Integrin-Mediated Adhesion Promotes Centrosome Separation in Early Mitosis
Abstract
:1. Introduction
2. Materials and Methods
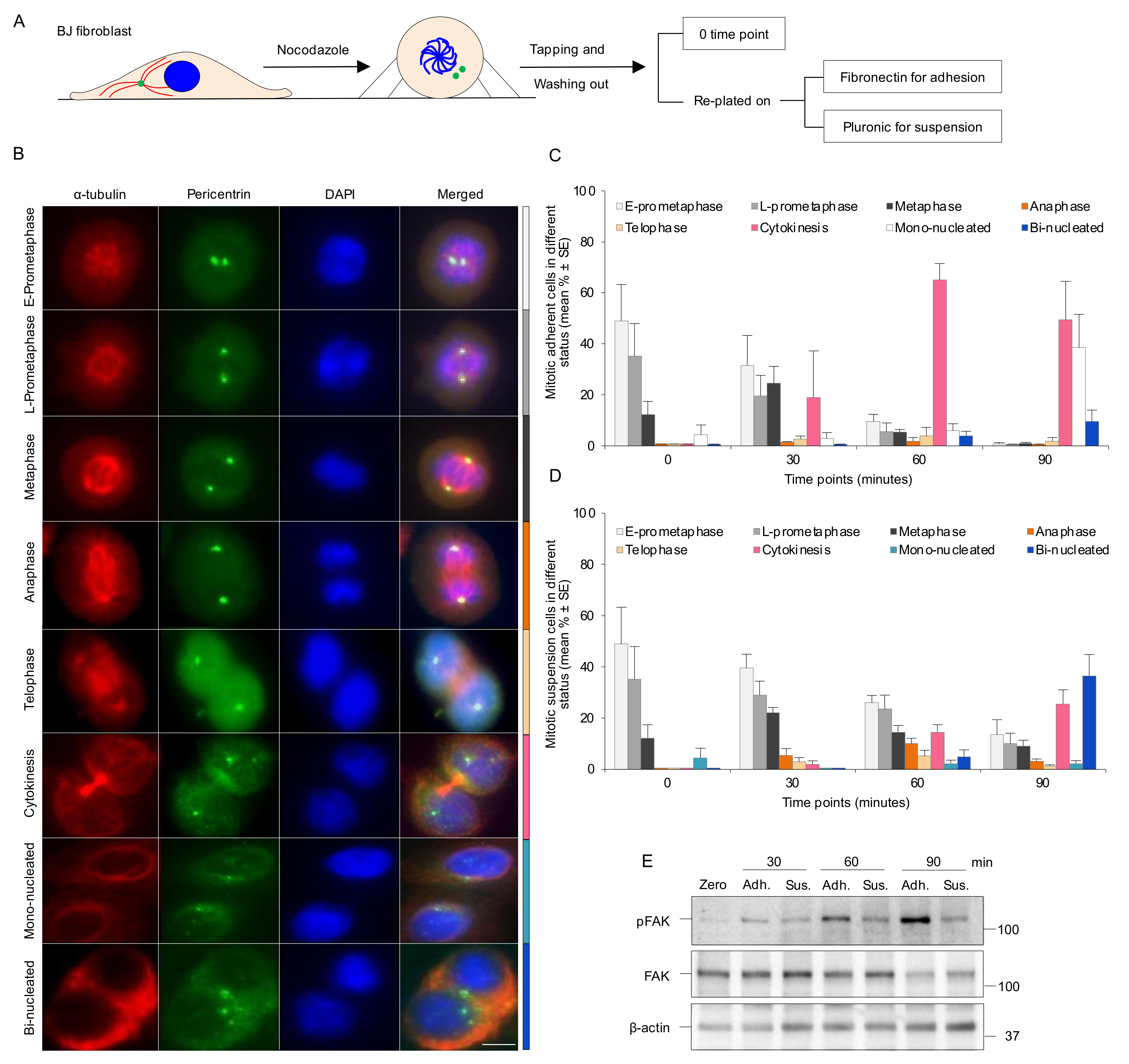
2.1. Cell Lines and Culturing of Mitotic Cells
2.2. Cell Synchronization
2.3. Live-Cell Imaging
2.4. Immunofluorescence Staining and Quantification of pPLk1 Signal Intensity
2.5. Metaphase Plate Analysis
2.6. SiRNA Transfection
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
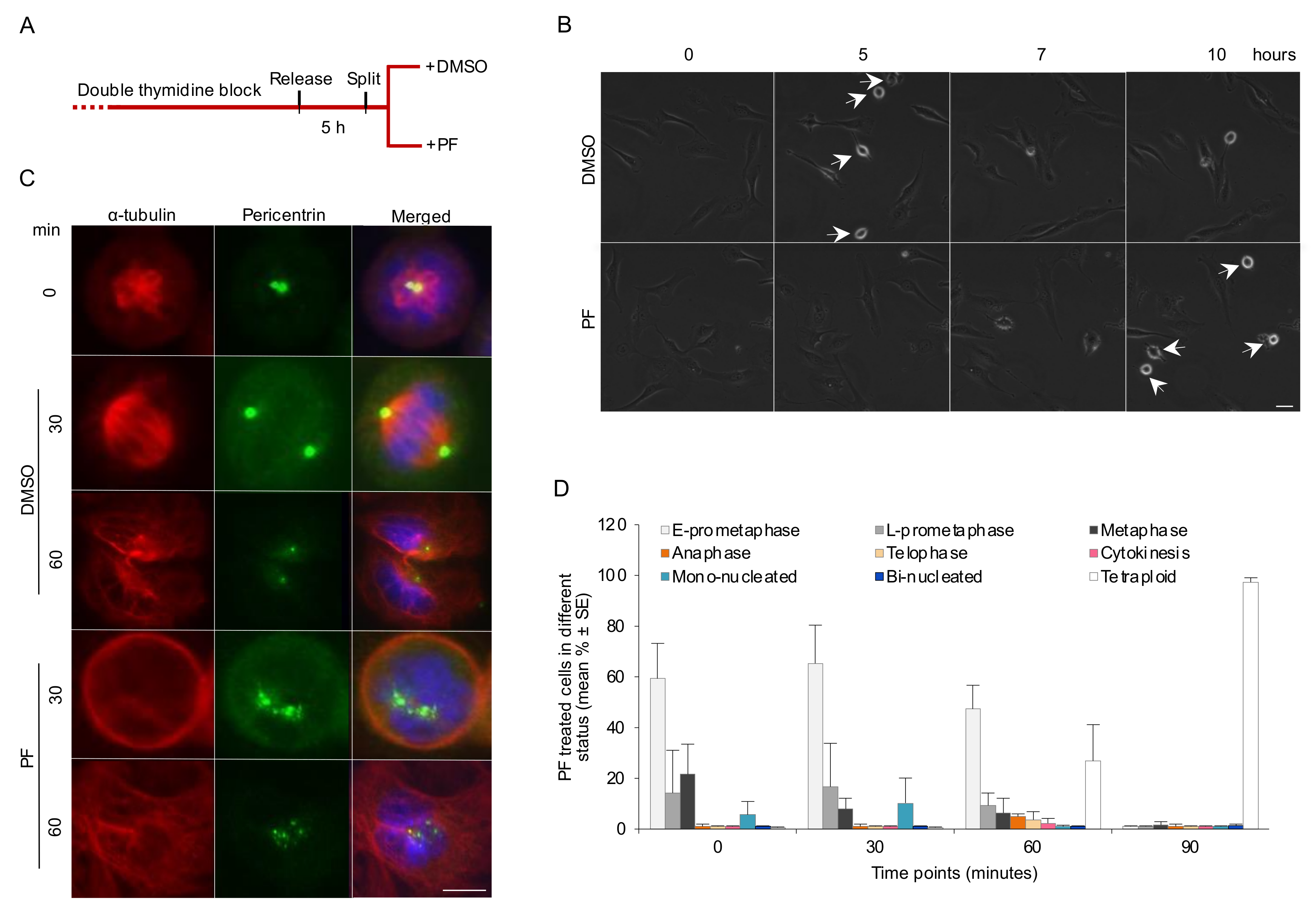
3.1. Absence of Integrin-Mediated Cell Adhesion Prolongs G2 Phase and Delays Mitotic Progression in the Early Stages
3.2. Absence of Integrin-Mediated Cell Adhesion Causes Centrosome Abnormality
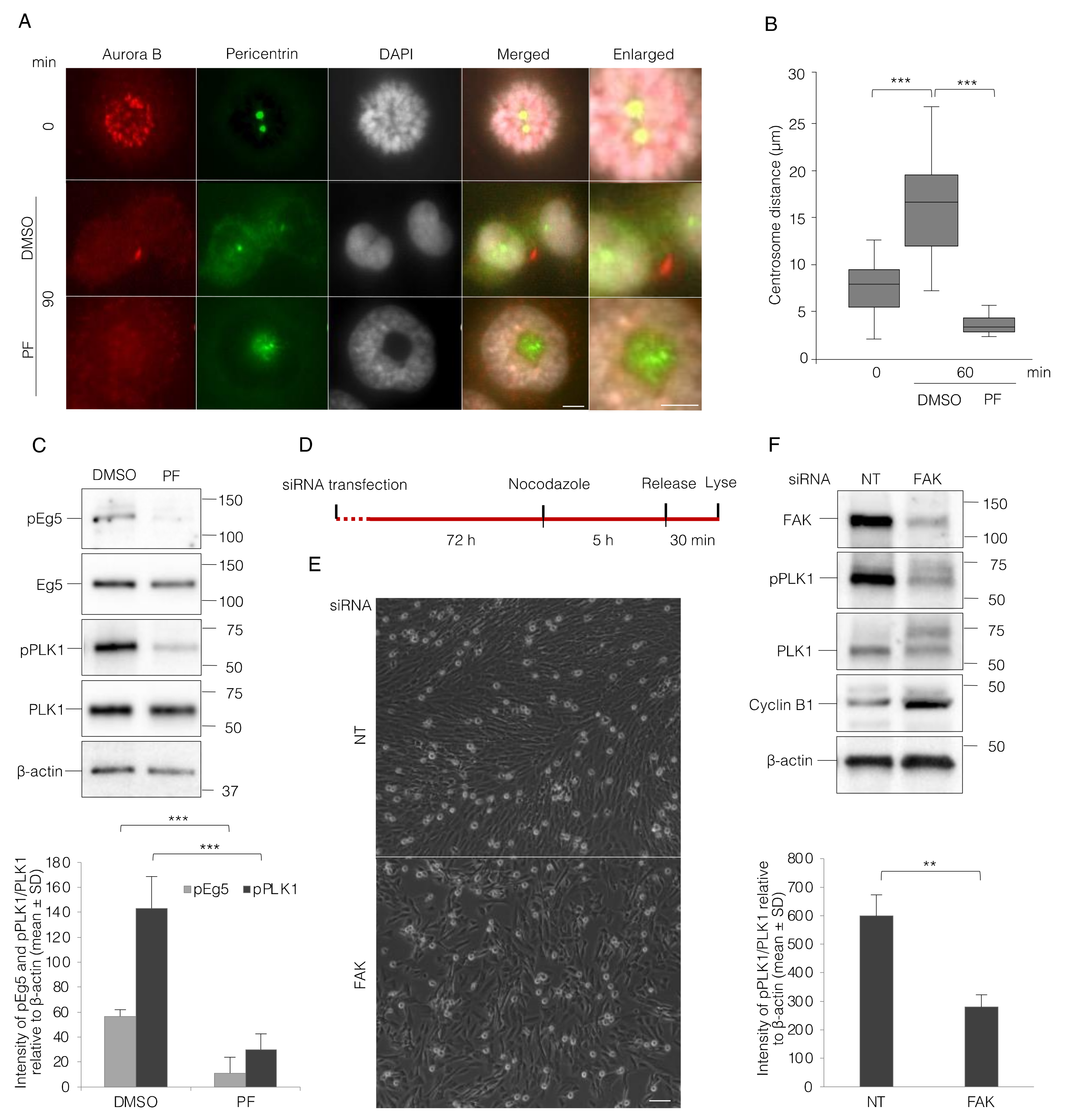
3.3. FAK Activity Promotes G2 to M Transition and Progression in Early Mitosis
3.4. FAK Inhibition Reduces PLK1 Activity and Thereby Impairs Centrosome Separation and Bipolar Spindle Assembly
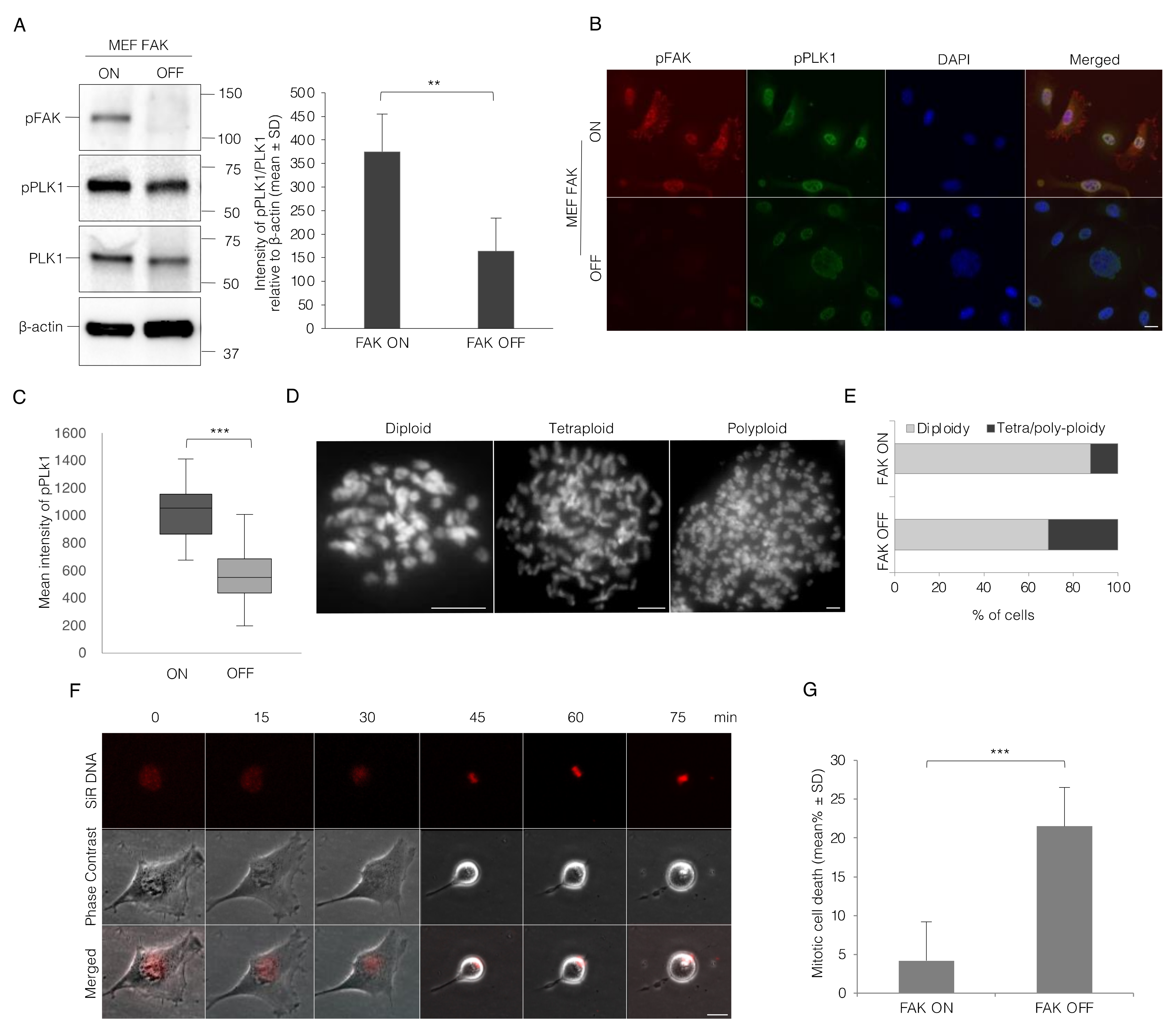
3.5. Lack of FAK Expression Resembles the Effect of Integrin-Mediated Adhesion on Mitotic Progression in MEF Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between the extracellular matrix—Cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Acebron, I.; Righetto, R.D.; Schoenherr, C.; de Buhr, S.; Redondo, P.; Culley, J.; Rodríguez, C.F.; Daday, C.; Biyani, N.; Llorca, O.; et al. Structural basis of Focal Adhesion Kinase activation on lipid membranes. EMBO J. 2020, 39, e104743. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase, in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Gough, R.E.; Jones, M.C.; Zacharchenko, T.; Le, S.; Yu, M.; Jacquemet, G.; Muench, S.P.; Yan, J.; Humphries, J.D.; Jørgensen, C.; et al. Talin mechanosensitivity is modulated by a direct interaction with cyclin-dependent kinase-1. J. Biol. Chem. 2021, 297, 100837. [Google Scholar] [CrossRef]
