Stem-Cell Therapy for Bronchopulmonary Dysplasia (BPD) in Newborns
Abstract
1. Introduction
2. Mechanism of Lung Injury in Premature Newborns
3. Limitation of the Current Therapy for BPD
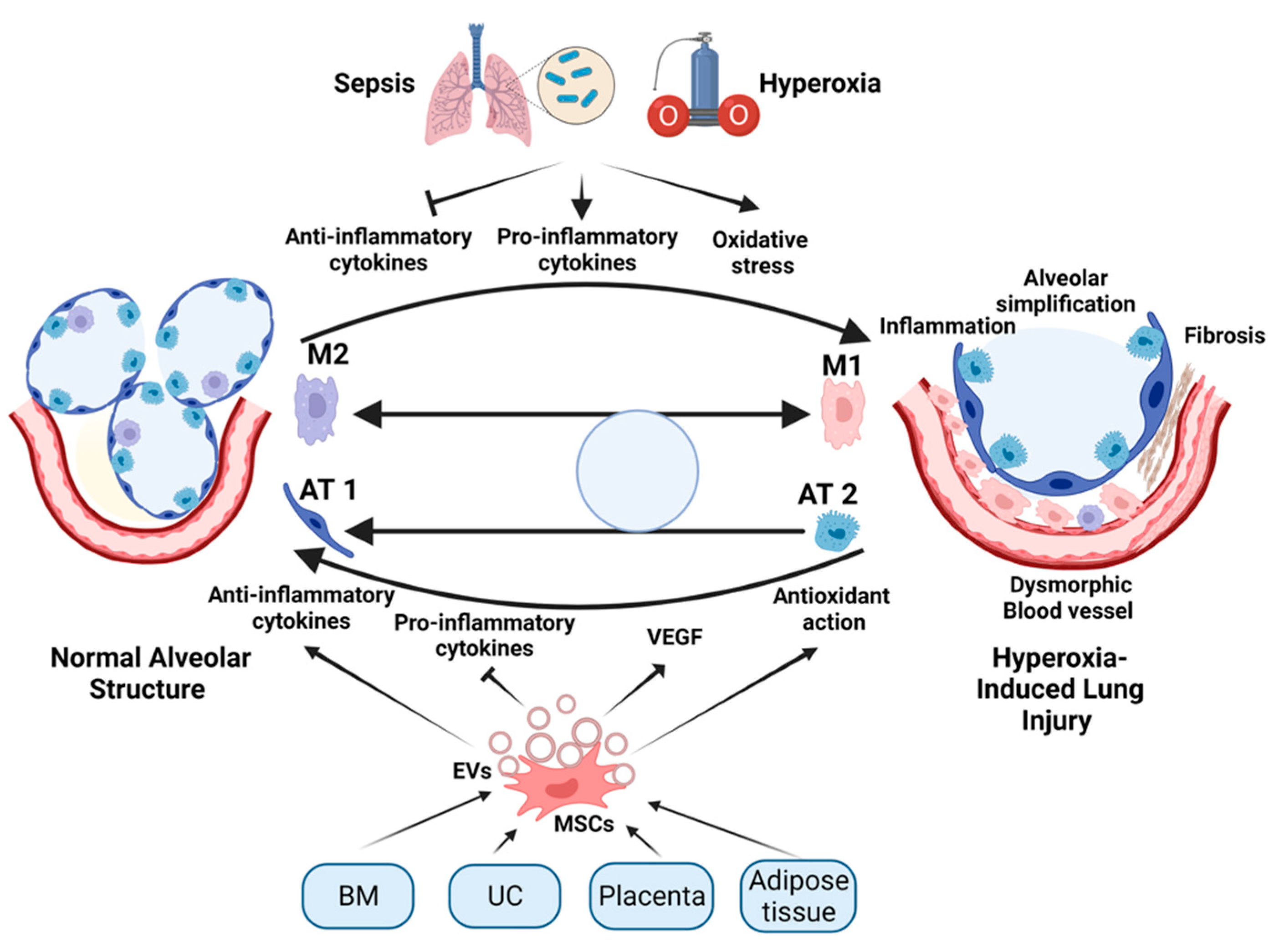
4. The Rationale for Stem-Cell Therapy for BPD
4.1. Changes in the Number of Stem Cells in Adult and Neonatal Lung Injury
4.2. Role of Nitric Oxide (NO) and EPCs in Promoting Angiogenesis in Hyperoxia-Induced Lung Injury
4.3. Role of Lung-Resident Stem/Progenitor Cells in the Development of BPD
5. Animal Studies on Mesenchymal Stem-Cell Therapy for BPD
5.1. MSC Engraftment and Improved Pulmonary Architecture in BPD Models
5.2. Therapeutic Effects of MSC-Secreted Products in BPD Models
5.3. Anti-Inflammatory Role of MSCs in BPD Models
5.4. MSC Effects on Alveolar Apoptosis and Angiotensin System in BPD Models
5.5. Sources of Mesenchymal Stem Cells (MSCs) for BPD Animal Studies
5.5.1. Bone-Marrow-Derived Mesenchymal Stem/Stromal Cells
5.5.2. Human Umbilical Cord Mesenchymal Stem/Stromal Cells (hUC-MSCs)
5.5.3. Placental Mesenchymal Stem/Stromal Cells (P-MSCs)
6. Other Sources of Stem-Cell Therapy for Prevention of Lung Injury
7. Human Clinical Trials of Stem Cells for Prevention of BPD
8. Paracrine Effect of MSC Therapy in BPD
8.1. Extracellular Vesicles (EVs)
8.2. Role of Extracellular Vesicles (EVs) in Mediating the Paracrine Effect of MSCs in Hyperoxia-Induced Lung Injury and BPD
9. Challenges and Limitations for the Use of MSC-Based Therapies for BPD in Clinical Settings
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.B.; Kinney, M.; Lawn, J. Born Too Soon: The Global Epidemiology of 15 Million Preterm Births. Reprod. Health 2013, 10 (Suppl. S1), S2. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.; Abdul-Hafez, A.; Gewolb, I.H.; Uhal, B.D. Oxygen Injury in Neonates: Which Is Worse? Hyperoxia, Hypoxia, or Alternating Hyperoxia/Hypoxia. Lung Pulm. Respir. Res. 2020, 7, 4–13. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Mervis, C.F.; Maxey, A.M.; Markham, N.E.; Abman, S.H. Hyperoxia Reduces Bone Marrow, Circulating, and Lung Endothelial Progenitor Cells in the Developing Lung: Implications for the Pathogenesis of Bronchopulmonary Dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L1073–L1084. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.P.; Greenough, A.; Abman, S.H. Bronchopulmonary Dysplasia. Lancet 2006, 367, 1421–1431. [Google Scholar] [CrossRef]
- Bancalari, E.; infancy, A.G.-C. Clinical Course and Lung Function Abnormalities during Development of Neonatal Chronic Lung Disease. In Chronic Lung Disease in Early Infancy, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 41–64. [Google Scholar]
- van Marter, L.J. Epidemiology of Bronchopulmonary Dysplasia. Semin. Fetal Neonatal Med. 2009, 14, 358–366. [Google Scholar] [CrossRef]
- Smith, L.J.; van Asperen, P.P.; McKay, K.O.; Selvadurai, H.; Fitzgerald, D.A. Reduced Exercise Capacity in Children Born Very Preterm. Pediatrics 2008, 122, e287–e293. [Google Scholar] [CrossRef]
- Ralser, E.; Mueller, W.; Haberland, C.; Fink, F.M.; Gutenberger, K.H.; Strobl, R.; Kiechl-Kohlendorfer, U. Rehospitalization in the First 2 Years of Life in Children Born Preterm. Acta Paediatr. 2012, 101, e1–e5. [Google Scholar] [CrossRef]
- Narang, I.; Rosenthal, M.; Cremonesini, D.; Silverman, M.; Bush, A. Longitudinal Evaluation of Airway Function 21 Years after Preterm Birth. Am. J. Respir. Crit. Care Med. 2008, 178, 74–80. [Google Scholar] [CrossRef]
- Filippone, M.; Bonetto, G.; Corradi, M.; Frigo, A.C.; Baraldi, E. Evidence of Unexpected Oxidative Stress in Airways of Adolescents Born Very Pre-Term. Eur. Respir. J. 2012, 40, 1253–1259. [Google Scholar] [CrossRef]
- Doyle, L.W.; Faber, B.; Callanan, C.; Freezer, N.; Ford, G.W.; Davis, N.M. Bronchopulmonary Dysplasia in Very Low Birth Weight Subjects and Lung Function in Late Adolescence. Pediatrics 2006, 118, 108–113. [Google Scholar] [CrossRef]
- Broström, E.B.; Thunqvist, P.; Adenfelt, G.; Borling, E.; Katz-Salamon, M. Obstructive Lung Disease in Children with Mild to Severe BPD. Respir. Med. 2010, 104, 362–370. [Google Scholar] [CrossRef]
