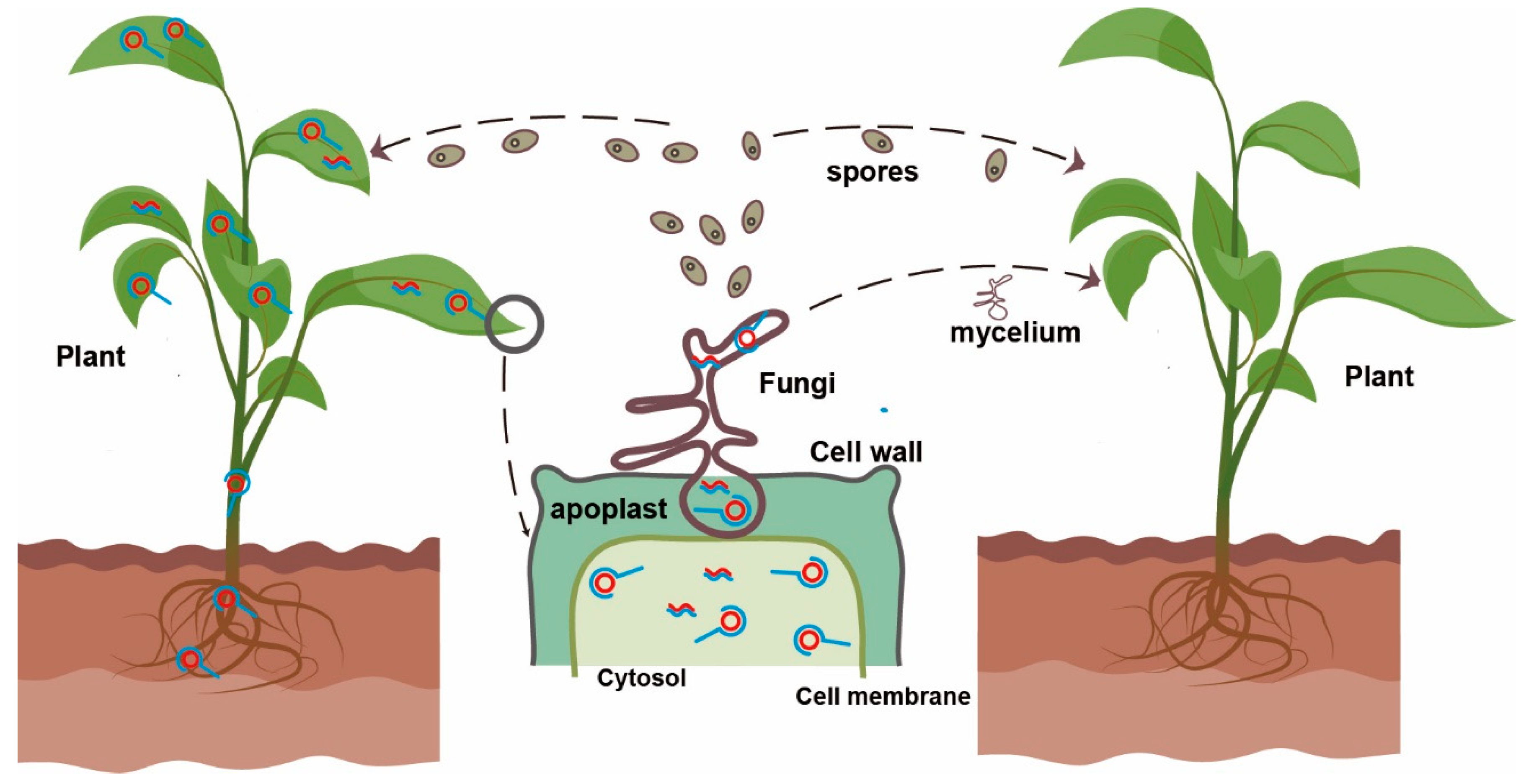
Mycoviroids: Fungi as Hosts and Vectors of Viroids
Funding
Conflicts of Interest
References
- Diener, T.O. Potato spindle tuber virus with properties of a free nucleic acid. III. Subcellular location of PSTV-RNA and the question of whether virions exist in extracts or in situ. Virology 1971, 43, 75–89. [Google Scholar] [CrossRef]
- Diener, T.O. Potato spindle tuber “virus”. IV. A replicating, low molecular weight RNA. Virology 1971, 45, 411–428. [Google Scholar] [CrossRef]
- Diener, T.O. Foreword. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: Oxford, UK, 2017; p. xxix. [Google Scholar]
- Smith, R.L.; Shukla, M.; Creelman, A.; Lawrence, J.; Singh, M.; Xu, H.; Li, X.; Gardner, K.; Nie, X. Coleus blumei viroid 7: A novel viroid resulting from genome recombination between Coleus blumei viroids 1 and 5. Arch. Virol. 2021, 166, 3157–3163. [Google Scholar] [CrossRef] [PubMed]
- Navarro, B.; Flores, R. Chrysanthemum chlorotic mottle viroid: Unusual structural properties of a subgroup of self-cleaving viroids with hammerhead ribozymes. Proc. Natl. Acad. Sci. USA 1997, 94, 11262–11267. [Google Scholar] [CrossRef] [Green Version]
- The International Committee of Taxonomy on Viruses (ICTV). Viroids: Virus Taxonomy: 2020 Release EC52, Online Meeting, October 2020. Email Ratification March 2021 (MSL #36). Available online: https://talk.ictvonline.org/taxonomy (accessed on 8 April 2022).