- Jones, M.C.; Askari, J.A.; Humphries, J.D.; Humphries, M.J. Cell adhesion is regulated by CDK1 during the cell cycle. J. Cell Biol. 2018, 217, 3203–3218. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.C.; Zha, J.; Humphries, M.J. Connections between the cell cycle, cell adhesion and the cytoskeleton. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2019, 374, 20180227. [Google Scholar] [CrossRef]
- Lock, J.G.; Jones, M.C.; Askari, J.A.; Gong, X.; Oddone, A.; Olofsson, H.; Göransson, S.; Lakadamyali, M.; Humphries, M.J.; Strömblad, S. Reticular adhesions are a distinct class of cell-matrix adhesions that mediate attachment during mitosis. Nat. Cell Biol. 2018, 20, 1290–1302. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, S.; Montani, F.; Deflorian, G.; D’Antuono, R.; Cuomo, A.; Bologna, S.; Mazzoccoli, C.; Bonaldi, T.; Di Fiore, P.P.; Nicassio, F. DEPDC1B coordinates de-adhesion events and cell-cycle progression at mitosis. Dev. Cell 2014, 31, 420–433. [Google Scholar] [CrossRef] [Green Version]
- Dix, C.L.; Matthews, H.K.; Uroz, M.; McLaren, S.; Wolf, L.; Heatley, N.; Win, Z.; Almada, P.; Henriques, R.; Boutros, M.; et al. The Role of Mitotic Cell-Substrate Adhesion Re-modeling in Animal Cell Division. Dev. Cell 2018, 45, 132–145.e133. [Google Scholar] [CrossRef] [Green Version]
- Dao, V.T.; Dupuy, A.G.; Gavet, O.; Caron, E.; de Gunzburg, J. Dynamic changes in Rap1 activity are required for cell retraction and spreading during mitosis. J. Cell Sci. 2009, 122, 2996–3004. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Layseca, P.; Streuli, C.H. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014, 34, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Sambandamoorthy, S.; Mathew-Steiner, S.; Varney, S.; Zuidema, J.M.; Gilbert, R.J.; Van De Water, L.; LaFlamme, S.E. Matrix compliance and the regulation of cytokinesis. Biol. Open 2015, 4, 885–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Ohtsubo, M.; Bohmer, R.M.; Roberts, J.M.; Assoian, R.K. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 1996, 133, 391–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugasundaram, K.; Block, K.; Nayak, B.K.; Livi, C.B.; Venkatachalam, M.A.; Sudarshan, S. PI3K regulation of the SKP-2/p27 axis through mTORC2. Oncogene 2013, 32, 2027–2036. [Google Scholar] [CrossRef] [Green Version]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Hognas, G.; Tuomi, S.; Veltel, S.; Mattila, E.; Murumagi, A.; Edgren, H.; Kallioniemi, O.; Ivaska, J. Cytokinesis failure due to derailed integrin traffic induces aneuploidy and oncogenic transformation in vitro and in vivo. Oncogene 2012, 31, 3597–3606. [Google Scholar] [CrossRef] [Green Version]
- Mierzwa, B.; Gerlich, D.W. Cytokinetic abscission, molecular mechanisms and temporal control. Dev. Cell 2014, 31, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Kamranvar, S.A.; Gupta, D.K.; Huang, Y.; Gupta, R.K.; Johansson, S. Integrin signaling via FAK-Src controls cytokinetic abscission by decelerating PLK1 degradation and subsequent recruitment of CEP55 at the midbody. Oncotarget 2016, 7, 30820–30830. [Google Scholar] [CrossRef] [Green Version]
- Bastos, R.N.; Barr, F.A. Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission. J. Cell Biol. 2010, 191, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Petronczki, M.; Lenart, P.; Peters, J.M. Polo on the Rise-from Mitotic Entry to Cytokinesis with Plk1. Dev. Cell 2008, 14, 646–659. [Google Scholar] [CrossRef] [Green Version]
- Roshak, A.K.; Capper, E.A.; Imburgia, C.; Fornwald, J.; Scott, G.; Marshall, L.A. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal. 2000, 12, 405–411. [Google Scholar] [CrossRef]