- Jobe, A.H. Mechanisms of Lung Injury and Bronchopulmonary Dysplasia. Am. J. Perinatol. 2016, 33, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Muljadi, R.; Koulaeva, E.; Vosdoganes, P.; Chan, S.T.; Acharya, R.; Gurusinghe, S.; Ritvos, O.; Pasternack, A.; Wallace, E.M. Activin A Contributes to the Development of Hyperoxia-Induced Lung Injury in Neonatal Mice. Pediatr. Res. 2015, 77, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Angusamy, S.; Mansour, T.; Abdulmageed, M.; Han, R.; Schutte, B.C.; LaPres, J.; Harkema, J.R.; Omar, S.A. Altered Thymocyte and T Cell Development in Neonatal Mice with Hyperoxia-Induced Lung Injury. J. Perinat. Med. 2017, 46, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.L. Defining Neonatal Sepsis. Curr. Opin. Pediatr. 2016, 28, 135–140. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Menon, R.T.; El-Saie, A.; Barrios, R.; Reynolds, C.; Shivanna, B. Interactive and Independent Effects of Early Lipopolysaccharide and Hyperoxia Exposure on Developing Murine Lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L981–L996. [Google Scholar] [CrossRef] [PubMed]
- Gortner, L.; Monz, D.; Mildau, C.; Shen, J.; Kasoha, M.; Laschke, M.W.; Roolfs, T.; Schmiedl, A.; Meier, C.; Tutdibi, E. Bronchopulmonary Dysplasia in a Double-Hit Mouse Model Induced by Intrauterine Hypoxia and Postnatal Hyperoxia: Closer to Clinical Features? Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2013, 195, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.B.; Kolodziej, M.; Lamanna, E.; Elgass, K.; Sehgal, A.; Rudloff, I.; Schwenke, D.O.; Tsuchimochi, H.; Kroon, M.A.G.M.; Cho, S.X.; et al. Interleukin-1 Receptor Antagonist Protects Newborn Mice Against Pulmonary Hypertension. Front. Immunol. 2019, 10, 1480. [Google Scholar] [CrossRef]
- Shrestha, D.; Ye, G.X.; Stabley, D.; Betal, S.G.; Zhu, Y.; Glazewski, L.; Holbrook, J.; Sethi, M.; Hesek, A.; Shaffer, T.H.; et al. Pulmonary Immune Cell Transcriptome Changes in Double-Hit Model of BPD Induced by Chorioamnionitis and Postnatal Hyperoxia. Pediatr. Res. 2021, 90, 565–575. [Google Scholar] [CrossRef]
- Saugstad, O.D. Oxygen and Oxidative Stress in Bronchopulmonary Dysplasia. J. Perinat. Med. 2010, 38, 571–577. [Google Scholar] [CrossRef]
- Plötz, F.B.; Slutsky, A.S.; van Vught, A.J.; Heijnen, C.J. Ventilator-Induced Lung Injury and Multiple System Organ Failure: A Critical Review of Facts and Hypotheses. Int. Care Med. 2004, 30, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Terragni, P.; Ranieri, V.M.; Brazzi, L. Novel Approaches to Minimize Ventilator-Induced Lung Injury. Curr. Opin. Crit. Care 2015, 21, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Vlahakis, N.E.; Schroeder, M.A.; Limper, A.H.; Hubmayr, R.D. Stretch Induces Cytokine Release by Alveolar Epithelial Cells in Vitro. Am. J. Physiol. 1999, 277, L167–L173. [Google Scholar] [CrossRef]
- Jobe, A.H.; Hillman, N.; Polglase, G.; Kramer, B.W.; Kallapur, S.; Pillow, J. Injury and Inflammation from Resuscitation of the Preterm Infant. Neonatology 2008, 94, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Abman, S.H. Lung Vascular Development: Implications for the Pathogenesis of Bronchopulmonary Dysplasia. Annu. Rev. Physiol. 2005, 67, 623–661. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, M.E.; Greco, D.; Mao, Q. Angiogenesis-Related Gene Expression Profiling in Ventilated Preterm Human Lungs. Exp. Lung Res. 2010, 36, 399–410. [Google Scholar] [CrossRef]
- De Paepe, M.E.; Patel, C.; Tsai, A.; Gundavarapu, S.; Mao, Q. Endoglin (CD105) Up-Regulation in Pulmonary Microvasculature of Ventilated Preterm Infants. Am. J. Respir. Crit. Care Med. 2008, 178, 180–187. [Google Scholar] [CrossRef]
- de Wijs-Meijler, D.P.; Duncker, D.J.; Tibboel, D.; Schermuly, R.T.; Weissmann, N.; Merkus, D.; Reiss, I.K.M. Oxidative Injury of the Pulmonary Circulation in the Perinatal Period: Short- and Long-Term Consequences for the Human Cardiopulmonary System. Pulm. Circ. 2017, 7, 55–66. [Google Scholar] [CrossRef]
- Yeh, T.F.; Lin, Y.J.; Lin, H.C.; Huang, C.C.; Hsieh, W.S.; Lin, C.H.; Tsai, C.H. Outcomes at School Age after Postnatal Dexamethasone Therapy for Lung Disease of Prematurity. N. Engl. J. Med. 2004, 350, 1304–1313. [Google Scholar] [CrossRef]
- Körbling, M.; Estrov, Z. Adult Stem Cells for Tissue Repair—A New Therapeutic Concept? N. Engl. J. Med. 2003, 349, 570–582. [Google Scholar] [CrossRef]
- Burnham, E.L.; Taylor, W.R.; Quyyumi, A.A.; Rojas, M.; Brigham, K.L.; Moss, M. Increased Circulating Endothelial Progenitor Cells Are Associated with Survival in Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2005, 172, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.; Maxey, A.M.; Morgan, D.B.; Markham, N.E.; Abman, S.H. Inhaled NO Restores Lung Structure in ENOS-Deficient Mice Recovering from Neonatal Hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L119–L127. [Google Scholar] [CrossRef] [PubMed]
