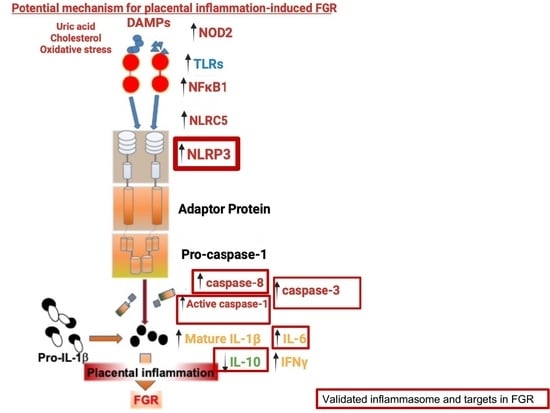
3.1. Placental Inflammasomes in FGR
3.1.1. Patient Demography of the Samples
3.1.2. Clinical Criteria of FGR Samples
3.1.3. Screening for the Presence or Absence of Inflammasomes by Fluidigm BiomarkTM Array
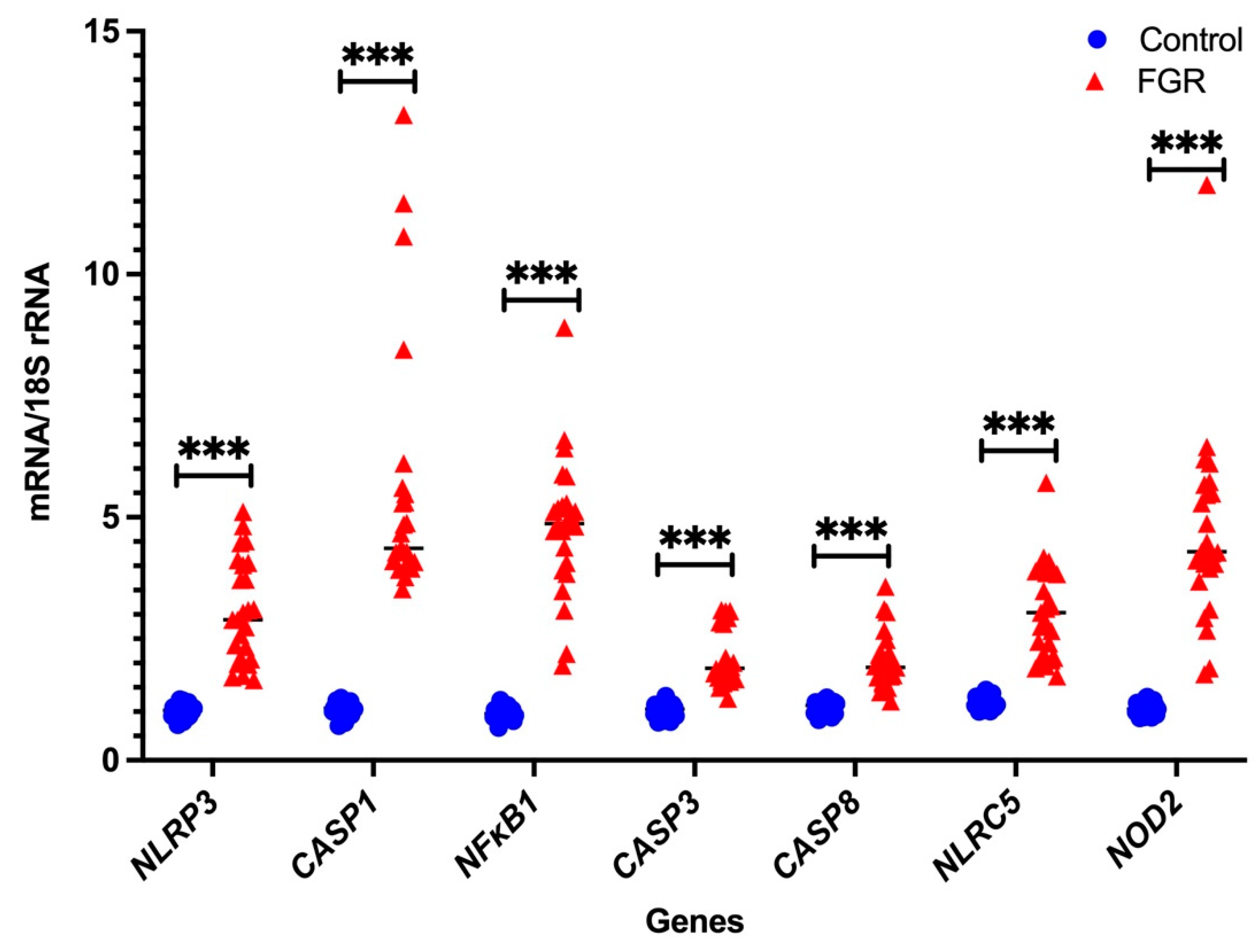
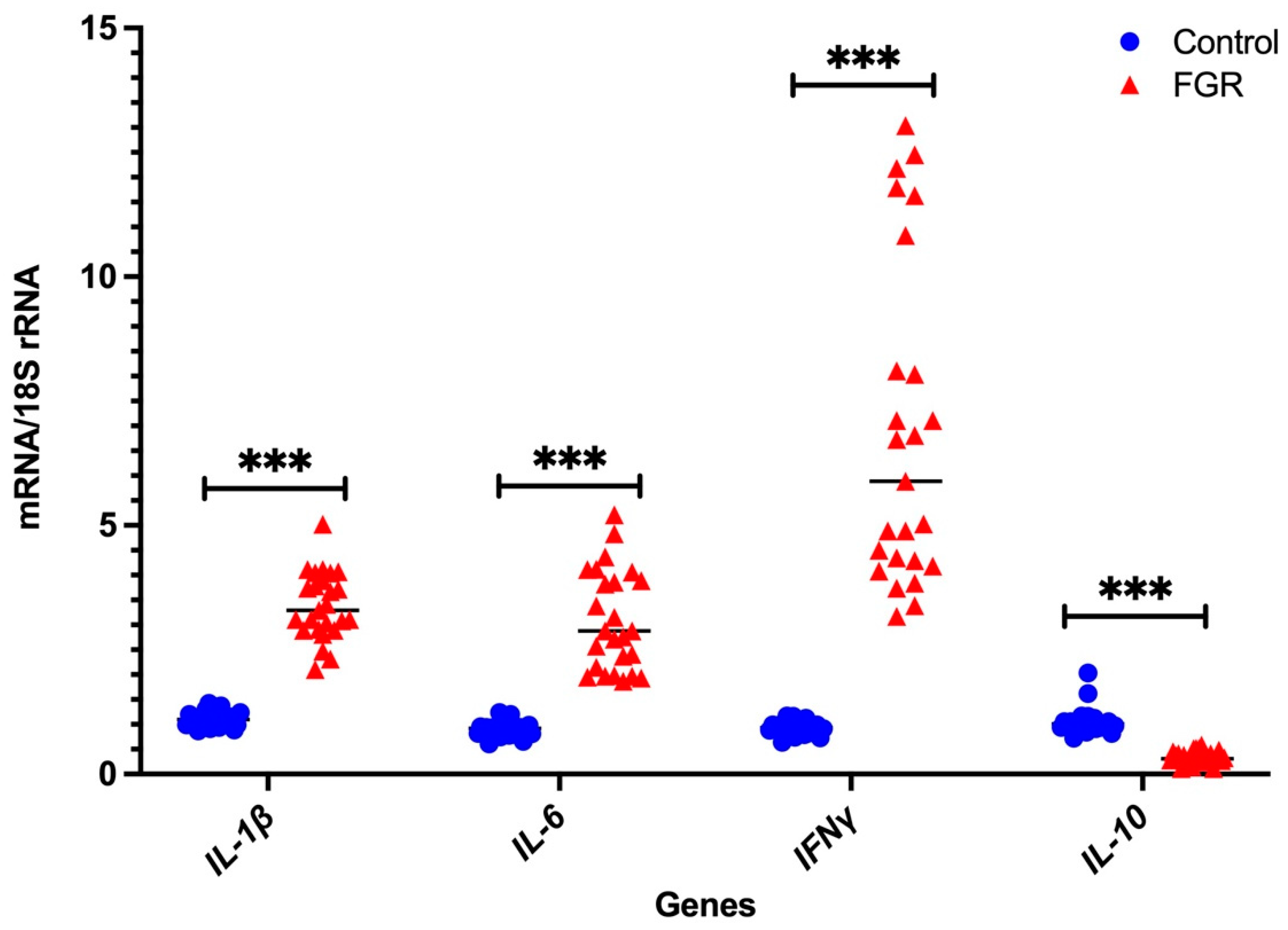
3.2. Independent Validation by Real-Time PCR
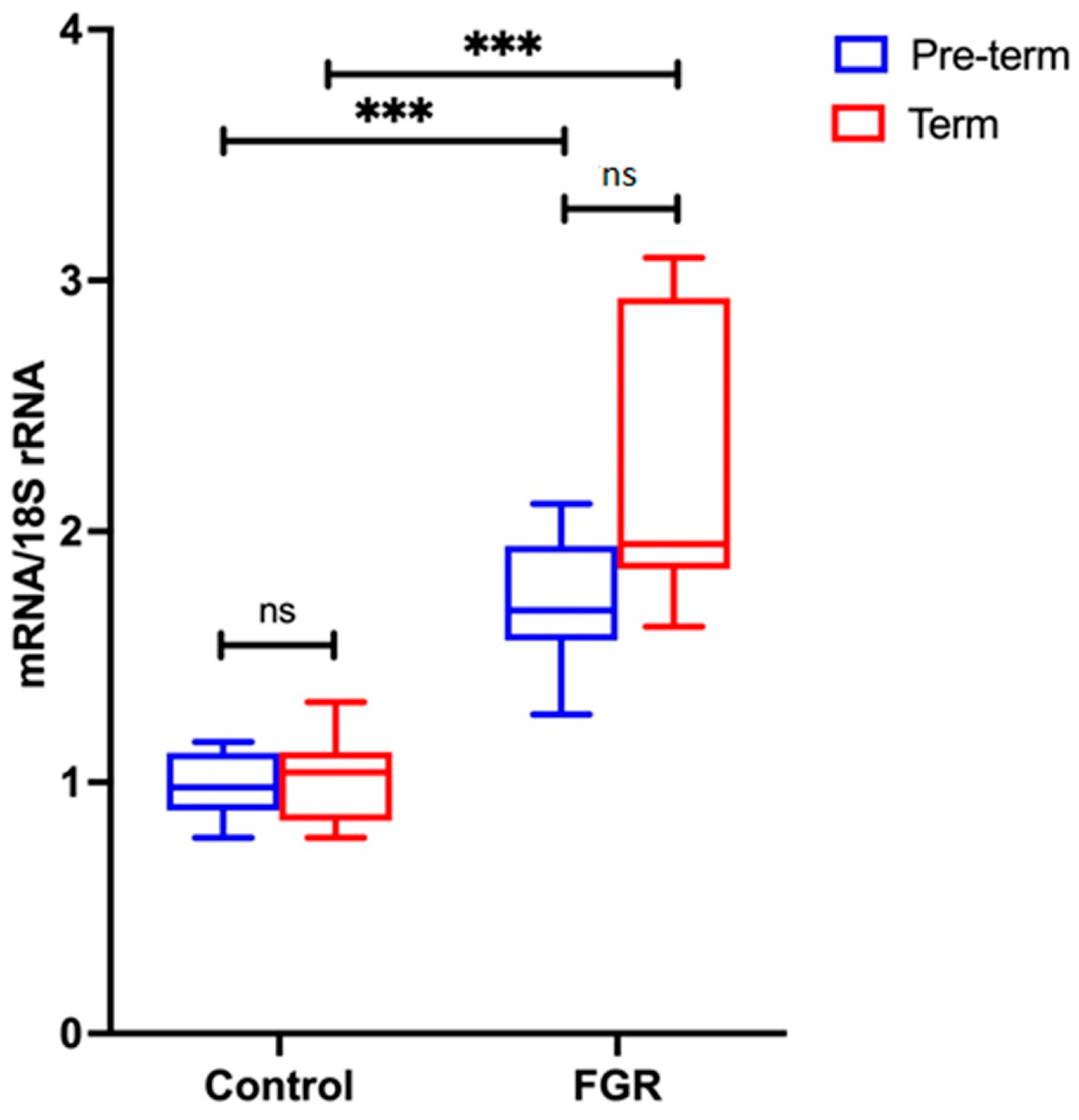
Placental NLRP3 mRNA is Increased in FGR Pregnancies
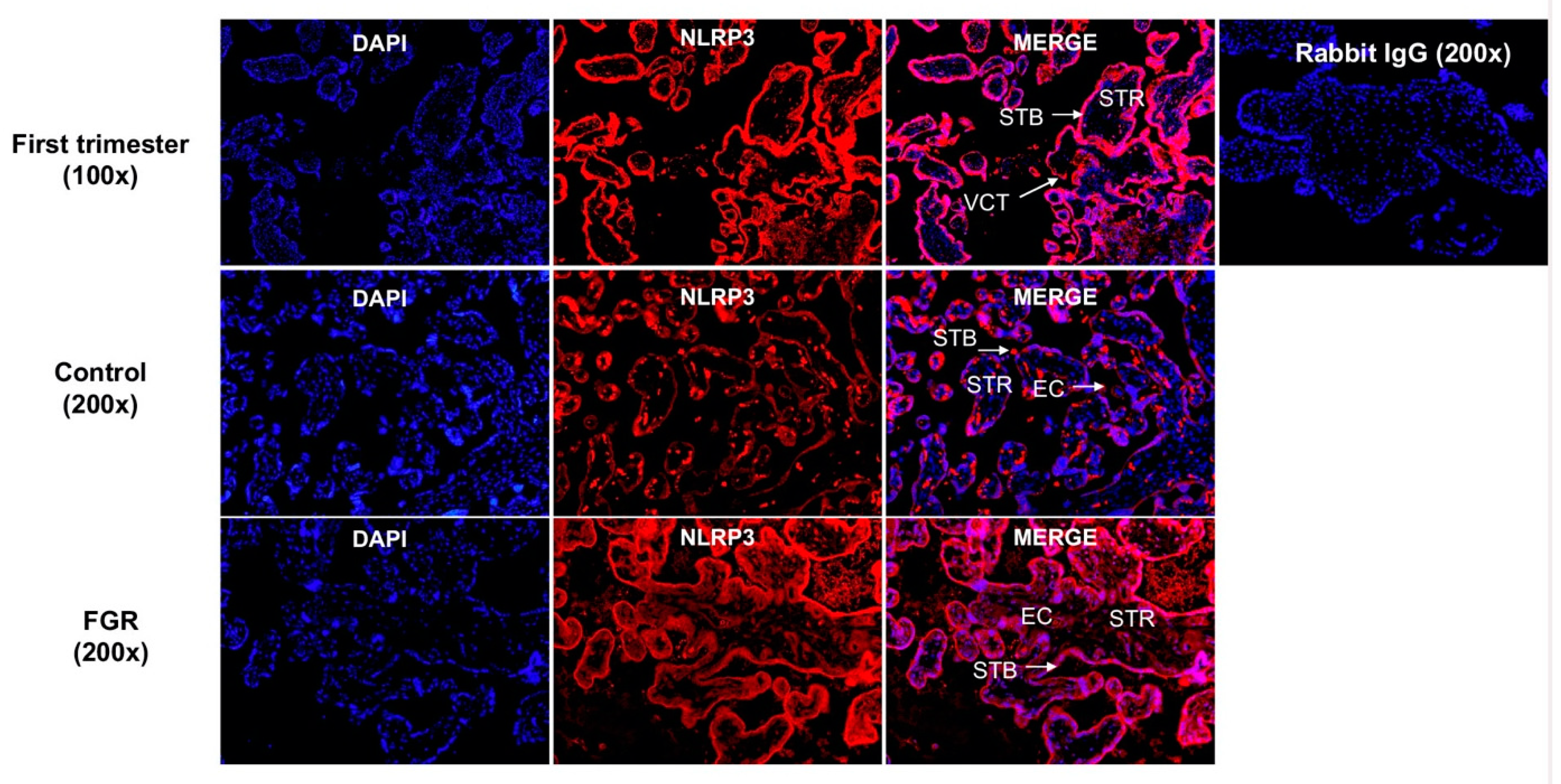
3.3. Placental NLRP3 Protein is Increased in FGR Pregnancies
3.4. NLRP3 Protein is Expressed in the First Trimester and Term Trophoblasts
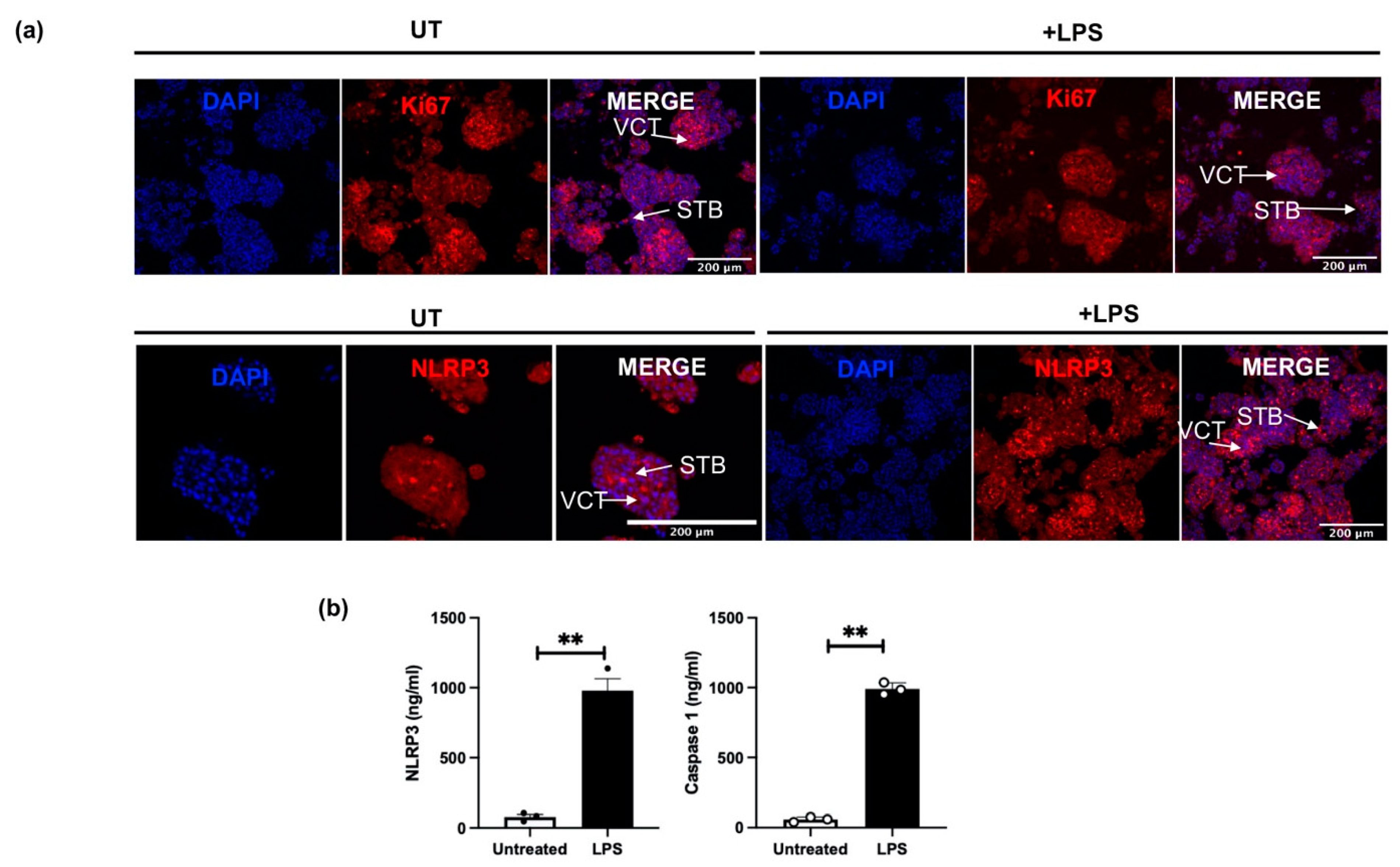
3.5. NLRP3 Protein Expression in an Inflammation-Induced Model of First Trimester Trophoblast Organoid Cultures In Vitro
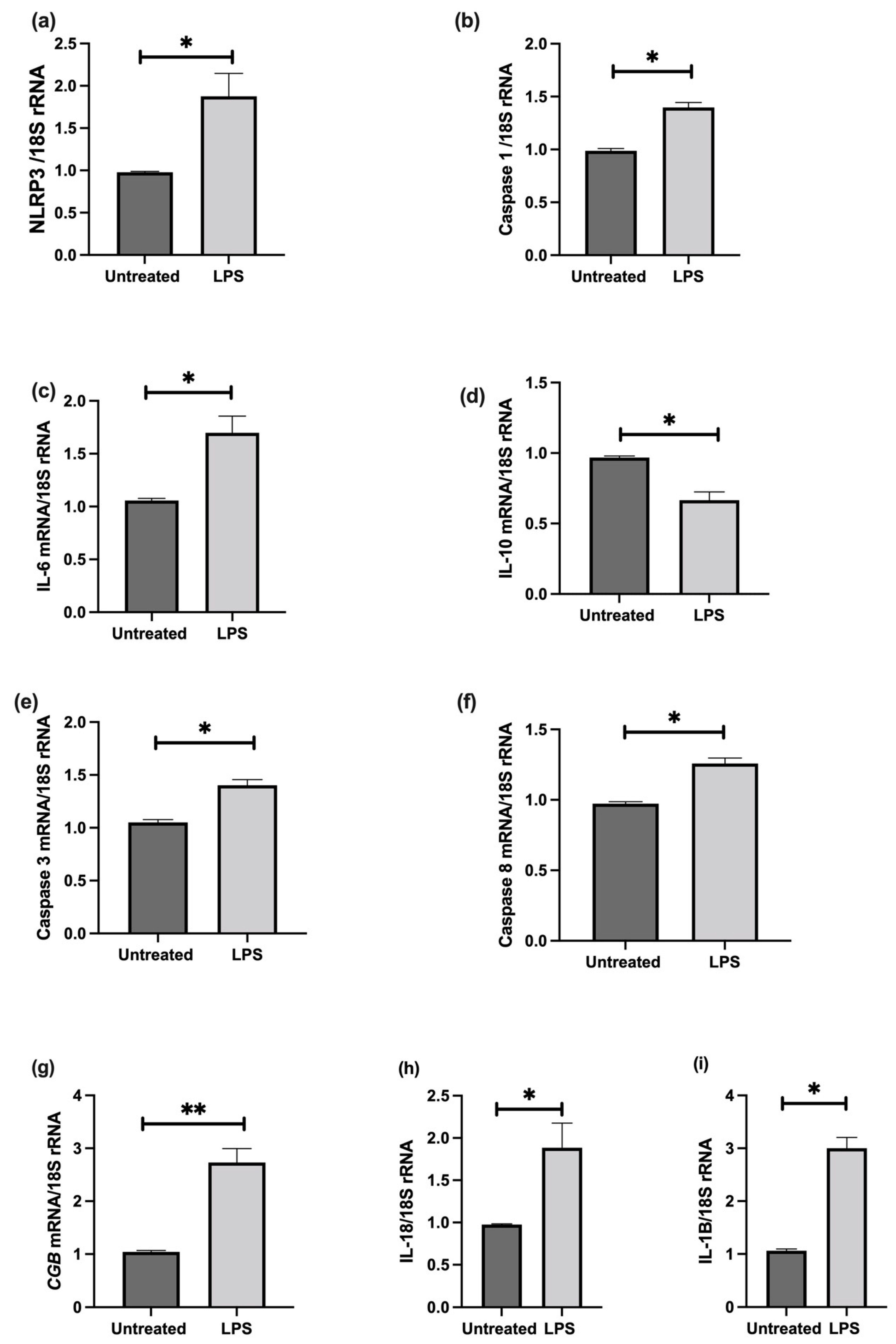
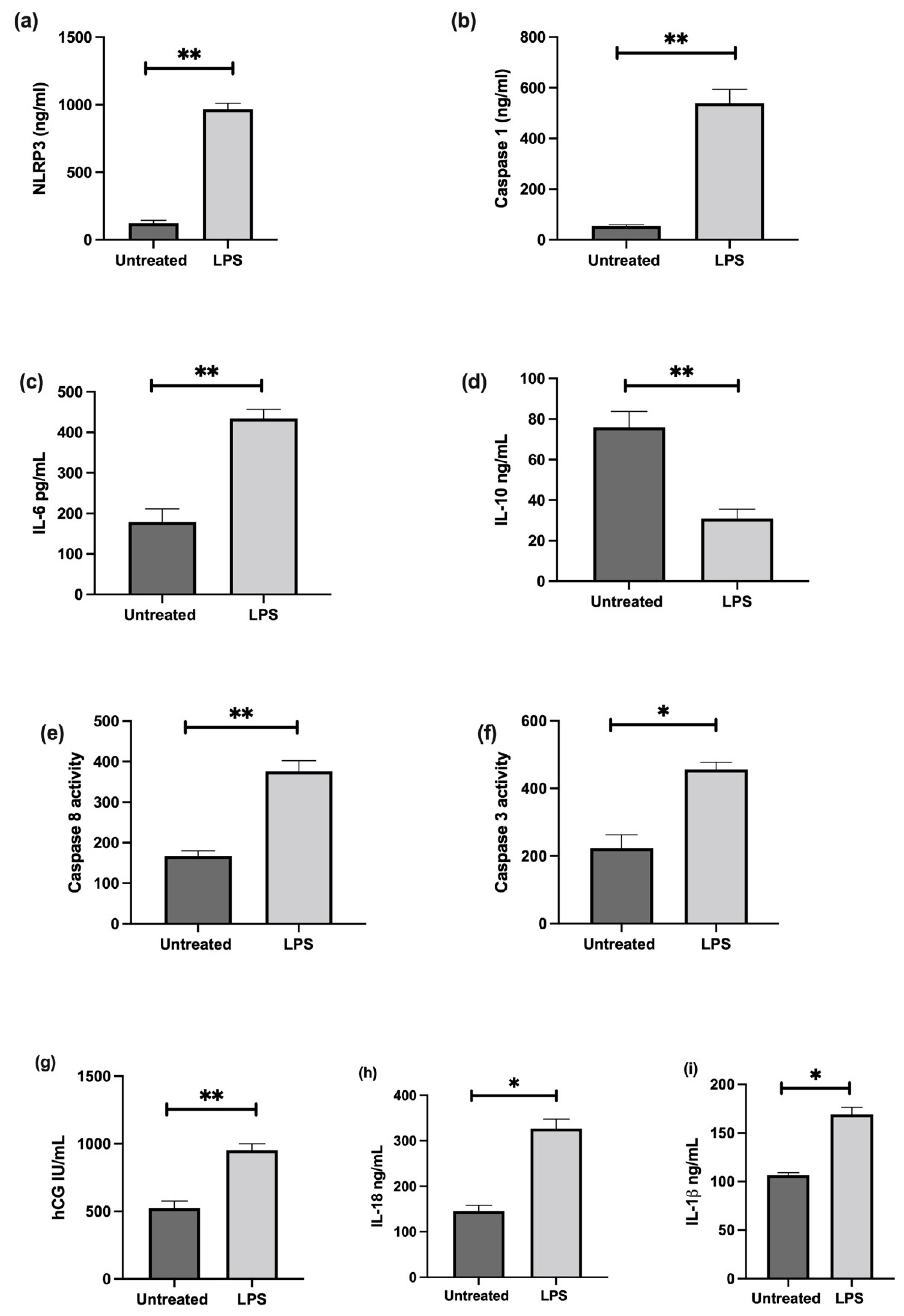
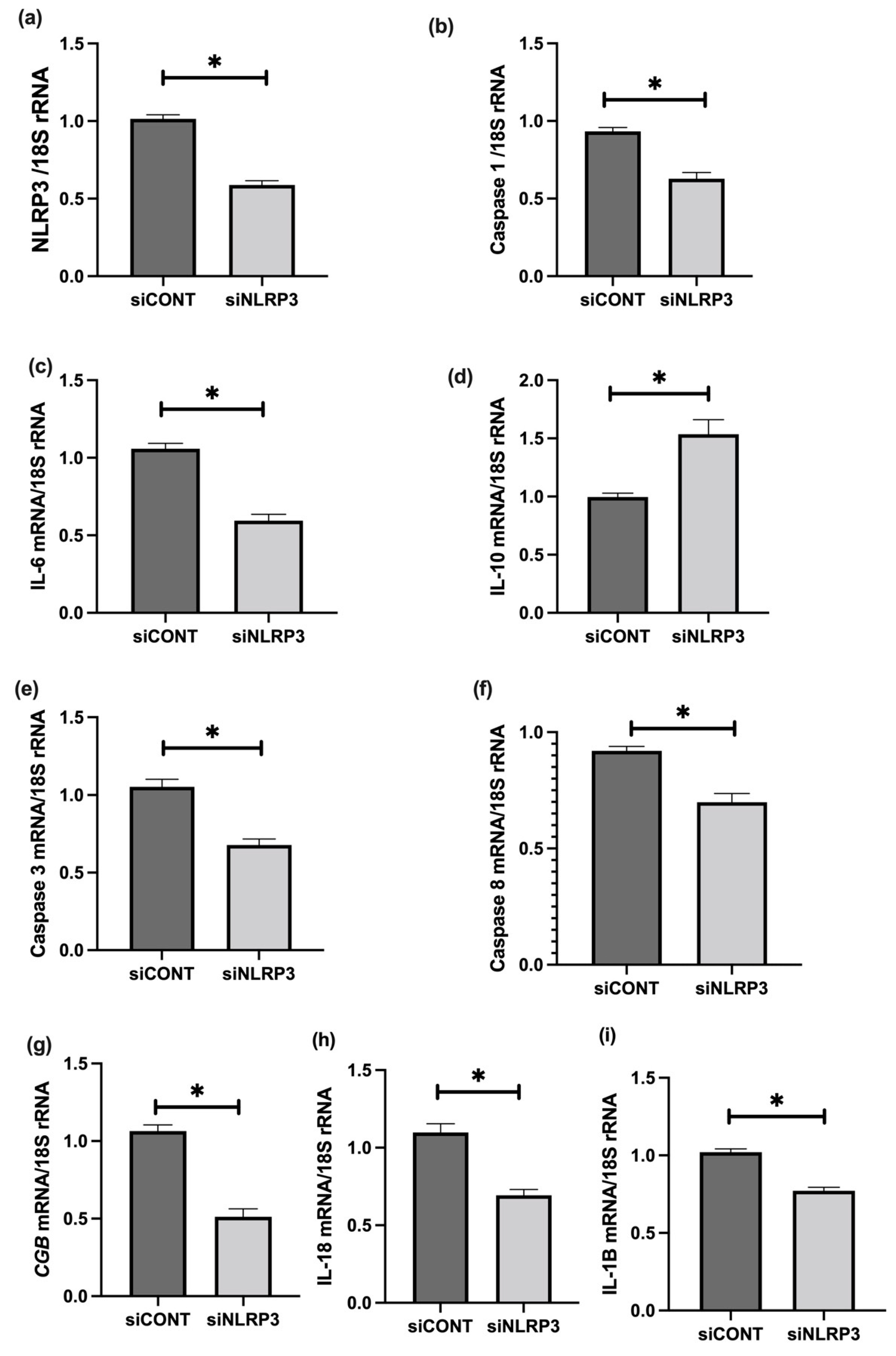
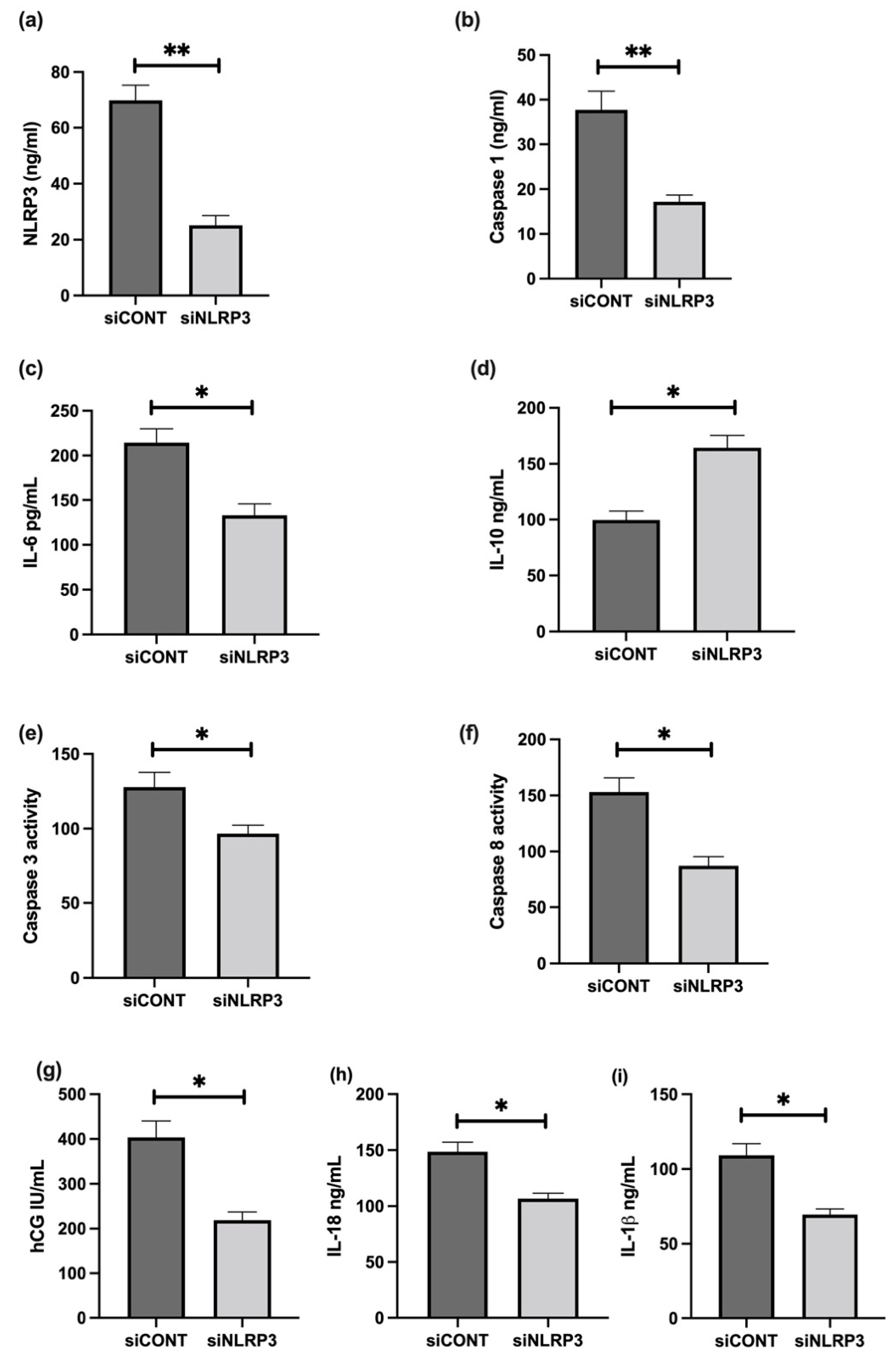
3.6. The Effect of LPS on NLRP3 Mediated BeWo Cell Function In Vitro
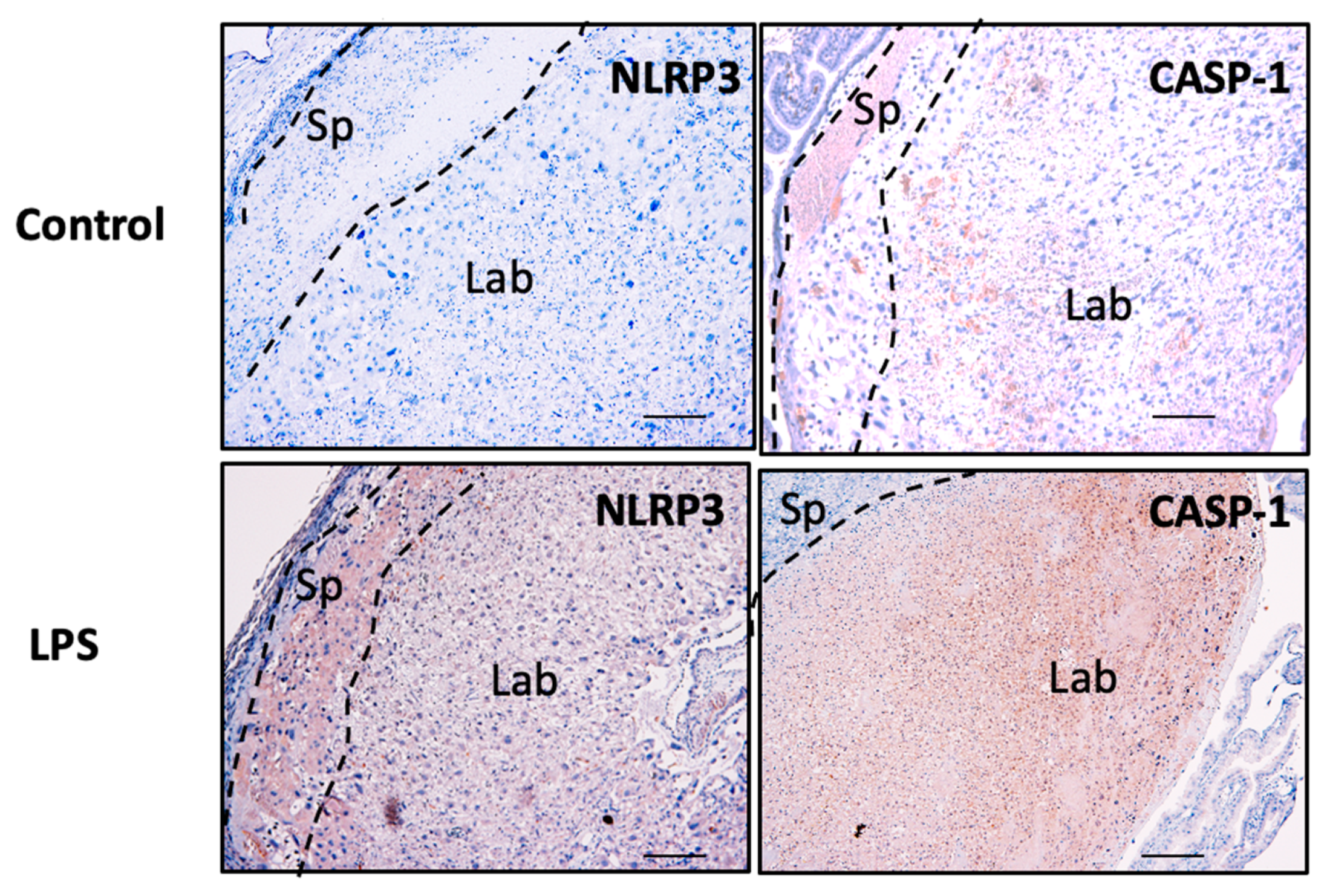
3.7. NLRP3 and Caspase-1 Expression is Increased in the Murine Model of Inflammation In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beune, I.M.; Bloomfield, F.H.; Ganzevoort, W.; Embleton, N.D.; Rozance, P.J.; van Wassenaer-Leemhuis, A.G.; Wynia, K.; Gordijn, S.J. Consensus Based Definition of Growth Restriction in the Newborn. J. Pediatr. 2018, 196, 71–76.e1. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 2006, 49, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Gardosi, J. Intrauterine growth restriction: New concepts in antenatal surveillance, diagnosis, and management. Am. J. Obstet. Gynecol. 2011, 204, 288–300. [Google Scholar] [CrossRef]
- Sun, C.; Groom, K.M.; Oyston, C.; Chamley, L.W.; Clark, A.R.; James, J.L. The placenta in fetal growth restriction: What is going wrong? Placenta 2020, 96, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Kyle, P.M. Aetiology and pathogenesis of IUGR. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, A. Idiopathic fetal growth restriction: A pathophysiologic approach. Obstet. Gynecol. Surv. 1996, 51, 376–382. [Google Scholar] [CrossRef]
- Krebs, C.; Macara, L.M.; Leiser, R.; Bowman, A.W.; Greer, I.A.; Kingdom, J.C. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am. J. Obstet. Gynecol. 1996, 175, 1534–1542. [Google Scholar] [CrossRef]
- Ishihara, N.; Matsuo, H.; Murakoshi, H.; Laoag-Fernandez, J.B.; Samoto, T.; Mauro, T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am. J. Obstet. Gynecol. 2002, 186, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Smith, S.D.; Yusuf, K.; Huettner, P.C.; Kraus, F.T.; Sadovsky, Y.; Nelson, D.M. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am. J. Obstet. Gynecol. 2002, 186, 1056–1061. [Google Scholar] [CrossRef]
