HLA-G and CD152 Expression Levels Encourage the Use of Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells as an Alternative for Immunosuppressive Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Expansion
2.2. Cell Characterization
2.3. Labelling of CD152
2.4. Labelling of HLA-G1
2.5. HLA-G5 Detection
2.6. Lymphocyte Inhibition Assay
2.7. HLA-G Genotyping
2.8. Statistical Analysis
3. Results
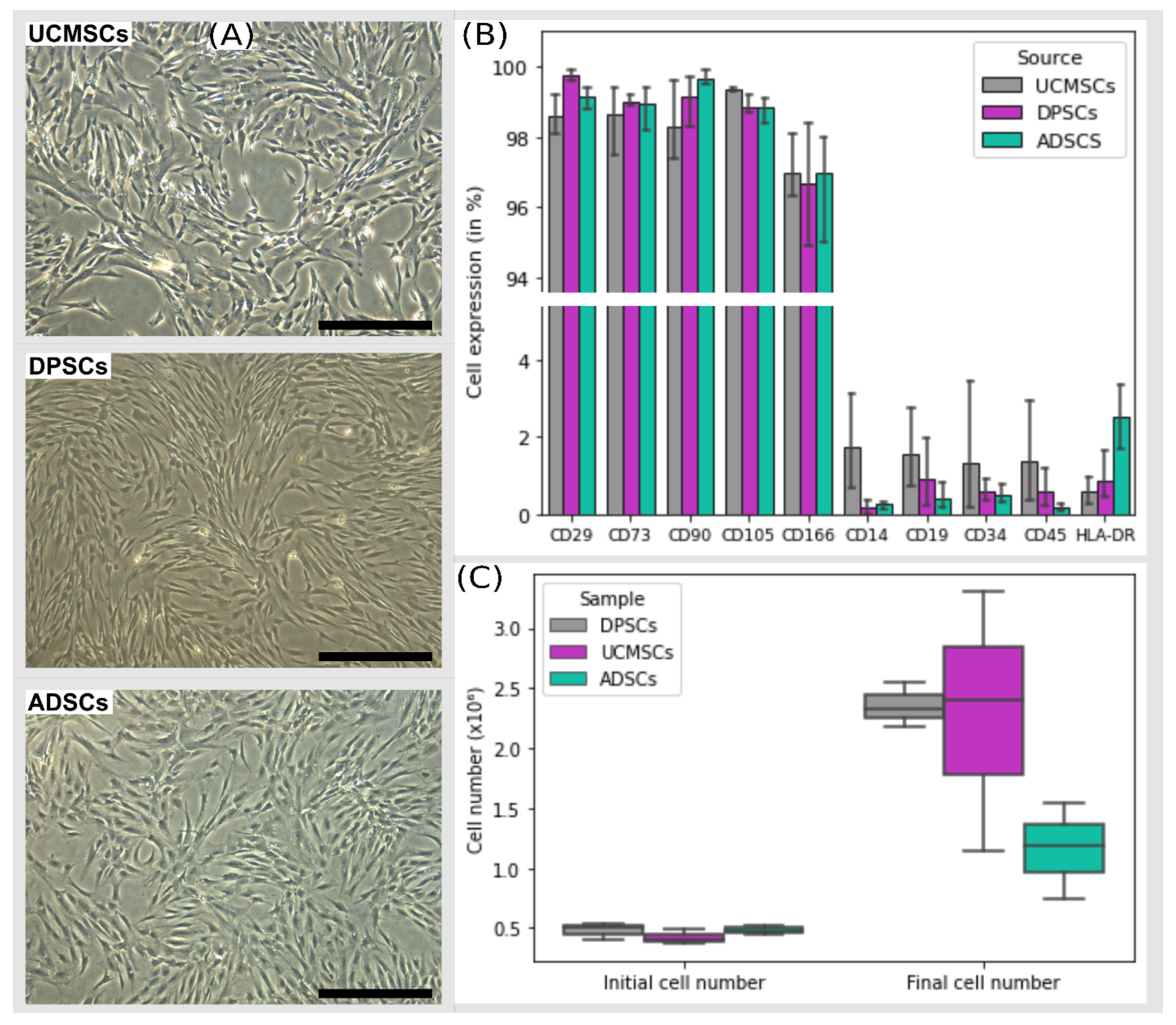
3.1. Cell Characterization
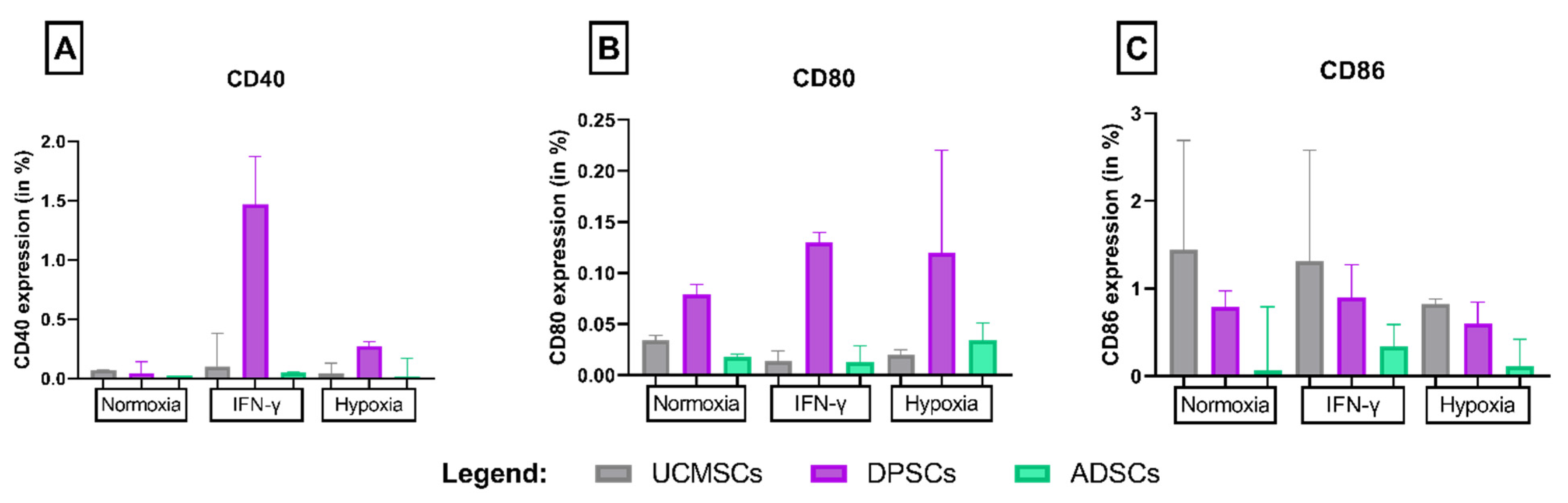
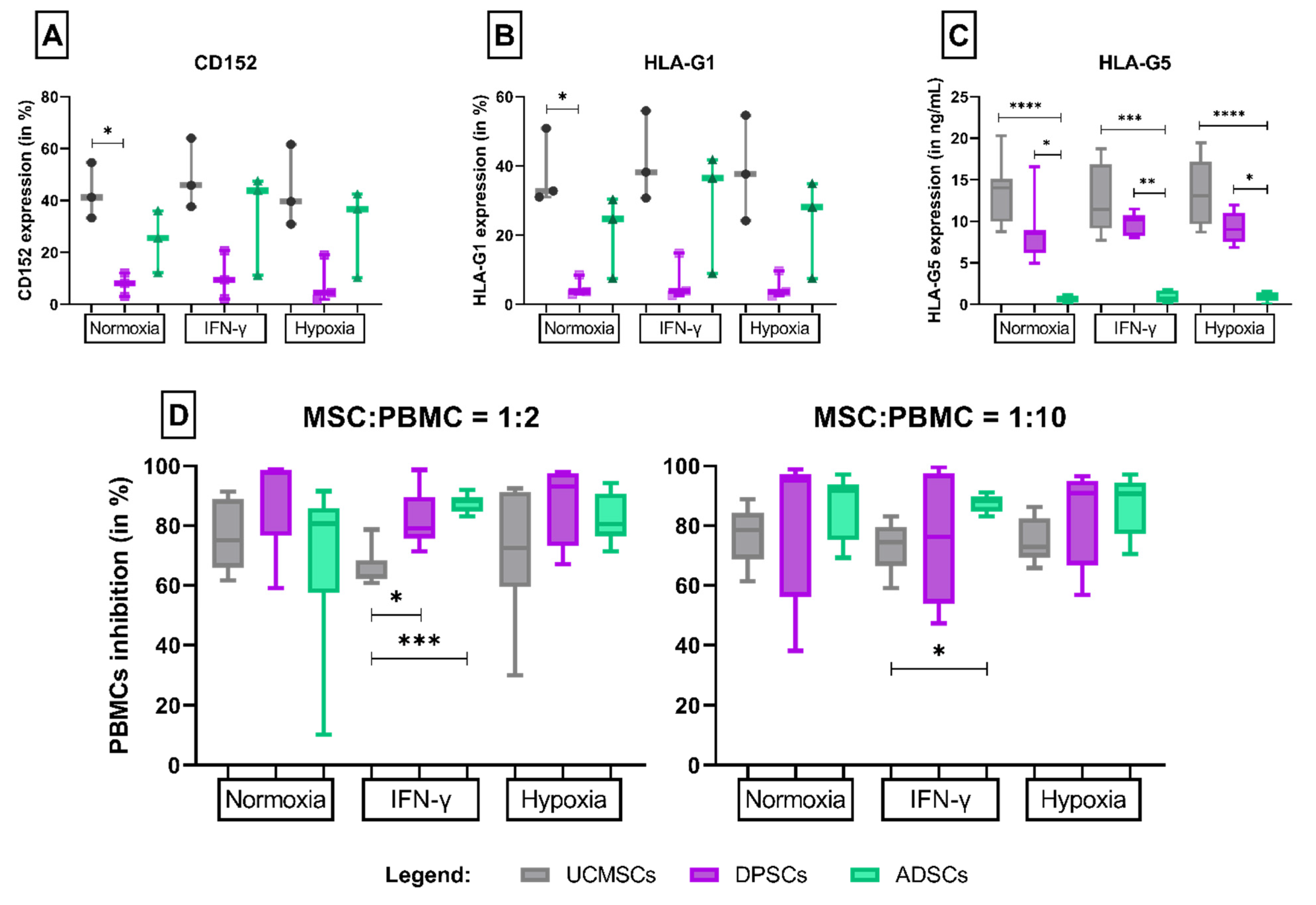
3.2. CD152
3.3. HLA-G: -G1 and -G5 Isoforms
3.4. Lymphocyte Inhibition Assay
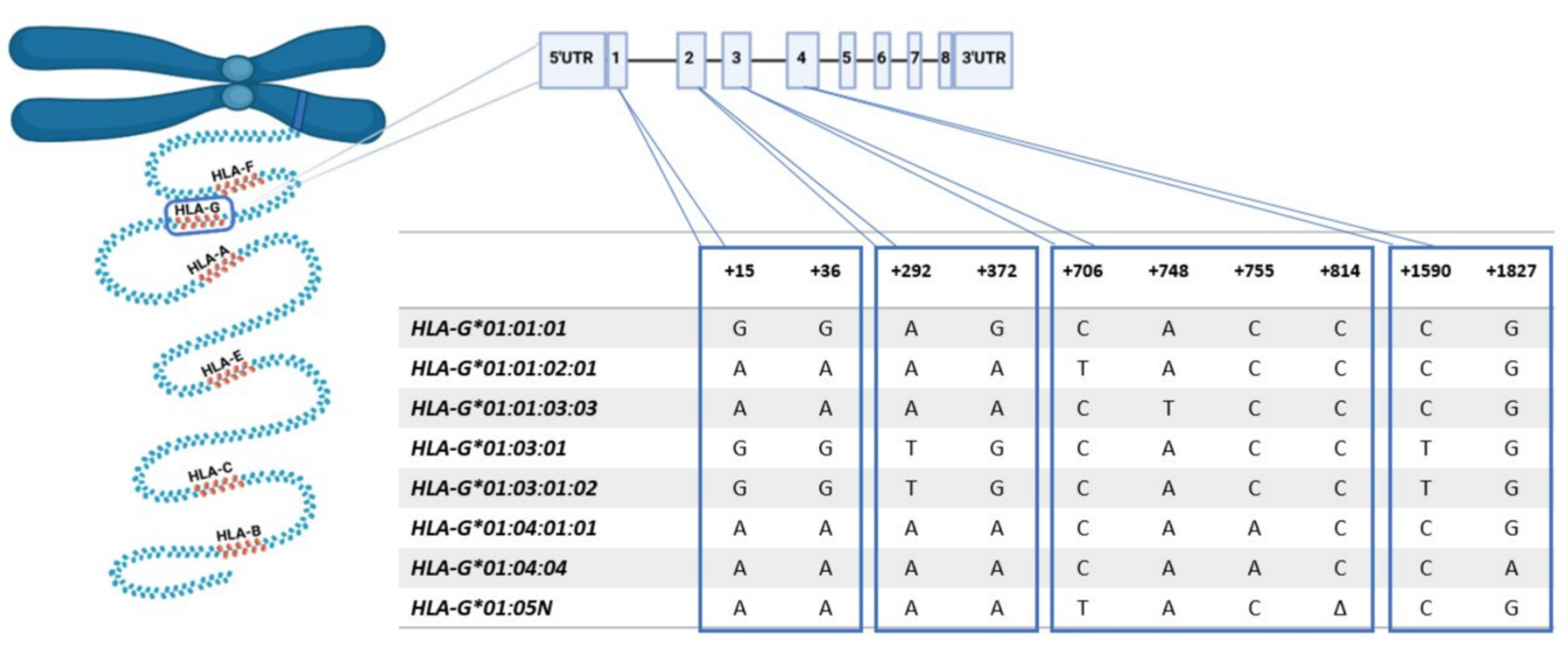
3.5. HLA-G Expression Associated with Genotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rad, F.; Ghorbani, M.; Mohammadi Roushandeh, A.; Habibi Roudkenar, M. Mesenchymal Stem Cell-Based Therapy for Autoimmune Diseases: Emerging Roles of Extracellular Vesicles. Mol. Biol. Rep. 2019, 46, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological Functions of Mesenchymal Stem Cells and Clinical Implications. Cell. Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.R. Immunotherapy of Autoimmunity and Cancer: The Penalty for Success. Nat. Rev. Immunol. 2008, 8, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, H.; Kong, W.; Deng, W.; Wang, D.; Feng, X.; Zhao, C.; Hua, B.; Wang, H.; Sun, L. Safety Analysis in Patients with Autoimmune Disease Receiving Allogeneic Mesenchymal Stem Cells Infusion: A Long-Term Retrospective Study. Stem Cell Res. Ther. 2018, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Nagamura-Inoue, T.; Takahashi, A.; Mori, Y.; Yamamoto, Y.; Shimazu, T.; Tsunoda, H.; Tojo, A. Immunosuppressive Properties of Wharton’s Jelly-Derived Mesenchymal Stromal Cells In Vitro. Int. J. Hematol. 2015, 102, 368–378. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jo, C.H.; Kim, H.-R.; Hwang, Y. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018, 2018, 8429042. [Google Scholar] [CrossRef]
- Yang, H.-M.; Sung, J.-H.; Choi, Y.-S.; Lee, H.-J.; Roh, C.-R.; Kim, J.; Shin, M.; Song, S.; Kwon, C.-H.; Joh, J.-W.; et al. Enhancement of the Immunosuppressive Effect of Human Adipose Tissue-Derived Mesenchymal Stromal Cells through HLA-G1 Expression. Cytotherapy 2012, 14, 70–79. [Google Scholar] [CrossRef]