- McCurnin, D.C.; Pierce, R.A.; Ling, Y.C.; Gibson, L.L.; Osborne-Lawrence, S.; Yoder, B.A.; Kerecman, J.D.; Albertine, K.H.; Winter, V.T.; Coalson, J.J.; et al. Inhaled NO Improves Early Pulmonary Function and Modifies Lung Growth and Elastin Deposition in a Baboon Model of Neonatal Chronic Lung Disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L450–L459. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, A.; Aschner, J.L.; Rey-Parra, G.J.; Magarik, J.; Zeng, H.; Summar, M.; Eaton, F.; Thébaud, B. L-Citrulline Attenuates Arrested Alveolar Growth and Pulmonary Hypertension in Oxygen-Induced Lung Injury in Newborn Rats. Pediatr. Res. 2010, 68, 519–525. [Google Scholar] [CrossRef]
- Grisafi, D.; Tassone, E.; Dedja, A.; Oselladore, B.; Masola, V.; Guzzardo, V.; Porzionato, A.; Salmaso, R.; Albertin, G.; Artusi, C.; et al. L-Citrulline Prevents Alveolar and Vascular Derangement in a Rat Model of Moderate Hyperoxia-Induced Lung Injury. Lung 2012, 190, 419–430. [Google Scholar] [CrossRef]
- Ekekezie, I.I.; Thibeault, D.W.; Rezaiekhaligh, M.H.; Norberg, M.; Mabry, S.; Zhang, X.; Truog, W.E. Endostatin and Vascular Endothelial Cell Growth Factor (VEGF) in Piglet Lungs: Effect of Inhaled Nitric Oxide and Hyperoxia. Pediatr. Res. 2003, 53, 440–446. [Google Scholar] [CrossRef][Green Version]
- Aicher, A.; Heeschen, C.; Mildner-Rihm, C.; Urbich, C.; Ihling, C.; Technau-Ihling, K.; Zeiher, A.M.; Dimmeler, S. Essential Role of Endothelial Nitric Oxide Synthase for Mobilization of Stem and Progenitor Cells. Nat. Med. 2003, 9, 1370–1376. [Google Scholar] [CrossRef]
- Cao, L.; Qiao, L.-L.; Zhu, Y.-R.; Guo, C.-B.; Gong, X.-H.; Sun, B. Regulation of Activity of Nuclear Factor-KappaB and Activator Protein-1 by Nitric Oxide, Surfactant and Glucocorticoids in Alveolar Macrophages from Piglets with Acute Lung Injury. Acta Pharmacol. Sin. 2016, 24, 1316323. [Google Scholar]
- Bloomfield, G.L.; Holloway, S.; Ridings, P.C.; Fisher, B.J.; Blocher, C.R.; Sholley, M.; Bunch, T.; Sugerman, H.J.; Fowler, A.A. Pretreatment with Inhaled Nitric Oxide Inhibits Neutrophil Migration and Oxidative Activity Resulting in Attenuated Sepsis-Induced Acute Lung Injury. Crit. Care Med. 1997, 25, 584–593. [Google Scholar] [CrossRef]
- Bloomfield, G.L.; Sweeney, L.B.; Fisher, B.J.; Blocher, C.R.; Sholley, M.M.; Sugerman, H.J.; Fowler, A.A. Delayed Administration of Inhaled Nitric Oxide Preserves Alveolar-Capillary Membrane Integrity in Porcine Gram-Negative Sepsis. Arch. Surg. 1997, 132, 65–75. [Google Scholar] [CrossRef]
- Ródenas, J.; Mitjavila, M.T.; Carbonell, T. Nitric Oxide Inhibits Superoxide Production by Inflammatory Polymorphonuclear Leukocytes. Am. J. Physiol. 1998, 274, C827–C830. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Sun, B.; Qian, L. Combined INO and Endothelial Progenitor Cells Improve Lung Alveolar and Vascular Structure in Neonatal Rats Exposed to Prolonged Hyperoxia. Pediatr. Res. 2015, 77, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.K.; Cheah, F.C.; Abdul Aziz, N.H.; Kampan, N.C.; Shuib, S.; Khong, T.Y.; Tan, G.C.; Wong, Y.P. A Review of Placenta and Umbilical Cord-Derived Stem Cells and the Immunomodulatory Basis of Their Therapeutic Potential in Bronchopulmonary Dysplasia. Front. Pediatr. 2021, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Fu, J.; Yang, H.; Zhu, Y.; Pan, Y.; Xu, S.; Xue, X. Hyperoxia Stimulates the Transdifferentiation of Type II Alveolar Epithelial Cells in Newborn Rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L861–L872. [Google Scholar] [CrossRef] [PubMed]
- Uhal, B.D. Cell Cycle Kinetics in the Alveolar Epithelium. Am. J. Physiol. 1997, 272, L1031–L1045. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.J.P.; Thébaud, B. Progenitor Cells of the Distal Lung and Their Potential Role in Neonatal Lung Disease. Birth Defects Res. Part A Clin. Mol. Teratol. 2014, 100, 217–226. [Google Scholar] [CrossRef]
- Collins, J.J.P.; Thébaud, B. Lung Mesenchymal Stromal Cells in Development and Disease: To Serve and Protect? Antioxid. Redox Signal. 2014, 21, 1849–1862. [Google Scholar] [CrossRef]
- Kumar, M.E.; Bogard, P.E.; Espinoza, F.H.; Menke, D.B.; Kingsley, D.M.; Krasnow, M.A. Mesenchymal Cells. Defining a Mesenchymal Progenitor Niche at Single-Cell Resolution. Science 2014, 346, 6211. [Google Scholar] [CrossRef]