- Chen, C.P.; Bajoria, R.; Aplin, J.D. Decreased vascularization and cell proliferation in placentas of intrauterine growth-restricted fetuses with abnormal umbilical artery flow velocity waveforms. Am. J. Obstet. Gynecol. 2002, 187, 764–769. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best. Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, B.C.; Heazell, A.E.P.; Sibley, C.; Wright, R.; Bischof, H.; Beards, F.; Guevara, T.; Girard, S.; Jones, R.L. Hypoxia and oxidative stress induce sterile placental inflammation in vitro. Sci. Rep. 2021, 11, 7281. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kanneganti, T.D. Inflammasome activation and assembly at a glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Abi Nahed, R.; Reynaud, D.; Borg, A.J.; Traboulsi, W.; Wetzel, A.; Sapin, V.; Brouillet, S.; Dieudonne, M.N.; Dakouane-Giudicelli, M.; Benharouga, M.; et al. NLRP7 is increased in human idiopathic fetal growth restriction and plays a critical role in trophoblast differentiation. J. Mol. Med. 2019, 97, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Abi Nahed, R.; Mikhael, M.E.; Reynaud, D.; Collet, C.; Lemaitre, N.; Michy, T.; Hoffmann, P.; Sergent, F.; Marquette, C.; Murthi, P.; et al. Role of NLRP7 in Normal and Malignant Trophoblast Cells. Biomedicines 2022, 10, 252. [Google Scholar] [CrossRef]
- Reynaud, D.; Abi Nahed, R.; Lemaitre, N.; Bolze, P.A.; Traboulsi, W.; Sergent, F.; Battail, C.; Filhol, O.; Sapin, V.; Boufettal, H.; et al. NLRP7 Promotes Choriocarcinoma Growth and Progression through the Establishment of an Immunosuppressive Microenvironment. Cancers 2021, 13, 2999. [Google Scholar] [CrossRef]
- Murthi, P.; Doherty, V.; Said, J.; Donath, S.; Brennecke, S.P.; Kalionis, B. Homeobox gene HLX1 expression is decreased in idiopathic human fetal growth restriction. Am. J. Pathol. 2006, 168, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Murthi, P.; Doherty, V.L.; Said, J.M.; Donath, S.; Brennecke, S.P.; Kalionis, B. Homeobox gene ESX1L expression is decreased in human pre-term idiopathic fetal growth restriction. Mol. Hum. Reprod. 2006, 12, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Murthi, P.; Said, J.M.; Doherty, V.L.; Donath, S.; Nowell, C.J.; Brennecke, S.P.; Kalionis, B. Homeobox gene DLX4 expression is increased in idiopathic human fetal growth restriction. Mol. Hum. Reprod. 2006, 12, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ranzil, S.; Ellery, S.; Walker, D.W.; Vaillancourt, C.; Alfaidy, N.; Bonnin, A.; Borg, A.; Wallace, E.M.; Ebeling, P.R.; Erwich, J.J.; et al. Disrupted placental serotonin synthetic pathway and increased placental serotonin: Potential implications in the pathogenesis of human fetal growth restriction. Placenta 2019, 84, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Murthi, P.; Fitzpatrick, E.; Borg, A.J.; Donath, S.; Brennecke, S.P.; Kalionis, B. GAPDH, 18S rRNA and YWHAZ are suitable endogenous reference genes for relative gene expression studies in placental tissues from human idiopathic fetal growth restriction. Placenta 2008, 29, 798–801. [Google Scholar] [CrossRef]
