Assessment of BPV-1 Mediated Matrix Metalloproteinase Genes Deregulation in the In Vivo and In Vitro Models Designed to Explore Molecular Nature of Equine Sarcoids
Abstract
:1. Introduction
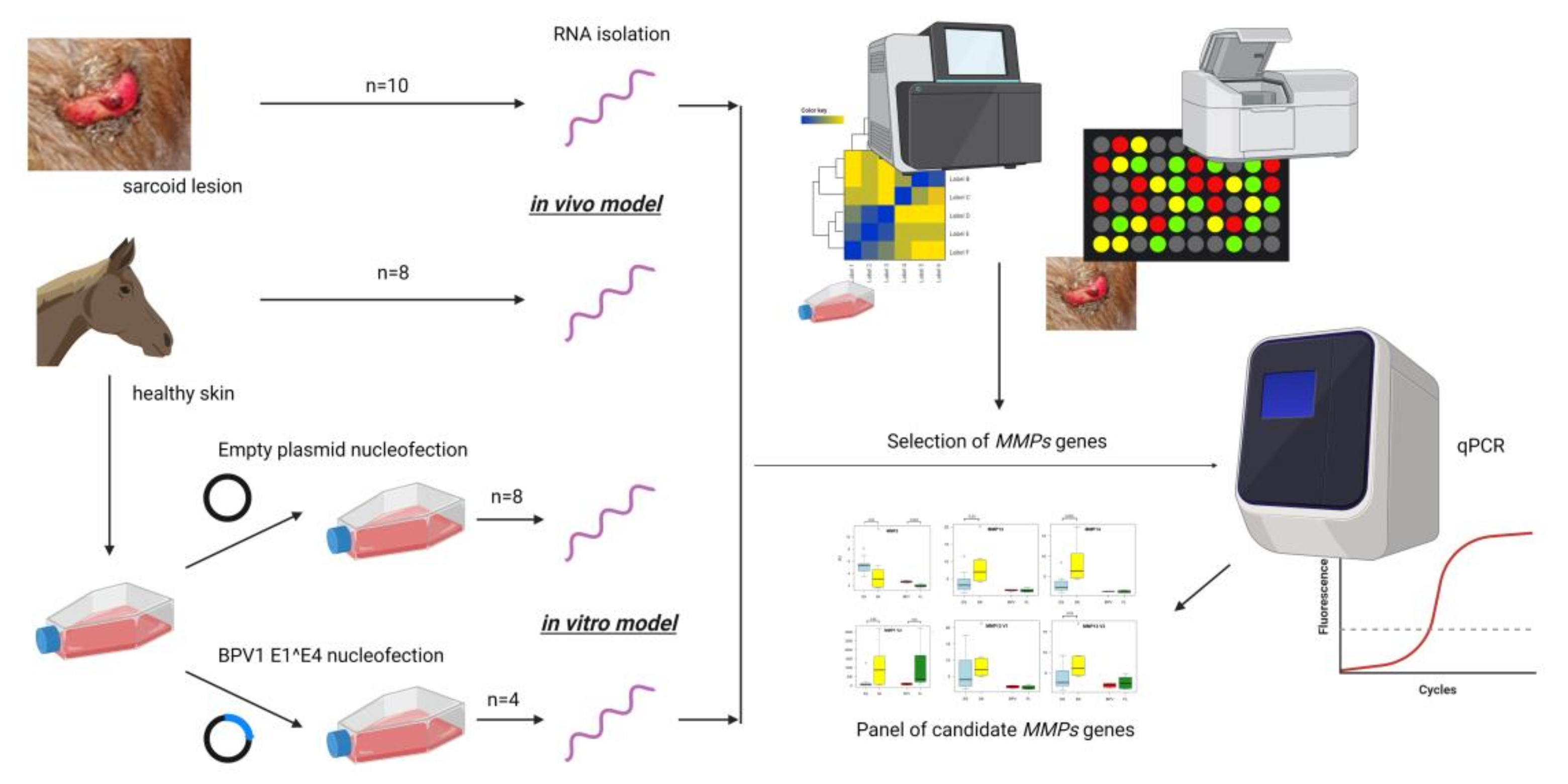
2. Materials and Methods
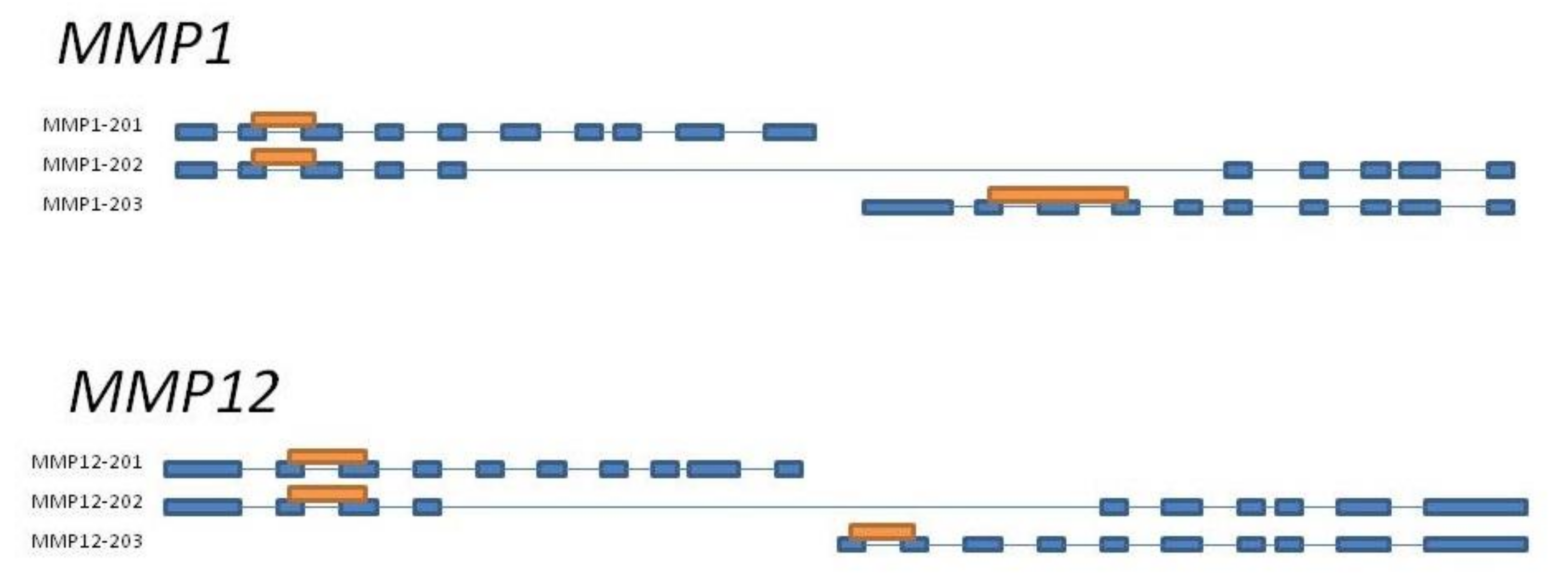
2.1. MMP Genes Selection
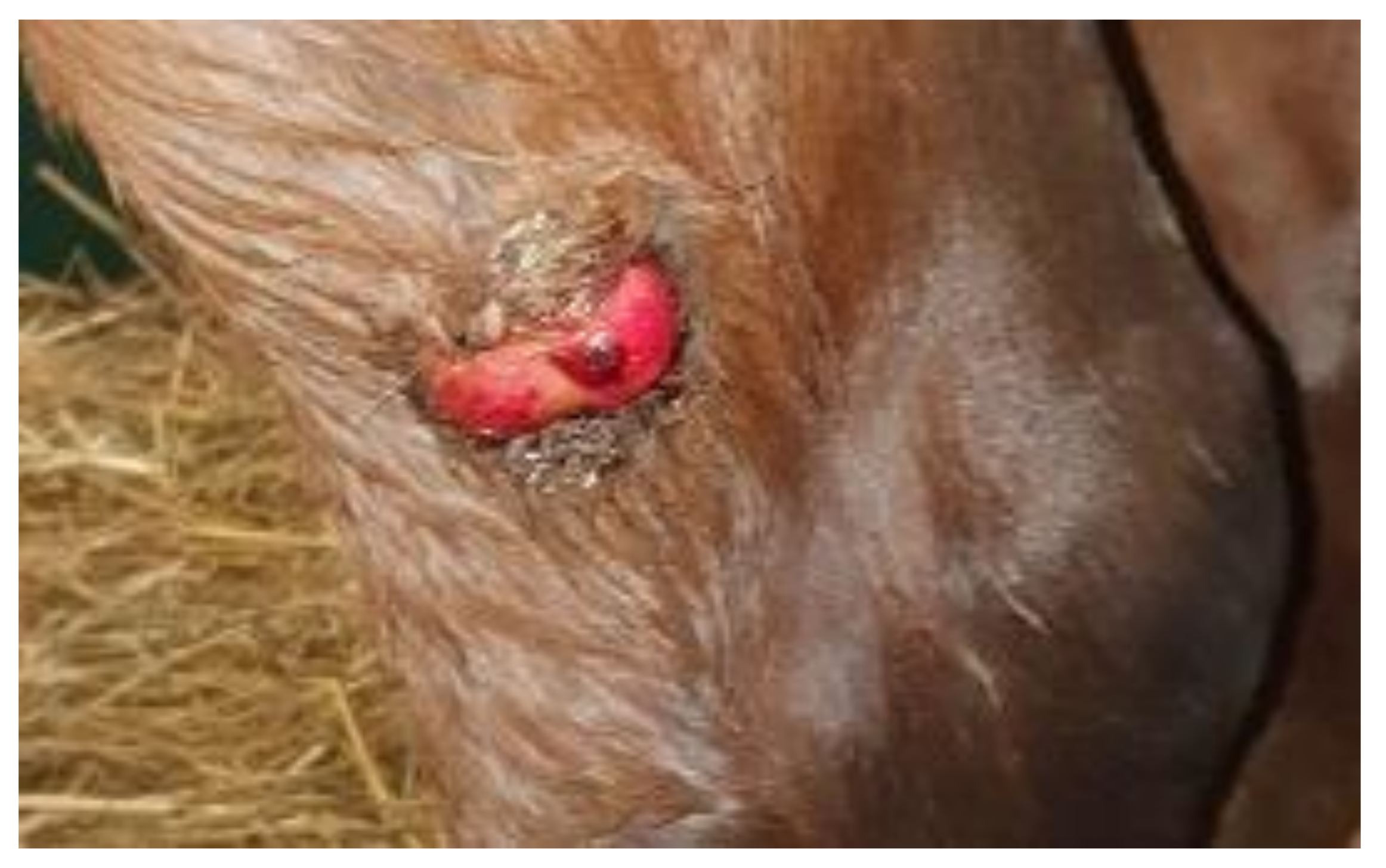
2.2. Sample Preparation
2.3. Quantitative PCR Analysis
2.4. Statistical Analysis
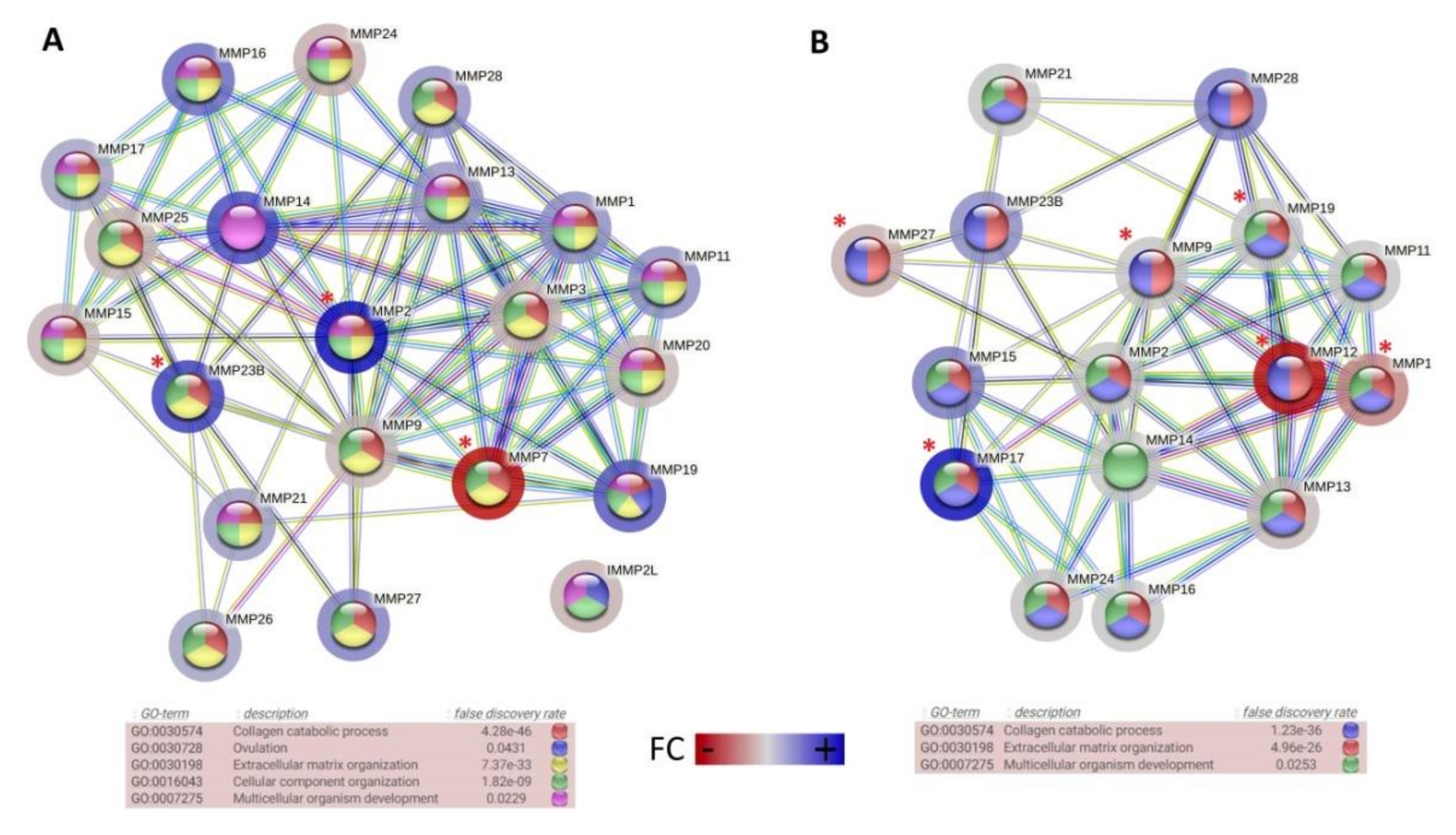