- Van Vugt, M.A.; Bras, A.; Medema, R.H. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol. Cell 2004, 15, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Conduit, P.T.; Wainman, A.; Raff, J.W. Centrosome function and assembly in animal cells. Nat. Rev. Mol. Cell Biol. 2015, 16, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Rhee, K. PLK1 phosphorylation of pericentrin initiates centrosome maturation at the onset of mitosis. J. Cell Biol. 2011, 195, 1093–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigg, E.A.; Holland, A.J. Once and only once, mechanisms of centriole duplication and their deregulation in disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Mardin, B.R.; Agircan, F.G.; Lange, C.; Schiebel, E. Plk1 controls the Nek2A-PP1gamma antagonism in centrosome disjunction. Curr. Biol. 2011, 21, 1145–1151. [Google Scholar] [CrossRef] [Green Version]
- Tanenbaum, M.E.; Medema, R.H. Mechanisms of centrosome separation and bipolar spindle assembly. Dev. Cell 2010, 19, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Bertran, M.T.; Sdelci, S.; Regue, L.; Avruch, J.; Caelles, C.; Roig, J. Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J. 2011, 30, 2634–2647. [Google Scholar] [CrossRef] [Green Version]
- Vitre, B.D.; Cleveland, D.W. Centrosomes, chromosome instability (CIN) and aneuploidy. Curr. Opin. Cell Biol. 2012, 24, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Lens, S.M.A.; Medema, R.H. Cytokinesis defects and cancer. Nat. Rev. Cancer 2019, 19, 32–45. [Google Scholar] [CrossRef]
- Toyoshima, F.; Nishida, E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J. 2007, 26, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Petridou, N.I.; Skourides, P.A. FAK transduces extracellular forces that orient the mitotic spindle and control tissue morphogenesis. Nat. Commun. 