- DeLaney, A.A.; Berry, C.T.; Christian, D.A.; Hart, A.; Bjanes, E.; Wynosky-Dolfi, M.A.; Li, X.; Tummers, B.; Udalova, I.A.; Chen, Y.H.; et al. Caspase-8 promotes c-Rel-dependent inflammatory cytokine expression and resistance against Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 2019, 116, 11926–11935. [Google Scholar] [CrossRef] [Green Version]
- Paniri, A.; Akhavan-Niaki, H. Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: Role of lncRNAs in cytokine storm modulation. Life Sci. 2020, 257, 118114. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Haider, S.; Meinhardt, G.; Saleh, L.; Kunihs, V.; Gamperl, M.; Kaindl, U.; Ellinger, A.; Burkard, T.R.; Fiala, C.; Pollheimer, J.; et al. Self-Renewing Trophoblast Organoids Recapitulate the Developmental Program of the Early Human Placenta. Stem Cell Rep. 2018, 11, 537–551. [Google Scholar] [CrossRef] [Green Version]
- Rezanejad, H.; Lock, J.H.; Sullivan, B.A.; Bonner-Weir, S. Generation of Pancreatic Ductal Organoids and Whole-Mount Immunostaining of Intact Organoids. Curr. Protoc. Cell Biol. 2019, 83, e82. [Google Scholar] [CrossRef]
- Murthi, P.; Sarkis, R.; Lim, R.; Nguyen-Ngo, C.; Pratt, A.; Liong, S.; Lappas, M. Endocan expression is increased in the placenta from obese women with gestational diabetes mellitus. Placenta 2016, 48, 38–48. [Google Scholar] [CrossRef]
- Panagodage, S.; Yong, H.E.; Da Silva Costa, F.; Borg, A.J.; Kalionis, B.; Brennecke, S.P.; Murthi, P. Low-Dose Acetylsalicylic Acid Treatment Modulates the Production of Cytokines and Improves Trophoblast Function in an in Vitro Model of Early-Onset Preeclampsia. Am. J. Pathol. 2016, 186, 3217–3224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonar, S.L.; Brydges, S.D.; Mueller, J.L.; McGeough, M.D.; Pena, C.; Chen, D.; Grimston, S.K.; Hickman-Brecks, C.L.; Ravindran, S.; McAlinden, A.; et al. Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS ONE 2012, 7, e35979. [Google Scholar] [CrossRef] [PubMed]
- Brydges, S.D.; Mueller, J.L.; McGeough, M.D.; Pena, C.A.; Misaghi, A.; Gandhi, C.; Putnam, C.D.; Boyle, D.L.; Firestein, G.S.; Horner, A.A.; et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity 2009, 30, 875–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 2014, 211, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Shimazaki, S.; Suzuki, S.; Henmi, Y.; Komiyama, H.; Kuwayama, T.; Iwata, H.; Karasawa, T.; Takahashi, M.; Takahashi, H.; et al. Beta-hydroxybutyrate suppresses NLRP3 inflammasome-mediated placental inflammation and lipopolysaccharide-induced fetal absorption. J. Reprod. Immunol. 2021, 148, 103433. [Google Scholar] [CrossRef]
