RMR-Related DNAJC6 Expression Suppresses Adipogenesis in 3T3-L1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. DNAJC6 Transfection
2.3. RT-PCR to Confirm Transfection
2.4. Cell Staining
2.5. Adipogenesis and Lipolysis Evaluation
2.6. Preparation of Protein Extracts and Western Blot Analysis
2.7. Oxygen Consumption Test (Mitochondrial Function Test)
2.8. Statistical Analysis
3. Results
3.1. DNAJC6 Transfection Confirmation and Cell Proliferation
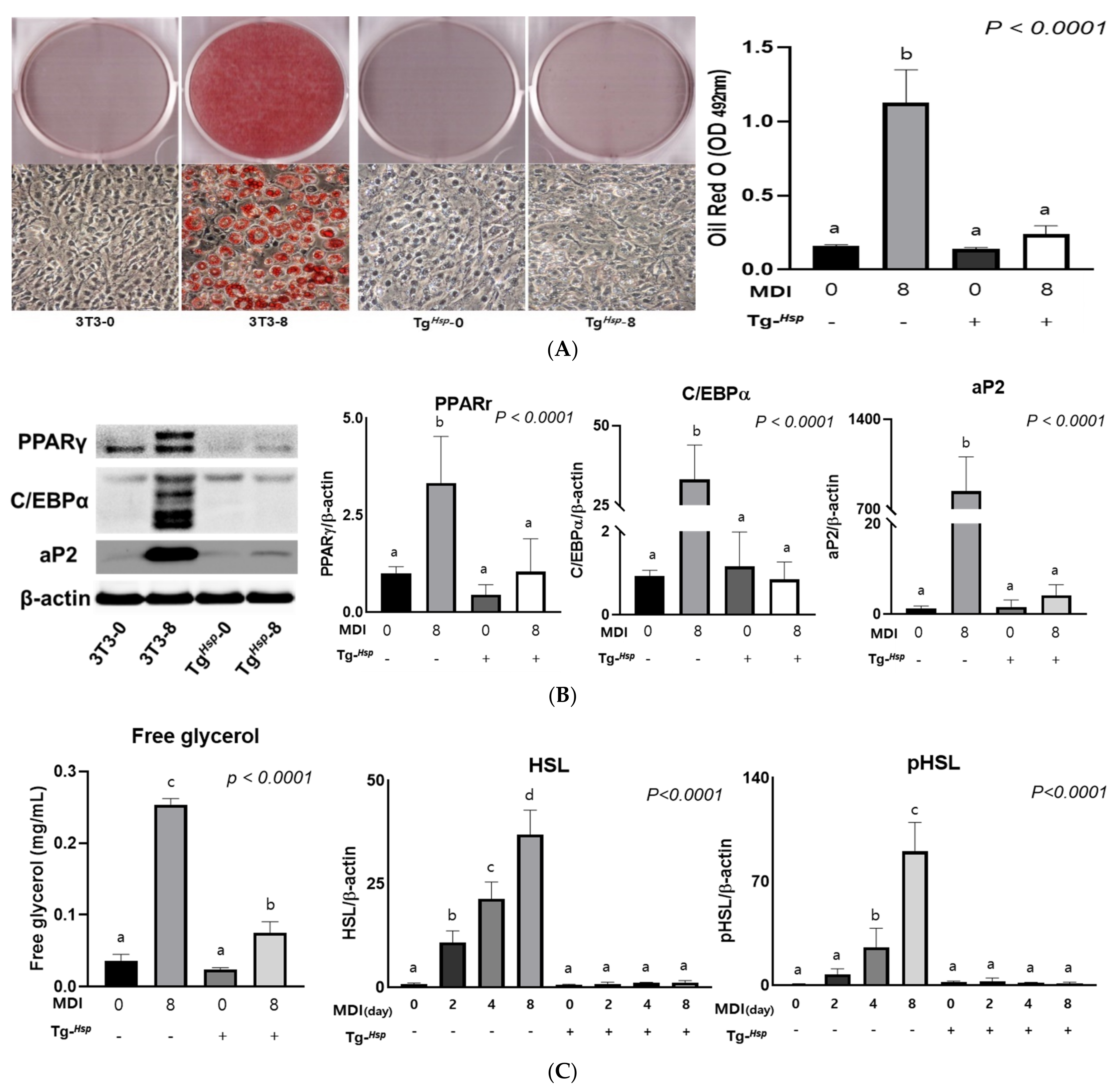
3.2. Adipogenesis and Lipolysis
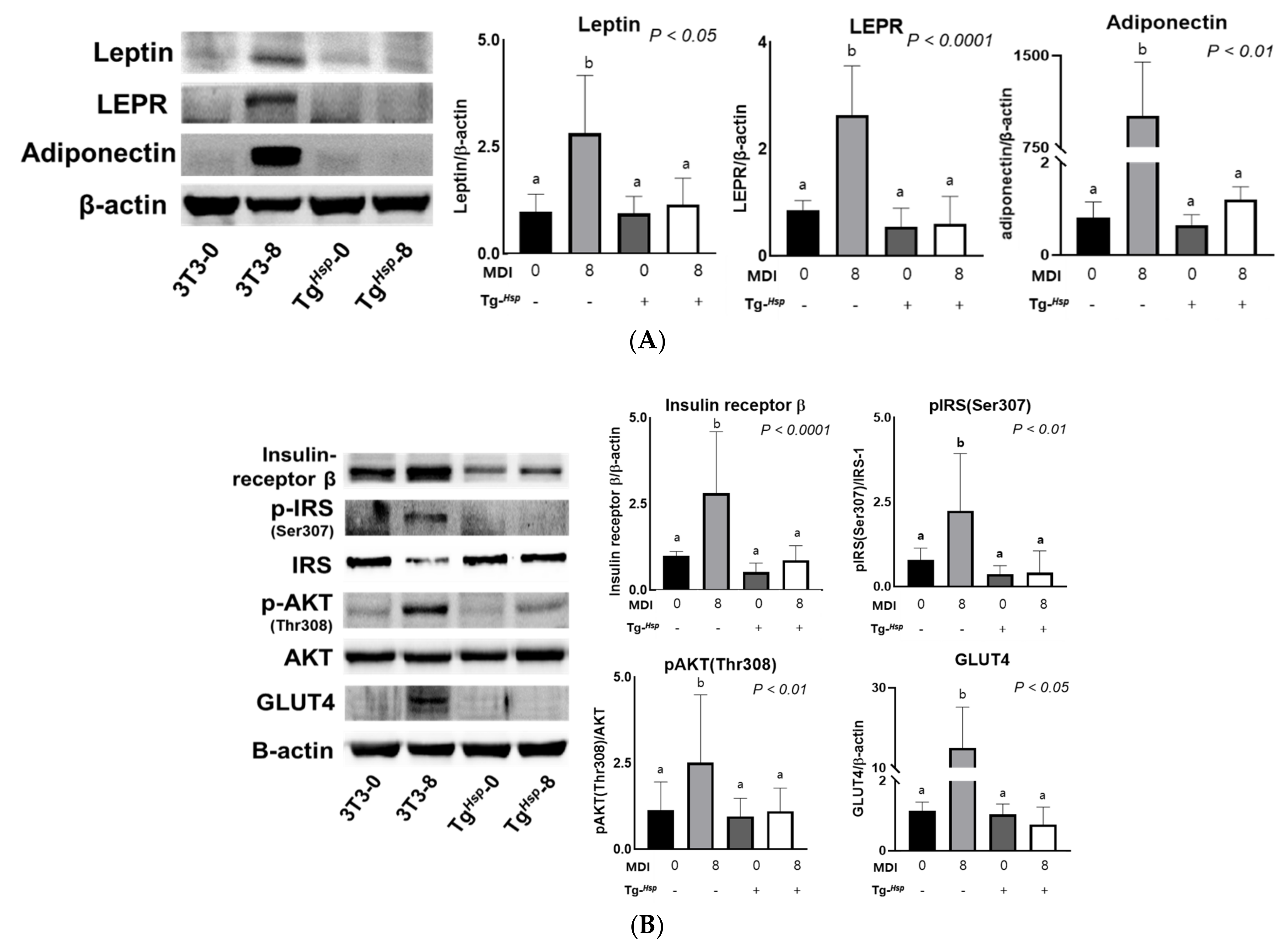
3.3. Adipokine Production and Insulin Signaling
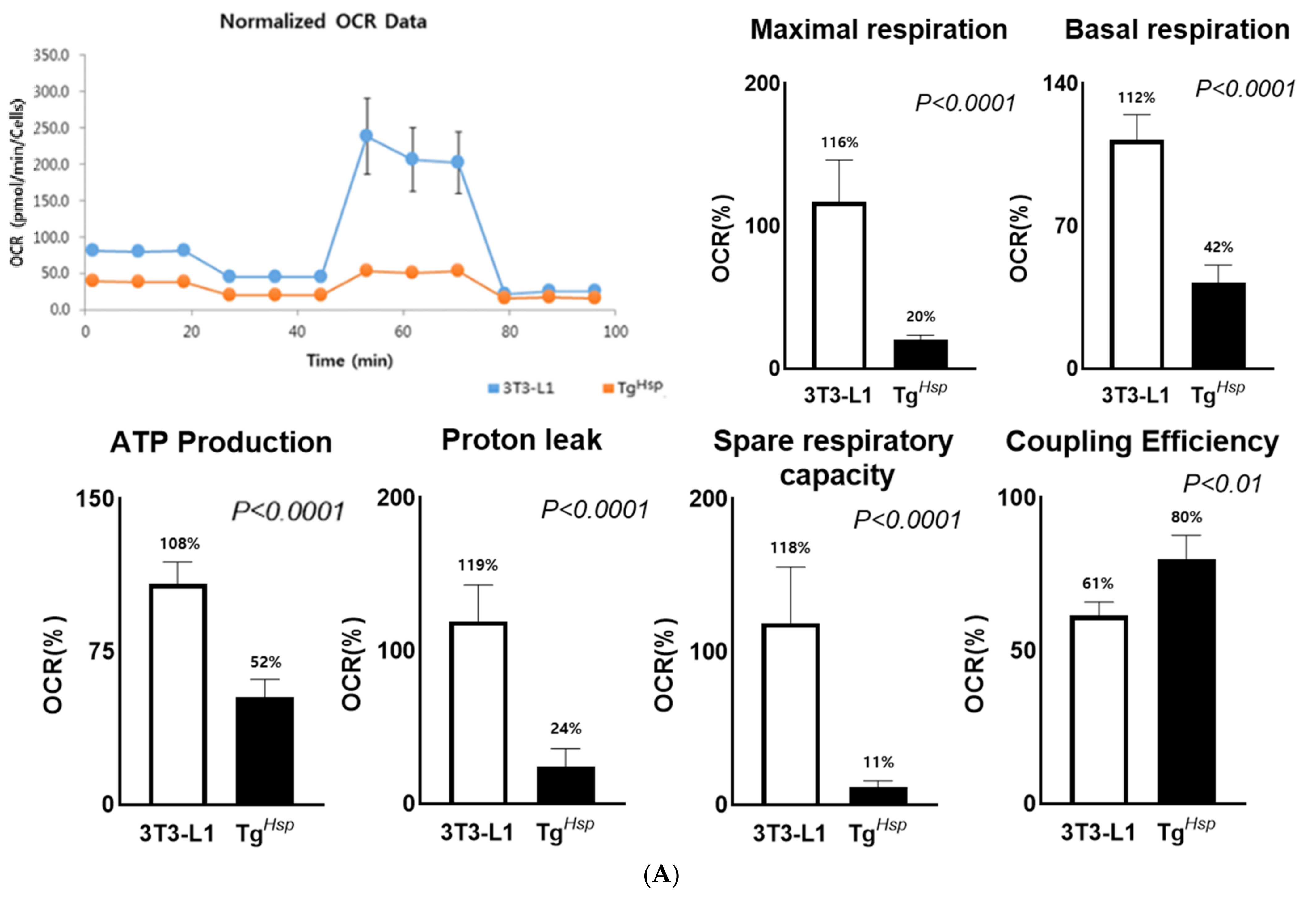
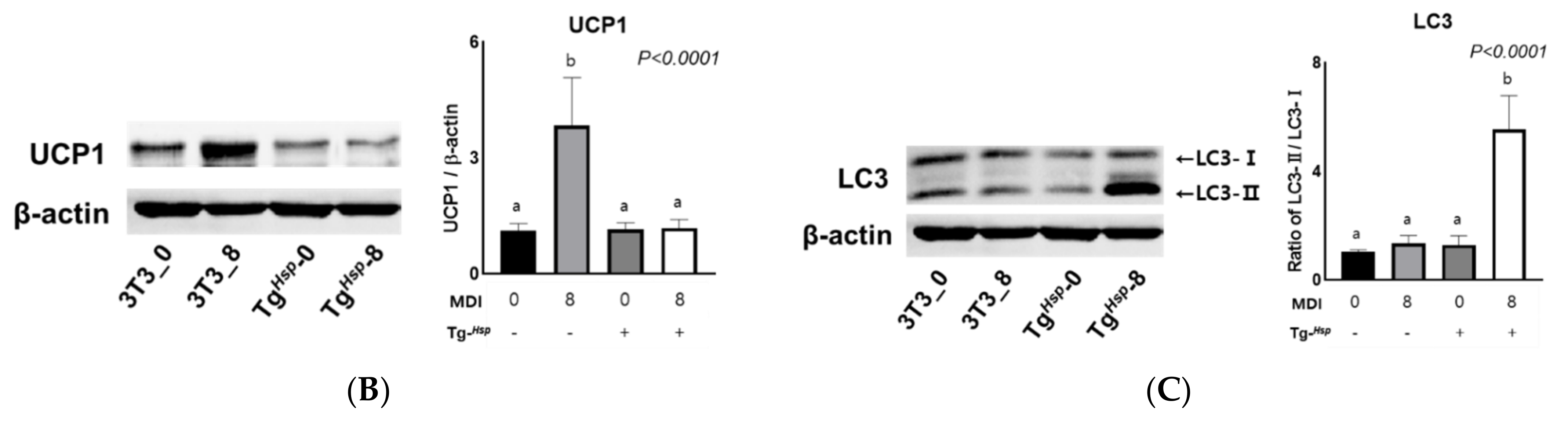
3.4. Mitochondrial Function (Thermogenesis) and Autophagy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, L.; Cho, Y.A. Gene-environment interaction and obesity. Nutr. Rev. 2008, 66, 684–694. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Clevio, N.; Licinio, M.; Cristina, P. The contribution of genetics and environment to obesity. Br. Med. Bull. 2017, 123, 159–173. [Google Scholar]
- Day, F.R.; Loos, R.J.F. Developments in obesity genetics in the era of Genome-wide association studies. J. Nutrigenet. Nutrigenom. 2011, 4, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Munoz Yanez, C.; Garcia Vargas, G.G.; Perez Morales, R. Monogenic, polygenic and multifactorial obesity in children: Genetic and Environmental factors. Austin J. Nutr. Metab. 2017, 4, 1052–1064. [Google Scholar]