2014, 5, 5240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverte, C.G.; Benware, A.; Jones, C.W.; LaFlamme, S.E. Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J. Cell Biol. 2006, 174, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colello, D.; Mathew, S.; Ward, R.; Pumiglia, K.; LaFlamme, S.E. Integrins regulate microtubule nucleating activity of centrosome through mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signaling. J. Biol. Chem. 2012, 287, 2520–2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, H.; Ong, Y.M.; Shah, W.A.; Holland, P.C.; Carbonetto, S. Integrins regulate centrosome integrity and astrocyte polarization following a wound. Dev. Neurobiol. 2013, 73, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.D.; Ruest, P.J.; Fry, D.W.; Hanks, S.K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell Biol. 1999, 19, 4806–4818. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.H.; Read, R.A.; Bedford, J.S. Comparison of synchronized Chinese hamster ovary cells obtained by mitotic shake-off, hydroxyurea, aphidicolin, or methotrexate. Cytometry 1987, 8, 315–320. [Google Scholar] [CrossRef]
- Rapley, J.; Nicolas, M.; Groen, A.; Regue, L.; Bertran, M.T.; Caelles, C.; Avruch, J.; Roig, J. The NIMA-family kinase Nek6 phosphorylates the kinesin Eg5 at a novel site necessary for mitotic spindle formation. J. Cell Sci. 2008, 121, 3912–3921. [Google Scholar] [CrossRef] [Green Version]
- Vakifahmetoglu, H.; Olsson, M.; Zhivotovsky, B. Death through a tragedy, mitotic catastrophe. Cell Death Differ. 2008, 15, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Yamakita, Y.; Totsukawa, G.; Yamashiro, S.; Fry, D.; Zhang, X.; Hanks, S.K.; Matsumura, F. Dissociation of FAK/p130(CAS)/c-Src complex during mitosis, role of mitosis-specific serine phosphorylation of FAK. J. Cell Biol. 