- Pathirage, N.A.; Cocquebert, M.; Sadovsky, Y.; Abumaree, M.; Manuelpillai, U.; Borg, A.; Keogh, R.J.; Brennecke, S.P.; Evain-Brion, D.; Fournier, T.; et al. Homeobox gene transforming growth factor beta-induced factor-1 (TGIF-1) is a regulator of villous trophoblast differentiation and its expression is increased in human idiopathic fetal growth restriction. Mol. Hum. Reprod. 2013, 19, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Al-Azemi, M.; Raghupathy, R.; Azizieh, F. Pro-inflammatory and anti-inflammatory cytokine profiles in fetal growth restriction. Clin. Exp. Obstet. Gynecol. 2017, 44, 98–103. [Google Scholar] [CrossRef]
- Liu, H.J.; Liu, P.C.; Hua, J.; Zhao, Y.; Cao, J. Placental weight and size in relation to fetal growth restriction: A case-control study. J. Matern. Fetal. Neonatal Med. 2021, 34, 1356–1360. [Google Scholar] [CrossRef]
- Roberts, D.J.; Post, M.D. The placenta in pre-eclampsia and intrauterine growth restriction. J. Clin. Pathol. 2008, 61, 1254–1260. [Google Scholar] [CrossRef]
- Freedman, A.A.; Hogue, C.J.; Marsit, C.J.; Rajakumar, A.; Smith, A.K.; Goldenberg, R.L.; Dudley, D.J.; Saade, G.R.; Silver, R.M.; Gibbins, K.J.; et al. Associations Between the Features of Gross Placental Morphology and Birthweight. Pediatr. Dev. Pathol. 2019, 22, 194–204. [Google Scholar] [CrossRef]
- Chui, A.; Kalionis, B.; Abumaree, M.; Cocquebert, M.; Fournier, T.; Evain-Brion, D.; Brennecke, S.P.; Murthi, P. Downstream targets of the homeobox gene DLX3 are differentially expressed in the placentae of pregnancies affected by human idiopathic fetal growth restriction. Mol. Cell. Endocrinol. 2013, 377, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chui, A.; Tay, C.; Cocquebert, M.; Sheehan, P.; Pathirage, N.A.; Donath, S.; Fournier, T.; Badet, J.; Evain-Brion, D.; Brennecke, S.P.; et al. Homeobox gene Distal-less 3 is a regulator of villous cytotrophoblast differentiation and its expression is increased in human idiopathic foetal growth restriction. J. Mol. Med. 2012, 90, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, G.; Murthi, P.; Brennecke, S.P.; Kalionis, B. Homeobox gene HLX is a regulator of HGF/c-met-mediated migration of human trophoblast-derived cell lines. Biol. Reprod. 2010, 83, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, G.; Murthi, P.; Pathirage, N.; Brennecke, S.P.; Kalionis, B. Downstream targets of homeobox gene HLX show altered expression in human idiopathic fetal growth restriction. Am. J. Pathol. 2010, 176, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthi, P.; Brouillet, S.; Pratt, A.; Borg, A.; Kalionis, B.; Goffin, F.; Tsatsaris, V.; Munaut, C.; Feige, J.J.; Benharouga, M.; et al. An EG-VEGF-Dependent Decrease in Homeobox Gene NKX3.1 Contributes to Cytotrophoblast Dysfunction: A Possible Mechanism in Human Fetal Growth Restriction. Mol. Med. 2015, 21, 645–656. [Google Scholar] [CrossRef]