- Chen, B.; Xu, D.; Lin, A.; Yan, W. NK Cytolysis Is Dependent on the Proportion of HLA-G Expression. Hum. Immunol. 2013, 74, 286–289. [Google Scholar] [CrossRef]
- Chen, C.; Liang, J.; Yao, G.; Chen, H.; Shi, B.; Zhang, Z.; Zhao, C.; Zhang, H.; Sun, L. Mesenchymal Stem Cells Upregulate Treg Cells via SHLA-G in SLE Patients. Int. Immunopharmacol. 2017, 44, 234–241. [Google Scholar] [CrossRef]
- Contini, P.; Ghio, M.; Poggi, A.; Filaci, G.; Indiveri, F.; Ferrone, S.; Puppo, F. Soluble HLA-A,-B,-C and -G Molecules Induce Apoptosis in T and NK CD8+ Cells and Inhibit Cytotoxic T Cell Activity through CD8 Ligation. Eur. J. Immunol. 2003, 33, 125–134. [Google Scholar] [CrossRef]
- Lemaoult, J.; Zafaranloo, K.; Le Danff, C.; Carosella, E.D. HLA-G Up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in Antigen Presenting Cells, NK Cells, and T Cells. FASEB J. 2005, 19, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Menier, C.; Morandi, F.; Agaugue, S.; Maki, G.; Ferretti, E.; Bruel, S.; Pistoia, V.; Carosella, E.D.; Rouas-Freiss, N. Binding of HLA-G to ITIM-Bearing Ig-like Transcript 2 Receptor Suppresses B Cell Responses. J. Immunol. 2014, 192, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Ristich, V.; Liang, S.; Zhang, W.; Wu, J.; Horuzsko, A. Tolerization of Dendritic Cells by HLA-G. Eur. J. Immunol. 2005, 35, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Queirolo, P.; Boero, S.; Salvi, S.; Piccioli, P.; Boccardo, S.; Minghelli, S.; Morabito, A.; Fontana, V.; Pietra, G.; et al. The Engagement of CTLA-4 on Primary Melanoma Cell Lines Induces Antibody-Dependent Cellular Cytotoxicity and TNF-α Production. J. Transl. Med. 2013, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Zhang, F.; Sun, D.; Zhang, Z.H.; Dong, S.W.; Xu, J.Z. CTLA4 Enhances the Osteogenic Differentiation of Allogeneic Human Mesenchymal Stem Cells in a Model of Immune Activation. Braz. J. Med. Biol. Res. 2015, 48, 629–636. [Google Scholar] [CrossRef]
- Gaber, T.; Schönbeck, K.; Hoff, H.; Tran, C.; Strehl, C.; Lang, A.; Ohrndorf, S.; Pfeiffenberger, M.; Röhner, E.; Matziolis, G.; et al. CTLA-4 Mediates Inhibitory Function of Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2018, 19, 2312. [Google Scholar] [CrossRef]