- Alphonse, R.S.; Vadivel, A.; Fung, M.; Shelley, W.C.; Critser, P.J.; Ionescu, L.; O’Reilly, M.; Ohls, R.K.; McConaghy, S.; Eaton, F.; et al. Existence, Functional Impairment, and Lung Repair Potential of Endothelial Colony-Forming Cells in Oxygen-Induced Arrested Alveolar Growth. Circulation 2014, 129, 2144–2157. [Google Scholar] [CrossRef]
- Borghesi, A.; Massa, M.; Campanelli, R.; Bollani, L.; Tzialla, C.; Figar, T.A.; Ferrari, G.; Bonetti, E.; Chiesa, G.; de Silvestri, A.; et al. Circulating Endothelial Progenitor Cells in Preterm Infants with Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2009, 180, 540–546. [Google Scholar] [CrossRef]
- Fujinaga, H.; Baker, C.D.; Ryan, S.L.; Markham, N.E.; Seedorf, G.J.; Balasubramaniam, V.; Abman, S.H. Hyperoxia Disrupts Vascular Endothelial Growth Factor-Nitric Oxide Signaling and Decreases Growth of Endothelial Colony-Forming Cells from Preterm Infants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L1160–L1169. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.D.; Ryan, S.L.; Ingram, D.A.; Seedorf, G.J.; Abman, S.H.; Balasubramaniam, V. Endothelial Colony-Forming Cells from Preterm Infants Are Increased and More Susceptible to Hyperoxia. Am. J. Respir. Crit. Care Med. 2009, 180, 454–461. [Google Scholar] [CrossRef]
- Möbius, M.A.; Thébaud, B. Bronchopulmonary Dysplasia: Where Have All the Stem Cells Gone?: Origin and (Potential) Function of Resident Lung Stem Cells. Chest 2017, 152, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bonadies, L.; Zaramella, P.; Porzionato, A.; Perilongo, G.; Muraca, M.; Baraldi, E. Present and Future of Bronchopulmonary Dysplasia. J. Clin. Med. 2020, 9, 1539. [Google Scholar] [CrossRef]
- Wagner, D.E.; Cardoso, W.v.; Gilpin, S.E.; Majka, S.; Ott, H.; Randell, S.H.; Thébaud, B.; Waddell, T.; Weiss, D.J. An Official American Thoracic Society Workshop Report 2015. Stem Cells and Cell Therapies in Lung Biology and Diseases. Ann. Am. Thorac. Soc. 2016, 13, S259–S278. [Google Scholar] [CrossRef] [PubMed]
- Nitkin, C.R.; Bonfield, T.L. Concise Review: Mesenchymal Stem Cell Therapy for Pediatric Disease: Perspectives on Success and Potential Improvements. Stem Cells Transl. Med. 2017, 6, 539–565. [Google Scholar] [CrossRef]
- Curley, G.F.; Hayes, M.; Ansari, B.; Shaw, G.; Ryan, A.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Mesenchymal Stem Cells Enhance Recovery and Repair Following Ventilator-Induced Lung Injury in the Rat. Thorax 2012, 67, 496–501. [Google Scholar] [CrossRef]
- Aslam, M.; Baveja, R.; Liang, O.D.; Fernandez-Gonzalez, A.; Lee, C.; Mitsialis, S.A.; Kourembanas, S. Bone Marrow Stromal Cells Attenuate Lung Injury in a Murine Model of Neonatal Chronic Lung Disease. Am. J. Respir. Crit. Care Med. 2009, 180, 1122–1130. [Google Scholar] [CrossRef]
- Tropea, K.A.; Leder, E.; Aslam, M.; Lau, A.N.; Raiser, D.M.; Lee, J.H.; Balasubramaniam, V.; Fredenburgh, L.E.; Mitsialis, S.A.; Kourembanas, S.; et al. Bronchioalveolar Stem Cells Increase after Mesenchymal Stromal Cell Treatment in a Mouse Model of Bronchopulmonary Dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L829–L837. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, J.; Su, H.; Yang, M.; Lai, W.; Mai, Y.; Wu, Y. Bone Marrow Mesenchymal Stem Cells Attenuate Lung Inflammation of Hyperoxic Newborn Rats. Pediatr. Transplantat. 2012, 16, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fang, J.; Wu, Y.; Mai, Y.; Lai, W.; Su, H. Mesenchymal Stem Cells Protect against Neonatal Rat Hyperoxic Lung Injury. Expert Opin. Biol. Ther. 2013, 13, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, G.; Fernandez-Gonzalez, A.; Aslam, M.; Vitali, S.H.; Martin, T.; Alex Mitsialis, S.; Kourembanas, S. Mesenchymal Stem Cell-Mediated Reversal of Bronchopulmonary Dysplasia and Associated Pulmonary Hypertension. Pulm. Circ. 2012, 2, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Chou, H.C. Human Mesenchymal Stem Cells Attenuate Hyperoxia-Induced Lung Injury through Inhibition of the Renin-Angiotensin System in Newborn Rats. Am. J. Transl. Res. 2018, 10, 2628. [Google Scholar] [PubMed]
- van Haaften, T.; Byrne, R.; Bonnet, S.; Rochefort, G.Y.; Akabutu, J.; Bouchentouf, M.; Rey-Parra, G.J.; Galipeau, J.; Haromy, A.; Eaton, F.; et al. Airway Delivery of Mesenchymal Stem Cells Prevents Arrested Alveolar Growth in Neonatal Lung Injury in Rats. Am. J. Respir. Crit. Care Med. 2009, 180, 1131–1142. [Google Scholar] [CrossRef]
- Sutsko, R.P.; Young, K.C.; Ribeiro, A.; Torres, E.; Rodriguez, M.; Hehre, D.; Devia, C.; McNiece, I.; Suguihara, C. Long-Term Reparative Effects of Mesenchymal Stem Cell Therapy Following Neonatal Hyperoxia-Induced Lung Injury. Pediatr. Res. 2013, 73, 46–53. [Google Scholar] [CrossRef]