- Reis, A.S.; Barboza, R.; Murillo, O.; Barateiro, A.; Peixoto, E.P.M.; Lima, F.A.; Gomes, V.M.; Dombrowski, J.G.; Leal, V.N.C.; Araujo, F.; et al. Inflammasome activation and IL-1 signaling during placental malaria induce poor pregnancy outcomes. Sci. Adv. 2020, 6, eaax6346. [Google Scholar] [CrossRef] [Green Version]
- Benyo, D.F.; Smarason, A.; Redman, C.W.; Sims, C.; Conrad, K.P. Expression of inflammatory cytokines in placentas from women with preeclampsia. J. Clin. Endocrinol. Metab. 2001, 86, 2505–2512. [Google Scholar] [CrossRef]
- Brien, M.E.; Duval, C.; Palacios, J.; Boufaied, I.; Hudon-Thibeault, A.A.; Nadeau-Vallee, M.; Vaillancourt, C.; Sibley, C.P.; Abrahams, V.M.; Jones, R.L.; et al. Uric Acid Crystals Induce Placental Inflammation and Alter Trophoblast Function via an IL-1-Dependent Pathway: Implications for Fetal Growth Restriction. J. Immunol. 2017, 198, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Bo, Q.L.; Gan, Y.; Chen, Y.H.; Zhao, H.; Tao, F.B.; Xu, D.X. Association among placental 11beta-HSD2, PPAR-gamma, and NF-kappaB p65 in small-for-gestational-age infants: A nested case-control study. Am. J. Reprod. Immunol. 2020, 83, e13231. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Motomura, K.; Miller, D.; Garcia-Flores, V.; Galaz, J.; Romero, R. Inflammasomes: Their Role in Normal and Complicated Pregnancies. J. Immunol. 2019, 203, 2757–2769. [Google Scholar] [CrossRef]
- Hanna, N.; Bonifacio, L.; Reddy, P.; Hanna, I.; Weinberger, B.; Murphy, S.; Laskin, D.; Sharma, S. IFN-gamma-mediated inhibition of COX-2 expression in the placenta from term and preterm labor pregnancies. Am. J. Reprod. Immunol. 2004, 51, 311–318. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Nunez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weel, I.C.; Romao-Veiga, M.; Matias, M.L.; Fioratti, E.G.; Peracoli, J.C.; Borges, V.T.; Araujo, J.P., Jr.; Peracoli, M.T. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J. Reprod. Immunol. 2017, 123, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthi, P.; Pinar, A.A.; Dimitriadis, E.; Samuel, C.S. Inflammasomes-A Molecular Link for Altered Immunoregulation and Inflammation Mediated Vascular Dysfunction in Preeclampsia. Int. J. Mol. Sci. 2020, 21, 1406. [Google Scholar] [CrossRef] [Green Version]
- Shirasuna, K.; Karasawa, T.; Takahashi, M. Role of the NLRP3 Inflammasome in Preeclampsia. Front. Endocrinol. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Socha, M.W.; Malinowski, B.; Puk, O.; Dubiel, M.; Wicinski, M. The NLRP3 Inflammasome Role in the Pathogenesis of Pregnancy Induced Hypertension and Preeclampsia. Cells 2020, 9, 1642. [Google Scholar] [CrossRef] [PubMed]