- Hegel, J.K.; Knieke, K.; Kolar, P.; Reiner, S.L.; Brunner-Weinzierl, M.C. CD152 (CTLA-4) Regulates Effector Functions of CD8 + T Lymphocytes by Repressing Eomesodermin. Eur. J. Immunol. 2009, 39, 883–893. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A Review of Therapeutic Effects of Mesenchymal Stem Cell Secretions and Induction of Secretory Modification by Different Culture Methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Wang, Y.; Markel, T.A.; Wang, M.; Lahm, T.; Meldrum, D.R. Human Mesenchymal Stem Cells Stimulated by TNF-α, LPS, or Hypoxia Produce Growth Factors by an NFκB- but Not JNK-Dependent Mechanism. Am. J. Physiol. Physiol. 2008, 294, C675–C682. [Google Scholar] [CrossRef]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.-H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine 2018, 28, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Jiang, E.; Yao, J.; Wang, M.; Chen, S.; Zhou, Z.; Zhai, W.; Ma, Q.; Feng, S.; Han, M. Interferon-γ Mediates the Immunosuppression of Bone Marrow Mesenchymal Stem Cells on T-Lymphocytes in Vitro. Hematology 2018, 23, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Mennan, C.; Brown, S.; McCarthy, H.; Mavrogonatou, E.; Kletsas, D.; Garcia, J.; Balain, B.; Richardson, J.; Roberts, S. Mesenchymal Stromal Cells Derived from Whole Human Umbilical Cord Exhibit Similar Properties to Those Derived from Wharton’s Jelly and Bone Marrow. FEBS Open Bio 2016, 6, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.E.; Carrión, F.; Villanueva, S.; Khoury, M. Mesenchymal Stem Cell Treatment for Autoimmune Diseases: A Critical Review. Biol. Res. 2012, 45, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Fracaro, L.; Senegaglia, A.C.; Herai, R.H.; Leitolis, A.; Boldrini-Leite, L.M.; Rebelatto, C.L.K.; Travers, P.J.; Brofman, P.R.S.; Correa, A. The Expression Profile of Dental Pulp-Derived Stromal Cells Supports Their Limited Capacity to Differentiate into Adipogenic Cells. Int. J. Mol. Sci. 2020, 21, 2753. [Google Scholar] [CrossRef]
- Robert, A.W.; Schittini, A.V.; Marchini, F.K.; Batista, M.; Affonso Da Costa, M.B.; Senegaglia, A.C.; Brofman, P.R.S.; Abud, A.P.R.; Stimamiglio, M.A. Tissue-Derived Signals for Mesenchymal Stem Cell Stimulation: Role of Cardiac and Umbilical Cord Microenvironments. Cells Tissues Organs 2017, 203, 173–182. [Google Scholar] [CrossRef]