- Gülaşi, S.; Atici, A.; Yilmaz, Ş.N.; Polat, A.; Yilmaz, M.; Laçin, M.T.; Örekici, G.; Çelik, Y. Mesenchymal Stem Cell Treatment in Hyperoxia-Induced Lung Injury in Newborn Rats. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2016, 58, 206–213. [Google Scholar] [CrossRef]
- Pierro, M.; Ionescu, L.; Montemurro, T.; Vadivel, A.; Weissmann, G.; Oudit, G.; Emery, D.; Bodiga, S.; Eaton, F.; Péault, B.; et al. Short-Term, Long-Term and Paracrine Effect of Human Umbilical Cord-Derived Stem Cells in Lung Injury Prevention and Repair in Experimental Bronchopulmonary Dysplasia. Thorax 2013, 68, 475–484. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Shi, Y.; Peng, W.; Zhang, S.; Zhang, W.; Xu, J.; Mei, Y.; Feng, Z. Role of Bone Marrow-Derived Mesenchymal Stem Cells in the Prevention of Hyperoxia-Induced Lung Injury in Newborn Mice. Cell Biol. Int. 2012, 36, 589–594. [Google Scholar] [CrossRef]
- Braun, R.K.; Chetty, C.; Balasubramaniam, V.; Centanni, R.; Haraldsdottir, K.; Hematti, P.; Eldridge, M.W. Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem. Biophys. Res. Commun. 2018, 503, 2653–2658. [Google Scholar] [CrossRef]
- Moreira, A.; Winter, C.; Joy, J.; Winter, L.; Jones, M.; Noronha, M.; Porter, M.; Quim, K.; Corral, A.; Alayli, Y.; et al. Intranasal Delivery of Human Umbilical Cord Wharton’s Jelly Mesenchymal Stromal Cells Restores Lung Alveolarization and Vascularization in Experimental Bronchopulmonary Dysplasia. Stem Cells Transl. Med. 2020, 9, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Peluzzo, A.M.; Autieri, M.V. Challenging the Paradigm: Anti-Inflammatory Interleukins and Angiogenesis. Cells 2022, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Capelari, D.N.; Sánchez, S.I.; Ortega, H.H.; Ciuffo, G.M.; Fuentes, L.B. Effects of Maternal Captopril Treatment during Late Pregnancy on Neonatal Lung Development in Rats. Regul. Pept. 2012, 177, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lasaitiene, D.; Chen, Y.; Nannmark, U.; Wollmer, P.; Friberg, P. Neonatal ACE Inhibition in Rats Interferes with Lung Development. Clin. Physiol. Funct. Imaging 2004, 24, 65–68. [Google Scholar] [CrossRef]
- Wagenaar, G.T.M.; Laghmani, E.H.; Fidder, M.; Sengers, R.M.A.; de Visser, Y.P.; de Vries, L.; Rink, R.; Roks, A.J.M.; Folkerts, G.; Walther, F.J. Agonists of MAS Oncogene and Angiotensin II Type 2 Receptors Attenuate Cardiopulmonary Disease in Rats with Neonatal Hyperoxia-Induced Lung Injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L341–L351. [Google Scholar] [CrossRef]
- Abdul-Hafez, A.; Mohamed, T.; Omar, H.; Shemis, M.; Uhal, B.D. The Renin Angiotensin System in Liver and Lung: Impact and Therapeutic Potential in Organ Fibrosis. J. Lung Pulm. Respir. Res. 2018, 5, 42–47. [Google Scholar] [CrossRef]
- Abdul-Hafez, A.; Mohamed, T.; Uhal, B.D. Activation of Mas Restores Hyperoxia-Induced Loss of Lung Epithelial Barrier Function Through Inhibition of Apoptosis. J. Lung Pulm. Respir. Res. 2019, 6, 58–62. [Google Scholar] [CrossRef]
- Thiruvenkataramani, R.; Abdul-Hafez, A.; Gewolb, I.H.; Uhal, B.D. The Mas Activator AVE0991 Increases Surfactant Protein-C (SP-C) in Human Lung A549 Cells. In Proceedings of the Pediatric Academic Societies (PAS) Meeting, Baltimore, MD, USA, 24 April–1 May 2019. [Google Scholar]
- Mohamed, T.L.; Nguyen, H.T.; Abdul-Hafez, A.; Dang, V.X.; Dang, M.T.; Gewolb, I.H.; Uhal, B.D. Prior Hypoxia Prevents Downregulation of ACE-2 by Hyperoxia in Fetal Human Lung Fibroblasts. Exp. Lung Res. 2016, 42, 121–130. [Google Scholar] [CrossRef][Green Version]
- Batsali, A.K.; Kastrinaki, M.-C.; Papadaki, H.A.; Pontikoglou, C. Mesenchymal Stem Cells Derived from Wharton’s Jelly of the Umbilical Cord: Biological Properties and Emerging Clinical Applications. Curr. Stem Cell Res. Ther. 2013, 8, 144–155. [Google Scholar] [CrossRef]
- Mayani, H.; Alvarado-Moreno, J.A.; Flores-Guzmán, P. Biology of Human Hematopoietic Stem and Progenitor Cells Present in Circulation. Arch. Med. Res. 2003, 34, 476–488. [Google Scholar] [CrossRef]