- Stodle, G.S.; Silva, G.B.; Tangeras, L.H.; Gierman, L.M.; Nervik, I.; Dahlberg, U.E.; Sun, C.; Aune, M.H.; Thomsen, L.C.V.; Bjorge, L.; et al. Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts. Clin. Exp. Immunol. 2018, 193, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Lopez, N.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Miller, D.; Hassan, S.S.; Hsu, C.D.; Panaitescu, B. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomesdagger. Biol. Reprod. 2019, 100, 1306–1318. [Google Scholar] [CrossRef]
- Faro, J.; Romero, R.; Schwenkel, G.; Garcia-Flores, V.; Arenas-Hernandez, M.; Leng, Y.; Xu, Y.; Miller, D.; Hassan, S.S.; Gomez-Lopez, N. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasomedagger. Biol. Reprod. 2019, 100, 1290–1305. [Google Scholar] [CrossRef]
- Rogers, L.M.; Serezani, C.H.; Eastman, A.J.; Hasty, A.H.; Englund-Ogge, L.; Jacobsson, B.; Vickers, K.C.; Aronoff, D.M. Palmitate induces apoptotic cell death and inflammasome activation in human placental macrophages. Placenta 2020, 90, 45–51. [Google Scholar] [CrossRef]
- Sano, M.; Shimazaki, S.; Kaneko, Y.; Karasawa, T.; Takahashi, M.; Ohkuchi, A.; Takahashi, H.; Kurosawa, A.; Torii, Y.; Iwata, H.; et al. Palmitic acid activates NLRP3 inflammasome and induces placental inflammation during pregnancy in mice. J. Reprod. Dev. 2020, 66, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontillo, A.; Girardelli, M.; Agostinis, C.; Masat, E.; Bulla, R.; Crovella, S. Bacterial LPS differently modulates inflammasome gene expression and IL-1beta secretion in trophoblast cells, decidual stromal cells, and decidual endothelial cells. Reprod. Sci. 2013, 20, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Plazyo, O.; Unkel, R.; Leng, Y.; Than, N.G.; Chaiworapongsa, T.; Panaitescu, B.; Dong, Z.; et al. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod. Sci. 2017, 24, 1382–1401. [Google Scholar] [CrossRef]
- Huppertz, B.; Bartz, C.; Kokozidou, M. Trophoblast fusion: Fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron 2006, 37, 509–517. [Google Scholar] [CrossRef]
- Newhouse, S.M.; Davidge, S.T.; Winkler-Lowen, B.; Demianczuk, N.; Guilbert, L.J. In vitro differentiation of villous trophoblasts from pregnancies complicated by intrauterine growth restriction with and without pre-eclampsia. Placenta 2007, 28, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Maneta, E.; Warren, A.Y.; Hay, D.P.; Khan, R.N. Caspase-1-mediated cytokine release from gestational tissues, placental, and cord blood. Front. Physiol. 2015, 6, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Zhu, J.; He, M.; Ma, C.; Peng, F.; Su, Y.; Huang, L. Expression and Clinical Significance of NOD-Like Receptor Protein 3 (NLRP3) and Caspase-1 in Fetal Membrane and Placental Tissues of Patients with Premature Rupture of Membrane. Med. Sci. Monit. 2018, 24, 1560–1566. [Google Scholar] [CrossRef] [Green Version]