- Lee, M.; Lee, Y.; Kang, I.; Shin, J.; Sorn, S.R. RMR-Related MAP2K6 Gene Variation on the Risk of Overweight/Obesity in Children: A 3-Year Panel Study. J. Pers. Med. 2021, 11, 91. [Google Scholar] [CrossRef]
- Kang, S.J.; Lee, M. Beiging Modulates Inflammatory Adipogenesis in Salt-Treated and MEK6–Transfected Adipocytes. Cells 2021, 10, 1106. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M. MEK6 Overexpression Exacerbates Fat Accumulation and Inflammatory Cytokines in High-Fat Diet-Induced Obesity. Int. J. Mol. Sci. 2021, 22, 13559. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yoshida, S.; Sugeno, N.; Kobayshi, J.; Aoki, M. DnaJ/Hsp40 family and Parkinson’s disease. Front. Neurosci. 2018, 11, 743. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Hata, M. Mammalian HSP40/DNAJ homologs: Cloning of novel cDNAs and a proposal for their classification and nomenclature. Cell Stress Chaperones 2000, 5, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Olgiati, S.; Quadri, M.; Fang, M.; Rood, J.P.M.A.; Saute, J.A.; Chien, H.F.; Bouwkamp, C.G.; Graafland, J.; Minneboo, M.; Breedvled, G.J.; et al. DNAJC6 Mutations Associated with Early-Onset Parkinson’s Disease. Ann. Neurol. 2016, 79, 244–256. [Google Scholar] [CrossRef]