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A Challenging Paradigm of Cancer Management. Semin. Cancer Biol. 2019, 56, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Clutterbuck, A.L.; Harris, P.; Allaway, D.; Mobasheri, A. Matrix Metalloproteinases in Inflammatory Pathologies of the Horse. Vet. J. 2010, 183, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix Metalloproteinases Participation in the Metastatic Process and Their Diagnostic and Therapeutic Applications in Cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix Metalloproteinases and Tumor Metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Knottenbelt, D.C. A Suggested Clinical Classification for the Equine Sarcoid. Clin. Tech. Equine Pract. 2005, 4, 278–295. [Google Scholar] [CrossRef]
- Nasir, L.; Reid, S.W.J. Bovine Papillomaviral Gene Expression in Equine Sarcoid Tumours. Virus Res. 1999, 61, 171–175. [Google Scholar] [CrossRef]
- Lunardi, M.; de Alcântara, B.K.; Otonel, R.A.A.; Rodrigues, W.B.; Alfieri, A.F.; Alfieri, A.A. Bovine Papillomavirus Type 13 DNA in Equine Sarcoids. J. Clin. Microbiol. 2013, 51, 2167–2171. [Google Scholar] [CrossRef] [Green Version]
- Semik, E.; Gurgul, A.; Ząbek, T.; Ropka-Molik, K.; Koch, C.; Mählmann, K.; Bugno-Poniewierska, M. Transcriptome Analysis of Equine Sarcoids. Vet. Comp. Oncol. 2017, 15, 1370–1381. [Google Scholar] [CrossRef]
- Podstawski, P.; Samiec, M.; Skrzyszowska, M.; Szmatoła, T.; Semik-Gurgul, E.; Ropka-Molik, K. The Induced Expression of BPV E4 Gene in Equine Adult Dermal Fibroblast Cells as a Potential Model of Skin Sarcoid-like Neoplasia. Int. J. Mol. Sci. 2022, 23, 1970. [Google Scholar] [CrossRef]
- BioRender. Available online: https://biorender.com/ (accessed on 31 March 2022).