- Amati, E.; Perbellini, O.; Rotta, G.; Bernardi, M.; Chieregato, K.; Sella, S.; Rodeghiero, F.; Ruggeri, M.; Astori, G. High-Throughput Immunophenotypic Characterization of Bone Marrow- and Cord Blood-Derived Mesenchymal Stromal Cells Reveals Common and Differentially Expressed Markers: Identification of Angiotensin-Converting Enzyme (CD143) as a Marker Differentially Expr. Stem Cell Res. Ther. 2018, 9, 10. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Kuo, T.-L.; Kuo, K.-K.; Hsiao, C.-C. Human Adipose-Derived Stem Cells: Isolation, Characterization and Current Application in Regeneration Medicine. Genom. Med. Biomark. Health Sci. 2011, 3, 53–62. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, M.; Hou, K.; Zhang, L.; Zheng, X.; Zhao, B.; Sui, X.; Xu, W.; Lu, S.; Guo, Q. Immune Characterization of Mesenchymal Stem Cells in Human Umbilical Cord Wharton’s Jelly and Derived Cartilage Cells. Cell. Immunol. 2012, 278, 35–44. [Google Scholar] [CrossRef]
- van Megen, K.M.; van’t Wout, E.T.; Lages Motta, J.; Dekker, B.; Nikolic, T.; Roep, B.O. Activated Mesenchymal Stromal Cells Process and Present Antigens Regulating Adaptive Immunity. Front. Immunol. 2019, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.L.; Ruszymah, B.H.I.; Ng, M.H.; Law, J.X. Characteristics and Clinical Applications of Wharton’s Jelly-Derived Mesenchymal Stromal Cells. Curr. Res. Transl. Med. 2020, 68, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ta, M.; Venugopal; Balasubramanian; Sen Majumdar. Isolation, Characterization, and Gene Expression Analysis of Wharton & Rsquo’s Jelly-Derived Mesenchymal Stem Cells under Xeno-Free Culture Conditions. Stem Cells Cloning Adv. Appl. 2011, 4, 39. [Google Scholar] [CrossRef][Green Version]
- Raicevic, G.; Najar, M.; Najimi, M.; El Taghdouini, A.; van Grunsven, L.A.; Sokal, E.; Toungouz, M. Influence of Inflammation on the Immunological Profile of Adult-Derived Human Liver Mesenchymal Stromal Cells and Stellate Cells. Cytotherapy 2015, 17, 174–185. [Google Scholar] [CrossRef]