- Park, C.-J.; Seo, Y.-S. Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Köroğlu, C.; Baysal, L.; Cetinkaya, M.; Karasoy, H.; Tolun, A. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Park. Relat. Disord. 2013, 19, 320–324. [Google Scholar] [CrossRef]
- Vauthier, V.; Jaillard, S.; Journel, H.; Dubourg, C.; Jockers, R.; Dam, J. Homozygous deletion of an 80kb region comprising part of DNAJC6 and LEPR genes on chromosome 1P31.3 is associated with early onset obesity, mental retardation and epilepsy. Mol. Genet. Metab. 2012, 106, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Yim, Y.-I.; Sun, T.; Wu, L.-G.; Raimondi, A.; De Camilli, P.; Eisenberg, E.; Greene, L.E. Endocytosis and clathrin-uncoating defects at synapses of auxilin knockout mice. Proc. Natl. Acad. Sci. USA 2010, 107, 4412–4417. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Srivastava, P. Heat shock proteins. Curr. Protoc. Immunol. 2004, 58, 1–6. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.-N.; Li, X.-G.; Li, M.; Gao, P.-Z. DNAJC6 promotes hepatocellular carcinoma progression through induction of epithelial–mesenchymal transition. Biochem. Biophys. Res. Commun. 2014, 455, 298–304. [Google Scholar] [CrossRef]
- Zhao, X.; Greener, T.; Al-Hasani, H.; Cushman, S.; Eisenberg, E.; Greene, L. Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: Evidence for formation of nascent pits containing AP1 or AP2 but not clathrin. J. Cell Sci. 2001, 114, 353–365. [Google Scholar] [CrossRef]