- Hadidi, A.; Flores, R.; Randles, J.W.; Semancik, J.S. (Eds.) Viroids; CSIRO Publishing: Collingwood, Australia, 2003; 370p. [Google Scholar]
- Hadidi, A.; Flores, R.; Randles, J.W.; Palukaitis, P. (Eds.) Viroids and Satellites; Academic Press: Oxford, UK; Elsevier: Cambridge, MA, USA, 2017; 716p. [Google Scholar]
- Hadidi, A.; Sun, L.; Randles, J.W. Modes of viroid transmission. Cells 2022, 11, 719. [Google Scholar] [CrossRef]
- Delan-Forino, C.; Maurel, M.-C.; Torchet, C. Replication of avocado sunblotch viroid in the yeast Saccharomyces cerevisiae. J. Virol. 2011, 85, 3229–3238. [Google Scholar] [CrossRef] [Green Version]
- Friday, D.; Mukkara, P.; Owens, R.A.; Baumstark, T.; Bruist, M.F. Processing of potato spindle tuber viroid RNAs in yeast, a nonconventional host. J. Virol. 2017, 91, e01078-17. [Google Scholar] [CrossRef] [Green Version]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R.; et al. A higher- level phylogenetic classification of the fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef]
- Ghabrial, S.A. Origin, adaptation and evolutionary pathways of fungal viruses. Virus Genes 1998, 16, 119–131. [Google Scholar] [CrossRef]
- Hillman, B.I.; Suzuki, N. Viruses of the chestnut blight fungus, Cryphonectria parasitica. Adv. Virus Res. 2004, 63, 423–472. [Google Scholar]
- Nuss, D.L. Hypovirulence: Mycoviruses at the fungal-plant interface. Nat. Rev. Microbiol. 2005, 3, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Ghabrial, S.A.; Caston, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-plus years of fungal viruses. Virology 2015, 479–480, 356–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.; Bian, R.; Andika, I.B.; Niu, E.; Liu, Q.; Kondo, H.; Yang, L.; Zhou, H.; Pang, T.; Lian, Z.; et al. Symptomatic plant viroid infections in phytopathogenic fungi. Proc. Natl. Acad. Sci. USA 2019, 116, 13042–13050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andika, I.B.; Wei, S.; Cao, C.; Salaipeth, L.; Kondo, H.; Sun, L. Phytopathogenic fungus hosts a plant virus: A naturally occurring cross-kingdom viral infection. Proc. Natl. Acad. Sci. USA 2017, 114, 12267–12272. [Google Scholar] [CrossRef] [Green Version]
- Bian, R.; Andika, I.B.; Pang, T.; Lian, Z.; Wei, S.; Niu, E.; Wu, Y.; Kondo, H.; Liu, X.; Sun, L. Facilitative and synergistic interactions between fungal and plant viruses. Proc. Natl. Acad. Sci. USA 2020, 117, 3779–3788. [Google Scholar] [CrossRef]
- Martinez, G.; Castellano, M.; Tortosa, M.; Pallas, V.; Gomez, G. A pathogenic non-coding RNA induces changes in dynamic DNA methylation of ribosomal RNA genes in host plants. Nucleic Acids Res. 2014, 42, 1553–1562. [Google Scholar] [CrossRef]
- Marquez-Mollens, J.; Gomez, G.; Pallas, V. Hop stunt viroid: A polyphagous pathogenic RNA that has shed light on viroid-host interactions. Mol. Plant Pathol. 2021, 22, 153–163. [Google Scholar] [CrossRef]
- Earley, K.W.; Pontvianne, F.; Wierzbicki, A.T.; Blevins, T.; Tucker, S.; Costa-Nunes, P.; Pontes, O.; Pikaard, C.S. Mechanisms of HDA6-mediated rRNA gene silencing: Suppression of intergenic Pol II transcription and differential effects on maintenance versus siRNA-directed cytosine methylation. Genes Dev. 2010, 24, 1119–1132. [Google Scholar] [CrossRef] [Green Version]
- Castellano, M.; Martinez, G.; Pallás, V.; Gómez, G. Alterations in host DNA methylation in response to constitutive expression of hop stunt viroid RNA in Nicotiana benthamiana plants. Plant Pathol. 2015, 64, 1247–1257. [Google Scholar] [CrossRef]
- Castellano, M.; Pallas, V.; Gomez, G. A pathogenic long noncoding RNA redesigns the epigenetic landscape of the infected cells by subverting host histone deacetylase 6 activity. New Phytol. 2016, 211, 1311–1322. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Bian, R.; Andika, I.B.; Niu, E.; Liu, Q.; Kondo, H.; Yang, L.; Zhou, H.; Pang, T.; Lian, Z.; et al. Nucleotide substitutions in plant viroid genomes that multiply in phytopathogenic fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 10129–10130. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Hadidi, A. Mycoviroids: Fungi as Hosts and Vectors of Viroids. Cells 2022, 11, 1335. https://doi.org/10.3390/cells11081335
Sun L, Hadidi A. Mycoviroids: Fungi as Hosts and Vectors of Viroids. Cells. 2022; 11(8):1335. https://doi.org/10.3390/cells11081335
Chicago/Turabian StyleSun, Liying, and Ahmed Hadidi. 2022. "Mycoviroids: Fungi as Hosts and Vectors of Viroids" Cells 11, no. 8: 1335. https://doi.org/10.3390/cells11081335
APA StyleSun, L., & Hadidi, A. (2022). Mycoviroids: Fungi as Hosts and Vectors of Viroids. Cells, 11(8), 1335. https://doi.org/10.3390/cells11081335