- Lippens, C.; Duraes, F.V.; Dubrot, J.; Brighouse, D.; Lacroix, M.; Irla, M.; Aubry-Lachainaye, J.-P.; Reith, W.; Mandl, J.N.; Hugues, S. IDO-Orchestrated Crosstalk between PDCs and Tregs Inhibits Autoimmunity. J. Autoimmun. 2016, 75, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Langhans, B.; Alwan, A.W.; Krämer, B.; Glässner, A.; Lutz, P.; Strassburg, C.P.; Nattermann, J.; Spengler, U. Regulatory CD4+ T Cells Modulate the Interaction between NK Cells and Hepatic Stellate Cells by Acting on Either Cell Type. J. Hepatol. 2015, 62, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Da Silva, I.P.; Palendira, U.; Scolyer, R.A.; Long, G.V.; Wilmott, J.S. Targeting NK Cells to Enhance Melanoma Response to Immunotherapies. Cancers 2021, 13, 1363. [Google Scholar] [CrossRef]
- Stojanovic, A.; Fiegler, N.; Brunner-Weinzierl, M.; Cerwenka, A. CTLA-4 Is Expressed by Activated Mouse NK Cells and Inhibits NK Cell IFN-γ Production in Response to Mature Dendritic Cells. J. Immunol. 2014, 192, 4184–4191. [Google Scholar] [CrossRef]
- Ding, D.-C.; Chou, H.-L.; Chang, Y.-H.; Hung, W.-T.; Liu, H.-W.; Chu, T.-Y. Characterization of HLA-G and Related Immunosuppressive Effects in Human Umbilical Cord Stroma-Derived Stem Cells. Cell Transplant. 2016, 25, 217–228. [Google Scholar] [CrossRef]
- Selmani, Z.; Naji, A.; Gaiffe, E.; Obert, L.; Tiberghien, P.; Rouas-Freiss, N.; Carosella, E.D.; Deschaseaux, F. HLA-G Is a Crucial Immunosuppressive Molecule Secreted by Adult Human Mesenchymal Stem Cells. Transplantation 2009, 87, S62–S66. [Google Scholar] [CrossRef]
- Zoehler, B.; Fracaro, L.; Senegaglia, A.C.; Bicalho, M.D.G. Infusion of Mesenchymal Stem Cells to Treat Graft Versus Host Disease: The Role of HLA-G and the Impact of Its Polymorphisms. Stem Cell Rev. Rep. 2020, 16, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.L.; Pinheiro, C.C.G.; Tanikawa, D.Y.S.; Ferreira, J.R.M.; Amano, M.T.; Bueno, D.F. Mesenchymal Stem Cells from Human Exfoliated Deciduous Teeth and the Orbicularis Oris Muscle: How Do They Behave When Exposed to a Proinflammatory Stimulus? Stem Cells Int. 2020, 2020, 3670412. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.; Sayed, A.; Jeppesen, H.B.; Persson, G.; Weisdorf, I.; Funck, T.; Hviid, T. Characterization of HLA-G Regulation and HLA Expression in Breast Cancer and Malignant Melanoma Cell Lines upon IFN-γ Stimulation and Inhibition of DNA Methylation. Int. J. Mol. Sci. 2020, 21, 4307. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Evans, J.H.; Crespo, Â.C.; Strominger, J.L. The HLA-G Cycle Provides for Both NK Tolerance and Immunity at the Maternal–Fetal Interface. Proc. Natl. Acad. Sci. USA 2015, 112, 13312–13317. [Google Scholar] [CrossRef]