- Aye, I.L.M.H.; Lager, S.; Powell, T.L. The Role of Placental Inflammasomes in Linking the Adverse Effects of Maternal Obesity on Fetal Development. In Metabolic Syndrome and Complications of Pregnancy: The Potential Preventive Role of Nutrition; Ferrazzi, E., Sears, B., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 77–90. [Google Scholar]
- Crowley, L.C.; Waterhouse, N.J. Detecting Cleaved Caspase-3 in Apoptotic Cells by Flow Cytometry. Cold Spring Harb. Protoc. 2016. [Google Scholar] [CrossRef]
- Salvesen, G.S.; Walsh, C.M. Functions of caspase 8: The identified and the mysterious. Semin. Immunol. 2014, 26, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Allam, R.; Lawlor, K.E.; Yu, E.C.; Mildenhall, A.L.; Moujalled, D.M.; Lewis, R.S.; Ke, F.; Mason, K.D.; White, M.J.; Stacey, K.J.; et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014, 15, 982–990. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, D.I.; Kolobov, A.V.; Lesnichija, M.V.; Kostiouchek, I.N.; Stepanova, O.I.; Kvetnoy, I.M.; Selkov, S.A. Regulatory mechanisms for apoptosis in placental tissue during normal pregnancy and gestosis-complicated pregnancy. Bull. Exp. Biol. Med. 2009, 148, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, P.; Eckert, R.L.; Reece, E.A. Caspase-8: A key role in the pathogenesis of diabetic embryopathy. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019, 33, e22834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, V.R.; Romao-Veiga, M.; Nunes, P.R.; de Oliveira, L.R.C.; Romagnoli, G.G.; Peracoli, J.C.; Peracoli, M.T.S. Immunomodulatory effect of vitamin D on the STATs and transcription factors of CD4(+) T cell subsets in pregnant women with preeclampsia. Clin. Immunol. 2022, 234, 108917. [Google Scholar] [CrossRef]
- Rivera, D.L.; Olister, S.M.; Liu, X.; Thompson, J.H.; Zhang, X.J.; Pennline, K.; Azuero, R.; Clark, D.A.; Miller, M.J. Interleukin-10 attenuates experimental fetal growth restriction and demise. FASEB J. 1998, 12, 189–197. [Google Scholar] [CrossRef]

| Characteristics | Control (n = 25) | FGR (n = 25) | Significance |
|---|---|---|---|
| Gestational age (weeks) | 34.44 ± 3.959 | 36.12 ± 3.232 | p = 0.107 |
| Maternal age (years) | 34 ± 5.323 | 31.2 ± 5.008 | p = 0.061 |
| Parity | p = 0.089 | ||
| Primaparous | 9 (36%) | 16 (64%) | |
| Multiparous | 16 (64%) | 9 (36%) | |
| Mode of delivery | p = 0.300 | ||
| Vaginal delivery | 6 (24%) | 9 (36%) | |
| Caesarean in labour | 1 (4%) | 3 (12%) | |
| Caesarean not in labour | 18 (72%) | 13 (52%) | |
| Newborn Characteristics | |||
| Gender | p ≥ 0.999 | ||