- Reichert, M.; Eick, D. Analysis of cell cycle arrest in adipocyte differentiation. Oncogene 1999, 18, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.J.; Pessin, J.E. Adipokines Mediate Inflammation and Insulin Resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef] [Green Version]
- Inoue, G.; Cheatham, B.; Emkey, R.; Kahn, C.R. Dynamics of Insulin Signaling in 3T3-L1 Adipocytes. J. Biol. Chem. 1998, 273, 11548–11555. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; DeFea, K.; Roth, R.A. Modulation of Insulin Receptor Substrate-1 Tyrosine Phosphorylation by an Akt/Phosphatidylinositol 3-Kinase Pathway. J. Biol. Chem. 1999, 274, 9351–9356. [Google Scholar] [CrossRef] [Green Version]
- Rui, L.; Aguirre, V.; Kim, J.K.; Shulman, G.I.; Lee, A.; Corbould, A.; Dunaif, A.; White, M.F. Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Investig. 2001, 107, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Ronnett, G.V.; Knutson, V.P.; Lane, M.D. Insulin-induced Down-Regulation of Insulin Receptors in 3T3-L1 Adipocytes. Altered Rate of Receptor Inactivation. J. Biol. Chem. 1982, 257, 4285–4291. [Google Scholar] [CrossRef]
- Glasow, A.; Kiess, W.; Anderegg, U.; Berthold, A.; Bottner, A.; Kratzsch, J. Expression of Leptin (Ob) and Leptin Receptor (Ob-R) in Human Fibroblasts: Regulation of Leptin Secretion by Insulin. J. Clin. Endocrinol. Metab. 2001, 86, 4472–4479. [Google Scholar] [CrossRef]