- Chang, Y.; Choi, S.; Sung, D.; Kim, S.; Oh, W.; Yang, Y.; Park, W. Intratracheal Transplantation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Dose-Dependently Attenuates Hyperoxia-Induced Lung Injury in Neonatal Rats. Cell Transplant. 2011, 20, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Oh, W.; Choi, S.J.; Sung, D.K.; Kim, S.Y.; Choi, E.Y.; Kang, S.; Jin, H.J.; Yang, Y.S.; Park, W.S. Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Attenuate Hyperoxia-Induced Lung Injury in Neonatal Rats. Cell Transplant. 2009, 18, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Choi, S.J.; Ahn, S.Y.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Oh, W., II; Park, W.S. Timing of Umbilical Cord Blood Derived Mesenchymal Stem Cells Transplantation Determines Therapeutic Efficacy in the Neonatal Hyperoxic Lung Injury. PLoS ONE 2013, 8, e52419. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Park, W.S.; Sung, D.K.; Ahn, S.Y.; Sung, S.I.; Yoo, H.S.; Chang, Y.S. Intratracheal Transplantation of Mesenchymal Stem Cells Simultaneously Attenuates Both Lung and Brain Injuries in Hyperoxic Newborn Rats. Pediatr. Res. 2016, 80, 415–424. [Google Scholar] [CrossRef]
- Oreilly, M.; Möbius, M.A.; Vadivel, A.; Ionescu, L.; Fung, M.; Eaton, F.; Greer, J.J.; Thébaud, B. Late Rescue Therapy with Cord-Derived Mesenchymal Stromal Cells for Established Lung Injury in Experimental Bronchopulmonary Dysplasia. Stem Cells Dev. 2020, 29, 364–371. [Google Scholar] [CrossRef]
- Hou, C.; Peng, D.; Gao, L.; Tian, D.; Dai, J.; Luo, Z.; Liu, E.; Chen, H.; Zou, L.; Fu, Z. Human Umbilical Cord-Derived Mesenchymal Stem Cells Protect from Hyperoxic Lung Injury by Ameliorating Aberrant Elastin Remodeling in the Lung of O2-Exposed Newborn Rat. Biochem. Biophys. Res. Commun. 2018, 495, 1972–1979. [Google Scholar] [CrossRef]
- Liu, L.; Mao, Q.; Chu, S.; Mounayar, M.; Abdi, R.; Fodor, W.; Padbury, J.F.; de Paepe, M.E. Intranasal versus Intraperitoneal Delivery of Human Umbilical Cord Tissue-Derived Cultured Mesenchymal Stromal Cells in a Murine Model of Neonatal Lung Injury. Am. J. Pathol. 2014, 184, 3344–3358. [Google Scholar] [CrossRef]
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R.; Trounson, A. Human Umbilical Cord Mesenchymal Stem Cells Reduce Fibrosis of Bleomycin-Induced Lung Injury. Am. J. Pathol. 2009, 175, 303–313. [Google Scholar] [CrossRef]
- Chou, H.C.; Li, Y.T.; Chen, C.M. Human Mesenchymal Stem Cells Attenuate Experimental Bronchopulmonary Dysplasia Induced by Perinatal Inflammation and Hyperoxia. Am. J. Transl. Res. 2016, 8, 342. [Google Scholar]
- Cargnoni, A.; Gibelli, L.; Tosini, A.; Signoroni, P.B.; Nassuato, C.; Arienti, D.; Lombardi, G.; Albertini, A.; Wengler, G.S.; Parolini, O. Transplantation of Allogeneic and Xenogeneic Placenta-Derived Cells Reduces Bleomycin-Induced Lung Fibrosis. Cell Transplant. 2009, 18, 405–422. [Google Scholar] [CrossRef]
- Moodley, Y.; Ilancheran, S.; Samuel, C.; Vaghjiani, V.; Atienza, D.; Williams, E.D.; Jenkin, G.; Wallace, E.; Trounson, A.; Manuelpillai, U. Human Amnion Epithelial Cell Transplantation Abrogates Lung Fibrosis and Augments Repair. Am. J. Respir. Crit. Care Med. 2010, 182, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Lim, R.; Dickinson, H.; Acharya, R.; Rosli, S.; Jenkin, G.; Wallace, E. Human Amnion Epithelial Cells Prevent Bleomycin-Induced Lung Injury and Preserve Lung Function. Cell Transplant. 2011, 20, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Vosdoganes, P.; Wallace, E.M.; Chan, S.T.; Acharya, R.; Moss, T.J.M.; Lim, R. Human Amnion Epithelial Cells Repair Established Lung Injury. Cell Transplant. 2013, 22, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Papagianis, P.C.; Ahmadi-Noorbakhsh, S.; Lim, R.; Wallace, E.; Polglase, G.; Pillow, J.J.; Moss, T.J. The Effect of Human Amnion Epithelial Cells on Lung Development and Inflammation in Preterm Lambs Exposed to Antenatal Inflammation. PLoS ONE 2021, 16, e0253456. [Google Scholar] [CrossRef]
- Vosdoganes, P.; Hodges, R.J.; Lim, R.; Westover, A.J.; Acharya, R.Y.; Wallace, E.M.; Moss, T.J.M. Human Amnion Epithelial Cells as a Treatment for Inflammation-Induced Fetal Lung Injury in Sheep. Am. J. Obstet. Gynecol. 2011, 205, 156.e26–156.e33. [Google Scholar] [CrossRef]
- Hodges, R.J.; Jenkin, G.; Hooper, S.B.; Allison, B.; Lim, R.; Dickinson, H.; Miller, S.L.; Vosdoganes, P.; Wallace, E.M. Human Amnion Epithelial Cells Reduce Ventilation-Induced Preterm Lung Injury in Fetal Sheep. Am. J. Obstet. Gynecol. 2012, 206, 448.e8–448.e15. [Google Scholar] [CrossRef]
- Zhu, D.; Tan, J.; Maleken, A.S.; Muljadi, R.; Chan, S.T.; Lau, S.N.; Elgass, K.; Leaw, B.; Mockler, J.; Chambers, D.; et al. Human Amnion Cells Reverse Acute and Chronic Pulmonary Damage in Experimental Neonatal Lung Injury. Stem Cell Res. Ther. 2017, 8, 257. [Google Scholar] [CrossRef]
- Home—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 13 February 2022).