- Teifke, J.P.; Kidney, B.A.; Löhr, C.V.; Yager, J.A. Detection of Papillomavirus-DNA in Mesenchymal Tumour Cells and Not in the Hyperplastic Epithelium of Feline Sarcoids. Vet. Dermatol. 2003, 14, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Podstawski, P.; Witarski, W.; Szmatoła, T.; Bugno-Poniewierska, M.; Ropka-Molik, K. Mobility and Invasion Related Gene Expression Patterns in Equine Sarcoid. Animals 2020, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of Stable Housekeeping Genes, Differentially Regulated Target Genes and Sample Integrity: BestKeeper—Excel-Based Tool Using Pair-Wise Correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, L.; Van Poucke, M.; De Baere, C.; Peelman, L.; Gasthuys, F.; Martens, A. Selection of a Set of Reliable Reference Genes for Quantitative Real-Time PCR in Normal Equine Skin and in Equine Sarcoids. BMC Biotechnol. 2006, 6, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 21 January 2022).
- String: Functional Protein Association Networks. Available online: https://string-db.org/ (accessed on 21 January 2022).
- Martano, M.; Corteggio, A.; Restucci, B.; De Biase, M.E.; Borzacchiello, G.; Maiolino, P. Extracellular Matrix Remodeling in Equine Sarcoid: An Immunohistochemical and Molecular Study. BMC Vet. Res. 2016, 12, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- English, W.R.; Puente, X.S.; Freije, J.M.P.; Knäuper, V.; Amour, A.; Merryweather, A.; López-Otín, C.; Murphy, G. Membrane Type 4 Matrix Metalloproteinase (MMP17) Has Tumor Necrosis Factor-α Convertase Activity but Does Not Activate Pro-MMP2. J. Biol. Chem. 2000, 275, 14046–14055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Z.Q.; Gault, E.A.; Gobeil, P.; Nixon, C.; Campo, M.S.; Nasir, L. Establishment and Characterization of Equine Fibroblast Cell Lines Transformed in Vivo and in Vitro by BPV-1: Model Systems for Equine Sarcoids. Virology 2008, 373, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Köhrmann, A.; Kammerer, U.; Kapp, M.; Dietl, J.; Anacker, J. Expression of Matrix Metalloproteinases (MMPs) in Primary Human Breast Cancer and Breast Cancer Cell Lines: New Findings and Review of the Literature. BMC Cancer 2009, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Zarrabi, K.; Dufour, A.; Li, J.; Kuscu, C.; Pulkoski-Gross, A.; Zhi, J.; Hu, Y.; Sampson, N.S.; Zucker, S.; Cao, J. Inhibition of Matrix Metalloproteinase 14 (MMP-14)-Mediated Cancer Cell Migration. J. Biol. Chem. 2011, 286, 33167–33177. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Oshima, T.; Yoshihara, K.; Tamura, S.; Kanazawa, A.; Inagaki, D.; Yamamoto, N.; Sato, T.; Fujii, S.; Numata, K.; et al. Overexpression of MMP-13 Gene in Colorectal Cancer with Liver Metastasis. Anticancer Res. 2010, 30, 2693–2699. [Google Scholar]
- Ameli, F.; Ghafourina Nassab, F.; Masir, N.; Kahtib, F. Tumor-Derived Matrix Metalloproteinase-13 (MMP-13) Expression in Benign and Malignant Breast Lesions. Asian Pac. J. Cancer Prev. 2021, 22, 2603–2609. [Google Scholar] [CrossRef] [PubMed]

| Gene | Accesion Number | Primers | Product Length [bp] | Localisation | Splice Variants | Splice Variants Accesion Number |