1999, 144, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.; Hegarat, N.; Vesely, C.; Roseboom, I.; Larch, C.; Streicher, H.; Straatman, K.; Flynn, H.; Skehel, M.; Hirota, T.; et al. Differential control of Eg5-dependent centrosome separation by Plk1 and Cdk1. EMBO J. 2011, 30, 2233–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, A.Y.; Shen, T.L.; Chien, S.; Guan, J.L. Role of focal adhesion kinase Ser-732 phosphorylation in centrosome function during mitosis. J. Biol. Chem. 2009, 284, 9418–9425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, D.K.; Du, J.; Kamranvar, S.A.; Johansson, S. Tension-induced cytokinetic abscission in human fibroblasts. Oncotarget 2018, 9, 8999–9009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maroto, B.; Ye, M.B.; von Lohneysen, K.; Schnelzer, A.; Knaus, U.G. P21-activated kinase is required for mitotic progression and regulates Plk1. Oncogene 2008, 27, 4900–4908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zheng, H.; Li, X.; Wang, S.; Meyerson, H.J.; Yang, W.; Neel, B.G.; Qu, C.K. Gain-of-function mutations of Ptpn11 (Shp2) cause aberrant mitosis and increase susceptibility to DNA damage-induced malignancies. Proc. Natl. Acad. Sci. USA 2016, 113, 984–989. [Google Scholar] [CrossRef] [Green Version]
- Du, R.; Huang, C.; Liu, K.; Li, X.; Dong, Z. Targeting AURKA in Cancer, molecular mechanisms and opportunities for Cancer therapy. Mol. Cancer 2021, 20, 15. [Google Scholar] [CrossRef]
- Tavernier, N.; Sicheri, F.; Pintard, L. Aurora A kinase activation, Different means to different ends. J. Cell Biol. 2021, 220, e202106128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.S.; Lim, J.P.; Ng, Y.W.; Lim, L.; Manser, E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol. Cell 2005, 20, 237–249. [Google Scholar] [CrossRef]