- Wan, R.; Wang, Z.-W.; Li, H.; Peng, X.-D.; Liu, G.-Y.; Ou, J.-M.; Cheng, A.-Q. Human Leukocyte Antigen-G Inhibits the Anti-Tumor Effect of Natural Killer Cells via Immunoglobulin-Like Transcript 2 in Gastric Cancer. Cell. Physiol. Biochem. 2017, 44, 1828–1841. [Google Scholar] [CrossRef]
- Gonen-Gross, T.; Goldman-Wohl, D.; Huppertz, B.; Lankry, D.; Greenfield, C.; Natanson-Yaron, S.; Hamani, Y.; Gilad, R.; Yagel, S.; Mandelboim, O. Inhibitory NK Receptor Recognition of HLA-G: Regulation by Contact Residues and by Cell Specific Expression at the Fetal-Maternal Interface. PLoS ONE 2010, 5, e8941. [Google Scholar] [CrossRef]
- Yen, B.L.; Hwa, H.L.; Hsu, P.J.; Chen, P.M.; Wang, L.T.; Jiang, S.S.; Liu, K.J.; Sytwu, H.K.; Yen, M.L. HLA-G Expression in Human Mesenchymal Stem Cells (Mscs) Is Related to Unique Methylation Pattern in the Proximal Promoter as Well as Gene Body DNA. Int. J. Mol. Sci. 2020, 21, 5075. [Google Scholar] [CrossRef]
- Yaghi, L.; Poras, I.; Simoes, R.T.; Donadi, E.A.; Tost, J.; Daunay, A.; de Almeida, B.S.; Carosella, E.D.; Moreau, P. Hypoxia Inducible Factor-1 Mediates the Expression of the Immune Checkpoint HLA-G in Glioma Cells through Hypoxia Response Element Located in Exon 2. Oncotarget 2016, 7, 63690–63707. [Google Scholar] [CrossRef]
- Wobma, H.M.; Kanai, M.; Ma, S.P.; Shih, Y.; Li, H.W.; Duran-Struuck, R.; Winchester, R.; Goeta, S.; Brown, L.M.; Vunjak-Novakovic, G. Dual IFN-γ/Hypoxia Priming Enhances Immunosuppression of Mesenchymal Stromal Cells through Regulatory Proteins and Metabolic Mechanisms. J. Immunol. Regen. Med. 2018, 1, 45–56. [Google Scholar] [CrossRef]
- Kay, A.G.; Treadwell, K.; Roach, P.; Morgan, R.; Lodge, R.; Hyland, M.; Piccinini, A.M.; Forsyth, N.R.; Kehoe, O. Therapeutic Effects of Hypoxic and Proinflammatory Priming of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Inflammatory Arthritis. Int. J. Mol. Sci. 2021, 23, 126. [Google Scholar] [CrossRef]