- Myers, M.G., Jr.; Leibel, R.L.; Seeley, R.J.; Schwartz, M.W. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol. Metab. 2010, 21, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Frühbeck, G. A heliocentric view of leptin. Proc. Nutr. Soc. 2001, 60, 301–318. [Google Scholar] [CrossRef] [Green Version]
- Ceddia, R.B.; Koistinen, H.A.; Zierath, J.; Sweeney, G. Analysis of paradoxical observations on the association between leptin and insulin resistance. FASEB J. 2002, 16, 1163–1176. [Google Scholar] [CrossRef]
- Martin, S.; Okano, S.; Kistler, C.; Fernandez-Rojo, M.A.; Hill, M.M.; Parton, R. Spatiotemporal Regulation of Early Lipolytic Signaling in Adipocytes. J. Biol. Chem. 2009, 284, 32097–32107. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, A.S.; Shen, W.-J.; Muliro, K.; Patel, S.; Souza, S.C.; Roth, R.A.; Kraemer, F.B. Stimulation of Lipolysis and Hormone-sensitive Lipase via the Extracellular Signal-regulated Kinase Pathway. J. Biol. Chem. 2001, 276, 45456–45461. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.-J.; Yu, Z.; Patel, S.; Jue, D.; Liu, L.-F.; Kraemer, F.B. Hormone-sensitive lipase modulates adipose metabolism through PPARγ. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 9–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, E.; Jung, W.; Joo, H.-Y.; Park, E.-R.; Kim, M.-Y.; Kim, S.-B.; Kim, K.S.; Bin Lim, Y.; Lee, K.H.; Shin, H.J. Cluh plays a pivotal role during adipogenesis by regulating the activity of mitochondria. Sci. Rep. 2019, 9, 6820. [Google Scholar] [CrossRef] [PubMed]