| Male | 11 (44%) | 12 (48%) | |
| Female | 14 (56%) | 13 (52%) | |
| Placental weight (g) | 506 ± 144.1 | 395.9 ± 124 | p = 0.006 |
| Birth weight (g) | 2474 ± 875.9 | 1968 ± 662.9 | p = 0.026 |
| Birth weight percentile | |||
| 10th–90th | 25 (100%) | 0 | |
| 5th–10th | 12/25 (48%) | ||
| 3th–5th | 11/25 (44%) | ||
| <3th | 2/25 (8%) |
| Clinical Characteristics | Number of Samples (%) |
|---|---|
| BW < 10th percentile | 25/25 (100%) |
| Abnormal umbilical artery Doppler velocimetry | |
| Elevated | 5/25 (20%) |
| Reversed | 6/25 (24%) |
| Absent | 8/25 (32%) |
| Normal | 4/25 (16%) |
| Not recorded | 2/25 (8%) |
| Asymmetric growth | |
| HC:AC ratio > 1.2 | 25/25 (100%) |
| Amniotic fluid index (AFI) | |
| Normal (AFI = 7) | 7/25 (28%) |
| Polyhydramnios (AFI > 7) | 7/25 (28%) |
| Oligohydramnios (AFI < 7) | 11/25 (44%) |
| Genes | Control (n = 25) | FGR (n = 25) |
|---|---|---|
| NLRP3 | 1.213 ± 0.851 | 1.099 ± 1.204 |
| CASP1 | 1.001 ± 1.305 | 1.552 ± 10.474 |
| NFκB1 | 1.303 ± 0.905 | 0.937 ± 1.189 |
| CASP3 | 1.384 ± 1.423 | 0.529 ± 0.845 |
| CASP8 | 1.256 ± 0.982 | 0.549 ± 0.397 |
| NLRC5 | 1.197 ± 1.427 | 1.508 ± 2.138 |
| NOD2 | 1.259 ± 1.117 | 1.767 ± 6.981 |
| Genes | Control (n = 25) | FGR (n = 25) |
|---|---|---|
| TLR2 | 0.958 ± 2.138 | 1.093 ± 4.707 |
| TLR5 | 0.685 ± 1.180 | 0.397 ± 4.318 |
| TLR6 | 0.620 ± 1.469 | 0.135 ± 0.658 |
| Genes | Control (n = 25) | FGR (n = 25) |
|---|---|---|
| IL-1β | 0.822 ± 1.208 | 2.724 ± 15.483 |
| IL-6 | 1.204 ± 0.579 | 0.900 ± 4.033 |
| IFNγ | 0.958 ± 1.596 | 11.7 ± 119.944 |
| IL-10 | 0.969 ± 0.950 | 1.163 ± 28.943 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfian, I.; Chakraborty, A.; Yong, H.E.J.; Saini, S.; Lau, R.W.K.; Kalionis, B.; Dimitriadis, E.; Alfaidy, N.; Ricardo, S.D.; Samuel, C.S.; et al. The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction. Cells 2022, 11, 1413. https://doi.org/10.3390/cells11091413
Alfian I, Chakraborty A, Yong HEJ, Saini S, Lau RWK, Kalionis B, Dimitriadis E, Alfaidy N, Ricardo SD, Samuel CS, et al. The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction. Cells. 2022; 11(9):1413. https://doi.org/10.3390/cells11091413
Chicago/Turabian StyleAlfian, Irvan, Amlan Chakraborty, Hannah E. J. Yong, Sheetal Saini, Ricky W. K. Lau, Bill Kalionis, Evdokia Dimitriadis, Nadia Alfaidy, Sharon D. Ricardo, Chrishan S. Samuel, and et al. 2022. "The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction" Cells 11, no. 9: 1413. https://doi.org/10.3390/cells11091413
APA StyleAlfian, I., Chakraborty, A., Yong, H. E. J., Saini, S., Lau, R. W. K., Kalionis, B., Dimitriadis, E., Alfaidy, N., Ricardo, S. D., Samuel, C. S., & Murthi, P. (2022). The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction. Cells, 11(9), 1413. https://doi.org/10.3390/cells11091413