- Copland, I.B.; Qayed, M.; Garcia, M.A.; Galipeau, J.; Waller, E.K. Bone Marrow Mesenchymal Stromal Cells from Patients with Acute and Chronic Graft-versus-Host Disease Deploy Normal Phenotype, Differentiation Plasticity, and Immune-Suppressive Activity. Biol. Blood Marrow Transpl. 2015, 21, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Torres, M.J.; Noël, D.; Fernandez, A.; Toupet, K.; Alcayaga-Miranda, F.; Tejedor, G.; Jorgensen, C.; Illanes, S.E.; Figueroa, F.E.; et al. The Immunosuppressive Signature of Menstrual Blood Mesenchymal Stem Cells Entails Opposite Effects on Experimental Arthritis and Graft versus Host Diseases. Stem Cells 2016, 34, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.G.; Ontoria-Oviedo, I.; Ricardo, C.P.; Harding, S.E.; Sacedon, R.; Varas, A.; Zapata, A.; Sepulveda, P.; Vicente, A. Overexpression of Hypoxia-Inducible Factor 1 Alpha Improves Immunomodulation by Dental Mesenchymal Stem Cells. Stem Cell Res. Ther. 2017, 8, 208. [Google Scholar] [CrossRef]
- Paganelli, A.; Trubiani, O.; Diomede, F.; Pisciotta, A.; Paganelli, R. Immunomodulating Profile of Dental Mesenchymal Stromal Cells: A Comprehensive Overview. Front. Oral Health 2021, 2, 635055. [Google Scholar] [CrossRef] [PubMed]
- de Witte, S.F.H.; Merino, A.M.; Franquesa, M.; Strini, T.; van Zoggel, J.A.A.; Korevaar, S.S.; Luk, F.; Gargesha, M.; O’Flynn, L.; Roy, D.; et al. Cytokine Treatment Optimises the Immunotherapeutic Effects of Umbilical Cord-Derived MSC for Treatment of Inflammatory Liver Disease. Stem Cell Res. Ther. 2017, 8, 140. [Google Scholar] [CrossRef]
- Zimmermann, J.A.; Hettiaratchi, M.H.; McDevitt, T.C. Enhanced Immunosuppression of T Cells by Sustained Presentation of Bioactive Interferon-γ Within Three-Dimensional Mesenchymal Stem Cell Constructs. Stem Cells Transl. Med. 2017, 6, 223–237. [Google Scholar] [CrossRef]
- Rebmann, V.; Van Der Ven, K.; Päßler, M.; Pfeiffer, K.; Krebs, D.; Grosse-Wilde, H. Association of Soluble HLA-G Plasma Levels with HLA-G Alleles. Tissue Antigens 2001, 57, 15–21. [Google Scholar] [CrossRef]
- Amodio, G.; Gregori, S. HLA-G Genotype/Expression/Disease Association Studies: Success, Hurdles, and Perspectives. Front. Immunol. 2020, 11, 1178. [Google Scholar] [CrossRef]
- Hakki, S.S.; Turaç, G.; Bozkurt, S.B.; Kayis, S.A.; Hakki, E.E.; Şahin, E.; Subaşı, C.; Karaoz, E. Comparison of Different Sources of Mesenchymal Stem Cells: Palatal versus Lipoaspirated Adipose Tissue. Cells Tissues Organs 2017, 204, 228–240. [Google Scholar] [CrossRef]