- Ahn, S.Y.; Chang, Y.S.; Lee, M.H.; Sung, S.I.; Lee, B.S.; Kim, K.S.; Kim, A.R.; Park, W.S. Stem Cells for Bronchopulmonary Dysplasia in Preterm Infants: A Randomized Controlled Phase II Trial. Stem Cells Transl. Med. 2021, 10, 1129–1137. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W., II; Park, W.S. Mesenchymal Stem Cells for Bronchopulmonary Dysplasia: Phase 1 Dose-Escalation Clinical Trial. J. Pediatr. 2014, 164, 966–972. [Google Scholar] [CrossRef]
- Powell, S.B.; Silvestri, J.M. Safety of Intratracheal Administration of Human Umbilical Cord Blood Derived Mesenchymal Stromal Cells in Extremely Low Birth Weight Preterm Infants. J. Pediatr. 2019, 210, 209–213. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Kim, J.H.; Sung, S.I.; Park, W.S. Two-Year Follow-Up Outcomes of Premature Infants Enrolled in the Phase I Trial of Mesenchymal Stem Cells Transplantation for Bronchopulmonary Dysplasia. J. Pediatr. 2017, 185, 49–54. [Google Scholar] [CrossRef] [PubMed]
- A Safety Study of IV Stem Cell-Derived Extracellular Vesicles (UNEX-42) in Preterm Neonates at High Risk for BPD—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03857841?term=vesicle&cond=Bronchopulmonary+Dysplasia&draw=2&rank=1 (accessed on 13 February 2022).
- Namba, F. Mesenchymal Stem Cells for the Prevention of Bronchopulmonary Dysplasia. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2019, 61, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Khan, Y.S. Histology, Extracellular Vesicles. StatPearls. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562256/ (accessed on 13 February 2022).
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.R.; Reis, M.; Gheinani, A.H.; Fernandez-Gonzalez, A.; Taglauer, E.S.; Yeung, V.; Liu, X.; Ericsson, M.; Haas, E.; Alex Mitsialis, S.; et al. Extracellular Vesicles Protect the Neonatal Lung from Hyperoxic Injury through the Epigenetic and Transcriptomic Reprogramming of Myeloid Cells. Am. J. Respir. Crit. Care Med. 2021, 204, 1418–1432. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Shen, J.; Sun, J.; Zhu, Z.; Gao, R.; Du, Y.; Yuan, L.; Chen, C.; Zhou, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Suppress Hyperoxia-Induced Transdifferentiation of Rat Alveolar Type 2 Epithelial Cells. Stem Cells Dev. 2021, 31, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Z.; Li, J.; Zhong, H.; Yuan, R.; Deng, Z.; Wu, X. Mechanism of Adipose-Derived Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying MiR-21-5p in Hyperoxia-Induced Lung Injury. Stem Cell Rev. Rep. 2022, 18, 1007–1024. [Google Scholar] [CrossRef]
- You, J.; Zhou, O.; Liu, J.; Zou, W.; Zhang, L.; Tian, D.; Dai, J.; Luo, Z.; Liu, E.; Fu, Z.; et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Alleviate Lung Injury in Rat Model of Bronchopulmonary Dysplasia by Affecting Cell Survival and Angiogenesis. Stem Cells Dev. 2020, 29, 1520–1532. [Google Scholar] [CrossRef]
- Porzionato, A.; Zaramella, P.; Dedja, A.; Guidolin, D.; Wemmel, K.V.; Macchi, V.; Jurga, M.; Perilongo, G.; Caro, R.D.; Baraldi, E.; et al. Intratracheal Administration of Clinical-Grade Mesenchymal Stem Cell-Derived Extracellular Vesicles Reduces Lung Injury in a Rat Model of Bronchopulmonary Dysplasia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L6–L19. [Google Scholar] [CrossRef]
- Porzionato, A.; Zaramella, P.; Dedja, A.; Guidolin, D.; Bonadies, L.; Macchi, V.; Pozzobon, M.; Jurga, M.; Perilongo, G.; Caro, R.D.; et al. Intratracheal Administration of Mesenchymal Stem Cell-Derived Extracellular Vesicles Reduces Lung Injuries in a Chronic Rat Model of Bronchopulmonary Dysplasia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 320, L688–L704. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Kim, Y.E.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Vascular Endothelial Growth Factor Mediates the Therapeutic Efficacy of Mesenchymal Stem Cell-Derived Extracellular Vesicles against Neonatal Hyperoxic Lung Injury. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Li, Z.; Gong, X.; Li, D.; Yang, X.; Shi, Q.; Ju, X. Intratracheal Transplantation of Amnion-Derived Mesenchymal Stem Cells Ameliorates Hyperoxia-Induced Neonatal Hyperoxic Lung Injury via Aminoacyl-Peptide Hydrolase. Int. J. Stem Cells 2020, 13, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wang, C.; Zhang, J.; Zhang, J.; Gu, Y.; Guo, X.; Zuo, X.; Pan, H.; Hsu, A.C.-Y.; Wang, G.; et al. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells Attenuate the Inflammation of Severe Steroid-Resistant Asthma by Reshaping Macrophage Polarization. Stem Cell Res. Ther. 2021, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Kadota, T.; Fujita, Y.; Araya, J.; Watanabe, N.; Fujimoto, S.; Kawamoto, H.; Minagawa, S.; Hara, H.; Ohtsuka, T.; Yamamoto, Y.; et al. Human Bronchial Epithelial Cell-Derived Extracellular Vesicle Therapy for Pulmonary Fibrosis via Inhibition of TGF-β-WNT Crosstalk. J. Extracell. Vesicles 2021, 10, e12124. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wu, L.; Liu, M.; Zhu, L.; Chen, Y.; Zhou, H.; Shi, X.; Wei, J.; Zheng, L.; Hu, X.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Attenuate LPS-Induced ARDS by Modulating Macrophage Polarization Through Inhibiting Glycolysis in Macrophages. Shock 2020, 54, 828–843. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Wang, X.; Groot, M.; Sharma, L.; dela Cruz, C.S.; Jin, Y. A Potential Role of Microvesicle-Containing MiR-223/142 in Lung Inflammation. Thorax 2019, 74, 865–874. [Google Scholar] [CrossRef]
- Li, J.W.; Wei, L.; Han, Z.; Chen, Z. Mesenchymal Stromal Cells-Derived Exosomes Alleviate Ischemia/Reperfusion Injury in Mouse Lung by Transporting Anti-Apoptotic MiR-21-5p. Eur. J. Pharmacol. 2019, 852, 68–76. [Google Scholar] [CrossRef]
- Zhu, Z.; Lian, X.; Su, X.; Wu, W.; Zeng, Y.; Chen, X. Exosomes Derived from Adipose-Derived Stem Cells Alleviate Cigarette Smoke-Induced Lung Inflammation and Injury by Inhibiting Alveolar Macrophages Pyroptosis. Respir. Res. 2022, 23, 5. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, Y.; Xu, J.; Ge, X.; Hu, Z.; Xiao, J.; Ning, Y.; Dong, Y.; Bai, C. Exosomes from Mmu_circ_0001359-Modified ADSCs Attenuate Airway Remodeling by Enhancing FoxO1 Signaling-Mediated M2-like Macrophage Activation. Mol. Ther. Nucleic Acids 2020, 19, 951–960. [Google Scholar] [CrossRef]
- Kim, G.; Lee, Y.; Ha, J.; Han, S.; Lee, M. Engineering Exosomes for Pulmonary Delivery of Peptides and Drugs to Inflammatory Lung Cells by Inhalation. J. Control. Release 2021, 330, 684–695. [Google Scholar] [CrossRef]
- el Baradie, K.B.Y.; Nouh, M.; O’Brien, F.; Liu, Y.; Fulzele, S.; Eroglu, A.; Hamrick, M.W. Freeze-Dried Extracellular Vesicles From Adipose-Derived Stem Cells Prevent Hypoxia-Induced Muscle Cell Injury. Front. Cell Dev. Biol. 2020, 8, 181. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, H.J.; Kim, W.; Kim, O.S.; Lee, S.; Han, S.Y.; Jeong, E.J.; Park, H.S.; Kim, H.W.; Moon, K.S. Tumorigenicity Evaluation of Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Toxicol. Res. 2016, 32, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.T.; Lai, R.C.; Padmanabhan, J.; Sim, W.K.; Choo, A.B.H.; Lim, S.K. Assessment of Tumorigenic Potential in Mesenchymal-Stem/Stromal-Cell-Derived Small Extracellular Vesicles (MSC-SEV). Pharmaceuticals 2021, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of Tumorigenicity in Mesenchymal Stromal Cell-Based Therapies--Bridging Scientific Observations and Regulatory Viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Røsland, G.V.; Svendsen, A.; Torsvik, A.; Sobala, E.; McCormack, E.; Immervoll, H.; Mysliwietz, J.; Tonn, J.C.; Goldbrunner, R.; Lønning, P.E.; et al. Long-Term Cultures of Bone Marrow-Derived Human Mesenchymal Stem Cells Frequently Undergo Spontaneous Malignant Transformation. Cancer Res. 2009, 69, 5331–5339. [Google Scholar] [CrossRef]
- He, J.; Yao, X.; Mo, P.; Wang, K.; Yang, Z.; Tian, N.; Zhu, X.; Zhao, J.; Pang, R.; Ruan, G.; et al. Lack of Tumorigenesis and Protumorigenic Activity of Human Umbilical Cord Mesenchymal Stem Cells in NOD SCID Mice. BMC Cancer 2022, 22, 307. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, S.A.; Abdul-Hafez, A.; Ibrahim, S.; Pillai, N.; Abdulmageed, M.; Thiruvenkataramani, R.P.; Mohamed, T.; Madhukar, B.V.; Uhal, B.D. Stem-Cell Therapy for Bronchopulmonary Dysplasia (BPD) in Newborns. Cells 2022, 11, 1275. https://doi.org/10.3390/cells11081275
Omar SA, Abdul-Hafez A, Ibrahim S, Pillai N, Abdulmageed M, Thiruvenkataramani RP, Mohamed T, Madhukar BV, Uhal BD. Stem-Cell Therapy for Bronchopulmonary Dysplasia (BPD) in Newborns. Cells. 2022; 11(8):1275. https://doi.org/10.3390/cells11081275
Chicago/Turabian StyleOmar, Said A., Amal Abdul-Hafez, Sherif Ibrahim, Natasha Pillai, Mohammed Abdulmageed, Ranga Prasanth Thiruvenkataramani, Tarek Mohamed, Burra V. Madhukar, and Bruce D. Uhal. 2022. "Stem-Cell Therapy for Bronchopulmonary Dysplasia (BPD) in Newborns" Cells 11, no. 8: 1275. https://doi.org/10.3390/cells11081275
APA StyleOmar, S. A., Abdul-Hafez, A., Ibrahim, S., Pillai, N., Abdulmageed, M., Thiruvenkataramani, R. P., Mohamed, T., Madhukar, B. V., & Uhal, B. D. (2022). Stem-Cell Therapy for Bronchopulmonary Dysplasia (BPD) in Newborns. Cells, 11(8), 1275. https://doi.org/10.3390/cells11081275