|---|---|---|---|---|---|---|
| MMP1 V1V2 | ENSECAG00000023733 | F: GCTGAAAGTGACTGGGAAGC R: GGTCCACATCTGCTCTTGGT | 179 | Exon 2 and 3 | 2/3 | ENSECAT00000022856.2 ENSECAT00000067257.1 |
| MMP1 V3 | ENSECAG00000023733 | F: CCCAAGTGGGAACGAAATAA R: ATTCCATTGGGTCCATCAAA | 224 | Exon 12 and 14 | 1/3 | ENSECAT00000025715.2 |
| MMP2 | ENSECAG00000000953 | F: TCCCTTTCCTCTTCAACGGC R: CCGTATTTGCCGTCCTTGTC | 112 | Exon 4 | 2/2 | ENSECAT00000044147.1 ENSECAT00000004278.3 |
| MMP9 | ENSECAG00000013081 | F: CGTGTTTCCCTTCACCTTCG R: GGTCGTAGTTGGCGGTAGT | 141 | Exon 6 | 3/3 | ENSECAT00000056131.1 ENSECAT00000014089.3 ENSECAT00000068636.1 |
| MMP12 V1V2 | ENSECAG00000019445 | F: TGGACATGATGCACAGACCT R: CAGCCTCGCCTGTGTTAATC | 222 | Exon 2 and 3 | 2/3 | ENSECAT00000002326.3 ENSECAT00000077256.1 |
| MMP12 V3 | ENSECAG00000019445 | F:GATCTGCAAGGGACGAGGAT R:CACCTCCACAGTGTCAGAGT | 194 | Exon 11 and 12 | 1/3 | ENSECAT00000020742.2 |
| MMP13 | ENSECAG00000005506 | F:GCTCCGAGAAATGCAGTCTT R: GCCATTTGAGAGTTCGAGGG | 140 | Exon 2 | 1/1 | ENSECAT00000007039.2 |
| MMP14 | ENSECAG00000008351 | F: CATGATCTTCTTCGCTGAGGG R: GGTGTCGCCTCCAATGTTG | 109 | Exon 4 | 1/1 | ENSECAT00000008899.3 |
| MMP17 | ENSECAG00000013201 | F: CACTACGCCCTCAAAGTCTG R: AGGGATAGCGGTCATTGTGG | 118 | Exon 4 | 2/2 | ENSECAT00000013924.2 ENSECAT00000013881.2 |
| MMP27 | ENSECAG00000019404 | F: CGGTGACTGGAAAACTGGAT R: CCCTTGGAAATCTTGGTGAA | 238 | Exon 2 and 3 | 1/1 | ENSECAT00000020635.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podstawski, P.; Ropka-Molik, K.; Semik-Gurgul, E.; Samiec, M.; Skrzyszowska, M.; Podstawski, Z.; Szmatoła, T.; Witkowski, M.; Pawlina-Tyszko, K. Assessment of BPV-1 Mediated Matrix Metalloproteinase Genes Deregulation in the In Vivo and In Vitro Models Designed to Explore Molecular Nature of Equine Sarcoids. Cells 2022, 11, 1268. https://doi.org/10.3390/cells11081268
Podstawski P, Ropka-Molik K, Semik-Gurgul E, Samiec M, Skrzyszowska M, Podstawski Z, Szmatoła T, Witkowski M, Pawlina-Tyszko K. Assessment of BPV-1 Mediated Matrix Metalloproteinase Genes Deregulation in the In Vivo and In Vitro Models Designed to Explore Molecular Nature of Equine Sarcoids. Cells. 2022; 11(8):1268. https://doi.org/10.3390/cells11081268
Chicago/Turabian StylePodstawski, Przemysław, Katarzyna Ropka-Molik, Ewelina Semik-Gurgul, Marcin Samiec, Maria Skrzyszowska, Zenon Podstawski, Tomasz Szmatoła, Maciej Witkowski, and Klaudia Pawlina-Tyszko. 2022. "Assessment of BPV-1 Mediated Matrix Metalloproteinase Genes Deregulation in the In Vivo and In Vitro Models Designed to Explore Molecular Nature of Equine Sarcoids" Cells 11, no. 8: 1268. https://doi.org/10.3390/cells11081268
APA StylePodstawski, P., Ropka-Molik, K., Semik-Gurgul, E., Samiec, M., Skrzyszowska, M., Podstawski, Z., Szmatoła, T., Witkowski, M., & Pawlina-Tyszko, K. (2022). Assessment of BPV-1 Mediated Matrix Metalloproteinase Genes Deregulation in the In Vivo and In Vitro Models Designed to Explore Molecular Nature of Equine Sarcoids. Cells, 11(8), 1268. https://doi.org/10.3390/cells11081268