- Sakha, Y.A.; Ehsani, E.; Roshandel, E.; Jalili, A.; Vahdani, N.; Hajifathali, A. Assessment of the Effect of Infliximab on Immunomodulation Properties of Mesenchymal Stem Cells in Vitro. Adv. Pharm. Bull. 2020, 11, 739–745. [Google Scholar] [CrossRef]
- Mrahleh, M.A.; Matar, S.; Jafar, H.; Wehaibi, S.; Aslam, N.; Awidi, A. Human Wharton’s Jelly-Derived Mesenchymal Stromal Cells Primed by Tumor Necrosis Factor-α and Interferon-γ Modulate the Innate and Adaptive Immune Cells of Type 1 Diabetic Patients. Front. Immunol. 2021, 12, 732549. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Horie, N.; Satoh, K.; Ishikawa, T.; Mori, T.; Maeda, H.; Fukuda, Y.; Ishizaka, S.; Hiu, T.; Morofuji, Y.; et al. Age of donor of human mesenchymal stem cells affects structural and functional recovery after cell therapy following ischaemic stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoehler, B.; Fracaro, L.; Boldrini-Leite, L.M.; da Silva, J.S.; Travers, P.J.; Brofman, P.R.S.; Bicalho, M.d.G.; Senegaglia, A.C. HLA-G and CD152 Expression Levels Encourage the Use of Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells as an Alternative for Immunosuppressive Therapy. Cells 2022, 11, 1339. https://doi.org/10.3390/cells11081339
Zoehler B, Fracaro L, Boldrini-Leite LM, da Silva JS, Travers PJ, Brofman PRS, Bicalho MdG, Senegaglia AC. HLA-G and CD152 Expression Levels Encourage the Use of Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells as an Alternative for Immunosuppressive Therapy. Cells. 2022; 11(8):1339. https://doi.org/10.3390/cells11081339
Chicago/Turabian StyleZoehler, Bernardo, Letícia Fracaro, Lidiane Maria Boldrini-Leite, José Samuel da Silva, Paul J. Travers, Paulo Roberto Slud Brofman, Maria da Graça Bicalho, and Alexandra Cristina Senegaglia. 2022. "HLA-G and CD152 Expression Levels Encourage the Use of Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells as an Alternative for Immunosuppressive Therapy" Cells 11, no. 8: 1339. https://doi.org/10.3390/cells11081339
APA StyleZoehler, B., Fracaro, L., Boldrini-Leite, L. M., da Silva, J. S., Travers, P. J., Brofman, P. R. S., Bicalho, M. d. G., & Senegaglia, A. C. (2022). HLA-G and CD152 Expression Levels Encourage the Use of Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells as an Alternative for Immunosuppressive Therapy. Cells, 11(8), 1339. https://doi.org/10.3390/cells11081339