- Rea, K.; Sensi, M.; Anichini, A.; Canevari, S.; Tomassetti, A. EGFR/MEK/ERK/CDK5-dependent integrin-independent FAK phosphorylated on serine 732 contributes to microtubule depolymerization and mitosis in tumor cells. Cell Death Dis. 2013, 4, e815. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Q.; Zhang, X.; Zhang, B.; Zhuo, X.; Liu, J.; Jiang, Q.; Zhang, C. PCM1 recruits Plk1 to the pericentriolar matrix to promote primary cilia disassembly before mitotic entry. J. Cell Sci. 2013, 126, 1355–1365. [Google Scholar] [CrossRef] [Green Version]
- Oshimori, N.; Ohsugi, M.; Yamamoto, T. The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity. Nat. Cell Biol. 2006, 8, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Karki, M.; Keyhaninejad, N.; Shuster, C.B. Precocious centriole disengagement and centrosome fragmentation induced by mitotic delay. Nat. Commun. 2017, 8, 15803. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamranvar, S.A.; Gupta, D.K.; Wasberg, A.; Liu, L.; Roig, J.; Johansson, S. Integrin-Mediated Adhesion Promotes Centrosome Separation in Early Mitosis. Cells 2022, 11, 1360. https://doi.org/10.3390/cells11081360
Kamranvar SA, Gupta DK, Wasberg A, Liu L, Roig J, Johansson S. Integrin-Mediated Adhesion Promotes Centrosome Separation in Early Mitosis. Cells. 2022; 11(8):1360. https://doi.org/10.3390/cells11081360
Chicago/Turabian StyleKamranvar, Siamak A., Deepesh Kumar Gupta, Anishia Wasberg, Liangwen Liu, Joan Roig, and Staffan Johansson. 2022. "Integrin-Mediated Adhesion Promotes Centrosome Separation in Early Mitosis" Cells 11, no. 8: 1360. https://doi.org/10.3390/cells11081360
APA StyleKamranvar, S. A., Gupta, D. K., Wasberg, A., Liu, L., Roig, J., & Johansson, S. (2022). Integrin-Mediated Adhesion Promotes Centrosome Separation in Early Mitosis. Cells, 11(8), 1360. https://doi.org/10.3390/cells11081360