- Rousset, S.; Alves-Guerra, M.-C.; Mozo, J.; Miroux, B.; Cassard-Doulcier, A.-M.; Bouillaud, F.; Ricquier, D. The Biology of Mitochondrial Uncoupling Proteins. Diabetes 2004, 53, S130–S135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar]
- Norman, A.W.; Henny, H.L. Thyroid hormones. Hormones 2015, 5, 89–107. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T. How to Interpret LC3 Immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef]
- Thorburn, A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis 2007, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, M. RMR-Related DNAJC6 Expression Suppresses Adipogenesis in 3T3-L1 Cells. Cells 2022, 11, 1331. https://doi.org/10.3390/cells11081331
Kim J, Lee M. RMR-Related DNAJC6 Expression Suppresses Adipogenesis in 3T3-L1 Cells. Cells. 2022; 11(8):1331. https://doi.org/10.3390/cells11081331
Chicago/Turabian StyleKim, Juhee, and Myoungsook Lee. 2022. "RMR-Related DNAJC6 Expression Suppresses Adipogenesis in 3T3-L1 Cells" Cells 11, no. 8: 1331. https://doi.org/10.3390/cells11081331
APA StyleKim, J., & Lee, M. (2022). RMR-Related DNAJC6 Expression Suppresses Adipogenesis in 3T3-L1 Cells. Cells, 11(8), 1331. https://doi.org/10.3390/cells11081331






